Leptin-dependent co-regulation of bone and energy metabolism
Abstract
The adipocyte-derived hormone leptin inhibits appetite and bone mass accrual. To fulfill these two functions leptin requires the integrity of hypothalamic neurons but not the expression of its receptor, ObRb on these neurons. These results suggested that leptin acts first elsewhere in the brain to mediate these functions. However, this neuroanatomical site of leptin action in the brain remained elusive. Recent mouse genetic, electrophysiological and neuroanatomical studies provide evidence that leptin inhibits appetite and bone mass accrual through a two-step pathway: it decreases synthesis and the release by brainstem neurons of serotonin that in turn targets hypothalamic neurons to regulate appetite and bone mass accrual.
Skeleton in vertebrates serves multiple mechanical,
hematopoietic and endocrine functions [1,2]. In order
to perform its functions properly, the skeleton continuously renews itself
through a homeostatic process known as bone remodeling [1,3]. Bone
remodeling occurs constantly and simultaneously in numerous parts of skeleton
spread throughout the body and requires considerable inflow and utilization of
energy [4]. Any perturbance
in energy homeostasis of the body can therefore result in dramatic changes in
skeletal metabolism [5,6]. For
example, obesity or high body mass index often reduces fracture risk, whereas
on the other hand anorexia enhances it [5,6]. These
observations provide clinical evidence that bone and energy metabolism are
balanced with each other and are likely co-regulated.
Serotonin (5-hydroxytryptamine) is a
biogenic amine that functions both as a neurotransmitter in central nervous
system and as a hormone in the periphery where most of it (95%) is produced [7,8]. Serotonin
is generated through an enzymatic pathway in which L-tryptophan is converted
into L-5OH-tryptophan by an enzyme called tryptophan hydroxylase (Tph); this inter
mediate product is then converted to serotonin by an aromatic L-aminoacid decarboxylase [7,8]. There
are two Tph genes: Tph1 and Tph2. Tph1 is expressed
mostly in cells of the gut and is responsible for the production of peripheral
serotonin [9]. Tph2
is expressed exclusively in neurons of the brainstem and is responsible for the
production of serotonin in the brain [8]. Moreover,
serotonin does not cross the blood brain barrier; therefore it should be viewed
from a functional point of view as two distinct molecules [7].
Brain-derived serotonin (BDS) acts as a neurotransmitter, while gut-derived
serotonin (GDS) acts as a hormone and regulates a wide variety of processes [10]. The
importance of serotonin in the regulation of bone mass is underscored by two
clinical observations. First, depressed patients, that allegedly have low
serotonergic tone, also have low bone mass [11]; and
second, serotonin reuptake inhibitors (SSRI's) when taken chronically can
either increase or more often decrease bone mass [12].
Our studies with loss and gain of function mutations
of low-density lipoprotein receptor-related protein 5 demonstrated that GDS is
a powerful inhibitor of osteoblast proliferation and bone formation that does
not affect bone resorption [13]. Although
correlative in nature, these studies showed that an increase in extracellular
concentration of blood serotonin in patients on SSRI's may explain their often
observed low bone mass phenotype [12]. However,
the influence of this gut-bone axis on bone mass could not explain the increase
in bone mass observed in another study with SSRI's [14]. In our
quest to understand the serotonin regulation of bone mass in vertebrates we inactivatedTph2, the gene that catalyzes the rate-limiting step in the biosynthesis
of BDS. The absence of serotonin in the brain resulted in a severe low bone
mass phenotype affecting the axial (vertebrae) and appendicular (long bones)
skeleton [15]. This
phenotype was secondary to a decrease in bone formation parameters (osteoblast
numbers and bone formation rate) and to an increase in bone resorption
parameters (osteoclast surface and circulating Dpd levels) [15]. Hence, BDS
is a positive and powerful regulator of bone mass accrual acting on both arms
of bone remodeling despite accounting for >5% of total serotonin pool in the
body it overrides the GDS regulation of bone mass [15].
While we were doing these studies we noticed, upon
opening the abdominal cavities, that Tph2-deficient animals had a
dramatic decrease in their adipose mass [15]. This
prompted us to analyze in great detail their energy metabolism phenotype. The
decrease in their fat mass was due, in part, to the fact that these mice ate
less and spent much more energy compared to their wild type littermates [15]. This
observation was not entirely surprising since serotonin is known to play
important roles in many other physiological processes. However what caught our attention was the fact that the
three most notable phenotypes of adult Tph2-deficient
animals i.e., decrease in bone mass and appetite, and an increase in the energy
expenditure are a mirror image of what is observed in mice that lack leptin [16,17].
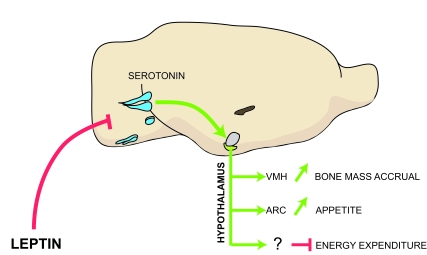
Figure 1. Model of the leptin-dependent central control of bone mass, appetite and energy expenditure.
Leptin inhibits release of brainstem-derived serotonin, which favors bone
mass accrual and appetite through its action on hypothalamic neurons.
Serotonergic neurons are in blue; VMH, ventromedial hypothalamus; ARC,
arcuate; VMH is in purple and arcuate is in green.
Three correlative experiments suggested that leptin
might signal in the serotonin neurons to regulate some of its downstream
functions. First, leptin receptor is expressed on serotonin neurons located in
the raphe nuclei of brainstem and is functional [15]. Second,
serotonin neurons project to the key hypothalamic nuclei responsible for the
regulation of appetite, energy expenditure and bone mass [15]. Third,
patients on SSRI's have been reported to have changes in their appetite and
bone mass [12,18]. To
explore that leptin may signal through the serotonin neurons to achieve these
three functions, we inactivated leptin receptors in different nuclei of the
hypothalamus or the serotonergic neurons of the brainstem [15]. Mice
lacking ObRb either in Sf1-expressing neurons of the ventromedial
hypothalamus (VMH) nuclei or in Pomc-expressing neurons of the arcuate
(ARC) nuclei had normal sympathetic activity, bone remodeling parameters and
bone mass; they also had normal appetite and energy expenditure, and when fed a
normal diet, did not develop an obesity phenotype [19,20]. In
contrast, mice that lack ObRb in serotonin neurons (ObRbSERT-/-)
developed a high bone mass phenotype; they had a similar increase in appetite
as ob/ob mice and had low energy expenditure. As a result, ObRbSERT-/-
mice, when fed a normal diet, developed an obesity phenotype. These genetic
studies demonstrated that leptin signals in the serotonin neurons of the
brainstem to regulate, to the most part, bone mass, appetite and energy
expenditure (Figure 1).
The demonstration that leptin-dependent central
control of bone mass, appetite and energy expenditure occurs through its
ability to inhibit serotonin production raised questions about the location and
identity of serotonin receptors on hypothalamic neurons mediating these
function. Double fluorescence in situ hybridization and nuclei-specific gene
inactivation experiments revealed that serotonin promotes bone mass accrual
through Htr2c receptors expressed on the VMH nuclei, while appetite through
Htr2b and Htr1a receptors expressed on ARC nuclei of the hypothalamus. Further
analysis revealed that Htr2c is upstream of the sympathetic center of the brain
while Htr1a and Htr2b achieve their functions on appetite through modulation of
melanocortin signaling (Figure 1).
In summary, these studies provide new insights into
the central control of appetite and bone mass accrual and they identify
serotonin as a focal point in the leptin-dependent common central control of
bone and energy metabolisms.
Acknowledgement
This work was supported by NIH grants
(VKY, GK) and a Rodan fellowship from IBMS (VKY).
Conflicts of Interest
The authors of this
manuscript have no conflict of interest to declare.
References
-
1.
Rodan
GA
and Martin
TJ.
Therapeutic approaches to bone diseases.
Science.
2000;
289:
1508
-1514.
[PubMed]
.
-
2.
Lee
NK
, Sowa
H
, Hinoi
E
, Ferron
M
, Ahn
JD
, Confavreux
C
, Dacquin
R
, Mee
PJ
, McKee
MD
, Jung
DY
, Zhang
Z
, Kim
JK
, Mauvais-Jarvis
F
, Ducy
P
and Karsenty
G.
Endocrine regulation of energy metabolism by the skeleton.
Cell.
2007;
130:
456
-469.
[PubMed]
.
-
3.
Zaidi
M
Skeletal remodeling in health and disease.
Nat Med.
2007;
13:
791
-801.
[PubMed]
.
-
4.
Karsenty
G
Convergence between bone and energy homeostases: Leptin regulation of bone mass.
Cell Metab.
2006;
4:
341
-348.
[PubMed]
.
-
5.
Felson
DT
, Zhang
Y
, Hannan
MT
and Anderson
JJ.
Effects of weight and body mass index on bone mineral density in men and women: the Framingham study.
J Bone Miner Res.
1993;
8:
567
-573.
[PubMed]
.
-
6.
Tremollieres
FA
, Pouilles
JM
and Ribot
C.
Vertebral postmenopausal bone loss is reduced in overweight women: a longitudinal study in 155 earlypostmenopausal women.
J Clin Endocrinol Metab.
1993;
77:
683
-686.
[PubMed]
.
-
7.
Mann
JJ
, McBride
PA
, Brown
RP
, Linnoila
M
, Leon
AC
, DeMeo
M
, Mieczkowski
T
, Myers
JE
and Stanley
M.
Relationship between central and peripheral serotonin indexes in depressed and suicidal psychiatric inpatients.
Arch Gen Psychiatry.
1992;
49:
442
-446.
[PubMed]
.
-
8.
Walther
DJ
, Peter
JU
, Bashammakh
S
, Hortnagl
H
, Voits
M
, Fink
H
and Bader
M.
Synthesis of serotonin by a second tryptophan hydroxylase isoform.
Science.
2003;
299:
76
[PubMed]
.
-
9.
Gershon
MD
and Tack
J.
The serotonin signaling system: from basic understanding to drug development for functional GI disorders.
Gastroenterology.
2007;
132:
397
-414.
[PubMed]
.
-
10.
Heath
MJ
and Hen
R.
Serotonin receptors: Genetic insights into serotonin function.
Curr Biol.
1995;
5:
997
-999.
[PubMed]
.
-
11.
Eskandari
F
, Martinez
PE
, Torvik
S
, Phillips
TM
, Sternberg
EM
, Mistry
S
, Ronsaville
D
, Wesley
R
, Toomey
C
, Sebring
NG
, Reynolds
JC
, Blackman
MR
, Calis
KA
, Gold
PW
and Cizza
G.
Low bone mass in premenopausal women with depression.
Arch Intern Med.
2007;
167:
2329
-2336.
[PubMed]
.
-
12.
Richards
JB
, Papaioannou
A
, Adachi
JD
, Joseph
L
, Whitson
HE
, Prior
JC
and Goltzman
D.
Effect of selective serotonin reuptake inhibitors on the risk of fracture.
Arch Intern Med.
2007;
167:
188
-194.
[PubMed]
.
-
13.
Yadav
VK
, Ryu
JH
, Suda
N
, Tanaka
KF
, Gingrich
JA
, Schutz
G
, Glorieux
FH
, Chiang
CY
, Zajac
JD
, Insogna
KL
, Mann
JJ
, Hen
R
, Ducy
P
and Karsenty
G.
Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum.
Cell.
2008;
135:
825
-837.
[PubMed]
.
-
14.
Battaglino
R
, Vokes
M
, Schulze-Spate
U
, Sharma
A
, Graves
D
, Kohler
T
, Muller
R
, Yoganathan
S
and Stashenko
P.
Fluoxetine treatment increases trabecular bone formation in mice.
J Cell Biochem.
2007;
100:
1387
-1394.
[PubMed]
.
-
15.
Yadav
VK
, Oury
F
, Suda
N
, Liu
ZW
, Gao
XB
, Confavreux
C
, Klemenhagen
KC
, Tanaka
KF
, Gingrich
JA
, Guo
XE
, Tecott
LH
, Mann
JJ
, Hen
R
, Horvath
TL
and Karsenty
G.
A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure.
Cell.
2009;
138:
976
-989.
[PubMed]
.
-
16.
Friedman
JM
and Halaas
JL.
Leptin and the regulation of body weight in mammals.
Nature.
1998;
395:
763
-770.
[PubMed]
.
-
17.
Spiegelman
BM
and Flier
JS.
Obesity and the regulation of energy balance.
Cell.
2001;
104:
531
-543.
[PubMed]
.
-
18.
Michelson
D
, Amsterdam
JD
, Quitkin
FM
, Reimherr
FW
, Rosenbaum
JF
, Zajecka
J
, Sundell
KL
, Kim
Y
and Beasley
CM Jr.
Changes in weight during a 1-year trial of fluoxetine.
Am J Psychiatry.
1999;
156:
1170
-1176.
[PubMed]
.
-
19.
Dhillon
H
, Zigman
JM
, Ye
C
, Lee
CE
, McGovern
RA
, Tang
V
, Kenny
CD
, Christiansen
LM
, White
RD
, Edelstein
EA
, Coppari
R
, Balthasar
N
, Cowley
MA
, Chua
S Jr
, Elmquist
JK
and Lowell
BB.
Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis.
Neuron.
2006;
49:
191
-203.
[PubMed]
.
-
20.
Balthasar
N
, Coppari
R
, McMinn
J
, Liu
SM
, Lee
CE
, Tang
V
, Kenny
CD
, McGovern
RA
, Chua
SC Jr
, Elmquist
JK
and Lowell
BB.
Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis.
Neuron.
2004;
42:
983
-991.
[PubMed]
.