TAp63: The fountain of youth
Abstract
The mechanisms controlling organismal aging have yet to be clearly defined. In our recent paper [1], we revealed thatTAp63, the p53 family member, is a critical gene in preventing organismal aging by controlling the maintenance of dermal and epidermal precursor and stem cells critical for wound healing and hair growth. In the absence of TAp63, dermal stem cells (skin-derived precursors or SKPs) in young mice are hyperproliferative. As early as one month of age, SKPs and epidermal precursor cells exhibit signs of premature aging including a marked increase in senescence, DNA damage, and genomic instability resulting in an exhaustion of these cells and an overall acceleration in aging. Here, we discuss our findings and its relevance to longevity, regenerative medicine, and tumorigenesis.
TAp63
maintains adult stem cells
The mysterious mechanisms that regulate
aging are an area of active research. The induction of senescence or apoptosis
in stem and progenitor cells is thought to trigger premature organismal aging [2]. Consistent
with this idea, we found that the TAp63-/- mice had a significantly
shortened life span compared to its wild-type littermates [1]. These mice
exhibited phenotypes associated with premature aging including kyphosis,
impaired wound healing, alopecia, epithelial and muscular atrophy, and chronic
nephritis. These phenotypes suggest a critical role for TAp63 in the
maintenance of adult stem cells in multiple epithelial and non-epithelial
tissues. Indeed, we found that TAp63 maintains dermal stem cells by
transcriptionally activating the cyclin dependent kinase inhibitor, p57,
thereby preventing hyperproliferation of these cells (Figure 1A) [1,3]. Similar
to the phenotype identified in dermal and epidermal progenitor and stem cells,
other adult stem cells in the TAp63-/- mice may be hyperproliferative early in life and through
similar senescence
mechanisms that we delineated may result in a
depletion of these stem cells and premature organismal aging (Figure 1B) [1].
The complex roles of the p53 family in aging
Increased p53 activity has been previously implicated
in aging [4,5]. Although
some mouse models with increased p53 activity exhibit signs of premature aging,
others show conflicting results [6,7]. The
important difference between these models is the alleles of p53 present
in these mice. The mice exhibiting signs of premature aging contain truncated
p53 mutants [4,5] while
those that display a normal lifespan upregulate p53 by other mechanisms, such
as the expression of a p53 transgene in addition to the endogenous p53
alleles or a hypomorphic allele of mdm2 [6,7]. One
potential explanation of the discrepancy in the phenotypes of these mice is
that TAp63 interacts with point mutant p53 rendering TAp63 functionally
inactive. Consequently, mice expressing mutant p53 would exhibit phenotypes
similar to those observed in the TAp63-/- mice. Previous studies have
shown this to occur in the context of tumorigenesis and metastasis [8,9]. Mice
engineered to express point mutants of p53 in Li-Fraumeni Syndrome inactivate
p63 and p73 in tumors by binding to them and preventing the transactivation of
their target genes [8,9,10]. These
mouse models exhibit a metastatic phenotype similar to that observed in p53+/-;p63+/-
and p53+/-;p73+/- mice illustrating an intricate relationship between
the p53 family members [11,12].
Yet, another unexplored and possible explanation is
that expression levels of the p53 family members change in mice that lack one
or more of the family members, i.e. gene compensation. Such family member
compensation has been observed in other families of genes including the Rb
family [13,14,15]. In
mouse models expressing abnormally high levels of p53, TAp63 levels may be
dampened commensurate with an increase in p53 protein expression. p53 protein
levels are known to be high in mice expressing mutated versions of p53 [8,9,10]. Thus,
loss of TAp63 in these mouse models may again result in an acceleration
of organismal aging. Furthermore, other
isoforms of p63 and p73 have been implicated in premature aging [16,17].
Therefore, careful characterization of the expression of the other p53 family
members, including the individual isoforms of p63 and p73, is necessary in
mouse models expressing altered levels of p53 in order to understand the
complex interplay and potential compensation between the p53 family
members in processes that regulate longevity (Figure 1).
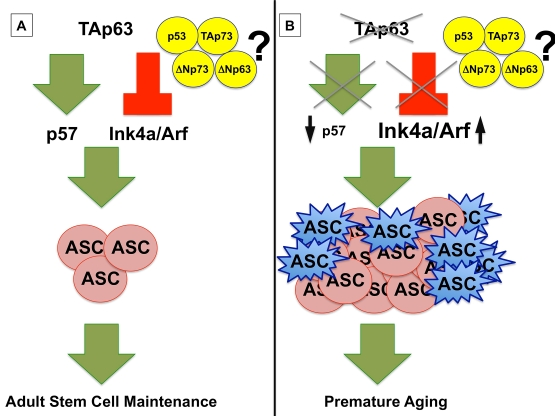
Figure 1. (A) TAp63
maintains adult stem cells (ASC) by transcriptionally activating p57
and repressing Ink4a/Arf, preventing premature aging. (B)
In the absence of TAp63, p57 mRNA levels are low, leading to
hyperproliferation of ASCs (shown in pink), and Ink4a/Arf levels are
high, resulting in a concomitant senescence of ASCs (shown in blue) and a
premature aging phenotype in TAp63 deficient mice. The interplay of
the p53 family, including TAp73, ΔNp73, and ΔNp63, remains to be
elucidated.
Loss of TAp63 triggers senescence and cannot be
reversed by concomitant loss of p53
Interestingly and surprisingly,
senescence triggered in TAp63-/- epidermal precursors is p53-independent.
In fact, we found a higher proportion of senescent cells in TAp63-/-;p53-/-
epidermal cells than in those lacking TAp63 only, indicating that loss
of p53 does not bypass senescence in this tissue [1]. This further indicates that TAp63
directly regulates senescence in epidermal precursor cells by transcriptionally
repressing Ink4a and Arf as has been observed in the epidermis of
mice deficient for p63 [1,18]. The mechanisms employed by TAp63
to induce senescence have important implications for deciphering its role as a
tumor suppressor gene.
TAp63 is induced in response to stress
p63 evolved to have several isoforms that can be divided
into two categories: the TA (transactivation competent isoforms) and the ΔN (those that lack the transactivation domain). The most highly
expressed isoforms of p63 in the skin are the ΔNp63
isoforms, thus the prevailing view is that ΔNp63, and more
specifically ΔNp63α, are the isoforms that play
critical roles in maintaining the epidermis [19,20].
However, it is important to note that the TAp63 isoforms structurally resemble
p53 and have been shown in other systems to be induced in response to DNA
damage and stress [21,22].
Importantly, although TAp63 protein expression is undetectable in the normal
epidermis, we found that TAp63 expression increased dramatically in response to
stress induced by wounding, indicating that much like p53, TAp63
serves to protect cells from damage [1]. This is a
novel and unrecognized role for TAp63 in maintaining the dermis and the
integrity of the epidermis.
TAp63: The key to longevity?
Mice lacking TAp63
also develop severe skin erosions that do not heal [1]. These
erosions result from trauma or ruptured blisters that form in the majority of TAp63-/- mice. The failure of these mice to appropriately heal their
wounds results from a depletion of SKP cells known to be required for wound
healing [1].
Additionally, the TAp63-/- mice exhibited patches where there was a
diminution in the number of hair follicles resulting in alopecia in these
mice. Some of these defects are similar to those seen in patients with Hay-Wells syndrome or ankyloblepharon-ectodermal
dysplasia-clefting (AEC) syndrome [23].
These pa-tients develop alopecia and skin erosions with impaired wound healing
indicating that the TAp63-/- mouse may be useful as a preclinical model
to test therapies for these disfiguring and painful diseases.
In addition, given the
critical function of TAp63 in wound healing and hair growth,
reactivation of TAp63 in tissues of patients with degenerative diseases
has important therapeutic implications not only in patients with AEC syndrome
but also in those with impaired wound healing, like diabetes. Important areas
for future investigation include developing models and therapies whereby TAp63
can be reactivated in adult dermal stem cells to determine whether senescence
and premature aging can be reversed in these cells to aid in the wound healing
process and hair regeneration.
The impact of the TAp63-/- aging phenotype on
cancer
p63 is an
important suppressor of tumorigenesis and metastasis; however, at first glance,
the role of p63 in senescence and aging may seem at odds with its role
as a tumor suppressor. It is important to note that adult dermal stem cells
are initially hyperproliferative prior to acquiring a senescent phenotype (Figure 1B). By extension, in tumor formation, cancer stem or precursor cells that
lose TAp63 may likewise be hyper-proliferative. With the high levels of
DNA damage and genomic instability that are detected in dermal and epidermal
stem cells lacking TAp63 [1], these cancer stem cells will likely
acquire new mutations that allow escape from senescence, an ideal formula for
tumor formation. In addition to further investigation on how TAp63
affects cancer stem cells, the milieu in which cancer cells reside must also be
closely examined in the TAp63-/- mouse model. Cancer incidence
increases with age, and it is possible that the prematurely aged environment of
the TAp63-/- mouse provides an ideal environment for tumor formation and
metastasis. Further investigation on the effects of premature aging in the TAp63
deficient mouse model on tumor formation is critical to obtain an understanding
of the roles of TAp63 as a tumor suppressor gene.
In summary, we have revealed a critical role for TAp63in preventing premature aging and further complexity of the p53
family, underscoring a need to understand the family as a whole and its roles
in human diseases. A clear understanding of the intimate and complex relationship
between the p53 family of genes is essential to target this pathway in
degenerative diseases and tumorigenesis.
Acknowledgments
E.R.F. is a scholar of the American Cancer Society
(RSG-07-082-01-MGO), Rita Allen Foundation, V Foundation for Cancer Research,
and March of Dimes (Basil O'Connor Scholar). We thank Kenneth Y. Tsai for critical reading of the manuscript.
Conflicts of Interest
The authors have no conflict of interests to declare.
References
-
1.
Su
X
, Paris
M
, Gi
YJ
, Tsai
KY
, Cho
MS
, Lin
YL
, Biernaskie
JA
, Sinha
S
, Prives
C
, Pevny
LH
, Miller
FD
and Flores
ER.
TAp63 prevents premature aging by promoting adult stem cell maintenance.
Cell Stem Cell.
2009;
5:
64
-75.
[PubMed]
.
-
2.
Finkel
T
, Serrano
M
and Blasco
MA.
The common biology of cancer and ageing.
Nature.
2007;
448:
767
-774.
[PubMed]
.
-
3.
Beaudry
VG
and Attardi
LD.
SKP-ing TAp63: stem cell depletion, senescence, and premature aging.
Cell Stem Cell.
2009;
5:
1
-2.
[PubMed]
.
-
4.
Maier
B
, Gluba
W
, Bernier
B
, Turner
T
, Mohammad
K
, Guise
T
, Sutherland
A
, Thorner
M
and Scrable
H.
Modulation of mammalian life span by the short isoform of p53.
Genes Dev.
2004;
18:
306
-319.
[PubMed]
.
-
5.
Tyner
SD
, Venkatachalam
S
, Choi
J
, Jones
S
, Ghebranious
N
, Igelmann
H
, Lu
X
, Soron
G
, Cooper
B
, Brayton
C
, Hee
Park S
, Thompson
T
and Karsently
G.
p53 mutant mice that display early ageing-associated phenotypes.
Nature.
2002;
415:
45
-53.
[PubMed]
.
-
6.
Garcia-Cao
I
, Garcia-Cao
M
, Martin-Caballero
J
, Criado
LM
, Klatt
P
, Flores
JM
, Wells
JC
, Blasco
MA
and Serrano
M.
"Super p53" mice exhibit enhanced DNA damage response, are tumor resistant and age normally.
EMBO J.
2002;
21:
6225
-6235.
[PubMed]
.
-
7.
Mendrysa
SM
, O'Leary
KA
, McElwee
MK
, Michalowski
J
, Eisenman
RN
, Powell
DA
and Perry
ME.
Tumor suppression and normal aging in mice with constitutively high p53 activity.
Genes Dev.
2006;
20:
16
-21.
[PubMed]
.
-
8.
Lang
GA
, Iwakuma
T
, Suh
YA
, Liu
G
, Rao
VA
, Parant
JM
, Valentin-Vega
YA
, Terzian
T
, Caldwell
LC
, Strong
LC
, El-Naggar
AK
and Lozano
G.
Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome.
Cell.
2004;
119:
861
-872.
[PubMed]
.
-
9.
Olive
KP
, Tuveson
DA
, Ruhe
ZC
, Yin
B
, Willis
NA
, Bronson
RT
, Crowley
D
and Jacks
T.
Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome.
Cell.
2004;
119:
847
-860.
[PubMed]
.
-
10.
Iwakuma
T
, Lozano
G
and Flores
ER.
Li-Fraumeni syndrome: a p53 family affair.
Cell Cycle.
2005;
4:
865
-867.
[PubMed]
.
-
11.
Flores
ER
The roles of p63 in cancer.
Cell Cycle.
2007;
6:
300
-304.
[PubMed]
.
-
12.
Flores
ER
, Sengupta
S
, Miller
JB
, Newman
JJ
, Bronson
R
, Crowley
D
, Yang
A
, McKeon
F
and Jacks
T.
Tumor predisposition in mice mutant for p63 and p73: evidence for broader tumor suppressor functions for the p53 family.
Cancer Cell.
2005;
7:
363
-373.
[PubMed]
.
-
13.
Mulligan
G
and Jacks
T.
The retinoblastoma gene family: cousins with overlapping interests.
Trends Genet.
1998;
14:
223
-229.
[PubMed]
.
-
14.
Mulligan
GJ
, Wong
J
and Jacks
T.
p130 is dispensable in peripheral T lymphocytes: evidence for functional compensation by p107 and pRB.
Mol Cell Biol.
1998;
18:
206
-220.
[PubMed]
.
-
15.
Sage
J
, Miller
AL
, Perez-Mancera
PA
, Wysocki
JM
and Jacks
T.
Acute mutation of retinoblastoma gene function is sufficient for cell cycle re-entry.
Nature.
2003;
424:
223
-228.
[PubMed]
.
-
16.
Keyes
WM
, Wu
Y
, Vogel
H
, Guo
X
, Lowe
SW
and Mills
AA.
p63 deficiency activates a program of cellular senescence and leads to accelerated aging.
Genes Dev.
2005;
19:
1986
-1999.
[PubMed]
.
-
17.
Wetzel
MK
, Naska
S
, Laliberte
CL
, Rymar
VV
, Fujitani
M
, Biernaski
JA
, Cole
CJ
, Lerch
JP
, Spring
S
, Wang
SH
, Frankland
PW
, Henkelman
RM
and Josselyn
SA.
p73 regulates neuro-degeneration and phospho-tau accumulation during aging and Alzheimer's disease.
Neuron.
2008;
59:
708
-721.
[PubMed]
.
-
18.
Su
X
, Cho
MS
, Gi
YJ
, Ayanga
BA
, Sherr
CJ
and Flores
ER.
Rescue of key features of the p63-null epithelial phenotype by inactivation of Ink4a and Arf.
EMBO J.
2009;
28:
1904
-1915.
[PubMed]
.
-
19.
Candi
E
, Rufini
A
, Terrinoni
A
, Dinsdale
D
, Ranalli
M
, Paradisi
A
, De
Laurenzi V
, Spagnoli
LG
, Catani
MV
, Ramadan
S
, Knight
RA
and Melino
G.
Differential roles of p63 isoforms in epidermal development: selective genetic complementation in p63 null mice.
Cell Death Differ.
2006;
13:
1037
-1047.
[PubMed]
.
-
20.
Senoo
M
, Pinto
F
, Crum
CP
and McKeon
F.
p63 Is essential for the proliferative potential of stem cells in stratified epithelia.
Cell.
2007;
129:
523
-536.
[PubMed]
.
-
21.
Flores
ER
, Tsai
KY
, Crowley
D
, Sengupta
S
, Yang
A
, McKeon
F
and Jacks
T.
p63 and p73 are required for p53-dependent apoptosis in response to DNA damage.
Nature.
2002;
416:
560
-564.
[PubMed]
.
-
22.
Jacobs
WB
, Govoni
G
, Ho
D
, Atwal
JK
, Barnabe-Heider
F
, Keyes
WM
, Mills
AA
, Miller
FD
and Kaplan
DR.
p63 is an essential proapoptotic protein during neural development.
Neuron.
2005;
48:
743
-756.
[PubMed]
.
-
23.
McGrath
JA
, Duijf
PH
, Doetsch
V
, Irvine
AD
, de Waal
R
, Vanmolkot
KR
, Wessagowit
V
, Kelly
A
, Atherton
DJ
, Griffiths
WA
, Orlow
SJ
, van
Haeringen A
and Ausems
MG.
Hay-Wells syndrome is caused by heterozygous missense mutations in the SAM domain of p63.
Hum Mol Genet.
2001;
10:
221
-229.
[PubMed]
.