Introduction
Aortic dissection (AD) is among the most elusive and life-threatening vascular diseases. When the ascending aorta is involved, the dissection is termed a Stanford type A aortic dissection (STAAD) [1]. In the natural history of aortic dissection, the mortality of untreated patients with acute STAAD is 32% in the first 24 hours, 50% at the end of the third day, and up to 80% two weeks after onset [2]. The molecular pathogenesis of STAAD is not yet well understood.
The ascending aortic wall consists of collagen, vascular smooth muscle cell (VSMC), and approximately 50 elastic laminas [3]. Previous studies have shown that histopathological and genetic factors lead to reduced elasticity and media degeneration of the aortic wall, which disrupt the homeostasis of extracellular matrix (ECM) mechanical stress [4,5]. Thus, pre-existing weaknesses of the aorta are considered the basis for aortic dissection [6,7]. Mature VSMCs play a role in contractile function during homoeostasis. VSMC apoptosis has been implicated as a major event in many aortic diseases [8–10] and especially in the pathogenesis of aortic dissection [11]. VSMC functioning is also associated with changes in mechanical stress [12,13]. However, how disorganized mechanical stress contributes to VSMC apoptosis and the development of STAAD remains unclear.
Yes-associated protein (YAP) is ubiquitous in vivo and involved in the regulation of cell proliferation and apoptosis [14–16]. Increased YAP expression and activation result in the initiation of proliferation and the suppression of apoptosis in hepatocellular carcinoma [17–20] and pancreatic progenitor cells [21]. Our previous study found that enlargement of cardiomyocytes, which is induced by YAP up-regulation, led to cardiac hypertrophy [22]. Recently, the ablation of smooth muscle–specific Yap in mice resulted in embryonic lethality with abnormal aorta development [23]. Thus, the functional role of YAP in cardiac/SMC proliferation during cardiovascular development cannot be overemphasized [23]. Moreover, altered mechanical stress reportedly affects YAP expression in tumor tissues [24,25].
In this study, we investigated the relationship between YAP down-regulation and VSMC apoptosis during the development of STAAD. Furthermore, in a BAPN (β-aminopropionitrile monofumarate)-induced mouse STAAD model, we found that the changes in YAP expression observed in VSMCs are similar to those observed in clinical samples.
Results
Maximum aortic wall velocity was decreased in STAAD ascending aorta
To determine whether there was a reduction of aortic wall elasticity, we collected samples from STAAD patients undergoing ascending aorta replacement and from heart transplantation donors (HTD). Representative computed tomography and intraoperative images of selected STAAD patients and HTDs illustrating typical STAAD features are presented in Figure 1A. The ascending aortas of STAAD patients were demonstrably enlarged according to the intraoperative images and computed tomography angiography (CTA) (Figure 1A); typical true and false cavities were observed using CTA (Figure 1A). We also compared the STAAD patients to age- and gender-matched healthy volunteers: echocardiography revealed highly significant maximum aortic wall velocity (Vmax) differences (including aortic wall systolic velocity, late diastolic retraction velocity and early diastolic retraction velocity) between healthy subjects (Figure 1B, C-E) and patients with STAAD (Figure 1B, C-E). The mean aortic wall systolic velocity of the ascending aorta was significantly lower (p=0.0417) in patients with STAAD (4.80±0.32 cm/s) than that in healthy volunteers (5.73±0.25 cm/s). The mean late- and early-diastolic retraction velocities of the ascending aorta were 5.73±0.37 cm/s and 4.30±0.42 cm/s, respectively, in healthy volunteers and 4.68±0.17 cm/s and 3.08±0.15 cm/s, respectively, in patients with STAAD (late, p=0.0478; early, p=0.0407).
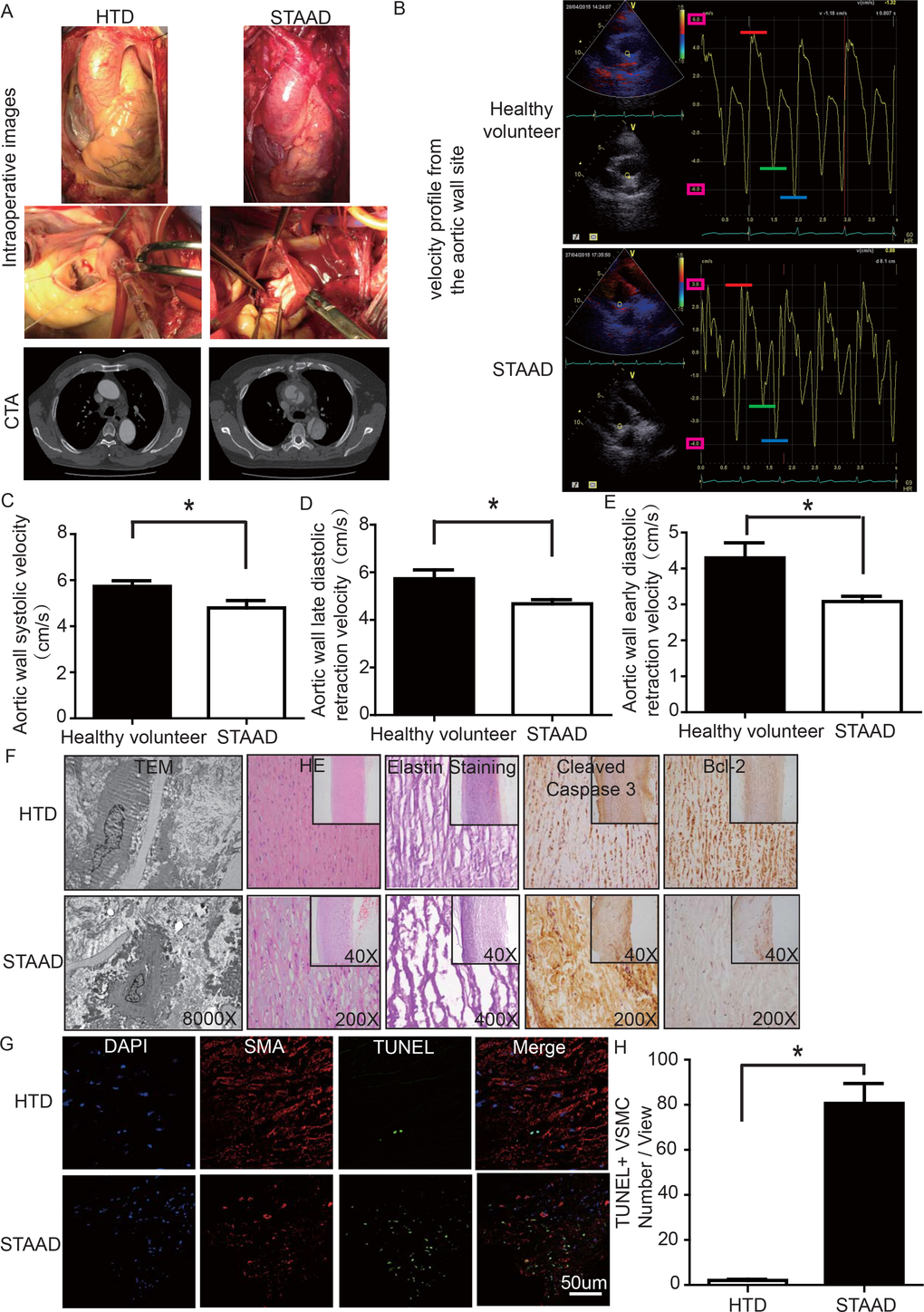
Figure 1. (A) Intraoperative images and CTA results showing the enlarged ascending aorta and typical true and false cavities in STAAD. (B) Echocardiography showing the Vmax of healthy volunteers and patients with STAAD (including aortic wall systolic velocity (red line), late diastolic retraction velocity (blue line) and early diastolic retraction velocity (green line)). (C) The mean aortic wall systolic velocity of the ascending aorta was significantly lower in patients with STAAD than in healthy volunteers (n=5 in healthy volunteer group, n=5 in STAAD group, *p=0.0417). (D) The mean late-diastolic retraction velocity of the ascending aorta was significantly lower in patients with STAAD than in healthy volunteers (n=5 in Healthy volunteer group, n=5 in STAAD group, *p=0.0478). (E) The mean early-diastolic retraction velocity of the ascending aorta was significantly lower in patients with STAAD than in healthy volunteers (n=5 in healthy volunteer group, n=5 in STAAD group, *p=0.0407). (F) TEM showed partly fragmented and reduplicated elastic lamina and abnormal VSMCs, together with an electron-dense amorphous material peripheral cell membrane in the ascending aortic wall of patients with STAAD, H&E and elastin staining showed obvious ascending aorta tissue structure disorganization in patients with STAAD, and immunohistochemistry showed that cleaved caspase-3 was present at high levels and bcl-2 was present at low levels in the ascending aortic wall of patients with STAAD relative to that of HTDs. (G, H) Confocal fluorescence microscopy showed that the numbers of double stained (TUNEL and α-SMA) cells was higher in the ascending aortic wall of patients with STAAD (n=19 in HTD group, n=23 in STAAD group, *p=0.0009).
The ascending aortic walls of patients with STAAD presented ECM disorders and increased VSMC apoptosis
We used histology to determine whether there were ECM disorders and increased VSMC apoptosis. H&E and elastin staining showed obvious VSMC disorganization and elastic lamellae dissection in the STAAD samples (Figure 1F). Relative to VSMCs in HTDs, the arrangement of the VSMCs in STAAD samples was sparse and irregular, the number of elastic lamellae decreased and the gaps among elastic lamellae were larger (Figure 1F). transmission electron microscope (TEM) analysis of human ascending aortic wall samples obtained from patients with STAAD showed obvious abnormalities compared to the normal control samples (Figure 1F). The aortic tunica media of the normal ascending aorta presented a regular appearance, and smooth muscle cells were observed between the elastic lamina within a homogenous interstitial matrix. The STAAD samples exhibited severe alterations in the vascular wall structure. The aortic media of STAAD showed irregular VSMCs between partly fragmented and reduplicated elastic lamina. In the STAAD samples, abnormal VSMCs were frequently observed together with an electron-dense amorphous material in the peripheral cell membrane; this finding is consistent with apoptosis (Figure 1F). Moreover, we also detected the VSMC apoptosis in the STAAD samples by confocal fluorescence microscopy analysis of terminal deoxynucleotidyl transferase-mediated nick-end labeling (TUNEL) and α-SMA staining (Figure 1G, H), as well as cleaved caspase-3 and Bcl-2 expression (Figure 1F). The results obtained were consistent with those obtained using TEM.
YAP expression decreased in ascending aortic wall in patients with STAAD
To observe YAP expression, we obtained quantitative data using western blotting and real-time PCR. We observed significant decreases in YAP RNA and protein expression in the ascending aortic wall of patients with STAAD (Figure 2A-C). Confocal fluorescence microscopy analysis of the co-staining of α-SMA and YAP in the tunica media indicated that YAP is mainly expressed in the VSMCs in normal aortas; in STAAD, the expression level of YAP in the middle layer is reduced (Figure 2D). Moreover, SMA expression was lower in STAAD, demonstrating that VSMCs were lost (Figure 2D). Additionally, YAP expression was negatively correlated with the ascending aorta diameter (Figure 2E), indicating that YAP may be involved in STAAD development.
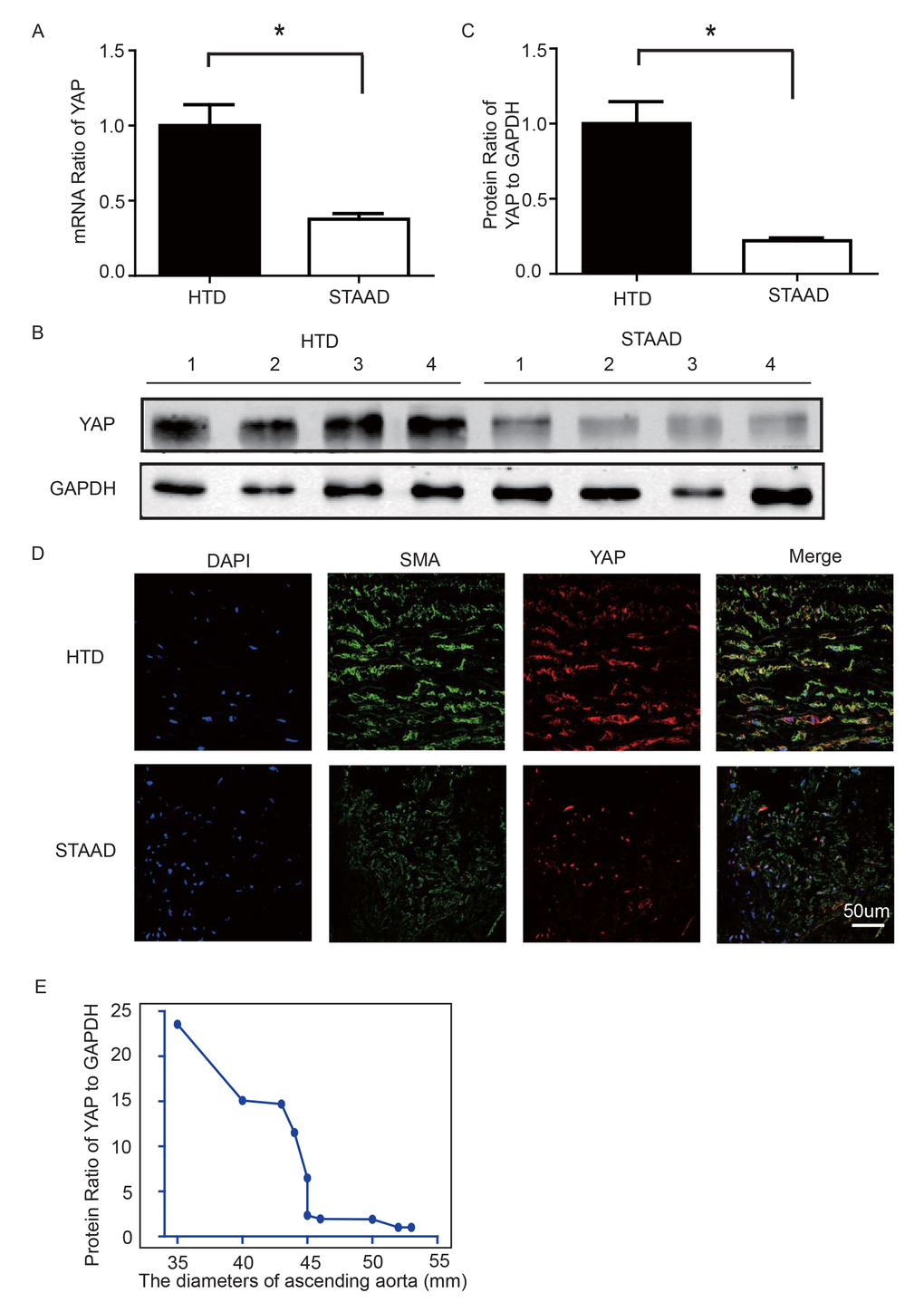
Figure 2. (A) Real-time PCR results showing that YAP was significantly down-regulated in the ascending aortic wall of patients with STAAD compared to HTDs (n=19 in HTD group, n=23 in STAAD group, *p=0.0003). (B, C) Western blotting showed that the expression of total YAP proteins was significantly lower in the ascending aortic wall of patients with STAAD compared to HTDs (n=19 in HTD group, n=23 in STAAD group, *p=0.0019). (D) Confocal fluorescence microscopy showing that YAP and α-SMA double stained cells were present at lower numbers and less quantity in patients with STAAD compared to HTDs. (E) Quantified and statistical analyzed of each patient sample’s Western blotting result showed that YAP expression was negatively correlated with the ascending aorta diameter (n=10).
BAPN-induced mouse STAAD model recapitulated human phenotype, showing damaged ECM and VSMC apoptosis
Lysyl oxidase (LOX), which crosslinks tropoelastin monomers to form elastic fibers, reportedly has a significant role in regulating ECM homeostasis [26]. Thus, BAPN (β-aminopropionitrile monofumarate, Sigma–Aldrich, St. Louis, MO), a LOX inhibitor, was used to build the model of aortic dissection in mice [4,27]. Through echocardiography and anatomical observation, we evaluated the ascending aortic dissection induced by BAPN after 4 weeks. After 4 weeks of BAPN treatment, the animal models presented with the features of STAAD, as shown in Figure 3A, B. We performed H&E and elastin staining and found VSMC disorganization and elastic lamella dissection that were similar to those observed in the clinical STAAD samples (Figure 3C). We also detected VSMC apoptosis in the ascending aortic wall of mice with BAPN-induced ascending aortic dissection based on TUNEL and α-SMA staining (Figure 3D, E). These results confirmed that the BAPN-induced mouse STAAD model successfully recapitulated the human phenotype, including ECM damage and VSMC apoptosis.
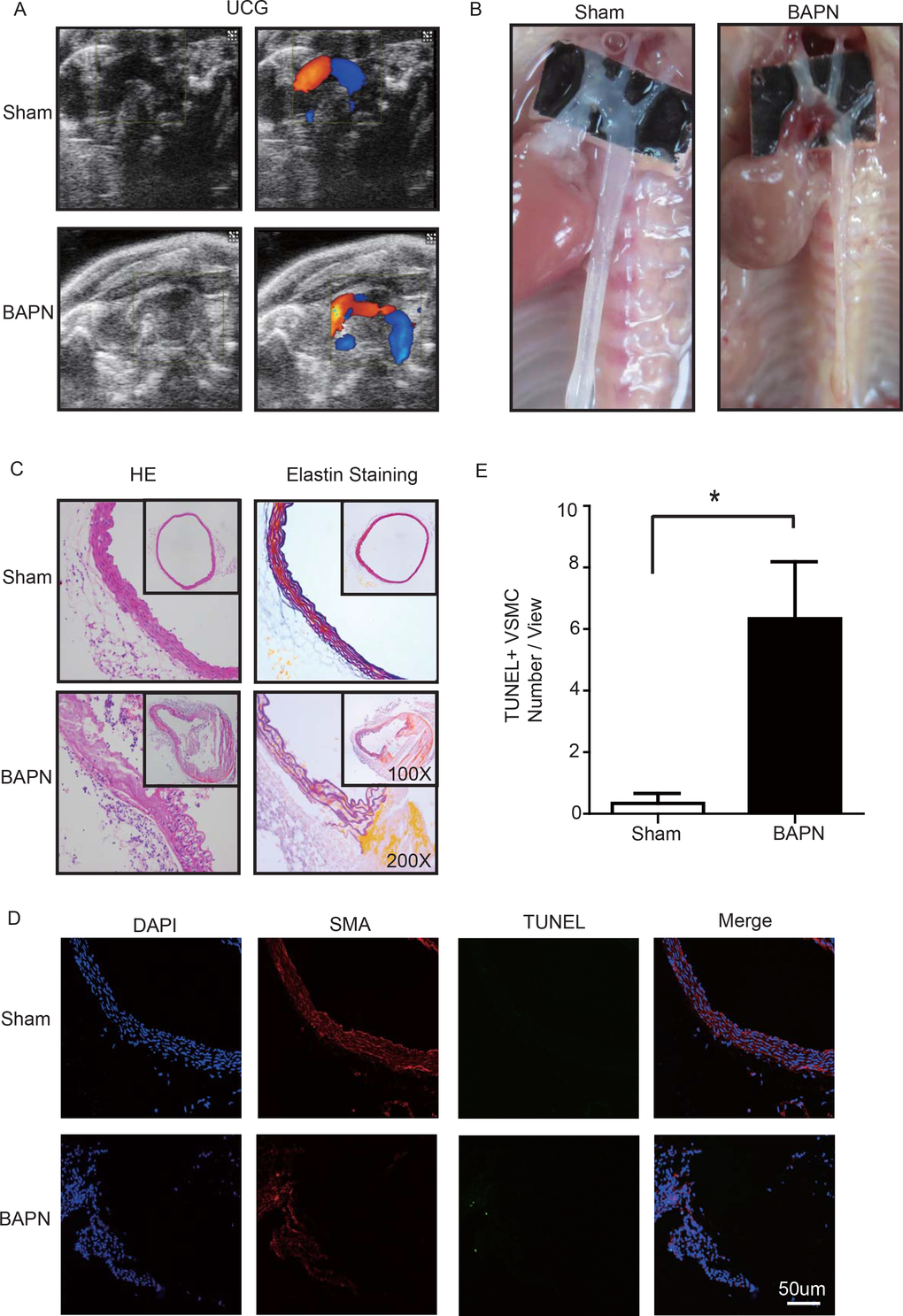
Figure 3. (A) Echocardiographic results showing that the area of the ascending aorta without colorful blood flow in the BAPN model suggesting a false cavity of dissection. (B) Gross examination revealing mural hematomas in the BAPN model, suggesting a false cavity of dissection. (C) H&E and elastin staining showing obvious disorganization of the aortic tissue structure in the BAPN model. (D, E) Confocal fluorescence microscopy showing that the cell number of double stained (TUNEL and α-SMA) cells was significantly more in the BAPN-induced ascending aortic dissection model (n=10 in Sham group, n=10 in BAPN group, *p=0.0335).
YAP was down-regulated in ascending aorta of BAPN-induced mouse STAAD model
We used the BAPN-induced mouse STAAD model to verify the relationship between down-regulated YAP and STAAD. Consistent with the patient samples, we found that YAP was significantly reduced in the ascending aortic wall of BAPN-induced STAAD mice at both the RNA and protein levels (Figure 4A-C). Confocal fluorescence microscopy analysis of the co-staining of α-SMA and YAP in the tunica media indicated that YAP is mainly expressed in VSMCs in normal aortas; In the BAPN models, the YAP expression level was reduced in the middle layer (Figure 4D). Finally, SMA expression was significantly lower, similarly to the results observed in human STAAD (Figure 4D).
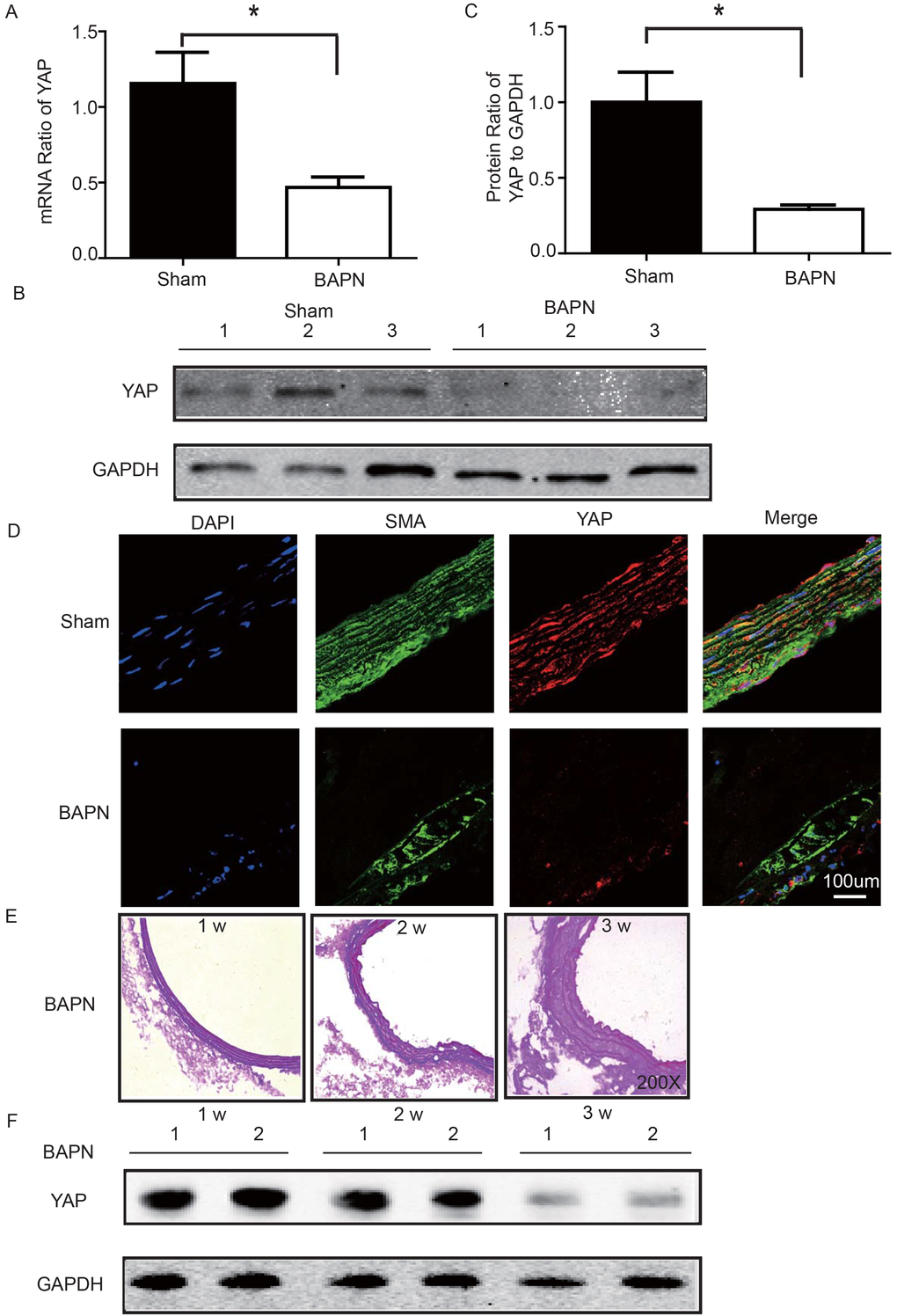
Figure 4. (A) Real-time PCR showing that YAP was expressed at significantly lower levels in the ascending aortic wall of the BAPN-induced STAAD mouse compared to that of the sham control (n=10 in Sham group, n=10 in BAPN group, *p=0.0337). (B, C) Western blotting showed that the total YAP protein expression was significantly lower in the ascending aortic wall of the BAPN-induced STAAD mouse compared to that of the sham control (n=10 in Sham group, n=10 in BAPN group, *p=0.0088). (D) Confocal fluorescence microscopy showing that YAP and α-SMA double stained cells were present at lower number and less expression of YAP in the ascending aortic wall of BAPN-induced STAAD mice. (E) Elastin staining of the ascending aortas of mice that were treated with BAPN for different times (1, 2 and 3 weeks) showing that the ascending aortas of mice presented significant elastin disorganization after 3 weeks of BAPN administration compared to mice receiving BAPN for 1 or 2 weeks. (F) Western blotting showing that YAP expression in the ascending aorta was lower after feeding with BAPN for 3 weeks than after feeding with BAPN for 2 weeks.
YAP decreased during development of ECM damage in BAPN-induced mouse STAAD model
To investigate the role of YAP in STAAD development, we examined its temporal expression in the ascending aortas of mice after BAPN administration at various time points (1, 2 and 3 weeks). To assess the extent of ECM damage, we graded elastin degradation (4 grades) [28,29]. The ascending aortas of mice after BAPN administration presented significant elastin disorganization at 3 weeks compared to 1 week or 2 weeks (Figure 4E). We then confirmed YAP down-regulation in the ascending aorta over time using western blotting. The observed trends were consistent with the ECM damage (Figure 4F).
YAP knockdown induced VSMC apoptosis under static conditions
To determine whether YAP down-regulation induced VSMC apoptosis, an in vitro experiment was performed. CRL-1999 VSMCs were infected with a lentivirus expressing either YAP short hairpin RNAs (shRNA) or a scrambled control. Western blotting analysis showed that YAP was successfully knocked down in the PLKO-YAP group compared to the scrambled control group (Figure 5A). Moreover, flow cytometry showed increased VSMC apoptosis after YAP knockdown in vitro (Figure 5B). The mean ratio of apoptotic VSMCs was significantly higher in the PLKO-YAP group (12.43±0.50%) compared to the scrambled group (8.37±0.09%, p=0.0013, Figure 5C).
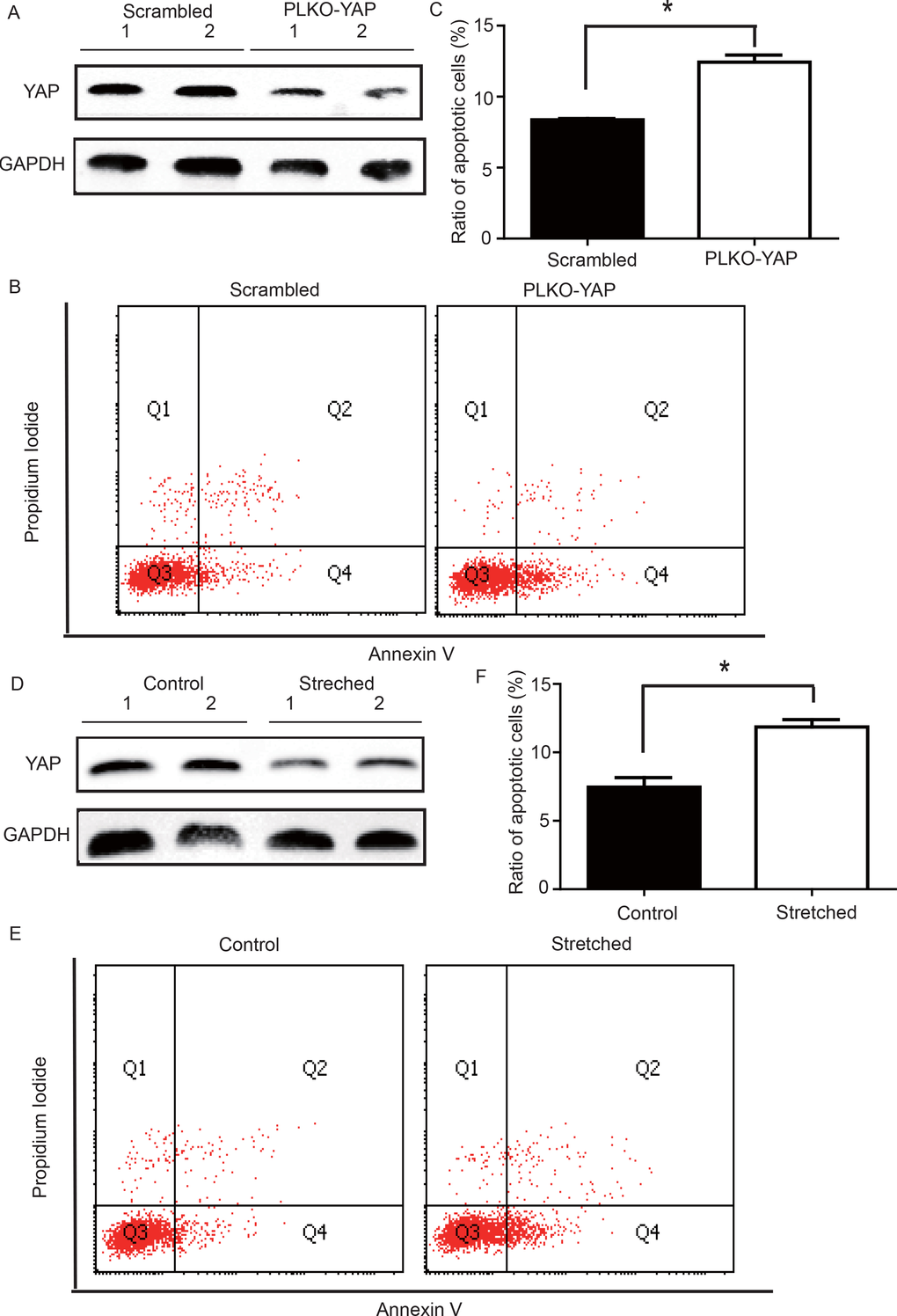
Figure 5. (A) Western blot showing YAP down-regulation in the PLKO-YAP group compared to the scrambled group. (B, C) Flow cytometry showing increased VSMC apoptosis in the PLKO-YAP group compared to the scrambled group (repeated 3 times for statistical analysis, *p=0.0013). (D) Western blotting showing YAP down-regulation in the experimental group compared to the control group after cyclic stretching. (E, F) Flow cytometry showing increased VSMC apoptosis compared to the control group after cyclic stretching in vitro (the lower right quadrant (Q4) represents the apoptotic VSMCs, and repeated 3 times for statistical analysis, *p=0.0010).
Altered mechanical stress induced YAP down-regulation and apoptosis in cultured VSMCs
To determine the possible relationship between the stress-induced YAP down-regulation and VSMC apoptosis, a mechanical strain unit was used to simulate altered mechanical stress in the damaged ECM. CRL-1999 VSMCs in the experimental group were subjected to cyclic mechanical stretching using a computer-controlled mechanical strain unit (18% elongation for 36 hours); CRL-1999 VSMCs in the control group were cultured under normal conditions. After cyclic stretching, western blotting analysis showed that YAP was down-regulated in the experimental group compared to the control (Figure 5D). In addition, flow cytometry showed increased VSMC apoptosis after cyclic stretching in vitro (Figure 5E). The mean percentage of apoptotic VSMCs in the experimental group (11.86±0.53%) was significantly larger than that in the control group (7.46±0.49%, p=0.0010, Figure 5F).
Discussion
Our main finding was the observation of a unique mechanism of altered mechanical stress caused by the damaged ECM. This altered stress induced YAP down-regulation and VSMC apoptosis, which is related to STAAD development (Figure 6). The elastic properties of the vascular system might represent a true measure of aortic wall weakness. In the clinic, these properties are evaluated based on the stiffness index of aorta, which is negatively related to Vmax (including aortic wall systolic, late diastolic retraction and early diastolic retraction velocities) [5,30]. Decreased Vmax is predictive of aortic dissection [5,30]. In this study, the Vmax of the ascending aorta was significantly decreased in patients with STAAD, suggesting that pre-existing weakness represents the histopathological basis of STAAD formation.
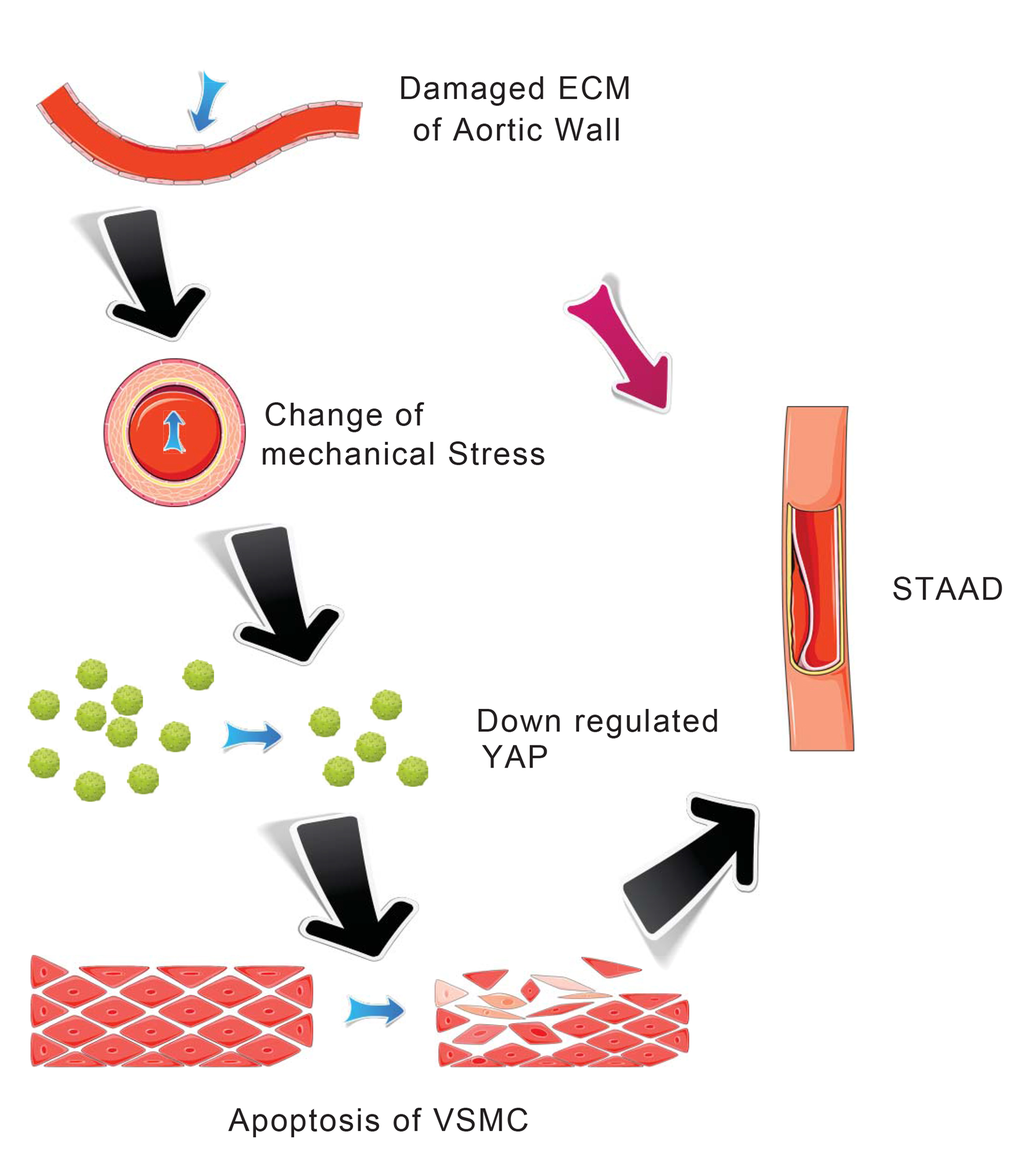
Figure 6. Schematic model showing that the disruption of mechanical stress in ECM induces STAAD and is related to the down-regulation of YAP.
VSMC plays a key role in maintaining aortic wall function and substantially contribute to the elastic lamellar architecture of the arterial wall, both directly and indirectly through their production of matrix proteins [31,32]. Several studies have found that apoptosis was related to the pathology of aortic medial degeneration and in the pathogenesis of aortic aneurysm and dissection [4,33,34]. Using TEM, we observed a distinct VSMC apoptotic ultrastructure in the ascending aortic wall of patients with STAAD. Some previous studies have also concluded that the α-SMA-positive cells in the middle layer of the aorta are mainly VSMCs [4,35,36]. We found that the α-SMA-positive cells, which were primarily VSMCs, showed an increased intracellular frequency of characteristic TUNEL apoptosis and increased expression of cleaved caspase-3, which were key markers of apoptosis, in STAAD patients’ ascending aortic wall. These results were similar to those found using TEM. Thus, VSMC apoptosis was confirmed to be accompanied by ECM damage. ECM damage can lead to reduced aortic wall tensile strength and elevated vascular wall mechanical stress [4]. In addition, VSMC apoptosis might be induced by cyclic stretching, similarly to the changes induced by mechanical stress [4]. This was also supported by the results found in our mouse BAPN model, in which the failure of elastin crosslinking in the ascending aorta and VSMC apoptosis following exacerbation of ECM damage were observed.
The functional role of YAP has been identified in cardiomyocytes [22] and VSMCs [37]. During cardiovascular development, YAP affects cardiac/SMC proliferation by regulating the expression of some cell cycle suppressors, such as G-protein–coupled receptor 132 (Gpr132) [23]. YAP is also a central regulator of the phenotypic switch of VSMCs [37]. Our previous study found that YAP also plays a key role in hypertrophic cardiomyopathy [22]. Thus, the regulation of cardiovascular development by YAP cannot be overemphasized. YAP is inhibited by culturing cancer cells, such as HeLa cells, on a soft matrix [38]. However, whether a similar phenomenon is conserved in VSMCs is still unknown. In the present study, the results of clinical samples showed that clearly disrupted elastic lamellae of variable widths in STAAD softened the ECM of the ascending aortic wall, possibly inducing YAP down-regulation.
Although we observed an association between ECM damage and YAP expression in STAAD patients’ samples, we were still unable to estimate whether changes in mechanical stress caused the YAP down-regulation. To solve this problem, we developed a mouse BAPN model in which the ECM was disturbed [26]; these mice were prone to aortic dissection [4,27]. In the present study, ECM damage occurred as early as 2 weeks after BAPN treatment, though decreases in YAP expression did not appear until 3 weeks after BAPN treatment. Gradual decreases in YAP expression were observed following ECM damage in the ascending aortas of the BAPN-treated mice; this finding supports the hypothesis that altered mechanical stress induces YAP down-regulation.
Some researchers have reported that YAP down-regulation can induce apoptosis in cells such as podocytes and PC-3 cells [39,40]. However, there are no studies showing whether YAP down-regulation induces VSMC apoptosis. To explore this issue, an in vitro experiment was used. After YAP knockdown, VSMC apoptosis increased significantly, demonstrating that YAP knockdown under static conditions causes VSMC apoptosis. Considering the complexity of the in vivo situation, cyclic stretching has been used to establish an in vitro apoptosis model in VSMC [41]. To test the direct effects of mechanical stress on VSMCs, we employed CRL-1999 VSMCs. Changed mechanical stretching induced YAP down-regulation in VSMCs. This suggested that cyclic stretching, which mimics altered mechanical stress in the ascending aorta, led to decreased YAP expression in the cyclic stretch-treated CRL-1999 VSMCs. These results demonstrated that the changes in mechanical stretching led to YAP down-regulation and VSMC apoptosis. Several researchers have reported that knockdown of YAP enhanced contractile phenotype-specific gene expression in VSMCs, including myocardin, SMA, SM22, and SMMHC [37,42]. Usually, an enhanced smooth muscle contractile phenotype would be beneficial to the function of the aorta. However, given the crucial role of YAP in the proliferation of VSMCs, knockdown of YAP in VSMCs after artery injury instead attenuated the injury-induced conversion of the smooth muscle phenotype [42]. Our present data confirmed this conclusion from a different perspective: knockdown of YAP abolished smooth muscle contractile phenotype-specific gene expression in VSMCs by inducing the apoptosis of VSMCs (Figure 2D), ultimately impairing the function of the aorta and promoting the development of STAAD.
The limitation of this study, the role of major targets of YAP in the pathogenesis of ECM mechanical stress-induced STAAD, will be examined in our future research. In fact, many YAP targets, including transcriptional coactivator with PDZ-binding motif (TAZ) [43,44], TEAD family transcription factors [44], and the myocardin-SRF complex [37], have been studied in the context of other arterial diseases. All the studies noted above focused on the role of YAP targets in the proliferation of VSMCs: inhibition of TAZ and TEAD reduced VSMCs proliferation [43,44], and YAP promoted the conversion of VSMCs from a proliferative phenotype to a contractile phenotype by interacting with the myocardin-SRF complex [37]. Additionally, the major YAP targets influencing the apoptosis of VSMCs should be investigated, especially with respect to their roles in ECM mechanical stress-induced STAAD pathogenesis.
In a conclusion, this study reported for the first time that altered YAP expression is associated with ECM damage in STAAD. YAP down-regulation caused by the disruption of mechanical stress is related to the development of STAAD through its induction of aortic VSMC apoptosis.
We thank Dr. Yuan Zengqiang for providing the lentivirus expressing either YAP shRNA and scrambled control.
This study was supported by the National Natural Science Foundation of China (NO. 81170283 NO. 81470580 and NO. 81422003).
No conflicts of interest, financial or otherwise, are declared by the authors.