The mRNA decay factor tristetraprolin (TTP) induces senescence in human papillomavirus-transformed cervical cancer cells by targeting E6-AP ubiquitin ligase
Abstract
The RNA-binding protein tristetraprolin (TTP) regulates expression of many cancer-associated and proinflammatory factors through binding AU-rich elements (ARE) in the 3'-untranslated region (3'UTR) and facilitating rapid mRNA decay. Here we report on the ability of TTP to act in an anti-proliferative capacity in HPV18-positive HeLa cells by inducing senescence. HeLa cells maintain a dormant p53 pathway and elevated telomerase activity resulting from HPV-mediated transformation, whereas TTP expression counteracted this effect by stabilizing p53 protein and inhibiting hTERT expression. Presence of TTP did not alter E6 and E7 viral mRNA levels indicating that these are not TTP targets. It was found that TTP promoted rapid mRNA decay of the cellular ubiquitin ligase E6-associated protein (E6-AP). RNA-binding studies demonstrated TTP and E6-AP mRNA interaction and deletion of the E6-AP mRNA ARE-containing 3'UTR imparts resistance to TTP-mediated downregulation. Similar results were obtained with high-risk HPV16-positive cells that employ the E6-AP pathway to control p53 and hTERT levels. Furthermore, loss of TTP expression was consistently observed in cervical cancer tissue compared to normal tissue. These findings demonstrate the ability of TTP to act as a tumor suppressor by inhibiting the E6-AP pathway and indicate TTP loss to be a critical event during HPV-mediated carcinogenesis.
Introduction
Cervical cancer is the second most common
cancer among women worldwide [1]. A necessary
factor in the development of nearly all cases of cervical cancer is infection
with the high-risk human papillomavirus (HPV) types 16 and 18 [2]. These
subtypes of HPV promote cellular transformation through
expression of the early viral genes E6 and E7. The HPV E7 protein
neutralizes the retinoblastoma (Rb) tumor suppressor pathway by sequestering
Rb from E2F and promoting its destabilization [3,4], while the E6 protein promotes degradation of the p53 tumor
suppressor and activates transcription of the human telomerase reverse
transcriptase gene (hTERT) [5,6]. The oncogenic functions of E6 occur through its interaction with a
number of cellular regulatory proteins and one of the best characterized
E6-binding partners is the E6-associated protein (E6-AP) [7,8]. E6-AP
belongs to a class of HECT ubiquitin-protein ligases [9] and its
interaction with E6 facilitates cell transformation through enhanced p53
protein degradation and activation of hTERT gene expression [10].
Deregulation of these critical factors through the combined action of E6 and E7
oncoproteins allows for continued cell proliferation and genomic instability
ultimately leading to HPV-mediated cellular transformation.
Messenger RNA turnover is a tightly regulated process
that plays a central role in controlling mammalian gene expression. The significance of this is evident in disease and
tumorigenesis where loss of post-transcriptional gene regulation accounts for
the aberrant overexpression of a variety of genes encoding growth factors,
inflammatory cytokines and proto-oncogenes [11,12]. A majority of cancer-associated
immediate-early response genes that control growth and inflammation display
conserved cis-acting adenylate- and uridylate (AU)-rich elements (ARE)
in the mRNA 3' untranslated region (3'UTR). A primary function of the ARE is to
target mRNAs for rapid decay through interaction with trans-acting
RNA-binding proteins that have high affinity for AREs. Among the best
characterized ARE-binding proteins involved in promoting ARE-mediated mRNA
decay is tristetraprolin (TTP, ZFP36, TIS11). TTP is a member of a small family
of tandem Cys3His zinc finger proteins originally identified as an inducible
immediate-early response gene [13]. Initially thought to be a
transcription factor, various studies have established the role of TTP as an
mRNA decay protein that binds to AREs in the mRNA of various inflammatory
mediators (e.g. TNF-α, GM-CSF, COX-2) [14-16]. The binding of TTP to ARE-mRNAs
targets them for rapid degradation through association with various decay
enzymes [14,17-21]. The physiological role of TTP
is significant as TTP deficient mice develop a number of inflammatory
syndromes. These abnormalities have been shown to be due to excessive levels
of pro-inflammatory factors resulting from defects in ARE-mediated decay in
these mice [22,23].
In this study, we examined the role of TTP in
HPV-mediated cervical carcinogenesis. Expression of TTP in HPV18-positive HeLa
cells dramatically inhibited cell growth by inducing cellular senescence
through a mechanism involving p53 protein stabilization and inhibition of
telomerase expression. It was found that TTP induced cellular senescence
through rapid decay of E6-AP ubiquitin ligase mRNA that was mediated through
the ARE-containing 3'UTR of E6-AP. Furthermore,
we demonstrate that TTP expression is lost in cervical cancer compared to
normal tissue, implying a tumor suppressor function for TTP in cervical tissue.
These novel findings not only add another attribute to the already established
anti-inflammatory role of this ARE-binding protein but also bring new insights
into the mechanism of HPV-mediated cervical carcinogenesis.
Results
TTP-mediated induction of senescence in HeLa cells
Based on its ability to control expression of ARE-containing mRNAs
associated with various aspects of cellular
transformation and tumorigenesis, TTP can serve in
a tumor suppressor capacity. To test this in HPV-transformed cervical carcinoma
cells, a tetracycline (Tet)-regulated TTP expression system in HeLa cells was
developed. HeLa Tet-Off cells were stably transfected with a Flag
epitope-tagged TTP cDNA in a Tet-regulated expression vector such that cells
grown in the
absence of doxycycline (Dox) allow for the expression of TTP (Figure 1A).
Consistent with other findings [24,25], endogenous TTP
expression was undetectable in HeLa cells and in HeLa Tet-Off parental cells
grown in the presence or absence of Dox (Figure 1A and data not shown).
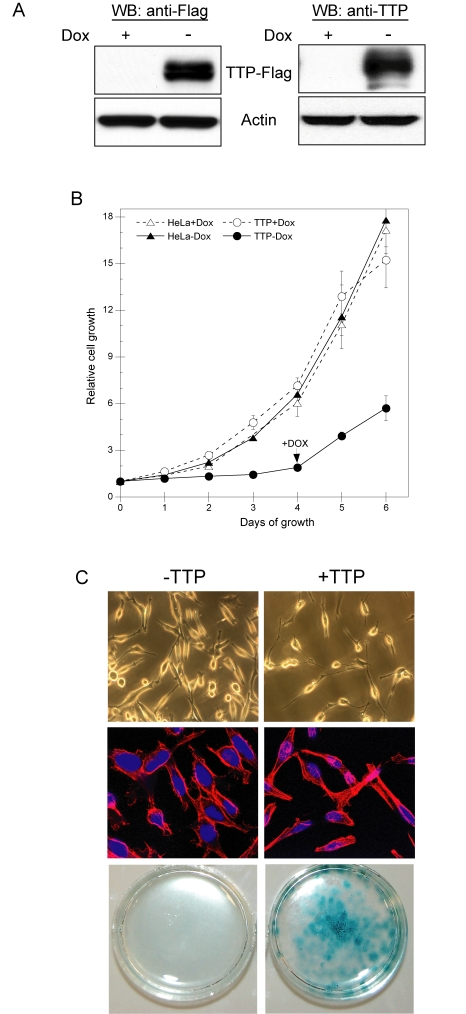
Figure 1. TTP inhibits HeLa cell proliferation through induction of senescence. (A) HeLa
Tet-Off/TTP-Flag cells grown in the presence or absence of 2 μg/ml Dox for
48 hr. The expression of TTP-Flag was detected by western blot (WB) using
antibodies against the Flag epitope (left panel) or TTP (right panel).
Actin was used as a loading control. (B) Growth curves of HeLa
Tet-Off/TTP-Flag (circles) and parental HeLa Tet-Off (triangles) cells in
the presence (open symbols) or absence (filled symbols) of 2 μg/ml Dox. On
day 4 of growth, Dox was added to HeLa Tet-Off/TTP-Flag cells to repress
TTP expression. Each point represents the mean of 4 replicates. (C)
HeLa Tet-Off/TTP-Flag cells were grown in the presence or absence of Dox to
repress (- TTP) or induce (+ TTP) TTP, respectively. Phase contrast (top
panels) and fluorescence (middle panels) microscopy of cells after 48 hr of
TTP expression; original magnification 200X and
400X, respectively. Nuclei (blue) and cytoskeleton (red) are shown in
fluorescent micrographs. HeLa-Tet-Off/TTP-Flag cells were stained for
SA-β-gal activity (bottom panels) after 12 days of TTP expression.
To
determine the consequence of TTP expression, we evaluated the ability of TTP to
attenuate HeLa cell growth and proliferation. As shown in Figure 1B, HeLa Tet-Off/TTP-Flag cells grown in the absence of Dox
showed a marked reduction in proliferation and this effect was dependent on
TTP; re-addition of Dox to turn-off TTP expression allowed for increased cell
growth. Consistent with this, a decreased rate of DNA synthesis was observed in
HeLa cells expressing TTP and similar results were obtained from 3 other
independent HeLa Tet-Off/TTP-Flag clones (data not shown). Interestingly, HeLa
cells grown in the presence of TTP for 48 hr exhibited a flattened morphology
resembling cells that had undergone replicative senescence (Figure 1C, [26]). Upon longer exposure to TTP (12 days), these cells
contained elevated levels of senescence-associated β-galactosidase (SA β-gal) [27] further indicating the ability of TTP to attenuate HeLa
cell growth through a mechanism involving cellular senescence.
TTP promotes p53 expression through protein stabilization
HPV oncogenicity is
mediated through the interaction between HPV E6 protein and the tumor
suppressor p53 with E6 promoting accelerated ubiquitin-mediated degradation of
p53 [7,10]. Based on this, we sought to determine if the growth inhibitory effect
exerted by TTP was being modulated through p53 activation. As shown in Figure 2A, induction of TTP in HeLa Tet-Off/TTP-Flag cells resulted in increased
expression of p53 protein. Similarly, infection of HeLa cells with an
adenovirus expressing TTP resulted in enhanced p53 protein expression as
compared to cells infected with control adenovirus expressing GFP (Figure 2A).
The ability of TTP to promote p53 expression appeared to be through protein
stabilization since p53 mRNA levels were not respectively increased with TTP
induction (Figure 2B). To specifically test this, HeLa Tet-Off/TTP-Flag cells
were grown in presence or absence of TTP for 48 hr and then treated with cycloheximide
(CHX) to inhibit protein synthesis. In the presence of TTP, the half-life of
p53 protein was increased 3-fold (Figure 2C), indicating the ability of TTP to
inhibit p53 protein turnover.
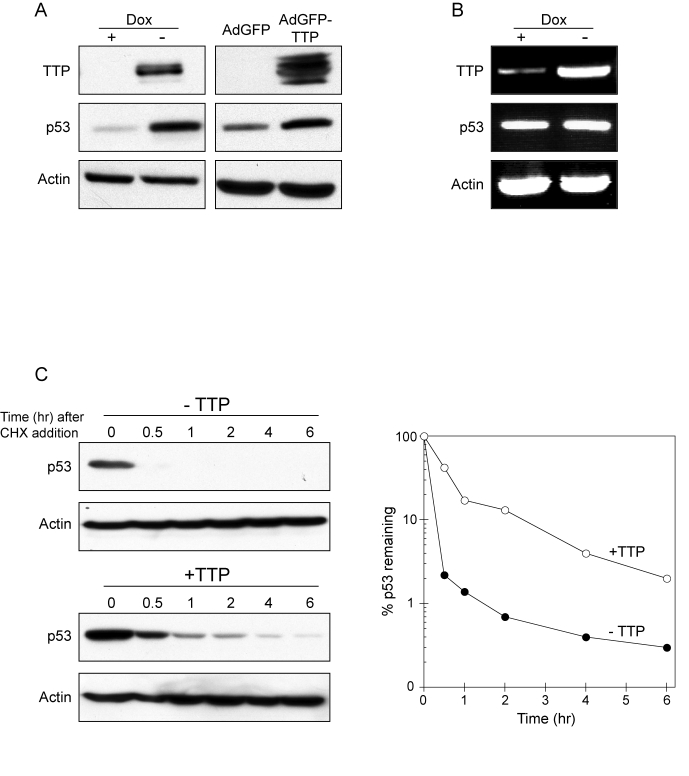
Figure 2. TTP promotes p53 expression through protein stabilization.
(A) HeLa Tet-Off/TTP-Flag cells grown in presence or absence of Dox for 48 hr
(left panel) and HeLa cells infected with AdGFP or AdGFP/TTP virus for 48 hr
(right panel) were examined for TTP and p53 expression by western blotting.
Actin was used as a loading control. (B) RT-PCR analysis of p53 mRNA
expression in HeLa Tet-Off/TTP-Flag cells grown in presence or absence of
Dox for 48 hr. Induction TTP-Flag mRNA is shown along with loading control
GAPDH. (C) TTP promotes
increased stability of p53 protein. HeLa Tet-Off/TTP-Flag cells grown in
presence (- TTP) or absence (+ TTP) of Dox for 48 hr were incubated with 20
μg/ml cycloheximide (CHX) to inhibit
protein synthesis for the indicated times. Decay of p53 protein was
examined by western blot (left panels) using actin as a loading control.
Decay curves of p53 protein (right panel) in the presence (open circles)
and absence (filled circles) of TTP was obtained by western blot analysis
and normalized to the internal control actin.
Activation of p53 promotes its
accumulation in the nucleus and transcription of p53-responsive promoters [28,29]. To
determine if nuclear localization of p53 is occurring in cells expressing TTP,
HeLa Tet-Off/TTP-Flag cells were examined for p53 localization by immunofluorescence.
In cells grown in the presence of TTP, p53 was detected in both the nucleus and
cytoplasm with a high level of p53 localized to the nucleus (Figure 3A). In
parallel experiments, a reporter construct containing a p53-dependent promoter
was transfected into HeLa Tet-Off/TTP-Flag cells and its activity was examined
in the presence and absence of TTP (Figure 3B). The magnitude of promoter
activity was significantly increased in the presence of TTP, consistent with
observed p53 protein stabilization and nuclear localization promoted by TTP.
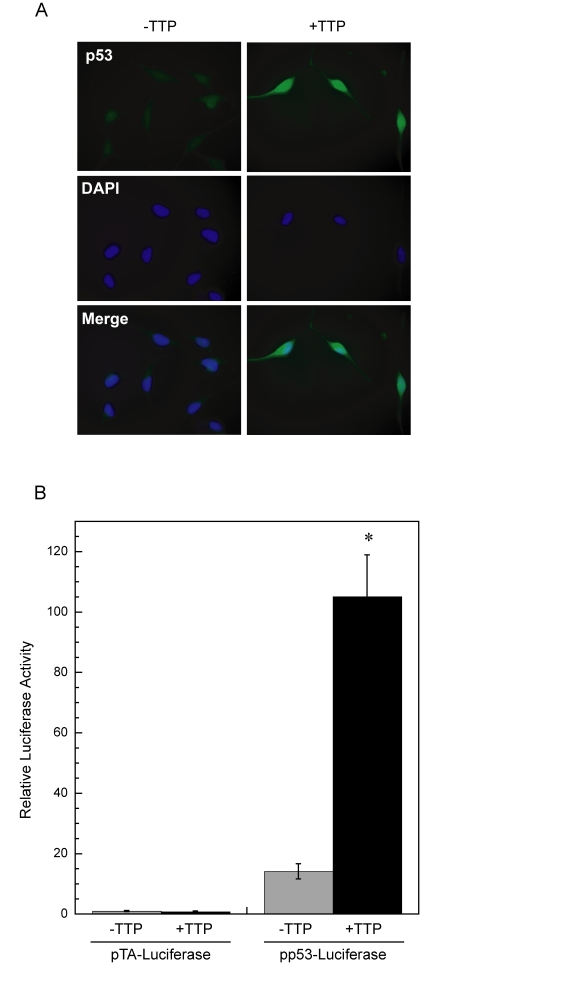
Figure 3. Enhanced p53 activity in HeLa cells expressing TTP. (A)
Immunofluorescent detection of p53, shown in green, in HeLa
Tet-Off/TTP-Flag cellsin the absence or
presence of TTP for 48 hr. DAPI nuclear staining and merged images are
shown. (B) Expression of TTP induces p53 transcriptional activity.
Luciferase reporter constructs containing either a p53-dependent promoter
(pp53-Luciferase) or control vector (pTA-Luciferase) were transfected into
HeLa Tet-Off/TTP-Flag cells and allowed to grow without (grey bars) or with
(black bars) TTP induction for 48 hr. Relative activity was assessed as
luciferase activity normalized to renilla activity and are the averages of
3 experiments. (*) P < 0.01
TTP expression downregulates telomerase activity
Elevated telomerase activity is
associated with approximately 85% of human cancers [30]. In
cervical cancers, the HPV E6 protein induces telomerase activity by promoting
expression of the catalytic subunit of telomerase, hTERT [31]. To
determine if TTP expression impacted hTERT levels, HeLa Tet-Off/TTP-Flag cells
were grown in the presence and absence of Dox for 48 hr and hTERT mRNA and
protein was evaluated. As shown in Figure 4A, steady state levels of both hTERT
mRNA and protein were dramatically reduced in presence of TTP. Consistent with
this inhibition, a decrease in telomerase activity was also detected (Figure 4B). In cells expressing TTP, a decrease in the characteristic laddering using
a telomeric repeat amplification protocol (TRAP) assay was observed indicating
that TTP inhibits telomerase activity through inhibition of hTERT expression.
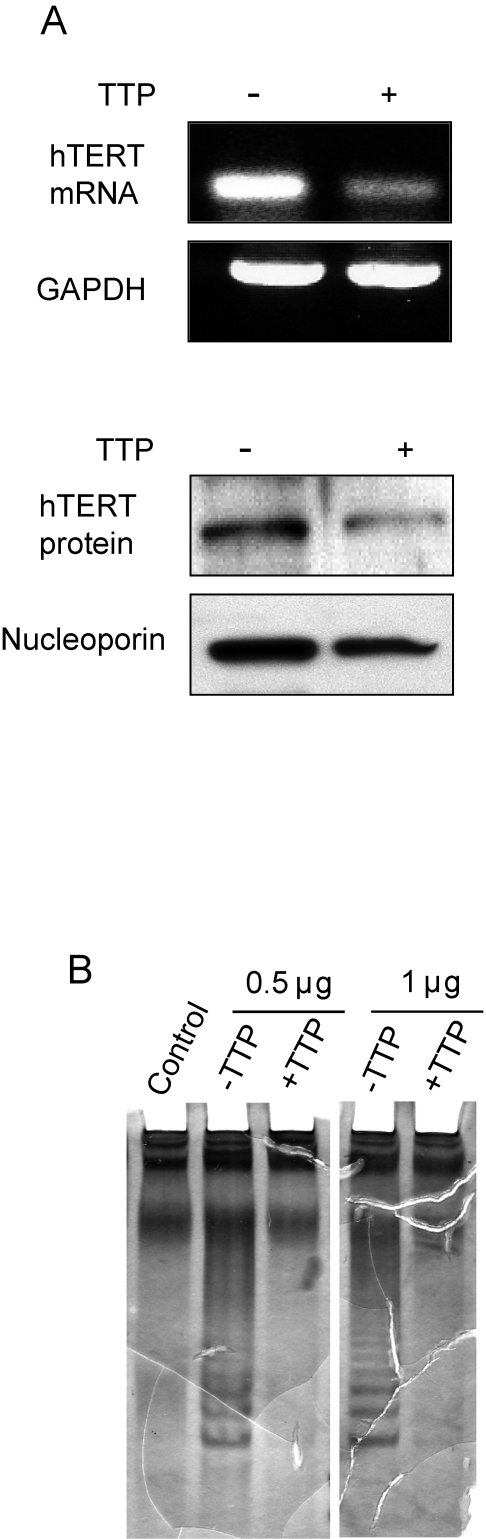
Figure 4. TTP-mediated inhibition of hTERT expression. (A) hTERT
expression in HeLa Tet-Off/TTP-Flag cells growing in absence or presence of
TTP for 48 hr was examined by RT-PCR analysis (top panel) and western blot
using nuclear lysates (bottom panel). GAPDH and nucleoporin were detected
as loading controls, respectively. (B) TRAP assay showing inhibition
of telomerase activity in TTP-expressing cells. 0.5 and 1 μg of lysate from
cells grown in the absence or presence of TTP was used for TRAP assay as
described in Methods. Control reaction lacks Taq polymerase.
TTP promotes downregulation of E6-AP ubiquitin ligase
In
high-risk HPV-transformed cells, the cellular ubiquitin ligase E6-associated
protein (E6-AP) plays a central role in mediating the oncogenic functions of
E6. E6-AP couples with E6 to target p53 for proteasomal degradation [32]. This complex
also degrades the 91 kDa isoform of NFX1 (NFX1-91) which is a repressor for the
hTERT promoter, allowing for constitutive hTERT expression in HPV-positive
cells [5,33]. Based on this,
we examined if TTP could inhibit E6-AP expression in order to establish a
molecular explanation underlying TTP's ability to promote senescence. As shown
in Figure 5AB, HeLa Tet-Off/TTP-Flag cells grown in the presence of TTP showed
downregulation of both E6-AP mRNA and protein. Similarly, HeLa cells infected
with adenovirus expressing TTP also showed inhibition of E6-AP expression
(Figure 5B, right panel). As a control, the RNA levels of HPV18-E6 and -E7 were
assayed (Figure 5A, right panel) and no change was observed in the presence of
TTP, indicating that the viral transcript is not a target of TTP. Furthermore,
re-addition of Dox to TTP-expressing HeLa Tet-Off/TTP-Flag cells to suppress
TTP expression allowed for rapid recovery of E6-AP expression (Figure 5C).
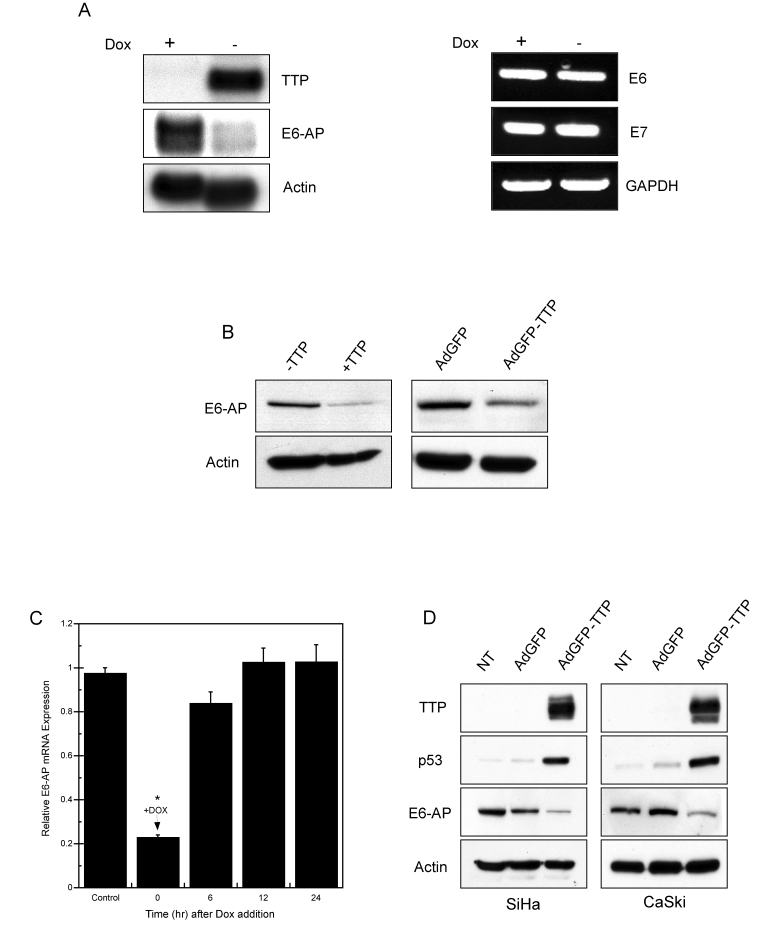
Figure 5. TTP downregulates E6-AP mRNA and protein expression. (A)
Northern blot (left panel) of TTP and E6-AP mRNA in HeLa Tet-Off/TTP-Flag
cells 48 hr after TTP induction. RT-PCR assay (right panel) of HPV18-E6 and
-E7 RNA levels in TTP-expressing cells. Actin and GAPDH were used as
loading controls. (B) Western blot of E6-AP protein in HeLa
Tet-Off/TTP-Flag cells (left panel) and adenovirus-infected HeLa cells
(right panel) expressing TTP for 48 hr. Actin was used as a loading
control. (C) TTP-dependent downregulation of E6-AP mRNA. HeLa
Tet-Off/TTP-Flag cells were initially grown without Dox for 48 hr to induce
TTP. At time zero, Dox was added to the culture medium and E6-AP mRNA
levels were evaluated by qPCR over the indicated time course. E6-AP mRNA
levels were normalized to control GAPDH mRNA. Cells grown in presence of
Dox were used as control. All values shown are normalized to E6-AP
expression of control-treated cells and are the averages of 3 experiments.
(*) P < 0.01 (D) HPV 16-positive cells, SiHa (left panel)
and CaSki (right panel) were infected with control AdGFP or AdGFP/TTP virus
at an MOI of 100 or left untreated (NT). 48 hr after infection, cell
lysates were examined for TTP, p53, and E6-AP expression by western blot.
Actin was used as a loading control.
TTP-mediated stabilization of p53 via E6-AP downregulation
occurs in high-risk HPV positive cell lines
HPV16
and HPV18 high-risk types are most frequently associated with cervical
carcinomas [2]. To determine
if these results extended to HPV16-positive cervical cancer cells, SiHa
(HPV16+) and CaSki (HPV16+) cells were infected with adenovirus expressing TTP
or control GFP. As shown in Figure 5D, endogenous TTP was not detected in
either SiHa and CaSki cells, whereas adenoviral delivery of TTP led to E6-AP
downregulation and elevated levels of p53 protein in both HPV16+ cell lines to
a similar extent to that observed in HPV18+ HeLa cells.
E6-AP
is a novel target for TTP-mediated mRNA decay
Rapid mRNA decay mediated by TTP occurs through cis-acting
AU-rich RNA elements (AREs) present in the 3'UTR of target transcripts [22,34]. Within
the 3'UTR of E6-AP, we detected multiple overlapping copies of AUUUA motif
characteristic of Class II AREs (Figure 6A, [35]) suggesting
E6-AP mRNA to be a target of ARE-mediated decay. To evaluate this, the
half-life of E6-AP mRNA was assessed by qPCR after actinomycin-D (ActD) was
added to HeLa Tet-Off/TTP-Flag cells to halt transcription. In cells expressing
TTP rapid E6-AP mRNA decay was observed yielding a half-life of approximately 90
min (Figure 6B). In contrast, E6-AP mRNA was stable in cells without TTP with
an estimated half-life of 460 min.
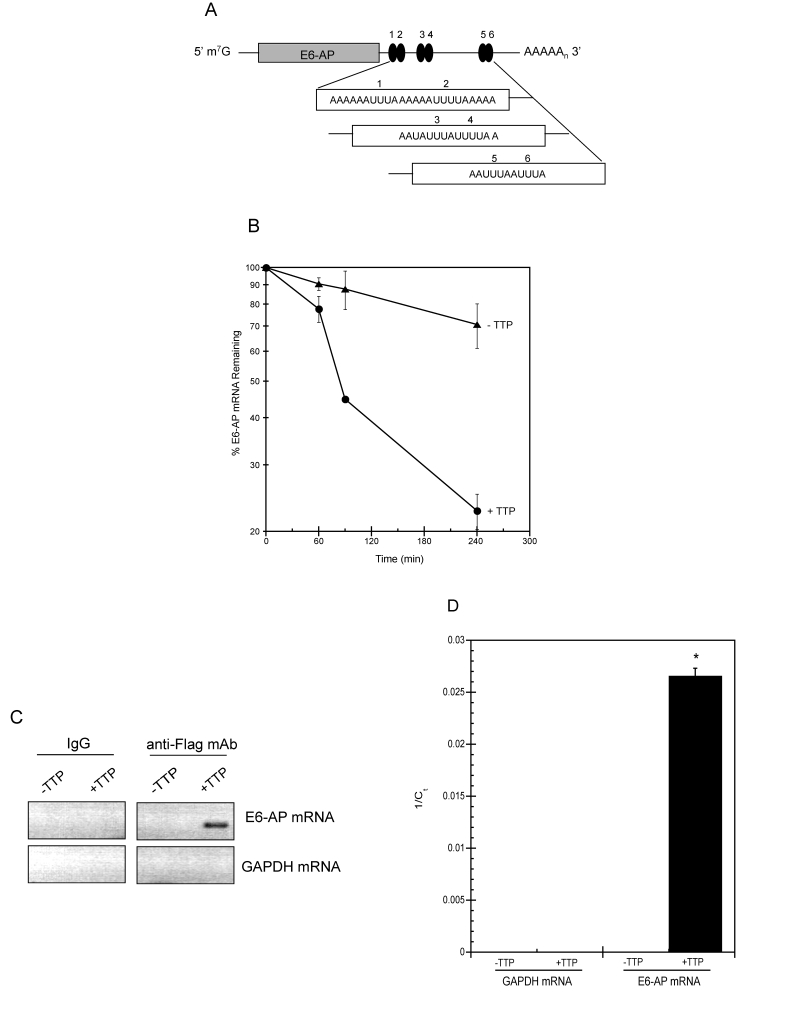
Figure 6. TTP binds E6-AP mRNA and targets it for rapid decay.
(A) Schematic representation of E6-AP mRNA. The grey bar
corresponds to E6-AP coding region and number-labeled black ovals
represent putative 3' UTR AU-rich elements (AREs). m7G, 7-methyl-guanosine
cap; AAAAn, polyadenylated tail. (B) E6-AP mRNA half-life
was assayed in HeLa Tet-Off/TTP-Flag cells grown in the presence
(triangles; labeled -TTP) or absence (circles; labeled +TTP) of
Dox to induce TTP expression. After 48 hr, 5 μg/ml of actinomycin
D was added to the cells and E6-AP mRNA decay was analyzed by qPCR
using GAPDH mRNA as a normalization control. The data shown is the
average of triplicate experiments. (C, D) Binding of TTP
and E6-AP mRNA. Control and TTP-expressing (48 hr) HeLa Tet-Off/TTP-Flag
cells were lysed and immunoprecipitation was performed on equal
amounts of cytoplasmic lysates using control IgG or anti-Flag mAb.
RNA purified from immuno-precipitates was subjected to RT-PCR (C)
or qPCR (D) to detect E6-AP and GAPDH mRNA. The ethidium
bromide-stained agarose gel depicting the 292bp E6-AP PCR product
is shown in reverse image. The relative amounts of immuno-precipitated
E6-AP mRNA is reported as the average 1/Ct value of triplicate experiments.
(*) P < 0.01
To determine if this shortening of half-life was a
result of TTP binding to E6-AP mRNA, cytoplasmic extracts from HeLa
Tet-Off/TTP-Flag cells grown in the presence and absence of TTP were subjected
to immunoprecipitation using anti-Flag antibody or control IgG.
Co-immunoprecipitated mRNA was reverse transcribed and PCR amplified using
primers specific for E6-AP and GAPDH. Shown in Figure 6C, E6-AP was amplified
from TTP expressing cells while no products were detected in control reactions.
Samples were also analyzed by qPCR and the Ct values were
used to detect the presence of a specific mRNA (Figure 6D). Ct value
for E6-AP was 38 in the mRNA pool from TTP expressing cells and undetectable in
cells absent of TTP. Ct values were undetectable for GAPDH
mRNA indicating its absence in both experimental and control
immunoprecipitations.
To determine if the ARE-containing 3'UTR
of E6-AP mediated post-transcriptional regulation through TTP, HeLa
Tet-Off/TTP-Flag cells were transiently transfected with a cDNA expression construct
containing the 2.5 kb coding region of E6-AP (E6-APΔ3'UTR), and protein expression was assayed in the presence or absence
of TTP. We found no TTP-dependent changes in the amount of E6-AP protein
expressed when the 3'UTR was absent (Figure 7A) and expression of E6-APΔ3'UTR completely abrogated TTP-mediated stabilization of p53 and
subsequent p53-dependent transcriptional activity (data not shown). The ability
of the E6-AP 3'UTR to confer TTP-dependent mRNA instability to a reporter was
tested by transfecting HeLa cells with a luciferase reporter containing the 1.6
kb E6-AP 3'UTR (Luc+E6-AP 3'UTR) along with a TTP expression construct. As seen
in Figure 7B, the E6-AP 3'UTR significantly inhibited luciferase expression in
presence of TTP, whereas control transfections using luciferase without a 3'UTR
was inhibited by TTP to a much lesser extent. Taken together, these results
indicate E6-AP mRNA to be a novel target of TTP-mediated mRNA decay through its
ARE-containing 3'UTR.
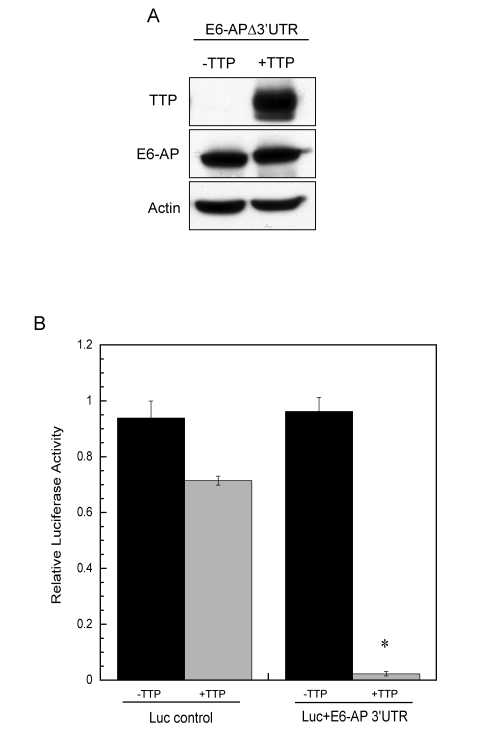
Figure 7. E6-AP 3' UTR is necessary for TTP-mediated decay. (A) HeLa
Tet-Off/TTP-Flag cells were transfected with an expression vector
containing the coding region of E6-AP (E6-APΔ3'UTR). Cells were grown in the absence or presence
of TTP for 48 hr and lysates were analyzed for E6-AP and TTP protein
expression by western blot. Actin was detected as a loading control. (B)
HeLa cells were transfected with a luciferase-reporter construct containing
the 1.6 kb E6-AP 3'UTR (Luc+E6-AP 3'UTR) or the control luciferase vector
(no 3'UTR) along with a TTP expression construct (pcDNA3-TTP-Flag) or empty
vector. Relative luciferase reporter activity in the absence (black bars)
or presence (grey bars) of TTP is shown. Relative activity was assessed as
luciferase activity normalized to its respective protein concentration for
each transfection in the absence or presence of TTP. The data shown is the
average of duplicate experiments. (*) P < 0.01
TTP
expression is lost in cervical cancer
Based on its ability to target E6-AP mRNA for rapid
decay, these results suggested that the presence of TTP would be inhibitory to
HPV-mediated tumorigenesis and loss of TTP would be observed in cervical
cancer. To test this, TTP expression was evaluated by immunohistochemistry
using human tissue arrays containing cervical tissue sections from both normal
and squamous cell carcinoma (Figure 8A). In normal cervical tissue (left panel)
strong cytoplasmic staining of TTP was observed in the cells of squamous
epithelium, whereas TTP immunoreactivity was negative or substantially
decreased in tissue sections from squamous cell carcinomas (right panel). Tissue
sections were assigned immunoreactivity scores (IRS) and grouped as low IRS of
0 to 6 or high IRS of 7 to 12 (Figure 8B). In normal tissue, TTP immuno-reactivity
was high (median IRS of 10) in 8 of 10 (80%) samples, while expression was
significantly lower (median IRS of 2) in 40 of 56 squamous cell carcinoma
samples (71%, P < 0.001). These results are consistent with recent
findings demonstrating elevated TTP mRNA levels to be present in normal cervix
tissue [36] and suggest
that loss of TTP expression in cervical cancer cells allows for aberrant mRNA
stabilization and enhanced expression of E6-AP.
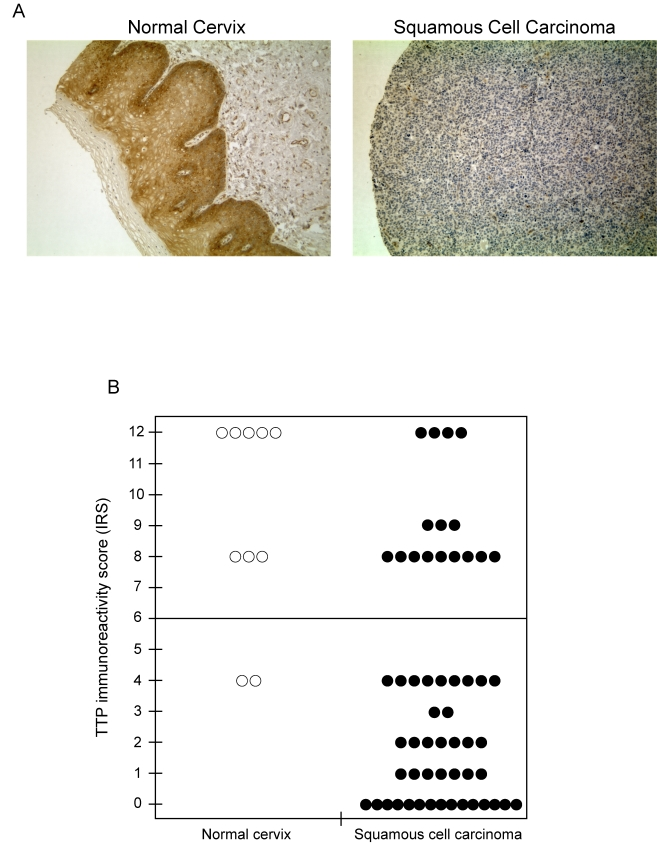
Figure 8. TTP protein expression is lost in human cervical cancer. (A)
Immunohistochemical detection of TTP expression in normal cervix and
squamous cell carcinoma. Representative tissue sections were examined for
TTP expression and counterstained with hematoxylin. Original magnification
x 200. (B) Immunoreactivity scores (IRS) for TTP expression in
tissue sections of normal cervix and squamous cell carcinoma. The line
indicates the division of samples with high IRS from 7-12 and low IRS from
0-6.
Discussion
Normal cellular growth is associated with rapid decay
of ARE-containing mRNAs and targeted mRNA decay is an essential way of controlling
their pathogenic overexpression. However, a number of observations have
implicated loss of ARE-mediated post-transcriptional regulation in the
neoplastic transformation of cells [11]. Based upon
the inherent genetic instability of tumor cells, it might be expected that
mutations in AREs are a frequent event. However, few naturally occurring
mutations in AREs have been described [37]. This
implies that loss of ARE function in tumor cells is primarily due to altered
recognition of AU-rich sequences by trans-acting RNA-binding regulatory
factors.
Through their ability to
selectively bind and control expression of many cancer-associated transcripts [12], ARE RNA-binding proteins are
being acknowledged as central regulators influencing various aspects of
tumorigenesis. Along with its recognized ability to target rapid decay of an
array of inflammatory mediators, the ARE-binding protein TTP has been shown to
inhibit expression of a wide range of cancer-associated factors [25,38-44]. Consistent with this,
expression of TTP was shown to inhibit cell growth and tumorigenesis in a mast
cell tumor model [45] and attenuate colon cancer cell
growth and proliferation [44]. These aspects, taken together
with the results presented here, indicate that TTP can serve in a tumor
suppressor capacity by controlling ARE-containing gene expression.
Through
its ability to promote rapid mRNA decay, the tumor suppressor ability of TTP
should reflect the ARE-containing mRNAs needed for enhanced tumor cell growth
and survival. Previous findings have indicated that TTP overexpression can
promote apoptosis in various cells lines [46]. The results
presented here using HPV-positive cervical cancer cells demonstrate the ability
of TTP to inhibit cell growth. However, in this TTP-inducible system evidence
of apoptosis was not observed as indication of caspase-3 activation, nuclear
condensation, and DNA fragmentation was not apparent in HeLa cells expressing
TTP over a 7 day time course (data not shown) indicating that TTP-mediated
growth inhibition was occurring through an alternate mechanism in HeLa cells.
In our findings, HeLa cells expressing TTP exhibited a flattened morphology and
elevated levels of β-galactosidase
activity indicating they have undergone replicative senescence. These findings
are in agreement with recent results demonstrating TTP-mediated growth
inhibition using a similar TTP-inducible HeLa cell model [25]. These
differences in phenotypic outcome resulting from TTP expression in cells may
reflect a specific variation in the ARE-containing mRNAs targeted for
TTP-mediated decay in the differing cell types.
In
HeLa cells, repression of viral E6 and E7 oncogene expression can trigger
endogenous senescence pathways [47-50]. Although our
results could be explained through the
ability of TTP to inhibit E6 or E7 expression, we
did not observe any TTP-dependent changes in E6/E7 transcript level (Figure 5A).
Interestingly, an AU-rich region has been identified within the 3'UTR of HPV16
E6/E7 RNA that can mediate rapid decay [51]. The results
presented here (Figure 8) and that of others [36] demonstrate TTP
to be abundantly expressed in normal uterine cervix. Based on these
observations, it is plausible that that TTP may play a protective role in the
early stages of HPV infection by targeting E6/E7 RNA for rapid degradation.
However, this viral ARE is lost in cells containing integrated HPV16 genomes
through the process of viral DNA linearization and host genome integration [51] and the
consequences of this would make E6/E7 RNA resistant to TTP-mediated decay. This
loss of post-transcriptional control, coupled with disruption of the viral E2
transcriptional repressor [52], would
potentiate persistent E6/E7 oncogene expression needed for cell transformation.
Actively growing HeLa cells maintain a dormant p53
pathway and elevated telomerase activities [49]. The
results presented here demonstrate the ability of TTP to promote p53 protein
expression, which is consistent with senescent growth arrest that is often
associated with an active p53 pathway [53]. In normal
cells, p53 levels are under negative regulation of Mdm2 ubiquitin ligase and
p53 pathway activation primarily involves signal-dependent escape from
degradation [54,55].
Whereas in high-risk HPV-transformed cervical cancer cells, the viral
oncoprotein E6 binds to p53 and with the help of the cellular ubiquitin ligase
E6-AP, p53 is targeted for constitutive degradation through the ubiquitin
proteasomal pathway [6,32,56].
Replicative senescence in somatic cells
is in part attributed to gradual loss of telomeres, while high telomerase
activity is observed in a majority of cancer cells [30]. Another
characteristic of cervical cancer cell transformation is reactivation of hTERT
gene expression, which is the catalytic component of telomerase. Although the
mechanism of E6-dependent activation of hTERT is not entirely defined in
HPV-transformed cells, current observations indicate the involvement of E6-AP
in targeting a regulator of hTERT expression [5,33,57].
Furthermore, p53 can serve as a negative regulator of hTERT expression [58], suggesting
that E6/E6-AP-dependent degradation of p53 may also play a causal role in hTERT
promoter activation.
Central to the deregulation of these factors in
cervical cancer is E6-AP and the results presented here are readily explained
with E6-AP being a novel target of TTP-mediated post-transcriptional regulation
(Figure 9). Within the 3'UTR of E6-AP, the presence of AU-rich elements provide
a binding site for TTP and this functional interaction targets E6-AP mRNA for
rapid ARE-mediated decay. These results are supported by the observations that
the presence of E6-AP 3'UTR to a luciferase reporter renders it susceptible to
TTP-mediated downregulation and deletion of the 3'UTR from E6-AP makes it
resistant to TTP-mediated mRNA decay (Figure 7). The functional consequences of
TTP-mediated suppression of E6-AP leads to p53 stabilization, hTERT
inhibition, and cellular senescence. These results are consistent with those
using RNA interference to downregulate E6-AP expression indicating the central
role E6-AP has in promoting HPV-associated cervical cancer [59].
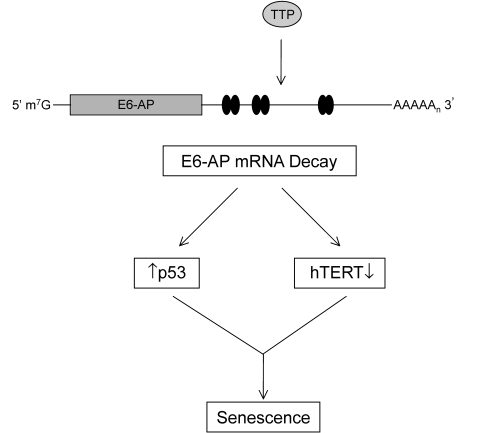
Figure 9. TTP-mediated regulation of E6-AP in cervical cancer cells. The binding of
TTP to the ARE-containing E6-AP mRNA targets it for rapid degradation.
Black ovals represent putative 3' UTR AU-rich elements (AREs). The
subsequent loss of E6-AP expression allows for concurrent p53 protein
stabilization and inhibition of hTERT transcription leading to
cellular senescence.
Recent findings have demonstrated that loss of TTP
expression is observed in a variety of tumor types [25,36,44,60].
Consistent with this, we also observe a similar loss of TTP in cervical cancer
cells and tumors. This loss of TTP expression appears to be a critical factor
in the progression of high-risk HPV-associated cervical cancer, since the
presence of TTP in cervical tumor cells impedes their tumorigenic potential
through rapid decay of E6-AP mRNA. Also observed with the loss of TTP
expression is increased expression of the ARE-mRNA stabilization factor HuR in
cervical cancers [61]. Through
these combined defects of TTP loss-of-function and HuR gain-of-function,
aberrant mRNA stabilization can occur leading to over-expression of
cancer-associated factors in cervical cancer similar to what is seen in colon
cancer [44]. Moreover,
the potential of TTP to promote senescence may be through its ability to
antagonize HuR-mediated stabilization of proliferative ARE-mRNAs [44,62] similar
to observations showing that reduction in HuR levels in fibroblasts promoted a
senescent phenotype [63].
The mechanisms underlying the loss of TTP expression
in cervical cancer cells and tumors are largely undefined. The TTP gene (ZFP36)
is located on 19q13.1 and does not appear to
be a target of genomic loss or rearrangement in cervical cancer [64]. One
explanation for the lack of TTP expression observed in tumor tissue may reside
in epigenetic silencing of the TTP promoter. Within the proximal 3' region of
the human TTP promoter lies a putative CpG island and the presence of
hypermethylation of this region was observed in HeLa cells (unpublished
observations). Based on this we hypothesize that epigenetic alterations
occurring through changes in DNA methylation and altered chromatin structure
promote TTP gene silencing in cervical tumors. This is consistent with
observations demonstrating that various tumor suppressor genes have been
silenced or display decreased expression resulting from abnormal promoter hypermethylation
in HPV-associated cervical carcinoma [65].
The results presented here provide a novel link
between post-transcriptional gene regulation and HPV-associated cervical
tumorigenesis. Based on these findings we conclude
that TTP promotes cellular senescence in cervical cancer cells through rapid
decay of E6-AP mRNA leading to p53 protein stabilization and inhibition of hTERT
transcription. Moreover, absence of TTP
expression in cervical cancer strongly implicates that loss of TTP expression
is a critical step that occurs early in HPV-mediated carcinogenesis. These
findings demonstrate the novel ability of TTP to servein
a tumor suppressor capacity by regulating ARE-mRNA gene expression and identify
how defects in post-transcriptional regulation can contribute to tumorigenesis.
Methods
Cell culture, DNA transfection, and adenoviral
infection.
Human cervical cancer cell lines HeLa (HPV18+), SiHa
(HPV16+) and CaSki (HPV16+) cells were obtained from ATCC; HeLa Tet-Off cells
were purchased from BD Clontech. Cells were maintained in DMEM supplemented
with 10% fetal bovine serum (FBS; Hyclone); HeLa Tet-Off cell media was
supplemented with 100 μg/ml G418 (Cellgro). The Tet-responsive pTRE2hyg/TTP-Flag
vector was created by cloning an N-terminal Flag epitope-tagged TTP cDNA from
pcDNA3-Flag-TTP (kindly provided by N. Kedersha, Brigham and Women's Hospital,
Boston, MA) into pTRE2hyg (Clontech). HeLa Tet-Off cells were stably
transfected with pTRE2hyg/TTP-Flag using Lipofectamine Plus (Invitrogen)
according to the vendor's protocol. Stably transfected cells were selected in
normal growth medium containing 100 μg/ml G418, 200 μg/ml hygromycin B
(Invitrogen), and 2 μg/ml doxycycline (Dox) (Clontech) for 2-3 weeks.
Individual clones were isolated by limiting dilution in 96-well plates.
Positive HeLa-Tet-Off/TTP-Flag clones were screened by growing cells in the
presence or absence of Dox (2 μg/ml) to induce expression of
TTP-Flag, respectively; the absence of Dox allows for TTP-Flag expression. For
stable cell maintenance the hygromycin B concentration was reduced to 100
μg/ml. Unless otherwise indicated, HeLa Tet-Off/TTP-Flag cells were grown in
the absence of Dox for 48 hr to induce TTP-Flag expression.
HeLa Tet-Off/TTP-Flag cells were transiently
transfected with p53-responsive promoter luciferase reporter vector pp53-Luc or
control vector pTA-Luc (Clontech) along with control pRL-TK renilla vector
(Promega) using Lipofectamine Plus. The E6-AP coding region or 3'UTR were PCR
amplified from HeLa cDNA as described [66]. E6-AP
coding region was cloned into the expression vector pcDNA3.1/Zeo (Invitrogen)
to generate pcDNA3.1/E6-APΔ3'UTR. Luciferase reporter construct containing the E6-AP 3'UTR was prepared by cloning E6-AP 3'UTR into pcDNA3.1/Zeo containing the luciferase cDNA [66]. Cells were
transfected in DMEM for 3 hr after which cells were grown in complete medium in
the presence or absence of 2 μg/ml Dox for 48 hr. Transfected cells were lysed
in reporter lysis buffer (Promega) and assayed for luciferase and renilla
activities using the Dual-Luciferase Assay System (Promega). Luciferase
reporter gene activities were normalized to renilla activity and all results represent
the average of triplicate experiments.
TTP-Flag expressing adenovirus was created by cloning TTP-Flag cDNA into the shuttle vector Dual-CCM-CMV-EGFP
(Vector Biolabs) that contains dual CMV promoters to drive expression of
TTP-Flag and GFP. Construction of TTP-expressing adenoviral vector (AdGFP/TTP)
and production of viral stocks were conducted by Vector Biolabs. Control
GFP-expressing adenovirus (AdGFP) was purchased from Vector Biolabs.
HeLa, SiHa and CaSki cells were infected with AdGFP or AdGFP/TTP using a MOI of
100 in serum-free DMEM for 2 hr after which FBS was added to a final
concentration of 10%. 48 hr after infection, cells were harvested in SDS-PAGE
lysis buffer for western blot analysis.
Immunoblot analysis.
Cells were lysed in SDS-PAGE lysis buffer (50 mM Tris-HCl, pH 6.8, 100
mM DTT, 2% SDS, 0.1% bromophenol blue, 10% glycerol) and protein content was
determined using a BCA protein assay with BSA as standard (Pierce
Biotechnology). Where indicated, nuclear lysates were prepared by resuspending
cells in lysis buffer (10 mM HEPES pH 7.9, 2 mM MgCl2, 10 mM KCl,
0.1 mM EDTA, 1 mM DTT, 0.5% NP-40) containing 0.5 mM PMSF and protease
inhibitor cocktail (Sigma) and incubated on ice for 10 min. Cells were
centrifuged at 13000 rpm for 10 min and the nuclear pellet was washed 3 times
with lysis buffer. Nuclei were lysed in RIPA buffer (50 mM Tris-Cl pH 8.0, 156
mM NaCl, 4 mM EDTA, 1% SDS, 1 % Triton X-100, 1% Na-deoxycholate). Lysates (50
μg) were separated by 10% SDS-PAGE, transferred to PVDF membranes (Bio-Rad),
and probed with antibodies against Flag epitope (M2; Sigma), TTP (Ab-36558,
Abcam), p53 (DO-1, Calbiochem), hTERT (Ab-1, Calbiochem), and E6-AP (BD
Biosciences) at dilutions specified by the vendor. Blots were stripped and then
probed with antibodies against β-actin (Clone C4, MP
Biomedicals) or nucleoporin (BD Biosciences). Detection and quantitation of
blots were performed as described [66].
mRNA analysis.
Total RNA was extracted from
cells using Trizol reagent (Invitrogen). Northern blotting was performed as
previously described [67] and probed
with P32-labeled DNA probes synthesized for TTP, E6-AP and actin
(Promega). cDNA synthesis and RT-PCR analysis of mRNA was accomplished as
described [66]. The
sequences for PCR primers used were: TTP sense, 5'-TCCACAACCCTAGCGAAGAC-3' and
TTP anti-sense, 5'-GAGAAGGCAGAGGGTGACAG-3'; p53 sense,
5'-CAGCCAAGTCTGTGACTTGCACGTAC-3' and p53 antisense, 5'-CTATGTCGAAAAGTGTTT CTGTCATC-3';
hTERT sense, 5'-GTGACCGTGGTT TCTGTGTG-3' and hTERT antisense, 5'-TCGCCTGA GGAGTAGAGGAA-3';
HPV18 E6 sense, 5'-CGCGC TTTGAGGATCCAA-3' and HPV18 E6 antisense,
5'-TATGGCATGCAGCATGCG-3'; HPV18 E7 sense, 5'-TATGCATGGACCTAAGGCAAC-3' and HPV18
E7 antisense, 5'-TTACTGCTGGGATGCACACC-3'; E6-AP sense,
5'-GCTTGAGGTTGAGCCTTTTG-3' and E6-AP antisense, 5'-CCAATTTCTCCCTTCCTTCC-3';
GAPDH sense, 5'-CCACCCATGGCAAATTCCAT GGCA-3' and GAPDH antisense,
5'-TCTAGACGGCA GGTCAGGTCCACC-3'. Real-time PCR (qPCR) was performed using the
7300 Real-Time PCR Assay System (Applied Biosystems) with SYBR green PCR master
mix (Applied Biosystems) and primers for E6-AP and GAPDH according to the
vendor's protocol.
Cell growth and senescence.
Cell growth
was assayed using the MTT-based cell growth determination kit (Sigma) as
previously described [68]. For
cellular senescence studies, 1 x 104 HeLa Tet-Off/TTP-Flag cells
were grown in 35mm diameter dishes in the presence or absence of 2 μg/ml Dox.
Twelve days later, the cells were stained at pH 6.0 with X-Gal
(5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside; Cell Signaling Technology)
to visualize senescence associated-β-galactosidase (SA-β-gal) activity.
Fluorescence microscopy.
HeLa
Tet-Off/TTP-Flag cells were plated on coverslips in a 24-well plate and grown
in the presence or absence of 2 μg/ml Dox. After 48 hrs, the cells were fixed
in 4% paraformaldehyde for 10 min and permeabilized with 0.1% Triton X-100 in
PBS for 5 min. The cells were blocked with 10% normal goat serum and 3% BSA
diluted in PBST (PBS + 0.1% Tween-20) for 1 hr. Cells were incubated for 1 hr
at RT with anti-p53 antibody (DO-1, Calbiochem; 1:100) diluted in blocking
buffer. After washing, the cells were incubated with fluorescein-conjugated
goat anti-mouse secondary antibody (MP Biomedicals; 1:150) for 1 hr at RT. DAPI
(Invitrogen) was used for nuclear counter-staining. Coverslips were mounted on
glass slides and visualized using an Axiovert 200 inverted microscope (Zeiss).
Cell morphology was examined by staining fixed and permeabilized cells with
DAPI and rhodamine phalloidin (Invitrogen) according to the vendor's
instructions.
Telomerase activity.
Telomerase
activity was determined in HeLa Tet-Off/TTP-Flag lysates 48 hr after TTP
induction using the PCR-based TRAP assay as previously described [69]. PCR
products were resolved on a 10% non-denaturing polyacrylamide gel and
visualized by silver staining [70].
Immunoprecipitations.
HeLa Tet-Off/TTP-Flag (1.25 x 105
cells) were grown in 100 mm diameter dishes in the presence or absence of 2
μg/ml Dox for 48 hr. Cells were lysed in polysome lysis buffer (100 mM KCl, 5mM
MgCl2, 10mM HEPES pH 7.0, 0.5% NP-40, and 1 mM DTT) containing 100
U/ml RNase inhibitor (Ambion) and protease inhibitor cocktail (Sigma).
Cytoplasmic extracts were separated from nuclei by centrifugation at 20,000g
for 30 min. 700 μl of IP buffer (50 mM Tris-Cl, pH 7.4, 150 mM NaCl, 1 mM MgCl2,
0.05% NP-40) was added to 500 μg of lysate and immunoprecipitation of TTP-bound
RNA was accomplished by incubating lysates with equal amounts (30 μg) of
anti-Flag mAb or mouse IgG pre-coated to protein A/G PLUS agarose (Santa Cruz
Biotechnology) overnight at 4°C. Immunoprecipitates were collected by brief
centrifugation and washed 4 times with IP buffer. Total RNA was isolated using
1 ml Trizol per IP reaction and then used
for cDNA synthesis [66]. Real-time PCR
reactions were performed using 1 μl of cDNA. Data was plotted as 1/Ct
to represent the abundance of E6-AP or GAPDH mRNA in each IP sample.
Immunohistochemical analysis.
Immunohistochemical
analysis of TTP expression was performed using cervical cancer tissue array
CXC96101 (Pantomics) that contained 12 cases of normal and inflammatory tissues
of cervix and 36 cases of cervical cancer graded by histology. TTP
immunostaining was performed using TTP polyclonal antibody (Ab-36558, Abcam) at
8 μg/ml (1:400). Standard staining protocol was performed
and stained tissue sections were evaluated for intensity of staining as
described [44] using two
blinded investigators (S.S and V.K.). For each tissue section, the percentage
of positive cells was scored on a scale of 0 to 4 : 0 (0% positive cells), 1
(< 25%), 2 (25-50%), 3 (50-75%) or 4 (> 75%). Staining intensity was
scored on a scale of 0 to 3; 0-negative, 1-weak, 2-moderate, or 3-strong. The
two scores were multiplied to give an immunoreactivity score (IRS) ranging from
0 to 12, with scores in the range of 0-6 grouped in the category of Low IRS and
those in the range of 7-12 representing High IRS.
Statistical analysis.
The data are expressed as the mean +/- SD. Student's t-test
was used to determine significant differences. P-values less than 0.05
were considered significant.
Acknowledgments
We thank Lucia Pirisi-Creek and Kim Creek for
assistance with cervical tissue analysis and Ulus Atasoy for technical advice.
This work was supported by the NIH/NCI grant P20-RR17698 and the American Cancer Society Research Scholar grant
RSG-06-122-01-CNE.
Conflicts of Interest
The authors declare no conflict of interests.
References
-
1.
Bedford
S
Cervical cancer: physiology, risk factors, vaccination and treatment. British J.
Nursing.
2009;
18:
80
-84.
.
-
2.
Walboomers
JM
, Jacobs
MV
, Manos
MM
, Bosch
FX
, Kummer
JA
, Shah
KV
, Snijders
PJ
, Peto
J
, Meijer
CJ
and Munoz
N.
Human papillomavirus is a necessary cause of invasive cervical cancer worldwide.
J Pathol.
1999;
189:
12
-19.
[PubMed]
.
-
3.
Dyson
N
, Howley
PM
, Munger
K
and Harlow
E.
The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product.
Science.
1989;
243:
934
-937.
[PubMed]
.
-
4.
Boyer
SN
, Wazer
DE
and Band
V.
E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through the ubiquitin-proteasome pathway.
Cancer Res.
1996;
56:
4620
-4624.
[PubMed]
.
-
5.
Liu
X
, Yuan
H
, Fu
B
, Disbrow
GL
, Apolinario
T
, Tomaic
V
, Kelley
ML
, Baker
CC
, Huibregtse
J
and Schlegel
R.
The E6AP ubiquitin ligase is required for transactivation of the hTERT promoter by the human papillomavirus E6 oncoprotein.
J Biol Chem.
2005;
280:
10807
-10816.
[PubMed]
.
-
6.
Scheffner
M
, Werness
BA
, Huibregtse
JM
, Levine
AJ
and Howley
PM.
The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53.
Cell.
1990;
63:
1129
-1136.
[PubMed]
.
-
7.
Tungteakkhun
SS
and Duerksen-Hughes
PJ.
Cellular binding partners of the human papillomavirus E6 protein.
Arch Virol.
2008;
153:
397
-408.
[PubMed]
.
-
8.
Beaudenon
S
and Huibregtse
JM.
HPV E6, E6AP and cervical cancer.
BMC Biochem.
2008;
9 Suppl 1:
S1
-S4.
[PubMed]
.
-
9.
Huibregtse
JM
, Scheffner
M
, Beaudenon
S
and Howley
PM.
A family of proteins structurally and functionally related to the E6-AP ubiquitin-protein ligase.
Proc Natl Acad Sci U S A.
1995;
92:
2563
-2567.
[PubMed]
.
-
10.
Mantovani
F
and Banks
L.
The human papillomavirus E6 protein and its contribution to malignant progression.
Oncogene.
2001;
20:
7874
-7887.
[PubMed]
.
-
11.
Lopez
de Silanes I
, Quesada
MP
and Esteller
M.
Aberrant regulation of messenger RNA 3'-untranslated region in human cancer.
Cell Oncol.
2007;
29:
1
-17.
.
-
12.
Benjamin
D
and Moroni
C.
mRNA stability and cancer: an emerging link.
Expert Opin Biol Ther.
2007;
7:
1515
-1529.
[PubMed]
.
-
13.
Taylor
GA
, Thompson
MJ
, Lai
WS
and Blackshear
PJ.
Mitogens stimulate the rapid nuclear to cytosolic translocation of tristetraprolin, a potential zinc-finger transcription factor.
Mol Endocrinol.
1996;
10:
140
-146.
[PubMed]
.
-
14.
Carballo
E
, Lai
WS
and Blackshear
PJ.
Evidence that tristetraprolin is a physiological regulator of granulocytemacrophage colony-stimulating factor messenger RNA dea-denylation and stability.
Blood.
2000;
95:
1891
-1899.
[PubMed]
.
-
15.
Lai
WS
and Blackshear
PJ.
Interactions of CCCH zinc finger proteins with mRNA: tristetraprolin-mediated AU-rich element-dependent mRNA degradation can occur in the absence of a poly(A) tail.
J Biol Chem.
2001;
276:
23144
-23154.
[PubMed]
.
-
16.
Sawaoka
H
, Dixon
DA
, Oates
JA
and Boutaud
O.
Tristetrapolin binds to the 3' untranslated region of cyclooxygenase-2 mRNA: A polyadenylation variant in a cancer cell line lacks the binding site.
J Biol Chem.
2003;
278:
13928
-13935.
[PubMed]
.
-
17.
Fenger-Gron
M
, Fillman
C
, Norrild
B
and Lykke-Andersen
J.
Multiple processing body factors and the ARE binding protein TTP activate mRNA decapping.
Mol Cell.
2005;
20:
905
-915.
[PubMed]
.
-
18.
Lykke-Andersen
J
and Wagner
E.
Recruitment and activation of mRNA decay enzymes by two ARE-mediated decay activation domains in the proteins TTP and BRF-1.
Genes Dev.
2005;
19:
351
-361.
[PubMed]
.
-
19.
Hau
HH
, Walsh
RJ
, Ogilvie
RL
, Williams
DA
, Reilly
CS
and Bohjanen
PR.
Tristetraprolin recruits functional mRNA decay complexes to ARE sequences.
J Cell Biochem.
2007;
100:
1477
-1492.
[PubMed]
.
-
20.
Chen
CY
, Gherzi
R
, Ong
SE
, Chan
EL
, Raijmakers
R
, Pruijn
GJ
, Stoecklin
G
, Moroni
C
, Mann
M
and Karin
M.
AU binding proteins recruit the exosome to degrade ARE-containing mRNAs.
Cell.
2001;
107:
451
-464.
[PubMed]
.
-
21.
Mukherjee
D
, Gao
M
, O'Connor
JP
, Raijmakers
R
, Pruijn
G
, Lutz
CS
and Wilusz
J.
The mammalian exosome mediates the efficient degradation of mRNAs that contain AU-rich elements.
EMBO J.
2002;
21:
165
-174.
[PubMed]
.
-
22.
Carballo
E
, Lai
WS
and Blackshear
PJ.
Feedback inhibition of macrophage tumor necrosis factor-a production by tristetraprolin.
Science.
1998;
281:
1001
-1005.
[PubMed]
.
-
23.
Taylor
GA
, Carballo
E
, Lee
DM
, Lai
WS
, Thompson
MJ
, Patel
DD
, Schenkman
DI
, Gilkeson
GS
, Broxmeyer
HE
, Haynes
BF
and Blackshear
PJ.
A pathogenetic role for TNF alpha in the syndrome of cachexia, arthritis, and autoimmunity resulting from tristetraprolin (TTP) deficiency.
Immunity.
1996;
4:
445
-454.
[PubMed]
.
-
24.
Sully
G
, Dean
JL
, Wait
R
, Rawlinson
L
, Santalucia
T
, Saklatvala
J
and Clark
AR.
Structural and functional dissection of a conserved destabilizing element of cyclo-oxygenase-2 mRNA: evidence against the involvement of AUF-1, AUF-2, tristetraprolin, HuR or FBP1.
Biochem J.
2004;
377:
629
-639.
[PubMed]
.
-
25.
Brennan
SE
, Kuwano
Y
, Alkharouf
N
, Blackshear
PJ
, Gorospe
M
and Wilson
GM.
The mRNA-Destabilizing Protein Tristetraprolin Is Suppressed in Many Cancers, Altering Tumorigenic Phenotypes and Patient Prognosis.
Cancer Res.
2009;
69:
5168
-5176.
[PubMed]
.
-
26.
Goodwin
EC
, Yang
E
, Lee
CJ
, Lee
HW
, DiMaio
D
and Hwang
ES.
Rapid induction of senescence in human cervical carcinoma cells.
Proc Natl Acad Sci U S A.
2000;
97:
10978
-10983.
[PubMed]
.
-
27.
Lee
BY
, Han
JA
, Im
JS
, Morrone
A
, Johung
K
, Goodwin
EC
, Kleijer
WJ
, DiMaio
D
and Hwang
ES.
Senescence-associated beta-galactosidase is lysosomal beta-galactosidase.
Aging Cell.
2006;
5:
187
-195.
[PubMed]
.
-
28.
Liang
SH
and Clarke
MF.
Regulation of p53 localization.
Eur J Biochem.
2001;
268:
2779
-2783.
[PubMed]
.
-
29.
Oren
M
Decision making by p53: life, death and cancer.
Cell Death Differ.
2003;
10:
431
-442.
[PubMed]
.
-
30.
Pendino
F
, Tarkanyi
I
, Dudognon
C
, Hillion
J
, Lanotte
M
, Aradi
J
and Segal-Bendirdjian
E.
Telomeres and telomerase: Pharmacological targets for new anticancer strategies.
Curr Cancer Drug Targets.
2006;
6:
147
-180.
[PubMed]
.
-
31.
Veldman
T
, Horikawa
I
, Barrett
JC
and Schlegel
R.
Transcriptional activation of the telomerase hTERT gene by human papillomavirus type 16 E6 oncoprotein.
J Virol.
2001;
75:
4467
-4472.
[PubMed]
.
-
32.
Scheffner
M
, Huibregtse
JM
, Vierstra
RD
and Howley
PM.
The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53.
Cell.
1993;
75:
495
-505.
[PubMed]
.
-
33.
Gewin
L
, Myers
H
, Kiyono
T
and Galloway
DA.
Identification of a novel telomerase repressor that interacts with the human papillomavirus type-16 E6/E6-AP complex.
Genes Dev.
2004;
18:
2269
-2282.
[PubMed]
.
-
34.
Lai
WS
, Carballo
E
, Thorn
JM
, Kennington
EA
and Blackshear
PJ.
Interactions of CCCH zinc finger proteins with mRNA. Binding of tristetraprolin-related zinc finger proteins to AU-rich elements and destabilization of mRNA.
J Biol Chem.
2000;
275:
17827
-17837.
[PubMed]
.
-
35.
Bakheet
T
, Williams
BR
and Khabar
KS.
ARED 3.0: the large and diverse AU-rich transcriptome.
Nucleic Acids Res.
2006;
34:
D111
-114.
[PubMed]
.
-
36.
Carrick
DM
and Blackshear
PJ.
Comparative expression of tristetraprolin (TTP) family member transcripts in normal human tissues and cancer cell lines.
Arch Biochem Biophys.
2007;
462:
278
-285.
[PubMed]
.
-
37.
Mendell
JT
and Dietz
HC.
When the message goes awry: disease-producing mutations that influence mRNA content and performance.
Cell.
2001;
107:
411
-414.
[PubMed]
.
-
38.
Ogilvie
RL
, Abelson
M
, Hau
HH
, Vlasova
I
, Blackshear
PJ
and Bohjanen
PR.
Tristetraprolin down-regulates IL-2 gene expression through AU-rich element-mediated mRNA decay.
J Immunol.
2005;
174:
953
-961.
[PubMed]
.
-
39.
Briata
P
, Ilengo
C
, Corte
G
, Moroni
C
, Rosenfeld
MG
, Chen
CY
and Gherzi
R.
The Wnt/beta-catenin-->Pitx2 pathway controls the turnover of Pitx2 and other unstable mRNAs.
Mol Cell.
2003;
12:
1201
-1211.
[PubMed]
.
-
40.
Marderosian
M
, Sharma
A
, Funk
AP
, Vartanian
R
, Masri
J
, Jo
OD
and Gera
JF.
Tristetraprolin regulates Cyclin D1 and c-Myc mRNA stability in response to rapamycin in an Akt-dependent manner via p38 MAPK signaling.
Oncogene.
2006;
25:
6277
-6290.
[PubMed]
.
-
41.
Fechir
M
, Linker
K
, Pautz
A
, Hubrich
T
, Forstermann
U
, Rodriguez-Pascual
F
and Kleinert
H.
Tristetraprolin regulates the expression of the human inducible nitric-oxide synthase gene.
Mol Pharmacol.
2005;
67:
2148
-2161.
[PubMed]
.
-
42.
Suswam
E
, Li
Y
, Zhang
X
, Gillespie
GY
, Li
X
, Shacka
JJ
, Lu
L
, Zheng
L
and King
PH.
Tristetraprolin down-regulates interleukin-8 and vascular endothelial growth factor in malignant glioma cells.
Cancer Res.
2008;
68:
674
-682.
[PubMed]
.
-
43.
Stoecklin
G
, Ming
XF
, Looser
R
and Moroni
C.
Somatic mRNA turnover mutants implicate tristetraprolin in the interleukin-3 mRNA degradation pathway.
Mol Cell Biol.
2000;
20:
3753
-3763.
[PubMed]
.
-
44.
Young
LE
, Sanduja
S
, Bemis-Standoli
K
, Pena
EA
, Price
RL
and Dixon
DA.
The mRNA Binding Proteins HuR and Tristetraprolin Regulate Cyclooxygenase 2 Expression During Colon Carcinogenesis.
Gastroenterology.
2009;
136:
1669
-1679.
[PubMed]
.
-
45.
Stoecklin
G
, Gross
B
, Ming
XF
and Moroni
C.
A novel mechanism of tumor suppression by destabilizing AU-rich growth factor mRNA.
Oncogene.
2003;
22:
3554
-3561.
[PubMed]
.
-
46.
Johnson
BA
, Geha
M
and Blackwell
TK.
Similar but distinct effects of the tristetraprolin/TIS11 immediate-early proteins on cell survival.
Oncogene.
2000;
19:
1657
-1664.
[PubMed]
.
-
47.
Goodwin
EC
and DiMaio
D.
Repression of human papillomavirus oncogenes in HeLa cervical carcinoma cells causes the orderly reactivation of dormant tumor suppressor pathways.
Proc Natl Acad Sci U S A.
2000;
97:
12513
-12518.
[PubMed]
.
-
48.
DeFilippis
RA
, Goodwin
EC
, Wu
L
and DiMaio
D.
Endogenous human papillomavirus E6 and E7 proteins differentially regulate proliferation, senescence, and apoptosis in HeLa cervical carcinoma cells.
J Virol.
2003;
77:
1551
-1563.
[PubMed]
.
-
49.
Goodwin
EC
and DiMaio
D.
Induced senescence in HeLa cervical carcinoma cells containing elevated telomerase activity and extended telomeres.
Cell Growth Differ.
2001;
12:
525
-534.
[PubMed]
.
-
50.
Gu
W
, Putral
L
, Hengst
K
, Minto
K
, Saunders
NA
, Leggatt
G
and McMillan
NA.
Inhibition of cervical cancer cell growth in vitro and in vivo with lentiviral-vector delivered short hairpin RNA targeting human papillomavirus E6 and E7 oncogenes.
Cancer Gene Ther.
2006;
13:
1023
-1032.
[PubMed]
.
-
51.
Jeon
S
and Lambert
PF.
Integration of human papillomavirus type 16 DNA into the human genome leads to increased stability of E6 and E7 mRNAs: implications for cervical carcinogenesis.
Proc Natl Acad Sci U S A.
1995;
92:
1654
-1658.
[PubMed]
.
-
52.
Pett
M
and Coleman
N.
Integration of high-risk human papillomavirus: a key event in cervical carcinogenesis.
J Pathol.
2007;
212:
356
-367.
[PubMed]
.
-
53.
Campisi
J
and d'Adda
di Fagagna F.
Cellular senescence: when bad things happen to good cells.
Nat Rev Mol Cell Biol.
2007;
8:
729
-740.
[PubMed]
.
-
54.
Haupt
Y
, Maya
R
, Kazaz
A
and Oren
M.
Mdm2 promotes the rapid degradation of p53.
Nature.
1997;
387:
296
-299.
[PubMed]
.
-
55.
Ashcroft
M
, Taya
Y
and Vousden
KH.
Stress signals utilize multiple pathways to stabilize p53.
Mol Cell Biol.
2000;
20:
3224
-3233.
[PubMed]
.
-
56.
Hengstermann
A
, Linares
LK
, Ciechanover
A
, Whitaker
NJ
and Scheffner
M.
Complete switch from Mdm2 to human papillomavirus E6-mediated degradation of p53 in cervical cancer cells.
Proc Natl Acad Sci U S A.
2001;
98:
1218
-1223.
[PubMed]
.
-
57.
Gewin
L
and Galloway
DA.
E box-dependent activation of telomerase by human papillomavirus type 16 E6 does not require induction of c-myc.
J Virol.
2001;
75:
7198
-7201.
[PubMed]
.
-
58.
Shats
I
, Milyavsky
M
, Tang
X
, Stambolsky
P
, Erez
N
, Brosh
R
, Kogan
I
, Braunstein
I
, Tzukerman
M
, Ginsberg
D
and Rotter
V.
p53-dependent down-regulation of telomerase is mediated by p21waf1.
J Biol Chem.
2004;
279:
50976
-50985.
[PubMed]
.
-
59.
Hengstermann
A
, D'Silva
M A
, Kuballa
P
, Butz
K
, Hoppe-Seyler
F
and Scheffner
M.
Growth suppression induced by downregulation of E6-AP expression in human papillomavirus-positive cancer cell lines depends on p53.
J Virol.
2005;
79:
9296
-9300.
[PubMed]
.
-
60.
Gebeshuber
CA
, Zatloukal
K
and Martinez
J.
miR-29a suppresses tristetraprolin, which is a regulator of epithelial polarity and metastasis.
EMBO Rep.
2009;
10:
400
-405.
[PubMed]
.
-
61.
Fay
J
, Kelehan
P
, Lambkin
H
and Schwartz
S.
Increased expression of cellular RNA-binding proteins in HPV-induced neoplasia and cervical cancer.
J Med Virol.
2009;
81:
897
-907.
[PubMed]
.
-
62.
Al-Ahmadi
W
, Al-Ghamdi
M
, Al-Haj
L
, Al-Saif
M
and Khabar
KS.
Alternative polyadenylation variants of the RNA binding protein, HuR: abundance, role of AU-rich elements and auto-regulation.
Nucleic Acids Res.
2009;
37:
3612
-3624.
[PubMed]
.
-
63.
Wang
W
, Yang
X
, Cristofalo
VJ
, Holbrook
NJ
and Gorospe
M.
Loss of HuR is linked to reduced expression of proliferative genes during replicative senescence.
Mol Cell Biol.
2001;
21:
5889
-5898.
[PubMed]
.
-
64.
Wilting
SM
, de Wilde
J
, Meijer
CJ
, Berkhof
J
, Yi
Y
, van
Wieringen WN
, Braakhuis
BJ
, Meijer
GA
, Ylstra
B
, Snijders
PJ
and Steenbergen
RD.
Integrated genomic and transcriptional profiling identifies chromosomal loci with altered gene expression in cervical cancer.
Genes Chromosomes Cancer.
2008;
47:
890
-905.
[PubMed]
.
-
65.
Duenas-Gonzalez
A
, Lizano
M
, Candelaria
M
, Cetina
L
, Arce
C
and Cervera
E.
Epigenetics of cervical cancer. An overview and therapeutic perspectives.
Mol Cancer.
2005;
4:
38
[PubMed]
.
-
66.
Dixon
DA
, Kaplan
CD
, McIntyre
TM
, Zimmerman
GA
and Prescott
SM.
Post-transcriptional control of cyclooxygenase-2 gene expression. The role of the 3'-untranslated region.
J Biol Chem.
2000;
275:
11750
-11757.
[PubMed]
.
-
67.
Dixon
DA
, Tolley
ND
, Bemis-Standoli
K
, Martinez
ML
, Weyrich
AS
, Morrow
JD
, Prescott
SM
and Zimmerman
GA.
Expression of COX-2 in platelet-monocyte interactions occurs via combinatorial regulation involving adhesion and cytokine signaling.
J Clin Invest.
2006;
116:
2727
-2738.
[PubMed]
.
-
68.
Dixon
DA
, Tolley
ND
, King
PH
, Nabors
LB
, McIntyre
TM
, Zimmerman
GA
and Prescott
SM.
Altered expression of the mRNA stability factor HuR promotes cyclooxygenase-2 expression in colon cancer cells.
J Clin Invest.
2001;
108:
1657
-1665.
[PubMed]
.
-
69.
Kim
NW
, Piatyszek
MA
, Prowse
KR
, Harley
CB
, West
MD
, Ho
PL
, Coviello
GM
, Wright
WE
, Weinrich
SL
and Shay
JW.
Specific association of human telomerase activity with immortal cells and cancer.
Science.
1994;
266:
2011
-2015.
[PubMed]
.
-
70.
Qu
L
, Li
X
, Wu
G
and Yang
N.
Efficient and sensitive method of DNA silver staining in polyacrylamide gels.
Electrophoresis.
2005;
26:
99
-101.
[PubMed]
.