Why human lifespan is rapidly increasing: solving "longevity riddle" with "revealed-slow-aging" hypothesis
Abstract
Healthy life span is rapidly increasing and human aging seems to be postponed. As recently exclaimed in Nature, these findings are so perplexing that they can be dubbed the 'longevity riddle'. To explain current increase in longevity, I discuss that certain genetic variants such as hyper-active mTOR (mTarget of Rapamycin) may increase survival early in life at the expense of accelerated aging. In other words, robustness and fast aging may be associated and slow-aging individuals died prematurely in the past. Therefore, until recently, mostly fast-aging individuals managed to survive into old age. The progress of civilization (especially 60 years ago) allowed slow-aging individuals to survive until old age, emerging as healthy centenarians now. I discuss why slow aging is manifested as postponed (healthy) aging, why the rate of deterioration is independent from aging and also entertain hypothetical use of rapamycin in different eras as well as the future of human longevity.
Unexpected increase in longevity
Death from aging is technically death from age-related
diseases, which are manifestations of advanced aging [1]. But, historically,
most people died young and, of course, not from age-related diseases but,
rather, from starvation and epidemics (cholera, smallpox, tuberculosis and many
currently rare infections) as well as from physical violence. Just three
centuries ago, life expectancy was less than 16 years and 75% of people born in
London in 1662 died before they reached the age of 26 (Graunt's life table).
The progress of civilization eliminated many causes of death that killed young
people in the past. This dramatically increased the average lifespan. In
addition, modern medicine extended lifespan of old people by treating
age-related diseases. But maximal lifespan seemed to be not affected. It was
assumed that human life span is close to its upper limits. However, surprising
demographists and gerontologists, it was shown that life
expectancy continues to increase at an astonishing pace [2,3]. In
the countries with the highest life expectancies, the long term increase in
life expectancy proceeds at a pace of 2.5 years per 10 years, or six hours per
day [4]. A century ago, the chance to become centenarian (a person older than
100 years) was a hundred times lower. Furthermore, as calculated, most babies
born since 2000 in countries with long life expectancies will celebrate their
100th birthdays [5]. Most astonishingly, people are reaching very old age in
better health. But then they deteriorate fast, seemingly indicating that the
rate of aging was not changed but just aging was postponed [3]. "Taken
together, these findings are so perplexing that they can be dubbed the
‘longevity riddle': why do the evolutionary forces that shaped human aging
provide a license to alter the level of health but not the rate of
debilitation?" [3]. So why can aging be delayed but not slowed? Or can aging be
slowed? In order to solve the longevity riddle, we should turn gerontology on
its head. It has been always assumed that aging is caused by damage. As
recently argued, aging is not driven by damage, but, in contrast, leads to
damage (organ damage) [6-8]. And aging is driven in part by mTOR (mammalian
target of rapamycin).
TOR-driven quasi-programmed aging and age-related
diseases
The mTOR intracellular signaling pathway is activated
by numerous signals including glucose, amino acids, fat acids and other
nutrients, insulin and some other hormones, growth factors and cytokines
[9-11]. In response, it increases cellular functions and cellular mass growth
[12]. When the cell cycle is blocked, mTOR drives cellular senescence [13].
Cellular aging can be defined as over-activation of signaling pathways (such as
mTOR) with secondary signal resistance [14]. In turn this slowly leads to
diseases of aging (hypertension, atherosclerosis, macular degeneration, insulin
resistance, obesity, neurodegeneration, cancer, osteoporosis, organ
hypertrophy). For example, TOR-dependent activation of osteoclasts causes bone
resorption (osteoporosis) [15]. But these aging processes are relatively silent
(subclinical, no obvious deterioration) until aging culminates in
"catastrophes" - organ damage. For example, osteoporosis can lead to broken hip
and atherosclerosis can lead to infarction. Then deterioration can be quick,
leading to death in a mater of hours or years or decades, depending on the
level of medical care.
Morbid phase
When diseases become clinical
then deterioration may be fast. For example, high blood pressure, thrombosis
and atherosclerosis can culminate in stroke. This will initiate a chain of
deteriorations (immobility - pneumonia, etc.) that are TOR-independent. The
duration of this morbid (deterioration) phase is almost solely determined by
the level of medical care. Furthermore, age-related blindness and Alzheimer's
disease are rarely lethal anymore. Medicine may dramatically prolong the
morbidity phase, delaying death. Thus, the speed of deterioration is almost
independent from the aging process and cannot serve as a marker of aging or the
rate of aging. The rate of aging is actually determined by the age of the onset
of age-related diseases. Slowing down the aging process (by calorie
restriction, rapamycin or genetic manipulation) delays diseases.
"Thought experiment": how would rapamycin affect
longevity in 1667 versus 1967
Rapamycin is an anti-aging drug, which is
currently used to prevent donor organ rejections [16]. Rapamycin delays cancer
in animals and humans (see for review [17]). It also delays other age-related
diseases in animal models of accelerated diseases. For example, rapamycin and
its analogs delay atherosclerosis [18-23]. mTOR is involved in age-related
diseases exactly because it is involved in aging. In fact, rapamycin prolongs
life span in mice and flies [24-27]. It is expected that, in adult humans, rapamycin
(at correct doses and schedules) will prolong healthy and maximal lifespan
[16]. But consider rapamycin administered for life, starting from childhood.
Then its effect on longevity will depend on the level of civilization and will
be opposite in the 17th and 20th centuries.
Scenario 1.
Assume that in 1667, 3 out of 4 newborns were randomly prescribed rapamycin for
life. Rapamycin would slow down developmental growth (a disadvantage for
survival, especially for orphans). Malnutrition and stresses would be less
tolerated, because the nutrient sensing pathway is deactivated by rapamycin.
Reduced muscle mass and fat stores would increase chances of death from
violence and famine. In infants with natural immunotolerance, rapamycin would
further decrease immunity against infections, which were numerous, incurable
and non-preventable in 17th century. So, if 3 out of 4 people must
die before the age of 26 (1667 in London), they would be those who were treated
with rapamycin. The control group would survive and develop diseases of aging
at normal (early) age.
Scenario 2.
In 20th century London, sanitation, vaccination and other measures
have greatly reduced epidemics. The discovery of antibiotics has further
prevented death from infections. Famine and violent death are not common
either. Those who were treated with rapamycin for life will survive into
adulthood and then will age slowly. In the rapamycin-treated group, diseases
will be delayed. Furthermore, even its ability to cause immunologic tolerance
(‘rejuvenate' immunity) will be beneficial in the elderly by decreasing
hyper-immunity and autoimmunity. (Note: rapamycin improves immunity in old
animals [28]). So, now, the rapamycin treated group becomes centenarians in
good heath. But because deterioration is mTOR-independent, this group will
deteriorate at the same rate (but later in life) as the control group, assuming
that the medical treatment is equal in both groups (in reality, younger
patients are treated more intensively.)
The revealed-slow-aging hypothesis
Thus, while slow aging was a disadvantage in 1667, it
became an advantage in 1967. In the past, mostly fast-aging individuals could
survive into chronologically old age (Figure 2A). Now, slow-aging individuals
can survive into chronologically old age (Figure 2B). Therefore, demographists
observe an increasing number of individuals who are healthy at advanced
chronological ages with delayed onset of diseases, who then deteriorate at the
same rate as younger patients (Figure 1A vs 1B).
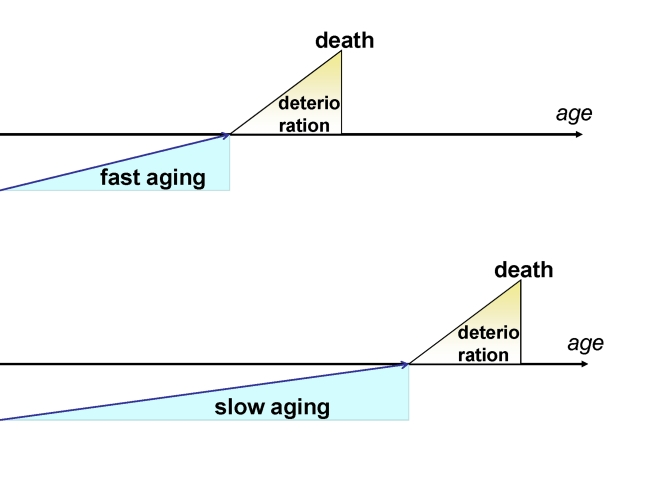
Figure 1. Fast and slow aging. In slow aging, the onset of
deterioration is postponed but the rate of deterioration is not changed.
Importantly, current increase in healthy lifespan
(increased longevity with late onset of age-related diseases) is not caused by
natural selection. It happens in the same generation. Slow aging was not
selected but was simply revealed (Figure 2 B). Until recently, most slow-aging
individuals died prematurely. They (we) did not necessarily die young but nevertheless
died not from aging. For example, at the same chronological age when
fast-aging individuals died from heart attack, healthy slow-aging individuals
died from malnutrition and infections, for instance. Elimination of premature
death greatly enriched chronologically old population with slow-aging
(biologically young) individuals (Figure 2).
To be possibly correct, the hypothesis requires
a high proportion of slow-aging individuals at birth (Figure 2). Otherwise,
there would be too few slow-aging individuals to make a difference later (Figure 2 A vs B). Why was not slow aging selected out? Slow aging must be beneficial
for women, by increasing their reproductive period. In fact, female's fertility
is decreasing early in life (starting from late twenties, long before
menopause). This reproductive aging is one of the earliest manifestations of
aging in females. So slow aging benefits females. Also, as I will discuss
elsewhere, women do not need to be as robust as men, so can afford to age
slower (see forthcoming article "Why men age faster but reproduce longer: mTOR
perspective"). In turn, males inherit genes for longevity too, explaining a
high proportion of slow-aging individuals at birth.
The revealed-slow-aging hypothesis predicts that
certain very harsh conditions may result in a decrease in healthy lifespan
decades later. For example, perhaps it is robust (and therefore fast-aging
later) young men who predominantly survived wars, camps and orphanages. (If so,
the death of weak slow-aging young men during 1940th-1950th
might explain a drop in healthy lifespan of Russian men 50 years later.) Also,
the hypothesis explains data on early-age mortality and subsequent mortality in
the same cohorts. Thus Finch and Crimmins showed that increasing longevity and
declining mortality in the elderly occurred among the same birth cohorts that
experienced a reduction in mortality at younger ages [29,30]. The
revealed-slow-aging hypothesis suggests that high levels of infection early in
life eliminate young individuals with a ‘weak' mTOR (slow-aging individuals,
who otherwise would live longer).
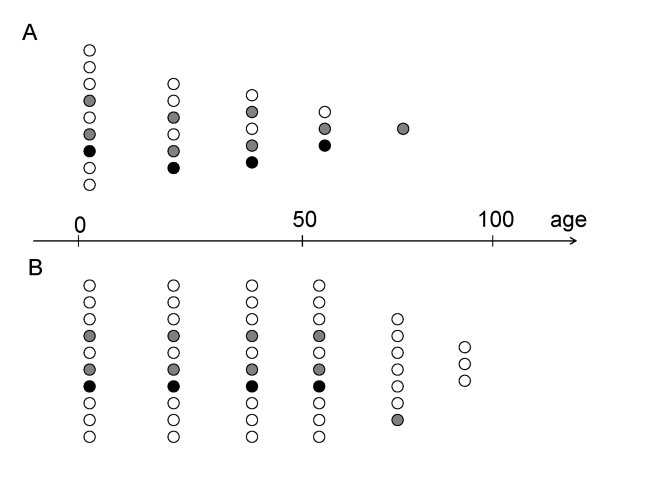
Figure 2. Preferential survival fast- versus slow-aging individuals.
(A) In the past, slow-aging individuals (open circles) died prematurely
and fast-aging individuals (closed circles) survived into old age.
(B) Now, slow-aging individuals (open circles) survived into old
age as healthy (biologically young) and outlive faster aging individuals (closed
circles).
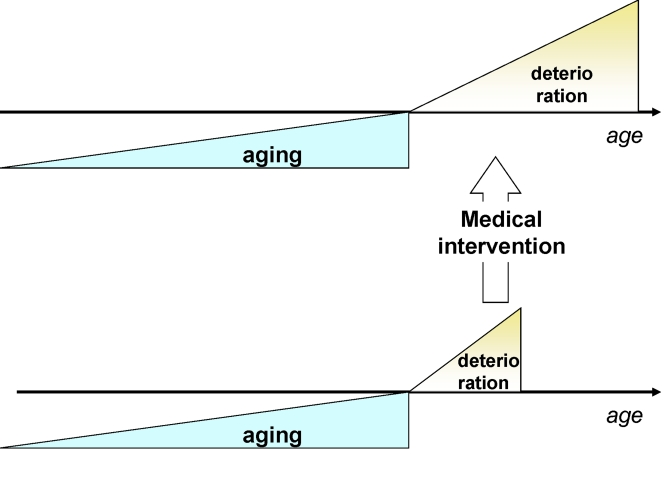
Figure 3. Traditional medicine increases survival (extends deterioration phase) without affecting the onset of deterioration.
The prospect of longevity
Today, most slow-aging individuals, with less active
mTOR, do not die early in life from malnutrition and infections and can reach
chronologically old age. Exactly because they are slow-aging (young
biologically), they are able to reach old age in good health. This may explain
the current increase in longevity. But this trend is probably close to
saturation and will be saturated by 2050 (a century after invention of
antibiotics) in the countries with the highest longevity. The reason is that
the rate of aging was not affected by elimination of death from famine and
infections.
Yet, aging could be slowed by rapamycin, a drug
currently approved to prevent organ rejection. (Note: rapamycin, as an
anti-aging drug, perhaps should not be administrated until after growth is
completed). Based on data with calorie restriction and rapamycin in mice,
lifespan might be increased on 30 percent. Then we will observe 140-150 years
old individuals and average lifespan will exceed 100.
Solution of heath care crisis and further prospect on
longevity
Currently, by treating each disease
individually and focusing on advanced diseases, traditional medical interventions
lengthen the morbidity phase (Figure 3).
So, traditional medicine increases number of old
people in bad health. However, extension of lifespan by lengthening only the
morbidity phase will make the cost of medical care unsustainable for society.
Anti-aging medicine can solve this crisis by delaying the morbidity
(deterioration) phase (Figure 4).
There is incorrect perception that anti-aging drugs
would increase a number of people suffering with age-related diseases. In contrast,
such old people will be healthy because they will be only
chronologically old but biologically young. They will be healthier for longer
(until they reach biological age of deterioration). Biological age is by itself
determined by the sum of all diseases of aging [1]. In other words, diseases of
aging are manifestations of biological aging. It is impossible to dissociate
biological aging and diseases of aging. Healthy aging is healthy non-aging
(or slow aging).
Deceleration of aging, manifested as "healthy aging",
increases the ratio of healthy to unhealthy people (Figure 4). Furthermore, the
ability to work is determined by biological age. Slow aging may delay
retirement until later in life (as also suggested by Vaupel [3]) and in turn
may provide the means for society to support further development of
increasingly powerful (and expensive) conventional medicine. Then lifespan can
be extended by both anti-aging medical intervention (to delay morbidity) and
specialized medical intervention (to prolong morbidity stage).
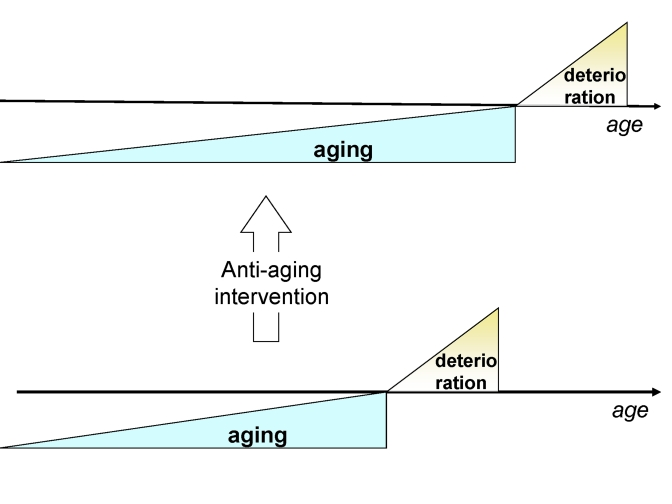
Figure 4. Anti-aging drugs will delay the onset of deterioration without affecting deterioration.
In conclusion, the progress of medicine
60-100 years ago (in prevention and treatment of non-age-related diseases)
allowed slow-aging individuals to survive long enough to die from late onset
age-related diseases (in other words to die from postponed aging). Civilization
increased a proportion of slow-aging persons among the elderly, without actually
slowing the aging process. Rapamycin will be used to slow down aging itself,
further extending healthy lifespan. The extent of lifespan extension will
depend on the future discoveries. And future discoveries are predictably
unpredictable [31].
Acknowledgments
I thank Vera Gorbunova, Valter Longo and
Jay Caplan for comments on the manuscript.
Conflicts of Interest
The author of this manuscript has no conflict of
interests to declare.
References
-
1.
Blagosklonny
MV
Validation of anti-aging drugs by treating age-related diseases.
Aging.
2009;
1:
281
-288.
[PubMed]
.
-
2.
Wilmoth
JR
, Deegan
LJ
, Lundstrom
H
and Horiuchi
S.
Increase of maximum life-span in Sweden, 1861-1999.
Science.
2000;
289:
2366
-2368.
[PubMed]
.
-
3.
Vaupel
JW
Biodemography of human ageing.
Nature.
2010;
464:
536
-542.
[PubMed]
.
-
4.
Oeppen
J
and Vaupel
JW.
Demography. Broken limits to life expectancy.
Science.
2002;
296:
1029
-1031.
[PubMed]
.
-
5.
Christensen
K
, Doblhammer
G
, Rau
R
and Vaupel
JW.
Ageing populations: the challenges ahead.
Lancet.
2009;
374:
1196
-1208.
[PubMed]
.
-
6.
Blagosklonny
MV
Aging and immortality: quasi-programmed senescence and its pharmacologic inhibition.
Cell Cycle.
2006;
5:
2087
-2102.
[PubMed]
.
-
7.
Blagosklonny
MV
Aging: ROS or TOR.
Cell Cycle.
2008;
7:
3344
-3354.
[PubMed]
.
-
8.
Blagosklonny
MV
TOR-driven aging: speeding car without brakes.
Cell Cycle.
2009;
8:
4055
-4059.
[PubMed]
.
-
9.
Hay
N
and Sonenberg
N.
Upstream and downstream of mTOR.
Genes Dev.
2004;
18:
1926
-1945.
[PubMed]
.
-
10.
Wullschleger
S
, Loewith
R
and Hall
MN.
TOR signaling in growth and metabolism.
Cell.
2006;
124:
471
-484.
[PubMed]
.
-
11.
Hands
SL
, Proud
CG
and Wyttenbach
A.
mTOR's role in ageing: protein synthesis or autophagy.
Aging.
2009;
586
-597.
[PubMed]
.
-
12.
Blagosklonny
MV
and Hall
MN.
Growth and aging: a common molecular mechanism.
Aging.
2009;
1:
357
-362.
[PubMed]
.
-
13.
Demidenko
ZN
and Blagosklonny
MV.
Growth stimulation leads to cellular senescence when the cell cycle is blocked.
Cell Cycle.
2008;
7:
3355
-3361.
[PubMed]
.
-
14.
Blagosklonny
MV
Aging-suppressants: cellular senescence (hyperactivation) and its pharmacological deceleration.
Cell Cycle.
2009;
8:
1883
-1887.
[PubMed]
.
-
15.
Kneissel
M
, Luong-Nguyen
NH
, Baptist
M
, Cortesi
R
, Zumstein-Mecker
S
, Kossida
S
, O'Reilly
T
, Lane
H
and Susa
M.
Everolimus suppresses cancellous bone loss, bone resorption, and cathepsin K expression by osteoclasts.
Bone.
2004;
35:
1144
-1156.
[PubMed]
.
-
16.
Blagosklonny
MV
An anti-aging drug today: from senescence-promoting genes to anti-aging pill.
Drug Discov Today.
2007;
12:
218
-224.
[PubMed]
.
-
17.
Blagosklonny
MV
Prevention of cancer by inhibiting aging.
Cancer Biol Ther.
2008;
7:
1520
-1524.
[PubMed]
.
-
18.
Elloso
MM
, Azrolan
N
, Sehgal
SN
, Hsu
PL
, Phiel
KL
, Kopec
CA
, Basso
MD
and Adelman
SJ.
Protective effect of the immunosuppressant sirolimus against aortic atherosclerosis in apo E-deficient mice.
Am J Transplant.
2003;
3:
562
-569.
[PubMed]
.
-
19.
Castro
C
, Campistol
JM
, Sancho
D
, Sanchez-Madrid
F
, Casals
E
and Andres
V.
Rapamycin attenuates atherosclerosis induced by dietary cholesterol in apolipoprotein-deficient mice through a p27 Kip1 -independent pathway.
Atherosclerosis.
2004;
172:
31
-38.
[PubMed]
.
-
20.
Pakala
R
, Stabile
E
, Jang
GJ
, Clavijo
L
and Waksman
R.
Rapamycin attenuates atherosclerotic plaque progression in apolipoprotein E knockout mice: inhibitory effect on monocyte chemotaxis.
J Cardiovasc Pharmacol.
2005;
46:
481
-486.
[PubMed]
.
-
21.
Gadioli
AL
, Nogueira
BV
, Arruda
RM
, Pereira
RB
, Meyrelles
SS
, Arruda
JA
and Vasquez
EC.
Oral rapamycin attenuates atherosclerosis without affecting the arterial responsiveness of resistance vessels in apolipoprotein E-deficient mice.
Braz J Med Biol Res.
2009;
42:
1191
-1195.
[PubMed]
.
-
22.
Zhao
L
, Ding
T
, Cyrus
T
, Cheng
Y
, Tian
H
, Ma
M
, Falotico
R
and Pratico
D.
Low-dose oral sirolimus reduces atherogenesis, vascular inflammation and modulates plaque composition in mice lacking the LDL receptor.
Br J Pharmacol.
2009;
156:
774
-785.
[PubMed]
.
-
23.
Mueller
MA
, Beutner
F
, Teupser
D
, Ceglarek
U
and Thiery
J.
Prevention of atherosclerosis by the mTOR inhibitor everolimus in LDLR-/- mice despite severe hypercholesterolemia.
Atherosclerosis.
2008;
198:
39
-48.
[PubMed]
.
-
24.
Harrison
DE
, Strong
R
, Sharp
ZD
, Nelson
JF
, Astle
CM
, Flurkey
K
, Nadon
NL
, Wilkinson
JE
, Frenkel
K
, Carter
CS
, Pahor
M
, Javors
MA
, Fernandezr
E
and Miller
RA.
Rapamycin fed late in life extends lifespan in genetically heterogenous mice.
Nature.
2009;
460:
392
-396.
[PubMed]
.
-
25.
Anisimov
VN
, Zabezhinski
MA
, Popovich
IG
, Piskunova
TS
, Semenchenko
AV
, Tyndyk
ML
, Yurova
MN
, Antoch
MP
and Blagosklonny
MV.
Rapamycin Extends Maximal Lifespan in Cancer-Prone Mice.
Am J Pathol.
2010;
In press
.
-
26.
Moskalev
AA
and Shaposhnikov
MV.
Pharmacological Inhibition of Phosphoinositide 3 and TOR Kinases Improves Survival of Drosophila melanogaster.
Rejuvenation Res.
2009;
In press
.
-
27.
Bjedov
I
, Toivonen
JM
, Kerr
F
, Slack
C
, Jacobson
J
, Foley
A
and Partridge
L.
Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster.
Cell Metab.
2010;
11:
35
-46.
[PubMed]
.
-
28.
Araki
K
, Turner
AP
, Shaffer
VO
, Gangappa
S
, Keller
SA
, Bachmann
MF
, Larsen
CP
and Ahmed
R.
mTOR regulates memory CD8 T-cell differentiation.
Nature.
2009;
460:
108
-112.
[PubMed]
.
-
29.
Finch
CE
and Crimmins
EM.
Inflammatory exposure and historical changes in human life-spans.
Science.
2004;
305:
1736
-1739.
[PubMed]
.
-
30.
Crimmins
EM
and Finch
CE.
Infection, inflammation, height, and longevity.
Proc Natl Acad Sci U S A.
2006;
103:
498
-503.
[PubMed]
.
-
31.
Taleb
NN
Random House
New York
The black swan: the impact of the highly improbable.
2007;
.