How to track cellular aging of mesenchymal stromal cells?
Abstract
Mesenchymal stromal cells (MSC) are currently tested in a large number of clinical trials and raise high hope in regenerative medicine. These cells have to be expanded in vitro before transplantation and several studies demonstrated that long-term culture evokes continuous changes in MSC: proliferation rate decays, the cell size increases, differentiation potential is affected, chromosomal instabilities may arise and molecular changes are acquired. Long-term culture of cell preparations might also have therapeutic consequences, although this has hardly been addressed in ongoing trials so far. Reliable therapeutic regimens necessitate quality control of cellular products. This research perspective summarizes available methods to track cellular aging of MSC. We have demonstrated that gene expression changes and epigenetic modifications are continuously acquired during replicative senescence. Molecular analysis of a suitable panel of genes might provide a robust tool to assess efficiency and safety of long-term expansion.
Quality
control for cellular therapeutics
There is growing interest in
transplantation of ex vivo amplified cell preparations for various
therapeutic applications. This has been fueled by novel insights from stem cell
biology, new molecular tools and promising preclinical model systems.
Mesenchymal stromal cells (MSC) can be isolated from various tissues including
bone marrow and adipose tissue, which contain a rare population of adult stem
cells (mesenchymal stem cells) with multilineage differentiation potential
towards at least adipogenic, osteogenic and chondrogenic lineage [3]. To date,
MSC are tested for a wide spectrum of diseases taking into
account their paracrine effect, immunomodulatory activity and differentiation
potential [4]. Hence, the use of MSC as cellular therapeutics necessitates stan-dardized
isolation and reliable quality control of cell preparations. This, however, is
greatly hampered by the multitude of different methods to prepare MSC [5].
Furthermore, there is a growing perception that even under highly standardized
culture conditions, continuous effects during long-term culture and eventually
replicative senescence need to be taken into account [2, 7].
MSC
can only be culture expanded for a limited time before they reach a senescent
state. This so called "Hayflick limit" is commonly observed in all primary cell
isolates [8]. Senescent cells are mitotically arrested, thus are not dead, and
remain metabolically active. However due to acquired functional and molecular changes,
MSC increase in size, they adopt "fried egg morphology", expression of specific
surface markers is attenuated [1] and adipogenic and osteogenic differentiation
potential is affected [9-13]. Recently, we have demonstrated, that long-term
culture is also associated with continuous changes in the global gene
expression profile [1] (Figure 1). Genes involved in cell cycle, DNA
replication, mitosis and DNA repair are significantly down-regulated in late
passages. This reproducible pattern of senescence associated gene expression
changes strengthens the hypothesis that cellular aging is driven by an
organized process rather than a random accumulation of cellular defects [14].
Interestingly, long-term culture associated gene expression changes were
related to age-associated changes in MSC from young versus elderly
donors [15]. This indicates that cellular aging might be related to aging of
the organism. The underlying molecular mechanisms of replicative senescence are
still unraveled but it evidently has consequences for cellular therapy [2, 16].
However, it is not a trivial question how to track cellular aging of MSC.
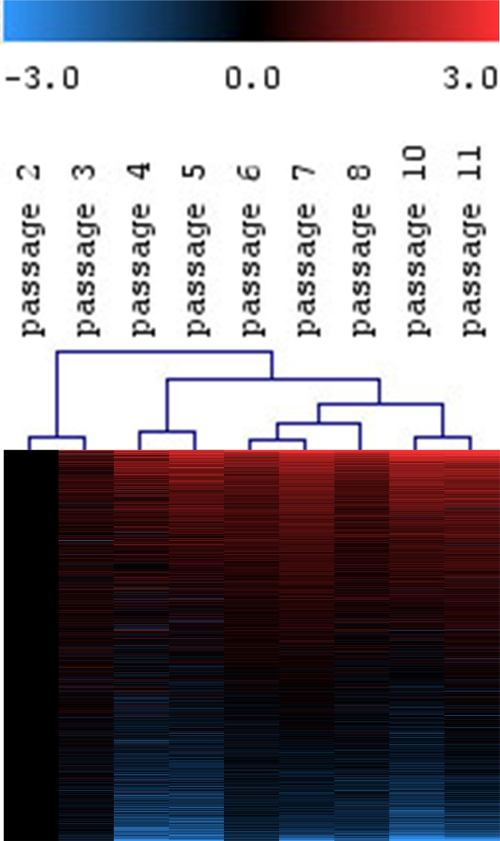
Figure 1. Continuous gene-expression changes in MSC upon long-term culture.
MSC from human bone marrow were expanded for 11 passages and analyzed by Affymetrix GeneChip technology.
Differential gene expression was always determined versus P2.
Hierarchical cluster analysis of all expressed genes (19,448 ESTs) revealed continuous changes with higher passages.
Hence, molecular changes in replicative senescence do not suddenly occur in late passages,
but are acquired in the course of long?term culture.
Restrictions of passage numbers and population doublings
Cells in culture can be continuously
observed and hence, it appears straightforward to determine proliferation and
the number of cell divisions. Obviously, the most convenient parameter for
documentation of long-term culture is simply counting the number of cell
passages. Under standardized culture conditions this procedure provides a
predictive indicator for replicative senescence. However, as seeding densities
often greatly vary between different laboratories (10 to 104
cells/cm2) and also confluence at the time of harvesting
[5, 2, 17, 18],
the sole recording of passage numbers may lead to deceptive
results in order to compare the state of senescence under non-standardized
conditions. In this respect, calculation of the number of cumulative population
doublings (PD) is more accurate [19]. MSC cultures are usually isolated by
plastic adherent growth and hence, the initial MSC number can only be estimated
by accounting fibroblastoid colony-forming unit (CFU-F) frequency based on the assumption that every colony
has been derived from a single clonogenic MSC. Thereafter, cell numbers have
to be exactly determined at all consecutive passages as any inaccuracy will be
carried over
to the next passage and falsify PD. Yet, analysis of PD excludes the likely
events of cells undergoing apoptosis, necrosis or loss during passaging. More
importantly, there are big variations between different donor samples. Taken
together, it is hard to predict at which passage or number of cell divisions
MSC are approaching either a replicative or stress-induced senescent state.
Surface
molecules and histochemical markers for senescence
To
date no specific molecular marker is available that prospectively reflects the
degree of cellular aging in MSC. For instance the leptin receptor (CD295)
increases with higher passages under hyperoxic culture conditions in MSC of
elderly donors [20]. Flow cytometric analysis of this surface marker
discriminates a CD295-positive subpopulation, but these cells also stained
positive for annexin V. CD295 therefore stains apoptotic cells that accumulate
at higher passages rather than senescent cells [20]. It is also possible to
stain the enlarged senescent cells based on the accumulation of
senescence-associated beta galactosidase (SA-β-gal). This lysosomal
protein is predominantly active in senescent fibroblasts and also, albeit to a
lower extent, in MSC [21]. The staining procedure is easy and reliable but the result can hardly be quantified
and almost exclusively
the very large senescent cells exhibiting a "fried egg morphology" stain
positive for SA-β-gal [[1, 22]. It should be mentioned, that SA-β-gal
itself is neither required nor causative for manifestation of senescence [23].
Despite limitations in quantification and prospective analysis of MSC, SA-β-gal
is the most widely used biomarker for senescent and aging cells.
Table 1. Methods to track changes upon long-term culture.
| Method | Advantage | Disadvantage |
| Number of passages |
Counting of passages can be easily documented.
|
Seeding density and expansion techniques vary
between different laboratories.
|
|
Under standardized culture conditions it provides an
indicator for long-term culture.
|
Even under standardized conditions there is
variation between different probes.
|
| Cumulative population doublings |
PD can be calculated based on precise cell numbers
at every passage and exact seeding densities.
|
The initial CFU-F frequency is required to estimate
initial PD.
|
|
This parameter is more robust for comparison between
different laboratories.
|
MSC are heterogeneous and the number of PD does not
correspond to the number of cell divisions in individual cells.
|
|
Prospective information on the senescent state is
hampered by large variation between different samples.
|
| SA-β-galactosidase |
Fast and easy method to stain activity of lysosomal,
senescence associated beta-galactosidase.
|
SA-β-gal is not required for senescence.
|
|
SA-β gal is
over-expressed and accumulates specifically in senescent cells.
|
Especially the large cells become beta-gal positive.
|
|
Quantitative analysis for quality control is
difficult.
|
| Karyotype / array-CGH |
May detect mutations and potentially immortalized
cell clones.
|
Human MSC appear to be relatively stable for
karyotypic aberrations.
|
|
Might prevent transplant-associated tumor formation.
|
No marker for normal cellular aging.
|
| Telomere length |
Might provide a direct measure for prospective
analysis of potential cell divisions.
|
Stress induced senescence might be independent of
cell cycle and telomere shortening.
|
|
Several techniques are available to quantify
telomere length.
|
It is yet unclear if analysis of telomere length
facilitates reliable quality control in different MSC preparations.
|
| Gene expression markers |
RT-PCR and microarray techniques facilitate fast and
reliable quantification.
|
Differential gene expression needs to be normalized
to "house keeping genes".
|
|
A panel of up-regulated and down-regulated genes may
be more robust than individual markers.
|
Suitable gene-sets need to be established and
cross-validated in different MSC preparations.
|
Genomic aberrations
Clinical
trials with MSC usually employ 1-2 x 106 MSC per kg bodyweight for
transplantation and therefore large-scale expansion is an indispensable
prerequisite. Proliferation under non-physiologic in vitro culture conditions
can result in mutations and chromosomal aberrations and eventually leads to malignant
transformations. Karyotypic aberrations are commonly observed in MSC from mice
and rats [24-26] whereas they have only been examined in few studies with human
MSC [27-29]. So far tumor formation has not been described in clinical trials
with MSC. Malignant transformation is obviously the "sword of Damocles"
hovering above therapeutic cell products. The risk can be reduced by
conventional karyotyping of MSC, however, minor genomic gains or losses may not
be detected. Array complete
genomic hybridization (CGH) analysisis more sensitive but this technique is incapable of
revealing balanced translocations or very small mutations. Furthermore,
malignant transformation may involve over-expression of c-myc, activation of
cyclin dependent kinases, deletion of tumor suppressor genes such as p16ink4a,
RB or p53 and re-expression of telomerase [30]. Initially, these changes may
only occur in a small subset of cell preparations. At that point, it remains to
be demonstrated whether routine karyotype analysis does actually reduce the
risk of transplant-related tumor formation. It is however generally accepted
that the stochastic effects of malignant transformation are no suitable markers
in order to determine normal senescence-associated changes in MSC.
Telomere
length
MSC do not express telomerase and
therefore telomere length decreases approximately 50-200 nucleotides per cell
cycle [31] and there is evidence, that telomere shortening occurs also upon
aging in vivo [33]. Ectopic expression of telomerase can immortalize MSC
while their differentiation potential is maintained [32]. It is still under
debate, if telomere loss really plays a causal role for replicative senescence
or aging. Either way, loss of telomere length might facilitate some kind of
internal clock to assess the state of cellular aging. Various methods are
available to determine telomere length including Southern hybridization, flow
cytometry based methods or quantitative PCR [34]. Therefore, telomere length
may serve as another good indicator for mitotic history and the prospective
additional life span. However, stress induced senescence may occur independent
of cell division and it needs to be demonstrated if quantitative analysis of
telomere length facilitates reliable and prospective quality control with
regard to cellular aging.
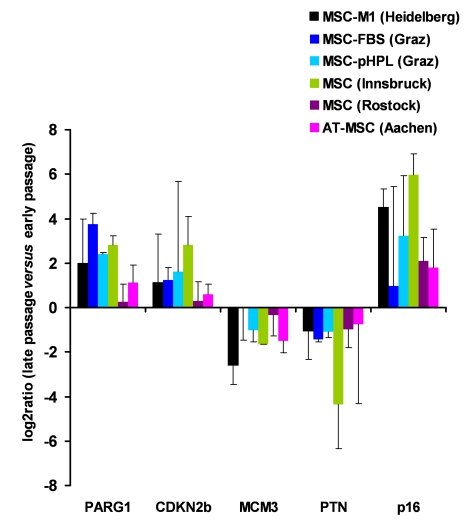
Figure 2. Gene expression markers for replicative senescence. MSC from human
bone marrow were either culture expanded as described before in medium-M1
with 2% fetal calf serum (M1, in Heidelberg, Germany [1]; n=3), in
culture medium with 10% fetal calf serum (FCS, n=2) or 10% pooled human
platelet lysate (pHPL, n=2; both in Graz, Austria
[38]),
in MEM supplemented with 20% FCS (Innsbruck, Austria [40];
n=2), and in MSCGM (Lonza) culture medium (Rostock, Germany; n=4).
Furthermore, MSC from adipose tissue were expanded with 10% pHPL (Aachen,
Germany,
n=4). RNA was isolated from corresponding early and late passages and
analyzed for differential gene expression in PARG1, CDKN2B, MCM3, PTN and p16ink4a. Primers and methods have
been described before [38]. These genes
did not facilitate reliable discrimination of senescent cells in all
samples but the tendency was consistent in all different MSC preparations.
Senescence
markers on gene expression level
Long-term
culture induces continuous changes in gene expression [1]. A clear-cut
characterization of distinct aberrations might facilitate determination when
cells are shifting into the final state of senescence. A prerequisite is the
reproducibility of senescence-associated gene expression changes in different
MSC preparations, whereas techniques for cell isolation, culture media and cell
culture methods have major impact on the composition of MSC and their gene
expression profiles [35-37]. Recently, we have compared gene expression changes
in MSC from human bone marrow, which had been isolated in two different
laboratories, grown in long-term culture with different culture media and
subsequently also analyzed with different microarray platforms [38]. Despite
these differences there was a high resemblance in senescence-associated gene
expression signatures. This led us to conclude, that these specific changes may
be suitable for analysis of cellular aging. A matrix of distinctly up- and
down-regulated genes thus provides a robust method for quality control. Taken
together, we found senescence-associated up-regulation of the
phosphate-associated RhoGAP protein-tyrosine (PARG1; alternatively termed
ARHGAP29) and of the cyclin-dependent kinase-inhibotor 2B (CDKN2B). Genes that
were down-regulated included pleiotrophin (PTN) and mini-chromosome maintenance
complex component 3 (MCM3) (patent pending) [38]. Furthermore, work from other
laboratories demonstrated that p16ink4a is up-regulated at higher
passages. We now performed quantitative RT-PCR analysis of these five genes in
five different types of MSC preparations and long-term cultures from different
laboratories (Figure 2). Overall, there were related changes when comparing early
and senescent passage. However, standard deviations were rather high in these
analyses and it was not always possible to discern MSC in late passage. As
microarray technology facilitates simultaneous analysis of thousands of genes,
a larger panel of genes most likely yields a more robust predictor for quality
control purposes. Further specification of senescence-associated markers and
cross-validation in different MSC preparations may pave the way for a reliable
quality control of cell preparations on gene expression level.
Besides
whole genome expression profiling, we could also demonstrate that DNA
methylation profiles are clearly affected by long-term culture [2]. Using
HumanMethylation27 BeadChip that represents 27,578 CpG sites in more than
13,500 annotated genes, it was shown that specific promoter regions become
either hyper- or hypo-methylated upon expansion of MSC. Some of these
deviations were also differentially methylated in fibroblasts (unpublished
data). Diploid cells have only two copies of DNA, whereas gene expression is
based on multiple copies of mRNA. Therefore, distinction of methylation changes
is a potent way to monitor cellular aging and this type of epigenetic analysis
could be more suitable for accounting the heterogeneity within primary MSC
preparations and also with regard to cellular aging.
Outlook
Cellular therapies are currently tested
for various novel therapeutic applications. At the same time requirements for
quality control of cell products have to be specified and standardized.
Establishing efficient quality control is challenging as it bases on trial and
error to accumulate knowledge on optimal culture conditions for therapeutic
applications. So far there are only limited numbers of reports available
tracking side effects of clinical application of MSC. Notably, some of the
preliminary observations are very promising [4, 39]. It is yet unclear how many
passages, population doublings or senescence-associated molecular changes are
acceptable to grant optimal therapeutic effect for the different applications.
Clearly, we need to establish a reliable method to track cellular aging of MSC.
Molecular changes either on gene expression or DNA methylation levels provide
powerful perspectives. Further bioinformatic analyses of datasets and
validation enrolling different MSC preparations will pave the way for a
reliable panel of distinct aging and senescence markers.
Acknowledgments
This work was supported by the German Ministry of
Education and Research (CB-HERMES), the Academy of Sciences and Humanities,
Heidelberg (WIN-Kolleg), by the FWF (SOP3 and N211-NAN), the Austrian Research Promotion Agency
(FFG: N200), by the Jubilee Fund of the
Austrian National Bank (P12518), by the excellence initiative of the German
federal and state governments (Pathfinder project - AGenFinder) and the Stem
Cell Network North Rhine Westphalia. The authors have no conflict of interest
to declare.
Conflicts of Interest
The authors of this manuscript have no
conflict of interest to declare.
References
-
1.
Wagner
W
, Horn
P
, Castoldi
M
, Diehlmann
A
, Bork
S
, Saffrich
R
, Benes
V
, Blake
J
, Pfister
S
, Eckstein
V
and Ho
AD.
Replicative Senescence of Mesenchymal Stem Cells - a Continuous and Organized Process.
PLoS ONE.
2008;
5:
e2213
[PubMed]
.
-
2.
Bork
S
, Pfister
S
, Witt
H
, Horn
P
, Korn
B
, Ho
AD
and Wagner
W.
DNA Methylation Pattern Changes upon Long-Term Culture and Aging of Human Mesenchymal Stromal Cells.
Aging Cell.
2010;
9:
54
-63.
[PubMed]
.
-
3.
Dominici
M
, Le
Blanc K
, Mueller
I
, Slaper-Cortenbach
I
, Marini
F
, Krause
D
, Deans
R
, Keating
A
, Prockop
D
and Horwitz
E.
Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement.
Cytotherapy.
2006;
8:
315
-317.
[PubMed]
.
-
4.
Sensebe
L
, Krampera
M
, Schrezenmeier
H
, Bourin
P
and Giordano
R.
Mesenchymal stem cells for clinical application.
Vox Sang.
2010;
98:
93
-107.
[PubMed]
.
-
5.
Wagner
W
and Ho
AD.
Mesenchymal stem cell preparations-comparing apples and oranges.
Stem Cell Rev.
2007;
3:
239
-248.
[PubMed]
.
-
6.
Wagner
W
, Ho
AD
and Zenke
M.
Different Facets of Aging in Human Mesenchymal Stem Cells.
Tissue Eng Part B Rev.
2010;
Epub ahead of print
.
-
7.
Roobrouck
VD
, Ulloa-Montoya
F
and Verfaillie
CM.
Self-renewal and differentiation capacity of young and aged stem cells.
Exp Cell Res.
2008;
314:
1937
-1944.
[PubMed]
.
-
8.
Hayflick
L
The limited in vitro lifetime of human diploid cell strains.
Exp Cell Res.
1965;
37:
614
-636.
[PubMed]
.
-
9.
Bonab
MM
, Alimoghaddam
K
, Talebian
F
, Ghaffari
SH
, Ghavamzadeh
A
and Nikbin
B.
Aging of mesenchymal stem cell in vitro.
BMC Cell Biol.
2006;
7:
14
[PubMed]
.
-
10.
Noer
A
, Boquest
AC
and Collas
P.
Dynamics of adipogenic promoter DNA methylation during clonal culture of human adipose stem cells to senescence.
BMC Cell Biol.
2007;
8:
18
[PubMed]
.
-
11.
Baxter
MA
, Wynn
RF
, Jowitt
SN
, Wraith
JE
, Fairbairn
LJ
and Bellantuono
I.
Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion.
Stem Cells.
2004;
22:
675
-682.
[PubMed]
.
-
12.
Lepperdinger
G
, Brunauer
R
, Jamnig
A
, Laschober
G
and Kassem
M.
Controversial issue: is it safe to employ mesenchymal stem cells in cell-based therapies.
Exp Gerontol.
2008;
43:
1018
-1023.
[PubMed]
.
-
13.
Kim
J
, Kang
JW
, Park
JH
, Choi
Y
, Choi
KS
, Park
KD
, Baek
DH
, Seong
SK
, Min
HK
and Kim
HS.
Biological characterization of long-term cultured human mesenchymal stem cells.
Arch Pharm Res.
2009;
32:
117
-126.
[PubMed]
.
-
14.
Blagosklonny
MV
TOR-driven aging: speeding car without brakes.
Cell Cycle.
2009;
8:
4055
-4059.
[PubMed]
.
-
15.
Wagner
W
, Bork
S
, Horn
P
, Krunic
D
, Walenda
T
, Diehlmann
A
, Benes
V
, Blake
J
, Huber
FX
, Eckstein
V
, Boukamp
P
and Ho
AD.
Aging and replicative senescence have related effects on human stem and progenitor cells.
PLoS ONE.
2009;
4:
e5846
[PubMed]
.
-
16.
Fehrer
C
and Lepperdinger
G.
Mesenchymal stem cell aging.
Exp Gerontol.
2005;
40:
926
-930.
[PubMed]
.
-
17.
Larson
BL
, Ylostalo
J
and Prockop
DJ.
Human multipotent stromal cells undergo sharp transition from division to development in culture.
Stem Cells.
2008;
26:
193
-201.
[PubMed]
.
-
18.
Bartmann
C
, Rohde
E
, Schallmoser
K
, Purstner
P
, Lanzer
G
, Linkesch
W
and Strunk
D.
Two steps to functional mesenchymal stromal cells for clinical application.
Transfusion.
2007;
47:
1426
-1435.
[PubMed]
.
-
19.
Cristofalo
VJ
, Allen
RG
, Pignolo
RJ
, Martin
BG
and Beck
JC.
Relationship between donor age andthe replicative lifespan of human cells in culture: a reevaluation.
Proc Natl Acad Sci U S A.
1998;
95:
10614
-10619.
[PubMed]
.
-
20.
Laschober
GT
, Brunauer
R
, Jamnig
A
, Fehrer
C
, Greiderer
B
and Lepperdinger
G.
Leptin receptor/CD295 is upregulated on primary human mesenchymal stem cells of advancing biological age and distinctly marks the subpopulation of dying cells.
Exp Gerontol.
2009;
44:
57
-62.
[PubMed]
.
-
21.
Dimri
G P
, Lee
X
, Basile
G
, Acosta
M
, Scott
G
, Roskelley
C
, Medrano
EE
, Linskens
M
, Rubelj
I
and Pereira-Smith
O.
A biomarker that identifies senescent human cells in culture and in aging skin in vivo.
Proc Natl Acad Sci U S A.
1995;
92:
9363
-9367.
[PubMed]
.
-
22.
Zhou
S
, Greenberger
JS
, Epperly
MW
, Goff
JP
, Adler
C
, Leboff
MS
and Glowacki
J.
Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentia-tion to osteoblasts.
Aging Cell.
2008;
7:
335
-343.
[PubMed]
.
-
23.
Lee
BY
, Han
JA
, Im
JS
, Morrone
A
, Johung
K
, Goodwin
EC
, Kleijer
WJ
, DiMaio
D
and Hwang
ES.
Senescence-associated beta-galactosidase is lysosomal beta-galactosidase.
Aging Cell.
2006;
5:
187
-195.
[PubMed]
.
-
24.
Josse
C
, Schoemans
R
, Niessen
NA
, Delgaudine
M
, Hellin
AC
, Herens
C
, Delvenne
P
and Bours
V.
Systematic chromosomal aberrations found in murine bone marrow-derived mesenchy-mal stem cells.
Stem Cells Dev.
2010;
Epub ahead of print
.
-
25.
Foudah
D
, Redaelli
S
, Donzelli
E
, Bentivegna
A
, Miloso
M
, Dalpra
L
and Tredici
G.
Monitoring the genomic stability of in vitro cultured rat bone-marrow-derived mesenchymal stem cells.
Chromosome Res.
2009;
17:
1025
-1039.
[PubMed]
.
-
26.
Furlani
D
, Li
W
, Pittermann
E
, Klopsch
C
, Wang
L
, Knopp
A
, Jungebluth
P
, Thedinga
E
, Havenstein
C
, Westien
I
, Ugurlucan
M
and Li
R.
K, Ma N, et al. A transformed cell population derived from cultured mesenchymal stem cells has no functional effect after transplantation into the injured heart.
Cell Transplant.
2009;
18:
319
-331.
[PubMed]
.
-
27.
Rubio
D
, Garcia-Castro
J
, Martin
MC
, de la
FR
, Cigudosa
JC
, Lloyd
AC
and Bernad
A.
Spontaneous human adult stem cell transformation.
Cancer Res.
2005;
65:
3035
-3039.
[PubMed]
.
-
28.
Rosland
G V
, Svendsen
A
, Torsvik
A
, Sobala
E
, McCormack
E
, Immervoll
H
, Mysliwietz
J
, Tonn
JC
, Goldbrunner
R
, Lonning
PE
, Bjerkvig
R
and Schichor
C.
Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation.
Cancer Res.
2009;
69:
5331
-5339.
[PubMed]
.
-
29.
Meza-Zepeda
LA
, Noer
A
, Dahl
JA
, Micci
F
, Myklebost
O
and Collas
P.
High-resolution analysis of genetic stability of human adipose tissue stem cells cultured to senescence.
J Cell Mol Med.
2008;
12:
553
-563.
[PubMed]
.
-
30.
Rubio
D
, Garcia
S
, Paz
MF
, De
la CT
, Lopez-Fernandez
LA
and Lloyd
A.
C, Garcia-Castro J, Bernad A. Molecular characterization of spontaneous mesenchymal stem cell transformation.
PLoS ONE.
2008;
3:
e1398
[PubMed]
.
-
31.
Shay
JW
, Zou
Y
, Hiyama
E
and Wright
WE.
Telomerase and cancer.
Hum Mol Genet.
2001;
10:
677
-685.
[PubMed]
.
-
32.
Simonsen
JL
, Rosada
C
, Serakinci
N
, Justesen
J
, Stenderup
K
, Rattan
SI
, Jensen
TG
and Kassem
M.
Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells.
Nat Biotechnol.
2002;
20:
592
-596.
[PubMed]
.
-
33.
Harley
CB
, Futcher
AB
and Greider
CW.
Telomeres shorten during ageing of human fibroblasts.
Nature.
1990;
345:
458
-460.
[PubMed]
.
-
34.
Fehrer
C
, Voglauer
R
, Wieser
M
, Pfister
G
, Brunauer
R
, Cioca
D
, Grubeck-Loebenstein
B
and Lepperdinger
G.
Techniques in gerontology: cell lines as standards for telomere length and telomerase activity assessment.
Exp Gerontol.
2006;
41:
648
-651.
[PubMed]
.
-
35.
Wagner
W
, Feldmann
RE Jr
, Seckinger
A
, Maurer
MH
, Wein
F
, Blake
J
, Krause
U
, Kalenka
A
, Burgers
HF
, Saffrich
R
, Wuchter
P
, Kuschinsky
W
and Ho
AD.
The heterogeneity of human mesenchymal stem cell preparations-Evidence from simulta-neous analysis of proteomes and transcriptomes.
Exp Hematol.
2006;
34:
536
-548.
[PubMed]
.
-
36.
Wagner
W
, Wein
F
, Seckinger
A
, Frankhauser
M
, Wirkner
U
, Krause
U
, Blake
J
, Schwager
C
, Eckstein
V
, Ansorge
W
and Ho
AD.
Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood.
Exp Hematol.
2005;
33:
1402
-1416.
[PubMed]
.
-
37.
Tanabe
S
, Sato
Y
, Suzuki
T
, Suzuki
K
, Nagao
T
and Yamaguchi
T.
Gene expression profiling of human mesenchymal stem cells for identification of novel markers in early- and late-stage cell culture.
J Biochem.
2008;
144:
399
-408.
[PubMed]
.
-
38.
Schallmoser
K
, Bartmann
C
, Rohde
E
, Bork
S
, Guelly
C
and Obenauf
A.
C, Reinisch A, Horn P, Ho AD, Strunk D, Wagner W. Replicative senescence-associated gene expression changes in mesenchymal stromal cells are similar under different culture conditions.
Haematologica.
2010;
Epub ahead of print
.
-
39.
Le
Blanc K
, Frassoni
F
, Ball
L
, Locatelli
F
, Roelofs
H
, Lewis
I
, Lanino
E
, Sundberg
B
, Bernardo
ME
, Remberger
M
, Dini
G
, Egeler
RM
and Bacigalupo
A.
Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study.
Lancet.
2008;
371:
1579
-1586.
[PubMed]
.
-
40.
Fehrer
C
, Brunauer
R
, Laschober
G
, Unterluggauer
H
, Reitinger
S
, Kloss
F
, Gully
C
, Gassner
R
and Lepperdinger
G.
Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan.
Aging Cell.
2007;
6:
745
-757.
[PubMed]
.