Ophthalmoscopy examination
Retinas from 20 day-aged OXYS rats did not revealed the presence of any pathological signs. The first AMD-like alterations in the fundus of the eye were found in 22% of the OXYS rats when they reached the age of 1.5 months (Figure 1). Alterations manifested itself as distorted reflectance of the fundus, swelling of the retina, the emergence of distinct foci of ischemia, and the signs of atrophy of choriocapillaris and RPE.
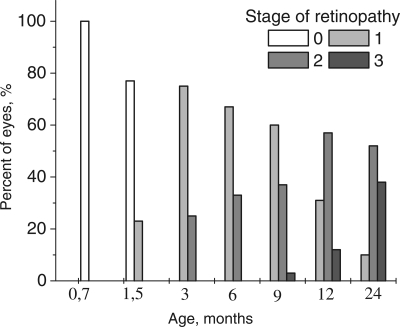
Figure 1. Distribution of the stages of development of retinopathy in eyes of OXYS rats. 0 − 3 – corresponding stage of the retinopathy according to Age-Related Eye Disease Study grade protocol (eyephoto.ophth.wisc.edu). 790 animals were examined in total.
Up to the age of 3-4 months, the morbidity had already reached 100% when OXYS rats exhibited the prevalent 1st stage of the disease: drusen with signs of atrophy of RPE and partial loss of choriocapillaris, whereas the major blood vessels of choroid remained unchanged. In some animals, we observed detachment of pigment epithelium in the form of dome elevation in the posterior pole of the eye. Four to five percent of the animals at this age have features of the 2nd stage: large soft drusen, edema in the central zone, enlargement of the zone, exudative detachment of pigmented and neuroepithelium of the retina. The proportion of animals with this stage of the disease increased with age (Figure 1), and by the age of 12 months, it reached 31%.
Up to the age of 12 months, clinical symptoms of retinopathy worsened. The third stage was registered in 3% of animals at 9 months, but at 12 months, it was apparent in 44% of the animals and was characterized by severe irreversible changes. The distinctive signs of this stage are hemorrhagic detachment of retina, dotted bleeding in retina, and new blood vessel formation and cicatrization. Importantly, neovascularization did not develop in all animals, but rather, in isolated cases, as it normally happens in people. By the age of 24 months, the changes were irreversible in nearly all animals. In contrast, first alteration in Wistar retina started to develop by the age of 24 months and could not be strictly classified as signs of AMD.
Histological analysis and electron microscopy
Light- and electron microscopy did not relive significant differences in structure of retina of OXYS and Wistar rats at 20 days and 3 months. The RPE layer has one line of cells with normal localized nucleus, endoplasmic reticulum, ribosome and mitochondria (Figure 2). On the Figure 2 endothelial cells, which formed the wall of choriocapillaries, cavity of choriocapillaries and erythrocyte in it are good visible. But already at the age of 20 days, along with normally functioning choriocapillaries, we observed appearance choriocapillaries with stasis of blood cells. (Quantity analysis of choroid vessels and RPE cells see below).
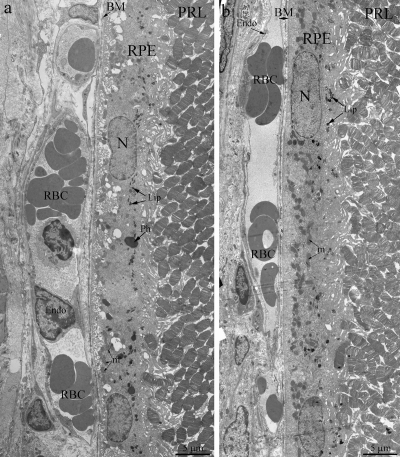
Figure 2. Outer retina of Wistar (a) and OXYS (b) rats at the age of 3 months. PRL – photoreceptors, RPE – retinal pigment epithelial cell, N – RPE nucleus, m – mitochondrion, Ph – phagosome, Lip – lipofuscin material, BM – Bruch's membrane, Endo – endothelial cell, RBC – red blood cell.
Towards the 12 months of age, destructive changes in OXYS rat's retina progressed and affect RPE/ Bruch's/choriocapillaris complex in contrast to Wistar rats (Figure 3a) with small anomaly in Bruch's membrane ultrastructure and open choriocapillaries. Main ultra-structural alterations are revealed in the cell apical part. First of all, this is the absence of a continuous layer of electron-dense inclusions as well as practically completes absence of phagosomes – debris of the photoreceptor outer segments phagocytized by pigment epithelium, whirling extensions of the basement membrane into the cytoplasm (Figure 3b). Underlying these areas was pronounced thickening of Bruch's membrane with disturbance of collagen and elastin layers. The choriocapillaris was found to have severe endothelial degeneration and transformation to fibrous tissue in the most severely affected regions.
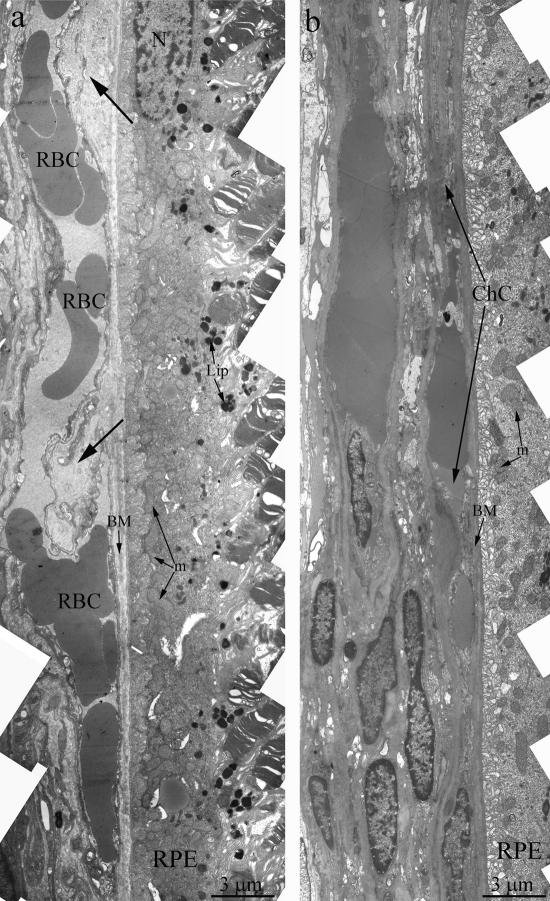
Figure 3. Outer retina of Wistar (a) and OXYS (b) rats at the age of 12 months. Arrows show thickening of Brunch membrane. RPE – retinal pigment epithelial cell, N – RPE nucleus, m – mitochondrion, BM – Bruch's membrane, ChC – choriocapillaris complex, RBC – red blood cell.
In the retina of 24 months-aged OXYS rats, there is almost complete fibrosis of choroid and destruction of endothelium and, as a result, obliteration of cavity of choriocapillaries. Ultrastructure analysis of OXYS eyes from 24-month-old animals shown, that area of degenerating retina is shown from the inner nuclear layer to retinal pigment epithelium/Bruch's/ choriocapillaris complex (Figure 4b). Outer nuclear layer and the photoreceptors are disorganized and fragmented. Changes of the RPE included significantly increased number of lipofuscin granules, atrophy of some RPE cells and vascularization (Figure 4a). Really, as the photoreceptor cells degenerated and the outer nuclear layer and photoreceptor cell layers disappeared, vessels migrated toward the RPE (arrow on Figure 4a). Thickening of Brunch membrane with disturbance of collagen and elastin layers were seen in all eyes (Figure 5a).Also there is almost complete fibrosis of choroid and destruction of endothelium and, as a result, obliteration of cavity of choriocapillaris.
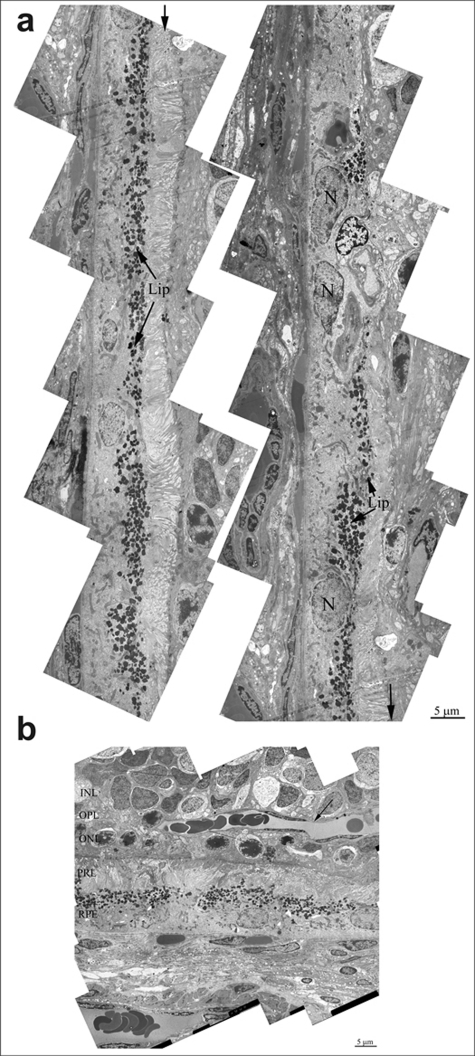
Figure 4. (a) Ultrastructure of pigment epithelium of 25-months-old OXYS rat. Selected area is shown under high magnification. Lip – lipofuscin material, N – retinal pigment epithelial cell nucleus. (b) Region of pigment epithelium of 25-months-old OXYS rat. Retina injure was estimated as 3 units. INL – inner nuclear layer, where the body and nucleus of interneurones is lied; OPL – outer plexiform layer – where axon of photoreceptor and dendrite of interneurone are in contact; ONL – outer nuclear layer, body and nucleus of photoreceptors; PRL – photoreceptors, RPE – retinal pigment epithelial cell. Arrow shown the blood vessel.
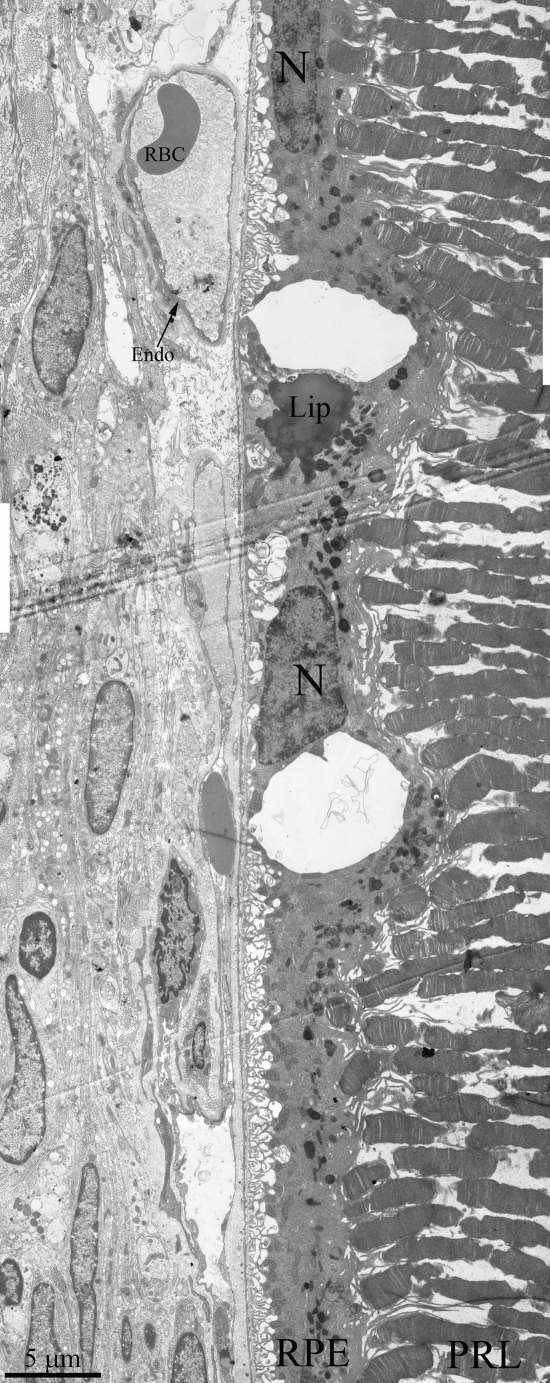
Figure 5. Ultrastructure of outer retina of Wistar rat at the age of 25 months. PRL – photoreceptors, RPE – retinal pigment epithelial cell, N – RPE nucleus, Lip – lipo-fuscin material, BM – Bruch's membrane, Endo – endothelial cell, RBC – red blood cell.
Ultrastructure of Wistar retina had some changes that typical for aging, but it was not so dramatic pathology (Figure 5) as in OXYS rats. We can see all outer retina layers. It is also increased amount of lipofuscin granules. Cavity of most choriocapillaries decreased, but vessels are functionally active, as it visible.
Quantitative analyses of the morphometry data showed that the average area of RPE cell was affected both by age (F4.414= 15.8, p<0.000) and by genotype (F1.39=73.4, p<0.000) and there was also a significant interaction between these two factors (F4.414=3.7, p<0.007). Overall, we observed well-pronounced growth with age the average area of RPE cells in both OXYS and Wistar rats from 20 days to 17 months of age (1.4- and 1.6-fold respectively, p<0.000). By the age of 24 months, the area extent of RPE cells decreased slightly compared to 17-month-old animals, but the decline was significant only in the Wistar rats (p<0.00004), while in the OXYS strain, we detected only a tendency for lowering (p<0.056) as shown on Figure 6a.
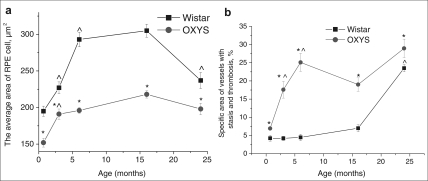
Figure 6. Age-related changes of the average area of RPE cells (a) and specific area of vessels with stasis and thrombosis (b) in the retinas' sections of OXYS and Wistar rats. *interstrain differences; ^differences in comparison with previous age; p<0.05. Data presented as mean ± S.E.M.
Pairwise comparisons within the strains revealed reduction of average area of RPE cell already in 20-day-old OXYS rats by 22% compared to Wistar rats (p<0.000). At ages 3, 6, 17 and 24 months, the differences remained and this parameter was lower in OXYS rats by 15% (p<0.023), 33% (p<0.000), 28% (p<0.001) and 16% (p<0.026), respectively.
ANOVA analysis of morphometry data revealed age- related increasing of the specific area of choriocapillaris with stasis and thrombosis (F4.68= 22.4, p<0.000), which was observed in rats of both strains. In addition, its quantity was affected by genotype (F1.68=56.7, p<0.000), and since the age of 20 days, specific area of vessels with signs of partial occlusion of retinal vessels was greater in OXYS rats compared to the age-matched Wistar controls (Figure 7b). There was also a significant interaction between these two factors (F4.68=4.8, p<0.002). As shown in the Figure 6b, in the Wistar retina, the number of vessels with thrombosis and stasis remained unchanged between 20 days and 17 months of age. Only at age 24 months the percentage of choriocapillaris with signs of abnormality became 5.6-fold greater than that in the 20-day-old animals.
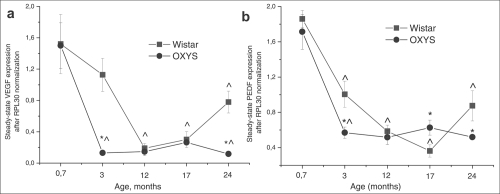
Figure 7. Messenger RNA (mRNA) expression of (a) vascular endothelium growth factor (VEGF) and (b) pigment epithelium-derived factor (PEDF) in retina determined by real-time polymerase chain reaction (PCR), N=5. Data presented as mean ± S.E.M. *interstrain differences; ^differences in comparison with previous age; p<0.05.
OXYS rats exhibited a relatively rapid expansion of specific area of choriocapillaris with stasis and thrombosis already by age 6 months. Up to the age of 24 months, these pathological changes progressed, but at a slower rate. As a result, at ages 3, 6, and 24 months, this parameter in the OXYS retina was 2.6-, 3.6-, and 4-fold higher compared to 20-day-old OXYS rats (p<0.006, p<0.0002, p<0.0001, respectively).
Gene Expression
Expression of VEGF gene was affected both by age (F4.39= 18.3, p<0.000) and by genotype (F1.39=10.0, p<0.003) and there was also a significant interaction between these two factors (F4.39=3.3, p<0.020). The same was observed for expression of PEDF gene: age – F4.39=46.9; p<0.000; genotype – F1.39=4.5; p<0.040. Factors also interacted with each other (F4.39=3.1; p<0.026), which is probably due to the differences in age-dependent changes of retinal VEGF and PEDF expression between Wistar and OXYS strains. Post hoc analysis revealed that the maximum level of genes mRNA was in the retina at the age of 20 days (Figure 6) and had not difference between strains. Expression of VEGF and PEDF genes decreased 10-fold (p<0.000) and 5-fold (p<0.001) respectively between 20 days and 24 months of age in both Wistar and OXYS rats. At the same time, in OXYS rats, the reduction progressed more rapidly than in Wistar (Figure 7).
At the age of 20 days, there was no differencebetween Wistar and OXYS rats in terms of expression of VEGF and PEDF. But already at the age of 3 months, VEGF expression (Figure 7a) in OXYS rats was almost 10-fold lower (F1.8=21.9, p<0.002) compared to 20-day-old animals and to age-matched Wistar rats (F1.8=21.7, p<0.002) and PEDF expression (Figure 7b) decreased three-fold (p<0.0001) from age 20 days to 3 months and was about two-fold lower (F1.8=7.29; p<0.027) compared to the age-matched Wistar animals (F1.8=7.29; p<0.027). Expression of both genes in OXYS rats remained without significant changes throughout the rest of the observation period.
VEGF expression at the age of 24 months was 4- (p<0.002) and 2.6-fold higher (p<0.008) than in 12 and 17-month-old Wistar rats respectively, and it reached half of the expression level at the age of 20 days (p<0.0003). By the age of 24 months, PEDF expression increased by 2.4-fold (p<0.013) compared to 17-month-old Wistar rats (Figure 7).