Are epidermal stem cells unique with respect to aging?
Abstract
Epidermal stem cells are a population of somatic stem cells responsible for maintaining and repairing the epidermis of the skin. A malfunctioning epidermal stem cell compartment results in loss of the epidermis and death of the whole organism. Since the epidermis continually renews itself by sloughing a layer of cells every day, it is in a constant state of cellular turnover and requires continual cell replacement for life. Thus, maintaining a pristine epidermal stem cell population is of prime importance, even during aging. Unlike stem cells from internal tissues, epidermal stem cells show little response to aging. They do not appear to decrease in number or functionality with age, and do not show changes in gene expression, developmental responsiveness, or age-associated increases of reactive oxygen species. Thus, epidermal stem cells may be a unique somatic stem cell.
Why epidermal stem cells might be unique
How an organism and its cells
age is an ongoing debate. One view is that organisms age because their cells
accumulate a series of accidental, but detrimental events throughout life. Another
view espouses that cells follow an established program of genetic and
epigenetic changes which slowly, but deliberately result in loss of cellular
repair mechanisms and ultimately in organismal death (for review see [1]). A
further point to consider is do organisms proceed through the aging process
because they gradually lose their stem cells as they age or because their stem
cells gradually change their function? Evidence from intestinal and
hematopoietic stem cells suggest that both may happen: the number of stem cells
decreases and their function changes with increasing age [2-5]. However, work
from our lab and others suggests that this is not the case for all somatic stem
cells, that mammalian epidermal stem cells appear to resist the aging process.
These adult stem cells show no loss in numbers, no changes in gene expression
or cellular function, and no changes in telomere length with respect to age
[6-8].
Perhaps epidermal stem cells are unique
among somatic stem cells because no matter how old the skin is, the epidermis
must continually replace itself with correctly functioning cells in order to
protect the organism from the outside world [9]. Mammalian epidermis sloughs
around one layer of cells every day. The sloughed cells are replaced through
proliferation of cells in the lower layers [10]. If epidermal cell replacement
ceases for any length of time, the mammal will die. This continual need for
replacement cells is met by epidermal stem cell proliferation followed by a
series of amplifying cell divisions of the daughter cells. Because the
epidermis lives a long time, epidermal stem cells are by default very long
lived. In fact, they can essentially "outlive" the mammal from which they came,
as evidenced by old skin grafted to young individuals living past the death of
the donor [11,12]. Having epidermal stem cells resist aging may be an
epidermal protective mechanism evolved against unexpected extension of life.
Epidermal stem cells remain
undifferentiated and functional into old age
We have known for nearly thirty years that
stratified squamous epithelia contain slowly or intermittently cycling
keratinocytes. These cells are identified as label-retaining cells (LRCs) by
the long term retention of a tritiated thymidine or bromodeoxyuridine (BrdU)
label [13-15]. Morphologically, LRCs look like undifferentiated primitive cells
with a large nuclear to cytoplasmic ratio and have the characteristics of
epidermal stem cells [15,16]. Furthermore, in neonatal epithelia the somatic
epidermal stem cells are morphologically the same as those in adult epithelia
[17]. It is thought that a somatic stem cell asymmetrically divides producing
one daughter identical to itself and one daughter cell that increases its
proliferative rate to maintain the tissue. Although there is no direct evidence
for this phenomenon in the epidermis, evidence does exist in the small
intestinal epithelium [18]. Asymmetric cell division was predicted more than
three decades ago as an intrinsic way for stem cells in continuously renewing
tissues, such as the epidermis, to protect their DNA by minimizing DNA
replication related defects [19]. This may explain why stem cells from both
neonatal and adult epidermis rarely enter the cell cycle even though the epidermis
requires continual cell replacement. Ninety-six percent of the epidermal stem
cells from both age groups remain in G1 of the cell cycle, whereas only 4% of
the cells are cycling in S-G2/M at any given time [17]. Instead it is the
transit amplifying daughter population in both age groups that is highly
proliferative, with 15% of these cells in S-G2/M [17]. Recent in vivo studies
confirm that the epidermal stem cell does not change its repopulating
characteristics with age; instead it is the aging transit amplifying daughter
cell that changes its kinetics [20].
Our method preferentially
selects for the LRC population, and thus highly enriches for the epidermal stem
cells [16]. It combines and refines two previous published methods The first
method showed that the long term repopulating hematopoietic stem cells were the
cells that excluded the vital dye Hoechst 33342 via the ABCG2 transporter [21,22]. The second method showed that the smallest epidermal keratinocytes were
the cells that produced the largest clonogenic potential in vitro [23].
Epidermal cells, isolated by combining these two methods, not only recapitulate
a functional epidermis, but also show multipotency when injected into a
developing mouse blastocyst [6,16]. This is irrespective of the cell's age.
Analysis shows that gene expression does not change between young adult and old
adult mouse epidermal cells [7]. These combined findings suggest that epidermal
stem cells maintain their functionality well into old age. Our results differ
from recent reports of hematopoietic stem and progenitor cells in which gene
expression not only changed with age in both populations, but was directly
associated with replicative senescence [5]. Such findings emphasize the
difference between epidermal stem cells and other somatic stem cells,
especially with respect to aging.
Epidermal stem cells remain
developmentally responsive irrespective of their age
We have shown that epidermal stem cells
isolated from newborn or aging mouse skin have a similar plasticity response
when injected in a developing blastocyst environment [6,7]. The labeled cells
are found incorporated into tissues from all three germ layers. The injected
stem cells alter their epidermal profile and express proteins of the tissues into
which they develop in vivo. Furthermore, the cells or their progeny are
retained for the life of the resultant mouse. Thus, something in the
developmental environment of the blastocyst is able to reprogram the injected
epidermal cells. This phenomenon is unique to the epidermal stem cells as none
of the other basal keratinocytes or their progeny are found in any of the adult
mouse tissues.
It was not determined in these experiments
whether it is contact with the cells in the blastocyst or a response to something
secreted by the cells that reprogram the epidermal cells. In vitro, exposure to
cell-free extracts or conditioned media from pluripotent cells results in
reprogramming of differentiated cells [24,25]. The treated cells increase
their developmental potency and can be specifically directed to differentiate
into neuronal cells or B lymphocytes in vitro [25,26]. In the B lymphocyte
experiment, the epidermal stem cells were shown to permanently change their
genome by deleting VDJ segments from the heavy chain immunoglobulin locus. Only
the epidermal stem cells respond to these stimuli, and their age is irrelevant
to their response. These findings indicate two things: first, that epidermal
stem cells have retained a remarkable developmental potency and second, this
ability to transform into other cell types in response to environmental stimuli
is not lost with age.
How epidermal stem cells maintain the
ability to be developmentally responsive into old age is not understood. One
potential mechanism might be to control the levels of reactive oxygen species
within the cellular borders. We have three reasons for posing this possible
scenario. First, oxidative stress has been associated with increased aging at
the molecular level as shown by the deletion of the superoxide dismutase 1
(Sod1) gene producing a decrease in the lifespan of mice [27]. Superoxide
dismutase 1 is an enzyme required to catalyze the dismutation of superoxide, a
reactive oxygen species (ROS). Second, it is believed that changes in levels of
oxygen can change the developmental potential of a cell via new epigenetic
programming (reviewed in [28]). Third, although we found no differences in gene
expression between young and old epidermal stem cells, we did find that these
cells had much higher expression of Sod1 than did the other basal keratinocytes
[7].
The redox state of the epidermal stem
cells, as indicated by changes in ROS such as superoxide, could affect the developmental
responsiveness (Figure 1 shows a diagram of ROS production in mammalian
cells). It has been speculated that generation of ROS can directly affect gene
expression by altering chromatin configuration [28]. Thus a low cellular
concentration of superoxide could directly affect DNA methylation states. This
idea has merit as the activity levels of the antioxidant enzymes, superoxide
dismutase (SOD), catalase, and glutathione peroxidase can change dynamically in
cells. These enzymes form the first-line of defense against ROS damage. SOD
converts superoxide anion to hydrogen peroxide, which is converted to water by
glutathione peroxidase and catalase (Figure 2). These antioxidant enzymes keep
ROS levels low in all cell types.
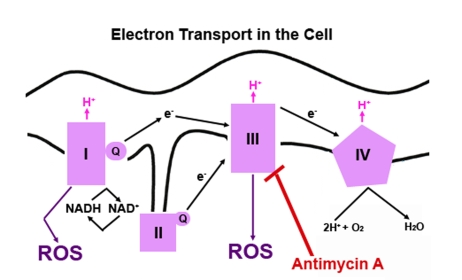
Figure 1. A simple diagram depicting electron transport in mammalian cells. The redox
potential increases as the electrons move through each complex located in
the inner membrane of the mitochondria. NADH, an electron donor, donates
two electrons. The electrons flow through the four complexes causing
hydrogen (H+) to be pumped across the inner mitochondrial
membrane to favor free energy. At each step, the free electron can be
picked up by oxygen (O2), which will convert O2 to
superoxide, a highly reactive oxygen species (ROS). Electron transfer can
be blocked at the complexes by several different compounds. Antimycin A
blocks transfer of electrons at complex III.
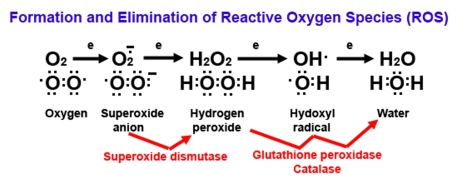
Figure 2. Diagram of reactive oxygen (ROS) formation.
Oxygen (O2) plays a major role in the formation of
ROS because O2 has unpaired electrons (represented by
single dots). When O2 picks up an electron, it becomes
superoxide, an extremely reactive anion. Superoxide dismutase
catalyzes the dismutation reaction of superoxide to hydrogen
peroxide, which is further catalyzed to the highly reactive hydroxyl
radical and ultimately to water by glutathione peroxidase and
catalase enzymes. Superoxide, hydrogen peroxide, and hydroxyl
radicals are considered to be ROS.
Epidermal stem cells subjected to analysis
of superoxide levels by dihydroethidium (DHE) show so little superoxide that it
is difficult to measure (Figure 3). This is not the case with dermal fibroblasts
or basal keratinocytes (Figure 3). Cells are stained with dihydroethidium
(DHE). In the presence of superoxide, the DHE is reduced to ethidium, which
intercalates into the DNA and fluoresces. Levels of superoxide are
substantially higher in the dermal fibroblasts and basal keratinocytes than in
the epidermal stem cells, as evidenced by the significant increase in
fluorescence after treatment with Antimycin A (Figure 3). Antimycin A blocks
electron transport at complex III, which results in a direct increase in
superoxide (Figure 1). The very low level of superoxide in the epidermal stem
cells is likely due to the high level of the expressed Sod1 gene previously
reported [7].
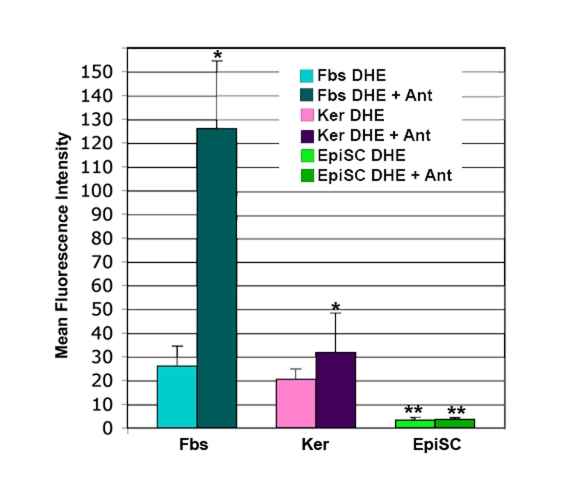
Figure 3. DHE staining of superoxide in skin cells. Cultures of
dermal fibroblasts (Fbs), epidermal keratinocytes (Ker), and epidermal stem
cells (EpiSC) were stained with dihydroethidium (DHE) in the presence or
absence of the electron transport chain blocker antimycin A (Ant).
Fluorescence for each cell type +/- Ant was determined by flow cytometry,
then normalized by comparison to a standard cell. EpiSCs show significantly
lower levels of DHE staining than the keratinocyte and fibroblast
populations (p<0.01). Increase in DHE staining in the Fbs+Ant samples
was significantly higher than that seen in Ker+Ant samples (p<0.05).
Lack of increase in DHE staining in the EpiSC after antimycin A treatment
was significant (p<0.01). Significant differences were determined by student's
T-test. n=5.
In conclusion, epidermal stem
cells have several characteristics that make them unique in the somatic stem
cell world: They appear to resist aging. They show no age-related changes in
gene expression. They maintain a developmental responsiveness to changes in
their environment. They show no effects associated with increasing levels of
reactive oxygen species found in aging cells by keeping levels of ROS low,
perhaps by maintaining high levels of superoxide dismutase (SOD1). Exactly how
these epidermal stem cells remain "young" requires further research.
Conflicts of Interest
The authors of this manuscript have no conflict of interest to declare.
References
-
1.
Hayflick
L
Biological aging is no longer an unsolved problem.
Ann N Y Acad Sci.
2007;
1100:
1
-13.
[PubMed]
.
-
2.
Martin
K
, Kirkwood
TB
and Potten
CS.
Age changes in stem cells of murine small intestinal crypts.
Exp Cell Res.
1998;
241:
316
-323.
[PubMed]
.
-
3.
Rossi
DJ
, Bryder
D
, Zahn
JM
, Ahlenius
H
, Sonu
R
, Wagers
AJ
and Weissman
IL.
Cell intrinsic alterations underlie hematopoietic stem cell aging.
Proc Natl Acad Sci U S A.
2005;
102:
9194
-9149.
[PubMed]
.
-
4.
Biteau
B
, Hochmuth
CE
and Jasper
H.
JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut.
Cell Stem Cell.
2008;
3:
442
-455.
[PubMed]
.
-
5.
Wagner
W
, Bork
S
, Horn
P
, Krunic
D
, Walenda
T
, Diehlmann
A
, Benes
V
, Blake
J
, Huber
FX
, Eckstein
V
, Boukamp
P
and Ho
AD.
Aging and replicative senescence have related effects on human stem and progenitor cells.
PLoS One.
2009;
4:
e5846
[PubMed]
.
-
6.
Lian
L
, Chinnathamb
S
, Ster
M
, Tomanek-Chalkle
A
, Manue
TD
and Bickenbac
JR.
As epidermal stem cells age they do not substantially change their characteristics.
J Investig Dermatol Symp Proc.
2004;
9:
229
-237.
.
-
7.
Stern
MM
and Bickenbac
JR.
Epidermal stem cells are resistant to cellular aging.
Aging Cell.
2007;
6:
439
-52.
[PubMed]
.
-
8.
Giangreco
A
, Qin
M
, Pintar
JE
and Watt
FM.
Epidermal stem cells are retained in vivo throughout skin aging.
Aging Cell.
2008;
7:
250
-259.
[PubMed]
.
-
9.
Webb
A
and Kaur
P.
Epidermal stem cells.
Front Biosci.
2006;
11:
1031
-1041.
[PubMed]
.
-
10.
Halprin
KM
Epidermal "turnover time"--a re-examination.
Br J Dermatol.
1972;
86(1):
14
-19.
[PubMed]
.
-
11.
Compton
CC
, Gill
JM
, Bradford
DA
, Regauer
S
, Gallico
GG
and O'Connor
NE.
Skin regenerated from cultured epithelial autografts on full-thickness burn wounds from 6 days to 5 years after grafting. A light, electron microscopic and immuno-histochemical study.
Lab Invest.
1989;
60:
600
-12.
[PubMed]
.
-
12.
Pellegrini
G
, Ranno
R
, Stracuzzi
G
, Bondanza
S
, Guerra
L
, Zambruno
G
, Micali
G
and De
Luca M.
The control of epidermal stem cells (holoclones) in the treatment of massive full-thickness burns with autologous keratinocytes cultured on fibrin.
Transplantation.
1999;
68:
868
-879.
[PubMed]
.
-
13.
Bickenbach
JR
Identification and behavior of label-retaining cells in oral mucosa and skin.
J Dent Res.
1981;
122C:
1611
-1620.
[PubMed]
.
-
14.
Bickenbach
JR
and Holbrook
KA.
Label-retaining cells in human embryonic and fetal epidermis.
J Invest Dermatol.
1987;
88:
42
-46.
[PubMed]
.
-
15.
Lavker
RM
and Sun
TT.
Epidermal stem cells: properties, markers, and location.
Proc Natl Acad Sci U S A.
2000;
97:
13473
-13475.
[PubMed]
.
-
16.
Dunnwal
M
, Tomanek-Chalkle
A
, Alexandruna
D
, Fishbaug
J
and Bickenbac
JR.
Isolating a pure population of epidermal stem cells for use in tissue engineering.
Exp Dermatol.
2001;
10:
45
-54.
[PubMed]
.
-
17.
Dunnwald
M
, Chinnathambi
S
, Alexandrunas
D
and Bickenbach
J.
Mouse epidermal stem cells proceed through the cell cycle.
J Cell Physiol.
2003;
195:
194
-201.
[PubMed]
.
-
18.
Potten
CS
, Owen
G
and Booth
D.
Intestinal stem cells protect their genome by selective segregation of template DNA strands.
J Cell Sci.
2002;
115:
2381
-2388.
[PubMed]
.
-
19.
Cairns
J
Mutation selection and the natural history of cancer.
Nature.
1975;
255:
197
-200.
[PubMed]
.
-
20.
Charruyer
A
, Barland
CO
, Yue
L
, Wessendorf
HB
, Lu
Y
, Lawrence
HJ
, Mancianti
ML
and Ghadially
R.
Transit-Amplifying Cell Frequency and Cell Cycle Kinetics Are Altered in Aged Epidermis.
J Invest Dermatol.
2009;
In press
.
-
21.
Goodell
MA
, Brose
K
, Paradis
G
, Conner
AS
and Mulligan
RC.
Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo.
J Exp Med.
1996;
183:
1797
-1806.
[PubMed]
.
-
22.
Zhou
S
, Schuetz
JD
, Bunting
KD
, Colapietro
AM
, Sampath
J
, Morris
JJ
, Lagutina
I
, Grosveld
GC
, Osawa
M
, Nakauchi
H
and Sorrentino
BP.
The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype.
Nat Med.
2001;
7:
1028
-1034.
[PubMed]
.
-
23.
Barrandon
Y
and Green
H.
Cell size as a determinant of the clone-forming ability of human keratinocytes.
Proc Natl Acad Sci U S A.
1985;
82:
5390
-5394.
[PubMed]
.
-
24.
Collas
P
, Taranger
CK
, Boquest
AC
, Noer
A
and Dahl
JA.
On the way to reprogramming cells to pluripotency using cell-free extracts.
Reprod Biomed Online.
2006;
12:
762
-770.
[PubMed]
.
-
25.
Grinnell
KL
and Bickenbach
JR.
Skin keratinocytes pretreated with embryonic stem cell conditioned medium or BMP4 can be directed to alternative cell lineages.
Cell Prolif.
2007;
40:
685
-705.
[PubMed]
.
-
26.
Stern
MM
, Tygrett
LT
, Waldschmidt
TJ
and Bickenbach
JR.
Cells isolated from the epidermis by Hoechst dye exclusion, small size, and negative selection for hematopoietic markers can generate B-lymphocyte precursors.
J Invest Dermatol.
2008;
128:
1386
-1396.
[PubMed]
.
-
27.
Perez
VI
, Bokov
A
, Remmen
HV
, Mele
J
, Ran
Q
, Ikeno
Y
and Richardson
A.
Is the oxidative stress theory of aging dead.
Biochim Biophys Acta.
2009;
In press
.
-
28.
Hitchler
MJ
and Domann
FE.
An epigenetic perspective on the free radical theory of development.
Free Radic Biol Med.
2007;
43:
1023
-1036.
[PubMed]
.