Rapamycin extends lifespan and delays tumorigenesis in heterozygous p53+/− mice
Abstract
The TOR (Target of Rapamycin) pathway accelerates cellular and organismal aging. Similar to rapamycin, p53 can inhibit the mTOR pathway in some mammalian cells. Mice lacking one copy of p53 (p53+/− mice) have an increased cancer incidence and a shorter lifespan. We hypothesize that rapamycin can delay cancer in heterozygous p53+/− mice. Here we show that rapamycin (given in a drinking water) extended the mean lifespan of p53+/− mice by 10% and when treatment started early in life (at the age less than 5 months) by 28%. In addition, rapamycin decreased the incidence of spontaneous tumors. This observation may have applications in management of Li-Fraumeni syndrome patients characterized by heterozygous mutations in the p53 gene.
Introduction
The mTOR (mammalian Target of Rapamycin) pathway plays a crucial role in the geroconversion from cell cycle arrest to senescence (geroconversion) [1]. Rapamycin suppresses or decelerates geroconversion, maintaining quiescence instead [2-8]. Furthemore, inhibition of the TOR pathway prolongs lifespan in model organisms, including mice [9-13]. In an organism, nutrients activate mTOR [14-16], whereas fasting or calorie restriction deactivates mTOR [17-19]. Calorie restriction slows down aging [20] and postpones tumorigenesis in several animal models [21,22], including p53-deficient mice [23-25].
Similar to other tumor suppressors, p53 can inhibit mTOR in mammalian cells [26-31]. While causing cell cycle arrest, p53 can suppress geroconversion, thus preventing a senescent phenotype in the arrested cells [30,31]. Therefore, it is not suprising that p53 inhibits hyper-secretory phenotype, a hallmark of senescence [32] whereas p53-deficiency resulted in pro-inflammatory phenotype [33,34]. Noteworthy, the activity of p53 is decreased with aging [35]. Lack of one p53-allele (p53+/−) accelerates carcinogenesis and shortens lifespan [36-41]. We propose that rapamycin can decelerate cancer development in p53+/− mice. Here we show experimental evidence supporting this hypothesis.
Results
Rapamycin (approximate dose, 1.5 mg/kg/day) was given in drinking water. 75 mice were divided into two groups: control (n=38) and rapamycin-treated (n=37). The mean lifespan of animals in control group was 373 days and the last 10% of survivals lived as long as 520 days (Fig. 1 A). In rapamycin-treated mice, the mean lifespan was 410 days and lifespan of the last 10% of survivals could not be determined (Fig. 1 A). Mice in both groups were also monitored for tumor development. The data presented in Fig. 1B demonstrate that carcinogenesis was significantly delayed in rapamycin-treated mice compared to control mice.
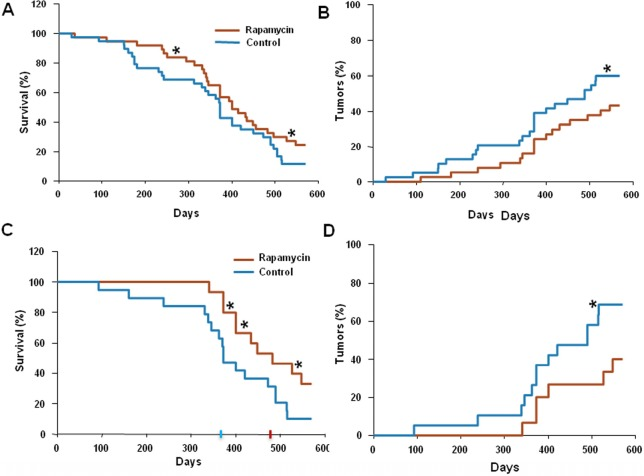
Figure 1. Administration of rapamycin extends lifespan and delays carcinogenesis in p53+/− male mice. (A) Kaplan Meier survival curve of rapamycin-treated (red line) and control (blue line) mice. (B) Incidence of tumors in rapamycin-treated (red) and control (blue) mice. Animals received rapamycin starting at various ages at 1.5 mg/kg per day in drinking water throughout entire life. * p<0.05. (C) Kaplan Meier survival curve of rapamycin-treated (red line) and control (blue line) mice that start receiving rapamycin early in life (<5 months). (D) Incidence of tumors in rapamycin-treated (red) and control (blue) mice that start receiving rapamycin early in life (<5 months). * p<0.05 toph
Since in our experiments animals started to receive rapamycin at different age, we sought to test whether this affected the outcome of the treatment.
For this, we further subdivided all mice used into two groups: “young” (receiving rapamycin from the age of 5 months or earlier) and “old” (receiving rapamycin starting at 5 months of age or older). Results of the data analysis for the “young” group are shown in Figure 1C and D. The mean lifespan in control group was 373 days, whereas in rapamycin-treated “young” mice the mean lifespan reached 480 days, 3.5 months increase over the control group. Furthermore, 40% of rapamycin-treated “young” mice survived 550 days (Fig. 1C) and by this age developed 2 times less tumors than control mice (Fig. 1D). In the “old” group the difference between control and treated group was blunted (data not shown).
Thus, the life-extending effect of rapamycin is more pronounced when treatment starts earlier in life. In order to confirm that rapamycin administered with drinking water has biological activity in vivo, we measured levels of phosphorylated ribosomal protein S6 (pS6), a marker of the mTOR activity in tissues of control and rapamycin-treated mice. After receiving rapamycin in drinking water for 2 days, mice were sacrificed and the levels of total S6 and pS6 were estimated by Western blot analysis and immuno-cytochemistry (Fig. 2).
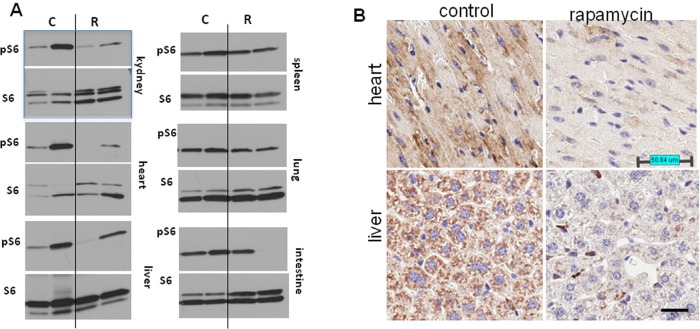
Figure 2. Administration of rapamycin in drinking water inhibits the mTOR pathway in p53+/− male mice. (A) Western blot analysis of whole cell lysates of 6 organs of rapamycin-treated and control mice probed with antibodies specific to S6 and phospho-S6 (Ser240/244). Mice received rapamycin in drinking water for 2 days. (B) Immunohistochemistry. pS6 in the heart and the liver. Mice received rapamycin in drinking water for 2 days.
As shown in Fig. 2A, levels of pS6 were reduced in the heart, kidney and liver of rapamycin-treated mice. Also, pS6/S6 ratios were lower in rapamycin-treated mice (Fig. S1).
These results were confirmed by immunohistochemical staining showing lower levels of pS6 in tissues of rapamycin-treated mice (Fig. 2B). The variability of pS6 levels among mice may explain the variability of biological effects of rapamycin.
Discussion
Previously it was shown that rapamycin prolongs lifespan in genetically heterogeneous mice [11], [12], inbred mice [42] and Her2-expressing mice [13]. In normal genetically heterogeneous mice, rapamycin extended life span even when its administration was started later in life [11]. Our data in p53+/− mice show that the effect of rapamycin was blunted when treatment started at the age of 5 months or older.
This indicates that the anti-cancer effect of rapamycin is likely to be indirect and is imposed via its systemic effect at the level of an organism rather than through direct inhibition of tumor growth. To further address this question we plan to test the effect of rapamycin on animals with established tumors (by measuring tumor growth) along with evaluating the functional status of mTOR and the ability of rapamycin to suppress it in tumors and normal tissues. As we report here, administration of rapamycin starting early in life increased mean lifespan in p53+/− male mice by 28%. Previous work has demonstrated that the life-extending effects of rapamycin [11,12] as well as metformin [43,44], calorie restriction [45] and genetic inhibition of the IGF-I/mTOR/S6K pathway [46,47] were less pronounced in male mice compared with female mice. Moreover, in some cases, life span extension was achieved in female mice only [43,47]. Therefore, the observed increase in the median lifespan is dramatic, taking into account that it was achieved in male mice. However, because of low bioavailability of rapamycin, it was given constantly (in drinking water) without interruptions, whereas intermittent schedules may be more appropriate for future clinical developments as cancer-preventive interventions. In fact, a novel formulation of rapamycin (Rapatar) may be given intermittently, which still reveal even more pronounced extension of life span in p53-deficient mice (Comas et al, Aging 2012; this issue).
Our study suggests that rapamycin can be considered for cancer prevention in patients with Li-Fraumeni syndrome. Li-Fraumeni syndrome is an autosomal dominant disorder with a germline p53 mutation [48]. The incidence of cancer in carriers of mutation reaches 50% at the age of 40 and 90% at the age 60. Children of affected parents have an approximate 50% risk of inheriting the familial mutation [48]. Although functional assays have been established allowing for easy genetic testing for TP53 mutation, no effective chemopreventive therapy is currently available. The p53 rescue compounds may hold some promise in the future [48-50]; however these are not clinically approved drugs. In contrast, rapamycin has been used in the clinic for over a decade mostly in renal transplant patients. It was reported that rapamycin significantly decreased cancer incidence in renal transplant patients [51-53]. Our data suggest that rapamycin or its analogs can be considered for cancer prevention in Li-Fraumeni syndrome.
Methods
Mice
All animal studies were conducted in accordance with the regulations of the Committee of Animal Care and Use at Roswell Park Cancer Institute. The colony of p53-knockout mice on a C57B1/6 background (originally obtained from Jackson Laboratories, Bar Habor, ME) was maintained by crossing p53+/− females with p53−/− males followed by genotyping of the progeny (PCR) as described previously [54]. Heterozygous p53+/− mice were generated by crossing p53−/− males with wild type p53 females. Male mice were kept in polypropelene cages (30×21×10 cm) under standard light/dark regimen (12 hours light: 12 hours darkness) at 22 ± 2°C, And received standard laboratory chow and water ad libitum.
Rapamycin treatment
Rapamycin (LC Laboratories, USA) was diluted in ethanol at concentration 15 mg/ml. Then the stock was diluted 1:1000 in drinking water. Drinking water was changed every week. Male mice were randomly divided into two groups. Mice of the first group (n=37) were given rapamycin in drinking water (approximately 1.5 mg/kg per day), whereas mice of the second group (n=38) were given tap water without rapamycin and served as control. Once a week all mice were palpated for detection of tumor mass appearance.
Pathomorphological examination
All animals were autopsied. Site, number and size of tumors were checked. All tumors, as well as the tissues and organs with suspected tumor development were excised and fixed in 10% neutral formalin. After the routine histological processing the tissues were embedded into paraffin. 5–7 μm thin histological sections were stained with haematoxylin and eosine and were microscopically examined. Tumors were classified according to International Agency for Research on Cancer recommendations.
Western blot analysis
Tissues were homogenized in Bullet blender using stainless steel 0.5 mm diameter beads (Next Advantage, Inc. NY, USA) and RIPA lysis buffer supplemented with protease and phosphatase inhibitors tablets (Roche Diagnostics, Indianopolis, IN, USA). Lysates were cleared by centrifugation at 4°C at 13000 rpm. Equal amounts of protein were separated on gradient Criterion gels (BioRad) and immunoblotting was performed with rabbit anti-phospho S6 (Ser 240/244) and mouse anti-S6 antibodies from Cell Signaling Biotechnology as described previously [55], [56].
Immunochemistry
Dissected tissue samples were fixed in 10% buffered formalin, embedded into paraffin. 5–7 μm thin histological sections were stained with anti-phospho S6 (Ser240/244) antibody (Cell Signaling) and counterstained with Hematoxylin.
Statistical analyses
The SigmaStat software package was used for analysis. The P values were calculated using Fisher's Exact Test (2-tail). P<0.05 was considered as statistically significant.
Conflicts of Interest
The authors of this manuscript have no conflict of interests to declare.
References
-
1.
Blagosklonny MV.
Cell cycle arrest is not yet senescence, which is not just cell cycle arrest: terminology for TOR-driven aging.
Aging (Albany NY).
2012;
4:
159
-165.
[PubMed]
.
-
2.
Demidenko ZN, Zubova SG, Bukreeva EI, Pospelov VA, Pospelova TV, Blagosklonny MV.
Rapamycin decelerates cellular senescence.
Cell Cycle.
2009;
8:
1888
-1895.
[PubMed]
.
-
3.
Gan B, Sahin E, Jiang S, Sanchez-Aguilera A, Scott KL, Chin L, Williams DA, Kwiatkowski DJ, DePinho RA.
mTORC1-dependent and -independent regulation of stem cell renewal, differentiation, and mobilization.
Proc Natl Acad Sci U S A.
2008;
105:
19384
-19389.
[PubMed]
.
-
4.
Gan B and DePinho RA.
mTORC1 signaling governs hematopoietic stem cell quiescence.
Cell Cycle.
2009;
8:
1003
-1006.
[PubMed]
.
-
5.
Chen C, Liu Y, Liu R, Ikenoue T, Guan KL, Zheng P.
TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species.
J Exp Med.
2008;
205:
2397
-2408.
[PubMed]
.
-
6.
Chen C, Liu Y, Zheng P.
mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells.
Sci Signal.
2009;
2:
ra75
[PubMed]
.
-
7.
Adhikari D, Zheng W, Shen Y, Gorre N, Hamalainen T, Cooney AJ, Huhtaniemi I, Lan ZJ, Liu K.
Tsc/mTORC1 signaling inoocytes governs the quiescence and activation of primordial follicles.
Human Molecular Genet.
2010;
19:
397
-410.
.
-
8.
Kolesnichenko M, Hong L, Liao R, Vogt PK, Sun P.
Attenuation of TORC1 signaling delays replicative and oncogenic RAS-induced senescence.
Cell Cycle.
2012;
11:
2391
-2401.
[PubMed]
.
-
9.
Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S.
Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway.
Curr Biol.
2004;
14:
885
-890.
[PubMed]
.
-
10.
Bjedov I, Toivonen JM, Kerr F, Slack C, Jacobson J, Foley A, Partridge L.
Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster.
Cell Metab.
2010;
11:
35
-46.
[PubMed]
.
-
11.
Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandezr E, Miller RA.
Rapamycin fed late in life extends lifespan in genetically heterogenous mice.
Nature.
2009;
460:
392
-396.
[PubMed]
.
-
12.
Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, et al.
Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice.
J Gerontol A Biol Sci Med Sci.
2011;
66:
191
-201.
[PubMed]
.
-
13.
Anisimov VN, Antoch M, Blagosklonny MV, et al.
Am J Pathol.
2010;
in press
.
-
14.
Khamzina L, Veilleux A, Bergeron S, Marette A.
Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance.
Endocrinology.
2005;
146:
1473
-1481.
[PubMed]
.
-
15.
Tremblay F, Krebs M, Dombrowski L, Brehm A, Bernroider E, Roth E, Nowotny P, WaldhŠusl W, Marette A, Roden M.
Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability.
Diabetes.
2005;
54:
2674
-2684.
[PubMed]
.
-
16.
Krebs M, Brunmair B, Brehm A, Artwohl M, Szendroedi J, Nowotny P, Roth E, FŸrnsinn C, Promintzer M, Anderwald C, Bischof M, Roden M.
The Mammalian target of rapamycin pathway regulates nutrient-sensitive glucose uptake in man.
Diabetes.
2007;
56:
1600
-1607.
[PubMed]
.
-
17.
Jiang W, Zhu Z, Thompson HJ.
Dietary energy restriction modulates the activity of AMP-activated protein kinase, Akt, and mammalian target of rapamycin in mammary carcinomas, mammary gland, and liver.
Cancer Res.
2008;
68:
5492
-5499.
[PubMed]
.
-
18.
Estep PWr, Warner JB, Bulyk ML.
Short-term calorie restriction in male mice feminizes gene expression and alters key regulators of conserved aging regulatory pathways.
PLoS One.
2009;
4:
e5242
[PubMed]
.
-
19.
Masternak MM, Panici JA, Bonkowski MS, Hughes LF, Bartke A.
Insulin sensitivity as a key mediator of growth hormone actions on longevity.
J Gerontol A Biol Sci Med Sci.
2009;
64:
516
-521.
[PubMed]
.
-
20.
Wang C, Maddick M, Miwa S, Jurk D, Czapiewski R, Saretzki G, Langie SA, Godschalk RW, Cameron K, von Zglinicki T.
Adult-onset, short-term dietary restriction reduces cell senescence in mice.
Aging (Albany NY).
2010;
2:
555
-566.
[PubMed]
.
-
21.
Dirx MJ, Zeegers MP, Dagnelie PC, van den Bogaard T, van den Brandt PA.
Energy restriction and the risk of spontaneous mammary tumors in mice: a meta-analysis.
Int J Cancer.
2003;
106:
766
-770.
[PubMed]
.
-
22.
Sarkar NH, Fernandes G, Telang NT, Kourides IA, Good RA.
Low-calorie diet prevents the development of mammary tumors in C3H mice and reduces circulating prolactin level, murine mammary tumor virus expression, and proliferation of mammary alveolar cells.
Proc Natl Acad Sci U S A.
1982;
79:
7758
-7762.
[PubMed]
.
-
23.
Berrigan D, Perkins SN, Haines DC, Hursting SD.
Adult-onset calorie restriction and fasting delay spontaneous tumorigenesis in p53-deficient mice.
Carcinogenesis.
2002;
23:
817
-822.
[PubMed]
.
-
24.
Hursting SD, Perkins SN, Phang JM.
Calorie restriction delays spontaneous tumorigenesis in p53-knockout transgenic mice.
Proc Natl Acad Sci U S A.
1994;
91:
7036
-7040.
[PubMed]
.
-
25.
Hursting SD, Perkins SN, Brown CC, Haines DC, Phang JM.
Calorie restriction induces a p53-independent delay of spontaneous carcinogenesis in p53-deficient and wild-type mice.
Cancer Res.
1997;
57:
2843
-2846.
[PubMed]
.
-
26.
Feng Z, Hu W, de Stanchina E, Teresky AK, Jin S, Lowe S, Levine AJ.
The regulation of AMPK beta1, TSC2, and PTEN expression by p53: stress, cell and tissue specificity, and the role of these gene products in modulating the IGF-1-AKT-mTOR pathways.
Cancer Res.
2007;
67:
3043
-3053.
[PubMed]
.
-
27.
Feng Z, Zhang H, Levine AJ, Jin S.
The coordinate regulation of the p53 and mTOR pathways in cells.
Proc Natl Acad Sci U S A.
2005;
102:
8204
-8209.
[PubMed]
.
-
28.
Levine AJ, Feng Z, Mak TW, You H, Jin S.
Coordination and communication between the p53 and IGF-1-AKT-TOR signal transduction pathways.
Genes Dev.
2006;
20:
267
-275.
[PubMed]
.
-
29.
Budanov AV and Karin M.
p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling.
Cell.
2008;
134:
451
-460.
[PubMed]
.
-
30.
Demidenko ZN, Korotchkina LG, Gudkov AV, Blagosklonny MV.
Paradoxical suppression of cellular senescence by p53.
Proc Natl Acad Sci U S A.
2010;
9660-4:
9660
-9664.
[PubMed]
.
-
31.
Korotchkina LG, Leontieva OV, Bukreeva EI, Demidenko ZN, Gudkov AV, Blagosklonny MV.
The choice between p53-induced senescence and quiescence is determined in part by the mTOR pathway.
Aging (Albany NY).
2010;
2:
344
-352.
[PubMed]
.
-
32.
CoppŽ JP, Patil CK, Rodier F, Sun Y, Mu-oz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J.
Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor.
PLoS Biol.
2008;
6:
2853
-2868.
[PubMed]
.
-
33.
Komarova EA, Krivokrysenko V, Wang K, Neznanov N, Chernov MV, Komarov PG, Brennan ML, Golovkina TV, Rokhlin OW, Kuprash DV, Nedospasov SA, Hazen SL, Feinstein E, Gudkov AV.
p53 is a suppressor of inflammatory response in mice.
FASEB J.
2005;
19:
1030
-1032.
[PubMed]
.
-
34.
Gudkov AV, Gurova KV, Komarova EA.
Inflammation and p53: A Tale of Two Stresses.
Genes Cancer.
2011;
2:
503
-516.
[PubMed]
.
-
35.
Feng Z, Hu W, Teresky AK, Hernando E, Cordon-Cardo C, Levine AJ.
Declining p53 function in the aging process: a possible mechanism for the increased tumor incidence in older populations.
Proc Natl Acad Sci U S A.
2007;
104:
16633
-16638.
[PubMed]
.
-
36.
Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA Jr., Butel JS, Bradley A.
Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours.
Nature.
1992;
356:
215
-221.
[PubMed]
.
-
37.
Harvey M, McArthur MJ, Montgomery CA Jr., Butel JS, Bradley A, Donehower LA.
Spontaneous and carcinogen-induced tumorigenesis in p53-deficient mice.
Nat Genet.
1993;
5:
225
-229.
[PubMed]
.
-
38.
Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, Weinberg RA.
Tumor spectrum analysis in p53-mutant mice.
Curr Biol.
1994;
4:
1
-7.
[PubMed]
.
-
39.
Donehower LA, Harvey M, Vogel H, McArthur MJ, Montgomery CA Jr., Park SH, Thompson T, Ford RJ, Bradley A.
Effects of genetic background on tumorigenesis in p53-deficient mice.
Mol Carcinog.
1995;
14:
16
-22.
[PubMed]
.
-
40.
Venkatachalam S, Shi YP, Jones SN, Vogel H, Bradley A, Pinkel D, Donehower LA.
Retention of wild-type p53 in tumors from p53 heterozygous mice: reduction of p53 dosage can promote cancer formation.
Embo J.
1998;
17:
4657
-4667.
[PubMed]
.
-
41.
Hinkal G, Parikh N, Donehower LA.
Timed somatic deletion of p53 in mice reveals age-associated differences in tumor progression.
PLoS One.
2009;
4:
e6654
[PubMed]
.
-
42.
Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Rosenfeld SV, Blagosklonny MV.
Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice.
Cell Cycle.
2011;
10:
4230
-4236.
[PubMed]
.
-
43.
Anisimov VN, Piskunova TS, Popovich IG, Zabezhinski MA, Tyndyk ML, Egormin PA, Yurova MV, Rosenfeld SV, Semenchenko AV, Kovalenko IG, Poroshina TE, Berstein LM.
Gender differences in metformin effect on aging, life span and spontaneous tumorigenesis in 129/Sv mice.
Aging (Albany NY).
2010;
2:
945
-958.
[PubMed]
.
-
44.
Blagosklonny MV.
Metformin and sex: Why suppression of aging may be harmful to young male mice.
Aging (Albany NY).
2010;
2:
897
-899.
[PubMed]
.
-
45.
Turturro A, Duffy P, Hass B, Kodell R, Hart R.
Survival characteristics and age-adjusted disease incidences in C57BL/6 mice fed a commonly used cereal-based diet modulated by dietary restriction.
J Gerontol A Biol Sci Med Sci.
2002;
57:
B379
-389.
[PubMed]
.
-
46.
Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, Cervera P, Le Bouc Y.
IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice.
Nature.
2003;
421:
182
-187.
[PubMed]
.
-
47.
Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, Woods A, Robinson IC, Schuster E, Batterham RL, Kozma SC, Thomas G, et al.
Ribosomal protein S6 kinase 1 signaling regulates mammalian life span.
Science.
2009;
326:
140
-144.
[PubMed]
.
-
48.
Upton B, Chu Q, Li BD.
Li-Fraumeni syndrome: the genetics and treatment considerations for the sarcoma and associated neoplasms.
Surg Oncol Clin N Am.
2009;
18:
145
-156.
ix
[PubMed]
.
-
49.
Glazer RI.
A new therapeutic basis for treating Li-Fraumeni Syndrome breast tumors expressing mutated TP53.
Oncotarget.
2010;
1:
470
-471.
[PubMed]
.
-
50.
Herbert BS, Chanoux RA, Liu Y, Baenziger PH, Goswami CP, McClintick JN, Edenberg HJ, Pennington RE, Lipkin SM, Kopelovich L.
A molecular signature of normal breast epithelial and stromal cells from Li-Fraumeni syndrome mutation carriers.
Oncotarget.
2010;
1:
405
-422.
[PubMed]
.
-
51.
Mathew T, Kreis H, Friend P.
Two-year incidence of malignancy in sirolimus-treated renal transplant recipients: results from five multicenter studies.
Clin Transplant.
2004;
18:
446
-449.
[PubMed]
.
-
52.
Campistol JM, Eris J, Oberbauer R, Friend P, Hutchison B, Morales JM, Claesson K, Stallone G, Russ G, Rostaing L, Kreis H, Burke JT, Brault Y, Scarola JA, Neylan JF.
Sirolimus Therapy after Early Cyclosporine Withdrawal Reduces the Risk for Cancer in Adult Renal Transplantation.
J Am Soc Nephrol.
2006;
17:
581
-589.
[PubMed]
.
-
53.
Blagosklonny MV.
Prevention of cancer by inhibiting aging.
Cancer Biol Ther.
2008;
7:
1520
-1524.
[PubMed]
.
-
54.
Leonova KI, Shneyder J, Antoch MP, Toshkov IA, Novototskaya LR, Komarov PG, Komarova EA, Gudkov AV.
A small molecule inhibitor of p53 stimulates amplification of hematopoietic stem cells but does not promote tumor development in mice.
Cell Cycle.
2010;
9:
1434
-1443.
[PubMed]
.
-
55.
Leontieva O, Gudkov A, Blagosklonny M.
Weak p53 permits senescence during cell cycle arrest.
Cell Cycle.
2010;
9:
4323
-4327.
[PubMed]
.
-
56.
Leontieva OV and Blagosklonny MV.
DNA damaging agents and p53 do not cause senescence in quiescent cells, while consecutive re-activation of mTOR is associated with conversion to senescence.
Aging (Albany NY).
2010;
2:
924
-935.
[PubMed]
.