"Shping 2" different cellular localizations - a potential new player in aging processes
Abstract
The functions of the ubiquitously expressed protein tyrosine phosphatase Shp-2 are dependent on its localization. Cytosolic Shp-2 is known to modulate different pathways involved in cell growth, cell development, tissue inflammation and cellular chemotaxis. But Shp-2 is also localized in the nucleus and the mitochondria. Nuclear Shp-2 forms a complex with the signal transducer and activator of transcription 5 (STAT5) which then binds to DNA and regulates transcription of milk genes. In contrast, nuclear Shp-2 dephosphorylates STAT1 and thereby inhibits gene transcription. In addition, it counteracts the oxidative stress dependent nuclear export of Telomerase Reverse Transcriptase (TERT) mediated by members of the Src kinase family, a process leading to replicative senescence. For the recently found mitochondrial Shp-2 an involvement in the regulation of the cellular redox balance is discussed. Shp-2 shows the ability to regulate reactive oxygen species formation in the mitochondria. There are hints that mitochondrial Shp-2 and Src are involved in the regulation of respiratory chain activity. Since a substantial fraction of TERT has been found in the mitochondria, it is hypothesized that mitochondrial Shp-2 acts as a positive regulator of TERT in the mitochondria, similar to its nuclear role. Taken together, Shp-2 seems to be a new player in aging processes.
Shp-2 is a ubiquitously expressed protein tyrosine
phosphatase, which contains two N-terminal Src homology 2 (SH2) domains and a
C-terminal protein tyrosine phosphatase domain. Several years of research
established an important role for cytosolic Shp-2. It is known to modulate
different pathways involved in cell growth, cell development, tissue
inflammation and cellular chemotaxis due to its well described function to
dephosphorylate receptor tyrosine kinases (reviewed in [1]). However, over the
last years it has become clear that Shp-2 is also localized in the nucleus and
in the mitochondria where it exerts different functions.
In 2002 Chughtai et al
reported a nuclear localization of Shp-2
associated with the signal transducer and activator of transcription 5 (STAT5). The stimulation of mammary
cells with prolactin induced the nuclear translocation of Shp-2 in a complex
with STAT5. Formation of this complex and tyrosine phosphorylation of STAT5 in
response to prolactin requires the SH2 domain closer to the C-terminus and the
catalytic activity of Shp-2. The authors speculated that the nuclear
Shp-2/STAT5 complex binds to DNA and regulates transcription of milk protein
genes [2], demonstrating a transcriptional regulation by nuclear Shp-2. This
provided for the first time evidence for a function of Shp-2 besides
dephosphorylation. In contrast, it has been demonstrated that Shp-2
dephosphorylates STAT1 at tyrosine and serine residues in the nucleus and
thereby inhibits its transcriptional activity
[3]. One may speculate that depending on the mode of its action Shp-2
differently regulates specific STAT proteins. Just recently, we discovered that
nuclear Shp-2 seems to be involved in aging processes. Previous findings from
our group demonstrated that the enzyme Telomerase Reverse Transcriptase
(TERT), which is important for maintaining telomere length and known to delay
aging processes, when overexpressed, is tyrosine phosphorylated by Src kinases
in the nucleus under conditions of oxidative stress in several cell types,
including endothelial cells [4,5]. This tyrosine phosphorylation triggers
nuclear export of TERT. Taking into account that cytosolic Shp-2 and the
cytosolic Src kinase family can regulate and antagonize each other under
certain conditions, we hypothesized that a nuclear Shp-2 also exists in
endothelial cells and that this may counteract the Src kinase dependent nuclear
export of TERT. Indeed, ablation of endogenous Shp-2 results in increased
tyrosine phosphorylation of nuclear TERT and a reduction of telomerase activity
in the nucleus. Moreover, overexpression of Shp-2 inhibited oxidative stress
induced tyrosine phosphorylation and export of TERT from the nucleus. It has to
be noted that this process requires the catalytic activity of Shp-2, since the
catalytically inactive mutant Shp-2(C459S) can not pre- vent nuclear export of TERT.
Interestingly, overexpression of Shp-2(C459S) reduced nuclear telomerase
activity already under basal conditions. This effect was dependent on tyrosine
707 in TERT [6]. One possible explanation for the nuclear export of TERT
induced by Shp-2(C459S) under basal conditions could be the significant
increase in reactive oxygen species (ROS), which are known to activate the Src
kinase family. Indeed, ROS formation is enhanced upon overexpression of
Shp-2(C459S) in endothelial cells (Figure 1). These data point to a regulatory
role of Shp-2 in the redox balance of cells.
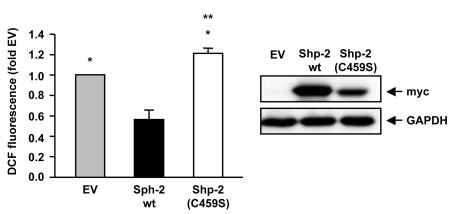
Figure 1. Shp-2 reduces endogenous ROS formation.
Endothelial cells were transfected with empty vector (EV), Shp-2 wt or
Shp-2(C459S) and endogenous ROS formation was measured using FACS analysis.
*p<0.05 versus Shp-2 wt. **p<0.05 versus EV. Data are means +/- SEM
(n=6).
The Western blot on the right demonstrates expression of Shp-2 wt and Shp-2(C459S), which were detected with anti-myc antibody.
ROS are important signalling molecules for cellular
signal transduction. An imbalance of the redox status with a reduced
antioxidative capacity and an increased ROS production has been described to
play an important role in aging processes as well as in several diseases.
Increased ROS can directly damage DNA, proteins and membrane lipids. This leads
among others to damage of the electron transport chain, which results in an
increased formation of ROS which in turn cause further damage to DNA, proteins and
lipids. This vicious cycle seems to play an important role in aging processes
and age-related diseases (for review see [7]).
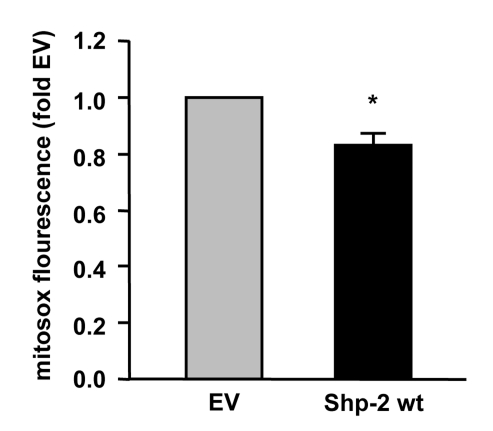
Figure 2.
Shp-2 reduces endogenous mitochondrial ROS formation. Endothelial cells
were transfected with empty vector (EV) and Shp-2 wt. Mitochondrial ROS
formation was measured using mitosox and FACS analysis. *p<0.05 versus
EV. Data are means +/- SEM (n=3).
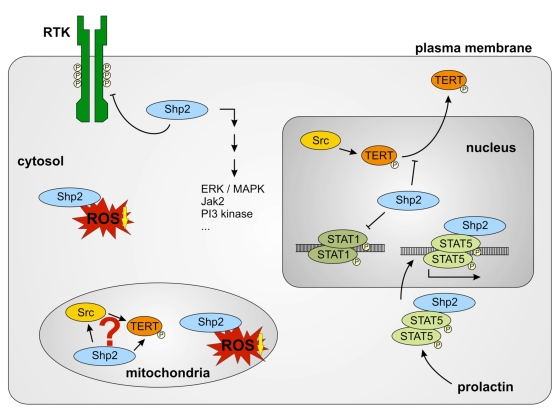
Figure 3.
Different functions of Shp-2 in different cell compartments. Cytosolic
Shp-2 modulates different pathways by dephosphorylation of receptor
tyrosine kinases (RTK). It also decreases cytosolic ROS levels. Nuclear
Shp-2 inhibits ROS induced nuclear export of TERT and DNA-binding of STAT1
dimers by dephosphorylation. Prolactin induces the association of
Shp-2 and STAT5 and nuclear import of this complex. Shp-2/STAT5 complex
binds to DNA and induces transcription of milk genes. Functions of
mitochondrial Shp-2 remain unclear. A connection between mitochondrial Src,
Shp-2 and TERT may exist. Reduction of mitochondrial ROS formation seems to
depend on Shp-2.
Therefore, controlling ROS formation seems to be an
interesting tool in delaying aging processes. It is tempting to speculate that
nuclear Shp-2 plays an important role in nuclear based aging processes by
reducing export of TERT from the nucleus and by reducing ROS formation (figure
1). However, we have also new hints, that Shp-2 may affect mitochondrial ROS
production and thus, aging processes which depend on reduced mitochondrial
function. New data from our laboratory demonstrate that overexpression of Shp-2
decreases not only ROS production in the cytosol (Figure 1) but also in the
mitochondria (Figure 2). Moreover, preliminary results suggest that ablation of
Shp-2 increases mitochondrial ROS levels. To specifically measure mitochondrial
ROS levels, we used mitosox, a redox-sensitive dye, which first has to enter
mitochondria before it can react with ROS. One can speculate, that the observed
reduction of mitochondrially derived ROS is connected to a localization of
Shp-2 in the mitochondria. Indeed, Salvi et al detected a tyrosine phosphatase
activity in the mitochondria of rat brains and identified the responsible
phosphatase as Shp-2 [8]. Recently, Arachiche et al showed also the
mitochondrial localization of Shp-2 and of the tyrosine kinase Src, which is
regulated by Shp-2 [9]. They demonstrated that the complexes of the respiratory
chain are substrates of Src, which indicates that respiratory chain activity is
partially dependent on tyrosine phosphorylation. Since Shp-2 is an important
regulator of Src, Shp-2 is possibly involved in regulation of mitochondrial
activity. In line with these findings, we recently demonstrated that TERT is
localized in the mitochondria and importantly contributes to respiratory chain
activity [10]. TERT deficient mice derived from heterozygous breeding pairs,
which show no reduction in telomere length and thus no premature aging
phenotype, demonstrated reduced respiratory chain activity in the heart,
suggesting an important role for TERT in respiration in vivo [10]. Given the
facts that Src kinase family members as well as Shp-2 show mitochondrial
localization [9], it is tempting to speculate that similar to nuclear TERT also
mitochondrial TERT is positively regulated by Shp-2 in these organelles. This
could implicate that mitochondrial Shp-2 in concert with TERT accounts for an
intact respiratory chain activity and for reduced mitochondrial ROS formation.
Therefore, mitochondrial Shp-2 and TERT could break the above mentioned vicious
cycle and thereby may delay aging processes, which depend on mitochondrial
dysfunction.
In summary, Shp-2 has the
potential to be a yet unknown new important key player in aging processes. Its
regulatory function seems to be dependent on its localization within the cell
(Figure 3). Nuclear localized Shp-2 counteracts replicative senescence induced
by nuclear TERT export and mitochondrial Shp-2 could delay aging processes
induced by elevated ROS levels. Therefore, Shp-2 could be an important target
for the therapy of diseases connected to aging processes. However, therapeutic
interventions aimed at the activation of Shp-2 should take into account the compartment
specific functions of this protein.
Acknowledgments
This work was supported by Deutsche Forschungs-gemeinschaft SFB 728 B5 and
HA2868/2-3 to J.H.
Conflicts of Interest
The
authors of this manuscript have no conflict of interests to declare.
References
-
1.
Chong
ZZ
and Maiese
K.
The Src homology 2 domain tyrosine phosphatases SHP-1 and SHP-2: diversified control of cell growth, inflammation, and injury.
Histol Histopathol.
2007;
22:
1251
-1267.
[PubMed]
.
-
2.
Chughtai
N
, Schimchowitsch
S
, Lebrun
JJ
and Ali
S.
Prolactin induces SHP-2 association with Stat5, nuclear translocation, and binding to the beta-casein gene promoter in mammary cells.
J Biol Chem.
2002;
277:
31107
-31114.
[PubMed]
.
-
3.
Wu
TR
, Hong
YK
, Wang
XD
, Ling
MY
, Dragoi
AM
, Chung
AS
, Campbell
AG
, Han
ZY
, Feng
GS
and Chin
YE.
SHP-2 is a dual-specificity phosphatase involved in Stat1 dephosphorylation at both tyrosine and serine residues in nuclei.
J Biol Chem.
2002;
277:
47572
-47580.
[PubMed]
.
-
4.
Haendeler
J
, Hoffmann
J
, Diehl
JF
, Vasa
M
, Spyridopoulos
I
, Zeiher
AM
and Dimmeler
S.
Antioxidants inhibit nuclear export of telomerase reverse transcriptase and delay replicative senescence of endothelial cells.
Circ Res.
2004;
94:
768
-775.
[PubMed]
.
-
5.
Haendeler
J
, Hoffmann
J
, Rahman
S
, Zeiher
AM
and Dimmeler
S.
Regulation of telomerase activity and anti-apoptotic function by protein-protein interaction and phosphorylation.
FEBS Lett.
2003;
536:
180
-186.
[PubMed]
.
-
6.
Jakob
S
, Schroeder
P
, Lukosz
M
, Büchner
N
, Spyridopoulos
I
, Altschmied
J
and Haendeler
J.
Nuclear protein tyrosine phosphatase Shp-2 is one important negative regulator of nuclear export of telomerase reverse transcriptase.
J Biol Chem.
2008;
283:
33155
-33161.
[PubMed]
.
-
7.
Mandavilli
BS
, Santos
JH
and Van
Houten B.
Mitochondrial DNA repair and aging.
Mutat Res.
2002;
509:
127
-151.
[PubMed]
.
-
8.
Salvi
M
, Stringaro
A
, Brunati
AM
, Agostinelli
E
, Arancia
G
, Clari
G
and Toninello
A.
Tyrosine phosphatase activity in mitochondria: presence of Shp-2 phosphatase in mitochondria.
Cell Mol Life Sci.
2004;
61:
2393
-2404.
[PubMed]
.
-
9.
Arachiche
A
, Augereau
O
, Decossas
M
, Pertuiset
C
, Gontier
E
, Letellier
T
and Dachary-Prigent
J.
Localization of PTP-1B, SHP-2, and Src exclusively in rat brain mitochondria and functional consequences.
J Biol Chem.
2008;
283:
24406
-24411.
[PubMed]
.
-
10.
Haendeler
J
, Dröse
S
, Büchner
N
, Jakob
S
, Altschmied
J
, Goy
C
, Spyridopoulos
I
, Zeiher
AM
, Brandt
U
and Dimmeler
S.
Mitochondrial telomerase reverse transcriptase binds to and protects mitochondrial DNA and function from damage.
Arterioscler Thromb Vasc Biol.
2009;
29:
929
-935.
[PubMed]
.