Xenohormetic, hormetic and cytostatic selective forces driving longevity at the ecosystemic level
Abstract
We recently found that lithocholic acid (LCA), a bile acid, extends yeast longevity. Unlike mammals, yeast do not synthesize bile acids. We therefore propose that bile acids released into the environment by mammals may act as interspecies chemical signals providing longevity benefits to yeast and, perhaps, other species within an ecosystem.
Bile acids
delay aging in yeast via two different mechanisms
We recently found that LCA greatly (and some other bile acids to a
lesser degree) increases the chronological life span of yeast under caloric
restriction (CR) conditions [1]. Our findings provided evidence that LCA
extends longevity of chronologically aging yeast through two different
mechanisms (Figure 1).
In one mechanism, this bile
acid targets longevity pathways that control chronological aging irrespective
of the number of calories available to yeast. Because these pathways modulate
longevity regardless of calorie availability, we called them "constitutive" or
"housekeeping" [1]. LCA modulates these housekeeping longevity assurance
pathways by suppressing lipid-induced necrosis, attenuating mitochondrial
fragmentation, altering oxidation-reduction processes in mitochondria,
enhancing resistance to oxidative and thermal stresses, suppressing
mitochondria-controlled apoptosis, and enhancing stability of nuclear and
mitochondrial DNA ([1]; Figure 1C). The housekeeping longevity pathways do not
overlap with the TOR (target of rapamycin) and cAMP/PKA (cAMP/protein kinase A)
signaling pathways ([1]; Figure 1A), both of which are "adaptable" by nature
because they are under the stringent control of calorie and/or nutrient availability ([2-6];
Figure 1B).
In the other mechanism, LCA targets the adaptable cAMP/PKA
pathway by unmasking an anti-aging potential of PKA under non-CR conditions,
perhaps by activating PKA-dependent phosphorylation of the cytosolic pool of
the key nutrient-sensory protein kinase Rim15p [1]. The phosphorylation of
Rim15p by PKA inactivates its protein kinase activity [7]. Hence, the
LCA-driven inactivation of Rim15p may reduce the phosphorylation status of its
known [8] target proteins in the cytosol, thereby lowering their pro-aging efficacy
([1]; Figure 1A).
Bile acids
are beneficial to health and longevity in animals
Although bile acids in mammals have been traditionally considered
only as trophic factors for the enteric epithelium and detergents for the
emulsification and absorption of dietary lipids [9-11], they are now also
recognized for their essential role as signaling molecules regulating lipid,
glucose and energy homeostasis and activating detoxification of xenobiotics
([9-14]; Figure 2A). Many of the numerous health-improving
metabolic effects caused by bile acids and their demonstrated ability to
protect mammals from xenobiotic toxins ([9-14]; Figure 2A) suggest that, by improving overall health, these
amphipathic molecules may delay the onset of age-related diseases and have
beneficial effect on longevity. Furthermore, because of the elevated levels of
several bile acids in the long-lived Ghrhrlit/lit mice and due to the ability of cholic acid
administered to food of wild-type mice to activate transcription of numerous
xenobiotic detoxification genes, it has been proposed that, by promoting
chemical hormesis in mammals, these mildly toxic molecules with detergent-like
properties may extend their longevity by acting as endobiotic regulators of aging [15-18].
Moreover, bile acid-like dafachronic acids (including 3-keto-LCA) in worms
function as endocrine regulators of aging by activating an anti-aging
transcriptional program governed by the DAF-12/DAF-16 signaling cascade ([19-21];
Figure 2B). Altogether, these findings support the notion that bile acids are
beneficial to health and longevity in animals because of their ability to
operate as potent signaling molecules that modulate a compendium health- and
longevity-related processes. Noteworthy, by modulating many of these processes
also in yeast, LCA extends their longevity [1]. It is likely therefore that the
life-extending capacity of LCA and other bile acids as well as, perhaps, the
mechanisms underlying their anti-aging action are conserved across animal
species and other phyla.
Bile acids may function as interspecies chemical signals extending
yeast longevity within ecosystems
Importantly, yeast do not
synthesize LCA or any other bile acid found in mammals [1,11,22]. We therefore
hypothesize that bile acids released into the environment by mammals may act as
interspecies chemical signals providing longevity benefits to yeast. In our
hypothesis, these mildly toxic compounds released into the environment by
mammals may create selective pressure for the evolution of yeast species that
can respond to the resulting mild cellular damage by developing the most
efficient stress protective mechanisms. Such mechanisms may provide effective
protection of yeast not only against cellular damage caused by bile acids (and,
perhaps, by other environmental xenobiotics) but also against molecular and
cellular damage accumulated with age. In our hypothesis, yeast species that
have been selected for the most effective mechanisms providing protection
against bile acids (and other environmental xenobiotics) are expected to evolve
the most effective anti-aging mechanisms that are sensitive to regulation by
bile acids (and, perhaps, by other environmental xenobiotics). Thus, the
ability of yeast to sense bile acids produced by mammals and then to respond by
undergoing certain life-extending changes to
their physiology (Figure 1) is expected to increase their chances of survival,
thereby creating selective force aimed at maintaining such ability.
Natural variations of bile acid levels within ecosystems may
modulate both housekeeping and adaptable longevity pathways in yeast
Noteworthy, the bulk quantity of bile acids in mammals exists as
an organismal pool which cycles between intestine and liver in the
enterohepatic circulation due to the efficient reabsorption of bile acids in
the terminal ileum [10,11]. However, about 5% (up to 600 mg/day) of this pool
escapes each reabsorption cycle, being continuously released into the large
intestine and ultimately into the environment [10,11]. Thus, yeast are
permanently exposed to bile acids due to their fecal loss by mammals. It is
conceivable therefore that, in yeast exposed to bile acids released by mammals,
these interspecies chemical signals modulate housekeeping longevity assurance
pathways that 1) regulate yeast longevity irrespective of the state of the
environment or food supply (i.e., the number of available calories and
nutrients); and 2) do not overlap (or only partially overlap) with the
adaptable TOR and cAMP/PKA longevity pathways that are under the stringent
control of calorie and nutrient availability.
It should be stressed, however, that the quantity
of bile acids released into the environment by mammals could vary due to
changes in the density of mammalian population and, perhaps, due to other
environmental factors (including the abundance of food available to mammals,
its nutrient and caloric content, and its fat mass and quality). In fact, the
organismal pool of bile acids in mammals is under the stringent control of
regulatory mechanisms operating in the liver during the fasting-refeeding
transition [9-11]. Hence, it is likely that, in addition to the ability of
yeast to respond to the permanently available exogenous pool of bile acids by
modulating some housekeeping longevity assurance pathways, they have also
evolved the ability to sense the environmental status-dependent variations of bile
acids abundance by modulating the adaptable TOR and cAMP/PKA longevity
pathways. Importantly, our recent study provided evidence for two mechanisms
underlying the life-extending effect of LCA in yeast; one mechanism involves
the calorie supply-independent modulation of a compendium of housekeeping
longevity assurance processes that are not regulated by the TOR and cAMP/PKA
pathways, whereas the other mechanism operates only in yeast on a calorie-rich
diet by unmasking the previously unknown anti-aging potential of the calorie
supply-dependent PKA [1].
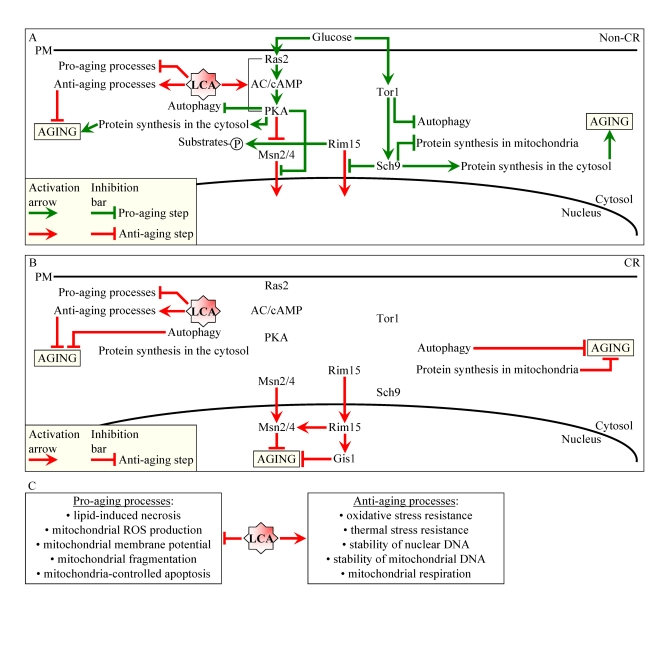
Figure 1. Lithocholic acid (LCA) extends longevity of chronologically aging yeast through two different mechanisms. (A
and B) Outline of pro- and anti-aging processes that are controlled
by the TOR and/or cAMP/PKA signaling pathways and are modulated by LCA in
yeast cells grown under non-CR (A) or CR (B) conditions.
Activation arrows and inhibition bars denote pro-aging (displayed in green
color) or anti-aging (displayed in red color) processes. Under both non-CR
and CR conditions, LCA targets housekeeping longevity assurance processes
listed in (C). Under non-CR conditions only, LCA also targets the
adaptable cAMP/PKA pathway. By activating PKA-dependent phosphorylation of
the cytosolic pool of the key nutrient-sensory protein kinase Rim15p, LCA
causes the inactivation of Rim15p. The resulting reduction of the
phosphorylation status of several Rim15p target proteins in the cytosol
lowers their pro-aging efficacy. Abbreviations: CR, caloric restriction;
PM, plasma membrane.
It remains to be seen if our hypothesis on the essential role of
bile acids as interspecies chemical signals regulating longevity in yeast is
applicable to other species routinely exposed to bile acids within an
ecosystem, such as plants and bacteria.
Rapamycin may also act as an interspecies chemical signal
modulating longevity at the ecosystemic level
Our hypothesis on longevity regulation by bile acids within ecosystems may explain the evolutionary
origin of the life-extending effect of another anti-aging compound, called
rapamycin. Synthesized by soil bacteria to inhibit growth of fungal
competitors, this macrocyclic lactone provides longevity benefit to yeast,
fruit flies and mice by specifically inhibiting TOR (Tor1p in yeast), a
nutrient-sensory protein kinase that operates as a master negative regulator of
the key adaptable longevity pathway [3,4,23-25]. Because rapamycin delays
proliferative growth of organisms across phyla by causing G1 cell cycle arrest
[3,4,26], it could be considered as a mildly cytotoxic compound, akin to bile
acids (Our recent unpublished data revealed that rapamycin is a more toxic
hormetic molecule than LCA and other bile acids). We propose therefore that,
following its release into the environment by soil bacteria, rapamycin may
create selective pressure for the evolution of yeast, fly and mammalian species
that can respond to rapamycin-induced growth retardation by developing certain
mechanisms aimed at such remodeling of their anabolic and catabolic processes
that would increase their chances of survival under conditions of slow growth.
It is plausible that some of these mechanisms delay aging by optimizing
essential longevity-related processes and remain sensitive to modulation by
rapamycin. Hence, the ability of yeast, fruit flies and mice to sense rapamycin
produced by soil bacteria and then to respond by undergoing certain
life-extending changes to their physiology is expected to increase their
chances of survival, thereby creating selective force for maintaining such
ability.
Interestingly, rapamycin has
been shown to increase life span in fruit flies under dietary restriction
conditions [25], when the TOR-governed adaptable pro-aging pathways are fully
suppressed and the TOR-governed adaptable anti-aging pathways are fully activated
[3,4]. It is plausible therefore that - similar to the proposed above
anti-aging mechanism of LCA in yeast - rapamycin in fruit flies can modulate
both the housekeeping (TOR-independent) and adaptable (TOR-dependent) longevity
pathways. Hence, it is tempting to speculate that, in addition to the ability
of fruit flies to respond to the permanently available exogenous pool of
rapamycin by modulating some housekeeping longevity assurance pathways, they
have also evolved the ability to sense the environmental status-dependent
variations of rapamycin abundance (due to, e.g., changes in the density
of soil bacteria population) by modulating the TOR-governed adaptable longevity
pathways. Of note, recent findings in yeast imply that - in addition to its
role as a master negative regulator of the key adaptable longevity pathway -
Tor1p may also operate as a positive longevity regulator, in particular by
stimulating nuclear import of the transcriptional factors Sfp1p, Rtg1 and Rtg3
in response to partial mitochondrial dysfunction or changes in the exogenous
and endogenous levels of glutamate and glutamine [27-29]. The ability of these
transcriptional factors to regulate metabolism, ribosome biogenesis and growth
is crucial for longevity [28,30,31].
The "xenohormesis" hypothesis: a case of xenohormetic
phytochemicals
Our hypothesis on longevity regulation by bile acids and rapamycin
within ecosystems complements the "xenohormesis" hypothesis, in which plants
and other autotrophic organisms respond to various environmental stresses (i.e.,
UV light, dehydration, infection, predation, cellular damage and nutrient
deprivation) by synthesizing a compendium of secondary metabolites [32-34].
Within plants and other autotrophs producing these phytochemicals in response
to environmental stresses, they activate defense systems protecting the host
organisms against such stresses. In addition, these phytochemicals constitute a
chemical signature of the environmental status of an ecosystem. As such, they
provide to heterotrophic organisms (i.e., animals and fungi) within the
ecosystem an advance warning about deteriorating environmental conditions [33].
By operating as interspecies chemical signals, they could create selective
pressure for the evolution of heterotrophic organisms that can sense these
signals and then to respond by altering their metabolism in defensive
preparation for the imminent adversity while conditions are still favorable.
The resulting metabolic remodeling causes such specific changes in physiology of heterotrophs that are beneficial to their health
and longevity [33]. Although xenohormetic phytochemicals are produced by
autotrophic organisms only in response to hormetic environmental stresses, it
is unlikely that they function as mildly toxic hormetic molecules within
heterotrophic organisms; rather, the xenohormesis hypothesis proposes that the beneficial
to health and longevity effects of xenohormetic phytochemicals are due to their
well known ability to modulate the key enzymes of stress-response pathways
governing numerous longevity-related processes in heterotrophic organisms
[33-42]. The xenohormetic mode of positive selection for the most efficient
longevity regulation mechanisms has been proposed to be driven by such
phytochemicals as resveratrol, butein, fisetin and other polyphenols, as well
as by curcumin [32-34]. The ability of caffeine to increase yeast
chronological life span by decreasing the catalytic activity of Tor1p [43] suggests that this
xanthine alkaloid could also operate as a xenohormetic phytochemical signal
providing an advance warning about deteriorating environmental conditions to
yeast, thereby driving the evolution of their longevity regulation mechanisms.
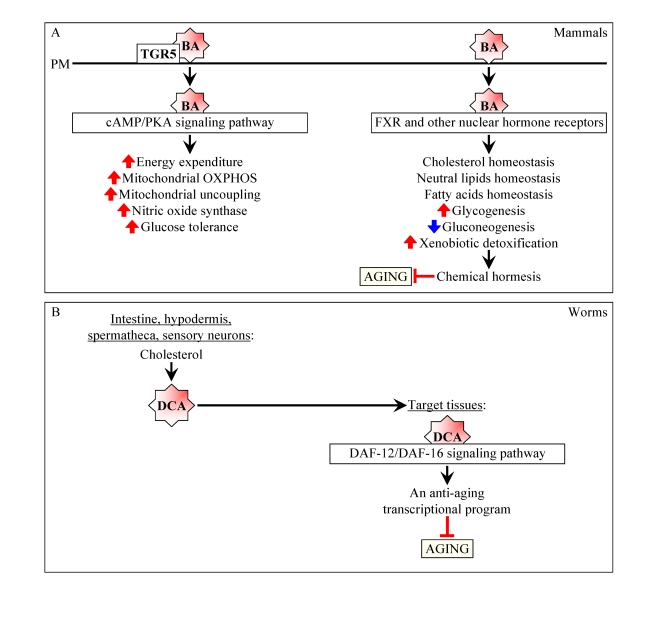
Figure 2. Bile acids are beneficial to health and longevity in animals. (A) In
mammals, bile acids (BA) function not only as
trophic factors for the enteric epithelium and detergents for the
emulsification and absorption of dietary lipids, but also as signaling
molecules that regulate lipid, glucose and energy homeostasis and activate
detoxification of xenobiotics.By improving overall health, BA may delay the onset of age-related
diseases and have beneficial effect on longevity. By activating
transcription of numerous xenobiotic detoxification genes and thus promoting chemical hormesis, BA may extend their
longevity by acting as endobiotic regulators
of aging. (B) In worms, following their synthesis from cholesterol
in the intestine, hypodermis, spermatheca and sensory neurons, bile
acid-like dafachronic acids (DCA) are delivered to other tissues where they
activate the DAF-12/DAF-16 signaling cascade, thereby orchestrating an
anti-aging transcriptional program and increasing the life span of the
entire organism.
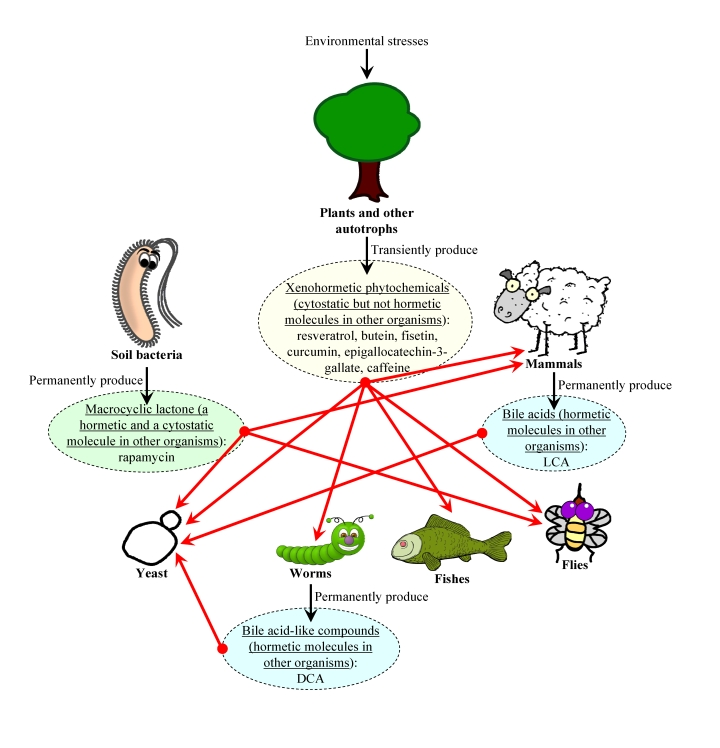
Figure 3. The xenohormetic, hormetic and cytostatic selective forces may drive the evolution of longevity regulation mechanisms within an ecosystem. We
propose that organisms from all domains of life within an ecosystem
synthesize chemical compounds that 1) are produced and then released into
the environment permanently or only in response to deteriorating
environmental conditions, increased population density
of competitors and/or predators, or changes in food availability and its
nutrient and/or caloric content; 2) are mildly toxic compounds that
trigger a hormetic response in an organism that senses them or,
alternatively, are not toxic for any organism within the ecosystem and do
not cause a hormetic response; 3) are cytostatic compounds that attenuate the TOR-governed signaling network or, alternatively,
do not modulate this growth-promoting network; and 4) extend
longevity of organisms that can sense these compounds (red arrows), thereby
increasing their chances of survival and creating selective force aimed at
maintaining the ability of organisms composing the ecosystem to respond to
these compounds by undergoing specific life-extending changes to their
physiology. In our hypothesis, the evolution of longevity regulation
mechanisms in each group of the organisms composing an ecosystem is driven
by the ability of this group of organisms to undergo specific
life-extending changes to their physiology in response to a compendium of
"critical" chemical compounds that are permanently or transiently released
to the ecosystem by other groups of organisms. Abbreviations: LCA, lithocholic
acid; DCA, bile acid-like dafachronic acids.
The "anti-aging side effect" hypothesis: delaying aging by
attenuating the growth-promoting TOR signaling pathway
A common feature of many anti-aging compounds - some of which are
mildly toxic hormetic molecules, whereas the others are non-toxic xenohormetic
phytochemicals - is that they exhibit a cytostatic effect by inhibiting TOR, a
nutrient-sensing signaling pathway that promotes proliferative growth in all heterotrophic
organisms. A recently proposed "anti-aging side effect" hypothesis envisions
that the primary objective for the synthesis of these cytostatic compounds by a
group of the organisms composing an
ecosystem is to suppress growth of other group(s) of organisms within this
ecosystem, thereby killing competitors and/or protecting themselves from
predators [39]. Due to its central role in promoting
proliferative growth of all heterotrophic
organisms, the TOR signaling pathway is a
preferable target of such cytostatic compounds [3,26,39,44, 45]. Because
the TOR pathway provides a molecular link between growth and aging by driving a
so-called quasi-programmed aging [3,44,45], these compounds exhibit a side
effect of suppressing aging [39]. In fact, soil
bacteria synthesize rapamycin to suppress growth of fungal competitors by inhibiting
the TOR protein kinase, a master positive regulator of the TOR signaling
pathway that drives developmental growth of young organisms [3,23-25]. However, since - according to the anti-aging side
effect hypothesis - in heterotrophic organisms across phyla this pathway also
drives aging after their developmental growth is completed [44,45], rapamycin
has a side effect of suppressing aging of all groups of heterotrophic organisms
within an ecosystem [39]. Moreover, the anti-aging side effect hypothesis predicts that plants
synthesize resveratrol in part to protect their grapes by inhibiting fungal
growth [39]. Yet, because this small polyphenol attenuates the TOR signaling
pathway by modulating key upstream regulators and downstream targets of
the TOR protein kinase [35-42], resveratrol also
displays a side effect of slowing down quasi-programmed TOR-driven aging of
various species of heterotrophic organisms within an ecosystem [39].
In the anti-aging side effect
hypothesis, cytostatic compounds attenuating the TOR pathway operate as
interspecies chemical signals that provide longevity benefits to a range of heterotrophic
organisms composing an ecosystem [39]. We propose that, following their release into the
environment by soil bacteria or plants, these growth suppressing chemical
compounds may create selective pressure for the evolution of yeast, worm, fly
and mammalian species that can respond to the resulting retardation of their
growth by developing certain mechanisms aimed at specific remodeling of the
TOR-governed signaling network. By targeting the TOR protein kinase itself
and/or its numerous upstream regulators
and downstream targets, such mechanisms may attenuate the hyper-activation of
TOR-governed cellular signaling pathways and cellular functions that -
according to the concept of quasi-programmed
TOR-driven aging [44,45] - are initiated after developmental growth of a heterotrophic
organism is completed. In our hypothesis, the species of heterotrophic
organisms that have been selected for the most efficient mechanisms preventing
the hyper-activation of TOR-governed cellular signaling pathways and
cellular functions following the completion of developmental
growth are expected to evolve the most effective anti-aging mechanisms. Such
mechanisms may be sensitive to various environmental
factors, including the density of organism population and abundance of
nutrients within an ecosystem.
The xenohormetic, hormetic and cytostatic selective forces
may drive the evolution of longevity regulation mechanisms within ecosystems
Unlike
xenohormetic phytochemicals that are non-toxic compounds transiently
synthesized and released by autotrophs only in response to environmental
stresses [33,34], bile acids are mildly toxic hormetic molecules that are permanently synthesized
and released by mammals [9-11,14-18]. Furthermore, rapamycin is a more toxic
hormetic molecule than bile acids (our unpublished data) that is permanently
synthesized and released by soil bacteria [46]. Moreover, many xenohormetic
phytochemicals and mildly toxic hormetic molecules exhibit a cytostatic effect
by attenuating TOR-governed cellular
signaling pathways and cellular functions [39]. Therefore,
by fusing the xenohormesis hypothesis [32-34], the anti-aging side effect
hypothesis [39] and the proposed here hypothesis on longevity regulation by
bile acids and rapamycin within ecosystems, we put forward a unified hypothesis
of the xenohormetic, hormetic and cytostatic selective forces driving the
evolution of longevity regulation mechanisms at the ecosystemic level.
In our unified
hypothesis (Figure 3), organisms from all domains of life (i.e., bacteria, fungi, plants and animals) within an
ecosystem are able to synthesize chemical
compounds that 1) are produced and then released into the environment
permanently or only in response to deteriorating environmental conditions,
increased population density of competitors and/or
predators, or changes in food availability and its nutrient and/or caloric
content; 2) are mildly toxic compounds that trigger a hormetic response
in an organism that senses them or, alternatively, are not toxic for any organism
within the ecosystem and do not cause a hormetic response; 3) are cytostatic
compounds that attenuate the TOR-governed signaling
network (e.g., rapamycin and resveratrol) or, alternatively, do not
modulate this growth-promoting network (e.g., LCA and other bile acid) and 4) extend longevity of
organisms that can sense these compounds, thereby increasing their chances of
survival and creating selective force aimed at maintaining the ability of
organisms composing the ecosystem to respond to these compounds by undergoing
specific life-extending changes to their physiology. Our
hypothesis implies that the evolution of longevity regulation mechanisms
in each group of the organisms composing an ecosystem is driven by the ability
of this group of organisms to undergo specific life-extending physiological
changes in response to a compendium of "critical" chemical compounds that are
permanently or transiently released to the ecosystem by other groups of
organisms.
Verification of our hypothesis
As the first step towards testing the
validity of our hypothesis of the xenohormetic, hormetic and cytostatic
selective forces driving the evolution of longevity regulation mechanisms
within ecosystems, we are currently carrying out the LCA-driven experimental
evolution of longevity regulation mechanisms in chronologically aging yeast
cultured under laboratory conditions. If we could select long-lived yeast
species following a long-term exposure of wild-type yeast to LCA, we would be
able to begin addressing the following intriguing questions: 1) what genes are
affected by mutations responsible for the extended longevity of selected
long-lived yeast species? 2) how these mutations influence a compendium of the
housekeeping longevity-related processes modulated by LCA in chronologically
aging yeast ([1]; Figure 1); 3) will these mutations affect the growth rate of
yeast in media with or without LCA? 4) will selected long-lived yeast species
be able to maintain their ability to live longer than wild-type yeast if they
undergo several successive passages in medium without LCA? - and, thus, is
there selective pressure aimed at maintaining of an "optimal" rather than a
"maximal" chronological life span of yeast (due to, e.g., a proposed
selective advantage of the envisioned "altruistic" program [47-52] of
chronological aging in yeast)? and 5) if mixed with an equal number of
wild-type yeast cells, will selected long-lived yeast species out-grow and/or
out-live them in medium without LCA or the opposite will happen (due to
selective pressure on yeast aimed at maintaining of the so-called "altruistic"
program [47-52] of their chronological aging)?
Acknowledgments
We are grateful
to current and former members of the Titorenko laboratory for discussions. We
are indebted to Dr. Mikhail V. Blagosklonny for the valuable insight into
important implications of the "anti-aging side effect" hypothesis and of the concept of quasi-programmed
TOR-driven aging. AAG was supported by a
doctoral scholarship from the CIHR. PK was supported by a Concordia University
Faculty of Arts and Science Graduate Fellowship and a Fonds québécois de la
recherche sur la nature et les technologies (FQRNT) Doctoral Research
Fellowship.
VIT is a
Concordia University Research Chair in Genomics, Cell Biology and Aging.
Conflicts of Interest
The authors of this manuscript have no conflict of
interests to declare.
References
-
1.
Goldberg
AA
Chemical genetic screen identifies lithocholic acid as an anti-aging compound that extends yeast chronological life span in a TOR-independent manner, by modulating housekeeping longevity assurance processes.
Aging.
2010;
2:
393
-414.
[PubMed]
.
-
2.
Greer
EL
and Brunet
A.
Signaling networks in aging.
J Cell Sci.
2008;
121:
407
-412.
[PubMed]
.
-
3.
Blagosklonny
MV
and Hall
MN.
Growth and aging: a common molecular mechanism.
Aging.
2009;
1:
357
-362.
[PubMed]
.
-
4.
Hands
SL
, Proud
CG
and Wyttenbach
A.
mTOR's role in ageing: protein synthesis or autophagy.
Aging.
2009;
1:
586
-597.
[PubMed]
.
-
5.
Narasimhan
SD
, Yen
K
and Tissenbaum
HA.
Converging pathways in lifespan regulation.
Curr Biol.
2009;
19:
R657
-R666.
[PubMed]
.
-
6.
Fontana
L
, Partridge
L
and Longo
VD.
Extending healthy life span - from yeast to humans.
Science.
2010;
328:
321
-326.
[PubMed]
.
-
7.
Smets
B
, Ghillebert
R
, De
Snijder P
, Binda
M
, Swinnen
E
, De
Virgilio C
and Winderickx
J.
Life in the midst of scarcity: adaptations to nutrient availability in Saccharomyces cerevisiae.
Curr Genet.
2010;
56:
1
-32.
[PubMed]
.
-
8.
Ptacek
J
, Devgan
G
, Michaud
G
, Zhu
H
, Zhu
X
, Fasolo
J
, Guo
H
, Jona
G
, Breitkreutz
A
, Sopko
R
, McCartney
RR
, Schmidt
MC
and Rachidi
N.
Global analysis of protein phosphorylation in yeast.
Nature.
2005;
438:
679
-684.
[PubMed]
.
-
9.
Thomas
C
, Pellicciari
R
, Pruzanski
M
, Auwerx
J
and Schoonjans
K.
Targeting bile-acid signalling for metabolic diseases.
Nat Rev Drug Discov.
2008;
7:
678
-693.
[PubMed]
.
-
10.
Hylemon
PB
, Zhou
H
, Pandak
WM
, Ren
S
, Gil
G
and Dent
P.
Bile acids as regulatory molecules.
J Lipid Res.
2009;
50:
1509
-1520.
[PubMed]
.
-
11.
Lefebvre
P
, Cariou
B
, Lien
F
, Kuipers
F
and Staels
B.
Role of bile acids and bile acid receptors in metabolic regulation.
Physiol Rev.
2009;
89:
147
-191.
[PubMed]
.
-
12.
Ramalho
RM
, Viana
RJ
, Low
WC
, Steer
CJ
and Rodrigues
CM.
Bile acids and apoptosis modulation: an emerging role in experimental Alzheimer's disease. Trends Mol.
Med.
2008;
14:
54
-62.
.
-
13.
Amaral
JD
, Viana
RJ
, Ramalho
RM
, Steer
CJ
and Rodrigues
CM.
Bile acids: regulation of apoptosis by ursodeoxycholic acid.
J Lipid Res.
2009;
50:
1721
-1734.
[PubMed]
.
-
14.
Vallim
TQ
and Edwards
PA.
Bile acids have the gall to function as hormones.
Cell Metab.
2009;
10:
162
-164.
[PubMed]
.
-
15.
Amador-Noguez
D
, Yagi
K
, Venable
S
and Darlington
G.
Gene expression profile of long-lived Ames dwarf mice and Little mice.
Aging Cell.
2004;
3:
423
-441.
[PubMed]
.
-
16.
Amador-Noguez
D
, Dean
A
, Huang
W
, Setchell
K
, Moore
D
and Darlington
G.
Alterations in xenobiotic metabolism in the long-lived Little mice.
Aging Cell.
2007;
6:
453
-470.
[PubMed]
.
-
17.
Gems
D
Long-lived dwarf mice: are bile acids a longevity signal.
Aging Cell.
2007;
6:
421
-423.
[PubMed]
.
-
18.
Gems
D
and Partridge
L.
Stress-response hormesis and aging: "that which does not kill us makes us stronger".
Cell Metab.
2008;
7:
200
-203.
[PubMed]
.
-
19.
Motola
DL
, Cummins
CL
, Rottiers
V
, Sharma
KK
, Li
T
, Li
Y
, Suino-Powell
K
, Xu
HE
, Auchus
RJ
, Antebi
A
and Mangelsdorf
DJ.
Identification of ligands for DAF-12 that govern dauer formation and reproduction in C. elegans.
Cell.
2006;
124:
1209
-1223.
[PubMed]
.
-
20.
Gerisch
B
, Rottiers
V
, Li
D
, Motola
DL
, Cummins
CL
, Lehrach
H
, Mangelsdorf
DJ
and Antebi
A.
A bile acid-like steroid modulates Caenorhabditis elegans lifespan through nuclear receptor signaling.
Proc Natl Acad Sci USA.
2007;
104:
5014
-5019.
[PubMed]
.
-
21.
Russell
SJ
and Kahn
CR.
Endocrine regulation of ageing.
Nat Rev Mol Cell Biol.
2007;
8:
681
-691.
[PubMed]
.
-
22.
Monte
MJ
, Marin
JJ
, Antelo
A
and Vazquez-Tato
J.
Bile acids: chemistry, physiology, and pathophysiology.
World J Gastroenterol.
2009;
15:
804
-816.
[PubMed]
.
-
23.
Powers
RW 3rd
, Kaeberlein
M
, Caldwell
SD
, Kennedy
BK
and Fields
S.
Extension of chronological life span in yeast by decreased TOR pathway signaling.
Genes Dev.
2006;
20:
174
-184.
[PubMed]
.
-
24.
Harrison
DE
, Strong
R
, Sharp
ZD
, Nelson
JF
, Astle
CM
, Flurkey
K
, Nadon
NL
, Wilkinson
JE
, Frenkel
K
, Carter
CS
, Pahor
M
, Javors
MA
, Fernandez
E
and Miller
RA.
Rapamycin fed late in life extends lifespan in genetically heterogeneous mice.
Nature.
2009;
460:
392
-395.
[PubMed]
.
-
25.
Bjedov
I
, Toivonen
JM
, Kerr
F
, Slack
C
, Jacobson
J
, Foley
A
and Partridge
L.
Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster.
Cell Metab.
2010;
11:
35
-46.
[PubMed]
.
-
26.
Wullschleger
S
, Loewith
R
and Hall
MN.
TOR signaling in growth and metabolism.
Cell.
2006;
124:
471
-484.
[PubMed]
.
-
27.
Dann
SG
and Thomas
G.
The amino acid sensitive TOR pathway from yeast to mammals.
FEBS Lett.
2006;
580:
2821
-2829.
[PubMed]
.
-
28.
Heeren
G
, Rinnerthaler
M
, Laun
P
, von
Seyerl P
, Kössler
S
, Klinger
H
, Jarolim
S
, Simon-Nobbe
B
, Hager
M
, Schüller
C
, Carmona-Gutierrez
D
, Breitenbach-Koller
L
, Mück
C
, Jansen-Dürr
P
, Criollo
A
, Kroemer
G
, Madeo
F
and Breitenbach
M.
The mitochondrial ribosomal protein of the large subunit, Afo1p, determines cellular longevity through mitochondrial back-signaling via TOR1.
Aging.
2009;
1:
622
-636.
[PubMed]
.
-
29.
Ralser
M
and Lehrach
H.
Building a new bridge between metabolism, free radicals and longevity.
Aging.
2009;
1:
836
-838.
[PubMed]
.
-
30.
Butow
RA
and Avadhani
NG.
Mitochondrial signaling: the retrograde response.
Mol Cell.
2004;
14:
1
-15.
[PubMed]
.
-
31.
Jazwinski
SM
Rtg2 protein: at the nexus of yeast longevity and aging.
FEMS Yeast Res.
2005;
5:
1253
-1259.
[PubMed]
.
-
32.
Howitz
KT
, Bitterman
KJ
, Cohen
HY
, Lamming
DW
, Lavu
S
, Wood
JG
, Zipkin
RE
, Chung
P
, Kisielewski
A
, Zhang
LL
, Scherer
B
and Sinclair
DA.
Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan.
Nature.
2003;
425:
191
-196.
[PubMed]
.
-
33.
Howitz
KT
and Sinclair
DA.
Xenohormesis: sensing the chemical cues of other species.
Cell.
2008;
133:
387
-391.
[PubMed]
.
-
34.
Lamming
DW
, Wood
JG
and Sinclair
DA.
Small molecules that regulate lifespan: evidence for xenohormesis.
Mol Microbiol.
2004;
53:
1003
-1009.
[PubMed]
.
-
35.
Baur
JA
, Pearson
KJ
, Price
NL
, Jamieson
HA
, Lerin
C
, Kalra
A
, Prabhu
VV
, Allard
JS
, Lopez-Lluch
G
, Lewis
K
, Pistell
PJ
, Poosala
S
, Becker
KG
, Boss
O
, Gwinn
D
, Wang
M
, Ramaswamy
S
, Fishbein
KW
, Spencer
RG
, Lakatta
EG
, Le
Couteur D
, Shaw
RJ
, Navas
P
, Puigserver
P
, Ingram
DK
, de Cabo
R
and Sinclair
DA.
Resveratrol improves health and survival of mice on a high-calorie diet.
Nature.
2006;
444:
337
-342.
[PubMed]
.
-
36.
Dasgupta
B
and Milbrandt
J.
Resveratrol stimulates AMP kinase activity in neurons.
Proc Natl Acad Sci USA.
2007;
104:
7217
-7222.
[PubMed]
.
-
37.
Armour
SM
, Baur
JA
, Hsieh
SN
, Land-Bracha
A
, Thomas
SM
and Sinclair
DA.
Inhibition of mammalian S6 kinase by resveratrol suppresses autophagy.
Aging.
2009;
1:
515
-528.
[PubMed]
.
-
38.
Demidenko
ZN
and Blagosklonny
MV.
At concentrations that inhibit mTOR, resveratrol suppresses cellular senescence.
Cell Cycle.
2009;
8:
1901
-1904.
[PubMed]
.
-
39.
Blagosklonny
MV
Inhibition of S6K by resveratrol: in search of the purpose.
Aging.
2009;
1:
511
-514.
[PubMed]
.
-
40.
Morselli
E
, Galluzzi
L
, Kepp
O
, Criollo
A
, Maiuri
MC
, Tavernarakis
N
, Madeo
F
and Kroemer
G.
Autophagy mediates pharmacological lifespan extension by spermidine and resveratrol.
Aging.
2009;
1:
961
-970.
[PubMed]
.
-
41.
Shakibaei
M
, Harikumar
KB
and Aggarwal
BB.
Resveratrol addiction: to die or not to die.
Mol Nutr Food Res.
2009;
53:
115
-128.
[PubMed]
.
-
42.
Morselli
E
, Maiuri
MC
, Markaki
M
, Megalou
E
, Pasparaki
A
, Palikaris
K
, Galluzzi
L
, Criollo
A
, Malik
SA
, Madeo
F
, Tavernarakis
N
and Kroemer
G.
Caloric restriction and resveratrol prolong longevity via the sirtuin-1 dependent induction of autophagy.
Cell Death Disease.
2010;
1:
e10
.
-
43.
Wanke
V
, Cameroni
E
, Uotila
A
, Piccolis
M
, Urban
J
, Loewith
R
and De
Virgilio C.
Caffeine extends yeast lifespan by targeting TORC1.
Mol Microbiol.
2008;
69:
277
-285.
[PubMed]
.
-
44.
Blagosklonny
MV
Aging and immortality: quasi-programmed senescence and its pharmacologic inhibition.
Cell Cycle.
2006;
5:
2087
-2102.
[PubMed]
.
-
45.
Blagosklonny
MV
Rapamycin and quasi-programmed aging: Four years later.
Cell Cycle.
2010;
9:
1859
-1862.
.
-
46.
Vezina
C
, Kudelski
A
and Sehgal
SN.
Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle.
J Antibiot.
1975;
28:
721
-726.
[PubMed]
.
-
47.
Fabrizio
P
, Battistella
L
, Vardavas
R
, Gattazzo
C
, Liou
LL
, Diaspro
A
, Dossen
JW
, Gralla
EB
and Longo
VD.
Superoxide is a mediator of an altruistic aging program in Saccharomyces cerevisiae.
J Cell Biol.
2004;
166:
1055
-1067.
[PubMed]
.
-
48.
Herker
E
, Jungwirth
H
, Lehmann
KA
, Maldener
C
, Frohlich
KU
, Wissing
S
, Buttner
S
, Fehr
M
, Sigrist
S
and Madeo
F.
Chronological aging leads to apoptosis in yeast.
J Cell Biol.
2004;
164:
501
-507.
[PubMed]
.
-
49.
Longo
VD
, Mitteldorf
J
and Skulachev
VP.
Programmed and altruistic ageing.
Nat Rev Genet.
2005;
6:
866
-872.
[PubMed]
.
-
50.
Vachova
L
and Palkova
Z.
Physiological regulation of yeast cell death in multicellular colonies is triggered by ammonia.
J Cell Biol.
2005;
169:
711
-717.
[PubMed]
.
-
51.
Büttner
S
, Eisenberg
T
, Herker
E
, Carmona-Gutierrez
D
, Kroemer
G
and Madeo
F.
Why yeast cells can undergo apoptosis: death in times of peace, love, and war.
J Cell Biol.
2006;
175:
521
-525.
[PubMed]
.
-
52.
Severin
FF
, Meer
MV
, Smirnova
EA
, Knorre
DA
and Skulachev
VP.
Natural causes of programmed death of yeast Saccharomyces cerevisiae.
Biochim Biophys Acta.
2008;
1783:
1350
-1353.
[PubMed]
.