Dopamine suppresses octopamine signaling in C. elegans: possible involvement of dopamine in the regulation of lifespan
Abstract
Amine neurotransmitters, such as dopamine, serotonin, and noradrenaline, play important roles in the modulation of behaviors and metabolism of animals. InC. elegans, it has been shown that serotonin and octopamine, an invertebrate equivalent of noradrenaline, also regulate lifespan through a mechanism related to food deprivation-mediated lifespan extension. We have shown recently that dopamine signaling, activated by the tactile perception of food, suppresses octopamine signaling and that the cessation of dopamine signaling in the absence of food leads to activation of octopamine signaling. Here, we discuss the apparent conservation of neural and molecular mechanisms for dopamine regulation of octopamine/noradrenaline signaling and a possible role for dopamine in lifespan regulation.
Amine neurotransmitter
regulation of life span
It is becoming clear from
studies in model animals that amine neurotransmitters can regulate the
longevity of animals. In Drosophila, it is shown that a quantitative
trait locus for the variation of longevity maps into the aromatic L-amino acid
decarboxylase gene, which is required for dopamine and serotonin synthesis [1].
Murakami et al. showed that, in C. elegans, the serotonin receptor
mutant ser-1 has increased lifespan whereas another serotonin receptor
mutant ser-4 has decreased life span, suggesting that serotonin can
affect lifespan in opposite ways depending on the receptor mechanism that is
invoked [2]. It is also reported that serotonin signaling is required for
reserpine-mediated lifespan extension [3].
Petrascheck et al. found through chemical
screening that mianserin, an antidepressant, extends life span of C. elegans
[4]. They demonstrated that mianserin is an
antagonist for the serotonin receptor SER-4 and the octopamine receptor SER-3
and that mianserin-mediated lifespan extension was dependent on each of these
receptors, suggesting that not only serotonin but also octopamine plays a role
in the regulation of lifespan. Octopamine is an amine neurotransmitter that is
considered to be a biological equivalent of noradrenaline [5]. It is shown that
in C. elegans exogenous serotonin induces behavioral changes that are
observed in the presence of food, whereas exogenous octopamine induces
behaviors of starved animals [6]. It has been proposed therefore that serotonin
and octopamine act as physiological antagonists and that serotonin signals the
presence of food, whereas octopamine signals the absence of food. Thus,
Petrascheck et al. tested the effect of mianserin under food deprivation since
food deprivation has been shown to extend lifespan in many animals including C.
elegans [7]. They found that mianserin did not further increase the
lifespan of food-deprived animals, indicating that mianserin extends lifespan
through aging mechanisms associated with food deprivation [4].
These results suggest that
octopamine along with serotonin regulates lifespan in C. elegans through
mechanisms that are related to food deprivation. We have recently elucidated a
mechanism for activation of octopamine signaling in the absence of food in C.
elegans and demonstrated the involvement of the amine neurotransmitter
dopamine in this regulation [8,9]. We review the findings and discuss potential
conservation with mammalian systems and a connection to aging.
Octopamine signaling is
activated in the absence of food
In C. elegans,
activation of CREB can be detected using a cre::gfp fusion gene, in
which the cyclic AMP response element (CRE) is fused to the gene encoding green
fluorescent protein (GFP) [10]. In a strain carrying cre::gfp, GFP is
expressed in cells in which CREB is activated. Using this reporter system, we
first found that the absence of food induces CREB activation in the cholinergic
SIA neurons [8]. To determine whether octopamine is involved in this signaling
mechanism, mutants of the tbh-1 gene were tested. tbh-1 encodes
tyramine-ƒΐ-hydroxylase which is required for octopamine synthesis and is
expressed only in the RIC neurons and the gonadal sheath cells (the latter are
unlikely to play a role in this food response) [11]. tbh-1 mutants
failed to respond to the absence of food, indicating that octopamine is
responsible for CREB activation. We also found that exogenous application of
octopamine in the presence of food induces CREB activation in the SIA neurons,
which also supports the involvement of octopamine. These results confirmed the
notion that octopamine signaling is activated in the absence of food.
Furthermore, we found that the octopamine receptor SER-3 is required for both
responses to the absence of food and to exogenous octopamine. Cell-specific
expression of SER-3 in the SIA neurons rescued exogenous octopamine and food responses
of ser-3 mutant animals, indicating that SER-3 works in the SIA neurons
to receive octopamine signaling. Given that there is no synaptic connection
between the RIC and SIA neurons, these results suggest that octopamine released
from the RIC neurons humorally activates SER-3 in the SIA neurons in the
absence of food.
Dopamine suppresses
octopamine signaling
Dopamine signaling in C. elegans
is important for food sensing [12]. Dopaminergic
neurons in C. elegans have sensory endings under the cuticle and sense
the presence of food by mechanosensation [12-14]. The mechanosensation of food
is believed to activate release of dopamine. Interestingly, one class of
dopaminergic neurons, the CEP neurons, is known to be presynaptic to both the
RIC and SIA neurons [13]. Considering that dopamine and octopamine are
regulated oppositely by food and that dopaminergic neurons are in a suitable
location to control octopamine signaling, we tested whether dopamine interacts
with octopamine signaling [9].
We first found that
exogenously applied dopamine suppresses exogenous octopamine-mediated CREB
activation in the SIA neurons. To determine whether endogenous dopamine also
suppresses octopamine signaling, we tested cat-2 mutants, which are
defective in dopamine synthesis since cat-2 encodes the tyrosine
hydroxylase, the rate limiting enzyme for dopamine synthesis [15]. cat-2
mutants exhibited spontaneous CREB activation in the SIA neurons even in the
presence of food. This spontaneous activation requires endogenous octopamine
since spontaneous CREB activation was suppressed in cat-2;tbh-1 double
mutants. These results indicate that octopamine-SER-3-CREB signaling pathway is
constitutively activated in cat-2 mutants and dopamine normally
suppresses this pathway in the presence of food.
To further demonstrate the
involvement of endogenous dopamine in the suppression of octopamine signaling,
we used the Sephadex beads. It was shown previously that the Sephadex beads
induce a dopamine-dependent behavioral change presumably by mimicking the
tactile attribute of food without providing nutritional or chemosensory cues
associated with bacteria (food) [12]. Addition of the Sephadex beads to the
culture plates completely suppressed CREB activation induced by the absence of
food [9]. This result suggests that octopamine-mediated CREB activation in the
absence of food is not initiated by the decrease in food intake (starvation)
but by the absence of tactile perception of food by the dopaminergic neurons.
We have tested all
identified dopamine receptors in C. elegans and found that two D2-like
dopamine receptors, DOP-2 and DOP-3 [16,17], work downstream of dopamine to
suppress octopamine signaling [9]. Cell-specific rescue experiments determined
that both DOP-2 and DOP-3 work in the SIA neurons to suppress
octopamine-mediated signaling. In addition, we found that DOP-3 also works in
the RIC neurons to suppress CREB activation in response to endogenous dopamine.
Therefore, it is likely that dopamine suppresses octopamine signaling in two
ways. One is by affecting release of octopamine from the RIC neurons and the
other is by negatively regulating the ability of octopamine to activate CREB in
the SIA neurons.
These studies suggests thatC. elegans uses a three-neuron-type circuit to control octopamine
signaling in response to food (Figure 1). In the presence of food, dopamine is
released by the dopaminergic neurons. The released dopamine activates DOP-3 in
the RIC neurons, possibly to decrease octopamine release. Simultaneous-ly,
dopamine also inhibits octopamine-mediated signaling in the SIA neurons through
DOP-2 and DOP-3. In the absence of food, dopamine is not released, which
inactivates DOP-3 in the RIC neurons, potentially increasing octopamine
release. The released octopamine activates the octopamine receptor SER-3 in the
SIA neurons, which results in activation of CREB because negative regulation by
dopamine receptors does not occur when dopamine is not released. An important
feature of this circuit is that octopamine signaling can be activated solely by
removal of suppression by dopamine signaling without any other signaling to
activate it.
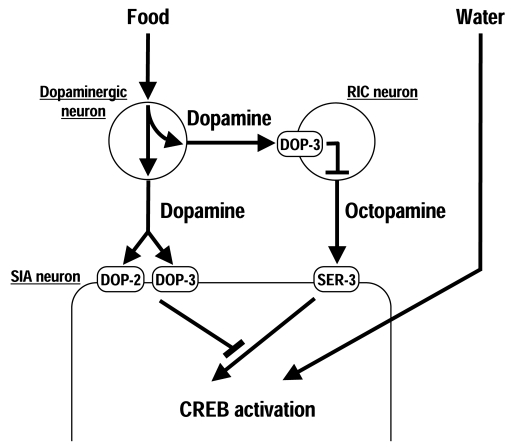
Figure 1. Regulation of CREB activation in the SIA neurons. In the presence of food, dopamine is
released from the dopaminergic neurons and activates the dopamine receptor
DOP-3 in the RIC neurons, possibly to decrease octopamine release. Dopamine
also inhibits octopamine-mediated signaling in the SIA neurons through the
dopamine receptors DOP-2 and DOP-3. In the absence of food, cessation of
dopamine signaling results in octopamine-mediated CREB activation through
the octopamine receptor SER-3. Exposure to water also induces CREB
activation in the SIA neurons independently of dopamine and octopamine.
There are striking
analogies between this three-neuron-type circuit in C. elegans and a
three-neuron-type circuit possibly involved in food response in the mammalian
brain. First, food stimuli increase dopamine and decrease noradrenaline release
in the mammalian brain [18,19]. Second, noradrenergic neurons in the locus
coeruleus receive projections from dopaminergic neurons in the ventral
tegmental area [20] and their firing rate is negatively regulated by dopamine
[21]. Third, both the noradrenergic neurons and the dopaminergic neurons
innervate basal forebrain cholinergic neurons [22]. This may be analogous to
the way the octopaminergic RIC neurons and dopaminergic neurons (e.g. the CEP
neurons) signals to the cholinergic SIA neurons in C. elegans.
Therefore, we postulate that the neuronal and molecular circuitry for food
sensing we have discovered in C. elegans is conserved in vertebrates, in
which cessation of dopamine signaling activates octopamine/noradrenaline
signaling.
Potential role of
dopamine in the lifespan regulation
It is unknown whether the
SIA neurons play any role in aging and it is highly possible that
mianserin-mediated lifespan extension work through its effect on SER-3 in other
cells. However, the finding that dopamine regulates the octopaminergic RIC
neurons suggests that octopamine signaling in the cells other than the SIA
neurons are also regulated by dopamine. This raises the possibility that
dopamine plays a role in the food-mediated regulation of lifespan.
It has been suggested that
food limits lifespan through at least two different mechanisms. One is by
providing nutrition and the other is by providing sensory perception [23].
Since dopamine regulates octopamine signaling in response to tactile perception
of food rather than ingestion of food, if dopamine plays a role in lifespan
regulation, it would be because of its involvement in food perception.
Murakami et al. showed that
dopamine-deficient cat-2 mutants have a normal lifespan when measured in
standard culture conditions [2]. However, this result does not rule out the possible
involvement of dopamine since molecular mechanisms that control lifespan are
highly context dependent [24]. In fact, mianserin-mediate lifespan extension is
not observed in the standard culture condition in which animals are grown on
solid agar but it is observed only in a liquid culture [25,26]. Intriguingly,
soaking animals in water also induces CREB activation in the SIA neurons just
as is seen in the absence of food (Figure 1) [8], suggesting the possible
existence of an interaction between food signaling and signaling mediated by
the exposure to liquid. Therefore, more detailed studies of the effect of
dopamine signaling on the regulation of lifespan in C. elegans would be
particularly enlightening, especially since it has been reported that a polymorphism
in the tyrosine hydroxylase gene, which is required for dopamine synthesis, is
associated with variation in human longevity [27,28].
Acknowledgement
This work was supported in
part by the Canadian Institutes of Health Research grant MOP-77722 and
MOP-82909 to J.G.C. J.G.C. and H.H.M.V.T. are holders of Canadian Research
Chairs. S.S. is a recipient of a Parkinson Society Canada Basic Research
Fellowship.
Conflicts of Interest
The authors of this manuscript have no conflict of interest to declare.
References
-
1.
De
Luca M
, Roshina
NV
, Geiger-Thornsberry
GL
, Lyman
RF
, Pasyukova
EG
and Mackay
TFC.
Dopa decarboxylase (Ddc) affects variation in Drosophila longevity.
Nat Genet.
2003;
34:
429
-433.
[PubMed]
.
-
2.
Murakami
H
and Murakami
S.
Serotonin receptors antagonistically modulate Caenorhabditis elegans longevity.
Aging Cell.
2007;
6:
483
-488.
[PubMed]
.
-
3.
Srivastava
D
, Arya
U
, SoundaraRajan
T
, Dwivedi
H
, Kumar
S
and Subramaniam
JR.
Reserpine can confer stress tolerance and lifespan extension in the nematode C. elegans.
Biogerontology.
2008;
9:
309
-316.
[PubMed]
.
-
4.
Petrascheck
M
, Ye
X
and Buck
LB.
An antidepressant that extends lifespan in adult Caenorhabditis elegans.
Nature.
2007;
450:
553
-556.
[PubMed]
.
-
5.
Roeder
T
Octopamine in invertebrates.
Prog Neurobiol.
1999;
59:
533
-561.
[PubMed]
.
-
6.
Horvitz
HR
, Chalfie
M
, Trent
C
, Sulston
JE
and Evans
PD.
Serotonin and octopamine in the nematode Caenorhabditis elegans.
Science.
1982;
216:
1012
-1014.
[PubMed]
.
-
7.
Piper
MDW
and Bartke
A.
Diet and aging.
Cell Metab.
2008;
8:
99
-104.
[PubMed]
.
-
8.
Suo
S
, Kimura
Y
and Van
Tol HHM.
Starvation induces cAMP response element-binding protein-dependent gene expression through octopamine-Gq signaling in Caenorhabditis elegans.
J Neurosci.
2006;
26:
10082
-10090.
[PubMed]
.
-
9.
Suo
S
, Culotti
JG
and Van
Tol HHM.
Dopamine counteracts octopamine signalling in a neural circuit mediating food response in C. elegans.
EMBO J.
2009;
28:
2437
-2448.
[PubMed]
.
-
10.
Kimura
Y
, Corcoran
EE
, Eto
K
, Gengyo-Ando
K
, Muramatsu
M
, Kobayashi
R
, Freedman
JH
, Mitani
S
, Hagiwara
M
, Means
AR
and Tokumitsu
H.
A CaMK cascade activates CRE-mediated transcription in neurons of Caenorhabditis elegans.
EMBO Rep.
2002;
3:
962
-966.
[PubMed]
.
-
11.
Alkema
MJ
, Hunter-Ensor
M
, Ringstad
N
and Horvitz
HR.
Tyramine Functions independently of octopamine in the Caenorhabditis elegans nervous system.
Neuron.
2005;
46:
247
-260.
[PubMed]
.
-
12.
Sawin
ER
, Ranganathan
R
and Horvitz
HR.
C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway.
Neuron.
2000;
26:
619
-631.
[PubMed]
.
-
13.
White
JG
, Southgate
E
, Thomson
JN
and Brenner
S.
The structure of the nervous system of the nematode C. elegans.
Philos Trans R Soc Lond B Biol Sci.
1986;
314:
1
-340.
.
-
14.
Sulston
J
, Dew
M
and Brenner
S.
Dopaminergic neurons in the nematode Caenorhabditis elegans.
J Comp Neurol.
1975;
163:
215
-226.
[PubMed]
.
-
15.
Lints
R
and Emmons
SW.
Patterning of dopaminergic neurotransmitter identity among Caenorhabditis elegans ray sensory neurons by a TGFbeta family signaling pathway and a Hox gene.
Development.
1999;
126:
5819
-5831.
[PubMed]
.
-
16.
Suo
S
, Sasagawa
N
and Ishiura
S.
Cloning and characterization of a Caenorhabditis elegans D2-like dopamine receptor.
J Neurochem.
2003;
86:
869
-878.
[PubMed]
.
-
17.
Sugiura
M
, Fuke
S
, Suo
S
, Sasagawa
N
, Van
Tol HHM
and Ishiura
S.
Characterization of a novel D2-like dopamine receptor with a truncated splice variant and a D1-like dopamine receptor unique to invertebrates from Caenorhabditis elegans.
J Neurochem.
2005;
94:
1146
-1157.
[PubMed]
.
-
18.
Hajnal
A
, Smith
GP
and Norgren
R.
Oral sucrose stimulation increases accumbens dopamine in the rat.
Am J Physiol Regul Integr Comp Physiol.
2004;
286:
R31
-37.
[PubMed]
.
-
19.
Hajnal
A
and Norgren
R.
Sucrose sham feeding decreases accumbens norepinephrine in the rat.
Physiol Behav.
2004;
82:
43
-47.
[PubMed]
.
-
20.
Beckstead
RM
, Domesick
VB
and Nauta
WJ.
Efferent connections of the substantia nigra and ventral tegmental area in the rat.
Brain Res.
1979;
175:
191
-217.
[PubMed]
.
-
21.
Guiard
BP
, El
Mansari M
, Merali
Z
and Blier
P.
Functional interactions between dopamine, serotonin and norepinephrine neurons: an in-vivo electrophysiological study in rats with monoaminergic lesions.
Int J Neuropsychopharmacol.
2008;
11:
625
-639.
[PubMed]
.
-
22.
Jones
BE
and Cuello
AC.
Afferents to the basal forebrain cholinergic cell area from pontomesencephalic--catecholamine, serotonin, and acetylcholine--neurons.
Neuroscience.
1989;
31:
37
-61.
[PubMed]
.
-
23.
Smith
ED
, Kaeberlein
TL
, Lydum
BT
, Sager
J
, Welton
KL
, Kennedy
BK
and Kaeberlein
M.
Age- and calorie-independent life span extension from dietary restriction by bacterial deprivation in Caenorhabditis elegans.
BMC Dev Biol.
2008;
8:
49
[PubMed]
.
-
24.
Greer
EL
and Brunet
A.
Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans.
Aging Cell.
2009;
8:
113
-127.
[PubMed]
.
-
25.
Petrascheck
M
, Ye
X
and Buck
LB.
A high-throughput screen for chemicals that increase the lifespan of Caenorhabditis elegans.
Ann N Y Acad Sci.
2009;
1170:
698
-701.
[PubMed]
.
-
26.
Zarse
K
and Ristow
M.
Antidepressants of the serotonin-antagonist type increase body fat and decrease lifespan of adult Caenorhabditis elegans.
PLoS ONE.
2008;
3 (12):
e4062
[PubMed]
.
-
27.
De
Luca M
, Rose
G
, Bonafè
M
, Garasto
S
, Greco
V
, Weir
BS
, Franceschi
C
and De
Benedictis G.
Sex-specific longevity associations defined by Tyrosine Hydroxylase-Insulin-Insulin Growth Factor 2 haplotypes on the 11p15.5 chromosomal region.
Exp Gerontol.
2001;
36:
1663
-1671.
[PubMed]
.
-
28.
De
Benedictis G
, Carotenuto
L
, Carrieri
G
, De
Luca M
, Falcone
E
, Rose
G
, Cavalcanti
S
, Corsonello
F
, Feraco
E
, Baggio
G
, Bertolini
S
, Mari
D
, Mattace
R
, Yashin
AI
, Bonafè
M
and Franceschi
C.
Gene/longevity association studies at four autosomal loci (REN, THO, PARP, SOD2).
Eur J Hum Genet.
1998;
6:
534
-541.
[PubMed]
.