Quality and quantity control of proteins in senescence
Abstract
Autophagy has been implicated in aging and age-related diseases but its roles in these processes are far from straightforward: the anti-aging effect of autophagy has been shown in lower eukaryotes, and both pro- and anti-tumorigenic effects of autophagy have also been demonstrated. The new link between autophagy and senescence provides an insight into the diversified downstream effects of autophagy in various cellular contexts.
Macroautophagy
(referred to as autophagy hereafter) is a highly conserved lysosome-mediated
catabolic process, which can deal with the bulk degradation of cytoplasmic
proteins as well as small organelles. Although the activation of autophagy can
be acutely induced by nutrient deprivation, it is also known that cells exhibit
a basal level of autophagy activity. Thus, autophagy plays important roles in
the fine-tuning of energy homeostasis and the quality control of proteins and
small organelles [1,2].
In
addition to metabolic stress, it has been shown that autophagy can also be
induced by various cytotoxic stresses. Not surprisingly, increasing evidence
has shown that autophagy is involved in a number of pathophysiologies,
including aging and age-related diseases (cancer, atherosclerosis, and neuro-degeneration),
and innate and adaptive immunity [3]. It is still not entirely clear, however,
how such a catabolic program contributes to the cytotoxic stress response.
Since autophagy is thought to be a survival as well as a non-apoptotic cell
death mechanism, it could be an effector for or against stress responsive
phenotypes depending on the context [4,5].
Replicative
senescence (RS) and oncogene-induced senescence (OIS)
Cellular
senescence was originally defined as ‘irreversible' cell cycle arrest caused by
replicative exhaustion in cultured human diploid fibroblasts (HDFs) [6]. Later,
it was shown that this ‘replicative exhaustion' is essentially telomere
shortening, which activates a persistent DNA damage response [7]. The
senescence trigger is, however, not restricted to telomere dysfunction. In
1997, Serrano et al. showed that oncogenic Ras, which can transform
immortalized cells, induces a senescence-like phenotype in normal HDFs [8].
This is rather paradoxical, but it was shown that the initial response of cells
to oncogenic Ras is hyper-proliferation. Thus, it was proposed that cells
somehow sense this abnormal proliferation, and undergo senescence as a delayed
response to counter the oncogenic signals [9]. It is conceivable that these
'delayed responses' would include effector mechanisms of senescence, and
understanding these mechanisms would provide insights into
senescence-associated pathophysiologies, including aging and cancer. Indeed, OIS in culture has been a very useful system for
the identification
and characterization of senescence effector mechanisms, such as epigenetic gene
regulation and chromatin modifications, DNA damage response, negative feedback
in the PI3K pathway, and senescence-associated secretory phenotype
(SASP)/senescence-mess secretome (SMS) [10-14]. Our recent study has added
autophagy to the list of OIS effector mechanisms [15].
Irrespective
of the triggers, senescence shares many, if not all, of the effector mechanisms
identified in OIS systems to some extent. Therefore it is not surprising that
autophagy is also implicated in RS [16]. However, despite the similarity of the
endpoint between RS and OIS, the modes of senescence establishment are
distinct: RS involves modest but long-term exposure of cells to stress and HDFs
reach a senescent state over several months, while OIS establishment is a more
acute and dynamic process. It remains to be addressed how these distinct
conditions share the regulatory mechanisms of autophagy and its downstream effects.
Based on the intensity of the stress and
acuteness of the process, RS and OIS may reflect natural aging and age-related
disease (e.g. cancer and atherosclerosis), respectively. Interestingly, many
senescence effector mechanisms, including autophagy, have also been implicated
in both aging and age-related disease [3,17-20]. Autophagy in lower eukaryotes
has been shown to be critical for the anti-aging effects of dietary
restriction and negative modulation of insulin-signalling [21-24]. In contrast
to its anti-aging effect, as shown in various models, autophagy can have either
pro- or anti-tumorigenic activity depending on the context [3,20]. Thus it is
possible that the same cellular machinery plays distinct roles ageing and
age-related diseases.
In
RS, Gamerdinger et al. (2009) showed that there is a gradual shift from the
proteasome pathway to autophagy within polyubiquitinated protein
degradation systems. This shift is mediated through at least two members of the BAG (Bcl-2-associated
athanogene) protein family,
which can bind to chaperones of the
Hsc/HSP70 family and thereby modulate protein quality control. They showed that BAG1 and BAG3 positively regulate the proteasomal and
autophagic pathways, respectively, and that BAG1 and BAG3 levels are reciprocally regulated during RS, in
which the BAG3/BAG1 ratio is elevated [16].
The increase of BAG3/BAG1 ratio and
activation of autophagy is also found in tissue aging, thus, it is not limited
to in vitro "cell aging". Gamerdinger
et al. (2009) found a similar age-related correlation between autophagy and the
BAG3/BAG1 ratio in rodent brains.
Considering the age-dependent accumulation of damaged proteins (particularly
due to oxidative stress), the role of autophagy in this case may be classic
‘quality control' of proteins and other macromolecules. This is also consistent
with the anti-aging role of autophagy as described earlier. However, it has
also been noted that global autophagy capacity declines with age in vivo
[25,26]. How can one reconcile the apparent discrepancy? First, it is possible
that the extent to which autophagy activity changes is different depending on cell type. It has been demonstrated in aged brains
that neurons, but not astrocytes, show upregulated autophagy [16]. Second, it
is also possible that it is the basal activity and metabolic regulation of
autophagy that decline during aging, but cytotoxic stress-induced autophagy may
not be severely affected particularly in long-lived cells, which are
susceptible to the accumulation of oxidative stress. Interestingly, it was
recently reported that progeroid mouse models exhibit an extensive activation
of the basal autophagy [27]. It still remains to be elucidated, however,
whether the chronic activation of autophagy in these mice is a protective
reaction against the causal elements associated with premature aging symptoms
or that autophagy actively contributes to the phenotype. This study, in
conjunction with the observations by Gamerdinger et al. (2009), suggests that alteration of autophagy activity
during aging and the functional implications of autophagy in age-associated
pathophysiologies can be more complex, at least in mammals.
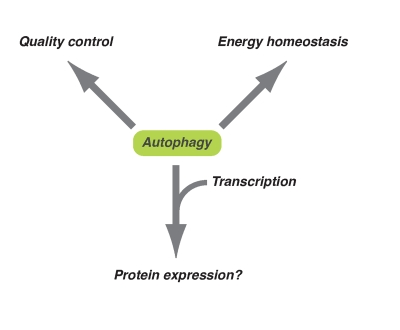
Figure 1. Diversified downstream effects of autophagy. Autophagy plays
an important role in energy homeostasis and quality control of
macromolecules at the basal level or occurs during long-term exposure to
oxidative stress. On the other hand, in response to acute cytotoxic
stresses (e.g. oncogenic stress), autophagy might contribute to the
expression of some proteins together with epigenetic transcriptional regulation.
From
transcription to proteins
If
autophagy is involved in the long-term quality control of cytoplasmic
macromolecules, as proposed in Gamerdinger
(2009), what is the acute role of
autophagy during OIS? To ask this question, we have focused on its highly
dynamic nature. This is more obvious when
inducible oncogenes are used, such as 4-hydroxytamoxifen (4-OHT)-inducible ER:Ras fusion protein, which
is comprised of a mutant form of the estrogen receptor ligand-binding domain
and constitutively active H-RasV12 [15].
This inducible system allows us to focus on the transition phase, which lies
between the initial mitotic burst after Ras-induction, and the static
senescence phase. It is possible that the most dramatic phenotypic remodelling
and cellular adjustments to the new environment occur during this transition
phase.
One
obvious mechanism that is responsible for this transition is a global
transcription change. We and others previously described a unique chromatin
structure - senescence associated heterochromatic foci (SAHFs) - which seem to
play a role in transcriptional regulation during senescence [10,28,29]. Indeed,
many senescence-associated genes are upregulated during the transition phase,
including a large number of secretory proteins. Among these, IL6 and IL8, an
inflammatory cytokine and chemokine respectively, have recently been shown to
reinforce the senescence phenotype, thus representing another senescence
effector mechanism - SASP/SMS [30-32]. The timing of IL6/8 induction has been
correlated with autophagy activation during the transition phase. Strikingly,
RNAi-mediated repression of Atg5 or Atg7 (essential genes for
autophagy) suppresses IL6/8 production, indicating a functional relevance of
autophagy in senescence. Although it is still unclear exactly how autophagy
facilitates IL6/8 production, we have shown that the transcription levels of
these genes are often even higher when Atg5 or Atg7 are
knocked-down, indicating that the positive regulation of these genes by
autophagy occurs at the post-transcriptional level. Thus IL6/8, which are
acutely produced en masse, seem to be regulated in a cooperative manner
by mRNA and protein synthesis (Figure 1). Massive induction of autophagy and
the resultant efficient protein turnover might provide another layer of gene
expression control - at least for some genes - to execute epigenetic
'blueprints' during OIS.
Perspective
Metabolism
is a very dynamic and robust process, thus interpreting 'snapshots' of
metabolic processes can be difficult. Our recent study focusing on the dynamic
phase of OIS highlighted the distinct role of autophagy in controlling protein
quantity in OIS. Extensive characterization of autophagy's distinct and shared
roles in RS and OIS would be beneficial to further understand the mechanisms by
which autophagy has diverse effects in different contexts. In addition to its
downstream effects, it is also important to understand how autophagy is
regulated during senescence. Consistent with a previous report [12], we have
shown that components of the PI3K pathway - including mTOR, a negative regulator
of autophagy - are attenuated after their acute activation following Ras
expression during the transition phase of OIS [15,33]. Although the long-term
fluctuation of mTOR activity during the senescence phase remains to be fully
characterized, our study raises an interesting question: how protein synthesis
(positively regulated by mTOR) and autophagy (negatively regulated by mTOR) are
activated during the senescence transition. Interestingly, recent reports show
that mTOR inhibition by rapamycin decelerates senescence [34,35].
mTOR-regulated catabolic and anabolic processes seems to be somehow coupled to
contribute to senescence, and perhaps aging.
Acknowledgments
MN is supported by the University of
Cambridge, Cancer Research UK and Hutchison Whampoa Limited. We thank Masako Narita for critical reading of the
manuscript and Laura Blackburn for editing.
Conflicts of Interest
The
author of this manuscript has no conflict of interests to declare.
References
-
1.
He
C
and Klionsky
DJ.
Regulation mechanisms and signaling pathways of autophagy.
Annu Rev Genet.
2009;
43:
67
-93.
[PubMed]
.
-
2.
Nakatogawa
H
, Suzuki
K
, Kamada
Y
and Ohsumi
Y.
Dynamics and diversity in autophagy mechanisms: lessons from yeast.
Nat Rev Mol Cell Biol.
2009;
10:
458
-467.
[PubMed]
.
-
3.
Mizushima
N
, Levine
B
, Cuervo
AM
and Klionsky
DJ.
Autophagy fights disease through cellular self-digestion.
Nature.
2008;
451:
1069
-1075.
[PubMed]
.
-
4.
Galluzzi
L
, Morselli
E
, Vicencio
JM
, Kepp
O
, Joza
N
, Tajeddine
N
and Kroemer
G.
Life, death and burial: multifaceted impact of autophagy.
Biochem Soc Trans.
2008;
36:
786
-790.
[PubMed]
.
-
5.
Mathew
R
, Karantza-Wadsworth
V
and White
E.
Role of autophagy in cancer.
Nat Rev Cancer.
2007;
7:
961
-967.
[PubMed]
.
-
6.
Hayflick
L
The limited in vitro lifetime of human diploid cell strains.
Exp Cell Res.
1965;
37:
614
-636.
[PubMed]
.
-
7.
Shay
JW
and Wright
WE.
Hayflick, his limit, and cellular ageing.
Nat Rev Mol Cell Biol.
2000;
1:
72
-76.
[PubMed]
.
-
8.
Serrano
M
, Lin
AW
, McCurrach
ME
, Beach
D
and Lowe
SW.
Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a.
Cell.
1997;
88:
593
-602.
[PubMed]
.
-
9.
Lin
AW
, Barradas
M
, Stone
JC
, van
Aelst L
, Serrano
M
and Lowe
SW.
Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling.
Genes & development.
1998;
12:
3008
-3019.
[PubMed]
.
-
10.
Narita M. Cellular senescence and chromatin organisation.
Br J Cancer.
2007;
96:
686
-691.
[PubMed]
.
-
11.
Campisi
J
and d'Adda
di Fagagna F.
Cellular senescence: when bad things happen to good cells.
Nat Rev Mol Cell Biol.
2007;
8:
729
-740.
[PubMed]
.
-
12.
Courtois-Cox
S
, Genther
Williams SM
, Reczek
EE
, Johnson
BW
, McGillicuddy
LT
, Johannessen
CM
, Hollstein
PE
, MacCollin
M
and Cichowski
K.
A negative feedback signaling network underlies oncogene-induced senescence.
Cancer cell.
2006;
10:
459
-472.
[PubMed]
.
-
13.
Kuilman
T
and Peeper
DS.
Senescence-messaging secretome: SMS-ing cellular stress.
Nat Rev Cancer.
2009;
9:
81
-94.
[PubMed]
.
-
14.
Acosta
JC
and Gil
J.
A role for CXCR2 in senescence, but what about in cancer.
Cancer research.
2009;
69:
2167
-2170.
[PubMed]
.
-
15.
Young
AR
, Narita
M
, Ferreira
M
, Kirschner
K
, Sadaie
M
, Darot
JF
, Tavare
S
, Arakawa
S
, Shimizu
S
, Watt
FM
and Narita
M.
. Autophagy mediates the mitotic senescence transition.
Genes & development.
2009;
23:
798
-803.
[PubMed]
.
-
16.
Gamerdinger
M
, Hajieva
P
, Kaya
AM
, Wolfrum
U
, Hartl
FU
and Behl
C.
Protein quality control during aging involves recruitment of the macroautophagy pathway by BAG3.
EMBO J.
2009;
28:
889
-901.
[PubMed]
.
-
17.
Lou
Z
, Wei
J
, Riethman
H
, Baur
JA
, Voglauer
R
, Shay
JW
and Wright
WE.
Telomere length regulates ISG15 expression in human cells.
Aging (Albany NY).
2009;
1:
608
-621.
[PubMed]
.
-
18.
De
Meyer GR
and Martinet
W.
Autophagy in the cardiovascular system.
Biochim Biophys Acta.
2009;
1793:
1485
-1495.
[PubMed]
.
-
19.
Dimauro
T
and David
G.
Chromatin modifications: the driving force of senescence and aging.
Aging (Albany NY).
2009;
1:
182
-190.
[PubMed]
.
-
20.
White
E
and DiPaola
RS.
The double-edged sword of autophagy modulation in cancer.
Clin Cancer Res.
2009;
15:
5308
-5316.
[PubMed]
.
-
21.
Melendez
A
, Talloczy
Z
, Seaman
M
, Eskelinen
EL
, Hall
DH
and Levine
B.
Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science (New York, N.
Y.
2003;
301:
1387
-1391.
.
-
22.
Toth
ML
, Sigmond
T
, Borsos
E
, Barna
J
, Erdelyi
P
, Takacs-Vellai
K
, Orosz
L
, Kovacs
AL
, Csikos
G
, Sass
M
and Vellai
T.
Longevity pathways converge on autophagy genes to regulate life span in Caenorhabditis elegans.
Autophagy.
2008;
4:
330
-338.
[PubMed]
.
-
23.
Simonsen
A
, Cumming
RC
, Brech
A
, Isakson
P
, Schubert
DR
and Finley
KD.
Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistance in adult Drosophila.
Autophagy.
2008;
4:
176
-184.
[PubMed]
.
-
24.
Morselli
E
, Galluzzi
L
, Kepp
O
, Criollo
A
, Maiuri
MC
, Tavernarakis
N
, Madeo
F
and Kroemer
G.
Autophagy mediates pharmacological lifespan extension by spermidine and resveratrol.
Aging (Albany NY).
2009;
1:
961
-970.
[PubMed]
.
-
25.
Donati
A
, Cavallini
G
, Paradiso
C
, Vittorini
S
, Pollera
M
, Gori
Z
and Bergamini
E.
Age-related changes in the autophagic proteolysis of rat isolated liver cells: effects of antiaging dietary restrictions.
J Gerontol A Biol Sci Med Sci.
2001;
56:
B375
-383.
[PubMed]
.
-
26.
Del Roso
A
, Vittorini
S
, Cavallini
G
, Donati
A
, Gori
Z
, Masini
M
, Pollera
M
and Bergamini
E.
Ageing-related changes in the in vivo function of rat liver macroautophagy and proteolysis.
Exp Gerontol.
2003;
38:
519
-527.
[PubMed]
.
-
27.
Marino
G
, Ugalde
AP
, Salvador-Montoliu
N
, Varela
I
, Quiros
PM
, Cadinanos
J
, van der Pluijm
I
, Freije
JM
and Lopez-Otin
C.
Premature aging in mice activates a systemic metabolic response involving autophagy induction.
Hum Mol Genet.
2008;
17:
2196
-2211.
[PubMed]
.
-
28.
Adams
PD
Remodeling of chromatin structure in senescent cells and its potential impact on tumor suppression and aging.
Gene.
2007;
397:
84
-93.
[PubMed]
.
-
29.
Funayama
R
and Ishikawa
F.
Cellular senescence and chromatin structure.
Chromosoma.
2007;
116:
431
-440.
[PubMed]
.
-
30.
Kuilman
T
, Michaloglou
C
, Vredeveld
LC
, Douma
S
, van
Doorn R
, Desmet
CJ
, Aarden
LA
, Mooi
WJ
and Peeper
DS.
Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network.
Cell.
2008;
133:
1019
-1031.
[PubMed]
.
-
31.
Acosta
JC
, O'Loghlen
A
, Banito
A
, Guijarro
MV
, Augert
A
, Raguz
S
, Fumagalli
M
, Da
Costa M
, Brown
C
, Popov
N
, Takatsu
Y
, Melamed
J
and d'Adda
di Fagagna F.
Chemokine signaling via the CXCR2 receptor reinforces senescence.
Cell.
2008;
133:
1006
-1018.
[PubMed]
.
-
32.
Coppé
JP
, Patil
CK
, Rodier
F
, Sun
Y
, Munoz
DP
, Goldstein
J
, Nelson
PS
, Desprez
PY
and Campisi
J.
Senescence-Associated Secretory Phenotypes Reveal Cell-Nonautonomous Functions of Oncogenic RAS and the p53 Tumor Suppressor.
PLoS biology.
2008;
6:
e301
.
-
33.
Young
AR
and Narita
M.
Connecting autophagy to senescence in pathophysiology.
Curr Opin Cell Biol.
2010;
22:
234
-240.
[PubMed]
.
-
34.
Demidenko
ZN
, Zubova
SG
, Bukreeva
EI
, Pospelov
VA
, Pospelova
TV
and Blagosklonny
MV.
Rapamycin decelerates cellular senescence.
Cell Cycle.
2009;
8:
1888
-1895.
[PubMed]
.
-
35.
Demidenko
ZN
and Blagosklonny
MV.
Growth stimulation leads to cellular senescence when the cell cycle is blocked.
Cell Cycle.
2008;
7:
3355
-3361.
[PubMed]
.