Cyclin A2 loss delays embryonic forebrain development
In order to quantitatively describe the neuropathology of Cyclin A2 loss in the VZ/SVZ, we performed high-resolution analyses of the CCNA2-/- brains using unbiased stereological methodologies. We generated CCNA2-null brains by intercrossing CCNA2fl/fl mice with Nestin-cre mice. Ablation of CCNA2 was confirmed by immunohistochemical staining (Supplemental Fig. S1). We focused our analyses on the VZ/SVZ of E14.5 and E17.5 mice. At E14.5, most radial glia divide symmetrically to expand the progenitor pool [13], while at later ages radial glia divide asymmetrically to self-renew and generate new neurons [14]. VZ/SVZ and cortical plate (CP) volumes and total number of cleaved caspase-3 positive cells in the entire VZ/SVZ and CP were determined in CCNA2fl/fl, Nestin-cre brains and compared to controls using unbiased stereology (Fig. 1A-C, Supplemental Table S1). E14.5 CCNA2fl/fl, Nestin-cre mice showed greater than 4-fold reduction in VZ/SVZ volume and greater than 2-fold reduction in CP volume (Fig. 1A-B).
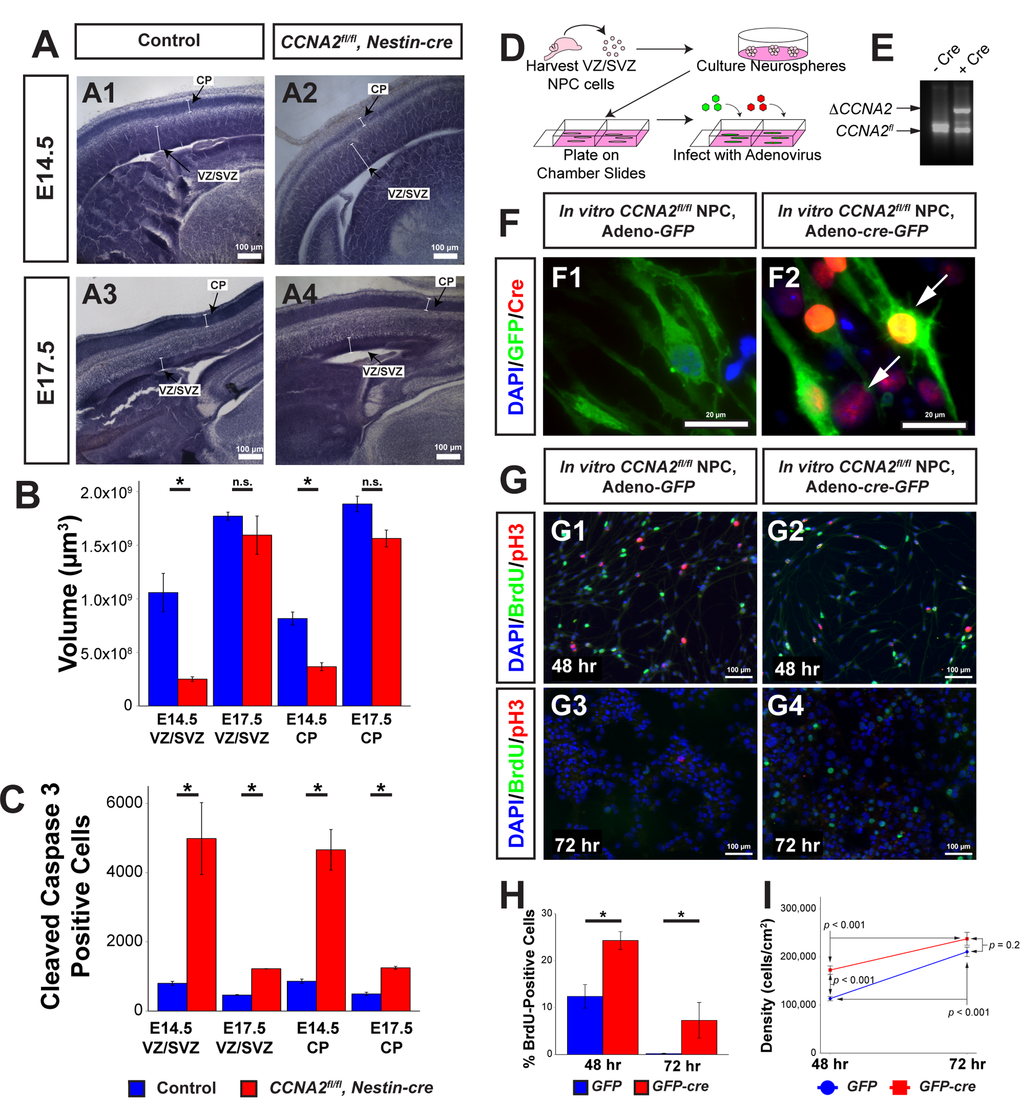
Figure 1. CCNA2 Loss delays embryonic forebrain development. (A) Representative low-magnification images used for unbiased stereology quantification. The VZ/SVZ and CP are noted by arrows. Experimental conditions are indicated above. (B) Total VZ/SVZ and CP volume. At E14.5, the volumes of both structures in CCNA2fl/fl, Nestin-cre animals were significantly reduced compared to controls. At E17.5, there was no statistical difference between groups. Quantifications represent Cavalieri unbiased stereology analysis of the entire brain. The y-axis is volume of the VZ/SVZ or CP. Unpaired t-test, * = p < 0.05, n.s. = not significant. For E14.5, n= 3 animals each for control and experimental groups. For E17.5, n=2 animals for control group and 3 animals for experimental group. (C) Total apoptotic cells in the VZ/SVZ and CP. At both ages, there was a significant increase in apoptosis in both structures. Quantifications represent Optical Fractionator unbiased stereology analysis of the entire brain at 100x magnification. The y-axis is total number of cleaved caspase 3-positive cells in the VZ/SVZ or CP. Unpaired t-test, * = p < 0.05. (D) Schematic of neural progenitor cell culture. Neural progenitors from the VZ/SVZ were dissected from P5 CCNA2fl/fl pups and cultured as neurospheres. Neurospheres were dissociated and infected with adenovirus encoding cre and GFP to excise CCNA2, or adenovirus encoding GFP only as a control. (E) CCNA2fl/fl ablation in vitro. DNA from infected cells was amplified by PCR. Alleles represented by each band are indicated on the left, and Cre condition is above. (F) Cells were stained for GFP and Cre recombinase. Infection of neural progenitor cells results in >90% infection. Arrows indicate Cre-positive cells in F2. (G) Cells were infected as shown in (F). Cells were pulsed with BrdU for 30 minutes before fixation 48 and 72 hours after plating. The proportion of BrdU and pH3-positive cells was increased in CCNA2-null cells. (H) Quantification of BrdU-positive cells 48 and 72 hours after plating. The x-axis is time after plating, and the y-axis is the percentage of cells that incorporated BrdU. Unpaired t-test, * = p < 0.05. (I) Quantification of cell density 48 and 72 hours after plating. Forty-eight hours after plating, CCNA2-null cells were less dense. There was no statistical difference between CCNA2-null and control cells 72 hours after plating. ANOVA with Tukey’s HSD, p values are noted on the plot. These data support that CCNA2-null neural stem cells are capable of reaching the carrying capacity of their respective stem cell niches. The x-axis is time after plating, and the y-axis is cell density. Error bars for all graphs represent standard errors of the mean (s.e.m.).
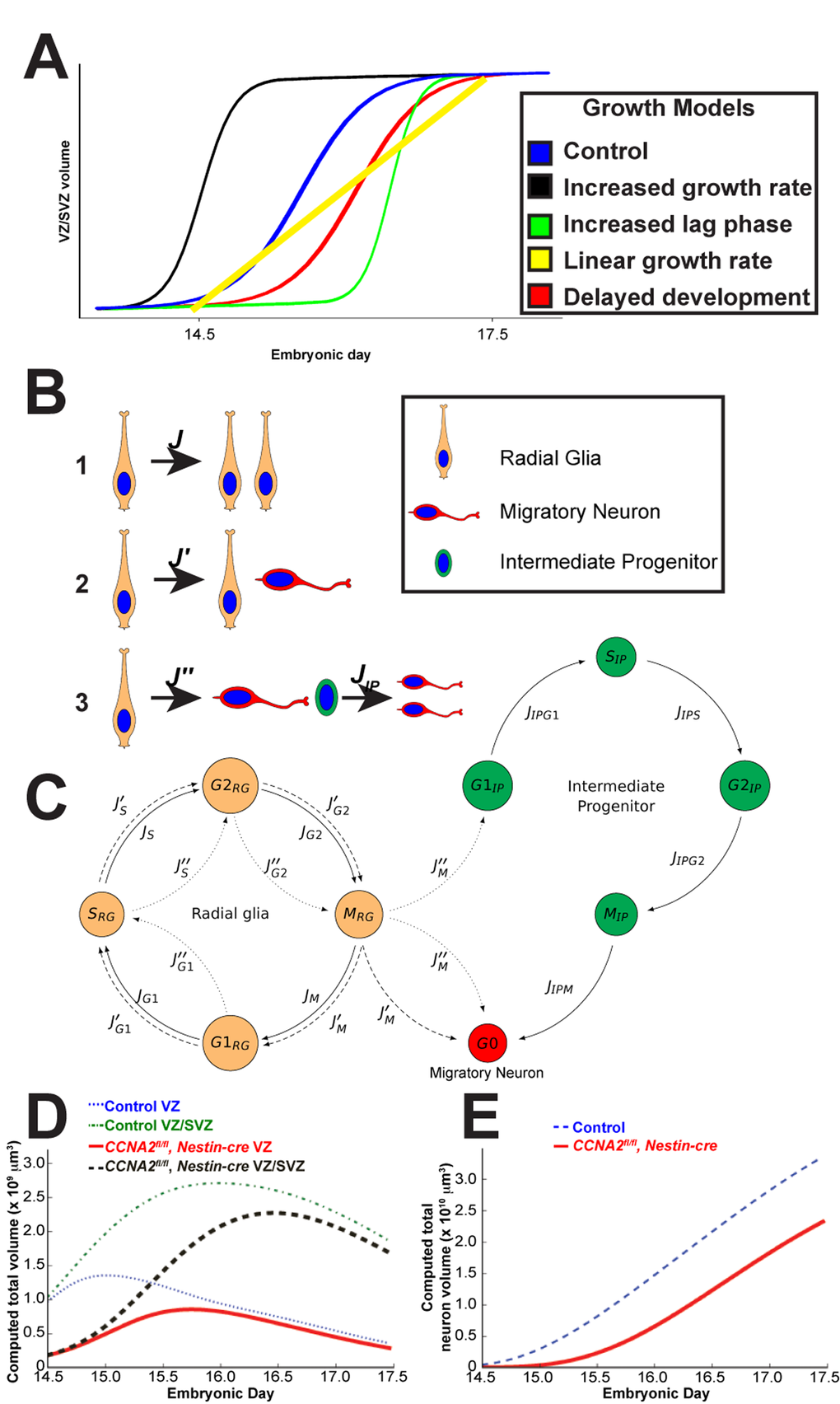
Figure 2. Mathematical modeling of forebrain growth. (A) Hypothetical models of forebrain growth. Various possible explanations of forebrain growth in CCNA2fl/fl, Nestin-cre brains are presented. (B) Schematic of the fates of a radial glia neural stem cell in our model. Radial glia can divide in (1) a symmetric self-renewal, (2) an asymmetric self-renewal generating a migratory neuron and a radial glia, or (3) an asymmetric, non-renewing division generating a migratory neuron and an intermediate progenitor, with the intermediate progenitor dividing into two migratory neurons. (C) Diagram of cell cycle progression corresponding to our mathematical model. Cells leave each phase of the cell cycle at different rates  depending on population size and mitotic age. These parameters are described in Supplemental Table S4. Cells are also able to enter an apoptotic state from each phase. The flows between the cell cycle phases for each type of division in (A) are noted by J, J′, J′′, or JIP. (D) Computed volumes of the VZ and combined VZ/SVZ for CCNA2fl/fl, Nestin-cre and control simulations. The control VZ volume plateaus at approximately E15, whereas it continues to grow in the CCNA2fl/fl, Nestin-cre until E15.5. Similar trends are seen in the combined VZ/SVZ volumes. The x-axis is the embryonic day, and the y-axis is the volume of the VZ or combined VZ/SVZ. (E) The cumulative neuronal output of the VZ/SVZ during E14.5-E17.5 of the CCNA2fl/fl, Nestin-cre simulations trails that of the control throughout the time period. The x-axis is embryonic day, and the y-axis is total neuronal volume.
depending on population size and mitotic age. These parameters are described in Supplemental Table S4. Cells are also able to enter an apoptotic state from each phase. The flows between the cell cycle phases for each type of division in (A) are noted by J, J′, J′′, or JIP. (D) Computed volumes of the VZ and combined VZ/SVZ for CCNA2fl/fl, Nestin-cre and control simulations. The control VZ volume plateaus at approximately E15, whereas it continues to grow in the CCNA2fl/fl, Nestin-cre until E15.5. Similar trends are seen in the combined VZ/SVZ volumes. The x-axis is the embryonic day, and the y-axis is the volume of the VZ or combined VZ/SVZ. (E) The cumulative neuronal output of the VZ/SVZ during E14.5-E17.5 of the CCNA2fl/fl, Nestin-cre simulations trails that of the control throughout the time period. The x-axis is embryonic day, and the y-axis is total neuronal volume.
By E17.5, the CP and VZ/SVZ volumes were not significantly different between groups (p = 0.068 and p = 0.5, respectively). We conclude that during the E14.5 ->E17.5 period, the amount of growth of the CCNA2fl/fl, Nestin-cre VZ/SVZ was greater than that of the control VZ/SVZ.
To investigate the underlying cause of the early size reduction, we examined apoptosis in the VZ/SVZ and CP of these embryos by measuring the total number of cleaved caspase 3-positive cells in the VZ/SVZ and CP. We found greater than 5-fold increase in apoptosis in the VZ/SVZ and CP of CCNA2fl/fl, Nestin-cre embryos compared to controls at E14.5. E17.5 mice displayed approximately 2.5-fold increase in apoptosis in both structures (Fig. 1C). Notably, the volume occupied by apoptotic cells in the VZ/SVZ was vastly increased by CCNA2 ablation compared to the increase observed in the cerebellar granule neuron progenitor cells of the external granule layer (Supplemental Table S1). These data indicate that forebrain neural progenitors are actually more sensitive to CCNA2 ablation than cerebellar neuron progenitors, yet cerebellar morphogenesis is more adversely affected post-CCNA2 ablation. To test if the observed growth delay was a property of neural progenitors in general, or was restricted to the embryonic period, we utilized an in vitro approach. We generated CCNA2-null neural progenitors dissected from the CCNA2fl/fl forebrain ganglionic eminence and expanded in vitro. CCNA2 ablation was accomplished by infection with adenovirus encoding cre-recombinase. CCNA2-null neural progenitors showed increased BrdU incorporation, as well as delayed growth (Fig. 1F-I). Comparing the overall apoptosis rates to the overall proliferation rates, we concluded that the increase in apoptosis in the VZ/SVZ had minimal impact on the overall growth of the embryonic forebrain whereas apoptosis has more detrimental effects on cerebellar morphogenesis. Furthermore, we were perplexed by our finding of compensatory growth in CCNA2-null forebrains despite loss of a crucial cell cycle gene.
Theoretical models of compensatory forebrain growth
We sought to understand the paradoxical effects of CCNA2 ablation on embryonic neurogenesis. In particular, we interpreted the results from Fig. 1 in terms of the interplay between proliferation, self-renewal, apoptosis, and differentiation. The volume trajectories for both lines suggest autoregulatory saturation of the neuronal population by day E15. This plateau is similar to that shown in previous observations in the literature [8,15]. The simplest model of such population dynamics is logistic growth*1, where proliferation rate decreases as a population approaches a limiting capacity. Logistic growth has been shown to well-explain the growth of many tissues including in the brain [16]. Since the main feature of logistic growth is a decrease in proliferation rate as a population approaches a certain threshold or carrying capacity*, two populations that initially grow at the same rate may converge to the same steady size over a sufficient amount of time. For this reason, it provides the most simplistic mechanism for the slow-down in the normal growth rate that would allow the CCNA2-null embryos to catch up to their CCNA2-intact counterparts.
In order to better understand the growth of CCNA2fl/fl, Nestin-cre brains, we considered several hypotheses to explain the data. These hypotheses are presented as theoretical growth curves in Fig. 2A. The blue curve represents logistic growth of CCNA2-intact brains. Growth mediated by more rapid cell cycle (black curve) cannot explain the growth of CCNA2fl/fl, Nestin-cre brains as it would require the CCNA2fl/fl, Nestin-cre VZ/SVZ volume to be increased at E14.5, in addition to showing more rapid cell cycle transit in cells lacking a critical S-phase cyclin. Elevated apoptosis would result in a prolonged lag phase (green curve), yet our measurements of apoptosis do not show a sufficiently elevated programmed cell death rate to cause such a lag. Thus, an increased lag phase model cannot explain the growth of CCNA2fl/fl, Nestin-cre brains because it would require the apoptosis rates to be much higher than what we observed in order to delay the transition from lag to log phase. The linear growth model (yellow curve), corresponding to a fixed-size proliferative pool, cannot explain the growth of CCNA2fl/fl, Nestin-cre brains because it would either require apoptosis rates to be high relative to proliferation rates, which is not the case, or it would require that the VZ/SVZ stem cell niche be populated only by a single cell type [1].
Our considerations of these alternative models left us with only our delayed development model (red curve) to explain the data. To test this hypothesis, we generated a mathematical model of mouse forebrain development using previously reported data on cell cycle timings available in the scientific literature, as well as data from our own unbiased estimates of VZ/SVZ size during embryonic development (Fig. 1 and 2, and Supplemental Tables S2-S4). The variables that we track in our model are the total volumes of cells in each of the G1/S/G2/M phases, existing within each of the VZ/SVZ niches, which have a given mitotic age. The parameters in this model are the rates of transitions between each stage of the cell cycle. We incorporated logistic growth mechanism in determining cell fate, with the rate of Type I pro-liferative divisions (Fig. 2B) decreasing linearly as a function of total volume.
Assuming a conservatively rapid clearance rate for apoptotic cells, we found that apoptosis did not significantly affect the population dynamics* in the E14.5 to E17.5 period. Although the apoptosis rate increases many-fold, the apoptosis rate remains mathematically insignificant relative to the growth rate (Supplemental Table S1). Since we have determined that apoptosis is insignificant, a decrease in growth rate is likely the determinant of the observed developmental delay. For this reason, we expected that CCNA2 loss in a neural stem cell lengthens the cell cycle, a finding that has previously been identified in CCNA2-null mouse embryonic fibroblasts [17]. We assume that such cell cycle lengthening occurs during S and G2 phases as these are the phases during which Cyclin A2 protein is present.
Simulations of the VZ/SVZ volume are given in Fig. 2D, where we assumed that the SVZ has no volume at E13.5, and that all radial glia present at E13.5 have the capability to divide (on average) approximately five more times before terminally differentiating. We arrived at the number five in order to match the characteristics of the observations in our unbiased measurements for the CCNA2-intact controls. We then tested simulations as to how a delayed forebrain stem cell niche would behave.
At E14.5, the measured control VZ/SVZ volume is approximately 4 times that of CCNA2fl/fl, Nestin-cre. Supposing that this cre expresses at E10.5 [18], and that the divisions in this period are primarily Type I symmetric divisions (Fig. 2B), which have a mean transit time of 9.2 hrs, then proliferative CCNA2fl/fl, Nestin-cre radial glia have a mean transit time of 10.6 hrs. Isolating the excess time of 1.4 hrs to the S and G2 phases suggests that they are elongated by 25%. This also suggests that at E13.5, CCNA2fl/fl Nestin-cre radial glia are capable on average of approximately 1 more division than in control animals. For this reason, we assumed that the radial glia are developmentally delayed, giving them the capability to divide six times before terminal differentiation during the E14.5-E17.5 time period. These simulations thus shed light as to why increased forebrain apoptosis does not affect overall growth rate in the CCNA2fl/fl, Nestin-cre animals.
In summary, the notion that developmental delay results in compensatory growth in CCNA2fl/fl Nestin-cre is best explained by a predominance of symmetric divisions (Type 1 in Fig. 2B) that expand the VZ/SVZ between E14.5 and E17.5, while neural progenitors in the control brains undergo more asymmetric and terminal divisions, slowing the growth of the control VZ/SVZ. Stated otherwise, the CCNA2fl/fl Nestin-cre brains behave similarly to control brains, although delayed by approximately one day.
Our developmental delay model’s predictions include (1) that the CCNA2-null neural progenitor cells have a prolonged cell cycle time, (2) the total output of intermediate progenitors would be decreased early in development, (3) more neurons would be produced at later time points, and (4) at later time points the mutants would have a higher overall level of proliferation. In order to test the predictions of our model, we examined VZ/SVZ cytoarchitecture and proliferation in E14.5 embryos and P0 pups. We injected an E14.5 timed-pregnant mouse with CldU, euthanized the pregnant dam 2.5 hours later, and harvested embryos. We first examined VZ/SVZ cytoarchitecture in these embryos. Neural progenitor cells are characterized by expression of Pax6, localized adjacent to the ventricle. As they divide and mature into intermediate progenitors, they express Tbr2 and are located deeper within the VZ/SVZ [19]. We observed appropriately-localized Pax6 and Tbr2, indicating that loss of CCNA2 does not perturb VZ/SVZ cytoarchitecture (Fig. 3A-C). We quantified Tbr2 and found that there was no change in the number of Tbr2-positive cells in individual sections (Fig. 3D). However, in concordance with our model, there was a reduction in the total number of Tbr2 cells when we normalized the volumes for the smaller size of the CCNA2fl/fl, Nestin-cre VZ/SVZ (Fig. 3D).
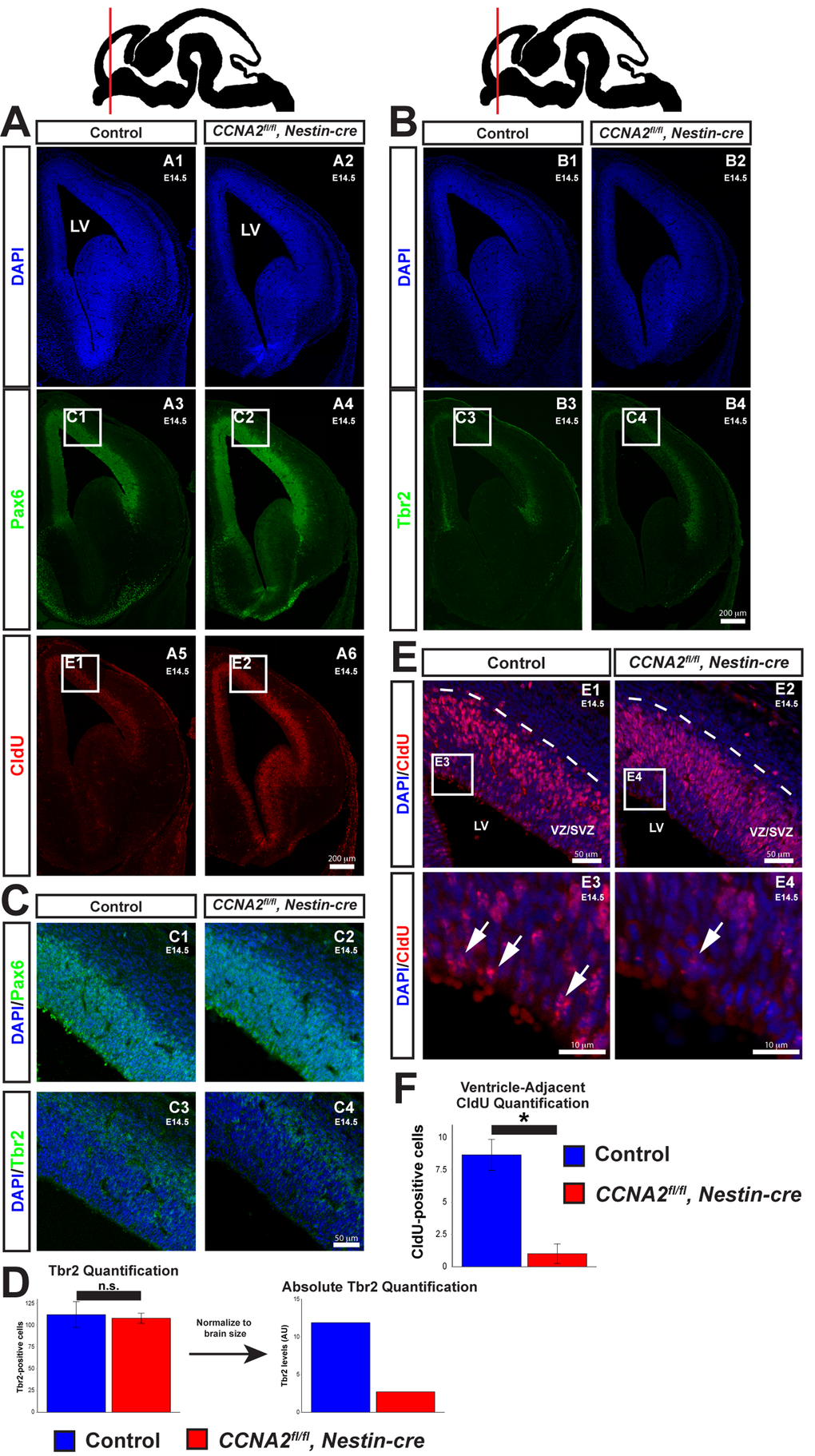
Figure 3. Delayed forebrain development in CCNA2fl/fl, Nestin-cre animals. (A) A timed-pregnant E14.5 dam was injected with CldU then euthanized. Embryos were stained for Pax6, CldU, and DAPI. Experimental condition is indicated above, molecular markers are color-coded on the left. LV = lateral ventricle. (B) Sections were stained for Tbr2. Experimental condition is indicated above, molecular markers are color-coded on the left. (C) Pax6 and Tbr2-positive cells are appropriately localized in CCNA2fl/fl, Nestin-cre forebrains, indicating preserved cytoarchitecture. Image locations are indicated in (A) and (B). (D) Quantification of Tbr2-positive cells. Cells were counted in a 100x180 μm counting frame in the VZ/SVZ. Tbr2-positive cells were unchanged within each counting frame. Accounting for the reduced size of the E14.5 VZ/SVZ there is a reduction in total Tbr2-positive cells. The y-axis is total Tbr2-positive cells. Unpaired t-test, n.s. = not significant, p > 0.05, n = 3 embryos per condition. (E) High magnification images of CldU staining in (A). Experimental condition is indicated above, molecular markers are color-coded on the left. Arrows indicate CldU-positive cells adjacent to the ventricle. (F) Quantification of CldU-positive cells adjacent to the lateral ventricle. CldU-positive cells were counted in a 100x10 μm bin adjacent to the ventricle. CldU-positive cells adjacent to the lateral ventricle were reduced in CCNA2fl/fl, Nestin-cre embryos. The y-axis is number of CldU-positive cells. Unpaired t-test, p < 0.05, n = 3 embryos per condition. Error bars for all graphs represent s.e.m.
To confirm that the cell cycle was lengthened in CCNA2fl/fl, Nestin-cre neural progenitors, we examined the localization of CldU-positive nuclei within the VZ/SVZ. Neural progenitors undergo interkinetic nuclear migration (INM), a process in which the localization of the nucleus within the cell depends upon cell cycle phase. During S-phase, the nucleus is located away from the ventricle, deep within the VZ/SVZ (Fig. 3A, E). Upon completion of DNA synthesis, the nucleus migrates back to the ventricular surface, where the cell divides. Cells located outside the prominent band of CldU-positive nuclei deep in the SVZ would therefore have completed S-phase and begun INM toward the ventricle. Increasing the duration of S phase would result in fewer CldU-positive nuclei that have completed DNA synthesis and begun their migration back to the ventricular surface. With this in mind, we measured the number of CldU-positive nuclei adjacent to the ventricle. Very few of these cells are noted in CCNA2fl/fl, Nestin-cre embryos compared to controls (Fig. 3E-F), indicative of a lengthened S-phase. In summary, CCNA2 loss results in increased brain growth during E14.5->E17.5, despite showing prolonged cell cycle time and increased apoptosis.
Between E14.5 and E17.5, wild-type VZ is known to plateau and decay due to differentiation [8,15]. Due to this normal growth plateau, the CCNA2fl/fl, Nestin-cre VZ recovers in volume relative to the control. This recovery, however, is misleading, as the cumulative neuronal output is predicted to be significantly depressed (Fig. 2E) in the mutant animals. Indeed, our model suggests that the CNS stem cell niche would exhibit behaviors at P0 characteristic of earlier embryonic stages. Stated otherwise, proliferation in CCNA2fl/fl, Nestin-cre animals would remain elevated and a significant number of neurons would be generated later in development from the forebrain stem cell niche.
To test these predictions, we examined proliferation in the VZ/SVZ of P0 mice. We injected P0 mice with BrdU and euthanized them 30 minutes later. We then stained for BrdU (Fig. 4A), pH3, and Ki67. Consistent with our model, we observed an increase in all three of these proliferation markers in the VZ/SVZ of CCNA2fl/fl, Nestin-cre mice (Fig. 4B). Furthermore, we measured neuronal output in newborn mice by determining the total number of NeuN-positive cells in the combined VZ/SVZ and intermediate zone (IZ) with unbiased stereological quantification of NeuN immunohistochemistry. We found that the total number of neurons in the combined VZ/SVZ and intermediate zone (IZ) was significantly increased, as predicted by our model (Fig. 4C-F). Thus, we conclude that CCNA2 loss does not affect stem cell self-renewal or migration to the CP and the forebrain develops appropriately, yet remains developmentally immature relative to a control brain. Furthermore, our experimental data and our mathematical model predict a maximal limit to the size of the forebrain stem cell niche, which we term the carrying capacity (Vmax). Vmax is constant between the control and CCNA2fl/fl, Nestin-cre brains. Since at E14.5 the CCNA2-null neural progenitor cells are at a different point in the growth curve toward Vmax, our model predicts that levels of proliferative cells would be elevated relative to controls as the CCNA2-null cells would not experience the autoinhibitory mechanisms acting on the control stem cell niche during equivalent epochs.
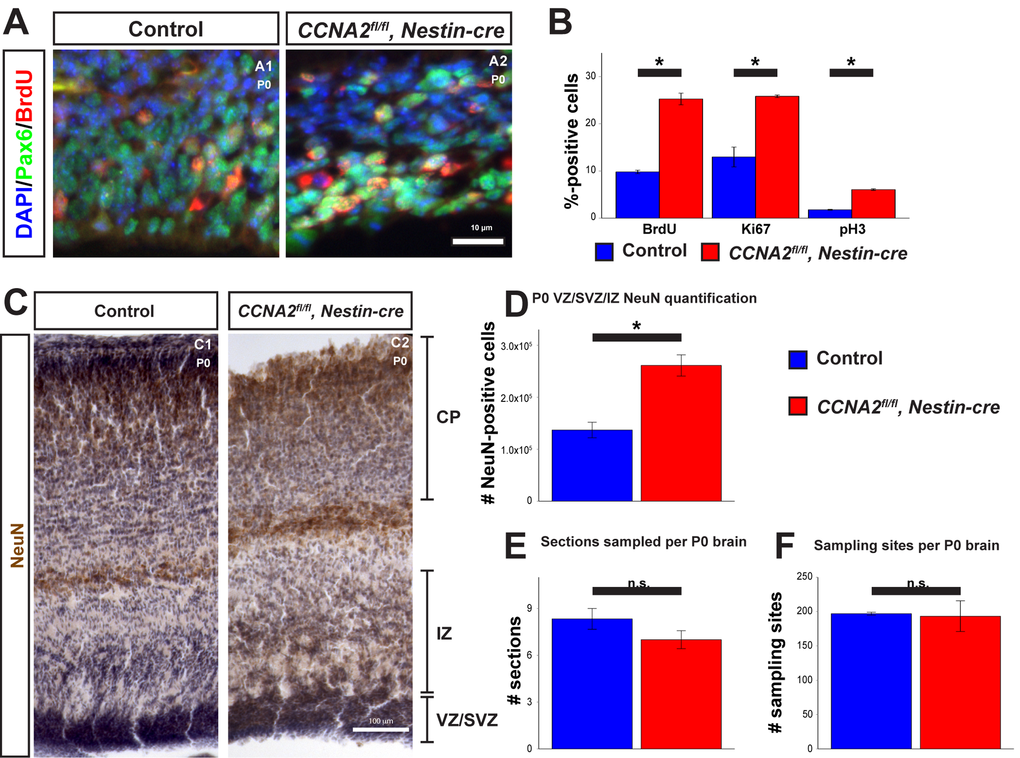
Figure 4. Newborn CCNA2fl/fl, Nestin-cre animals exhibit a developmental delay. (A) P0 animals were pulsed with BrdU for 30 minutes before euthanasia and tissue stained for BrdU and Pax6. (B) Quantifications of BrdU, Ki67, and pH3 were significantly elevated in CCNA2fl/fl, Nestin-cre mice. The y-axis is the percentage of cells positive for each marker in the VZ/SVZ. Unpaired t-test, * = p < 0.05. (C) P0 brains were sectioned for unbiased stereology, stained for NeuN, and counterstained with hematoxylin. NeuN-positive cells were quantified in the combined VZ/SVZ and intermediate zone (IZ). Experimental conditions are indicated above, molecular marker is color-coded on the left. VZ/SVZ, intermediate zone (IZ), and CP are indicated. (D) Optical fractionator measurements of NeuN-positive cells in the VZ/SVZ/IZ show increased neuronal output in CCNA2fl/fl, Nestin-cre animals compared to controls, indicative of a developmental delay. The y-axis is the number of NeuN-positive cells in the VZ/SVZ/IZ per brain. Unpaired t-test, * = p < 0.05, n = 3 brains per condition. (E) Measurement of the number of sections sampled and (F) measurement of the number of sampling sites. There was no significant difference in either metric, precluding the possibility that the increased NeuN was due to a difference in brain size. For (E), the y-axis is the number of sections sampled per brain. For (F), the y-axis is the number of sampling sites per brain. Unpaired t-test, n.s. = not significant, p > 0.05, n = 3 brains per condition. Error bars for all graphs represent s.e.m.
Mechanisms of increased cell cycle time in CCNA2 deficient cells
The lengthening of the cell cycle in CCNA2fl/fl, Nestin-cre mice is perhaps not surprising, given the loss of an important cell cycle regulator. However, Cyclin E has been shown previously to compensate for loss of Cyclin A2 [17], leading us to ask if there were other underlying reasons for the lengthened cell cycle. One of the major causes of cell cycle arrest leading to a lengthened cell cycle is DNA damage. The function of Cyclin A2 with its effector kinase CDK2 is blocked during the DNA damage checkpoint, which stops progression through the cell cycle [20]. However, our results suggest that Cyclin A2 may have a more active role in the DNA damage response. Therefore, we asked if Cyclin A2 was involved in promoting DNA repair in addition to its well-characterized cell cycle roles. To answer these biochemical questions, we utilized an in vitro cell culture model. First, we tested the extent to which Cyclin A2 localized to sites of DNA damage after ionizing radiation (IR), which induces DNA double-strand breaks (DSBs). Cells were exposed to 2 Gy of X-ray radiation, and the localization of Cyclin A2 with the DNA double-strand break marker γH2AX was tested by proximity ligation assay (PLA). Epitopes located within 30 nm of each other elicit fluorescent punctae [21], which we quantified by automated counting in ImageJ. We observed an approximately three-fold increase in PLA signals in the nuclei during recovery from X-irradiation (Fig. 5A-C), which is indicative of localization of Cyclin A2 at double-strand breaks. We conclude that Cyclin A2 is found at DSB sites after ionizing radiation.
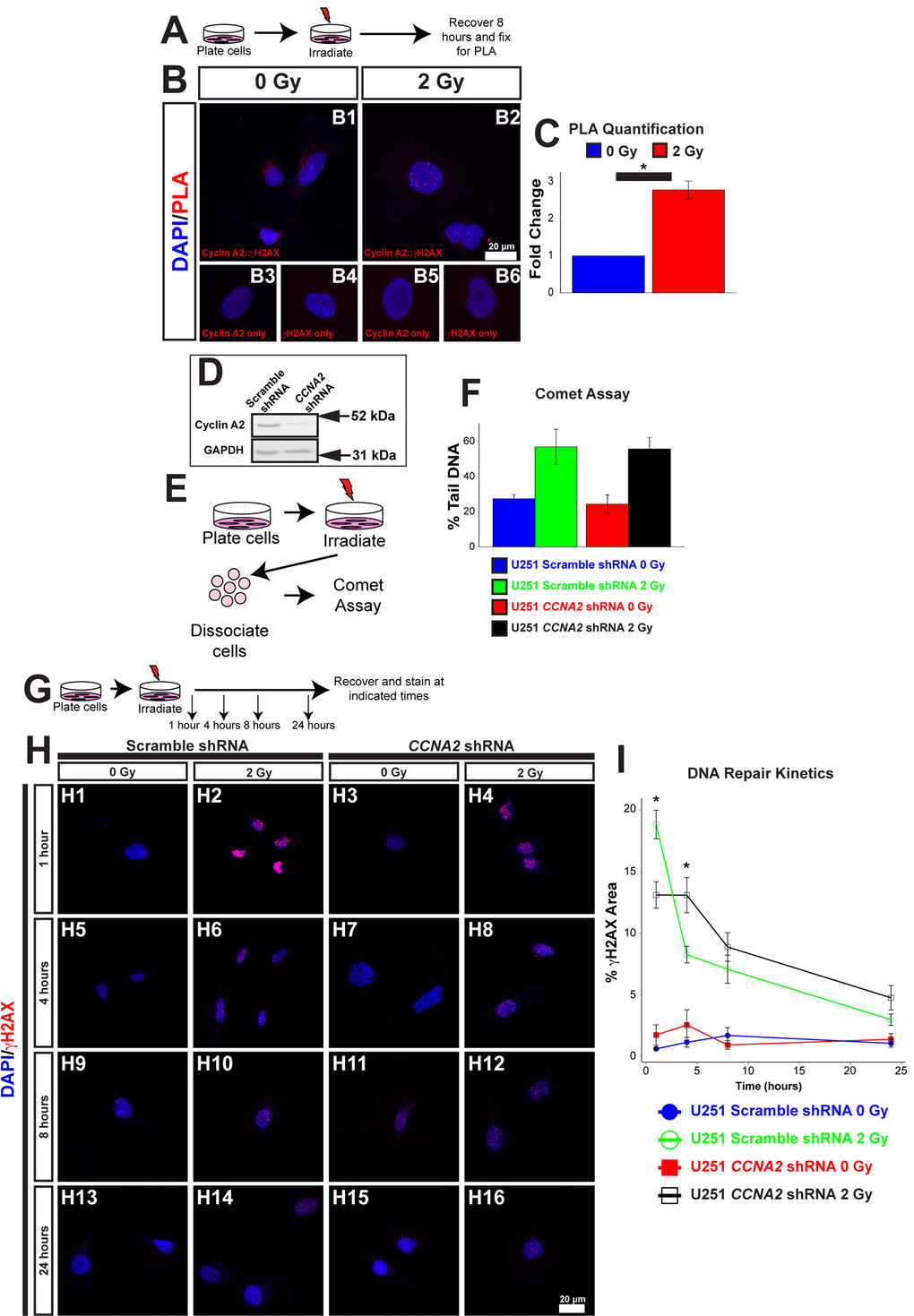
Figure 5. γH2AX is located at foci of DNA damage. (A) Schematic of experiment in (B) and (C). U251 cells were irradiated with 2 Gy. 8 hours after irradiation, cells were processed for PLA. (B) Association between Cyclin A2 and γH2AX. Positive PLA signals manifest as punctate foci in the nucleus. Single-labeled controls are presented below. Experimental conditions are indicated above, molecular markers are color-coded on the left. Antibodies used are indicated in each image. (C) Quantification of (B). The y-axis is fold-change of PLA signals induced by radiation. 2 Gy induces associations between γH2AX and Cyclin A2 approximately 3-fold. Unpaired t-test, * = p < 0.05, n=3 independent experiments, 45 total cells for 0 Gy condition, 44 total cells for 2 Gy condition. (D) Silencing of CCNA2 by shRNA confirmed by western blotting. (E) Schematic of comet assay. (F) Equivalent DNA damage after treatment with X-rays in CCNA2-silenced U251 cells. Comet assay was performed on cells with CCNA2 or scramble shRNA after treatment with 2 Gy X-rays. We did not observe a significant change in DNA damage (ANOVA, n=2). The y-axis shows percentage of DNA found in the tail of each comet. (G) Schematic of experiment in (H) and (I). U251 cells encoding either CCNA2-targeting or scrambled shRNA were irradiated with 2 Gy or mock-treated. Cells were allowed to recover and fixed the times indicated then stained for Rad51 and γH2AX. (H) Silencing CCNA2 reduces phosphorylation of H2AX after 2 Gy and slows DNA repair rates as measured by γH2AX signal as a percentage of the nuclear area. (I) CCNA2-silenced cells display decreased phosphorylation of H2AX and delayed DNA repair. The x-axis is the time after irradiation, and the y-axis is the area of each nucleus with γH2AX signal. ANOVA with Tukey’s HSD, * = p < 0.05 between the CCNA2 and scramble shRNA, n=3 independent experiments, 46-55 total cells per condition. Error bars for all graphs represent s.e.m.
We next tested the hypothesis that CCNA2 played a role in the signal transduction cascade of the DNA damage response. One of the main hallmarks of the DNA signal transduction cascade is phosphorylation of H2AX [22]. In normal cells, IR treatment results in acutely high levels of phosphorylated H2AX (γH2AX); the levels of this phosphorylated histone returns to baseline following DNA repair. We therefore asked if the phosphorylation of H2AX was affected by loss of Cyclin A2. We used a previously published and validated lentiviral approach to silence CCNA2 expression (Fig. 5D) [23]. Comet assay found no change in DNA damage between CCNA2-silenced cells and control cells (Fig. 5E-F). We then analyzed levels of γH2AX in CCNA2-silenced cells. Quantification of γH2AX normally involves counting of nuclear foci. In our case, we observed such a large γH2AX response to irradiation that we were unable to count distinct foci; we thus analyzed the percent of the nucleus that was positive for γH2AX signal. CCNA2-silenced cells phosphorylated H2AX post-IR at lower levels than did control cells. Furthermore, CCNA2-silenced cells showed delayed DNA repair kinetics 1 and 4 hours post-IR, indicating that the damaged DNA was repaired at a reduced rate (Fig. 5G-I). Thus, reduced Cyclin A2 protein levels results in decreased H2AX phosphorylation with a functional, albeit slowed, DNA damage response.
With this in mind, we tested the hypothesis that CCNA2 was a mediator of DSB repair by performing DNA repair assays in CCNA2-silenced cells. Specifically, we utilized the DRGFP homologous recombination (HR) and EJ5GFP non-homologous end joining (NHEJ) assays [24,25], which required us to modify our CCNA2-silencing methodology (Fig. 6A-B). In these assays, DSB resolution by the pathway in question is determined by manifestation of GFP fluorescence detected by flow cytometry. CCNA2-silenced cells showed a mean reduction of 40% in HR and a mean reduction of 67% in NHEJ (Fig. 6C-D and Supplemental Fig. S2). These data are in line with reductions in GFP fluorescence observed by silencing other DNA repair genes such as RNF8 [26]. In toto, our finding showing blunted γH2AX formation and deficient HR and NHEJ DSB repair in CCNA2-silenced cells support the notion that CCNA2 functions early in the DSB signal transduction cascade. Such a finding would result in radiation sensitivity in CCNA2-silenced cells. Lentiviral CCNA2-silenced cells were thus subjected to the clonogenic assay [27] after exposure to 0, 3, 6, or 9 Gy of X-rays. Cells deficient in CCNA2 were significantly more sensitive than control cells (Fig. 6E). We conclude that DSB repair is deficient in CCNA2 silenced cells.
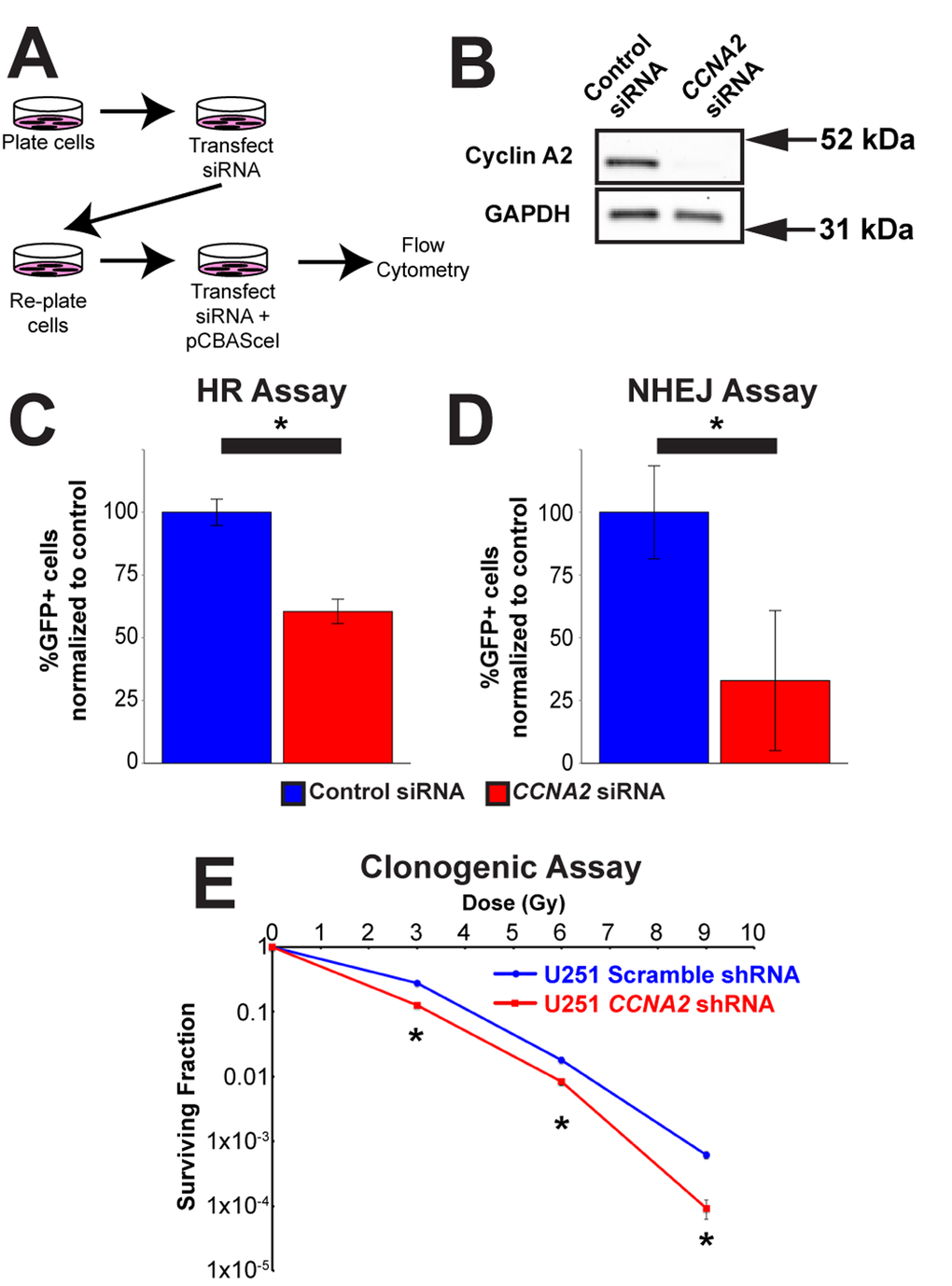
Figure 6. Cyclin A2 is involved in both homologous recombination and non-homologous end joining. (A) Schematic of experiments in (C) and (D). (B) Silencing of CCNA2 by siRNA was confirmed by western blot. (C) siRNA silencing of CCNA2 reduces HR. U251 cells with an integrated pDR-GFP plasmid were transfected with pCBASceI to induce a DSB in the DR-GFP locus. Cells that repaired this DSB by HR express GFP. Percentage CCNA2-silenced cells expressing GFP were normalized to percentage of control cells expressing GFP. Unpaired t-test, * = p < 0.05, n=3 independent experiments. (D) Silencing of CCNA2 reduces NHEJ. Experiments were performed as in (C), but with an integrated EJ5GFP plasmid. Unpaired t-test, * = p < 0.05, n=4 independent experiments. The y-axes in (C) and (D) are the percentage of GFP-positive cells in each condition normalized to the control siRNA condition transfected with pCBASceI plasmid. Error bars represent SEM for all graphs. (E) Lentiviral silencing CCNA2 sensitizes cells to IR by clonogenic assay. CCNA2-silenced cells demonstrate reduced survival compared to control cells with scrambled shRNA. The x-axis is dose, and the y-axis is surviving fraction. Unpaired t-test at each dose, * = p < 0.05, n=3 independent experiments. Error bars for all graphs represent s.e.m.
CCNA2-null cells of the hippocampus show evidence of intrinsic DNA repair defects
The above data indicates that Cyclin A2 is important in the development of the VZ/SVZ that gives rise to the cerebral cortex. We next asked if Cyclin A2 was necessary for the development of other stem cell niches in the brain. We previously demonstrated that Cyclin A2 is critically important for the development of the cerebellum [3]. Similar to the above results in the VZ/SVZ, growth of the cerebellar EGL stem cell niche was delayed with an increase in apoptosis. However, in contrast to the VZ/SVZ, the cerebellum fails to form appropriately. These contrasting results led us to hypothesize that Cyclin A2 was necessary for the development and function during adulthood of another stem cell niche in the CNS, the dentate gyrus of the hippocampus. To test this hypothesis, we used different cre drivers to ablate Cyclin A2 expression in the brain at different times during development. We used Nestin-cre for embryonic ablation, and CamkIIα-cre for ablation in the adult after the brain was fully developed. The CamkIIα-cre mouse was first developed to drive cre expression in the CA1 layer of the hippocampus, although it displays widespread expression in the hippocampus and cerebral cortex [32]. We performed unbiased stereological volume measurements of the dentate gyrus as we performed in the VZ/SVZ. We found that embryonic ablation of Cyclin A2 led to a drastic reduction in size of the dentate gyrus immediately after birth (Fig. 8A). Unlike the VZ/SVZ, the size of the dentate gyrus did not recover. However, loss of Cyclin A2 after the brain was fully formed by interbreeding with CamkIIα-cre mice had no effect on the size of the dentate gyrus at 4 or 8 months after birth (Fig. 8A).
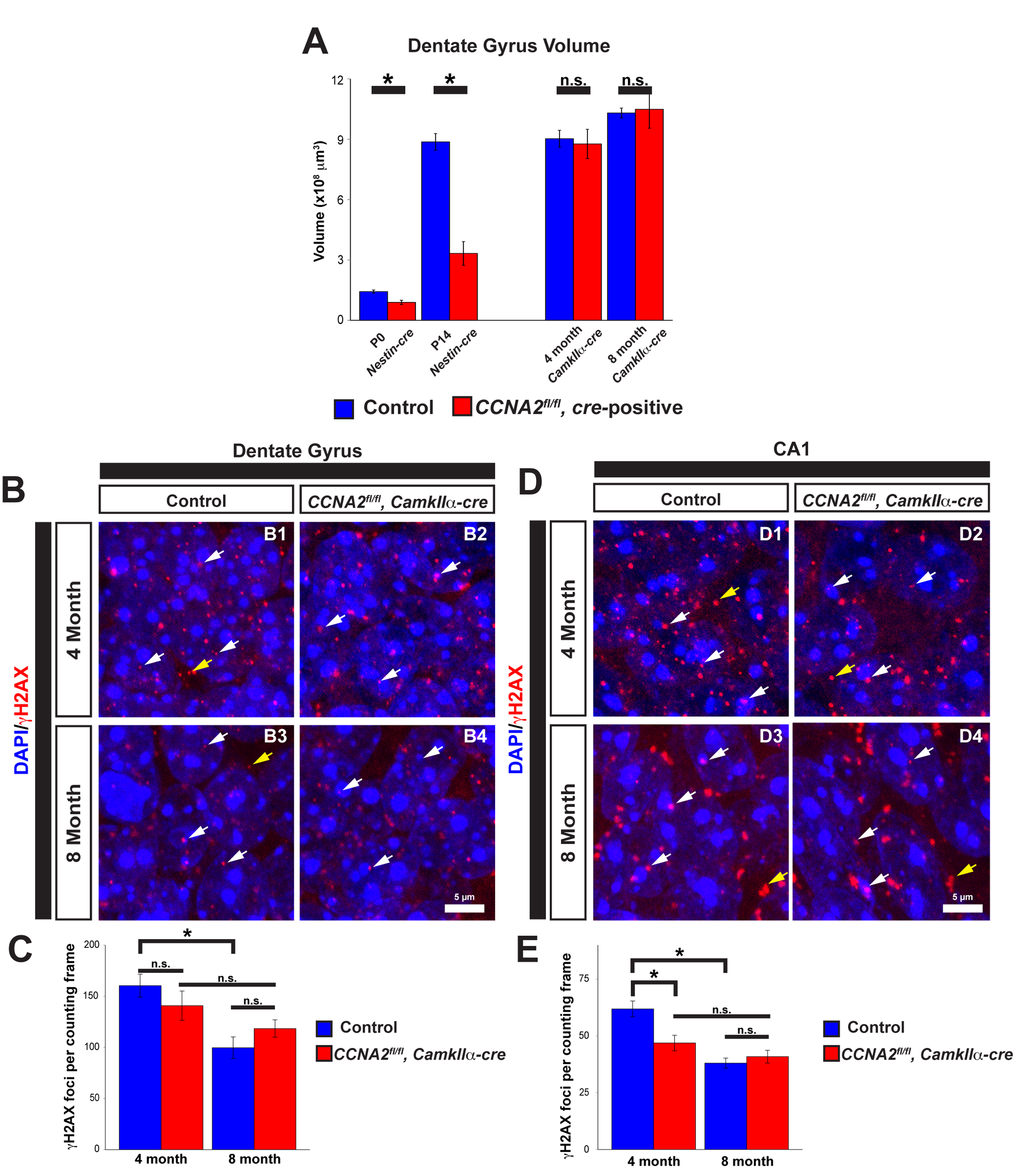
Figure 8. Cyclin A2 loss impairs hippocampal development. (A) Cavalieri estimations of the size of the dentate gyrus were performed in CCNA2fl/fl, Nestin-cre brains at P0 or P14, and in CCNA2fl/fl, CamkIIa-cre brains at 4 months or 8 months of age. The y-axis is volume of the dentate gyrus. Age and cre-driver are indicated below. Unpaired t-test, * = p < 0.05, n.s. = not significant, n=3 animals per condition for P0, P14, and 4 months; n=4 animals per condition for 8 month animals. (B, D) Cryosections of brains were stained for γH2AX as in Fig. 7 and foci counted in the dentate gyrus (B) or CA1 layer in 50x40 μm counting frames (D). White arrows represent γH2AX foci in the nucleus, yellow arrows represent background staining that was not counted. (C, E) Quantification of γH2AX in the dentate gyrus (C) or CA1 layer (E). The y-axis is γH2AX foci per counting frame. ANOVA with Tukey’s HSD, * = p < 0.05. n = 3 animals per experimental condition. Error bars represent s.e.m.
We next asked if, similar to the VZ/SVZ, loss of Cyclin A2 led to decreased γH2AX in the hippocampus. We examined both the dentate gyrus and the CA1 layer of the hippocampus, the original target for cre expression in the CamkIIα-cre mouse. We found no significant change in γH2AX in the dentate gyrus in 4 month old animals (Fig. 8B-C). However, we did observe a decrease in γH2AX foci in the CA1 layer in 4 month old animals, and observed a time-dependent decrease in γH2AX in controls in both hippocampal areas in the controls. This time-dependent decrease in γH2AX was much less pronounced in experimental animals (Fig. 8D-E).
Next, we asked if loss of Cyclin A2 in the developed brain led to appreciable behavioral changes. Based on our γH2AX results in the hippocampus, we hypothesized that these mice would have defects in learning and memory. We therefore subjected 8 month old animals to a battery of behavioral tests. Mice without Cyclin A2 performed significantly worse in the Barnes Maze [33], which tests spatial learning and memory, and fear conditioning [34], which tests contextual memory (Fig. 9A-D). We additionally observed a trend toward decreased performance in the passive avoidance test, although it did not meet our threshold for statistical significance. We did not observe differences in other behavioral tests including anxiety-like responses (open field and elevated plus maze tests), depressive-like responses (forced swim and tail suspension tests), sociability (social preference test), or in marble burying behavior, although there was a small decrease in pain sensitivity and a small increase in Rotarod performance (Supplemental Fig. S3). These results underscore the differential requirement of Cyclin A2 in the development of different structures of the brain. Furthermore, they support a novel role for Cyclin A2 in DNA repair in post-mitotic neurons, which is unexpected given its major role as an S-phase regulator in cycling cells.
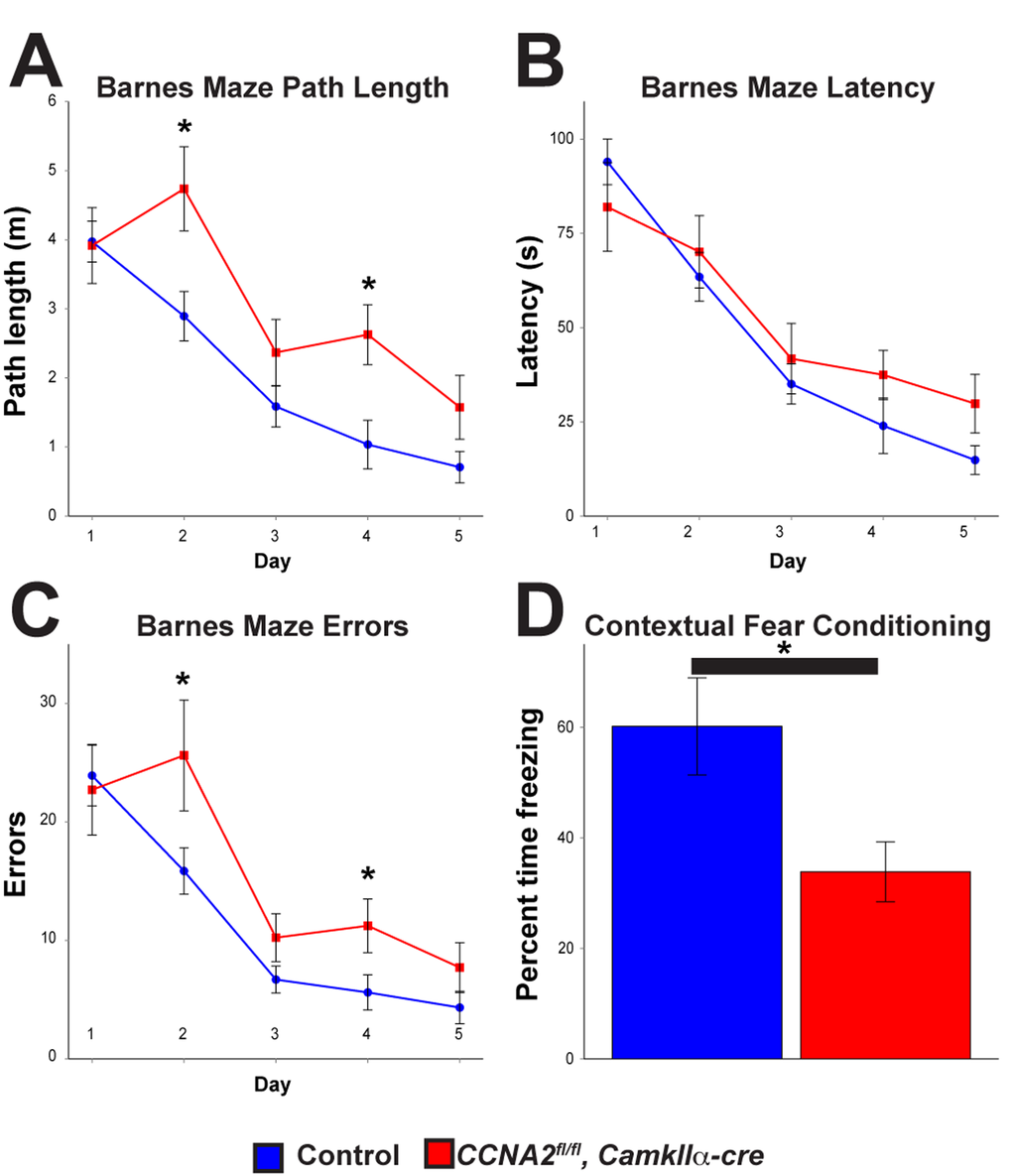
Figure 9. Cyclin A2 loss in the hippocampus leads to deficits in learning and memory.CCNA2fl/fl, CamkIIα-cre mice and control CCNA2-intact mice were subjected to a battery of behavioral tests. (A-C) Barnes maze path length, latency, and errors, respectively. The x-axis is testing day. The y-axis is path length (A), total time to escape (B), and total errors during the test (C). CCNA2fl/fl, CamkIIα-cre mice performed worse in the Barnes maze than did control mice. Mixed model ANOVA with post-hoc t-test, * = p < 0.05. n = 12 control mice and 7 CCNA2fl/fl, CamkIIα-cre mice. (D) Mice were subjected to a fear conditioning test. CCNA2fl/fl, CamkIIα-cre mice showed less of a response to contextual clues during the test. The y-axis is percent of the test time the mouse spent in the “frozen” fear response state. Error bars for all graphs represent s. e. m.