Sestrins at the crossroad between stress and aging
Abstract
Sestrins are a family of stress-inducible proteins that can function as antioxidants and as inhibitors of target of rapamycin complex 1. In this research perspective, we discuss the possible roles of Sestrins in diverse stress-induced patho-physiological contexts that can result in premature aging and age-related diseases. We suggest that Sestrins provide critical feedback regulation that adjust metabolic and stress responses to different environmental cues and evolutionary constraints.
The
discovery of Sestrins as p53 targets [1,2] suggested that these proteins are
stress-inducible because they protect cells against various insults [2].
Sestrins have dual biochemical functions, as antioxidants that control the
activity of peroxiredoxins which scavenge reactive oxygen species (ROS) [3] and
as inhibitors of target of rapamycin complex 1 (TORC1) signaling [4,5]. Both
ROS accumulation [6] and TORC1 activation [7,8] are associated with accelerated
aging and development of age-associated pathologies in diverse organs and
organisms, implicating Sestrins as anti-aging agents. Conversely, reduction of
ROS accumulation with antioxidants or as a result of TORC1 inhibition [7-12]
causes an extension of life span as well as health span. Indeed, we recently
confirmed Drosophila Sestrin (dSesn) prevents age-associated pathologies
including fat accumulation and cardiac and skeletal muscle degeneration by
providing a feedback loop that prevents excessive TORC1 activation and ROS
accumulation [4]. In this research perspective, we discuss the possible roles
of Sestrins in diverse stress-induced patho-physiological contexts that can
result in premature aging and age-related diseases. We suggest that Sestrins
provide critical feedback regulation that adjust metabolic
and stress responses to different environmental cues and evolutionary
constraints.
DNA
damage
Chronic
exposure to genotoxic stress is known to accelerate aging, and mutations that
disrupt proper DNA damage responses and interfere with DNA damage repair are
associated with premature aging in humans [13]. Many studies have established
that genotoxic stresses can inhibit protein and lipid synthesis, and that these
coordinated responses may be essential for survival because reducing energy
expenditure on macromolecule biosynthesis can divert scarce resources to the
repair of damaged DNA [14]. Sestrins, as DNA damage-inducible proteins, may
play a critical role in this process [15]. Both mammalian Sesn1 and Sesn2 are
induced upon DNA damage in response to activation of p53 [1,2], and dSesn is
also induced upon radiation-induced DNA damage (Figure 1). Increased Sestrin
abundance potentiates the activity of AMP-activated protein kinase (AMPK),
thereby diminishing TORC1 activity [5]. Reduced TORC1 activity inhibits
anabolic pathway including protein and lipid
synthesis [16,17]. Shutdown of TORC1-dependent anabolism upon genotoxic stress
is likely to be important for minimizing new protein and membrane synthesis and
use the energy that was thus saved to promote DNA repair. Therefore, DNA
damage-dependent induction of Sestrins may minimize the detrimental effects of
DNA damage that contribute to accelerated aging and various pathologies that
are associated with premature aging.
Age-dependent
accumulation of DNA damage can lead to cancer [13], one of the leading causes
of mortality worldwide [18]. Therefore, Sestrin induction in response to DNA
damage [1,2] may contribute to the many tumor suppressor functions carried out
by p53 [19]. In addition to inhibiting cell proliferation and promoting the
death of cells with excessive DNA damage, p53 was recently found to inhibit
TORC1 [14] and to suppress cell growth as well as cellular and organismal
senescence [19-21]. We found that Sesn1 and/or Sesn2 are critical mediators of
p53-induced TORC1 inhibition in cultured cells and in mouse liver [5]. In
addition, Sestrins can suppress the growth of some cancer cell lines [2] and
loss of Sesn2 makes immortalized cells more susceptible to oncogenic
transformation [5]. The SESN1 (6q21) and SESN2 (1p35) loci are frequently
deleted in a variety of human cancers [1,22,23], implicating loss of Sestrins
in tumor progression and suggesting that Sestrin-dependent inhibition of TORC1
is critical for suppressing tumorigenesis spurred by age-dependent accumulation
of damaged DNA.

Figure 1. Induction of dSesn after DNA damage. First instar
fly larvae were challenged with 4000 rads (R) of gamma radiation, and RNA
was extracted after 4 hrs. Northern blot analysis revealed that dSesn
mRNA is highly induced upon irradiation. rp49 mRNA was used as a
loading control.
Oxidative
stress
Oxidative
stress not only can interfere with the proper flow of genomic information by
oxidizing DNA and RNA, but also can damage other macromolecules such as
proteins and lipids [6]. Accumulation of oxidative macromolecular damage causes
cellular senescence, tissue aging and reduced life span [6], as well as
neurodegeneration [24] and metabolic disorders [25], which are diseases
associated with aging. Amongst the organelles that are affected by oxidative
stress, mitochondria appear to be the most sensitive [6,26]. Moreover, damaged
mitochondria are a major source of ROS [6], which escalates oxidative damage in
stressed cells. Extensive mitochondrial dysfunction causes cell death, and in
some cases can lead to neuronal or muscular degeneration [6,24,27]. To prevent
the detrimental consequences of mitochondrial dysfunction, cells eliminate
damaged mitochondria through an autophagic process, called mitophagy [28,29].
Sestrins
are transcriptionally induced upon oxidative stress [2], and are important for
cell survival under oxidative stress [2,3,30,31]. Sestrins can function as
oxidoreductases in vitro and in vivo that lead to the reactivation of
peroxiredoxin [3], and may be involved in reducing oxidative stress [30-32] by
scavenging ROS and/or regenerating reduced peroxiredoxin [3]. Independent of
their oxidoreductase activity, Sestrins induce autophagy by inhibition of TORC1
[5,33,34]. Enhanced autophagy results in more efficient elimination of
ROS-producing damaged mitochondria in stressed cells [28,29]. Sestrin-induced
activation of AMPK and inhibition of TORC1 can also reduce ROS production by
increasing the efficiency of mitochondrial respiration [11,12]. Therefore,
Sestrins have a key role in maintaining cellular integrity and homeostasis
during oxidative insults.
Hypoxia
Hypoxia
is another environmental stimulus that can induce Sestrin gene transcription
[2]. Sestrins protect cells from apoptosis during hypoxic conditions [2], and
Sestrin-induced shutdown of TORC1 signaling can reduce cellular energy
consumption that is likely to improve adaptation to hypoxic conditions.
Sestrin-stimulated autophagy can provide an additional energy source and at the
same time eliminate dysfunctional mitochondria generated by inefficient
respiration under low oxygen tension.
Ischemic
injury to heart muscles and neurons, which is caused by hypoxia, is one of the
major causes of death in elderly individuals [18]. In an experimental model of
acute stroke, Sesn2 was shown to be highly induced upon hypoxic injury [2],
suggesting that Sesn2 may exert its neuroprotective role during stroke. In the
heart, hypoxic injury and re-oxygenation cause bursts of ROS production, which
can cause irreversible damage to the heart muscle, resulting in cardiac
arrhythmia and heart failure [35,36]. In the Drosophila heart, both aging
[37-41] and hypoxia [39,42,43] cause cardiac dysfunction, and activation of
TORC1 pathway aggravates or accelerates age-associated arrhythmicity and heart
failure [44]. Loss of dSesn function results in a similar cardiac arrhythmicity
[4], suggesting a cardio-protective function of Sestrin in restraining TORC1
activity. Thus Sestrin expression retards the appearance of age-associated
cardiac pathologies, as was previously observed in response to genetic
reduction of TORC1 function [10,44].
Hypoxic
preconditioning can protect both heart and neuronal cells from severe ischemic
injury-induced cellular damage [36,45], but the underlying mechanisms were not
elucidated. Induction of autophagy upon preconditioning was suggested to be
required for protection of heart and neuronal cells from hypoxic insults
[36,46]. An intriguing possibility to investigate therefore is whether hypoxic
preconditioning induces Sestrin
to increase the level of autophagy that is required for the prevention of serious heart
attacks and neurological strokes.
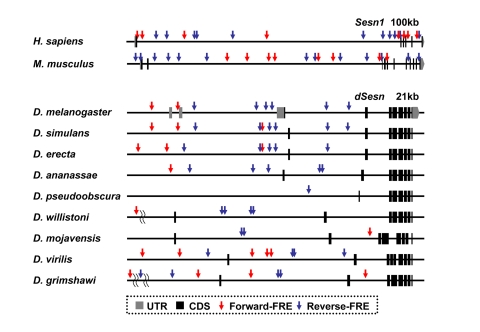
Figure 2. FoxO binding sites in the Sestrin locus vary among the species. Genomic
organization and location of FoxO response elements (FRE, GTAAACAA [57]) in the Sesn1
locus of human and mouse [58] and the dSesn
locus of various Drosophila species [59,60] with indicated
genomic span. Gray boxes indicate untranslated exons (UTR), and black boxes
indicate coding exons (CDS). For Drosophila species other than D.
melanogaster, untranslated regions of dSesn mRNAs are currently
unknown. Forward FREs are indicated by red arrows and reverse FREs by blue
arrows.
An
evolvable link to the environment
In
addition to the important role that Sestrins play in mediating essential
environmental inputs into metabolic regulation, these molecules may also play
central roles in responding to other environmental cues such as nutrient
supply, hydration status, temperature, chemical damage, and reproductive
signals. One might speculate that because these various cues would have
different impacts on different organisms, Sestrin genes should have evolved
complex cis-regulatory regions to place them under distinct regulatory controls
that vary from one organism to another. Indeed, our analysis of Sestrin genomic
loci revealed that these sequences are rapidly evolving among the various
Drosophila species. For example, the
disposition and number of FoxO binding sites in the Sestrin locus vary
significantly among species (Figure 2). Comparative studies of cis-regulatory
sequences between Drosophila species by swapping these sequences using
recombineering techniques may shed light on mechanisms by which selective
pressures sculpt the stress response during evolution.
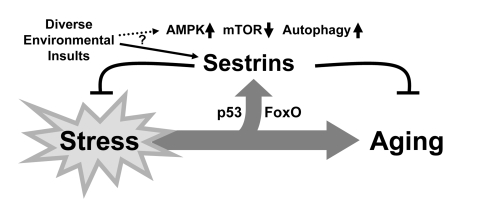
Figure 3. Schematic diagram hypothesizing the role of Sestrin as a brake of stress-accelerated aging processes. Various environmental insults increase
expression of Sestrins to affect Sestrin-mediated regulation of AMPK-TORC1
signaling.
Concluding
remarks
In
this research perspective, we briefly reviewed how Sestrins may act as
suppressors of aging that are responsive to stressful stimuli and insults that
can accelerate the aging process. By activating AMPK and inhibiting TORC1,
Sestrins can reprogram cells to adapt to stressful conditions by attenuating
anabolism and enhancing autophagic catabolism. Sestrins can suppress oxidative
damage by acting as either antioxidants, inducers of autophagy that eliminate
ROS-producing dysfunctioning mitochondria, or suppressors of ROS-producing
mitochondrial metabolism. Therefore, we can view Sestrins as physiological brakes
that can attenuate stress-dependent acceleration of aging (Figure 3).
In
addition, it is worth noting that Sestrins are also expressed under normal
unstressed conditions [1,2,4], and that dSesn knockout mutants show accelerated
aging phenotypes even in the absence of any environmental stress [4].
Therefore, it is possible that Sestrins provide a baseline protective function
that reduces the damage from physiological insults that are unavoidable
consequences of basic life processes such as oxidative respiration and DNA
replication.
Over-nutrition
and obesity can elevate the incidence and frequency of physiological insults by
stimulating TORC1 activity [47], which in turn accelerates anabolic metabolism
[16,17]. Hyperactive TORC1 can enhance the accumulation of unfolded and
aggregated proteins [48] and ROS [4,12,49], leading to stress responses that
increase Sestrin expression, thereby activating negative feedback
loops that readjust AMPK and TORC1 activity [4]. Therefore, Sestrins may also
function as metabolic brakes that attenuate the pathological consequences of
over-nutrition and its associated TORC1 hyperactivity [50]. An interesting
human evolutionary question in this regard is whether regulation of Sestrin
induction has been weakened in populations subject to frequent starvation
conditions since such populations have been shown to be particularly at risk for
obesity, presumably as a result of their altered response to nutrient cues
[51,52].
Although
environmental stress generally accelerates aging, it should be considered that
exposure to a low level of stress or stress adaptation can actually be
beneficial for the organism, increasing life span and preventing age-associated
degenerative diseases [53-56]. The beneficial effect of low-level stress
exposure, referred to as hormesis, was observed in both experimental animal
models and human subjects [53-56], but no decisive molecular explanation has
been provided to explain this paradoxical phenomenon. Given that Sestrins are
upregulated in response to a variety of stresses [1,2], it will be interesting
to investigate whether stress-induced Sestrins are also mediators of the
hormetic effect.
In
summary, we suggest that Sestrins are uniquely poised at the crossroads between
stress and aging to adjust the metabolic timbre of an organism to meet its
needs under normal conditions and to respond to predictable forms of
environmental stress. Future experiments should shed light on the specific
mechanisms by which the various effector functions of the Sestrins are
achieved.
Acknowledgments
The authors thank Charles L. Sanders and Myungjin Kim
for their encouragement and intuitive suggestions. Work was supported by grants
from the National Institutes of Health and the Superfund Research Program
(CA118165, ES006376 and P42-ES010337 to M.K., and NS29870 and AI070654 to
E.B.), National Science Foundation (IOS-0744662 to E.B.), Korea Research
Foundation (KRF-2007-357-C00096 to J.H.L.), and Human Frontier Science Program
Organzation (LT00653/2008-L to J.H.L). M.K. is an American Cancer Society
Professor.
Conflicts of Interest
The authors of this manuscript have no conflict of
interests to declare.
References
-
1.
Velasco-Miguel
S
, Buckbinder
L
, Jean
P
, Gelbert
L
, Talbott
R
, Laidlaw
J
, Seizinger
B
and Kley
N.
PA26, a novel target of the p53 tumor suppressor and member of the GADD family of DNA damage and growth arrest inducible genes.
Oncogene.
1999;
18:
127
-137.
[PubMed]
.
-
2.
Budanov
AV
, Shoshani
T
, Faerman
A
, Zelin
E
, Kamer
I
, Kalinski
H
, Gorodin
S
, Fishman
A
, Chajut
A
, Einat
P
, Skaliter
R
, Gudkov
AV
and Chumakov
PM.
Identification of a novel stress-responsive gene Hi95 involved in regulation of cell viability.
Oncogene.
2002;
21:
6017
-6031.
[PubMed]
.
-
3.
Budanov
AV
, Sablina
AA
, Feinstein
E
, Koonin
EV
and Chumakov
PM.
Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD.
Science.
2004;
304:
596
-600.
[PubMed]
.
-
4.
Lee
JH
, Budanov
AV
, Park
EJ
, Birse
R
, Kim
TE
, Perkins
GA
, Ocorr
K
, Ellisman
MH
, Bodmer
R
, Bier
E
and Karin
M.
Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies.
Science.
2010;
327:
1223
-1228.
[PubMed]
.
-
5.
Budanov
AV
and Karin
M.
p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling.
Cell.
2008;
134:
451
-460.
[PubMed]
.
-
6.
Finkel
T
and Holbrook
NJ.
Oxidants, oxidative stress and the biology of ageing.
Nature.
2000;
408:
239
-247.
[PubMed]
.
-
7.
Stanfel
MN
, Shamieh
LS
, Kaeberlein
M
and Kennedy
BK.
The TOR pathway comes of age.
Biochim Biophys Acta.
2009;
1790:
1067
-1074.
[PubMed]
.
-
8.
Kapahi
P
and Zid
B.
TOR pathway: linking nutrient sensing to life span.
Sci Aging Knowledge Environ.
2004;
2004:
PE34
[PubMed]
.
-
9.
Landis
GN
and Tower
J.
Superoxide dismutase evolution and life span regulation.
Mech Ageing Dev.
2005;
126:
365
-379.
[PubMed]
.
-
10.
Luong
N
, Davies
CR
, Wessells
RJ
, Graham
SM
, King
MT
, Veech
R
, Bodmer
R
and Oldham
SM.
Activated FOXO-mediated insulin resistance is blocked by reduction of TOR activity.
Cell Metab.
2006;
4:
133
-142.
[PubMed]
.
-
11.
Zid
BM
, Rogers
A
, Katewa
SD
, Au
Lu T
, Benzer
S
and Kapahi
P.
4E-BP modulates lifespan and mitochondrial translation upon dietary restriction in Drosophila.
Cell.
2009;
139:
149
-160.
[PubMed]
.
-
12.
Bonawitz
ND
, Chatenay-Lapointe
M
, Pan
Y
and Shadel
GS.
Reduced TOR signaling extends chronological life span via increased respiration and upregulation of mitochondrial gene expression.
Cell Metab.
2007;
5:
265
-277.
[PubMed]
.
-
13.
Hoeijmakers
JH
DNA damage, aging, and cancer.
N Engl J Med.
2009;
361:
1475
-1485.
[PubMed]
.
-
14.
Levine
AJ
, Feng
Z
, Mak
TW
, You
H
and Jin
S.
Coordination and communication between the p53 and IGF-1-AKT-TOR signal transduction pathways.
Genes Dev.
2006;
20:
267
-275.
[PubMed]
.
-
15.
Hay
N
p53 strikes mTORC1 by employing sestrins.
Cell Metab.
2008;
8:
184
-185.
[PubMed]
.
-
16.
Hay
N
and Sonenberg
N.
Upstream and downstream of mTOR.
Genes Dev.
2004;
18:
1926
-1945.
[PubMed]
.
-
17.
Laplante
M
and Sabatini
DM.
An emerging role of mTOR in lipid biosynthesis.
Curr Biol.
2009;
19:
R1046
-1052.
[PubMed]
.
-
18.
World
Health Organization
Geneva
World Health Organization
The world health report 2008: primary health care now more than ever.
2008;
.
-
19.
Vousden
KH
and Prives
C.
Blinded by the Light: The Growing Complexity of p53.
Cell.
2009;
137:
413
-431.
[PubMed]
.
-
20.
Demidenko
ZN
, Korotchkina
LG
, Gudkov
AV
and Blagosklonny
MV.
Paradoxical suppression of cellular senescence by p53.
Proc Natl Acad Sci U S A.
2010;
107:
9660
-9664.
[PubMed]
.
-
21.
Matheu
A
, Maraver
A
, Klatt
P
, Flores
I
, Garcia-Cao
I
, Borras
C
, Flores
JM
, Vina
J
, Blasco
MA
and Serrano
M.
Delayed ageing through damage protection by the Arf/p53 pathway.
Nature.
2007;
448:
375
-379.
[PubMed]
.
-
22.
Schwab
M
, Praml
C
and Amler
LC.
Genomic instability in 1p and human malignancies.
Genes Chromosomes Cancer.
1996;
16:
211
-229.
[PubMed]
.
-
23.
Ragnarsson
G
, Eiriksdottir
G
, Johannsdottir
JT
, Jonasson
JG
, Egilsson
V
and Ingvarsson
S.
Loss of heterozygosity at chromosome 1p in different solid human tumours: association with survival.
Br J Cancer.
1999;
79:
1468
-1474.
[PubMed]
.
-
24.
Emerit
J
, Edeas
M
and Bricaire
F.
Neurodegenerative diseases and oxidative stress.
Biomed Pharmacother.
2004;
58:
39
-46.
[PubMed]
.
-
25.
Roberts
CK
and Sindhu
KK.
Oxidative stress and metabolic syndrome.
Life Sci.
2009;
84:
705
-712.
[PubMed]
.
-
26.
Hyslop
PA
, Hinshaw
DB
, Halsey
WA Jr
, Schraufstatter
IU
, Sauerheber
RD
, Spragg
RG
, Jackson
JH
and Cochrane
CG.
Mechanisms of oxidant-mediated cell injury. The glycolytic and mitochondrial pathways of ADP phosphorylation are major intracellular targets inactivated by hydrogen peroxide.
J Biol Chem.
1988;
263:
1665
-1675.
[PubMed]
.
-
27.
Green
DR
and Kroemer
G.
The pathophysiology of mitochondrial cell death.
Science.
2004;
305:
626
-629.
[PubMed]
.
-
28.
Jin
S
Autophagy, mitochondrial quality control, and oncogenesis.
Autophagy.
2006;
2:
80
-84.
[PubMed]
.
-
29.
Yen
WL
and Klionsky
DJ.
How to live long and prosper: autophagy, mitochondria, and aging.
Physiology (Bethesda).
2008;
23:
248
-262.
[PubMed]
.
-
30.
Papadia
S
, Soriano
FX
, Leveille
F
, Martel
MA
, Dakin
KA
, Hansen
HH
, Kaindl
A
, Sifringer
M
, Fowler
J
, Stefovska
V
, McKenzie
G
, Craigon
M
and Corriveau
R.
Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses.
Nat Neurosci.
2008;
11:
476
-487.
[PubMed]
.
-
31.
Lipton
SA
NMDA receptor activity regulates transcription of antioxidant pathways.
Nat Neurosci.
2008;
11:
381
-382.
[PubMed]
.
-
32.
Nogueira
V
, Park
Y
, Chen
CC
, Xu
PZ
, Chen
ML
, Tonic
I
, Unterman
T
and Hay
N.
Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis.
Cancer Cell.
2008;
14:
458
-470.
[PubMed]
.
-
33.
Maiuri
MC
, Malik
SA
, Morselli
E
, Kepp
O
, Criollo
A
, Mouchel
PL
, Carnuccio
R
and Kroemer
G.
Stimulation of autophagy by the p53 target gene Sestrin2.
Cell Cycle.
2009;
8:
1571
-1576.
[PubMed]
.
-
34.
Chan
EY
mTORC1 phosphorylates the ULK1-mAtg13-FIP200 autophagy regulatory complex.
Sci Signal.
2009;
2:
pe51
[PubMed]
.
-
35.
Giordano
FJ
Oxygen, oxidative stress, hypoxia, and heart failure.
J Clin Invest.
2005;
115:
500
-508.
[PubMed]
.
-
36.
Gottlieb
RA
and Mentzer
RM.
Autophagy during cardiac stress: joys and frustrations of autophagy.
Annu Rev Physiol.
2010;
72:
45
-59.
[PubMed]
.
-
37.
Taghli-Lamallem
O
, Akasaka
T
, Hogg
G
, Nudel
U
, Yaffe
D
, Chamberlain
JS
, Ocorr
K
and Bodmer
R.
Dystrophin deficiency in Drosophila reduces lifespan and causes a dilated cardiomyopathy phenotype.
Aging Cell.
2008;
7:
237
-249.
[PubMed]
.
-
38.
Wessells
RJ
, Fitzgerald
E
, Cypser
JR
, Tatar
M
and Bodmer
R.
Insulin regulation of heart function in aging fruit flies.
Nat Genet.
2004;
36:
1275
-1281.
[PubMed]
.
-
39.
Ocorr
K
, Akasaka
T
and Bodmer
R.
Age-related cardiac disease model of Drosophila.
Mech Ageing Dev.
2007;
128:
112
-116.
[PubMed]
.
-
40.
Ocorr
K
, Reeves
NL
, Wessells
RJ
, Fink
M
, Chen
HS
, Akasaka
T
, Yasuda
S
, Metzger
JM
, Giles
W
, Posakony
JW
and Bodmer
R.
KCNQ potassium channel mutations cause cardiac arrhythmias in Drosophila that mimic the effects of aging.
Proc Natl Acad Sci U S A.
2007;
104:
3943
-3948.
[PubMed]
.
-
41.
Cammarato
A
, Dambacher
CM
, Knowles
AF
, Kronert
WA
, Bodmer
R
, Ocorr
K
and Bernstein
SI.
Myosin transducer mutations differentially affect motor function, myofibril structure, and the performance of skeletal and cardiac muscles.
Mol Biol Cell.
2008;
19:
553
-562.
[PubMed]
.
-
42.
Feala
JD
, Omens
JH
, Paternostro
G
and McCulloch
AD.
Discovering regulators of the Drosophila cardiac hypoxia response using automated phenotyping technology.
Ann N Y Acad Sci.
2008;
1123:
169
-177.
[PubMed]
.
-
43.
Akasaka
T
, Klinedinst
S
, Ocorr
K
, Bustamante
EL
, Kim
SK
and Bodmer
R.
The ATP-sensitive potassium (KATP) channel-encoded dSUR gene is required for Drosophila heart function and is regulated by tinman.
Proc Natl Acad Sci U S A.
2006;
103:
11999
-12004.
[PubMed]
.
-
44.
Wessells
R
, Fitzgerald
E
, Piazza
N
, Ocorr
K
, Morley
S
, Davies
C
, Lim
HY
, Elmen
L
, Hayes
M
, Oldham
S
and Bodmer
R.
d4eBP acts downstream of both dTOR and dFoxo to modulate cardiac functional aging in Drosophila.
Aging Cell.
2009;
8:
542
-552.
[PubMed]
.
-
45.
Gidday
JM
, Fitzgibbons
JC
, Shah
AR
and Park
TS.
Neuroprotection from ischemic brain injury by hypoxic preconditioning in the neonatal rat.
Neurosci Lett.
1994;
168:
221
-224.
[PubMed]
.
-
46.
Park
HK
, Chu
K
, Jung
KH
, Lee
ST
, Bahn
JJ
, Kim
M
, Lee
SK
and Roh
JK.
Autophagy is involved in the ischemic preconditioning.
Neurosci Lett.
2009;
451:
16
-19.
[PubMed]
.
-
47.
Dann
SG
, Selvaraj
A
and Thomas
G.
mTOR Complex1-S6K1 signaling: at the crossroads of obesity, diabetes and cancer.
Trends Mol Med.
2007;
13:
252
-259.
[PubMed]
.
-
48.
Ozcan
U
, Ozcan
L
, Yilmaz
E
, Duvel
K
, Sahin
M
, Manning
BD
and Hotamisligil
GS.
Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis.
Mol Cell.
2008;
29:
541
-551.
[PubMed]
.
-
49.
Kim
JH
, Chu
SC
, Gramlich
JL
, Pride
YB
, Babendreier
E
, Chauhan
D
, Salgia
R
, Podar
K
, Griffin
JD
and Sattler
M.
Activation of the PI3K/mTOR pathway by BCR-ABL contributes to increased production of reactive oxygen species.
Blood.
2005;
105:
1717
-1723.
[PubMed]
.
-
50.
Topisirovic
I
and Sonenberg
N.
Cell biology. Burn out or fade away.
Science.
2010;
327:
1210
-1211.
[PubMed]
.
-
51.
Carulli
L
, Rondinella
S
, Lombardini
S
, Canedi
I
, Loria
P
and Carulli
N.
Review article: diabetes, genetics and ethnicity.
Aliment Pharmacol Ther.
2005;
22 Suppl 2:
16
-19.
[PubMed]
.
-
52.
Neel
JV
Diabetes mellitus: a "thrifty" genotype rendered detrimental by "progress".
Am J Hum Genet.
1962;
14:
353
-362.
[PubMed]
.
-
53.
Mattson
MP
and Calabrese
EJ.
New York
Humana Press
(eds.) Hormesis: A Revolution in Biology, Toxicology and Medicine.
2009;
.
-
54.
Sanders
CL
New York
Springer
Radiation Hormesis and the Linear-No-Threshold Assumption.
2010;
.
-
55.
Le
Bourg E
Hormesis, aging and longevity.
Biochim Biophys Acta.
2009;
1790:
1030
-1039.
[PubMed]
.
-
56.
Minois
N
Longevity and aging: beneficial effects of exposure to mild stress.
Biogerontology.
2000;
1:
15
-29.
[PubMed]
.
-
57.
Furuyama
T
, Nakazawa
T
, Nakano
I
and Mori
N.
Identification of the differential distribution patterns of mRNAs and consensus binding sequences for mouse DAF-16 homologues.
Biochem J.
2000;
349:
629
-634.
[PubMed]
.
-
58.
Hubbard
TJ
, Aken
BL
, Ayling
S
, Ballester
B
, Beal
K
, Bragin
E
, Brent
S
, Chen
Y
, Clapham
P
, Clarke
L
, Coates
G
, Fairley
S
and Fitzgerald
S.
Ensembl 2009.
Nucleic Acids Res.
2009;
37:
D690
-697.
[PubMed]
.
-
59.
Clark
AG
, Eisen
MB
, Smith
DR
, Bergman
CM
, Oliver
B
, Markow
TA
, Kaufman
TC
, Kellis
M
, Gelbart
W
, Iyer
VN
, Pollard
DA
, Sackton
TB
and Larracuente
AM.
Evolution of genes and genomes on the Drosophila phylogeny.
Nature.
2007;
450:
203
-218.
[PubMed]
.
-
60.
Stark
A
, Lin
MF
, Kheradpour
P
, Pedersen
JS
, Parts
L
, Carlson
JW
, Crosby
MA
, Rasmussen
MD
, Roy
S
, Deoras
AN
, Ruby
JG
, Brennecke
J
and Hodges
E.
Discovery of functional elements in 12 Drosophila genomes using evolutionary signatures.
Nature.
2007;
450:
219
-232.
[PubMed]
.