Macronutrient balance and lifespan
Abstract
Dietary restriction (DR) without malnutrition is widely regarded to be a universal mechanism for prolonging lifespan. It is generally believed that the benefits of DR arise from eating fewer calories (termed caloric restriction, CR). Here we argue that, rather than calories, the key determinant of the relationship between diet and longevity is the balance of protein to non-protein energy ingested. This ratio affects not only lifespan, but also total energy intake, metabolism, immunity and the likelihood of developing obesity and associated metabolic disorders. Among various possible mechanisms linking macronutrient balance to lifespan, the nexus between the TOR and AMPK signaling pathways is emerging as a central coordinator.
Convincingly separating the effects of CR
on lifespan from more specific nutrient effects is not trivial and requires
experimental designs comprising multiple dietary regimes in which energy intake
and nutrient balance are considered both separately and interactively [1].
Building upon an earlier study questioning the role of CR in Drosophila
melanogaster [2], the first study to employ a design that unequivocally
disentangled CR from specific nutrient effects was that of Lee et al. [3].
Mated female flies were allowed ad libitum access to one of 28 diets, varying
in the ratio and concentration of yeast to sugar. Food intake was measured for
each fly and bi-coordinate intakes of protein and carbohydrate (the major
macronutrients in the diets) were plotted. Response surfaces for lifespan, age
of maximal mortality, rate of age-dependent increase in mortality, lifetime egg
production and rate of egg production were then fitted over the array of
protein-carbohydrate intake points (see Figure 1A for lifespan and lifetime egg
production surfaces). Flies lived longest on a diet containing a 1:16 P:C ratio
and lived progressively less long as the P:C ratio increased. The contours of the
longevity surface ran almost orthogonally to lines of equal caloric intake
(dotted lines in Figure 1A). Even allowing for possible differences in the
relative availability of energy in protein and carbohydrate or interactions
between protein and carbohydrate metabolism, the lifespan and caloric intake
isoclines in Figure 1A cannot be aligned. The data therefore prove that CR
could not account for the variation in lifespan. Rather, the balance of
carbohydrate to protein ingested was strongly correlated with longevity.
The response surface for
lifetime egg production peaked at a higher protein content than supported
maximal lifespan (1:4 P:C, Figure 1A). This demonstrates that the flies could
not maximize both lifespan and egg production rate on a single diet, and raises
the interesting question of what the flies themselves prioritized - extending
lifespan or maximizing lifetime egg production. Lee et al. [3] answered this by
offering one of 9 complementary food choices in the form of separate yeast and
sugar solutions differing in concentration. The flies mixed a diet such that
they converged upon a nutrient intake trajectory of 1:4 P:C, thereby maximizing
lifetime egg production and paying the price of a diminished lifespan.
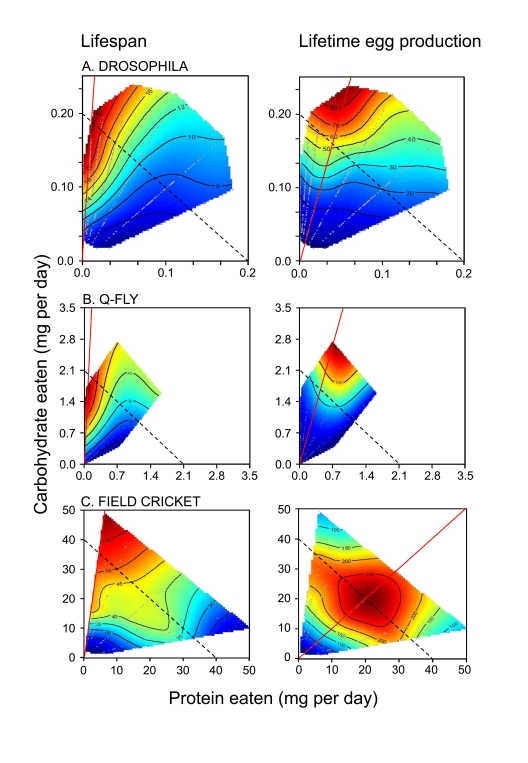
Figure 1. How the intake of protein and carbohydrate influence longevity and lifetime egg production in adults of three insect species. Individuals
were given ad libitum access to one of 28 (Drosophila and the
Queensland fruit fly, Q-fly) or 24 (field cricket) diets varying in the
ratio and total concentration of protein to carbohydrate (P:C). Plotted
onto arrays of points of nutrient intake are fitted surfaces for the two
performance variable, which rise in elevation from dark blue to dark red.
Unbroken red lines indicate the dietary P:C that maximized the response
variable, whereas the dotted lines indicate isocaloric intakes. In each
case, insects lived longest when the diet contained a low P:C, and lifespan
declined as P:C rose. Female reproductive output was maximal on higher P:C
diets than sustained greatest longevity, but fell as P:C rose further, even
at high total energy intakes. Data are replotted from Lee et al. [3]
(Drosophila), Maklakov et al. [11] (field crickets), and Fanson et
al. [4] (Q-fly).
Lee et al. [3] compared their data against a longevity
surface compiled from previously published studies, individually involving many
fewer dietary treatments and no measurement of long-term food intake. The two
surfaces corresponded closely, despite substantial procedural differences
across studies and differences in mean lifespan between capillary-fed, singly
housed flies in the study of Lee et al. [3] and flies housed in groups and fed
agar-based diets in the other experiments. To further demonstrate that the
nutritional associations were robust, traditional demography cage trials were
run for a selection of diets without measuring intake. These flies lived longer
than when housed singly and fed from capillaries, but the pattern of lifespan,
egg production and egg production rate in relation to dietary P:C ratio was the
same.
A parallel experiment was conducted by Fanson et al.
[4] on Queensland fruit fly, Bactrocera tryoni (another dipteran but
from a different family, Tephritidae rather than Drosophilidae) subjected to
one of 28 no-choice or 25 choice diet treatments. As can be seen in Figure 1B,
the results and conclusions were similar in all respects to those reported by
Lee et al. [3] for Drosophila. Once again, dietary P:C and not energy
intake was strongly associated with lifespan. The data were also consistent
with those from studies on another species of tephritid, the Mexican fruit fly,Anastrepha ludens [5].
Recently, Ja et al. [6] confirmed that increasing the
ratio of yeast to sugar (hence P:C) in the diet substantially reduced lifespan
in adult Drosophila, to an extent that maps precisely onto the data of
Lee et al. [3]. Additionally, these authors found that the more modest
shortening of lifespan found on concentrated relative to dilute versions of a
diet containing a 1:1 yeast to sugar ratio (the diet composition employed in
many previous studies) was absent when flies had access to free water; implying
that what has previously been reported as the beneficial effects of DR may
instead be the obverse of the deleterious consequences of water deprivation.
Providing a separate water source had no effect on the change in lifespan
associated with a change in yeast:sugar. Indeed, it can now be suggested with
some credence that perhaps the life-prolonging effects of DR, as traditionally
conceived, do not occur in Drosophila. It is interesting to note how a
recent study [7] denotes an increase in P:C combined with overall dilution as
‘diet restriction', rather than relying on dilution of a 1:1 yeast:sugar diet
as in the past.
In the studies of Lee et al. [3], Fanson et al. [4],
Ja et al. [6] and others, longevity was primarily associated with the ratio of
yeast to sugar eaten. Yeast is a complex food, containing micronutrients and
other chemicals in addition to protein and carbohydrate. To be sure that P:C is
influencing lifespan rather than some correlated component of yeast or another
confounding change in diet composition will require using chemically defined
diet formulations. No fully satisfactory such diet exists as yet for Drosophila,
although Troen et al. [8] used four chemically defined diets in which the amino
acid methionine and glucose were varied. Small but significant effects of
dietary methionine on lifespan were reported.
However, chemically defined diets do exist for other
insect species. It is well documented that lowering P:C in chemically defined
diets slows the development of juvenile insects [9,10], and the recent work of
Maklakov et al. [11,12] on adult crickets provides conclusive evidence that the
ratio of protein to carbohydrate is the primary dietary determinant of lifespan
in that insect (Figure 1C). Maklakov et al. fed field crickets, Teleogryllus
commodus, one of 24 chemically defined diets and measured intake, lifespan,
female lifetime egg production, daily egg production, male lifetime courtship
singing effort, and singing effort per night. As for tephritids and Drosophila,
crickets lived longest on low P:C ratio diets, and died progressively earlier
as P:C ratio increased. Males but not females demonstrated a reduction in
lifespan at high intakes of very low P:C
diets; a result which was consistent with their greater propensity to lay down
excess body fat on such diets and hence reflects the costs of obesity (a point
that we consider further below). Again as for flies, female lifetime egg
production was maximal at a higher P:C ratio than sustained maximal lifespan
(Figure 1C). Male courtship singing attained a maximum at a lower P:C ratio
than did female egg production.
The data for insects show that CR is not responsible
for lifespan extension, rather, dietary P:C is critical: is the same true for
mammals? It is widely held that CR, not specific nutrient effects, is
responsible for lifespan extension in mammals [13,14]. However, we have argued
previously [1] that it is not possible to estimate response surfaces such as
those in Figure 1 without using a much larger number of diet treatments than
have been employed to date in experiments on any mammal, including rodents.
Without such surfaces it is simply not possible to separate CR from the effects
of nutrient balance. Additionally, it has been reported over many years,
notably in the early work of Morris Ross, that protein restriction, and of
methionine in particular, extends lifespan in rodents [15-19]. Therefore, a
study akin to that of Lee et al. [3] is required on rodents.
Whereas the experiments on insects have
been able to concentrate on two macronutrient dimensions, protein and
carbohydrate, a full design for rodents would need to extend to three
dimensions by including variation in dietary lipid. An efficient initial design
would need to include around 30 dietary treatments (e.g. 10 P:C:L ratios and 3
total concentrations), which would need to be fed to mice throughout their
lives. This is challenging but by no means intractable - and would allow
surfaces for lifespan and all manner of histological, biochemical and molecular
variables, including those implicated in the process of aging, to be plotted
onto macronutrient intake arrays.
To this point we have concentrated on evidence that
increasing the ratio of protein and non-protein energy in the diet decreases
lifespan; but as seen in the example from male crickets discussed above, if
this ratio falls too far there is an increased risk of decreased longevity
associated with obesity. The reason for this is that in omnivores and
herbivores studied to date, protein intake is more strongly regulated than that
of carbohydrate and fat [20]. As a result, protein appetite drives
overconsumption of energy on low percent protein diets, promoting obesity and
metabolic disorders with consequent effects on longevity. Overconsumption of
energy on low percent protein diets has been reported for insects (e.g. [21]),
fish (e.g. [22]), birds [23], rodents [24,25], nonhuman primates [26] and
humans [20,27]. Fat deposition in response to excess ingested carbohydrate,
driven by low dietary percent protein, has been shown to be labile in
laboratory selection experiments in an insect - it increased in response to
habitual shortage of carbohydrate across successive generations and decreased
in the face of persisting carbohydrate excess in the diet [28]. One adaptive
mechanism that helps counteract the risk of developing obesity on low percent
protein diets is increased facultative diet-induced thermogenesis, whereby
excess ingested carbohydrates are removed via wastage metabolic cycles, e.g.
involving uncoupling proteins [29].
In the context of the
deleterious consequences of overconsumption it is interesting to note that the
major causes of increased longevity in studies on calorically restricted
primates (most recently [30]) is a reduction in the incidence of diabetes,
cancer and cardiovascular disease relative to ad libitum fed controls. This may
not result from benefits associated with CR per se, but rather reflect the
costs of nutrient imbalance when feeding ad libitum on a fixed diet. As the required balance of
nutrients changes over time (with time of day, season, growth and development,
and senescence), animals will be forced to overeat some nutrients to gain
enough of others. Even if a fixed diet is nutritionally balanced when
integrated across the entire lifespan (and worse if it is not), changes in
requirements at a finer timescale will result in accumulated damage from
short-term nutrient excesses, which may be ameliorated by modest diet
restriction [1].
When protein is eaten in higher then optimal
quantities relative to non-protein energy it shortens lifespan - in insects
certainly and perhaps too in mammals - but what might the underlying mechanisms
be? There are several possibilities, including enhanced production of mitochondrial
radical oxygen species [19,31], DNA and protein
oxidative modification, changes in membrane fatty acid composition and
mitochondrial metabolism [19,32], changes
in the relationship between insulin/IGF and amino acid signaling
pathways, including TOR [33-38], toxic effects of nitrogenous breakdown
products and capacity to deal with other dietary toxins [39,40], changes in
immune function to pathogen attack [41,42], and changed functioning of circadian
systems [43]. How these various components are interrelated will begin to
emerge from analyses in which multiple biomarkers and response variables are
mapped onto nutrient intake surfaces such as shown in Figure 1.
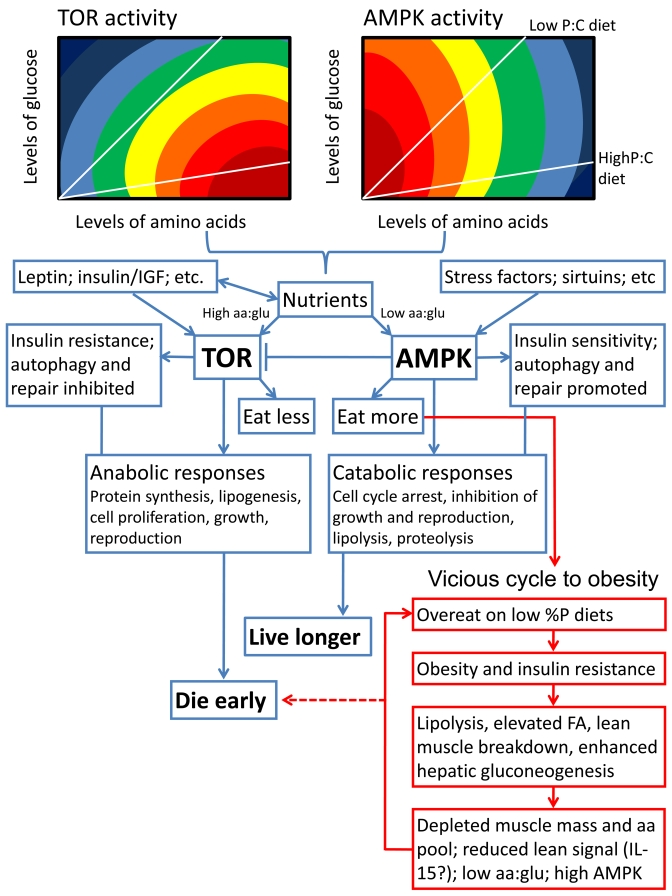
Figure 2. Schematic summarizing our hypothesis for how diet balance might affect lifespan via the TOR and AMPK signaling pathways. We propose that
both TOR and AMPK respond not only to the concentration of circulating
nutrients (with TOR activity stimulated and AMPK depressed either directly
or indirectly by increasing concentrations), but also to nutrient balance.
We show hypothetical response surfaces for TOR and AMPK in relation to
circulating concentrations and ratios of amino acids (aa) and glucose
(glu), with responses rising from dark blue to deep red. The red boxes
indicate what we have termed the vicious cycle to obesity, in which chronic
exposure to a low percent protein diet can drive overconsumption, metabolic
disorders and shortened lifespan unless excess ingested energy is
dissipated (see [20], and further supporting evidence from rodents in
[52,53]). Otherwise, low percent protein diets are life extending via the
normal actions of AMPK, whereas high percent protein diets shorten lifespan
and encourage aging via the TOR pathway.
If we were to propose one
candidate for the hub linking nutrient balance and other inputs to longevity it
would be the interplay between the TOR and AMPK signaling pathways. Both TOR and AMPK
serve as nutrient sensors and are linked to nutrient intake and metabolism.
Factors that directly or indirectly increase TOR signaling, including elevated
nutrients such a branch chain amino acids, glucose and fatty acids, are broadly
anabolic and life-shortening. In contrast low levels of nutrients, declining ATP:AMP,
and other influences that stimulate AMPK signaling are catabolic and
life-extending [34; 38; 44-48] (Figure 2); - except when overconsumption,
obesity and insulin resistance are driven by protein shortage on a habitually
low percent protein diet [20] (see Figure 2). Although it is not yet establish
whether TOR and AMPK are nutrient balance detectors, there are
suggestions that they may well be. For example, glucose activates TOR in an
amino acid-dependent manner [49] and elevated percent protein diet stimulates
TOR and inhibits AMPK (e.g. [50,51]). We predict that mapping the responses of
both TOR and AMPK onto nutrient intake arrays will provide fundamental new
insights not only into aging, but also a whole range of interlinked metabolic
phenomena, including obesity, type 2 diabetes, cancer risk and cardiovascular
disease. To illustrate this point, we have predicted response surfaces in
Figure 2 and linked aspects of nutrient balance, aging and obesity within a
single schema.
Conflicts of Interest
The authors have no conflict of interests to declare.
References
-
1.
Simpson
SJ
and Raubenheimer
D.
Caloric restriction and aging revisited: the need for a geometric analysis of the nutritional bases of aging.
J Gerontol A Biol Sci Med Sci.
2007;
62:
707
-713.
[PubMed]
.
-
2.
Mair
W
, Piper
MDW
and Partridge
L.
Calories do not explain extension of life span by dietary restriction in Drosophila.
PLoS Biol.
2005;
3:
1305
-1311.
.
-
3.
Lee
KP
, Simpson
SJ
, Clissold
FJ
, Brooks
R
, Ballard
JWO
, Taylor
PW
, Soran
N
and Raubenheimer
D.
Lifespan and reproduction in Drosophila: New insights from nutritional geometry.
PNAS.
2008;
105:
2498
-2503.
[PubMed]
.
-
4.
Fanson
BG
, Weldon
CW
, Pérez-Staples
D
, Simpson
SJ
and Taylor
PW.
Nutrients, not caloric restriction, extend lifespan in Queensland fruit flies (Bactrocera tryoni).
Aging Cell.
2009;
8:
514
-523.
[PubMed]
.
-
5.
Carey
JR
, Harshman
LG
, Liedo
P
, Müller
H-G
, Wang
J-L
and Zhang
Z.
Longevity-fertility trade-offs in the tephritid fruit fly, Anastrepha ludens, across dietary-restriction gradients.
Aging Cell.
2008;
7:
470
-477.
[PubMed]
.
-
6.
Ja
WW
, Carvalho
GB
, Zid
BM
, Mak
EM
, Brummel
T
and Benzer
S.
Dual modes of lifespan extension by dietary restriction in Drosophila.
PNAS.
2009;
In press
.
-
7.
Wong
R
, Piper
MDW
, Wertheim
B
and Partridge
L.
Quantification of food intake in Drosophila.
PLoS ONE.
2009;
4:
1
-10.
.
-
8.
Troen
AM
, French
EE
, Roberts
JF
, Selhub
J
, Ordovas
JM
, Parnell
LD
and Lai
CQ.
Lifespan modification by glucose and methionine in Drosophila melanogaster fed a chemically defined diet.
Age.
2007;
29:
29
-39.
[PubMed]
.
-
9.
Raubenheimer
D
and Simpson
SJ.
The geometry of compensatory feeding in the locust.
Anim Behav.
1993;
45:
953
-964.
.
-
10.
Lee
KP
, Behmer
ST
, Simpson
SJ
and Raubenheimer
D.
A geometric analysis of nutrient regulation in the generalist caterpillar Spodoptera littoralis (Boisduval).
J Insect Physiol.
2002;
48:
655
-665.
[PubMed]
.
-
11.
Maklakov
AA
, Simpson
SJ
, Zajitschek
F
, Hall
MD
, Dessmann
J
, Clissold
F
, Raubenheimer
D
, Bonduriansky
R
and Brooks
RC.
Sex-specific fitness effects of nutrient intake on reproduction and lifespan.
Curr Biol.
2008;
18:
1062
-1066.
[PubMed]
.
-
12.
Maklakov
AA
, Hall
MD
, Simpson
SJ
, Dessmann
J
, Clissold
F
, Zajitschek
F
, Lailvaux
SP
, Raubenheimer
D
, Bonduriansky
R
and Brooks
RC.
Sex differences in nutrient-dependent reproductive ageing.
Aging Cell.
2009;
8:
324
-330.
[PubMed]
.
-
13.
Weindruch
R
and Walford
RL.
Springfield, IL
Charles C Thomas
The retardation of aging and disease by dietary restriction.
1988;
.
-
14.
Masoro
EJ
Caloric restriction and aging: Controversial issues.
J Gerontol A Biol Sci Med Sci.
2006;
61:
14
-19.
[PubMed]
.
-
15.
Ross
MH
Length of life and nutrition in the rat.
J Nutr.
1961;
75:
197
-210.
[PubMed]
.
-
16.
Orentreich
N
, Matias
JR
, DeFelice
A
and Zimmerman
JA.
Low methionine ingestion by rats extends life span.
J Nutr.
1993;
123:
269
-274.
[PubMed]
.
-
17.
Miller
RA
, Buehner
G
, Chang
Y
, Harper
JM
, Sigler
R
and Smith-Wheelock
M.
Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance.
Aging Cell.
2005;
4:
119
-125.
[PubMed]
.
-
18.
Zimmerman
JA
, Malloy
V
, Krajcik
R
and Orentreich
N.
Nutritional control of aging.
Exp Gerontol.
2003;
38:
47
-52.
[PubMed]
.
-
19.
Ayala
V
, Naudí
A
, Sanz
A
, Caro
P
, Portero-Otin
M
, Barja
G
and Pamplona
R.
Dietary protein restriction decreases oxidative protein damage, Peroxidizability Index, and mitochondrial complex I content in rat liver.
J Gerontol A Biol Sci Med Sci.
2007;
62:
352
-360.
[PubMed]
.
-
20.
Simpson
SJ
and Raubenheimer
D.
Obesity: the protein leverage hypothesis.
Obesity Rev.
2005;
6:
133
-142.
.
-
21.
Simpson
SJ
, Sibly
RM
, Lee
KP
, Behmer
ST
and Raubenheimer
D.
Optimal foraging when regulating intake of multiple nutrients.
Anim Behav.
2004;
68:
1299
-1311.
.
-
22.
Ruohonen
K
, Simpson
SJ
and Raubenheimer
D.
A new approach to diet optimisation: A reanalysis using European whitefish (Coregonus lavaretus).
Aquaculture.
2007;
267:
147
-156.
.
-
23.
Raubenheimer
D
and Simpson
SJ.
Integrative models of nutrient balancing: Application to insects and vertebrates.
Nutr Res Rev.
1997;
10:
151
-179.
[PubMed]
.
-
24.
Simpson
SJ
and Raubenheimer
D.
The geometric analysis of feeding and nutrition in the rat.
Appetite.
1997;
28:
201
-213.
[PubMed]
.
-
25.
Sørensen
A
, Mayntz
D
, Raubenheimer
D
and Simpson
SJ.
Protein-leverage in mice: The geometry of macronutrient balancing and consequences for fat deposition.
Obesity.
2008;
16:
566
-571.
[PubMed]
.
-
26.
Felton
AM
, Felton
A
, Raubenheimer
D
, Simpson
SJ
, Foley
WJ
, Wood
JT
, Wallis
IR
and Lindenmayer
DB.
Protein content of diets dictates the daily energy intake of a free-ranging primate.
Behav Ecol.
2009;
20:
685
-690.
.
-
27.
Simpson
SJ
, Batley
R
and Raubenheimer
D.
Geometric analysis of macronutrient intake in humans: the power of protein.
Appetite.
2003;
41:
123
-140.
[PubMed]
.
-
28.
Warbrick-Smith
J
, Behmer
ST
, Lee
KP
, Raubenheimer
D
and Simpson
SJ.
Evolving resistance to obesity in an insect.
PNAS.
2006;
103:
14045
-14049.
[PubMed]
.
-
29.
Stock
MJ
Gluttony and thermogenesis revisited.
Int J Obesity.
1999;
23:
1105
-1117.
.
-
30.
Colman
RJ
, Anderson
RM
, Johnson
SC
, Kastman
EK
, Kosmatka
KJ
, Beasley
TM
, Allison
DB
, Cruzen
C
, Simmons
HA
, Kemnitz
JW
and Weindruch
R.
Caloric restriction delays disease onset and mortality in rhesus monkeys.
Science.
2009;
325:
201
-204.
[PubMed]
.
-
31.
Sanz
A
, Caro
P
and Barja
G.
Protein restriction without strong caloric restriction decreases mitochondrial oxygen radical production and oxidative DNA damage in rat liver.
J Bioenerg Biomembr.
2004;
36:
545
-552.
[PubMed]
.
-
32.
Toden
S
, Bird
AR
, Topping
DL
and Conlon
MA.
High red meat diets induce greater numbers of colonic DNA double-strand breaks than white meat in rats: attenuation by high-amylose maize starch.
Carcinogenesis.
2007;
28:
2355
-2362.
[PubMed]
.
-
33.
Kapahi
P
and Zid
BM.
TOR pathway: linking nutrient sensing to life span.
SAGE KE.
2004;
36:
pe34
[PubMed]
.
-
34.
Kapahi
P
, Zid
BM
, Harper
T
, Koslover
D
, Sapin
V
and Benzer
S.
Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol 2004; 14:885-890.
[See also Curr Biol.
2004;
14:
1789]
.
-
35.
Harrison
DE
, Strong
R
, Sharp
ZD
, Nelson
JF
, Astle
CM
, Flurkey
K
, Nadon
NL
, Wilkinson
JE
, Frenkel
K
, Carter
CS
, Pahor
M
, Javors
MA
, Fernandez
E
and Miller
RA.
Rapamycin fed late in life extends lifespan in genetically heterogeneous mice.
Nature.
2009;
460:
392
-395.
[PubMed]
.
-
36.
Powers
III RW
, Kaeberlein
M
, Caldwell
SD
, Kennedy
BK
and Fields
S.
Extension of chronological life span in yeast by decreased TOR pathway signaling.
Gene Dev.
2009;
20:
174
-184.
[PubMed]
.
-
37.
Blagosklonny
MV
Aging: ROS or TOR.
Cell Cycle.
2008;
7:
3344
-3354.
[PubMed]
.
-
38.
Blagosklonny
MV
and Hall
MN.
Growth and aging: a common molecular mechanism.
Aging.
2009;
1:
357
-362.
.
-
39.
Simpson
SJ
and Raubenheimer
D.
The geometric analysis of nutrient-allelochemical interactions: A case study using locusts.
Ecology.
2001;
82:
422
-439.
.
-
40.
Raubenheimer
D
and Simpson
SJ.
Nutritional PharmEcology: Doses, nutrients, toxins, and medicines.
Integr Comp Biol.
2009;
49:
329
-337.
.
-
41.
Lee
KP
, Cory
JS
, Wilson
K
, Raubenheimer
D
and Simpson
SJ.
Flexible diet choice offsets protein costs of pathogen resistance in a caterpillar.
Proc R Soc B Biol Sci.
2006;
273:
823
-829.
.
-
42.
Povey
S
, Cotter
SC
, Simpson
SJ
, Lee
K-P
and Wilson
K.
Can the protein costs of bacterial resistance be offset by altered feeding behaviour.
J Anim Ecol.
2009;
78:
437
-446.
[PubMed]
.
-
43.
Hirao
A
, Tahara
Y
, Kimura
I
and Shibata
S.
A balanced diet is necessary for proper entrainment signals of the mouse liver clock.
PLoS ONE.
2009;
4:
e6909
[PubMed]
.
-
44.
Cota
D
, Proulx
K
and Seeley
RJ.
The role of CNS fuel sensing in energy and glucose regulation.
Gastroenterol.
2007;
132:
2158
-2168.
.
-
45.
Meijer
AJ
and Codogno
P.
Nutrient sensing: TOR's ragtime.
Nature Cell Biol.
2008;
10:
881
-883.
[PubMed]
.
-
46.
Minokoshi
Y
, Shiuchi
T
, Lee
S
, Suzuki
A
and Okamoto
S.
Role of hypothalamic AMP-kinase in food intake regulation.
Nutrition.
2008;
24:
786
-790.
[PubMed]
.
-
47.
Levine
AJ
, Feng
Z
, Mak
TW
, You
H
and Jin
S.
Coordination and communication between the p53 and IGF-1-AKT-TOR signal transduction pathways.
Gen Dev.
2009;
20:
267
-275.
.
-
48.
Steinberg
GR
and Kemp
BE.
AMPK in health and disease.
Physiol Rev.
2009;
89:
1025
-1078.
[PubMed]
.
-
49.
Kwon
G
, Marshall
CA
, Pappan
KL
, Remedi
MS
and McDaniel
ML.
Signaling elements involved in the metabolic regulation of mTOR by nutrients, incretins, and growth factors in islets.
Diabetes.
2004;
53:
S225
-S232.
[PubMed]
.
-
50.
Ropelle
ER
, Pauli
JR
, Fernandes
MFA
, Rocco
SA
, Marin
RM
, Morari
J
, Souza
KK
, Dias
MM
, Gomes-Marcondes
MC
, Gontijo
JAR
, Franchini
KG
, Velloso
LA
, Saad
MJA
and Carvalheira
JBC.
A central role for neuronal AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) in high-protein diet-induced weight loss.
Diabetes.
2008;
57:
594
-605.
[PubMed]
.
-
51.
Newgard
CB
, An
J
, Bain
JR
, Muehlbauer
MJ
, Stevens
RD
, Lien
LF
, Haqq
AM
, Shah
SH
, Arlotto
M
, Slentz
CA
, Rochon
J
, Gallup
D
, Ilkayeva
O
, Wenner
BR
, Yancy
WS
, Eisenson
H
, Musante
G
, Surwit
RS
, Millington
DS
, Butler
MD
and Svetkey
LP.
A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance.
Cell Metab.
2009;
9:
311
-326.
[PubMed]
.
-
52.
Zhou
Q
, Du
J
, Hu
Z
, Walsh
K
and Wang
XH.
Evidence for adipose-muscle cross talk: Opposing regulation of muscle proteolysis by adiponectin and fatty acids.
Endocrinol.
2007;
148:
5696
-5705.
.
-
53.
Quinn
LS
Interleukin-15: A muscle-derived cytokine regulating fat-to-lean body composition.
J Anim Sci.
2008;
86:
E75
-E83.
[PubMed]
.