Abstract
Women live longer than men. Yet, it is believed that men do not age faster than women but simply are weaker at every age. In contrast, I discuss that men age faster. From evolutionary perspective, high accidental death rate in young males is compatible with fast aging. Mechanistically, hyper-activated mTOR (Target of Rapamycin) may render young males robust at the cost of accelerated aging. But if women age slower, why then is it women who have menopause? Some believe that menopause is programmed and purposeful (grandmother theory). In contrast, I discuss how menopause is not programmed but rather is an aimless continuation of the same program that initially starts reproduction at puberty. This quasi-program causes over-activation of female reproductive system, which is very vulnerable to over-activation. Mechanisms of aging and menopause are discussed.
Longevity: men and women
Women have lived longer than men in different
countries and in every era [1]. In 1980 in the USA, the estimated life
expectancy at birth was 70 years for men and 77.5 years for women [2]. In the
world, 75% and 90% of people older than 100 years and 110 years (respectively)
are women. And the longest living person (122 years old) was a woman. But do
women age slower than men? The conventional opinion is that women and men age
at the same rate but men are ‘less robust' than women [1]. Seemingly in
agreement, the mortality rate is lower in young women compared with young men.
In women, the mortality rate is lower at every age, even in childhood. In other
words, "women do not live longer than men because they age slowly, but because
they are more robust at every age" [1].
This reasoning would be correct if causes of death were the same at every
age. However, young and old men die from different causes. Young men die from
accidents, while old men die from aging (technically speaking, from age-related
diseases).
High
accidental death rate and fast aging (evolutionary perspective)
There is
a very noticeable jump of mortality in the late teens in men [1]. Young men are
often engaged in competitive, reckless, and dangerous activities. Therefore,
even in modern society, the accidental death rate is high in young men.
Historically, the accidental death rate in men was much higher than it is now.
(Due to a fierce competition for status and mates, due to fights and wars,
young men were killed at a very high rate). So, historically, men had lower
chances to survive into old age than women had. And, according to evolutionary
theory, a high accidental death rate determines fast aging [3-5]. If most men
died young from accidental death, then they could not live long enough to
experience aging. Then there was no natural selection to postpone aging. So
accelerated aging in men is predictable from evolutionary perspective. But
accelerated aging is also predictable mechanistically.
Mechanistic explanation:
antagonistic pleiotropy and mTOR
In males, muscle hypertrophy
and heavy body helps to compete with other males. (In fact, men are larger than
women.) Cellular growth and hypertrophy are stimulated by the mTOR (mammalian
Target of Rapamycin) intracellular signaling pathway. Insulin, growth factors,
amino acids, glucose lipoproteins, and testosterone all activate the mTOR
pathway [6-9]. In turn, the mTOR pathway stimulates protein synthesis and
cell size growth [10]. For example, skeletal muscle hypertrophy depends on the
mTOR pathway [11,12]. In addition, inhibition of the mTOR pathway decreases
testosterone levels and spermatogenesis [13]. Thus, activation of mTOR may
provide a selective advantage to young males.
On the other hand, the mTOR
pathway is required forcellular senescence of mammalian cells [14-18]. Cellular
aging is driven by the remaining activation of mitogenic signaling pathways in
post-mitotic cells [19,20]. In fact, mechanistically, aging is a continuation
of growth, driven in part by mTOR [21]. In agreement, mTOR is involved in
age-related diseases such as atherosclerosis, neurodegeneration, cancer, which
are deadly manifestations of aging (see for review [22-24]).
And rapamycin prolongs
lifespan in mammals [25].
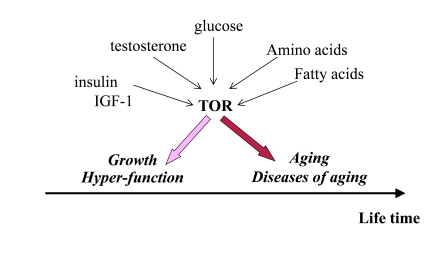
Figure 1. Program of growth and quasi-program of aging. The TOR pathway
is activated by growth factors, hormones and nutrients. This activation is
beneficial early in life by stimulating growth and muscle hypertrophy.
Evolutionary perspective: This was especially important for prehistoric
men, living in dangerous environment that required physical strength. mTOR
is involved in aging later in life, but most men died young from accidental
death. Thus, robustness early in life is associated with accelerated aging.
Thus, over-activation of
mTOR may provide an advantage (muscle hypertrophy, high levels of testosterone
and high spermatogenesis) in early life at the cost of accelerated aging later
in life. As an illuminating example, mice over-expressing growth hormone
exhibit increased levels of IGF-I and adult body size, reduced life span and
reproductive life span [26]. (Note: IGF-I stimulates mTOR, Figure 1).
Accelerated age-related
diseases in men
Humans
do not die from "healthy" aging. Humans die from age-related diseases. The mTOR
pathway is involved in age-related diseases such as cancer, atherosclerosis,
hypertension, heart failure, osteoporosis, type II diabetes [22,24,27].
These diseases are deadly manifestations of aging. When aging is accelerated,
age-related diseases occur earlier in life too. Healthy aging (a late onset of
diseases) is associated with longevity (see for discussion [28]). For example,
centenarians (100 years old or older) show a delay in the onset of age-related
diseases, including cardiovascular disease, type 2 diabetes, cancer and
Alzheimer's disease. In other words, those who age slower are healthier [29,30].
If women age slower than
men, then age-related diseases must be delayed in women. In fact, most
age-related diseases are delayed in women compared with men. For example,
coronary atherosclerosis is postponed in women. Not only atherosclerosis, but
also cancer and most other diseases of aging occur earlier in men than in women
[31]. Women also live more years than men free of each of these diseases with
the exception of arthritis [32]. Women rarely die from age-related diseases
before menopause. The later onset of diseases in women compared with men
suggests that women age slower than men.
Intriguingly,
slower erosion of human telomeres favor females [33] and, even further, the
rate of leukocyte telomere shortening predicts mortality from cardiovascular
disease in elderly men [34]. I speculate that high rate of telomere shortening
reflects cellular hyper-activation and may be suppressed by rapamycin.
Aging versus
reproductive aging
Yet common wisdom holds that
women age faster than men. One should not confuse aging and subjective
perception of youthfulness and sexual attractiveness,
which reflects fertility. Aging is an increase of the probability of death. And
a 50-year-old man has higher chances to die than a 50- year-old woman.
Furthermore, men acquire grey hair and wrinkles faster than women and thus men
even ‘look' older [35]. Although men age faster, they can reproduce longer. And
here is another puzzle: why women undergo menopause.
Like aging itself, menopause
is tolerated by natural selection, because women (until recently) did not live
long enough to experience it. (In modern society, there must be a very strong
natural selection for delayed menopause). So an evolutionary explanation is
simple: ancestral women did not live long enough to have menopause. But male
lifespan was even shorter: why then do men not have menopause? What is so
special about female reproduction?
Can
menopause be programmed?
There is common opinion among traditional
gerontologists that menopause is beneficial for women, has an evolutionary
advantage and is adaptive [36-38]. It was suggested, for instance, that
menopause prevents death of women in labor. The most popular is a "grandmother
hypothesis" that menopause frees older women to help their daughters to raise
grandchildren. This is a sort of group-selection hypothesis. Why do not daughters
delay reproduction just in order to help their mothers raise siblings? Or what
is the biological sense to stop reproduction, if a woman has no grandchildren
living with her? The crucial assumption of ‘grandmother' hypothesis is that
menopause occurs only in humans [37]. Yet, menopause was documented in
non-human primates, rodents, whales, dogs, rabbits, elephants and domestic
livestock [39]. It was shown, for instance, that mice eventually undergo
ovarian changes analogous to menopause in humans [40,41].
It was shown that
grandmothers may promote survival of their maternal grandchildren in Gambia
[37]. Grandmothers are useful but menopause is not. There is no experimental
evidence that menopause is beneficial even when women live with grandchildren
in Gambia. Menopause accelerates age-related diseases such as atherosclerosis,
osteoporosis and cancer [42,43]. Reproductive death provides no selective
benefit (unless group-selection theories of aging are correct) and ‘grandmother
hypothesis' contradicts the evolutionary theory. If aging is not programmed,
then reproductive aging is not programmed too.
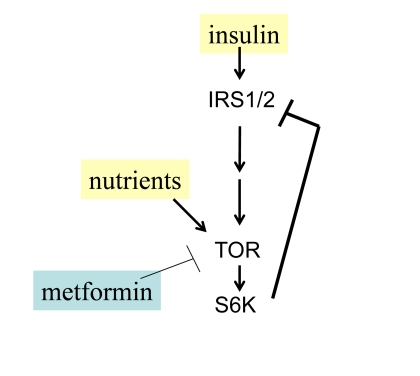
Figure 2. Negative feedback and insulin resistance. TOR is activated
by nutrients and insulin and in turn causes depletion of IRS1/2 and insulin
resistance. Whereas nutrients activate TOR, low nutrients and metformin
deactivate TOR.
TOR-driven
quasi-programmed aging
Aging
is not programmed but quasi-programmed [22,44-46]. ("Quasi-" means "as if,
resembling"). Quasi-program is an aimless continuation of a useful program that
was not switched off
upon its completion. Unlike a program, a
quasi-program has no purpose. Developmental programs become aimless
quasi-programs later in life. Quasi-programs are driven by antagonistic
pleiotropic genes, which are beneficial early in life on the cost of aging
later in life. Most genes that control aging and longevity constitute the mTOR
pathway [22,23]. mTOR is absolutely essential during embryonic development
[47,48]. In post-development, mTOR is involved in aging and age-related
diseases [22].
Nutrients activate mTOR and
cause insulin-resistance in cell culture [49,50] as well as systemically in rodents
and humans [51-54]. There is a negative feedback loop between insulin signaling
and TOR (Figure 2). When mTOR is activated, it blocks insulin signaling
(insulin resistance) [49,55]. Noteworthy, insulin resistance is associated
with premature menopause in some patients [56].
The
menstrual cycle is fragile
Since
aging is not programmed, it does not hurt on purpose. It does not cause ovarian
failure (menopause) on purpose. The logic of aging is simple: the most fragile systems fail first. A female reproductive system is
fragile because it depends on exact interactions between The hypothalamus and
ovaries, communicating via dozens of hormones. The menstrual cycle is regulated
by interplay of negative and positive feedback loops. The hypothalamus
stimulates the pituitary gland to secrete Follicle-Stimulating Hormone (FSH),
which in turn stimulates follicles in the ovaries (Figure 3). Follicles
maturate and secrete estrogens. Estrogens inhibit the hypothalamus, decreasing
secretion of FSH (a negative feedback loop). In turn, FSH stimulates ovarian
follicles, which produce estrogens, which in turn inhibit FSH production. Also,
estrogens stimulate secretion of Lutenizing Hormone (LH). LH in turn causes
ovulation. So for the normal menstrual cycle, the hypothalamus should have a
narrow range of sensitivity to estrogens. Both too high and too low
sensitivities are not compatible with menstrual cycles. In comparison, regulation
of reproduction in men is simpler. There is a gradual decrease in fertility in
men too (analogous to pre-menopause), although this usually does not result in
testicular failure during a man's lifetime [57,58].
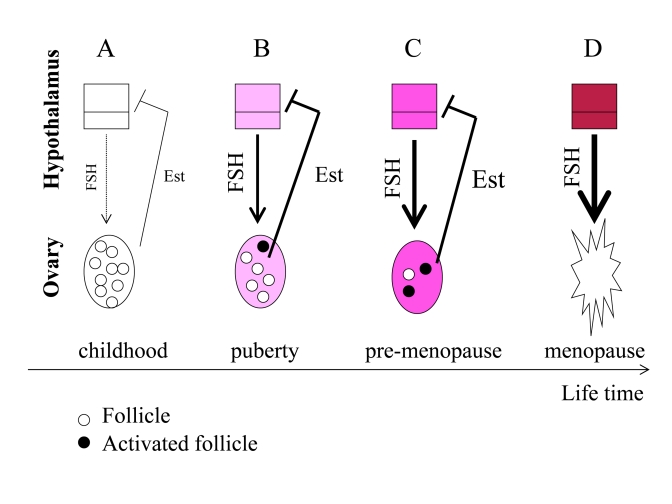
Figure 3. From programmed puberty to quasi-programmed menopause. For
simplicity, only the FSH-estrogen feedback loop is shown. FSH stimulates follicles
and production of estrogens (Est). Estrogens inhibit FSH production (negative
feedback). (A) In girls, the hypothalamus is extremely sensitive to
estrogens and even low levels of estrogens inhibit FSH. (B) The onset of
menstrual cycle. While the hypothalamus is becoming resistant to estrogens, FSH
stimulates the ovaries and estrogen production. Progressive activation of follicles
from the dormant pool serves as the source of fertilizable ova. (C) Pre-menopause.
While the hypothalamus is becoming progressively resistant to estrogens, FSH
progressively over-stimulates the ovaries. (D) The ovaries fail. Menopause
occurs when the primordial follicle pool is exhausted. Estrogen levels drop. The
feedback between hypothalamus and the ovaries is disrupted.
Quasi-programmed
menopause
A half century ago, Vladimir
Dilman proposed a "biological clock" that initially launches reproduction in
puberty and then causes menopause [59,60]. This idea is absolutely compatible
with quasi-programmed nature of menopause, as discussed herein.
Before puberty, the
hypothalamus is extremely sensitive to estrogens (Figure 3 A). Even low levels
of estrogens suppress FSH production and, therefore, levels of FSH are low. At
puberty, the hypothalamus becomes more resistant to estrogens. Then low levels
of estrogens cannot suppress FSH. FSH in turn stimulates the ovarian follicles.
Follicles produce estrogens, which in turn inhibit FSH production (Figure 3 B).
During lifetime, resistance to estrogens continues to increase (Figure 3C).
This ever-increasing resistance is an aim-less continuation of the same program
that initiated menstrual cycle at puberty. FSH is elevated in pre-menopause
and rising serum FSH levels is one of the earliest signs of human female
reproductive aging [61], [62]. Rising FSH levels over-stimulate the ovaries (Figure 3C), thus depleting follicles (Figure 3D).
FSH hyper-stimulates the
ovaries, causing more follicles to be recruited simultaneously (Figure 3 C).
This may explain the increased tendency of older mothers to have dizygotic
twins [63]. Due to hypothalamic resistance to estrogens, estrogens cannot
induce LH surges, which are necessary for ovulation. Therefore, follicles are
recruited without progression to ovulation. Therefore, fertility gradually decreases
long before menopause.
Hypothalamic
resistance to estrogens causes higher FSH levels and lower LH pulses, disturbed
feedback relationships and decrease in fertility [64]. Levels of estrogens tend
to be increased in pre-menopause [64], but even increased estrogens cannot
suppress FSH [61]. FSH over-stimulates follicle recruitment, leading eventually
to follicular depletion (Figure 3D). This process eventually results in ovarian
failure (Figure 3 D). Post-menopause is characterized by a drop in estrogen
levels because of the depletion of follicular oocytes that normally produce
estrogen (Figure 3 D).
Noteworthy, aged mouse ovaries possess
rare premeiotic germ cells that can generate oocytes following transplantation
into a young host environment [65], and even further young adult donor bone
marrow infusions into female mice postpone age-related reproductive failure
[66]. In other words, some follicles may become unresponsive due to
age-associated over-stimulation but can be rejuvenated.
Thus,
reproductive aging is set in motion at puberty by an ever-increasing
hypothalamic resistance to estrogens. By increasing resistance of the hypothalamus
to estrogens, the developmental program establishes the menstrual cycle at
puberty. There is no program to cause menopause. It simply happens because
resistance to estrogens (and some other hormones) is ever-increasing. This is
an example of a quasi-program, a continuation of a program that was not
switched off upon its completion (at puberty). The quasi-program interrupts the
same reproductive function that the program establishes. The same mechanism
(resistance of the hypothalamus to estrogen) first starts and then ends
reproduction in women. An increased resistance to estrogens can explain both
initiation and termination of the menstrual cycle.
How may we explain an increased resistance to estrogens?
Resistance may be secondary to hyper-stimulation by estrogens themselves. In
fact, in old acyclic mice, ovariectomy for 2 months partially reversed the
hypothalamic resistance [41]. Hyper-stimulation of the hypothalamus by
estrogens may cause resistance, in turn increasing stimulation of the ovary,
until failure occurs. Alternatively, overstimulation of the hypothalamus with
hormones and nutrients can cause estrogen-resistance. Is there a feedback
resistance to overstimulation as shown in Figure 2? Then over-stimulation, with
secondary resistance, is the driving cause of reproductive program and
quasi-program. And most importantly over-stimulation occurs simultaneously both
in the ovary and the brain.
mTOR
and menopause
I propose that the
increasing activation of mTOR (both in the hypothalamus and the ovary) drives
hormone resistance, causing the onset of reproduction and then
hyper-stimulation of the ovary and the hypothalamus and finally menopause (Figure 4). Let us bring together several pieces of data.
First, mTOR is a regulator
of puberty onset via modulation of the hypothalamus [67]. Also, both FSH and
estrogens activate the mTOR pathway [68], [69]. So if TOR is activated
constantly, it may not respond further to stimulation (hormone resistance).
Second, in mice lacking
PTEN in oocytes, the entire primordial follicle pool is activated.
Subsequently, all primordial follicles become depleted in early adulthood,
causing premature ovarian failure [70]. PTEN loss
results in suppression of Foxo, so the Foxo was a primer suspect [70]. Yet, in
theory loss of PTEN must also result in mTOR overactivation (Figure 1). I
suggest that premature ovarian failure is
caused by over-activation of TOR. (Note: this paper was initially written in 2008
and was ahead of its time and was not well received by conventional journals.
Now it can be updated). It was shown tuberous sclerosis complex (Tsc), which
negatively regulates mTOR, functions in oocytes to maintain the quiescence of
primordial follicles. In mutant mice lacking the Tsc1 gene in oocytes, the
entire pool of primordial follicles is activated prematurely due to elevated
mTORC1 activity in the oocyte, ending up with follicular depletion in early
adulthood and causing premature ovarian failure [71,72].
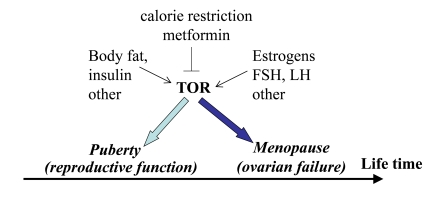
Figure 4. Program of puberty and quasi-program of menopause.
The TOR pathway in the hypothalamus and the ovary is activated by growth
factors, hormones (leptin, estrogens and FSH, LH, respectively) and nutrients.
This activation starts menarche and then leads to menopause.
Third, calorie restriction
(CR) prevents age-related increase in estrogen resistance in the hypothalamus
of old female mice [73]. As already discussed, CR de-activates TOR [74]. I
speculate that CR de-activates mTOR and delays estrogen resistance in the
hypothalamus. Simultaneously, by deactivating mTOR in the oocytes, it may delay
their depletion.
It was shown almost a century ago [75]
and then reproduced numerous times that CR extends lifespan and prevents
age-related infertility in rodents. In most of these studies, CR was initiated
at weaning, causing a delayed onset of sexual maturation. So, the same
condition (CR) delays both puberty and menopause. This is consistent with the
notion that a quasi-program (menopause) is a mere continuation of the program
(puberty). But quasi-programs can be manipulated, exactly like programs.
Recently it has been shown that a moderate caloric restriction initiated in
rodents during adulthood sustains reproductive function of the female
reproductive axis into advanced chronological age [76].
Fifth, metformin, an
antidiabetic drug, activates AMPK and thus inhibits mTOR [77]. Furthermore,
metformin inhibits mTOR in AMPK-independent manner too. Metformin restores
ovulations in patients with premature menopause associated with polycystic
ovary syndrome [56]. On the other hand, metformin delays a premature onset of
the menstrual cycle [78]. So the same agent that inhibits the onset of
reproductive function also inhibits its termination. This antagonistic
pleiotropic effect is consistent with the notion of the same mechanism
switching reproduction on and off. Metformin slowed down aging and the
age-related switch-off of estrous function in mice [79]. Thus menopause can be
delayed pharmacologically.
Conclusion
This article presents two
hypotheses. The first hypothesis explains (from both an evolutionary and
mechanistic perspective) why aging is accelerated in men. From the
evolutionary perspective, the high accidental death rate in young men
determines an accelerated aging. A model of TOR-driven aging provides a
mechanistic explanation. When the accidental death rate is high, it is
important to be bigger and stronger. And the mTOR pathway is involved in
growth and cellular hypertrophy. So, overactivated mTOR may be adaptive for
young men.
But this can accelerate
aging. At the cost of accelerated aging, over-stimulated mTOR pathway may
provide an advantage earlier in life. And vice versa as discussed, "weak mTOR"
provides disadvantage earlier in life and, vice versa, robustness and fast
aging are associated [28]. Noteworthy, "competitive, aggressive personality"
among men is associated with atherosclerosis and earlier death from age-related
coronary disease [80].
The second hypothesis
explains why menopause in women occurs despite
slow-aging. Simply, the
regulation of the menstrual cycle is fragile. There is a fine balance between
ovarian stimulation by FSH and feedback hypothalamic responsiveness to
estrogens. The menstrual cycle is vulnerable. Menopause is an example of a
quasi-program (a program that was not switched off after its completion). In
puberty, an increasing resistance to estrogen starts reproduction (a program).
A further increase in the resistance (a quasi-program) causes overactivation of
the ovary, decreasing fertility. This process can be treated pharmacologically (as any other age-related disease) to postpone menopause Potential therapeutic
interventions to postpone menopause (as well as abolishment of the harmful
consequences of menopause) will be discussed in forthcoming book The Origin
of Aging.
Conflicts of Interest
The author of this manuscript has no conflict of
interests to declare.
References
-
1.
Austad
SN
Why women live longer than men: sex differences in longevity.
Gend Med.
2006;
3:
79
-92.
[PubMed]
.
-
2.
Verbrugge
LM
and Wingard
DL.
Sex differentials in health and mortality.
Women Health.
1987;
12:
103
-145.
[PubMed]
.
-
3.
Medawar
PB
London
HK Lewis
An unsolved problem in biology.
1952;
.
-
4.
Keller
L
and Genoud
M.
Extraordinary lifespans in ants: a test of evolutionary theories of ageing.
Nature.
1997;
389:
958
-960.
.
-
5.
Gardner
MP
, Gems
D
and Viney
ME.
Extraordinary plasticity in aging in Strongyloides ratti implies a gene-regulatory mechanism of lifespan evolution.
Aging Cell.
2006;
5:
315
-323.
[PubMed]
.
-
6.
Kim
E
and Guan
KL.
RAG GTPases in nutrient-mediated TOR signaling pathway.
Cell Cycle.
2009;
8:
1014
-1018.
[PubMed]
.
-
7.
Xu
Y
, Chen
SY
, Ross
KN
and Balk
SP.
Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and post-transcriptional increases in cyclin D proteins.
Cancer Res.
2006;
66:
7783
-7792.
[PubMed]
.
-
8.
Glazer
HP
, Osipov
RM
, Clements
RT
, Sellke
FW
and Bianchi
C.
Hypercholesterolemia is associated with hyperactive cardiac mTORC1 and mTORC2 signaling.
Cell Cycle.
2009;
8:
1738
-1746.
[PubMed]
.
-
9.
Hands
SL
, Proud
CG
and Wyttenbach
A.
mTOR's role in ageing: protein synthesis or autophagy.
Aging.
2009;
586
-597.
[PubMed]
.
-
10.
Wullschleger
S
, Loewith
R
and Hall
MN.
TOR signaling in growth and metabolism.
Cell.
2006;
124:
471
-484.
[PubMed]
.
-
11.
Bodine
SC
, Stitt
TN
, Gonzalez
M
, Kline
WO
, Stover
GL
, Bauerlein
R
, Zlotchenko
E
, Scrimgeour
A
, Lawrence
JC
, Glass
DJ
and Yancopoulos
GD.
Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo.
Nat Cell Biol.
2001;
3:
1014
-1019.
[PubMed]
.
-
12.
Rommel
C
, Bodine
SC
, Clarke
BA
, Rossman
R
, Nunez
L
, Stitt
TN
, Yancopoulos
GD
and Glass
DJ.
Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways.
Nat Cell Biol.
2001;
3:
1009
-1013.
[PubMed]
.
-
13.
Skrzypek
J
and Krause
W.
Azoospermia in a renal transplant recipient during sirolimus (rapamycin) treatment.
Andrologia.
2007;
39:
198
-199.
[PubMed]
.
-
14.
Demidenko
ZN
and Blagosklonny
MV.
Growth stimulation leads to cellular senescence when the cell cycle is blocked.
Cell Cycle.
2008;
7:
3355
-3361.
[PubMed]
.
-
15.
Demidenko
ZN
, Zubova
SG
, Bukreeva
EI
, Pospelov
VA
, Pospelova
TV
and Blagosklonny
MV.
Rapamycin decelerates cellular senescence.
Cell Cycle.
2009;
8:
1888
-1895.
[PubMed]
.
-
16.
Demidenko
ZN
, Shtutman
M
and Blagosklonny
MV.
Pharmacologic inhibition of MEK and PI-3K converges on the mTOR/S6 pathway to decelerate cellular senescence.
Cell Cycle.
2009;
8:
1896
-1900.
[PubMed]
.
-
17.
Demidenko
ZN
and Blagosklonny
MV.
Quantifying pharmacologic suppression of cellular senescence: prevention of cellular hypertrophy versus preservation of proliferative potential.
Aging.
2009;
1:
1008
-1016.
[PubMed]
.
-
18.
Pospelova
TV
, Demidenk
ZN
, Bukreeva
EI
, Pospelov
VA
, Gudkov
AV
and Blagosklonny
MV.
Pseudo-DNA damage response in senescent cells.
Cell Cycle.
2009;
8:
4112
-4118.
[PubMed]
.
-
19.
Blagosklonny
MV
Cell senescence and hypermitogenic arrest.
EMBO Rep.
2003;
4:
358
-362.
[PubMed]
.
-
20.
Blagosklonny
MV
Cell senescence: hypertrophic arrest beyond restriction point.
J Cell Physiol.
2006;
209:
592
-7.
[PubMed]
.
-
21.
Blagosklonny
MV
and Hall
MN.
Growth and aging: a common molecular mechanism.
Aging.
2009;
1:
357
-362.
[PubMed]
.
-
22.
Blagosklonny
MV
Aging and immortality: quasi-programmed senescence and its pharmacologic inhibition.
Cell Cycle.
2006;
5:
2087
-2102.
[PubMed]
.
-
23.
Blagosklonny
MV
An anti-aging drug today: from senescence-promoting genes to anti-aging pill.
Drug Disc Today.
2007;
12:
218
-224.
.
-
24.
Blagosklonny
MV
Validation of anti-aging drugs by treating age-related diseases.
Aging.
2009;
1:
281
-288.
[PubMed]
.
-
25.
Harrison
DE
, Strong
R
, Sharp
ZD
, Nelson
JF
, Astle
CM
, Flurkey
K
, Nadon
NL
, Wilkinson
JE
, Frenkel
K
, Carter
CS
, Pahor
M
, Javors
MA
, Fernandezr
E
and Miller
RA.
Rapamycin fed late in life extends lifespan in genetically heterogenous mice.
Nature.
2009;
460:
392
-396.
[PubMed]
.
-
26.
Bartke
A
Insulin and aging.
Cell Cycle.
2008;
7:
3338
-3343.
[PubMed]
.
-
27.
Tsang
CK
, Qi
H
, Liu
LF
and Zheng
XFS.
Targeting mammalian target of rapamycin (mTOR) for health and diseases.
Drug Disc Today.
2007;
12:
112
-124.
.
-
28.
Blagosklonny
MV
Why human lifespan is rapidly increasing: solving "longevity riddle" with "revealed-slow-aging" hypothesis.
Aging.
2010;
2:
177
-182.
[PubMed]
.
-
29.
Perls
T
, Kunkel
L
and Puca
A.
The genetics of aging.
Curr Opin Genet Dev.
2002;
12:
362
-369.
[PubMed]
.
-
30.
Curtis
R
, Geesaman
BJ
and DiStefano
PS.
Ageing and metabolism: drug discovery opportunities.
Nat Rev Drug Discov.
2005;
4:
569
-80.
[PubMed]
.
-
31.
Giampaoli
S
Epidemiology of major age-related diseases in women compared to men.
Aging.
2000;
12:
93
-105.
[PubMed]
.
-
32.
Crimmins
EM
, Kim
JK
and Hagedorn
A.
Life with and without disease: women experience more of both.
J Women Aging.
2002;
14:
47
-59.
[PubMed]
.
-
33.
Moller
P
, Mayer
S
, Mattfeldt
T
, Muller
K
, Wiegand
P
and Bruderlein
S.
Sex-related differences in length and erosion dynamics of human telomeres favor females.
Aging.
2009;
1:
733
-739.
[PubMed]
.
-
34.
Epel
ES
, Merkin
SS
, Cawthon
R
, Blackburn
EH
, Adler
NE
, Pletcher
MJ
and Seeman
TE.
The rate of leukocyte telomere shortening predicts mortality from cardiovascular disease in elderly men.
Aging.
2009;
1:
81
-88.
[PubMed]
.
-
35.
Bulpitt
CJ
, Markowe
HL
and Shipley
MJ.
Why do some people look older than they should.
Postgrad Med J.
2001;
77:
578
-581.
[PubMed]
.
-
36.
Shanley
DP
and Kirkwood
TB.
Evolution of the human menopause.
Bioessays.
2001;
23:
282
-287.
[PubMed]
.
-
37.
Shanley
DP
, Sear
R
, Mace
R
and Kirkwood
TB.
Testing evolutionary theories of menopause.
Proc Biol Sci.
2007;
274:
2943
-2949.
[PubMed]
.
-
38.
Kirkwood
TB
Understanding ageing from an evolutionary perspective.
J Intern Med.
2008;
263:
117
-127.
[PubMed]
.
-
39.
Packer
C
, Tatar
M
and Collins
A.
Reproductive cessation in female mammals.
Nature.
1998;
392:
807
-811.
[PubMed]
.
-
40.
Gee
DM
, Flurkey
K
and Finch
CE.
Aging and the regulation of luteinizing hormone in C57BL/6J mice: impaired elevations after ovariectomy and spontaneous elevations at advanced ages.
Biol Reprod.
1983;
28:
598
-607.
[PubMed]
.
-
41.
Mobbs
CV
, Gee
DM
and Finch
CE.
Reproductive senescence in female C57BL/6J mice: ovarian impairments and neuroendocrine impairments that are partially reversible and delayable by ovariectomy.
Endocrinology.
1984;
115:
1653
-1662.
[PubMed]
.
-
42.
Horiuchi
S
Postmenopausal acceleration of age-related mortality increase.
J Gerontol A Biol Sci Med Sci.
1997;
52:
B78
-92.
[PubMed]
.
-
43.
Krum
SA
and Brown
M.
Unraveling estrogen action in osteoporosis.
Cell Cycle.
2008;
7:
1348
-1352.
[PubMed]
.
-
44.
Blagosklonny
MV
Program-like aging and mitochondria: instead of random damage by free radicals.
J Cell Biochem.
2007;
102:
1389
-1399.
[PubMed]
.
-
45.
Blagosklonny
MV
TOR-driven aging: speeding car without brakes.
Cell Cycle.
2009;
8:
4055
-4059.
[PubMed]
.
-
46.
Blagosklonny
MV
Rapamycin and quasi-programmed aging: four years later.
Cell Cycle.
2010;
9:
.
-
47.
Gangloff
YG
, Mueller
M
, Dann
SG
, Svoboda
P
, Sticker
M
, Spetz
JF
, Um
SH
, Brown
EJ
, Cereghini
S
, Thomas
G
and Kozma
SC.
Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development.
Mol Cell Biol.
2004;
24:
9508
-2516.
[PubMed]
.
-
48.
Murakami
M
, Ichisaka
T
, Maeda
M
, Oshiro
N
, Hara
K
, Edenhofer
F
, Kiyama
H
, Yonezawa
K
and Yamanaka
S.
mTOR is essential for growth and proliferation in early mouse embryos and embryonic stem cells.
Mol Cell Biol.
2004;
24:
6710
-6718.
[PubMed]
.
-
49.
Shah
OJ
, Wang
Z
and Hunter
T.
Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies.
Curr Biol.
2004;
14:
1650
-1656.
[PubMed]
.
-
50.
Tremblay
F
and Marette
A.
Amino acid and insulin signaling via the mTOR/p70 S6 kinase pathway. A negative feedback mechanism leading to insulin resistance in skeletal muscle cells.
J Biol Chem.
2001;
276:
38052
-38060.
[PubMed]
.
-
51.
Liu
Z
, Wu
Y
, Nicklas
EW
, Jahn
LA
, Price
WJ
and Barrett
EJ.
Unlike insulin, amino acids stimulate p70S6K but not GSK-3 or glycogen synthase in human skeletal muscle.
Am J Physiol Endocrinol Metab.
2004;
286:
E523
-528.
[PubMed]
.
-
52.
Tremblay
F
, Krebs
M
, Dombrowski
L
, Brehm
A
, Bernroider
E
, Roth
E
, Nowotny
P
, Waldhäusl
W
, Marette
A
and Roden
M.
Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability.
Diabetes.
2005;
54:
2674
-2684.
[PubMed]
.
-
53.
Krebs
M
, Brunmair
B
, Brehm
A
, Artwohl
M
, Szendroedi
J
, Nowotny
P
, Roth
E
, Fürnsinn
C
, Promintzer
M
, Anderwald
C
, Bischof
M
and Roden
M.
The Mammalian target of rapamycin pathway regulates nutrient-sensitive glucose uptake in man.
Diabetes.
2007;
56:
1600
-1607.
[PubMed]
.
-
54.
Khamzina
L
, Veilleux
A
, Bergeron
S
and Marette
A.
Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance.
Endocrinology.
2005;
146:
1473
-1481.
[PubMed]
.
-
55.
Briaud
I
, Dickson
LM
, Lingohr
MK
, McCuaig
JF
, Lawrence
JC
and Rhodes
CJ.
Insulin receptor substrate-2 proteasomal degradation mediated by a mammalian target of rapamycin (mTOR)-induced negative feedback down-regulates protein kinase B-mediated signaling pathway in beta-cells.
J Biol Chem.
2005;
280:
2282
-2293.
[PubMed]
.
-
56.
Cheang
KI
, Sharma
ST
and Nestler
JE.
Is metformin a primary ovulatory agent in patients with polycystic ovary syndrome.
Gynecol Endocrinol.
2006;
22:
595
-604.
[PubMed]
.
-
57.
Wu
FC
, Tajar
A
, Pye
SR
, Silman
AJ
, Finn
JD
, O'Neill
TW
, Bartfai
G
, Casanueva
F
, Forti
G
, Giwercman
A
, Huhtaniemi
IT
, Kula
K
, Punab
M
, Boonen
S
and Vanderschueren
DEMASG.
Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study.
J Clin Endocrinol Metab.
2008;
93:
2737
-2745.
[PubMed]
.
-
58.
Veldhuis
JD
, Keenan
DM
, Liu
PY
, Iranmanesh
A
, Takahashi
PY
and Nehra
AX.
The aging male hypothalamic-pituitary-gonadal axis: Pulsatility and feedback.
Mol Cell Endocrinol.
2009;
299:
14
-22.
[PubMed]
.
-
59.
Dilman
VM
Age-associated elevation of hypothalamic, threshold to feedback control, and its role in development, ageine, and disease.
Lancet.
1971;
1:
1211
-1219.
[PubMed]
.
-
60.
Dilman
VM
and Anisimov
VN.
Hypothalmic mechanisms of ageing and of specific age pathology--I. Sensitivity threshold of hypothalamo-pituitary complex to homeostatic stimuli in the reproductive system.
Exp Gerontol.
1979;
14:
161
-174.
[PubMed]
.
-
61.
Weiss
G
, Skurnick
JH
, Goldsmith
LT
, Santoro
NF
and Park
SJ.
Menopause and hypothalamic-pituitary sensitivity to estrogen.
JAMA.
2004;
292:
2991
-2996.
[PubMed]
.
-
62.
Sherman
BM
, West
JH
and Korenman
SG.
The menopausal transition: analysis of LH, FSH, estradiol, and progesterone concentrations during menstrual cycles of older women.
J Clin Endocrinol Metab.
1976;
42:
629
-636.
[PubMed]
.
-
63.
Beemsterboer
SN
, Homburg
R
, Gorter
NA
, Schats
R
, Hompes
PG
and Lambalk
CB.
The paradox of declining fertility but increasing twinning rates with advancing maternal age.
Hum Reprod.
2006;
21:
1531
-1532.
[PubMed]
.
-
64.
Prior
JC
Ovarian aging and the perimenopausal transition: the paradox of endogenous ovarian hyperstimulation.
Endocrine.
2005;
26:
297
-300.
[PubMed]
.
-
65.
Niikura
Y
, Niikura
T
and Tilly
JL.
Aged mouse ovaries possess rare premeiotic germ cells that can generate oocytes following transplantation into a young host environment.
Aging.
2009;
1:
971
-978.
[PubMed]
.
-
66.
Selesniemi
K
, Lee
HJ
, Niikura
T
and Tilly
JL.
Young adult donor bone marrow infusions into female mice postpone age-related reproductive failure and improve offspring survival.
Aging.
2009;
1:
49
-57.
[PubMed]
.
-
67.
Roa
J
, Garcia-Galiano
D
, Varela
L
, Sanchez-Garrido
MA
, Pineda
R
, Castellano
JM
, Ruiz-Pino
F
, Romero
M
, Aguilar
E
, Lopez
M
, Gaytan
F
, Dieguez
C
, Pinilla
L
and Tena-Sempere
M.
The mammalian target of rapamycin as novel central regulator of puberty onset via modulation of hypothalamic Kiss1 system.
Endocrinology.
2009;
150:
5016
-5026.
[PubMed]
.
-
68.
Kayampilly
PP
and Menon
KM.
Follicle-stimulating hormone increases tuberin phosphorylation and mammalian target of rapamycin signaling through an extracellular signal-regulated kinase-dependent pathway in rat granulosa cells.
Endocrinology.
2007;
148:
3950
-3957.
[PubMed]
.
-
69.
Yu
J
and Henske
EP.
Estrogen-induced activation of mammalian target of rapamycin is mediated via tuberin and the small GTPase Ras homologue enriched in brain.
Cancer Res.
2006;
66:
9461
-9466.
[PubMed]
.
-
70.
Reddy
P
, Liu
L
, Adhikari
D
, Jagarlamudi
K
, Rajareddy
S
, Shen
Y
, Du
C
, Tang
W
, Hämäläinen
T
, Peng
SL
, Lan
ZJ
, Cooney
AJ
, Huhtaniemi
I
and Liu
K.
Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool.
Science.
2008;
319:
611
-613.
[PubMed]
.
-
71.
Adhikari
D
, Flohr
G
, Gorre
N
, Shen
Y
, Yang
H
, Lundin
E
, Lan
Z
, Gambello
MJ
and Liu
K.
Disruption of Tsc2 in oocytes leads to overactivation of the entire pool of primordial follicles.
Mol Hum Reprod.
2009;
15:
765
-770.
[PubMed]
.
-
72.
Adhikari
D
, Zheng
W
, Shen
Y
, Gorre
N
, Hamalainen
T
, Cooney
AJ
, Huhtaniemi
I
, Lan
ZJ
and Liu
K.
Tsc/mTORC1 signaling in oocytes governs the quiescence and activation of primordial follicles.
Hum Mol Genet.
2010;
19:
397
-410.
[PubMed]
.
-
73.
Yaghmaie
F
, Saeed
O
, Garan
SA
, Freitag
W
, Timiras
PS
and Sternberg
H.
Caloric restriction reduces cell loss and maintains estrogen receptor-alpha immunoreactivity in the pre-optic hypothalamus of female B6D2F1 mice.
Neuro Endocrinol Lett.
2005;
26:
197
-203.
[PubMed]
.
-
74.
Blagosklonny
MV
Calorie restriction: decelerating mTOR-driven aging from cells to organisms (including humans).
Cell Cycle.
2010;
9:
683
-688.
[PubMed]
.
-
75.
Osborne
TB
, Mendel
LB
and Ferry
EL.
The effect of retardation of growth upon the breeding period and duration of life of rats.
Science.
1917;
45:
294
-295.
[PubMed]
.
-
76.
Selesniemi
K
, Lee
H-J
and Tilly
J.
Moderate caloric restriction initiated in rodents during adulthood sustains function of the female reproductive axis into advanced chronological age.
Aging Cell.
2008;
7:
622
-9.
[PubMed]
.
-
77.
Shaw
RJ
, Lamia
KA
, Vasquez
D
, Koo
SH
, Bardeesy
N
, Depinho
RA
, Montminy
M
and Cantley
LC.
The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin.
Science.
2005;
310:
1642
-1646.
[PubMed]
.
-
78.
Palomba
S
, Orio
FJ
, Falbo
A
, Russo
T
, Tolino
A
and Zullo
F.
Clomiphene citrate versus metformin as first-line approach for the treatment of anovulation in infertile patients with polycystic ovary syndrome.
J Clin Endocrinol Metab.
2007;
92:
3498
-3503.
[PubMed]
.
-
79.
Anisimov
VN
, Berstein
LM
, Egormin
PA
, Piskunova
TS
, Popovich
IG
, Zabezhinski
MA
, Tyndyk
ML
, Yurova
MV
, Kovalenko
IG
, Poroshina
TE
and Semenchenko
AV.
Metformin slows down aging and extends life span of female SHR mice.
Cell Cycle.
2008;
7:
2769
-2773.
[PubMed]
.
-
80.
Waldron
I
and Johnston
S.
Why do women live longer than men.
J Human Stress.
1976;
2:
19
-30.
.