Human insulin/IGF-1 and familial longevity at middle age
Abstract
Recently, we have shown that compared to controls, long-lived familial nonagenarians (mean age: 93.4 years) from the Leiden Longevity Study displayed a lower mortality rate, and their middle-aged offspring displayed a lower prevalence of cardio-metabolic diseases, including diabetes mellitus. The evolutionarily conserved insulin/IGF-1 signaling (IIS) pathway has been implicated in longevity in model organisms, but its relevance for human longevity has generated much controversy. Here, we show that compared to their partners, the offspring of familial nonagenarians displayed similar non-fasted serum levels of IGF-1, IGFBP3 and insulin but lower non-fasted serum levels of glucose, indicating that familial longevity is associated with differences in insulin sensitivity.
Introduction
In Western societies, life expectancy has
increased dramatically over the last century, but striking inter-individual
differences in life expectancy remain [1]. Ample
evidence has shown that healthy longevity is determined by a mix of genetic,
environmental and chance elements. Because the odds of exceptional longevity
runs in families, we designed the Leiden Longevity Study [2]. Recently,
we have shown that the nonagenarian siblings included in the Leiden Longevity
Study displayed a 41% lower risk of mortality compared to sporadic nonagenarians
[3]. Moreover, compared
to their partners, the offspring of nonagenarian
siblings displayed a significantly lower prevalence of myocardial infarction, hypertension and diabetes mellitus [3]. The
differences in clinical phenotype observed after selection for familial
longevity are in line with the lower prevalence of cardio-metabolic disease
previously detected when offspring from sporadic centenarians were compared to
offspring of parents who had died at average age [4] and when
offspring from sporadic centenarians were compared to their partners [5]. Moreover,
the observed lower mortality rate at high ages and better preservation of health
at middle age indicates that resilience against disease and death may have
similar underlying biological mechanisms that are influenced by genetic or
familial factors.
Of the genetically determined pathways that have been
implicated in longevity in a variety of different model organisms, the evolutionarily conserved insulin/IGF-1 signaling (IIS) pathway clearly stands out in current
literature (reviewed in [6]). Mutations
in IIS components were first found to affect reproduction, metabolism, stress
response and life span in C. elegans (reviewed in [7]). The link
between reduced IIS signaling and longevity was subsequently also observed in D.
melanogaster. Mutants in the D. melanogaster insulin receptorInR [8] and
in the insulin receptor substrate CHICO [9] are both
long-lived. Strikingly however, in both cases the long-lived phenotype was only
observed for females. In addition to being long-lived, these D. melanogaster
females are small, obese and infertile. In mice, selective disruption of the
insulin receptor in the adipose tissue leads to a reduction in fat mass and
extended longevity [10]. Increases
in lifespan were also reported in mice with deletion of insulin receptor
substrate 1 (IRS1) in whole body [11] or IRS2
only in the brain [12]. Moreover,
dwarf mice exhibiting GH deficiency
or resistance, including Prop1df/d[13], Pit1dw/dw[14], GHRHRlit/lit[14] and GHR-/-[15] all display
hypoinsulinemia and enhanced insulin sensitivity along with extended longevity.
In mice heterozygous for igf1r deletion (Igf1r+/-[16]) or
containing a hypomorphic igf1r mutation (Midi mice [11]), only
females, but not males, exhibited the long-lived phenotype.
Based on the similarities among the
insulin/IGF-1 pathways in animals and humans, the possibility that
modifications in the insulin/IGF-I signaling system could also extend lifespan
in humans has been suggested. However, separating the roles of insulin and
IGF-1 in mammals and their relevance for human healthy longevity has been
difficult and generated much controversy. In humans, relatively low IGF-I
levels have been associated with an increased risk of developing cardiovascular
disease and diabetes, while relatively high IGF-I levels have been associated
with an increased risk of developing cancer [17]. Moreover,
in humans, an age-related decline in IGF-1 levels occurs [18], and at old
age, low IGF-1 levels are associated with frailty [19], poor
nutrition and cognitive decline [20] and an
increased risk of death [21]. On the
other hand, genetic variation in genes associated with down-regulation of the IIS
pathway has been associated with human longevity in several instances,
although, when moving up the evolutionary ladder, together with an increase in
genome complexity, effect sizes became smaller [22]. Two
studies have shown evidence for a role for genetic variation in the IIS pathway
in body height as well as human longevity. First, earlier we found an association
between genetic variation associated with
reduced IIS pathway activity and shorter stature as well as improved old age
survival in sporadic female octogenarians [23]. Second,
offspring of sporadic female centenarians were shown to be smaller and display
higher IGF-1 levels, indicative of IGF-1 insensitivity, while rare IGF-1R
mutations associated with IGF-1 insensitivity were found enriched in
centenarians [24]. Here, to
investigate whether these results could be generalized to familial longevity,
we have compared key anthropometric
measures as well as serum parameters related to insulin/IGF-1 signaling in a
group of middle-aged offspring of nonagenarian siblings and a control group of
their partners of the Leiden Longevity Study.
Results
Metabolic characteristics of offspring compared to
partners
Table 1 depicts the demographic and metabolic
characteristics of the groups from the Leiden Longevity Study that were used
for the present study. The group of offspring proportionately contained less
diabetics than the group of partners (p = 0.001). After exclusion of diabetics,
the group of offspring had lower non-fasted serum levels of glucose (p = 0.002)
than the group of partners. In addition, the group of offspring had a slightly
more favorable lipid profile as compared to the group of partners.
IGF-1/IGFBP3 and non-fasted glucose
Next we assessed whether the lower glucose levels
observed among the group of offspring relative to thegroup of partners could be driven by
differences in IGF-1 axis parameters. Therefore we determined the association
between serum IGF-1 / IGFBP3 molar ratios and non-fasted serum glucose levels.
Higher ratios of IGF-1/ IGFBP3 were associated with lower serum glucose levels.
One standard deviation increase in IGF-1/IGFBP3 ratio was associated with a
decrease of 0.10 mmol/L serum glucose (SE: 0.05) among the group of partners (p
= 0.05). The difference between partners and offspring in the change of glucose
levels per standard deviation IGF-1/IGFBP3 ratio was not significant: 0.02 (SE:
0.06) nmol/L per year (p for interaction = 0.70).
Measures of the IGF-1 axis in offspring compared to
partners
Table 2 shows the comparison between the groups of offspring
and partners for various IGF-1 axis parameters for males and females
separately. In order to detect the effect of possible genetic differences in
IGF-1 signaling between offspring and partners, we also determined
anthropometrical characteristics in subjects of both study groups
(Table 2). With regard to serum IGF-1 axis parameters, no differences were
observed between the group of offspring and the group of partners in both
sexes. Likewise, the study groups showed no differences in terms of
sex-specific body stature, i.e. height, weight and body mass index.
Next, we determined whether the
distribution of serum IGF-1 axis parameters and anthropometrical parameters were different between offspring and partners. Figure 1 displays the cumulative distributions of IGF-1, IGFBP3
and height among partners and offspring for both sexes separately. No
differences in height were observed between offspring and partners in the tails
of the IGF-1 and IGFBP3 distribution curves. Taken together, the cumulative
distribution curves do not suggest enrichment of high or low IGF-1 axis
parameters nor large or short statures among the groups of offspring versus
partners.
Table 1. Comparison of demographics and serum parameters between offspring and partners for males and females combined.
*Age is presented as median with interquartile range. Serum parameters are presented as mean values with 95% confidence intervals. Mean values, 95% confidence intervals and p-values were calculated using a linear regression model, adjusted for age and sex. LDL denotes low-density lipoprotein and HDL high-density lipoprotein.
**Data are presented as geometric means with 95% confidence intervals.
† Mean values, standard error of the mean and p-value for Total cholesterol, LDL cholesterol, HDL cholesterol, , Triglycerides and Free Fatty Acids were adjusted for lipid lowering agents (fibrates, niacin, bile acid sequestrants, 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors).
|
Offspring
|
Partners
|
p-value
|
| Demographics | | | |
|
Participants - n
|
1171
|
542
| |
|
Diabetics - n (%)
|
46 (3.9)
|
42 (7.7)
| 0.001 |
|
Females - n (%)
|
633 (54.1)
|
302 (55.7)
|
0.57
|
|
Age (year)
|
59.2 (55.0 - 64.1)
|
58.8 (54.3 - 63.7)
|
0.15
|
| | | |
| Serum
parameters (non-diabetics)
| | | |
|
Participants - n
|
1125
|
500
| |
|
Glucose (mmol/L)
|
5.69 (5.62 - 5.76)
|
5.87 (5.76 - 5.97)
| 0.002 |
|
Insulin (mU/L )**
|
14.4 (13.6 - 15.4)
|
15.4 (14.0 -16.8)
|
0.21
|
| | | |
|
Total cholesterol (mmol/L)†
|
5.56 (5.47 - 5.65)
|
5.62 (5.52 - 5.72)
|
0.40
|
|
LDL cholesterol (mmol/L)†
|
3.32 (3.24 - 3.39)
|
3.37 (3.29 - 3.45)
|
0.33
|
|
HDL cholesterol (mmol/L)†
|
1.46 (1.42 - 1.49)
|
1.43 (1.39 - 1.47)
|
0.24
|
|
Triglycerides (mmol/L) **,†
|
1.50 (1.44 - 1.55)
|
1.57 (1.50 - 1.65)
|
0.09
|
|
Free fatty acids (mmol/L) **,†
|
0.27 (0.26 - 0.28)
|
0.27 (0.26 - 0.29)
|
0.38
|
|
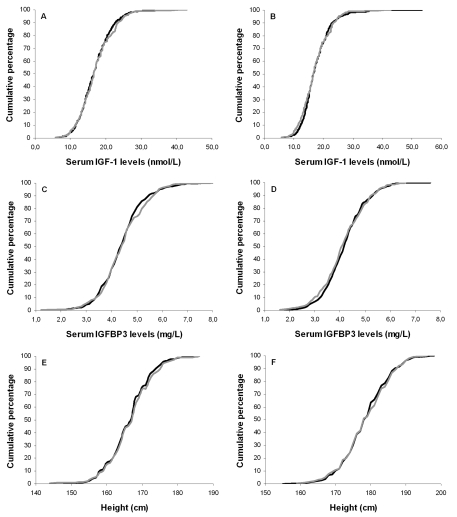
Figure 1. Cumulative distribution curves of serum IGF-1 levels, serum IGFBP3 levels and height.
Cumulative distribution curves of IGF-1 levels for offspring and partners
among females (A) and males (B); Cumulative distribution
curves of IGFBP3 levels for offspring and partners among females (C)
and males (D); Cumulative distribution curves of height for
offspring and partners among females (E) and males (F). Black
lines represent offspring, gray lines represent partners.
IGF-1 levels have been
consistently reported to progressively decline with age. To determine whether
this observation applied to the groups that were used in the present study, we
assessed the association between serum IGF-1 levels and serum IGFBP3 levels
with age. Figure 2 displays the sex-specific serum IGF-1 and IGFBP3 levels for
different age categories among offspring and partners. Serum IGF-1 levels
declined with age in both female partners
(-0.14 (SE: 0.04) nmol peryear increase; p<0.001) and male partners (-0.16
(SE: 0.05) nmol/L per year increase; p
=0.001). The difference in annual change in serum IGF-1 levels between partners
and offspring was not significant: 0.01 (SE: 0.05) nmol/L per year (p for
interaction= 0.79) for females and 0.01 nmol/L (SE: 0.06) per year (p for
interaction=0.83) for males. Similarly, no differences between partners and
offspring were observed in terms of annual change in serum IGFBP3 levels: 0.01
mg/L (SE: 0.01) (p for interaction = 0.47) for females and 0.02 mg/L (SE: 0.01)
(p for interaction =0.10) for males.
Table 2. Comparison of anthropometrics and IGF-1 axis parameters between offspring and partners for females and males separately.
Data are presented as means with 95% confidence intervals. All analyses were adjusted for age.
*Diabetic subjects were excluded from analyses.
|
Offspring*
|
Partners*
|
p-value
|
| Females (n) |
610
|
286
| |
|
IGF-1 axis serum parameters
| | | |
|
IGF-1 (nmol/L)
|
17.1 (16.7 - 17.5)
|
17.1 (16.5 - 17.7)
|
0.99
|
|
IFGBP3 (mg/L)
|
4.44 (4.36 - 4.53)
|
4.47 (4.36 - 4.57)
|
0.72
|
|
IGF-1/ IGFBP3 (molar ratio)
|
0.11 (0.11 - 0.11))
|
0.11 (0.11 - 0.11)
|
0.60
|
| | | |
|
Anthropometrics
| | | |
|
Height (m)
|
166.8 (166.2 - 167.3)
|
166.9 (166.1 - 167.7)
|
0.79
|
|
Weight (kg)
|
69.2 (68.2 - 70.3)
|
70.2 (68.9 - 71.6)
|
0.25
|
|
Body Mass Index (kg/m2)
|
24.9 (24.5 - 25.3)
|
25.2 (24.8 - 25.7)
|
0.25
|
| | | |
| Males (n) |
515
|
214
| |
|
IGF-1 axis serum parameters
| | | |
|
IGF-1 (nmol/L)
|
17.5 (17.0 - 17.9)
|
17.3 (16.6 - 18.0)
|
0.75
|
|
IGFBP3 (mg/L)
|
4.22 (4.13 - 4.30)
|
4.20 (4.08 - 4.32)
|
0.85
|
|
IGF-1/ IGFBP3 (molar ratio)
|
0.12 (0.12 - 0.12)
|
0.12 (0.12 - 0.12)
|
0.82
|
| | | |
|
Anthropometrics
| | | |
|
Height (m)
|
178.7 (178.1 - 179. 4)
|
179.1 (178.2 - 180.0)
|
0.44
|
|
Weight (kg)
|
82.0 (80.9 - 83.0)
|
82.4 (80.8 - 84.1)
|
0.61
|
|
Body Mass Index (kg/m2)
|
25.6 (25.4 - 25.9)
|
25.7 (25.2 - 26.1)
|
0.96
|
Discussion
The main findings of this study are
twofold. First, consistent with the lower prevalence of diabetes observed
earlier, non-fasted serum glucose levels were lower in the offspring of
familial nonagenarians when compared to their partners. Second, we did not
observe differences in non-fasted serum levels of IGF-1, IGFBP3 or in height
between the groups of offspring and partners, nor in the rate of the decline of
levels of IGF-1 or IGFBP3 over chronological age. Taken together, these data indicate that familial longevity is associated with differences in glucose handling, which
are not explained by major differences in IGF-1 and/or IGFBP3 levels.
The link between reduced IIS activity and longevity is
evolutionarily conserved from worms to rodents, with effects on longevity often
being stronger in the female sex. However, separating the roles of insulin and
IGF-1 in mammals has been very difficult and generated much controversy.
Because the actions of GH, insulin and IGF-1 are largely interwoven, genetic
modification of the GH/IGF-1 axis in mammals also entails differences in the
regulation of glucose metabolism. Interestingly, the hallmark phenotype of all
long-lived mouse models containing mutations that induce GH/IGF-1 deficiency or
resistance, is their enhanced insulin sensitivity [6]. Previously,
we observed a lower prevalence of diabetes in the offspring group [3]. Here, we
show that after exclusion of all diabetics, lower non-fasted glucose levels
were observed in the group of offspring of familial nonagenarians as compared
to the partners. The lower non-fasted glucose levels in offspring compared to
partners are suggestive of a better glucose handling and/or higher insulin
sensitivity in familial longevity, which is in line with the hallmark phenotype
observed in the many long-lived mammalian IIS mutants. Other data also support
a link between preserved insulin sensitivity and human longevity. While insulin
sensitivity generally declines with age in humans [25], sporadic
long-lived centenarians have been shown to exhibit an exquisite insulin
sensitivity, comparable to that of young adults [26].
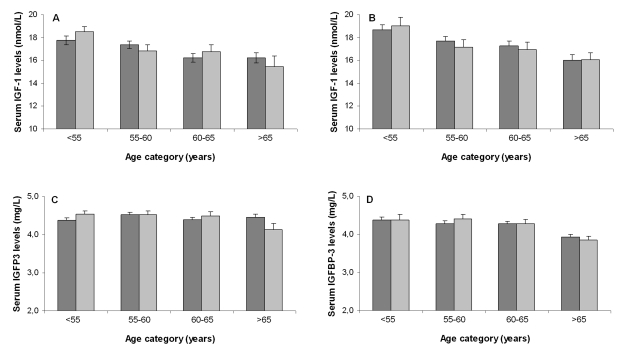
Figure 2. Association
between age categories and serum IGF-1 levels for offspring and partners
among females (A) and males (B) and association between age
categories and serum IGFBP3 levels for offspring and partners among females
(C) and males (D). Dark bars represent offspring, light bars
represent partners. Number of participants per age category for females
(offspring/ partners): category <55: 156/110; category 55-60: 194/83; category
60-65: 146/66; category >65: 114/27. Number of participants per age
category for males (offspring/ partners): category <55: 133/42; category
55-60: 140/49; category 60-65: 140/57; category >65: 102/66.
The preserved insulin sensitivity observed in
centenarians, co-occurred with relatively high levels of IGF-1/IGFBP3, which
has lead to the suggestion of causal link between the preserved insulin
sensitivity and levels of IGF-I/IGFBP3 [27]. In rats,
IGF-1 and IGFBP3 were shown to have opposing (centrally mediated) effects on glucose metabolism, with IGF-1 acting as an insulin
sensitizer, and IGFBP3 as an insulin inhibitor [28]. Similarly, in humans, IGF-1
administration was found to increase glucose uptake and inhibit hepatic glucose
production in healthy subjects [29], and low serum IGF-1 levels were
found associated with glucose intolerance [30]. In line with these findings, we
also observed a negative association between IGF-1/IGFBP3 levels and non-fasted
glucose levels in both our study groups, but neither this association nor the
mean levels of IGF-1 and IGFBP3 were different between the offspring and
partner groups. Our observation of improved glucose handling in the absence of
major differences in IGF-1/IGFBP3 levels resembles the Effects observed upon caloric restriction in humans.
In contrast to model organisms, in humans, IGF-1 levels were not found to be
decreased upon caloric restriction, while insulin sensitivity was increased
upon caloric restriction in humans as in model organisms [31]. The lack
of differences in BMI, as well as preliminary data on food intake, indicate
however that the observed difference in glucose handling between the groups of offspring
and partners can not be explained by a lower caloric intake in the offspring
group.
The observation of improved
glucose handling in the absence of major differences in IGF-1/IGFBP3 in
familial longevity does not rule out the possibility that genetic variations
affecting IGF-1/IGFBP3 levels do contribute to human longevity. Recently, it
was shown that centenarians exhibited a relative enrichment for rare genetic
variants in the IGF-1 receptor which resulted in high levels of IGF-1/IGFBP3
coexisting with low levels of IGF-1 signaling [24]. Also,
earlier we and others showed that common genetic variations affecting IGF-1
signaling might contribute to differences in mortality in the population at
large [23,32], but
the phenotypic effects associated with such variants (smaller stature,
differences in serum levels of IGF-1 and/or IGFBP3) do not form a distinctive
part of the hallmark phenotype of preserved glucose handling which we found
associated with familial longevity.
Methods
Leiden
Longevity Study.
In the Leiden Longevity Study, 420 families were recruited consisting
of long-lived Caucasian siblings together with their offspring and the partners
thereof [2,23].
Families were recruited if at least two long-lived siblings were alive and
fulfilled the age-criterion of 89 years or older for males and 91 year or older
for females. There were no selection criteria
on health or demographic characteristics. For 2465 of the offspring and their
partners, non-fasted serum samples taken at baseline were available for the
determination of endocrine and metabolic parameters. Between November 2006 and
May 2008, for 2235 of the offspring and their partners, information on medical
history was obtained from the participants' general practitioner (response:
91%). For 2255 of the offspring and their partners, information on the use of
medication was obtained from the participants' pharmacy (response: 92%). For
2184 of the offspring and partners a general questionnaire containing information
on lifestyle and self-reported height and weight was obtained (response: 89%).
For the present study, for a total of 1713 of the offspring and their partners,
serum as well as information on medical history on diabetes and information on
medication use and the general questionnaire were available (inclusion: 70%).
After exclusion of subjects with diabetes in medical history (n=87) and/or
non-fasted glucose lower than 11 mmol/L (n=1) and/or use of glucose lowering
medication (n=37), a sample of 1625 subjects was available for the current
study. The Medical Ethical Committee of the Leiden University Medical Centre
approved the study and informed consent was obtained from all subjects.
Biochemical analysis
. All serum measurements were performed with fully automated equipment.
For insulin‑like growth factor-1 (IGF-1), insulin-like growth factor
binding protein 3 (IGFBP3) and insulin, the Immulite 2500 from DPC (Los Angeles, CA, USA) was applied. CVs for these measurements were all below 8%. For
glucose, total cholesterol, HDL-cholesterol, triglycerides, free fatty acids
(FFA) the Hitachi Modular or the Cobas Integra 800, both from Roche, Almere,
the Netherlands were applied. CVs of these measurements were all below 5 %.
Medication use
. Lipid lowering agents were
defined as fibrates, niacin, bile acid sequestrants, 3-hydroxy-3-methylglutaryl-coenzyme
Areductase inhibitors (ATC code C10).
Calculations and statistical analysis.
For estimation of the level of LDL cholesterol the
Friedewald formula was applied (LDL cholesterol [mmol/l] = total cholesterol -
HDL cholesterol - [triglycerides/2.2]), whereby participants with a
triglyceride concentration higher than 443 mg/dl (5 mmol/l) were excluded. For
molar comparisons between IGF-1 and IGFBP3, the following molecular masses were
used in the calculation: IGF-1: 7.5 kDa and IGFBP3: 28.5 kDa.
Distributions of continuous variables were examined
for normality and logarithmically transformed, when appropriate and used in all
calculations. Geometric means (with 95% confidence intervals (CI)) are reported
for transformed variables (insulin, triglycerides and free fatty acids). All
differences in mean serum levels and anthropometrics between the groups of
offspring and partners were assessed with the use of linear regression,
adjusted for sex, age and correlation of sibling data using robust standard
errors in STATA. The relation between IGF-1/IGFBP3 molar ratio (expressed in
Z-scores and restricted to values within 3 standard deviations (SDs) from the
mean) and glucose was assessed with the use of a linear mixed model, adjusted
for sex, age and correlation of sibling data in SPSS. The cumulative
distributions of IGF-1, IGFBP3 and height were calculated in SPSS. The change
in levels of IGF-1, IGFBP3 over chronological age as a continuous variable was
assessed with the use of a linear mixed model, adjusted for age and correlation
of sibling data in SPSS. The Statistical Package for the Social Sciences (SPSS)
program for Windows, version 14.0, and STATA version 10.0 were used for data
analysis, and plots were drawn in Excel.
Acknowledgments
The LLS was funded by the Innovation Oriented research
Program on Genomics (SenterNovem; IGE01014 and IGE5007), the Centre for Medical
Systems Biology (CMSB), the Netherlands Genomics Initiative/Netherlands
Organization for scientific research (NGI/NWO; 05040202 and 050-060-810 (NCHA))
and the EU funded Network of Excellence Lifespan (FP6 036894). Rudi G.J.
Westendorp is supported by an unrestricted grant from the Netherlands Genomics
Initiative (NCHA 050-060-810). We thank all participants of the Leiden
Longevity Study for their consistent cooperation, as well all participating
general practitioners and pharmacists, the secretary staff (Meriam H van der
Star, Ellen H Bemer-Oorschot) and research nurses (Corrie J Groenendijk), data
managers (Karin H Herbschleb) for their expert contribution.
Conflicts of Interest
The authors of this manuscript have no conflict of
interests to declare.
References
-
1.
Oeppen
J
and Vaupel
JW.
Demography. Broken limits to life expectancy.
Science.
2002;
296:
1029
-1031.
[PubMed]
.
-
2.
Schoenmaker
M
, de Craen
AJ
, de Meijer
PH
, Beekman
M
, Blauw
GJ
, Slagboom
PE
and Westendorp
RG.
Evidence of genetic enrichment for exceptional survival using a family approach: the Leiden Longevity Study.
Eur J Hum Genet.
2006;
14:
79
-84.
[PubMed]
.
-
3.
Westendorp
RGJ
, van
Heemst D
, Rozing
MP
, Frohlich
M
, de Craen
AJM
, Beekman
M
, Heijmans
BT
, Mooijaart
SP
, Blauw
GJ
and Slagboom
PE.
Nonagenarian siblings and their offspring display lower risk of mortality and morbidity than sporadic nonagenarians: The Leiden Longevity Study.
J Am Geriatr Soc.
2009;
In press
.
-
4.
Terry
DF
, Wilcox
M
, McCormick
MA
, Lawler
E
and Perls
TT.
Cardiovascular advantages among the offspring of centenarians.
J Gerontol A Biol Sci Med Sci.
2003;
58:
M425
-M431.
[PubMed]
.
-
5.
Atzmon
G
, Schechter
C
, Greiner
W
, Davidson
D
, Rennert
G
and Barzilai
N.
Clinical phenotype of families with longevity.
J Am Geriatr Soc.
2004;
52:
274
-277.
[PubMed]
.
-
6.
Bartke
A
Impact of reduced insulin-like growth factor-1/insulin signaling on aging in mammals: novel findings.
Aging Cell.
2008;
7:
285
-290.
[PubMed]
.
-
7.
Kenyon
C
A conserved regulatory system for aging.
Cell.
2001;
105:
165
-168.
[PubMed]
.
-
8.
Tatar
M
, Kopelman
A
, Epstein
D
, Tu
MP
, Yin
CM
and Garofalo
RS.
A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function.
Science.
2001;
292:
107
-110.
[PubMed]
.
-
9.
Clancy
DJ
, Gems
D
, Harshman
LG
, Oldham
S
, Stocker
H
, Hafen
E
, Leevers
SJ
and Partridge
L.
Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein.
Science.
2001;
292:
104
-106.
[PubMed]
.
-
10.
Bluher
M
, Kahn
BB
and Kahn
CR.
Extended longevity in mice lacking the insulin receptor in adipose tissue.
Science.
2003;
299:
572
-574.
[PubMed]
.
-
11.
Selman
C
, Lingard
S
, Choudhury
AI
, Batterham
RL
, Claret
M
, Clements
M
, Ramadani
F
, Okkenhaug
K
, Schuster
E
, Blanc
E
, Piper
MD
, Al-Qassab
H
and Speakman
JR.
Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice.
FASEB J.
2008;
22:
807
-818.
[PubMed]
.
-
12.
Taguchi
A
, Wartschow
LM
and White
MF.
Brain IRS2 signaling coordinates life span and nutrient homeostasis.
Science.
2007;
317:
369
-372.
[PubMed]
.
-
13.
Brown-Borg
HM
, Borg
KE
, Meliska
CJ
and Bartke
A.
Dwarf mice and the ageing process.
Nature.
1996;
384:
33
[PubMed]
.
-
14.
Flurkey
K
, Papaconstantinou
J
, Miller
RA
and Harrison
DE.
Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production.
Proc Natl Acad Sci U S A.
2001;
98:
6736
-6741.
[PubMed]
.
-
15.
Chandrashekar
V
, Bartke
A
, Coschigano
KT
and Kopchick
JJ.
Pituitary and testicular function in growth hormone receptor gene knockout mice.
Endocrinology.
1999;
140:
1082
-1088.
[PubMed]
.
-
16.
Holzenberger
M
, Dupont
J
, Ducos
B
, Leneuve
P
, Geloen
A
, Even
PC
, Cervera
P
and Le
BY.
IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice.
Nature.
2003;
421:
182
-187.
[PubMed]
.
-
17.
Juul
A
Serum levels of insulin-like growth factor I and its binding proteins in health and disease.
Growth Horm IGF Res.
2003;
13:
113
-170.
[PubMed]
.
-
18.
Iranmanesh
A
, Lizarralde
G
and Veldhuis
JD.
Age and relative adiposity are specific negative determinants of the frequency and amplitude of growth hormone (GH) secretory bursts and the half-life of endogenous GH in healthy men.
J Clin Endocrinol Metab.
1991;
73:
1081
-1088.
[PubMed]
.
-
19.
Lamberts
SW
, van den Beld
AW
and van der Lely
AJ.
The endocrinology of aging.
Science.
1997;
278:
419
-424.
[PubMed]
.
-
20.
Arai
Y
, Hirose
N
, Yamamura
K
, Shimizu
K
, Takayama
M
, Ebihara
Y
and Osono
Y.
Serum insulin-like growth factor-1 in centenarians: implications of IGF-1 as a rapid turnover protein.
J Gerontol A Biol Sci Med Sci.
2001;
56:
M79
-M82.
[PubMed]
.
-
21.
Cappola
AR
, Xue
QL
, Ferrucci
L
, Guralnik
JM
, Volpato
S
and Fried
LP.
Insulin-like growth factor I and interleukin-6 contribute synergistically to disability and mortality in older women.
J Clin Endocrinol Metab.
2003;
88:
2019
-2025.
[PubMed]
.
-
22.
Kuningas
M
, Mooijaart
SP
, van
HD
, Zwaan
BJ
, Slagboom
PE
and Westendorp
RG.
Genes encoding longevity: from model organisms to humans.
Aging Cell.
2008;
7:
270
-280.
[PubMed]
.
-
23.
van
Heemst D
, Beekman
M
, Mooijaart
SP
, Heijmans
BT
, Brandt
BW
, Zwaan
BJ
, Slagboom
PE
and Westendorp
RG.
Reduced insulin/IGF-1 signalling and human longevity.
Aging Cell.
2005;
4:
79
-85.
[PubMed]
.
-
24.
Suh
Y
, Atzmon
G
, Cho
MO
, Hwang
D
, Liu
B
, Leahy
DJ
, Barzilai
N
and Cohen
P.
Functionally significant insulin-like growth factor I receptor mutations in centenarians.
Proc Natl Acad Sci U S A.
2008;
105:
3438
-3442.
[PubMed]
.
-
25.
Ferrannini
E
, Vichi
S
, Beck-Nielsen
H
, Laakso
M
, Paolisso
G
and Smith
U.
Insulin action and age. European Group for the Study of Insulin Resistance (EGIR).
Diabetes.
1996;
45:
947
-953.
[PubMed]
.
-
26.
Paolisso
G
, Gambardella
A
, Ammendola
S
, D'Amore
A
, Balbi
V
, Varricchio
M
and D'Onofrio
F.
Glucose tolerance and insulin action in healty centenarians.
Am J Physiol.
1996;
270:
E890
-E894.
[PubMed]
.
-
27.
Paolisso
G
, Ammendola
S
, Del
BA
, Gambardella
A
, Riondino
M
, Tagliamonte
MR
, Rizzo
MR
, Carella
C
and Varricchio
M.
Serum levels of insulin-like growth factor-I (IGF-I) and IGF-binding protein-3 in healthy centenarians: relationship with plasma leptin and lipid concentrations, insulin action, and cognitive function.
J Clin Endocrinol Metab.
1997;
82:
2204
-2209.
[PubMed]
.
-
28.
Muzumdar
RH
, Ma
X
, Fishman
S
, Yang
X
, Atzmon
G
, Vuguin
P
, Einstein
FH
, Hwang
D
, Cohen
P
and Barzilai
N.
Central and opposing effects of IGF-I and IGF-binding protein-3 on systemic insulin action.
Diabetes.
2006;
55:
2788
-2796.
[PubMed]
.
-
29.
Sherwin
RS
, Borg
WP
and Boulware
SD.
Metabolic effects of insulin-like growth factor I in normal humans.
Horm Res.
1994;
41 Suppl 2:
97
-101.
[PubMed]
.
-
30.
Sandhu
MS
, Heald
AH
, Gibson
JM
, Cruickshank
JK
, Dunger
DB
and Wareham
NJ.
Circulating concentrations of insulin-like growth factor-I and development of glucose intolerance: a prospective observational study.
Lancet.
2002;
359:
1740
-1745.
[PubMed]
.
-
31.
Redman
LM
, Martin
CK
, Williamson
DA
and Ravussin
E.
Effect of caloric restriction in non-obese humans on physiological, psychological and behavioral outcomes.
Physiol Behav.
2008;
94:
643
-648.
[PubMed]
.
-
32.
Bonafe
M
, Barbieri
M
, Marchegiani
F
, Olivieri
F
, Ragno
E
, Giampieri
C
, Mugianesi
E
, Centurelli
M
, Franceschi
C
and Paolisso
G.
Polymorphic variants of insulin-like growth factor I (IGF-I) receptor and phosphoinositide 3-kinase genes affect IGF-I plasma levels and human longevity: cues for an evolutionarily conserved mechanism of life span control.
J Clin Endocrinol Metab.
2003;
88:
3299
-3304.
[PubMed]
.