Abstract
miRNAs function as a critical regulatory layer in development, differentiation, and the maintenance of cell fate. Depletion of miRNAs from embryonic stem cells impairs their differentiation capacity. Total elimination of miRNAs leads to premature senescence in normal cells and tissues through activation of the DNA-damage checkpoint, whereas ablation of miRNAs in cancer cell lines results in an opposite effect, enhancing their tumorigenic potential. Here we compile evidence from the literature that point at miRNAs as key players in the maintenance of genomic integrity and proper cell fate. There is an apparent gap between our understanding of the subtle way by which miRNAs modulate protein levels, and their profound impact on cell fate. We propose that examining miRNAs in the context of the regulatory transcriptional and post-transcriptional networks they are embedded in may provide a broader view of their role in controlling cell fate.
miRNAs are key
regulators of cell fate
miRNAs have emerged in the past decade as
important players in numerous cellular and organismal processes in animals and
plants [1]. Deletion of the Dicer gene, encoding the critical enzyme
involved in miRNA processing and maturation, is embryonic lethal in both mice
[2] and zebrafish [3]. Accordingly, many studies showed, using conditional
elimination of Dicer, that miRNAs are crucial for the proper
spatiotemporal development of various tissues and organs ([2,4-9] and reviewed
in [10]). Further, mouse embryonic stem (ES) cells defective in miRNA processing
were shown to proliferate slower [11], and to be impaired in their ability to differentiate
[8]. In parallel, other studies have shown a major role for miRNAs in development,
indicating that many miRNAs are upregulated during the process of ES cell
differentiation ([12] and reviewed in [13]). Many miRNAs also play a role in differentiation
processes in the adult organism, including hematopoiesis
[14] and the germinal center response [15]. In fact, the
first miRNAs to be discovered, lin-4 and let-7 in C. elegans,
regulate epithelial cell differentiation [16,17]. In addition, manipulations
of individual miRNA genes were shown to result in marked defects at the
organismal level ([18,19] and reviewed in [20]). Based on these accumulated observations
it is plausible to suggest that in many cases miRNAs are indeed a part of the
driving force of differentiation processes. miRNAs were also shown to regulate many
cellular processes [21,22], such as cell growth and proliferation (reviewed
in [23,24]) and apoptosis (reviewed in [25]). It appears, therefore, that
miRNAs are crucial players in the regulation and determination of cell fate.
miRNAs - guardians of
genome integrity?
Lu et al. [26]
carried out an extensive analysis of miRNA expression in human cancer. This
study, that included a global expression profiling of miRNAs across a large set
of tumors, demonstrated that miRNA expression profiles can be used to classify
human cancers of unknown origin. In addition, the researchers made the very interesting
observation that, in general, tumors have lower levels of miRNAs than normal
tissues. The authors suggested that the observed low global levels of miRNAs may
be a reflection of the de-differentiated state of tumors.
An alternative,
complementary explanation might be that tumors evolve to silence the miRNA
pathway during the course of cancer progression. In other words, globally avoiding
regulation of gene expression by miRNAs may be one of the many ways of cancer
cells to enhance their proliferation and tumorigenic potential.
Several lines of evidence support the
idea that proliferating cells and cancer cells in particular, find many
different ways to avoid post-transcriptional regulation by miRNAs (Figure 1).
Some of these mechanisms are straightforward, and are in agreement with what we
know of tumor suppressors and oncogenes. For example, the MYC oncogenic
transcription factor (TF) was found in a lymphoma mouse model to mediate
widespread repression of a large set of miRNAs, contributing to tumorigenesis
[27]. Other mechanistic possibilities for tumors to avoid posttranscriptional regulation
by miRNAs include epigenetic silencing, mutation and deletion of genomic loci
encoding for miRNAs [28-33]. A prominent example is the miR-15a/16-1 cluster,
residing in the DLEU2 non-coding RNA, which was long known to be
frequently deleted in leukemia [34,35], and was later shown to harbor these
miRNAs [29]. Another newly described mechanism is the interruption of the miRNA
biogenesis pathway, by processes such as nuclear retention of unprocessed pre-miRNAs
[36], or pri- and pre-miRNA processing blockage such as in the case of inhibition
of maturation of the let-7 family by the Lin28 protein [37-39]. Lin28 was further shown to promote cancer, and this was attributed to its
repression of the let-7 miRNA family [40]. A recent report implicates p53 in the enhancement of miRNA maturation for many miRNAs following DNA damage
[41], attesting to global miRNA upregulation as a possible anti-cancer
mechanism. Additional highly intriguing phenomenon was reported by Sandberg et
al. [42], indicating that proliferating cells tend to employ alternative
polyadenylation or alternative splicing in order to express mRNAs with shorter
3' UTRs, having fewer miRNA binding sites. These shorter mRNAs avoid
post-transcriptional regulation by miRNAs, thus potentially enhancing their
protein level. This phenomenon represents another path by which proliferating
cells achieve the same goal - avoiding miRNA-mediated silencing, presumably in
order to accelerate proliferation.
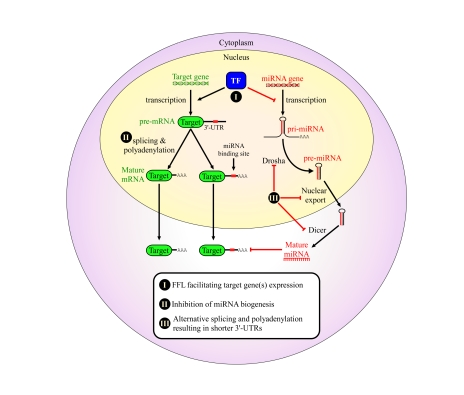
Figure 1. Proposed mechanisms for avoidance of regulation by miRNAs in cancer cells.
We propose that cancers may
evolve to avoid regulation by miRNAs in order to enhance their tumorigenic
potential. This might occur through a variety of mechanisms: (I) combined
transcriptional/post-transcriptional FFL wiring, which may enhance the
repression of several co-regulated miRNAs, thereby facilitating the
expression of the mutual target genes; (II) global avoidance of miRNA
regulation via expression of shorter 3' UTRs [42]; (III) global reduction
in miRNA levels by impairing miRNA biogenesis in various ways, some of
which were shown to happen in tumors, such as inhibition of Drosha processing [39,40] and pre-miRNA nuclear retention [36]. All of these are suggested as
means that developing tumors may evolve to enhance proliferation and
increase genome instability.
The most striking evidence
in support of the 'miRNA avoidance' strategy played by tumors is shown by two
seemingly contradictory studies, one focusing on cancer cells and the other on
normal cells. The study by Kumar et al. [43] reported that the ablation
of miRNAs in various cancer cell lines resulted in enhanced cellular transformation,
evident by increased colony formation efficiency in vitro and increased
tumor burden in vivo. On the other hand, Mudhasani et al. [44]
showed that the total elimination of miRNAs using conditional Dicer knock-out
results in premature senescence in normal mouse embryonic fibroblasts (MEFs).
This effect was also apparent at the level of the organism, as the knock-out ofDicer in keratinocytes and skin epidermis of adult mice resulted in
senescence-induced hairloss and skin aging [44].
At first glance, these two
studies seem to disagree. How is it possible that a similar manipulation would
enhance proliferation in one system, and cause a proliferation arrest or
senescence in the other? A potential solution to this conflict would consider
that the same event can lead to two opposite outcomes, depending on the
cellular context. For example, activation of an oncogene, such as RAS,
is one of the hallmarks of cancer, and when occurring in cancer cells will
cause the enhancement of their tumorigenic phenotype. However, in normal cells,
oncogene activation will often lead to genomic instability, which is sensed by
the DNA damage checkpoint, and leads to p53 and ARF-dependent
senescence, a phenomenon known as "oncogene-induced senescence" [45].
Importantly, the phenomenon described by Mudhasani et al. [44] was not a
classical case of oncogene-induced senescence, as it was not accompanied by the
upregulation of the oncogenes MYC or RAS, (two well known activators
of oncogene-induced senescence), even though they are documented miRNA targets
[46-48]. Interestingly, however, the depletion of miRNAs led to DNA damage, as
evident by γH2A.X staining, and consequently, through activation of
the p19ARF and p53-dependent DNA-damage checkpoint,
resulted in premature senescence.
Therefore, in this case
too, the same event of global miRNA depletion induced the DNA damage checkpoint
in normal cells due to proper p19ARF and p53 activation, while in
cancer cells it led to enhanced transformation, where these checkpoint response
pathways are frequently inactivated, and genomic instability enhances
tumorigenesis [49].
Importantly, as we outline here,
inactivation of miRNA-mediated silencing is not only capable in principle of
influencing cell fate, following genetic manipulations as shown by Mudhasani et
al. and Kumar et al. [43,44], but may actually occur in vivo during
tumorigenesis [26,42]. It therefore seems likely that miRNAs are not only necessary for proliferation and differentiation
in normal cells, but also act to maintain normal cell proliferation, and may
be thought of as "guardians" of genome integrity. In cancer cells, on the other
hand, inactivation of the miRNA-mediated silencing pathway and the avoidance of
miRNA regulation contribute to transformation (Figure 1). In principle we can
therefore consider miRNAs as a regulatory barrier whose removal may be part of
a series of events that ultimately lead to cancer.
A conceptual gap between
the influence of miRNAs on protein levels and their effects on cell fate
miRNAs can exert their
silencing effects by cleavage of their target mRNAs and by inhibition of their
translation. A common knowledge in the field was that animal miRNAs exert most
of their silencing through the inhibition of translation, rather than through
the degradation of their targets, and that this was due to a low overall degree
of sequence complementarity that animal miRNAs share with their target sites on
3' UTRs of mRNAs [1]. In fact, the first discovered miRNAs in C. elegans, lin-4, was shown to inhibit the translation of its target Lin-14,
without affecting its mRNA levels [50,51]. Mechanistically, it became evident
that the miRNA-effector protein complex, the RISC, is enzymatically
capable of both mRNA cleavage and inhibition of translation [52,53]. Lim et
al. then showed that miRNAs can influence the mRNA levels of their
target genes [54]. Using overexpression of miRNAs followed by global expression
profiling using microarrays, they demonstrated a modest but significant
downregulation of mRNA levels of genes that were enriched for the miRNA seed
sequence. This study and others that followed contributed to the overall view
that miRNAs exert silencing through both mechanisms simultaneously, but the
more major effect was expected at the protein level, rather than at the mRNA
levels.
Recent studies used high
throughput proteomics in order to both identify translationally inhibited
targets and to more accurately assess the extent of inhibition that a miRNA
exerts on mRNA levels and on protein levels [55,56]. These studies reported
that individual miRNAs affect hundreds of proteins in the human and mouse out
of thousands that were examined. However, the levels of these proteins were decreased
only to a relatively mild extent. miRNAs were often before considered as modulators
of expression, and their generally observed mild effect on protein levels (and
mRNA levels as well) promoted their suggested role as buffers for noise in protein
expression, which may confer robustness to developmental programs [57].
Overall, there seems to be
a discrepancy between the observation that miRNAs have such subtle effects on
protein levels and the fact that their effects on cell fate are so profound. We
would like to suggest here one possible model that might bridge this conceptual
gap.
Coupling transcriptional
and post-transcriptional miRNA regulation in the control of cell fate
One trivial way to resolve
the above discrepancy might argue that the multiplicity of miRNA targets and
the simultaneous down-regulation of many proteins might have a cumulative
effect, eventually exerting a significant impact on cell fate, even though
individual proteins are repressed to a very modest extent. This is a valid
argument, particularly since some miRNAs were predicted and shown to have
multiple targets within the same pathway [58-60], thus potentially having
greater effects on entire pathways than on individual proteins.
While miRNAs may exert modest effects,
yet on many targets, another possible answer to their significant effect on
cell fate may lie in the level of the regulatory networks that miRNAs take
central part in. miRNAs do not act in isolation, but rather they regulate
target genes combinatorially with one another, and are often embedded within
intricate regulatory networks together with TFs (Figure 2). In fact, it was
demonstrated that at the network level, there is tight coupling between
posttranscriptional regulation by miRNAs and the regulation of transcription by
TFs [61,62]. Examination of regulatory networks showed that in many cases the
same TF controls the transcription of both a miRNA and the targets of that
miRNA, or is regulated by the same miRNA with which it shares common targets,
forming a diversity of combined transcriptional/post-transcriptional
Feed-Forward Loops (FFLs). Collectively, such FFLs potentially regulate
thousands of target genes.
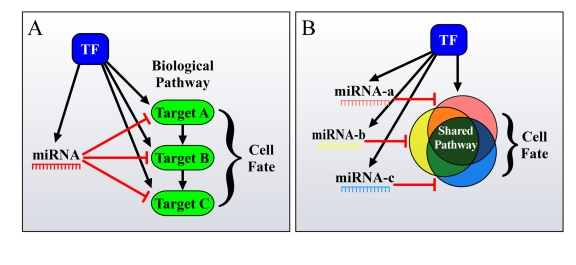
Figure 2. Different ways by which FFLs can account for the enhanced phenotypic effect of miRNAs on cell fate. (A) miRNAs
and TFs in FFLs tend to mutually target genes from the same pathway. (B)
Additionally, co-regulated miRNAs and miRNA families co-target many genes
in the same pathway, thus resulting in a significant total output, having a
major effect on cell fate.
Network analyses showed
that these FFLs constitute over-represented architectures in the mammalian
regulatory network [61,62]. Network FFLs, initially described by Alon and
colleagues, were shown to comprise a major component of the transcription
networks in bacteria and yeast [63,64]. The discovery that miRNAs and TFs also
constitute FFLs offered new possibilities for potential functions for these
regulatory units. Clues for the existence of coupling between transcription and
miRNA regulation emerged from a very intriguing concept, called miRNA-target
avoidance. Two parallel studies, one in Drosophila and the other in
mammals, showed that during development as well as in adult tissues, miRNA
targets often avoid being expressed in the same tissue, or at the same
developmental time, as their potential inhibitory miRNA [65,66]. In Drosophila,
it was shown for some cases that a miRNA and its targets are expressed in
adjacent tissues during development, or in consecutive developmental stages,
and that miRNAs serve as key players in the precise definition of
spatiotemporal differentiation boundaries [66]. This phenomenon was observed
also in adult tissues and organs in both Drosophila [66] and mouse [65].
Moreover, both studies indicated that this mutual exclusion of miRNAs and their
targets does not stem from target degradation by the miRNA. From these two
studies, it became evident that posttranscriptional regulation by miRNAs is
somehow coordinated with transcription. However, it was not shown originally
how, at the mechanistic level, such "miRNA-target spatiotemporal
avoidance" is achieved. Combined transcriptional/posttranscriptional FFLs,
where the same TF regulates the transcription of both a miRNA and its target
genes, or where the miRNA targets a TF and its target genes as well, could
serve just that purpose (Figure 3). Such FFLs are thus suggested as a simple mechanism
that might facilitate the miRNA-target avoidance phenomenon, where a TF that
activates the target genes also represses the miRNA transcription in the
tissues in which it is expressed, or the miRNA represses both the TF and its
target genes, thereby indirectly causing reduced transcription of its targets
in the tissue where it is expressed (Figure 3) [61]. In addition, such FFLs
were further suggested to enable the "canalization" and the
maintenance of fidelity of developmental processes in general [57].
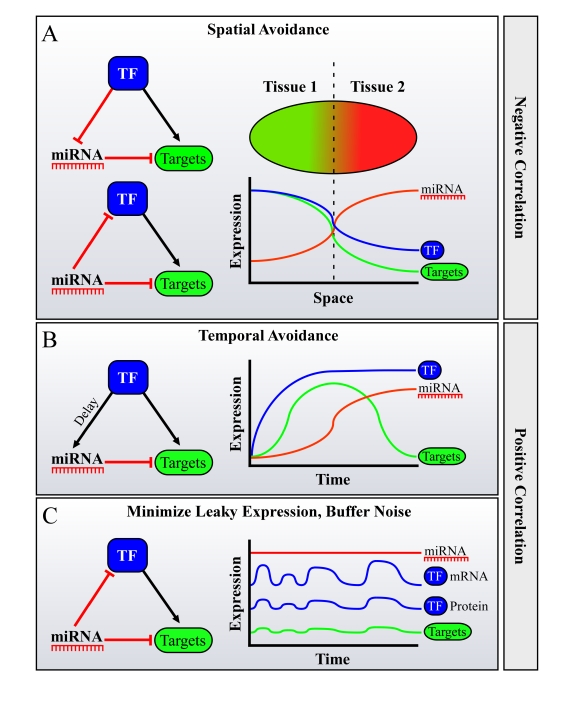
Figure 3. Possible roles for FFLs of miRNAs, Transcription Factors (TFs) and their mutual targets in facilitating spatiotemporal avoidance, or noise buffering. miRNAs are often
embedded in Feed-Forward loops (FFLs) with TFs, sharing mutual targets. It
was shown that in many cases during development, miRNAs and their targets
avoid expression in the same tissue or at the same developmental stage.
This phenome-non was termed "miRNA-target spatiotemporal
avoidance". The figure depicts how the network wiring of miRNAs in
combined transcriptional/posttranscriptional FFLs may explain the spatio-temporal
avoidance phenomenon. Different scenarios may facilitate spatial and
temporal avoidance, where the TF and the miRNA are either negatively
correlated in their expression across tissues (in A) or positively
correlated, namely are expressed in the same tissue (B or C).
(A) Spatial
avoidance may be facilitated by the presented FFLs when expression of a
miRNA and of a TF anti-correlates across tissues. (B) Temporal avoidance
may be facilitated by the presented FFL when a miRNA and a TF are
co-expressed in the same tissues, creating a temporal shut-down mechanism
for their mutual targets, when there is a delay between the activation of
the targets by the TF, and its activation of the miRNA. This delay may be
achieved for example by a lower affinity binding site of the TF to the
miRNA's promoter, by a natural miRNA processing time, etc. (C) Buffering of
noise in expression may also be facilitated by a FFL wiring when a miRNA and
a TF are co-expressed in the same tissues.
More recently, evidence has
been accumulating that such combined transcriptional post-transcriptional FFLs
indeed act as functional units in the regulation of cell fate in many cell
types and systems [48,58,67-71]. One striking example, recently published by
Marson et al. [69], demonstrated that miRNAs and TFs are involved
together in FFLs controlling the maintenance of mouse embryonic stem (ES) cell
identity. Consistent with the studies mentioned above [2,3,8,11], which
showed that complete miRNA ablation from ES cells eliminates their
differentiation capacity, Marson et al. showed that several FFLs
involving miRNAs and ES cell TFs act to regulate ES cell identity and
differentiation. For example, the miR-290-295 polycistronic cluster,
containing the most abundantly expressed miRNAs in mouse ES cells, is
positively regulated by the ES cell TF Oct4, whereas its promoter is co-occupied
by Oct4, Sox2, and Nanog. In addition, miR-290-295 co-regulate
mutual target genes along with these same TFs. Intriguingly, while miR-290-295 is a rodent specific cluster, a similar FFL involving Sox and Oct4 was computationally predicted in humans [61]. This FFL comprises miR-302,
which shares the same seed as the rodent-specific miR-290-295, and was
shown to be highly expressed in human ES cells [72],
perhaps serving as a miR-290-295 human ortholog.
Consideration of these results in the perspective of previous
studies on miRNAs role in ES cell differentiation
supports the conjecture that miRNA-involving FFLs might play an important
role in this context, and suggest potential conserved roles for similar FFLs in
the maintenance of human ES cell identity as well.
A
different perspective on miRNA-TF FFLs was recently provided by Brosh et al. [58].
In this study, a family of 15 homologous miRNAs transcribed as three polycistrons:
miR-106b/93/-25, miR-17-92 and miR-106a-363, were shown to form a proliferation-promoting
FFL together with the transcription factor E2F. These miRNAs were shown to
target a whole battery of anti-proliferative E2F target genes. Most
importantly, the study demonstrated that in normal fibroblasts p53 inhibits
this FFL as a central step towards cellular senescence. When this inhibition is
perturbed by overexpression of the miRNAs, normal cell fate is altered;
proliferation is accelerated and senescence is delayed. In agreement with these
results, breast cancer tumors bearing mutated p53 showed an elevation in the
levels of these miRNAs and were characterized by a high tumor grade, hinting at
the role of these miRNAs in promoting proliferation and aggressiveness also in
vivo in tumors. This miRNA family was
indeed reported in several independent studies to be related to promotion of
cancer [58,73,74] (also reviewed in [75]). The above study illustrates how deregulation
of the entire FFL may contribute to aberrant proliferation. It also reveals another
concept of network wiring of miRNAs, namely combinatorial regulation, and more
specifically combinatorial regulation by family-related miRNAs (Figure 2). Combinatorial
regulation by miRNAs was globally predicted based on co-occurrence of miRNA
target sites in common gene sets [61], and was also observed experimentally
[58,76].
miRNAs can be grouped by mature sequence
similarity into miRNA families. In some cases, as in the case of the miR-106b/93/-25 family mentioned above, these families are shown to represent paralogous
groups of miRNAs of a common evolutionary origin [77]. Just as paralogous genes
were duplicated during evolution but retained some degree of sequence
similarity, these paralogous miRNAs share similarity in their sequence, which
immediately suggests that they might also share common target genes. More
intriguingly, it seems that in many cases such families had not only retained
similar targets, but also retained similar transcriptional programs. As described
by Brosh et al. [58], the above family of 15 miRNAs retained their joint
transcriptional regulation by E2F. Coordinated transcriptional
regulation of a family of miRNAs, sharing similar targets, all of which are
part of the same pathway (in this case negative regulators of proliferation),
may have a cumulative effect on the overall levels of proteins in the pathway,
thus resulting in a strong effect on cell fate.
Coordinated regulation of
family miRNAs was also shown in other cases [78,79].
For example the miR-34 family, consisting of two transcription units and three mature family
members, were all shown to be transcriptionally activated by p53 and to
contribute to apoptosis [80,81], G1 cell-cycle arrest [82] and senescence
[83]. Moreover, miR-34a and miR-34c
were shown to target c-MYC [46, 84].
In addition, in both mouse and human ES cells, several related
miRNA families, often sharing similar seeds, were shown to be co-expressed [69,72]. Moreover, miRNAs from the same family were indeed verified experimentally
to have many shared targets [76].
Overall it seems that
combinatorial regulation of miRNAs, particularly from the same family, and
shared transcription programs for such miRNAs and their common targets portray
intricate network architecture (Figure 2). Such architecture is not only
over-represented [61], but may also cumulatively generate a strong output that
is likely to account for the observed effects on cell fate, and for its
alteration when the miRNAs are mis-regulated.
Concluding Remarks
It is intriguing that
despite a relatively mild influence of individual miRNAs on protein levels they
are indispensable to various cellular and organismal processes, including
control of cell fate and maintenance of genomic integrity. One possible explanation
for this may lie in the level of regulatory networks in which miRNAs are embedded.
Indeed, joint miRNA-TF FFLs are not only an over-represented architecture in
the network but a recurring principle of miRNA regulation of cell fate.
The connection between cell
fate and the wiring of miRNAs in coupled transcription/post-transcriptional
networks is appealing, and the multiple evidence outlines here serve to support
it.
Two principles are common
to the different examples discussed above: 1. miRNAs are embedded in
combined transcription-nal/post-transcriptional FFLs that co-target many genes.
2. Several co-regulated
miRNAs act together to exert their regulation on target genes involved in the
same pathway.
However, more studies should
be undertaken in order to fully establish the link between the network wiring of
miRNAs in transcriptional/post-transcriptional FFLs and their effect on cell
fate. A recent study demonstrated that the wiring of miR-7 in a network
of FFLs in the fly equips the network with robustness to environmental
perturbation [68]. Such approach suggests that when studying possible roles for
miRNAs, one should consider them as parts of a larger regulatory network,
rather than adopting the reductionist view of single miRNA - single target. Our
recognition of the centrality of miRNAs in the regulatory network may help us
to elucidate how miRNAs exert such profound impact on cell fate.
Conflicts of Interest
The authors declare no conflict of interests.
References
-
1.
Bartel
DP
MicroRNAs: genomics, biogenesis, mechanism, and function.
Cell.
2004;
116:
281
-297.
[PubMed]
.
-
2.
Bernstein
E
, Kim
SY
, Carmell
MA
, Murchison
EP
, Alcorn
H
, Li
MZ
, Mills
AA
, Elledge
SJ
, Anderson
KV
and Hannon
GJ.
Dicer is essential for mouse development.
Nat Genet.
2003;
35:
215
-217.
[PubMed]
.
-
3.
Wienholds
E
, Koudijs
MJ
, van
Eeden FJ
, Cuppen
E
and Plasterk
RH.
The microRNA-producing enzyme Dicer1 is essential for zebrafish development.
Nat Genet.
2003;
35:
217
-218.
[PubMed]
.
-
4.
Chen
JF
, Murchison
EP
, Tang
R
, Callis
TE
, Tatsuguchi
M
, Deng
Z
, Rojas
M
, Hammond
SM
, Schneider
MD
, Selzman
CH
, Meissner
G
, Patterson
C
and Hannon
GJ.
Targeted deletion of Dicer in the heart leads to dilated cardiomyopathy and heart failure.
Proc Natl Acad Sci U S A.
2008;
105:
2111
-2116.
[PubMed]
.
-
5.
Damiani
D
, Alexander
JJ
, O'Rourke
JR
, McManus
M
, Jadhav
AP
, Cepko
CL
, Hauswirth
WW
, Harfe
BD
and Strettoi
E.
Dicer inactivation leads to progressive functional and structural degeneration of the mouse retina.
J Neurosci.
2008;
28:
4878
-4887.
[PubMed]
.
-
6.
Davis
TH
, Cuellar
TL
, Koch
SM
, Barker
AJ
, Harfe
BD
, McManus
MT
and Ullian
EM.
Conditional loss of Dicer disruptscellular and tissue morphogenesis in the cortex and hippocampus.
J Neurosci.
2008;
28:
4322
-4330.
[PubMed]
.
-
7.
Volinia
S
, Calin
GA
, Liu
CG
, Ambs
S
, Cimmino
A
, Petrocca
F
, Visone
R
, Iorio
M
, Roldo
C
, Ferracin
M
, Prueitt
RL
, Yanaihara
N
and Lanza
G.
A microRNA expression signature of human solid tumors defines cancer gene targets.
Proc Natl Acad Sci U S A.
2006;
103:
2257
-2261.
[PubMed]
.
-
8.
Kanellopoulou
C
, Muljo
SA
, Kung
AL
, Ganesan
S
, Drapkin
R
, Jenuwein
T
, Livingston
DM
and Rajewsky
K.
Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing.
Genes Dev.
2005;
19:
489
-501.
[PubMed]
.
-
9.
Murchison
EP
, Stein
P
, Xuan
Z
, Pan
H
, Zhang
MQ
, Schultz
RM
and Hannon
GJ.
Critical roles for Dicer in the female germline.
Genes Dev.
2007;
21:
682
-693.
[PubMed]
.
-
10.
Bushati
N
and Cohen
SM.
microRNA functions.
Annu Rev Cell Dev Biol.
2007;
23:
175
-205.
[PubMed]
.
-
11.
Murchison
EP
, Partridge
JF
, Tam
OH
, Cheloufi
S
and Hannon
GJ.
Characterization of Dicer-deficient murine embryonic stem cells.
Proc Natl Acad Sci U S A.
2005;
102:
12135
-12140.
[PubMed]
.
-
12.
Houbaviy
HB
, Murray
MF
and Sharp
PA.
Embryonic stem cell-specific MicroRNAs.
Dev Cell.
2003;
5:
351
-358.
[PubMed]
.
-
13.
Gangaraju
VK
and Lin
H.
MicroRNAs: key regulators of stem cells.
Nat Rev Mol Cell Biol.
2009;
10:
116
-125.
[PubMed]
.
-
14.
Lu
J
, Guo
S
, Ebert
BL
, Zhang
H
, Peng
X
, Bosco
J
, Pretz
J
, Schlanger
R
, Wang
JY
, Mak
RH
, Dombkowski
DM
, Preffer
FI
and Scadden
DT.
MicroRNA-mediated control of cell fate in megakaryocyte-erythrocyte progenitors.
Dev Cell.
2008;
14:
843
-853.
[PubMed]
.
-
15.
Thai
TH
, Calado
DP
, Casola
S
, Ansel
KM
, Xiao
C
, Xue
Y
, Murphy
A
, Frendewey
D
, Valenzuela
D
, Kutok
JL
, Schmidt-Supprian
M
, Rajewsky
N
and Yancopoulos
G.
Regulation of the germinal center response by microRNA-155.
Science.
2007;
316:
604
-608.
[PubMed]
.
-
16.
Lee
RC
, Feinbaum
RL
and Ambros
V.
The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14.
Cell.
1993;
75:
843
-854.
[PubMed]
.
-
17.
Ambros
V
and Horvitz
HR.
Heterochronic mutants of the nematode Caenorhabditis elegans.
Science.
1984;
226:
409
-416.
[PubMed]
.
-
18.
Bonauer
A
, Carmona
G
, Iwasaki
M
, Mione
M
, Koyanagi
M
, Fischer
A
, Burchfield
J
, Fox
H
, Doebele
C
, Ohtani
K
, Chavakis
E
, Potente
M
and Tjwa
M.
MicroRNA-92a Controls Angiogenesis and Functional Recovery of Ischemic Tissues in Mice.
Science.
2009;
In press
.
-
19.
Ventura
A
, Young
AG
, Winslow
MM
, Lintault
L
, Meissner
A
, Erkeland
SJ
, Newman
J
, Bronson
RT
, Crowley
D
, Stone
JR
, Jaenisch
R
, Sharp
PA
and Jacks
T.
Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters.
Cell.
2008;
132:
875
-886.
[PubMed]
.
-
20.
Smibert
P
and Lai
EC.
Lessons from microRNA mutants in worms, flies and mice.
Cell Cycle.
2008;
7:
2500
-2508.
[PubMed]
.
-
21.
Alvarez-Garcia
I
and Miska
EA.
MicroRNA functions in animal development and human disease.
Development.
2005;
132:
4653
-4662.
[PubMed]
.
-
22.
Erson
AE
and Petty
EM.
MicroRNAs in development and disease.
Clin Genet.
2008;
74:
296
-306.
[PubMed]
.
-
23.
Bueno
MJ
, de Castro
IP
and Malumbres
M.
Control of cell proliferation pathways by microRNAs.
Cell Cycle.
2008;
7:
3143
-3148.
[PubMed]
.
-
24.
Chivukula
RR
and Mendell
JT.
Circular reasoning: microRNAs and cellcycle control.
Trends Biochem Sci.
2008;
33:
474
-481.
[PubMed]
.
-
25.
Jovanovic
M
and Hengartner
MO.
miRNAs and apoptosis: RNAs to die for.
Oncogene.
2006;
25:
6176
-6187.
[PubMed]
.
-
26.
Lu
J
, Getz
G
, Miska
EA
, Alvarez-Saavedra
E
, Lamb
J
, Peck
D
, Sweet-
Cordero A
, Ebert
BL
, Mak
RH
, Ferrando
AA
, Downing
JR
, Jacks
T
and Horvitz
HR.
MicroRNA expression profiles classify human cancers.
Nature.
2005;
435:
834
-838.
[PubMed]
.
-
27.
Chang
TC
, Yu
D
, Lee
YS
, Wentzel
EA
, Arking
DE
, West
KM
, Dang
CV
, Thomas-Tikhonenko
A
and Mendell
JT.
Widespread microRNA repression by Myc contributes to tumorigenesis.
Nat Genet.
2008;
40:
43
-50.
[PubMed]
.
-
28.
Bueno
MJ
, Perez
de Castro I
, Gomez
de Cedron M
, Santos
J
, Calin
GA
, Cigudosa
JC
, Croce
CM
, Fernandez-Piqueras
J
and Malumbres
M.
Genetic and epigenetic silencing of microRNA-203 enhances ABL1 and BCR-ABL1 oncogene expression.
Cancer Cell.
2008;
13:
496
-506.
[PubMed]
.
-
29.
Calin
GA
, Dumitru
CD
, Shimizu
M
, Bichi
R
, Zupo
S
, Noch
E
, Aldler
H
, Rattan
S
, Keating
M
, Rai
K
, Rassenti
L
, Kipps
T
and Negrini
M.
Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia.
Proc Natl Acad Sci U S A.
2002;
99:
15524
-15529.
[PubMed]
.
-
30.
Calin
GA
, Sevignani
C
, Dumitru
CD
, Hyslop
T
, Noch
E
, Yendamuri
S
, Shimizu
M
, Rattan
S
, Bullrich
F
, Negrini
M
and Croce
CM.
Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers.
Proc Natl Acad Sci U S A.
2004;
101:
2999
-3004.
[PubMed]
.
-
31.
Datta
J
, Kutay
H
, Nasser
MW
, Nuovo
GJ
, Wang
B
, Majumder
S
, Liu
CG
, Volinia
S
, Croce
CM
, Schmittgen
TD
, Ghoshal
K
and Jacob
ST.
Methylation mediated silencing of MicroRNA-1 gene and its role in hepatocellular carcinogenesis.
Cancer Res.
2008;
68:
5049
-5058.
[PubMed]
.
-
32.
Lujambio
A
, Calin
GA
, Villanueva
A
, Ropero
S
, Sanchez-Cespedes
M
, Blanco
D
, Montuenga
LM
, Rossi
S
, Nicoloso
MS
, Faller
WJ
, Gallagher
WM
, Eccles
SA
and Croce
CM.
A microRNA DNA methylation signature for human cancer metastasis.
Proc Natl Acad Sci U S A.
2008;
105:
13556
-13561.
[PubMed]
.
-
33.
Zhang
L
, Volinia
S
, Bonome
T
, Calin
GA
, Greshock
J
, Yang
N
, Liu
CG
, Giannakakis
A
, Alexiou
P
, Hasegawa
K
, Johnstone
CN
, Megraw
MS
and Adams
S.
Genomic and epigenetic alterations deregulate microRNA expression in human epithelial ovarian cancer.
Proc Natl Acad Sci U S A.
2008;
105:
7004
-7009.
[PubMed]
.
-
34.
Migliazza
A
, Bosch
F
, Komatsu
H
, Cayanis
E
, Martinotti
S
, Toniato
E
, Guccione
E
, Qu
X
, Chien
M
, Murty
VV
, Gaidano
G
, Inghirami
G
and Zhang
P.
Nucleotide sequence, transcription map, and mutation analysis of the 13q14 chromosomal region deleted in B-cell chronic lymphocytic leukemia.
Blood.
2001;
97:
2098
-2104.
[PubMed]
.
-
35.
Migliazza
A
, Cayanis
E
, Bosch-Albareda
F
, Komatsu
H
, Martinotti
S
, Toniato
E
, Kalachikov
S
, Bonaldo
MF
, Jelene
P
, Ye
X
, Rzhetsky
A
, Qu
X
and Chien
M.
Molecular pathogenesis of B-cell chronic lymphocytic leukemia: analysis of 13q14 chromosomal deletions.
Curr Top Microbiol Immunol.
2000;
252:
275
-284.
[PubMed]
.
-
36.
Lee
EJ
, Baek
M
, Gusev
Y
, Brackett
DJ
, Nuovo
GJ
and Schmittgen
TD.
Systematic evaluation of microRNA processing patterns in tissues, cell lines, and tumors.
Rna.
2008;
14:
35
-42.
[PubMed]
.
-
37.
Heo
I
, Joo
C
, Cho
J
, Ha
M
, Han
J
and Kim
VN.
Lin28 mediates the terminal uridylation of let-7 precursor MicroRNA.
Mol Cell.
2008;
32:
276
-284.
[PubMed]
.
-
38.
Newman
MA
, Thomson
JM
and Hammond
SM.
Lin-28 interaction with the Let-7 precursor loop mediates regulated microRNA processing.
Rna.
2008;
14:
1539
-1549.
[PubMed]
.
-
39.
Viswanathan
SR
, Daley
GQ
and Gregory
RI.
Selective blockade of microRNA processing by Lin28.
Science.
2008;
320:
97
-100.
[PubMed]
.
-
40.
Viswanathan
SR
, Powers
JT
, Einhorn
W
, Hoshida
Y
, Ng
TL
, Toffanin
S
, O'Sullivan
M
, Lu
J
, Phillips
LA
, Lockhart
VL
, Shah
SP
, Tanwar
PS
and Mermel
CH.
Lin28 promotes transformation and is associated with advanced human malignancies.
Nat Genet.
2009;
In press
.
-
41.
Suzuki
HI
, Yamagata
K
, Sugimoto
K
, Iwamoto
T
, Kato
S
and Miyazono
K.
Modulation of microRNA processing by p53.
Nature.
2009;
460:
529
-533.
[PubMed]
.
-
42.
Sandberg
R
, Neilson
JR
, Sarma
A
, Sharp
PA
and Burge
CB.
Proliferating cells express mRNAs with shortened 3' untranslated regions and fewer microRNA target sites.
Science.
2008;
320:
1643
-1647.
[PubMed]
.
-
43.
Kumar
MS
, Lu
J
, Mercer
KL
, Golub
TR
and Jacks
T.
Impaired microRNA processing enhances cellular transformation and tumorigenesis.
Nat Genet.
2007;
39:
673
-677.
[PubMed]
.
-
44.
Mudhasani
R
, Zhu
Z
, Hutvagner
G
, Eischen
CM
, Lyle
S
, Hall
LL
, Lawrence
JB
, Imbalzano
AN
and Jones
SN.
Loss of miRNA biogenesis induces p19Arf-p53 signaling and senescence in primary cells.
J Cell Biol.
2008;
181:
1055
-1063.
[PubMed]
.
-
45.
Serrano
M
, Lin
AW
, McCurrach
ME
, Beach
D
and Lowe
SW.
Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a.
Cell.
1997;
88:
593
-602.
[PubMed]
.
-
46.
Christoffersen
NR
, Shalgi
R
, Frankel
LB
, Klausen
M
, Pilpel
Y
, Nielsen
FC
, Oren
M
and Lund
AH.
p53-independent upregulation of miR-34a during oncogene-induced senescence represses MYC.
Cell Death Differ.
2009;
In press
.
-
47.
Johnson
SM
, Grosshans
H
, Shingara
J
, Byrom
M
, Jarvis
R
, Cheng
A
, Labourier
E
, Reinert
KL
, Brown
D
and Slack
FJ.
RAS is regulated by the let-7 microRNA family.
Cell.
2005;
120:
635
-647.
[PubMed]
.
-
48.
Sachdeva
M
, Zhu
S
, Wu
F
, Wu
H
, Walia
V
, Kumar
S
, Elble
R
, Watabe
K
and Mo
YY.
p53 represses c-Myc through induction of the tumor suppressor miR-145.
Proc Natl Acad Sci U S A.
2009;
106:
3207
-3212.
[PubMed]
.
-
49.
Hanahan
D
and Weinberg
RA.
The hallmarks of cancer.
Cell.
2000;
100:
57
-70.
[PubMed]
.
-
50.
Olsen
PH
and Ambros
V.
The lin-4 regulatory RNA controls developmental timing in Caenorhabditis elegans by blocking LIN-14 protein synthesis after the initiation of translation.
Dev Biol.
1999;
216:
671
-680.
[PubMed]
.
-
51.
Wightman
B
, Ha
I
and Ruvkun
G.
Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans.
Cell.
1993;
75:
855
-862.
[PubMed]
.
-
52.
Liu
J
, Carmell
MA
, Rivas
FV
, Marsden
CG
, Thomson
JM
, Song
JJ
, Hammond
SM
, Joshua-Tor
L
and Hannon
GJ.
Argonaute2 is the catalytic engine of mammalian RNAi.
Science.
2004;
305:
1437
-1441.
[PubMed]
.
-
53.
Meister
G
, Landthaler
M
, Patkaniowska
A
, Dorsett
Y
, Teng
G
and Tuschl
T.
Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs.
Mol Cell.
2004;
15:
185
-197.
[PubMed]
.
-
54.
Lim
LP
, Lau
NC
, Garrett-Engele
P
, Grimson
A
, Schelter
JM
, Castle
J
, Bartel
DP
, Linsley
PS
and Johnson
JM.
Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs.
Nature.
2005;
433:
769
-773.
[PubMed]
.
-
55.
Baek
D
, Villen
J
, Shin
C
, Camargo
FD
, Gygi
SP
and Bartel
DP.
The impact of microRNAs on protein output.
Nature.
2008;
455:
64
-71.
[PubMed]
.
-
56.
Selbach
M
, Schwanhausser
B
, Thierfelder
N
, Fang
Z
, Khanin
R
and Rajewsky
N.
Widespread changes in protein synthesis induced by microRNAs.
Nature.
2008;
455:
58
-63.
[PubMed]
.
-
57.
Hornstein
E
and Shomron
N.
Canalization of development by microRNAs.
Nat Genet.
2006;
38 Suppl:
S20
-24.
[PubMed]
.
-
58.
Brosh
R
, Shalgi
R
, Liran
A
, Landan
G
, Korotayev
K
, Nguyen
GH
, Enerly
E
, Johnsen
H
, Buganim
Y
, Solomon
H
, Goldstein
I
, Madar
S
and Goldfinger
N.
p53-Repressed miRNAs are involved with E2F in a feed-forward loop promoting proliferation.
Mol Syst Biol.
2008;
4:
229
[PubMed]
.
-
59.
John
B
, Enright
AJ
, Aravin
A
, Tuschl
T
, Sander
C
and Marks
DS.
Human MicroRNA targets.
PLoS Biol.
2004;
2:
e363
[PubMed]
.
-
60.
Stark
A
, Brennecke
J
, Russell
RB
and Cohen
SM.
Identification of Drosophila MicroRNA targets.
PLoS Biol.
2003;
1:
E60
[PubMed]
.
-
61.
Shalgi
R
, Lieber
D
, Oren
M
and Pilpel
Y.
Global and Local Architecture of the Mammalian microRNA-Transcription Factor Regulatory Network.
PLoS Comput Biol.
2007;
3:
e131
[PubMed]
.
-
62.
Tsang
J
, Zhu
J
and van
Oudenaarden A.
MicroRNA-mediated feedback and feedforward loops are recurrent network motifs in mammals.
Mol Cell.
2007;
26:
753
-767.
[PubMed]
.
-
63.
Milo
R
, Shen-Orr
S
, Itzkovitz
S
, Kashtan
N
, Chklovskii
D
and Alon
U.
Network motifs: simple building blocks of complex networks.
Science.
2002;
298:
824
-827.
[PubMed]
.
-
64.
Shen-Orr
SS
, Milo
R
, Mangan
S
and Alon
U.
Network motifs in the transcriptional regulation network of Escherichia coli.
Nat Genet.
2002;
31:
64
-68.
[PubMed]
.
-
65.
Farh
KK
, Grimson
A
, Jan
C
, Lewis
BP
, Johnston
WK
, Lim
LP
, Burge
CB
and Bartel
DP.
The widespread impact of mammalian MicroRNAs on mRNA repression and evolution.
Science.
2005;
310:
1817
-1821.
[PubMed]
.
-
66.
Stark
A
, Brennecke
J
, Bushati
N
, Russell
RB
and Cohen
SM.
Animal MicroRNAs confer robustness to gene expression and have a significant impact on 3'UTR evolution.
Cell.
2005;
123:
1133
-1146.
[PubMed]
.
-
67.
Cohen
EE
, Zhu
H
, Lingen
MW
, Martin
LE
, Kuo
WL
, Choi
EA
, Kocherginsky
M
, Parker
JS
, Chung
CH
and Rosner
MR.
A feed-forward loop involving protein kinase Calpha and microRNAs regulates tumor cell cycle.
Cancer Res.
2009;
69:
65
-74.
[PubMed]
.
-
68.
Li
X
, Cassidy
JJ
, Reinke
CA
, Fischboeck
S
and Carthew
RW.
A microRNA imparts robustness against environmental fluctuation during development.
Cell.
2009;
137:
273
-282.
[PubMed]
.
-
69.
Marson
A
, Levine
SS
, Cole
MF
, Frampton
GM
, Brambrink
T
, Johnstone
S
, Guenther
MG
, Johnston
WK
, Wernig
M
, Newman
J
, Calabrese
JM
, Dennis
LM
and Volkert
TL.
Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells.
Cell.
2008;
134:
521
-533.
[PubMed]
.
-
70.
O'Donnell
KA
, Wentzel
EA
, Zeller
KI
, Dang
CV
and Mendell
JT.
c-Myc regulated microRNAs modulate E2F1 expression.
Nature.
2005;
435:
839
-843.
[PubMed]
.
-
71.
Woods
K
, Thomson
JM
and Hammond
SM.
Direct regulation of an oncogenic micro-RNA cluster by E2F transcription factors.
J Biol Chem.
2007;
282:
2130
-2134.
[PubMed]
.
-
72.
Laurent
LC
, Chen
J
, Ulitsky
I
, Mueller
FJ
, Lu
C
, Shamir
R
, Fan
JB
and Loring
JF.
Comprehensive microRNA profiling reveals a unique human embryonic stem cell signature dominated by a single seed sequence.
Stem Cells.
2008;
26:
1506
-1516.
[PubMed]
.
-
73.
He
L
, Thomson
JM
, Hemann
MT
, Hernando-Monge
E
, Mu
D
, Goodson
S
, Powers
S
, Cordon-Cardo
C
, Lowe
SW
, Hannon
GJ
and Hammond
SM.
A microRNA polycistron as a potential human oncogene.
Nature.
2005;
435:
828
-833.
[PubMed]
.
-
74.
Li
Y
, Tan
W
, Neo
TW
, Aung
MO
, Wasser
S
, Lim
SG
and Tan
TM.
Role of the miR-106b-25 microRNA cluster in hepatocellular carcinoma.
Cancer Sci.
2009;
In press
.
-
75.
Mendell
JT
miRiad roles for the miR-17-92 cluster in development and disease.
Cell.
2008;
133:
217
-222.
[PubMed]
.
-
76.
Ivanovska
I
and Cleary
MA.
Combinatorial microRNAs: working together to make a difference.
Cell Cycle.
2008;
7:
3137
-3142.
[PubMed]
.
-
77.
Tanzer
A
and Stadler
PF.
Molecular evolution of a microRNA cluster.
J Mol Biol.
2004;
339:
327
-335.
[PubMed]
.
-
78.
He
L
, He
X
, Lim
LP
, de Stanchina
E
, Xuan
Z
, Liang
Y
, Xu
W
, Zender
L
, Magnus
J
, Ridzon
D
, Jackson
AL
, Linsley
PS
and Chen
C.
A microRNA component of the p53 tumour suppressor network.
Nature.
2007;
447:
1130
-1134.
[PubMed]
.
-
79.
Zhao
Y
, Samal
E
and Srivastava
D.
Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis.
Nature.
2005;
436:
214
-220.
[PubMed]
.
-
80.
Chang
TC
, Wentzel
EA
, Kent
OA
, Ramachandran
K
, Mullendore
M
, Lee
KH
, Feldmann
G
, Yamakuchi
M
, Ferlito
M
, Lowenstein
CJ
, Arking
DE
, Beer
MA
and Maitra
A.
Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis.
Mol Cell.
2007;
26:
745
-752.
[PubMed]
.
-
81.
Raver-Shapira
N
, Marciano
E
, Meiri
E
, Spector
Y
, Rosenfeld
N
, Moskovits
N
, Bentwich
Z
and Oren
M.
Transcriptional activation of miR- 34a contributes to p53-mediated apoptosis.
Mol Cell.
2007;
26:
731
-743.
[PubMed]
.
-
82.
Tarasov
V
, Jung
P
, Verdoodt
B
, Lodygin
D
, Epanchintsev
A
, Menssen
A
, Meister
G
and Hermeking
H.
Differential regulation of microRNAs by p53 revealed by massively parallel sequencing: miR-34a is a p53 target that induces apoptosis and G1-arrest.
Cell Cycle.
2007;
6:
1586
-1593.
[PubMed]
.
-
83.
Kumamoto
K
, Spillare
EA
, Fujita
K
, Horikawa
I
, Yamashita
T
, Appella
E
, Nagashima
M
, Takenoshita
S
, Yokota
J
and Harris
CC.
Nutlin-3a activates p53 to both down-regulate inhibitor of growth 2 and up-regulate mir-34a, mir- 34b, and mir-34c expression, and induce senescence.
Cancer Res.
2008;
68:
3193
-3203.
[PubMed]
.
-
84.
Kong
YW
, Cannell
IG
, de Moor
CH
, Hill
K
, Garside
PG
, Hamilton
TL
, Meijer
HA
, Dobbyn
HC
, Stoneley
M
, Spriggs
KA
, Willis
AE
and Bushell
M.
The mechanism of micro-RNA-mediated translation repression is determined by the promoter of the target gene.
Proc Natl Acad Sci U S A.
2008;
105:
8866
-8871.
[PubMed]
.