Introduction
Sea urchins belong to deuterostomes and as such are closer relatives to vertebrates than other invertebrate taxons such as insects and nematodes. Sea urchins are used as a convenient model for developmental biology. Fertilization of sea urchin eggs takes place in sea water and is followed by rapid development of a pluteus, a free floating larva possessing bilateral symmetry. Radially symmetrical adult body develops from the rudiment asymmetrically placed within the larva. Adult sea urchins possess calcite skeleton and live on seabed from tidal zone to the several kilometers deep.
Apart from complex development scheme, sea urchins attracted attention due to extreme longevity of some of their species. Red sea urchin, S. franciscanus, populating cold waters of Pacific coast of North America, was demonstrated to survive over a century [1]. Although S. franciscanus could not be cultivated in the lab for a century for direct observation, deposition pattern of radioactive carbon released to the Pacific upon nuclear tests [2] and skeleton growth rate studies using tetracycline labeling [1] allowed red sea urchin to climb the pedestal of the most long-lived marine animals [3]. At the same time, green sea urchin, L. variegatus, populating warm Caribbean sea hardly survive over four years [4]. Although direct difference in the senescence rates between red and green sea urchins is hard to demonstrate directly on the sole basis of field studies, these two related species might be the a convenient pair for comparative genetics of longevity.
In this report we aimed to obtain draft genome assemblies of S. franciscanus and L. variegatus and compare the sequence of their proteins related to longevity with longevity related proteins of other species. We used mapping of our sequencing data onto previously published complete genomic sequence of a purple sea urchin, Strongylocentrotus purpuratus [5].
Discussion
Phenotype is determined by a genotype. In this paradigm all explanations of the longevity difference between species and to certain extent even between individuals could be deduced from their genomes. In a golden dream, one could deduce a limited set of genetic variations which might be introduced to a certain genome to extend the lifespan of a creature. However, what sort of differences are we looking for? Even genomes of individuals who belong to the same species differ too much to allow easy determination of those differences that have an impact on longevity. E.g. genome-wide association studies, carried on different cohorts rarely came to the same candidate genes whose specific allelic variants are beneficial for longevity, ApoE and Foxo3A being only reproducible examples [10]. At the same time a number of single mutations or a small set of mutations may increase an average lifespan of a model organism by a substantial proportion, sometimes twice or even more. Mutations, affecting insulin related receptor/forkhead transcription factor pathway in C. elegans allowed to extend lifetime of a worm by a factor of five [11]. This finding correlates well with the observation that caloric restriction is one of the key environmental factors influencing longevity [12]. Do we have a hope that genetic difference between the related species that have drastically different lifespan could explain the longevity? If senescence is a consequence of entire developmental program of the individual [13] leading to the accumulation of undiluted poisonous by-products of metabolism [14] then genome as a whole encode the longevity. However, if senescence is a program beneficial for the survival of a population at the expense of individual, as was originally proposed by August Weizmann [15] and later rephrased by other scientists [16] than a limited set of genes might be found to determine longevity. In both cases, complete genomes of related species could be used to determine the basis of longevity.
A number of comparative studies were previously done to decipher genetic backgrounds of exceptional longevity. Naked mole rat, Heterocephalus glaber, has a lifespan of other 30 years and shows no increase in mortality with its age [17]. Sequencing of its genome allowed a direct comparison with the genome of related short living rodent, mouse [14]. Later on, the complete genomic sequence of exceptionally long living bat, Myotis brandtii, was deciphered [18]. Apart from genes obviously related to the adaptation to ecological niche, specific genetic variants were revealed for telomere maintenance and DNA integrity maintenance systems of naked mole rat [19]. Genes encoding a subset of insulin related receptor/forkhead transcription factor pathway components were found to differ Myotis brandtii from other species [14].
Complete genomes of a number of exceptionally long living species, including human [20] become available as well as genomic sequences of related short living species, which could be used for comparison. We decided to use genomic sequence of S. franciscanus and L. variegatus determined in our study to analyze variations distinguishing the species on the basis of their longevity. We selected a set of genes previously known to affect longevity (Table 1) of the model species and made alignments of their homologues from the set of organisms. We included human (Homo sapiens), naked mole rat (Heterocephalus glaber), bat (Myotis brandtii) and red sea urchin (S. franciscanus) into our set as long living organisms, while mouse (Mus musculus) and green sea urchin (L. variegatus) populated a list of short living species. Protein sequences of purple sea urchin S. purpuratus were also included into alignments as a reference. If a protein originally described to alter longevity was initially described in the species other than listed, its sequence is also included to the alignment. For all listed species the protein sequences most closely related to the query was taken for the alignment. It should be stated that some parts of the protein sequences might be misidentified due to the ambiguity in identification of juxtaposed contigs and that some marginally similar proteins could actually perform non-ortologues function.
Table 1. Proteins that could be related to longevity according to the literature data
| | Residues that co-vary with longevity | Commentaries |
|---|
| Category | Protein | H. sapiens | H. glaber | M. brandtii | S. franciscanus | L. variegatus | M. musculus | |
|---|
| Mitochondrial proteins encoded in mitochondria | ND1 | | | | | | | |
| ND2 | T156
W239 | S
I | | A
I | V
T | M
A | |
| COX1 | | | | | | | |
| ND4L | | | | | | | |
| COX2 | | | | | | | |
| ATP8 | | | | | | | |
| ATP6 | | | | | | | |
| COX3 | | | | | | | |
| ND3 | L12
L15 | S
S | | A
T | V
I | L
L | |
| ND4 | | | | | | | |
| ND5 | I283 | T | | T | I | L | |
| CYTB | | | | | | | |
| Mitochondrial proteins encoded in nucleus and could be related to longevity | CYTC | | | | | | | |
| COX4 | | | | | | | |
| COX5B | | | | | | | COX5 mutation in P. anserine increase lifespan 10-times [31] |
| COX6A | | | | | | | |
| COX6B | | | | | | | |
| COX6C | | | | | | | |
| COX7C | | | | | | | |
| p66Shc | | | | | | | Increases reactive oxygen species production [14] |
| Proteins involved in detoxification of reactive oxygen species | MnSOD | | | | | | | Overexpression extends lifespan of fly [35] |
| CuZnSOD | | | | | | | |
| CAT | | | | | | | |
| Prdx | | | | | | | |
| GPx | | | | | | | |
| Lipid transport proteins | ApoB | K720
I3433 | K
I | K
I | K
I | N
P | E
A | ApoE allelic variant is associated with increased lifespan in humans [39]. Since ApoE homologues were not identified in sea urchins, all apolipoproteins which are present in sea urchin were analyzed. |
| ApoA | | | | | | |
| ApoH | R203
N253 | R
N | R
N | K
E | I
S | L
T |
| ApoD | K75
I138 | K
I | K
V | R
L | Q
F | E
F |
| ApoO | | | | | | |
| LDLR | | | | | | |
| VLDLR | | | | | | |
| CETP | | | | | | |
| Proteins involved in amyloidogenesis | APP | | | | | | | Mutations cause predisposition to Alzheimer disease in humans [22]. |
| PSEN1 | R42 | R | R | R | Q | |
| BACE1 | | | | | | | |
| Telomere maintenance | TERT | G252
R342
T491
P702
D975 | G
R
T
P
D | | G
K
S
P
D | R
Q
I
N
S | R
N
L
Q
S | |
| POT1 | I198 | I | V | V | S | T | |
| TEP1 | | | | | | | |
| Insulin/IGF1 signaling pathway | INSR | | | | | | | |
| IGF1R | | | | | | | |
| IRS1 | | | | | | | |
| PTEN | | | | | | | |
| PI3K | H295 (γ isophorm)S275 (αisophorm) | H | H | K | Q | Q | |
| PDK1 | | | | | | | |
| AKT1 | | | | | | | |
| SGK | | | | | | | |
| FOXO1 | | | | | | | |
| FOXO3 | | | | | | | |
| FOXO4 | | | | | | | |
| MTOR | | | | | | | |
| SIRT1 | | | | | | | |
| SIRT2 | | | | | | | |
| YWHAG | | | | | | | |
| Other proteins associated with longevity | clk-1 | H
L | H
I | H
L | K
I | E
Y | N
F | Q117
F132 (C. elegans) |
| daf-9 | | | | | | | |
| Mth | | | | | | | |
| Indy | P
S | P
S | P
S | S
G | N
N | Q
N | E61
V193 (D. melanogaster) |
| EXO1 | | | | | | | |
We used the created alignments (see Supplementary material) for identification of the aminoacid positions co-varied with longevity. Although it might be naïve to expect that single positions within a limited set of proteins could determine longevity, we decided to perform such kind of analysis to suggest hypotheses for further studies.
Amyloid protein biogenesis
Alzheimer disease is one of the widely recognized factors limiting human longevity. In a brain of Alzheimer disease patients one can find an accumulation of beta-amyloid protein plaques [21] which are formed from a peptide excised from APP protein by β (BACE1) and γ-secretases (PSEN1). A number of mutations in APP and PSEN1 genes were identified as a cause of hereditary form of Alzheimer disease [22]. Although sea urchins have rather primitive nervous system we decided to search for APP, PSEN1 and BACE1 homologs in S. franciscanus and L. variegatus genomes. Only short patches of APP homolog in sea urchins display some similarity with mammalian APP preventing direct comparison of the β-amyloid part of the protein. However, both β- and γ-secretases could readily be identified in all sea urchins under study. Only one aminoacid residue was found to correlate with longevity in PSEN1 protein. Aminoacid corresponding to Arg42 of human PSEN1 is represented by arginine in other long living species, is substituted by glutamine in short living mice and green sea urchins. Position of this aminoacid residue is located in the area close to the region 79-291, carrying a number of mutation sites predisposing an individual for Alzheimer disease [22].
Mitochondrial proteins and proteins involved in detoxification of reactive oxygen species
One of the most recognized theories of aging is a theory of oxidative damage [23]. Although originally proposed variant of the theory underwent several rounds of modification [16, 24], the main postulate of negative influence of reactive oxygen species on longevity [25] could still face some exceptions [26]. Positive role of reactive oxygen species in regulatory networks may be more beneficial than potential damage imposed by those reactants [27]. However controversial might be the issue of oxidative damage for senescence we included a set of relevant proteins into our analysis. Among the sequences of proteins encoded in the mitochondrial genome, ND2 subunit of NADH dehydrogenase possesses two aminoacid residues whose identity co-varies with longevity. Aminoacid 156 (human numbering) is represented by small aminoacid in long living species, threonine in human, alanine in red sea urchin, serine in naked mole rat. In contrast, short living mouse and green sea urchin contains large hydrophobic methionine and valine at this position. Opposite specificity is attributed to the aminoacids at position 239 (human numbering). Human ND2 contains tryptophan at position 239, naked mole rat and red sea urchin contains isoleucine, while mice and green sea urchin have small alanine and threonine at this position. Substitutions of proximal aminoacids 150 and 259 in human cause genetically inherited Leber optic neuropathy [28, 29].
In ND3 subunit of NADH dehydrogenase position 12 is occupied by small aminoacids serine and alanine in ND3 of naked mole rat and red sea urchin, while mouse and green sea urchin possesses large hydrophobic leucine and valine at this position. It should be noted that human ND3 also has a leucine at position 12, while human belongs to the species that have an increased lifespan. Similar rules act for the aminoacids at position 15 of ND3. Naked mole rat and red sea urchin contains serine and threonine in this place, while human, mouse and green sea urchins contains leucine or isoleucine. Mutation Thr114Ala in human ND3 was found to be associated with reduced risk of Parkinson disease development [30]. While mouse also has threonine at the position 114, long living naked mole rat possesses aspartic acid and red sea urchins have alanine, similar to people with reduced predisposition to Parkinson disease. In a position 283 of ND5 one can find threonine in naked mole rat and red sea urchin, while human, green sea urchin and mouse contain bulky isoleucine and leucine in this place. Mutation in the cytochrome c oxidase subunit of fungi Podospora anserine resulted in a 10-times increase in the lifespan [31]. We checked for the aminoacid positions that correlate with the lifespan in our species set and could not identify any. However, we noted that COX6B mutation Arg20His which was found in a family with 5-time reduced cytochrome oxidase activity [32] could also be found in the long living bat, M. brandtii. Protein p66Shc was demonstrated to increase reactive oxygen species production in mitochondria [33, 34]. Analysis of p66Shc sequences in the set of long and short living organisms revealed only differences explained by phylogenetic relations, and not by longevity.
A number of proteins aim in detoxification of reactive oxygen species. Among them, superoxide dismutases MnSOD, localized in mitochondria and CuZnSOD residing in the cytoplasm. Ectopic expression of CuZnSOD in fly allowed to extend its lifespan [35]. Mutation Ala16Val in human MnSOD leads to a 30-40% reduction of its activity resulting in cardiomyopathy and nephropathy [36-38]. Long living naked mole rat, similar to human, contains Ala16 residue, while short living mouse contains Val16, similar to humans, predisposed to the pathology. Unfortunately, we were unable to identify the sequence corresponding to this region of MnSOD in sea urchin genomes. We found no substitutions correlated with the lifespan in CuZnSOD, catalase, peroxiredoxin and glutathione peroxidase.
Lipoprotein metabolism
Arthrosclerosis is an important human pathology with age dependent onset and high impact of human longevity. Accumulation of lipid plaques on the walls of blood vessels accompanied by local inflammation increases the risk of heart attack and stroke. Although lipid metabolism of sea urchins might be substantially different from those in mammals, sea urchins possess apolipoproteins which are used as lipid carriers. In humans, the main scaffold for lipid transport as low density lipoprotein particles is ApoB protein. To best of our knowledge none of the mutations in ApoB encoding gene are related to longevity. However, allelic variant of another lipoprotein scaffold protein, ApoE, was recognized as a marker of human longevity [39]. We included several apolipoproteins into our analysis. In the sequence of ApoB protein, aminoacid, corresponding to the aminoacid 620 (human ApoB numbering) is lysine in long living animals. Green sea urchins have asparagine in the equivalent position, while mouse has glutamic acid. Aminoacid residue 3433 of ApoB is isoleucine in long living organisms. This position is occupied by proline and alanine in short living green sea urchin and mouse. ApoH protein also contains two aminoacids, whose identity varies in consort with lifespan. Aminoacid 203 is occupied by positively charged arginine in human, naked mole rat and Myotis brandtii. Red sea urchin also has positively charged aminoacid, lysine, in the same position. Short living creatures, such as green sea urchin and mouse contain isoleucine and leucine at the same place. Aminoacid 253 is represented by asparagine in ApoH of human, naked mole rat and Myotis brandtii and glutamic acid in red sea urchin. In contrast, mouse and green sea urchins have threonine and serine in equivalent position. ApoD protein serves as a scaffold for high density lipoproteins. Its sequence harbors two aminoacids that are varied in concert with longevity. ApoD aminoacid 75 (human ApoD numbering) is positively charged in long living species. Human, naked mole rat and Myotis brandtii possess lysine, while red sea urchin has arginine at this place. Green sea urchin and mouse have glutamine and glutamic acid at this position. Aliphatic aminoacids isoleucine, valine and leucine could be found at the position 138 of human, naked mole rat, Myotis brandtii and red sea urchin. Green sea urchin and mouse have aromatic phenylalanine at this place. Other apolipoproteins analyzed in this study do not have any aminoacids co-varied with longevity.
Insulin/IGF1 signaling
Caloric restriction is one of the known factors of increase in the lifespan [12]. It is sensed through the insulin/IGF1 signaling pathway. Mutations of the components of this pathway could increase longevity of model organisms up to several fold [11]. We analyzed protein sequences of the IGF1 receptor, PI3K, PTEN, PDK, AKT, TOR, SIRT in a set of long and short living organisms. Phosphatidylinositol kinase PI3K is acting downstream of insulin/IGF receptor and leads to increased biosynthetic and antiapoptotic activity. Mutation of PI3K homolog in C. elegans, age-1, doubled lifespan of this organism [40]. Mutations in PI3K gene were found in numerous cancers [41] as well as in individuals predisposed to Cowden syndrome [42], syndrome CLOVES [43] and megalencephaly [44]. Protein sequence of PI3K contains a position which co-varies with longevity. Residue 275 (human PI3K alpha numbering) is occupied with positively charged residues histidine (human PI3K gamma isoform, H. glaber, M. brandtii) and lysine (red sea urchin). Short living mouse and green sea urchin contain neutral glutamine at this position. It should be noted, however, that human PI3K alpha isoform and nematode age-1 protein contains serine at this position. No other components of insulin/IGF1 signaling pathway contained positions which vary in concert with longevity.
Telomerase
Senescence of somatic cells in a culture [45] was insightfully associated with telomere shortening by A. Olovnikov [46], which was later demonstrated experimentally [47]. In the germ line, stem and cancer cells telomere length is maintained by telomerase [48]. Influence of telomerase activity on longevity is not as obvious as its influence on senescence of cell cultures. Telomerase is activated in majority of cancer cells and as such its excessive activity might cause increased risk of cancer development. Inhibition of telomerase activity in somatic tissues might be an evolutionary tradeoff between benefits of tissue renovation and risk of cancer. According to previously published work [49], telomerase activity is not ceased in somatic tissues of both long and short living sea urchins. Never the less, we analyzed genes encoding telomerase components in order to identify positions that co-vary with longevity.
The main catalytic component of telomerase is TERT, carrying enzymatic reverse transcriptase activity. In human, mutations Ala202Thr, His412Tyr, Val694Met, Tyr772Cys and Val1090Met leads to defect in bone marrow development [50]. Mutations Lys902Asn, Arg631Gln, Arg811Cys, Arg901Trp and Pro704Ser result in dyskeratosis [51-53], while yet another set of mutations cause pulmonary fibrosis: Arg865His, Val791Ile, Val867Met, Val170Met, Ala716Thr, Lys902Arg and Pro923Leu [14]. Comparison of TERT sequences of the long and short living organisms resulted in identification of several aminoacids that vary in concert with longevity. Position 252 is occupied by glycine in all long living organisms, while in short living organisms it is occupied by arginine. Positively charged aminoacids, lysine and arginine could be found in position 342 of human, naked mole rat and red sea urchin TERT, while green sea urchin and mouse TERT contain glutamine and asparagine at equivalent position. Position 491 is occupied by hydroxyl containing aminoacids, threonine and serine in TERT of human, naked mole rat and red sea urchin. Same position is populated by hydrophobic residues leucine and isoleucine in TERT of green sea urchin and mouse. Aminoacids 342 and 491 belong to the RNA binding domain of TERT. Catalytic, reverse transcriptase domain contains aminoacid 702, being proline in TERT of human, naked mole rat and red sea urchin. Short living green sea urchin and mouse contain asparagine and glutamine at the equivalent position. It is of note that mutations of neighboring proline 704 leads to dyskeratosis in human, which speaks in favor of functional value of the corresponding region of TERT. In C-terminal domain of TERT aminoacid 975 is represented by aspartic acid in long living organisms while short living organisms contain serine at this place. Thus, telomerase reverse transcriptase contains a largest set of positions that co-vary in agreement with longevity.
Pot1 protein binds telomeric repeats and protects telomeres from degradation [54]. Lack of Pot1 leads to senescence of cells in a culture due to telomere shortening. Aminoacid 198 of human and naked mole rat Pot1 is isoleucine. Another hydrophobic residue, valine, occupies the same position of Myotis brandtii and red sea urchin. At the same place in Pot1 of the short living mouse and green sea urchin we found threonine and serine.
Other proteins, related to longevity
In a genetic screen for Drosophila melanogaster with increased lifespan a mutation in a gene Indy (I'm not dead yet) was found [55]. This gene codes for the transporter of tricarboxylic acid-cycle intermediates [56]. Although involvement of this gene in longevity was a matter of debates [57, 58], we decided to check if any of aminoacid residues of this protein vary in concert with longevity. Aminoacid, equivalent for D. melanogaster aminoacid 61 is a proline in human, M. brandtii and naked mole rat. Red sea urchin harbors serine at equivalent position, while short lived green sea urchin and mouse contain asparagine and glutamine. In originally described Indy protein of fly, glutamic acid might be found at this place. Aminoacid 193 (D. melanogaster numbering) is serine in Indy protein of human, naked mole rat, M. brandtii and glycine in S. franciscanus. Both green sea urchin and mouse have asparagine at the same position. It should be mentioned, that original mutations, found in long lived D. melanogaster were mapped to noncoding regions and only affected expression level of the gene. In our work we were not able to check expression level of homologous genes in sea urchins. Another Drosophila gene, which was fond in selection experiments towards longer living flies, mth [59], was also checked for positions that co-vary with longevity. Unfortunately, no aminoacids that vary in accordance with lifespan were found in our study.
Mutations in a clk1 gene were found in experiments for selection of long living nematodes [11]. The product of this gene is involved in ubiquinone biosynthesis. Mutations of clk1 lead to decrease in respiration and as a consequence to increase of the nematode lifespan [60]. Position 117 (C. elegans numbering) is occupied by histidine in human, naked mole rat and M. brandtii, while red sea urchin has lysine in the equivalent place. C. elegans has glutamine in the same position of Clk1, mouse has asparagine and green sea urchin has a glutamic acid.
Another gene related to longevity in C. elegans is daf-9 [61]. This gene codes for cytochrome P450 that is involved in steroid hormone biosynthesis. Aminoacid 132 (C. elegans Daf9 numbering) was found to vary in concert with longevity. Aliphatic aminoacids leucine and isoleucine were found at this position of human, M. brandtii, naked mole rat and red sea urchin. Short living organisms contain aromatic residues at the same place, tyrosine in L. variegatus and phenylalanine in mouse and worm.
Categories of proteins enriched with positions that co-vary with longevity
Analysis of protein sequences in a representative set of species with high and low lifespan allowed us to reveal several aminoacid positions that co-vary with longevity. Although this approach is not guaranteed from mistakes originated from misalignment, identification of related proteins that have different function, it could present a framework of further hypothesis-driven experiments on longevity. Our analysis revealed (Figure 2) highly uneven distribution of proteins having aminoacid residues that co-vary with longevity among functional categories. Surprisingly, several categories of proteins were completely devoid of such positions. For example, nuclear encoded mitochondrial proteins and proteins involved in reactive oxygen species inactivation. Minimum of such aminoacids were found in the components of insulin/IGF1 pathway. Particularly enriched in positions that vary in coordination with longevity are categories of mitochondrial proteins, encoded in mitochondrial genome, lipid transport proteins, proteins involved in amyloidogenesis and system of telomere maintenance. Among other, catalytic subunit of telomerase, TERT holds absolute record of the frequency of such positions. Despite the fact, that somatic telomerase activity could be detected in short and long living sea urchins, telomerase reverse transcriptase might be involved in longevity due to more intricate mechanisms, such as maintaining the balance between support of tissue renovation and simultaneous restriction of unwanted proliferation of cancerous cells.
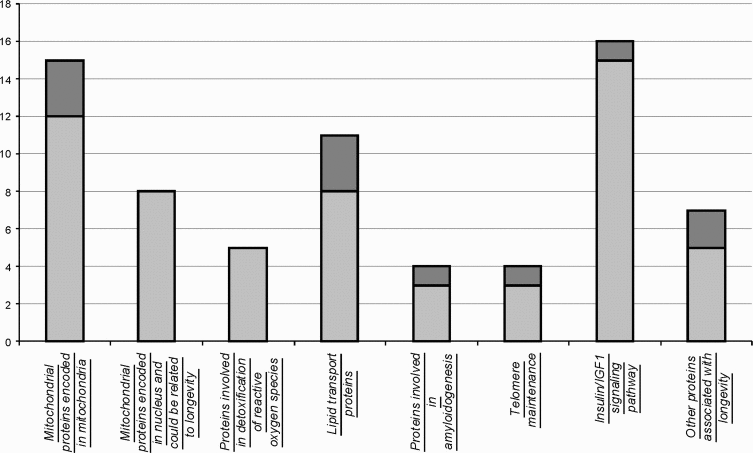
Figure 2. Number of proteins containing aminoacid positions that vary in agreement with longevity. Shown are numbers of analyzed proteins by category (light grey) and those that contained aminoacids that co-vary with longevity (dark grey).
Authors are very thankful to Vadim Gladyshev and Inge Seim for the help in data analysis and fruitful discussions.
The work was supported by ESN group, Russian Foundation for basic research 13-04-40211-H, 14-04-01061, 13-04-00836, 15-34-20139 and Russian Science Foundation grant 14-24-00061.
None of the authors has any conflict of interest to declare.