Abstract
The central nervous system (CNS) is the cellular substrate for the integration of complex, dynamic, constant, and simultaneous interactions among endogenous and exogenous stimuli across the entire human lifespan. Numerous studies on aging-related brain diseases show that some genes identified as risk factors for some of the most common neurodegenerative diseases - such as the allele 4 of APOE gene (APOE4) for Alzheimer’s disease (AD) - have a much earlier neuro-anatomical and neuro-physiological impact. The impact of APOE polymorphism appears in fact to start as early as youth and early-adult life. Intriguingly, though, those same genes associated with aging-related brain diseases seem to influence different aspects of the brain functioning much earlier actually, that is, even from the neonatal periods and earlier. The APOE4, an allele classically associated with later-life neurodegenerative disorders as AD, seems in fact to exert a series of very early effects on phenomena of neuroplasticity and synaptogenesis that begin from the earliest periods of life such as the fetal ones.
We reviewed some of the findings supporting the hypothesis that APOE polymorphism is an early modifier of various neurobiological aspects across the entire human lifespan - from the in-utero to the centenarian life - during both normal and pathological conditions of the brain.
Introduction
Some genes identified as risk factors for aging-related neurodegenerative diseases - such as the allele 4 of Apolipoprotein E gene (APOE4) for Alzheimer’s disease (AD) - can have an early impact on different neuronal and non-neuronal features of the human brain, even starting from the perinatal periods or earlier. The possible early influence of APOE polymorphism on various cognitive and non-cognitive aspects through the entire human lifespan [1–8] poses the biological rationale for a wider re-consideration on how identical genes - for example, the ones identified as risk factors for aging-related diseases - could differently interact with other genes and environmental factors from the beginning of the human life until the advanced or extreme age.
Essentially, in this context, the main scientific question is: how do these genetic risk factors for late-life brain diseases impact, or have impacted, the general development of the brain and specific cytoarchitectural aspects of neuronal and non-neuronal cells and generate, or have generated, meaningful changes on complex cerebral functions (e.g. episodic memory, visual attention) and ultimately determine divergent clinical outcomes across the entire human lifespan [9,10].
It is in fact more and more evident that a series of mutual and long-term interactions between genetic and environmental factors control, or might even determine, quantitative differences between healthy and pathologic neural tissues [11–13]. Accordingly, it would be only after a relatively long period of time, normally measured in decades, that global or more selective quantitative differences across different types of neural circuits (e.g. extrapyramidal motor system, limbic system, pyramidal motor system) or brain regions (e.g. hippocampus, amygdala, frontal cortex), would affect specific cerebral functions later in life. Specifically, these cellular quantitative differences could culminate later in life in a more diffuse and irreversibly progressive neurodegenerative process such as dementia (e.g. Alzheimer’s disease [AD], dementia with Lewy bodies [DLB], frontotemporal dementias [FTDs]); in some more selectively distributed deficits (e.g. Parkinson’s disease [PD], progressive supranuclear palsy [PSP]); or in a more focally-distributed functional impairment of a specific neuronal circuitry (e.g. amnestic mild cognitive impairment [a-MCI], essential tremor [ET]).
The concept about gene-environment influence on quantitative ratios between health and pathologic neural tissues implies that when certain specific amounts of neuronal and non-neuronal cells (e.g. glial), which we could term “cellular ratio thresholds” that support the global functioning of the brain (e.g. cardio-respiratory regulation through the cardio-respiratory circuits of the brainstem nuclei), or a cluster of specific cerebral functions (e.g. the executive functions through the signals elaboration in the frontal cortex, or the visual-spatial functions through the temporal lobe activation), or a single cerebral function (e.g. the memory through the hippocampal neuronal firing integration) in a specific individual (a subject carrying a specific set of genes and exposed to either beneficial or detrimental environmental factors along his/her previous life) have been crossed, a neurodegenerative process begins to be clinically manifest and, only in appearance, to be linked to the aging process, but actually being the effect of a long-lasting accrual of various pathogenic and detrimental elements accumulated in the previous decades of his/her life.
Maintaining adequate cellular ratios between healthy and pathologic neural tissues would consist in maintaining enough amounts of still-functioning (normal) vs. non-functioning (pathologic) cells able to keep and preserve a normal operational status of the brain. This normal operational status would be the one accounting for keeping motor, sensory, cognitive, emotional, and behavioral skills still functional enough in order to conduct the normal daily functions of life in an independent manner [14]. Importantly, these functional neural ratios (neuronal and non-neuronal cells) kept below the “dysfunctional cellular ratio thresholds” would not be simply determined by the passive accumulation of a single or multiple brain pathologies (e.g. accumulation of extracellular insoluble β-amyloid in the cerebral cortex or intracellular formation of hyperphosphorylated-tau neurofibrillary tangles as in AD) but also on how certain “predisposing genes” in a specific CNS/brain/person have mutually interacted with a specific group of environmental factors throughout his/her life.
One of the most striking examples of the possibility to maintain normally-functioning neural ratios despite the presence of brain lesions accumulation, it is observation that some older subjects can be categorized as asymptomatic AD subjects (ASYMAD). ASYMAD are cognitively normal older subjects that show, at autopsy, an equivalent or even higher amounts of AD pathology in comparison to those found in age-matched, or even younger subjects, which did receive a clinical diagnosis of AD [15–21]. These autopsy-confirmed findings greatly support the hypothesis that genetic or environmental factors might contribute to the clinical silencing of AD pathology, at least in a certain group of older subjects (e.g. the ASYMAD subjects). Moreover, these clinicopathologic discrepancies between brain pathology and clinical manifestations (of absence of them) in AD or generally in dementia, support, among others, the general concept of the “cognitive/brain reserve” [22–24].
The cognitive/brain reserve concept, though, needs to necessarily correspond to some biological reservoir of still-functioning cells and neural circuits, which ultimately result from the cumulative balance between beneficial and detrimental genetic and environmental factors that have impacted the architecture of the brain (e.g. synaptic contact distribution in the hippocampus) and that consequently have modified specific brain functions (e.g. memory) across all the previous periods of life [25]. Additionally, gene-environment interactions accounting for the cognitive/brain reserve capacities in a specific individual across his/her entire lifespan should consider possible prenatal genetic and behavioral factors originating from each parent since the fetal times [26–28].
APOE is one of the best examples that shows how a well-established genetic risk factor for an aging-related disease (AD) exerts its influence throughout the entire human lifespan and is part of complex metabolic and gene-environment interactions, especially in terms of brain cholesterol metabolism and synaptic formation [29]. The fact that the APOE polymorphism (presence of three possible alleles [APOE2, APOE3, APOE4] and for each individual the possibility to have only one of the six possible APOE genotypes [APOE2/2, APOE2/3, APOE2/4, APOE3/3, APOE3/4, APOE4/4]) correlates with different, sometimes divergent, clinical, pathologic, and survival outcomes, implies fundamental questions on which are the specific gene-environmental molecular mechanisms that determine those different effects across the entire human lifespan [30].
We aimed to briefly review some of the well-established findings on the APOE polymorphism associated with different, sometimes paradoxical, effects of this gene on the CNS structures and functions. We aimed to focus on human studies that evidence the effects of APOE polymorphism on the development of the brain, on different neuropsychiatric phenotypes, and on some higher cognitive functions present in humans only (e.g. language). Furthermore, we succinctly describe some of the possible molecular mechanistic aspects and biochemical pathways that have been proposed to explain the apparently paradoxical effects of APOE across the human lifespan [31].
ApoE (protein)
In humans, the apolipoproteins E (ApoE2, ApoE3 and ApoE4) are molecules expressed in peripheral tissues (liver, spleen, kidneys, and macrophages) [32,33] and within the CNS [34]. ApoE is an essential apolipoprotein for the catabolism of triglyceride-rich lipoprotein constituents and it is found in chylomicron, low- and very low-density lipoproteins (LDLs, VLDLs) [35]. While liver and macrophages are the primary peripheral tissues where ApoE is produced, astrocytes are the main, and apparently the only cells within the CNS, that produce ApoE. Functionally, in the peripheral tissues, ApoE is part of cholesterol metabolism, while in the CNS it has been recognized as the principal carrier of cholesterol [36]. ApoE transfers cholesterol to neurons and represents an essential molecule for neuronal growth, synaptic plasticity, and membrane reparative processes [37]. The transfer of ApoE (cholesterol) to neurons occurs through the interaction of ApoE receptors [38]. These receptors such as LDLR, VLDLR, ApoER2, and LRP1 receptors belong to the low-density lipoprotein receptor gene family [39]. While the different chemical differences across the three isoforms of ApoE proteins started to be lately better defined [40], their different roles and effects in each cellular type remain to be completely understood yet [41,42].
APOE (gene)
ApoE, the protein, is codified by a gene localized on the long arm (“q” arm) of the chromosome 19 in the sub-band 32 of region 13 (19q13.32): APOE gene (APOE). The APOE has three alleles (genetic polymorphism): APOEpsilon2 (APOE2), APOEpsilon3 (APOE3), and APOEpsilon4 (APOE4). Each allele codifies to corresponding protein isoforms (ApoE2, ApoE3, ApoE4) that differ in amino acid sequence at the residue 112 (also called “site A”) and 158 (also called “site B”). Specifically, APOE2, APOE3, APOE4 (the alleles) codify respectively for ApoE2, ApoE3, ApoE4 (the proteins), which at the position 112 and 158 of their amino acidic sequence, contain respectively cysteine/ cysteine, cysteine/arginine, and arginine/arginine [43]. These amino acid differences in the primary sequence of each ApoE protein determine a different number of charges (0, 1+, 2+) and account for their variant tertiary and quaternary conformations and electrophoretic differences [44]. Four individual mutations give electrophoretically separated bands at the E2 position. Using the isoelectric focusing techniques [45] at least four different bands (corresponding to different ApoE2 conformational status) have been identified: E2 (arg158-to-cys) [46], E2 (lys146-to-gln) [47], E2 (arg145-to-cys), and E2-Christchurch (arg136-to-ser) [48]. E2 (arg158-to-cys) is the most common of all four. In general, these “minimal” physical-chemical differences among ApoE proteins determine massive metabolic and structural consequences at tissue and metabolic level, especially within the human CNS [49,50].
Genetic epidemiology
In the general population, the distribution of APOE alleles can vary across different ethnic groups [51]. These variations seem to be related to different non-genetic factors such as geographical latitude [52], local temperature and altitude, metabolic rate [53], hypoxia [54], “local” availability of lipophilic nutrients [55], climate [56] and others [57–60].
Notably, from an evolutionary point of view, APOE4 has been determined to be the “oldest allele” (which is also the only allele found in non-human primates and other mammals) followed by the APOE3, and then by the APOE2, which is indeed the “most recent allele” [61]. APOE2 has been estimated to appear in humans only about 80,000 years ago [62–64]. The more recent appearance of APOE2 in humans could be explained by the relative advantage provided by the corresponding product of this allele (APOE2 protein), which seems to increase the neural plasticity, synaptic reparative and clearance capacities of the CNS [65], as well as human longevity [66]. Intriguingly, however, while carrying the APOE2 could represent a protective factor late in life, especially for delaying dementia and cognitive deficits during aging, this is not necessarily the case early in life, especially during the very early phases of life (see antagonistic pleiotropy paragraph).
APOE and antagonistic pleiotropy
APOE began to appear as one of the best examples of genetic “antagonistic pleiotropy” (AP), a concept applicable to human traits and diseases, and to human cognition, neurodegenerative diseases, and brain diseases in general [67–71]. AP is defined as a genetic phenomenon existing when a gene can control for more than one trait (pleiotropy) with at least one possible trait beneficial and another detrimental (antagonistic) to the fitness of that same organism [72,73]. Once considered a rare genetic phenomenon, AP seems rather to be a much more frequent event, especially when considering human diseases [74], and in particular aging-related diseases and human longevity [75–77]. Among the APOE polymorphism-associated traits that appear to have AP characteristics are included the different biochemical consequences that ApoE2, ApoE3, ApoE4 proteins have on the metabolism of cholesterol, which have important implications, among others, on the metabolism of β-amyloid and synaptogenesis resistance in younger vs. aged mammalians, and could so explain the “paradoxical” beneficial effects of APOE4 on specific cognitive skills (e.g. verbal memory in schizophrenic patients [78], memory performance [79], and attention in young [80]), and the observation that APOE4 is an advantageous factor on survival and infertility in infectious environments [81,82], or that APOE alleles are associated with conscientiousness personality-trait in relationship to gray matter volume [7] and greater cortical connectivity [83]. Figure 1 shows graphs describing the antagonistic pleiotropy concept applied to APOE polymorphism.
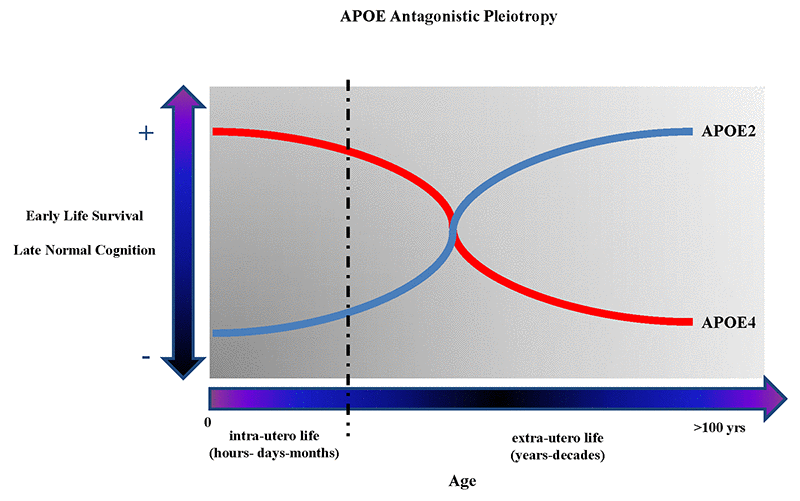
Figure 1. The figure shows the theoretical antagonistic pleotropic (AP) effect of APOE gene across the entire human lifespan. The increased probability of early survival and normal cognition later in life are alternatively correlated with the presence of APOE4 and APOE2 allele. However, other genes and environmental factors through their mutual interactions from the in-utero life until centenarian age can probably potentiate the beneficial or detrimental effects on the onset and manifestations of brain diseases and, respectively, reduce or potentiate the genetic predisposition toward more negative or positive clinical outcomes during different periods of life.
APOE polymorphism in normal and pathologic conditions of the brain across all ages
In general, APOE4 and its association with different measurable clinical variables such as dementia severity [84], neuropsychological scores [85], pathology burdens [86], cortical morphometry [5], morphometric-MRI [87,88], functional-MRI [89–91], electroencephalographic (EEG) [92,93] and magnetoencephalographic (MEG) signals [94], evoked potentials (EPs) changes [95,96], and other methods [97] has been investigated much more extensively than APOE2 [98–100]. In fact, studies focusing on APOE2 and its association to different clinical and subclinical parameters and possible molecular mechanisms of brain protection have been historically, less numerous than investigations comparing APOE4 vs. APOE3 for example. Furthermore, although APOE4 and APOE2 appear to have divergent effects on cognitive outcomes during aging, their corresponding protective or deleterious effects on other periods or conditions of life as brain development, birth prematurity, infancy, childhood cognitive and behavioral outcomes, as well as early-adult and mid-adult life predisposition for neurodegeneration have been generally much less investigated.
We organized the description of the reviewed findings on the effects of APOE polymorphism across the human lifespan using a bio-chronological order. We first described the effects of APOE polymorphism in normal and pathological conditions of the CNS during in-utero life followed by the neonatal, infancy, teenaging, and adult mid-life, and finally we describe data on APOE and the effect on elders and extreme old subjects including octogenarian, nonagenarian and centenarian populations.
APOE and brain during in-utero life (before birth) and in preterm babies (<38 weeks of gestation)
Studies focusing on the possible clinical correlations between APOE polymorphism and divergent cognitive or behavioral outcomes of in-utero life, preterm babies, and infant populations are scarce. Due to its historical predominant consideration as a gene negatively associated with later life brain diseases, especially AD, APOE and its possible effects on the normal brain development or as a genetic modifier of specific pathologic conditions such as motor-behavioral delay, cerebral palsy, prematurity, and in-utero life brain abnormalities has received little attention until recent times [101]. In-utero life events imply complex interactions among maternal and fetal APOE genotypes, which might determine a highly complex spectrum of different biochemical interactions [102,103]. For example, recent findings propose that maternal APOE genotype could be a relevant risk factor for poor outcomes at birth, premature delivery, and predisposition for preeclampsia [27,104,105]. These maternal-fetal gene-metabolism interactions during in-utero life seem to potentially determine specific patterns of biochemical imprinting events directly at cerebral level, or alternatively, on different organs and systems such as the vascular system, which could indirectly include the cerebral vasculature [106,107]. However, based on more recent observations about AP phenomenon associated with APOE polymorphism and on more consolidated data showing that the APOE2 is, per se, a protective allele against dementia and cognitive decline during aging even independently on the β-amyloid accumulation and “classic” AD pathology [109], it would be possible in the future to investigate on unprecedented aspects of the cholesterol metabolism related to genetic aspects of APOE polymorphism whom influence seem to go well behind specific cognitive aspects and to be actually a “general modulator” of neuronal structures and functions, synaptic formation, brain repair, and neural regenerative mechanisms [110,111]. Consequentially, the APOE polymorphism appears to acquire an important general role in terms of brain development, impact on neuroreparative capacities and neuroplasticity potentialities across the entire human lifespan. Moreover, coherently with the concept of an early impact of APOE on brain structures, Stoknes et al. [112] have hypothesized that APOE2-carriers have a higher risk of death in-utero life following brain injury probably due to an altered or abnormal metabolism of the cholesterol. Other investigators have shown similar results and proposed similar APOE-based pathogenetic mechanisms [113]. These initial observations could explain why some findings show a non-significant association between APOE polymorphism and cerebral palsy (CP) simply due the fact that APOE2 carriage would represent actually a major risk factors for in-utero life survival (increased prenatal mortality). Recently, another group of investigators showed that APOE gene and weight at birth are risk factors for future cardiovascular diseases, and these factors seem to be independent factors as well [114]. To note, nonetheless, that these latter findings make the possible mechanistic (molecular) correlations among APOE, cholesterol metabolism, and vascular diseases much less linear than what could have been expected earlier [115,116]. Furthermore, data in support of the interaction between APOE alleles and environment suggest that a specific APOE genotype is a risk factor able to potentiate the toxic effects of various environmental contaminants. For instance, it has been shown that APOE4-carriage implies lower cognitive scores in preterm neonates exposed to higher levels of mercury [117,118].
APOE and brain during normal neonatal (1 day-30 days full-term babies) and infancy (1-12 months full-term babies) periods
Studies on the impact of APOE polymorphism on various clinical outcomes in full-term normal neonates and infants are rare [119–121]. However, some investigations performed on selected types of neonate populations, suggest that APOE has indeed AP features. In fact, an over-representation of APOE 4/4 and 2/4 with a reduction in APOE 2/3 and 3/3 when compared with adult population [122] suggests that APOE4 is related to an increased risk of premature death. Furthermore, data from the same group of investigators found an over-representation of APOE2 in perinatal deaths populations [123]. This apparent paradoxical inversion of the protective effect of APOE2 vs. APOE4 in neonates, in contrast with the findings observed in elders, fits very well with the hypothesis that APOE has indeed antagonistic pleiotropic properties across the entire human lifespan [67,124,125]. Nevertheless, considering that the distributions of APOE alleles in healthy general populations are linked to various types of factors such as ancestry, geography, etc. (see paragraph Genetic epidemiology), much larger population cohorts stratified by the “local” APOE frequencies (corrected for all other identified influencing factors) together with a more precise definition of the periods of life to consider (perinatal deaths can have different definition across different countries) and large multi-institutional collaborative investigations are necessary to expand our knowledge on the possible pre-natal or peri-natal effects of APOE on early life events as well as on later life physiological or pathological events.
At a more speculative level, we retain that the possible early life effects associated with APOE polymorphism could primarily act on the cholesterol metabolism during the development of the brain and other organs and apparatuses (vascular system, liver, lungs, kidneys, etc.). Theoretically, APOE could also manifest a protective or detrimental effect on the brain development or its functions through indirect pathways [126,127]. Pivotal MRI-imaging studies focusing on morphometric aspects of gray matter (GM) in very preterm infants seem to predict some future aspects of neurodevelopmental outcomes [128]. While no imaging studies have been performed in large cohorts of normal full-term neonates or infants in the attempt to correlate GM morphometric aspects to APOE genotype, a link between APOE genotype and cortical thickness using neuroimaging techniques has been shown in young adults [129].
APOE and brain during pathological neonatal (1 day-30 days) and infancy (1-12 months) periods
Another example of the possible negative effect of APOE2 on brain functions is the detrimental association between APOE2 and neurodevelopmental dysfunction observed in neonate and infants after cardiac surgery [130,131]. However, this potentially detrimental effect of APOE2 early in life could be biased by the higher incidence of neonatal sudden death associated with APOE4 or other genetic variants [132,133]. In fact, the supposed detrimental APOE2 effect in infants after cardiac surgery could be simply due to a possible skewing effect toward APOE2 (risk of a reduced survival) in the absence of the chance to test an equivalent number of APOE4-carrier subjects with similar conditions underwent to cardiac surgery and subsequently assessed. However, if this after cardiac surgery detrimental APOE2-cognitive outcomes association will be confirmed, it will corroborate the hypothesis that APOE has AP properties.
To date, the AP properties in humans are not well timed, particularly in relationship to the various phases of the brain development. It has been suggested that the AP effects of the genes related to the brain function could begin to be assessed only after the first year of life. This could be due to the fact, for example, that specific skills (e.g. motor skills) are “localized and matured” in specific circuits of the brain, only after certain periods of the brain development [134,135]. Consequently, it would be difficult, or impossible, at clinical level, to evaluate or pre-evaluate the possible future outcomes of each type of motor or non-motor skill until the full genetically-determined neurodevelopmental program has been completed. Furthermore, it is possible to hypothesize that not only there is a life-lasting AP effect of APOE across the entire human lifespan, but that this AP effect is present in different areas or brain circuits at different times of the development as well as in the same brain at different ages. In addition, it is not unconceivable to exclude that the AP effects might be additionally modulated by non-genetic factors (e.g. nutrition, environmental toxins, infections, etc.), especially before the conclusion of the major process of maturation of the CNS [136–138]. Of course, APOE is not the only gene involved in various highly complex phenomena of the human brain maturation but seems to be part of a cluster of genes that reciprocally interact during the different phases of the neurodevelopment [139]. Accordingly, it would be through these multiple short- and long-term interactions between parental genes and gestational environments that APOE polymorphism could influence neurodevelopmental trajectories during early life periods and infancy and later, by pre-establishing a higher risk for “aging-related” conditions, or at least influencing, clinical onset or phenotype in neurodegenerative diseases such as AD, Parkinson’s disease (PD) [140] or ALS [141]. Therefore, it is not possible to exclude that those specific metabolic abnormalities or environmental events occurring during the very early period of life could trigger specific biochemical compensatory mechanisms (including synaptic formation and biochemistry) in preterm babies, which are different from those possibly activated during a regular gestation period ending in full-term birth [142]. For example, there are data showing that children exposed to environmental intoxication can have a different cognitive impact based on the APOE genotype [143], and that these ApoE-toxicants interactions can be activated during earlier periods of life and establish the “neural ground” for potential future abnormal reactions in the context of a later normal environment. Finally, other types of illnesses such as intra-uterine infections could directly or indirectly and differently affect cognitive outcomes of children based on their specific APOE genotype [144].
APOE and brain during normal childhood and teenaging
As investigations on the APOE polymorphism get performed considering human subjects with earlier age in life, the possible influence of each APOE genotype in these younger populations should consider that APOE2 and APOE4 can be part of AP phenomena occurring in the same individual (e.g. APOE2/E4) and across different individuals (e.g. APOE3/E2 vs. APOE3/E4) at different ages. The AP effect of APOE suggests that it would be fundamental to precisely define the specific periods of life to investigate and in which of those periods AP effects take place [145]. In fact, during earlier periods of life, the deleterious effects of APOE4 vs. APOE2 appear to be paradoxically reversed in some cases in respect to older age [31,129,146,147]. Although studies focusing on the allelic effects of APOE on young adults and children are relatively rare, a series of recent analyses evidenced an increased risk for poorer outcomes in APOE2- vs. APOE4-carriers in pediatric populations [130]. However, this effect is paradoxical only in appearance if the AP concept is considered. The “switched” effect of APOE4 (and alternatively of APOE2) during aging (in comparison to earlier periods of life), fits very well with the genetic AP concept for which the function of a gene can change during the time (i.e. from infancy to extreme age) or it can be otherwise activated in presence of different or mutated environmental conditions [148]. In support of the AP properties of APOE alleles, recent imaging studies have demonstrated a negative correlation between APOE4 and GM maturation in subjects between 3 and 20 years old [149]. However, more specific aspects of cortical connectivity and cognition - such as fluid intelligence - resulted to be lower in APOE4-carriers vs. non-APOE4-carriers [150]. Yet, in this last study, no direct analyses were performed to compare APOE4- vs. APOE2-carriers. Other studies, however, have also analyzed the interaction between APOE (e.g. non-APOE4 carriers) and other genes such as SORL1 showing that is the interaction between these two genes to determine different levels of hippocampal connectivity [151]. Again, when studying the APOE effects across different ages, it looks like of extreme relevance to define in a very precise way, the quantitative parameters or qualitative variables (including other genetic traits or genes) to analyze (e.g. clinical signs or symptoms, cognitive scores, imaging measures, neurophysiological values, etc.) and the specific system, organ, tissue or function to study.
APOE and brain during pathological conditions of childhood and teenaging
There have been various studies that focused on neurological disorders during childhood and adolescence that attempted to identify possible meaningful correlations between manifestation and progression of a disease, or its variable phenotypic characterization, and the APOE genotype. Initial findings by Treble-Barna et al. [152] suggest an APOE-environmental interaction in terms of long-term outcomes in children with a history of traumatic brain injury (TBI). This possible gene-environmental interaction has been further suggested by Kassam et al. [153] based on meta-analytic results showing that APOE4 is undoubtedly associated with a worse prognosis after a TBI in both children and young adults and that this effect might be due to mechanisms quite different from those linked to neurodegeneration during aging.
In support of the hypothesis that APOE4-related effects could be linked to different pathomechanisms than the ones leading to neurodegeneration when considering short- or long-term outcomes in the context of the same disease, for example traumatic brain injury (TBI) where there are data showing the possible association between APOE and post-concussive syndrome in children, which do not have any significant difference between APOE4- vs. non-APOE4 carriers, in particular when immediate or short-term outcomes are considered [154]. By contrast, in other types of brain diseases such as epilepsy, APOE genotype resulted to be a strong modifying factor in terms of both neuronal and glial neuropathological changes [155]. Nonetheless, depending on the specific type of disease associated with seizures or epileptic syndromes, the impact of APOE polymorphism seems to vary and it is not always associated with a direct epilepsy-inducing pathogenetic effect [156], but rather with other clinical aspects such as the age of onset of the disease [157].
In summary, APOE polymorphism during childhood and teenaging does not seem to be necessarily associated with specific structural brain changes but does seem to act in a much more complex way on a series of clinical outcomes characterizing the natural history of a specific disease such as cognitive decline, age of onset, or other associated co-morbidities related to the primary pathologic condition [113]. Though, earlier in life, the influence of APOE genotype seems to operate on a wider set of aspects either at the structural as functional level [158,159].
APOE and brain during normal adulthood (3rd-7th decade of life)
A series of APOE-associated white matter (WM) and gray matter (GM) microstructure differences in adult life have been described and they support the hypothesis that APOE polymorphism has indeed an early impact on various neurocytological aspects and different neurophenotypic aspects during normal and pathologic conditions of the CNS. For example, recent cognitive-morphological MRI investigations in normal adult populations described an earlier influence of the APOE genotype on specific morphometric aspects of different brain regions as an independent variable that is, as a variable that is not linked to any other known associated genetic or environmental risk factor [83,160–164]. Moreover, functional MRI (fMRI) studies showed significant differences in normal older as well young-adult populations when clustered based on APOE4 vs. non-APOE4 carriers [165–168]. In addition, other factors such as brain metabolism [169] and vascular reactivity [170] seem to be associated with an APOE genotype, which predisposes to cognitive impairment later in life.
Although studies using normal human brain tissue for comparisons across different APOE genotypes are very rare, Love et al. [171] were able to demonstrate different levels of synaptic proteins in the temporal cortex of normal brains between APOE4 vs. non-APOE4 carrier subjects.
APOE and brain during pathological conditions in adulthood (3rd-7th decade of life)
There are different investigations demonstrating that APOE genotype can influence the clinical phenotype of brain diseases during adulthood. For example, different levels of neuroinflammation associated with epilepsy were directly correlated with a specific APOE genotype in adult patients [172]. Specifically, APOE3 conferred a higher level of protection against neuroinflammatory and neurodegenerative processes associated with epilepsy [155]. In other brain diseases, such as TBI, it has been shown that different clinical outcomes are present in young adults (in contrast with findings in children) APOE4-carriers vs. non-APOE4 carriers [173,174]. In this case, it has been shown that a specific APOE allele can directly influence the reparative capacities of the brain and consequently the clinical outcomes based on a specific allele, the APOE4 in this case [175].
Another fascinating set of data proposed that there is a significant association between sleep disorders and APOE genotype [176,177], as well as among APOE, sleep-wake cycle, and deposition of β-amyloid [178]. This is a further indication that during adult age APOE4 confers negative effects to those subjects that have sleep-disordered breathing illnesses. By contrast, it has been evidenced that APOE2 confers a higher risk for intracranial hemorrhage in the contest of the natural history of brain arteriovenous malformations in young adults [179].
Then, importantly, as the mean age of the subjects analyzed in each different clinical or epidemiological study becomes lower, it is valuable to consider the possible AP effect of APOE alleles together with a clear distinction of the pathologic conditions to investigate, for example a clear distinction should be kept if a specific study is considering a vascular vs. non-vascular diseases. In fact, as the mean age of the subjects analyzed across different studies lowers also the different type of tissues, organs, or functional systems to target could vary together with the notion that an AP phenomenon can affect different organs or functions at different biological periods of life as well as different areas of the same organ (e.g. the brain) across those different periods of life.
APOE and brain during normal aging: successful octogenarians, nonagenarians, and centenarians
To date, APOE4 remains the best-established genetic risk factor associated with an increased risk of sporadic late-onset Alzheimer’s disease (LOAD) [180,181]. The possibility of APOE4 to determine an increased risk for other types of dementias is currently debated and under continued investigation [182–186]. While APOE4 has a deleterious effect on the onset and progression of AD as well as on cognition during pathological aging [187], APOE2 - the rarest allele of APOE gene in Caucasian populations - has been associated with a reduced risk of AD and increased longevity [77,188,189]. However, the detrimental effect of APOE4 on cognition (in both homo- and heterozygosis) has been largely confirmed by clinical [190], neuropsychological [191], neuroimaging [192], and neuropathological studies [193]. Conversely, fewer investigations have focused on the impact of APOE in normal brain aging conditions and specifically on APOE-related influence on cognition and other brain functions during normal brain aging. Furthermore, for statistical purposes, APOE4 effects have been much more frequently contrasted to APOE3 (the most frequent APOE allele in Caucasian populations) and much less to APOE2 due to its lower frequency in most human populations [194]. Very often, in fact, the rarity of APOE2 did not allow performing a correct and reliable statistical approach due to the marked differences in terms of population sample sizes to compare (e.g. numerosity of APOE4 vs. APOE2 subjects).
Moreover, the harmful effect of APOE4 has been associated not only to a higher risk of AD but also to a higher risk or different clinical outcomes for other neurological [195–198] and non-neurological conditions [199–203]. These latter findings support the hypothesis that APOE is very much indeed a possible general modifier of various biological and consequently, clinical phenomena. Curiously, APOE4 has also been associated with some apparently paradoxical effects [204–207]. For example, the absence of a higher risk for AD or cognitive deficits in some specific human populations has been found [206,207]. Nonetheless, the variability of APOE4-associated risk across different human populations not only reinforces the concept that APOE4 is “just” a risk factor (in contrast to a Mendelian mutation) but also that other environmental and genetic factors are possibly associated with a higher risk of dementia, its onset, and pathogenic progression [208,209]. Significantly, the detrimental or beneficial biological mechanisms of APOE alleles during premorbid normal adult life periods are far from being completely clarified.
In terms of APOE polymorphism-associated changes during normal aging, some studies have shown that APOE4 (vs. APOE3) is associated with a cortical thinning in a series of specific brain regions (e.g. medial and inferior temporal regions, including entorhinal cortex) [210]. These thinner cortical regions are the same cerebral regions more frequently affected in AD and particularly vulnerable to the AD pathology accumulation (e.g. β-amyloid accumulation). Interestingly, though, the APOE4-associated thinning of those AD-vulnerable cortical regions seems to be, per se, independent from the extracellular β-amyloid accumulation, especially when cognitively normal vs. early mild cognitive impairment (EMCI) or AD subjects are taken in account [210]. This newer perspective on APOE4 that is, a genetic factor having its own impact independently from β-amyloid accumulation (or other pathologies?) seems to confirm a direct and autonomous influence of APOE4 on the cortical architecture in specific regions of the brain [211,212].
The pathogenetic “independency” of APOE could be probably linked to basic cholesterol metabolic mechanisms related of the three different APOE alleles, their final protein products and cellular localizations [213]. In addition, the possible association among APOE genotypes, specific brain areas architecture and related brain functions, seems to go behind pure cognitive aspects and involve other non-cognitive functions such as the motor skills for example [214].
Finally, it is relevant to consider that the effect of APOE4 on the pathology and progression of AD, and probably on human cognition in general, appears to be mitigated by a series of other possible beneficial environmental factors [215]. However, it is not completely known which could be the possible environmental factors that could alternatively (beneficial or detrimental) potentiate the opposite effects of APOE4 and APOE2 during normal life conditions.
This brief description of some most recent neuroimaging and population-based studies of the APOE impact in humans illustrates either the complexity and intrinsic plastic capacities of the human brain to produce different clinical outcomes as consequence of the mutual interplay among genetic risk factors (e.g. APOE4, is not a determinant of disease) on the neural tissue (e.g. cortical thinning), independent pathogenetic factors (different for example, from β-amyloid extracellular accumulation or intracellular phosphorylated-tau formation) and other possible environmental variables, which are not identified or completely investigated yet such as lifestyle, nutritional habits, stress, or history of infectious diseases. Moreover, it is important to mention that there are recent findings from relatively large cohort studies that enrolled cognitively normal centenarians, which started to investigate cognitive aspects related to APOE genotype at a very advanced age and started to show unexpected and interesting results. For example, APOE4 in centenarians seems to be positively correlated with negative rather than positive affect in interaction with life events [216].
In general, these new findings seem to reinforce the concept that APOE not only influences some cognitive aspects until very late in life, until the 10th of life or later, but that there are much tighter possible links among cognition, emotions, and longevity in the human species [217].
APOE during pathological aging: Alzheimer’s Disease and other dementias in elders
In the context of neurodegenerative diseases, and dementia in particular, extensive analyses have shown that APOE genotype influences cognitive and non-cognitive phenotypes of subjects with a clinical diagnosis of MCI or AD (clinically probable AD). For example, APOE4 has been found to cluster at least two different groups of cognitive outcomes among subjects with dementia [5]. Moreover, in behavioral terms, APOE4 has been associated with different compartmental subtypes of AD [218]. Other investigations have further demonstrated that APOE4 can generate different endophenotypes in terms of neuropathology. For example, Murray et al. [219] were able to distinguish different subtypes of AD when APOE4 was clustered together with age of onset of the disease. However, in other non-AD dementias it seems that the detrimental link between APOE4 and cognitive decline is much more complex than in AD [220,221]. Moreover, previous and more recent investigations began showing that in the context of non-AD dementias such as FTD or cognitive decline in ALS, the modifying action of APOE polymorphism can be rather different from what is observed in classical AD [222,223]. For example, cognitive dysfunctions in ALS are associated with APOE genotype and other genes as well, such as SCNA gene. In this case, it seems that both genes cooperate for a higher risk of dementia in ALS only when related to the presence of Lewy bodies - a typical neuropathological feature of dementia with Lewy bodies (DLB) [224,225]. These recent findings support the possible mutual interactions between APOE and other genes and other non-genetic factors and that these more complex molecular interactions can produce different clinical phenotypes during the manifestations of age-related neurodegenerative diseases and probably earlier in life as well [226,227].
The “special” case of APOE2 and neurodegeneration during aging
As described earlier in this review, only few studies have focused on the relative impact, including possible molecular mechanisms, of APOE4 vs. APOE2 in terms of different cognitive outcomes during normal or cognitively successful aging [228–231]. Those few studies that have attempted to verify the possible associations between cognitively normal older subjects and APOE polymorphism have shown that the protective effect of APOE2 is not directly associated with mechanisms linked, for example, to the extracellular β-amyloid accumulation into the brain or to the alterations of synaptic and neuroinflammatory levels in the CNS [232,233]. These latter observations are of special interest since they could open other and different views on the possible protective mechanisms of APOE2 independent on the “classic” AD pathology [234,235]. These more recent findings allow hypothesizing that the protective effects of APOE2 on the brain and its functions are intrinsic to the molecular features of APOE alleles and its protein products. In fact, the beneficial effects of APOE2 later in life can be only partially, or indirectly, related to the reduction of those pathologic factors currently considered as the “pathogenetic” causes of AD: extracellular accumulation of β-amyloid pathology, hyperphosphorylated-tau formation and spreading, increased levels of neuroinflammation and synaptic loss [236,237]. Therefore, these more recent findings imply that also other factors linked to the intrinsic molecular advantage of APOE2 need to be taken in account [228,238,239]. However, the new molecular aspects of APOE2 need future confirmation by analyzing data from very large prospective studies aiming to measure the real effect of APOE2 protein in a large multi-factorial statistical model that would include both biological (genetic) and non-biological (environmental) factors [240,241]. These studies should include human brain donations to increase the chance to quantifying ratios between residual (normal/still-functional) and pathologic tissues (dysfunctional neuronal or non-neuronal) that could directly derive from the action of APOE2 vs. APOE4 on the neural tissue through probably its biochemical interaction with cholesterol metabolism. Therefore, it could be possible to hypothesize that the APOE2-related action on the cholesterol metabolism would be capable to delay the cognitive decline during normal aging by specific molecular mechanisms that could be modulated using pharmacological compounds or non-pharmacological treatments, or alternatively, offer the opportunity to potentiate their beneficial action in a more preventive or protective manner. Unfortunately, the specific APOE2 molecular mechanisms are currently not completely known [242]. Nonetheless, some molecular mechanisms have been proposed based on new and unexpected metabolic links between different biochemical pathways. For example, new possible links between homeostatic pathways of iron and lipids metabolism [243] or between APOE2 and disease-onset of other mutations through the interaction of APOE2 and genes involved in cellular proliferation, protein degradation, apoptotic and immune dysregulation processes has been shown [244]. Moreover, differential phenomena of neuroplasticity as based on APOE4 vs. APOE3 have been proposed [245]. Furthermore, as for possible differences across the three APOE proteins, recent pilot investigations started to show that APOE genotypes is associated with different plasmatic levels of APOE2 vs. APOE3 and APOE4 proteins as hourly measured in human subjects [246]. These newer biochemical findings on APOE have been made possible by recent methodological advancement especially those based on mass spectrometry [247] and highly complex techniques [248].
APOE, higher cognitive functions, and modulatory effects of personality-traits
APOE2 in older subjects, even in the presence of high levels AD pathology and clinically silent dementia, have shown a significant association with higher language skills acquired early in life [249]. Furthermore, recently, MRI findings showed that specific spectroscopy signals are associated with cognitive and language development at term-equivalent period in some of the identical brain areas (e.g. hippocampus) that are later often affected by aging-related diseases such as AD [250,251]. However, it is not known, yet, if these imaging findings are also directly linked to APOE or to other genetic polymorphisms. Nonetheless, it is highly possible that language skills, as well as other higher cognitive human functions, could be connected to more specific gene-environmental interactions during either in-utero life and during the very first months or years of life as based on a cluster of genes, including APOE, whose function or dysfunction, directly predispose an individual to a higher risk of neurological and psychiatric risk later in life due to the very early impact of those gene-environmental interactions on specific brain structures and functions “already set up for” at birth or earlier. These early gene-environmental neurobiological phenomena could predispose to specific types of neurophenotypes later in life during the youth, middle-age or aging of that individual [253].
Intriguingly, APOE genotype seems to be associated or interacting with specific traits of personality [254], and even with GM volumes in specific areas of the brain that can have a modulatory effect on cognition. Among the personality traits that seem to be associated with an APOE-personality modulatory interaction there is the conscientiousness [7]. Although many of these studies were limited by the relatively small number of subjects analyzed, they do represent initial and important findings of the more plastic relationship between APOE and personality-based behavioral aspects that could represent useful clinical tools in establishing a higher risk of dementia before any clinical manifestation [255,256].
Future perspectives
This necessarily succinct review focusing on the possible types of impact of APOE polymorphism on human brain structures, cerebral functions, and brain illnesses across different ages in normal and pathological conditions of the brain and possible modulatory effects of specific personality traits (e.g. conscientiousness, neuroticism) suggests that future larger longitudinal investigations would need to consider numerous genetic and environmental variables to fully identify the molecular mechanisms of APOE as well as the interactions between the APOE with other genes and other aspects such as environmental imprinting (nutritional, educational, behavioral, etc.) during the early phase of life [257,258].
APOE started to appear a gene associated with relevant antagonistic pleiotropic phenomena and it seems to be involved in different human diseases, not only brain diseases [259]. APOE appears, in fact, to have an important and modifying impact on lung diseases [200], infectious diseases [202,260], and on very specific pathologic processes [203]. Finally, APOE seems to be involved in the different behavioral outcomes and personality-traits modulatory effects whose molecular mechanisms still need to be completely elucidated [261].
Future studies, possibly stratified by individual APOE genotype, should be able to identify the precise molecular mechanisms by which a specific compound could modify or halt the detrimental effects of APOE4 during late-life period or, by “paradoxical” contrast, enhance its effects during the early or very early phases of life. Different types of molecules with possible pharmacological actions on the APOE gene products, and especially on APOE4 gene product, are under constant investigation [262,263], using newer technological sequencing and computational advancements that better inform us on the possible genome-based mechanisms of health maintenance [264,265]. Moreover, APOE4 has been identified as a ligand for the LDL receptors (LDLRs) family and that ApoE receptor 2 (ApoEr2) [266], together with VLDL receptor (VLDLR), have major impacts on brain development and adult synaptic plasticity [267,268]. ApoEr2 acts through the activation of Reelin [269–271], whose function could be modulated by specific compounds that secondarily would act or reduce the APOE4 detrimental effects on the CNS in general, or on dementia being the main genetic risk factor for AD [272,273].
Finally, new and exciting facets of CNS biology and aging such as those related to the glymphatic system [274], or the CNS-associated lymphatic system [275], have recently opened new ways to better understand the CNS biology, its clearance capacities, as well as the possibility to associate these new discoveries with acquired knowledge about APOE, its pathogenic contribution to neuropathology of dementia, and the effects of APOE alleles across the entire human lifespan.
Box 1. Possible APOE-linked biological trains associated with antagonistic pleiotropy feautures
Although this review aimed to describe the findings about different functional brain outcomes, in normal and pathological conditions of the brain, associated or influenced by the APOE allelic polymorphism in humans, we also briefly describe some of the possible APOE-molecular and non-molecular traits that could be associated with the proposed AP features of APOE. There is a plethora of animal studies (experiments employing transgenic animals, mainly rodents) that define various genetic (APOE), metabolic (ApoE proteins), and ApoE receptors (intracellular functional consequences) molecular aspects that would be impossible to describe here in an exhaustive way. For this reason, we preferred to focus on those possible APOE-traits that seem to have some antagonistic pleiotropic (AP) features across the lifespan in mammalians.
We retain that the following “APOE-linked traits” are among the most suitable candidates able to explain the antagonistic pleiotropic effects of the APOE polymorphism:
APOE4-carrier as “Higher β-amyloid accumulator subject”
Using different animal models and different biochemical approaches, it has been demonstrated that APOE4-animals have a reduced capacity to catabolize and consequently to reduce, the progressive accumulation of 1-42 β-amyloid, one of the main constituents of extracellular β-amyloid accumulation [276–278]. Furthermore, APOE4 seems also to indirectly exacerbate tau-mediated neurodegeneration [279]. Moreover, the APOE4 seems to determine this reduction of catabolic 1-42 β-amyloid (as well as of other forms of β-amyloid [280,281]) in a regionally-based manner [282–284]. If these deleterious effects of APOE4 on the β-amyloid metabolism during aging or adult life are deleterious as well for the brain or other tissues in early life is not known or, by contrast, if APOE2 has a beneficial effect in early-life is not completely understood [285,286]. Curiously, however, newer investigations both in animals and humans showed a “paradoxical” (due to actually to the AP?) effect of APOE2, specifically in case of APOE2 homozygosis (APOE2/E2) [287]. Zhao and colleagues, for example, show increased levels of tau lesions in cases of Progressive Supranuclear Palsy (PSP), a typical tauopathy, when associated with APOE2 homozygosis.
APOE4-carrier as “Lower synaptic/spine replacement/ repairing capacity subject”
A consistent series of experiments either in-vitro and in-vivo aiming to compare the formation of synapses or the capacity to repair synapses in the presence of APOE4 vs. APOE3 or APOE2 have now consolidated the evidence that APOE polymorphism is indeed directly linked to differential capacities of the brain, both in terms of synapses formation and repair, when one of those three alleles are expressed. For example, in the specific context of AD - where entorhinal cortex lesions represent some of the very initial pathogenetic events ending in the synaptic loss subjacent memory impairment - it has been demonstrated that APOE4 is associated to a reduced capacity to form new synapses as measured by GAP-43 and synaptophysin proteins, which are typical molecular markers of neo-synaptogenesis [288,289]. Furthermore, other investigators have demonstrated that APOE4 is specifically associated with a reduced capacity to produce spine and consequently contribute to the dendritic complexity [1,290]. Although these animal models show the detrimental effect of APOE4 on the synaptic compartment, which is coherent with the APOE4 effect later in life, paradoxically, or better in antagonistic pleiotropic terms, there are studies in humans showing a higher performance of specific cognitive tasks in younger subjects when carrying APOE4 in comparison to APOE2 [291–293]. However, some new recent studies in humans show that even in the presence of APOE4 there are some morphometric alterations of the dendritic spines (e.g. in the frontal cortex), which could support the maintenance of normal cognitive functions despite the presence of AD pathology or aging [294].
APOE4-carrier and personality-traits, “Higher genetic-personality-trait susceptible subject”
The neurohistologic structure as the neuronal and synaptic complexity associated to the different traits of personality defined in humans is not completely known [295]. The attempt to link specific connectomics aspects to each specific type of personality or behavior is still in its infancy [296–298]. However, initial studies are showing interesting findings when introducing the APOE polymorphism factor in the analyses of possible connectomics changes in APOE4 carrier individuals. Some recent studies [300,301] show that the default-mode network in APOE4 subjects differs from non-APOE4 in a way that APOE4 seems to establish the functional communications across different brain regions (connectomics), which could predispose to some cognitive disadvantage across the entire lifespan. Moreover, some investigations pointed out that there is indeed an association between cognition measurements and personality traits [302,303]. If these links are also based, influenced, or modulated by a specific genotype, such APOE4 vs. APOE2 is under investigation. However, initial studies, show that specific personality factors (e.g. neuroticism and extraversion) can actually moderate the cognitive outcomes in APOE4 carriers [255,261].
Conflicts of Interest
The authors have nothing to disclose and do not have any conflict of interests.
Funding
This manuscript was supported by intramural funds of the Biomedical Research Institute of New Jersey (BRInj), Cedar Knolls, NJ, USA.
References
-
1.
Dumanis SB, Tesoriero JA, Babus LW, Nguyen MT, Trotter JH, Ladu MJ, Weeber EJ, Turner RS, Xu B, Rebeck GW, Hoe HS. ApoE4 decreases spine density and dendritic complexity in cortical neurons in vivo. J Neurosci. 2009; 29:15317–22. https://doi.org/10.1523/JNEUROSCI.4026-09.2009 [PubMed]
-
2.
Jain S, Yoon SY, Leung L, Knoferle J, Huang Y. Cellular source-specific effects of apolipoprotein (apo) E4 on dendrite arborization and dendritic spine development. PLoS One. 2013; 8:e59478. https://doi.org/10.1371/journal.pone.0059478 [PubMed]
-
3.
Simonovitch S, Schmukler E, Bespalko A, Iram T, Frenkel D, Holtzman DM, Masliah E, Michaelson DM, Pinkas-Kramarski R. Impaired autophagy in APOE4 a strocytes. J Alzheimers Dis. 2016; 51:915–27. https://doi.org/10.3233/JAD-151101 [PubMed]
-
4.
Levi O, Lütjohann D, Devir A, von Bergmann K, Hartmann T, Michaelson DM. Regulation of hippocampal cholesterol metabolism by apoE and environmental stimulation. J Neurochem. 2005; 95:987–97. https://doi.org/10.1111/j.1471-4159.2005.03441.x [PubMed]
-
5.
Scheltens NM, Tijms BM, Koene T, Barkhof F, Teunissen CE, Wolfsgruber S, Wagner M, Kornhuber J, Peters O, Cohn-Sheehy BI, Rabinovici GD, Miller BL, Kramer JH, et al, and Alzheimer’s Disease Neuroimaging Initiative, and German Dementia Competence Network, and University of California San Francisco Memory and Aging Center, and Amsterdam Dementia Cohort. Cognitive subtypes of probable Alzheimer’s disease robustly identified in four cohorts. Alzheimers Dement. 2017; 13:1226–36. https://doi.org/10.1016/j.jalz.2017.03.002 [PubMed]
-
6.
Gardener SL, Rainey-Smith SR, Sohrabi HR, Weinborn M, Verdile G, Fernando WM, Lim YY, Harrington K, Burnham S, Taddei K, Masters CL, Macaulay SL, Rowe CC, et al, and AIBL Research Group. Increased Carbohydrate Intake is Associated with Poorer Performance in Verbal Memory and Attention in an APOE Genotype-Dependent Manner. J Alzheimers Dis. 2017; 58:193–201. https://doi.org/10.3233/JAD-161158 [PubMed]
-
7.
Kunz L, Reuter M, Axmacher N, Montag C. Conscientiousness is negatively associated with grey matter volume in young APOE ɛ4-carriers. J Alzheimers Dis. 2017; 56:1135–44. https://doi.org/10.3233/JAD-160854 [PubMed]
-
8.
Håkansson K, Soininen H, Winblad B, Kivipelto M. Feelings of hopelessness in midlife and cognitive health in later life: a prospective population-based cohort. PLoS One. 2015; 10:e0140261. https://doi.org/10.1371/journal.pone.0140261 [PubMed]
-
9.
Khan W, Giampietro V, Banaschewski T, Barker GJ, Bokde AL, Büchel C, Conrod P, Flor H, Frouin V, Garavan H, Gowland P, Heinz A, Ittermann B, et al, and Alzheimer–s Disease Neuroimaging Initiative, and AddNeuroMed Consortium, Australian, Imaging, Biomarkers, and Lifestyle Study Research Group, and IMAGEN consortium. A multi-cohort study of ApoE ɛ4 and Amyloid-β effects on the hippocampus in Alzheimer’s Disease. J Alzheimers Dis. 2017; 56:1159–74. https://doi.org/10.3233/JAD-161097 [PubMed]
-
10.
Krell-Roesch J, Vemuri P, Pink A, Roberts RO, Stokin GB, Mielke MM, Christianson TJ, Knopman DS, Petersen RC, Kremers WK, Geda YE. Association between mentally stimulating activities in late life and the outcome of incident mild cognitive impairment, with an analysis of the APOE ε4 genotype. JAMA Neurol. 2017; 74:332–38. https://doi.org/10.1001/jamaneurol.2016.3822 [PubMed]
-
11.
Pappas A, Chaiworapongsa T, Romero R, Korzeniewski SJ, Cortez JC, Bhatti G, Gomez-Lopez N, Hassan SS, Shankaran S, Tarca AL. Transcriptomics of maternal and fetal membranes can discriminate between gestational-age matched preterm neonates with and without cognitive impairment diagnosed at 18-24 months. PLoS One. 2015; 10:e0118573. https://doi.org/10.1371/journal.pone.0118573 [PubMed]
-
12.
Eidem HR, Ackerman WE4th, McGary KL, Abbot P, Rokas A. Gestational tissue transcriptomics in term and preterm human pregnancies: a systematic review and meta-analysis. BMC Med Genomics. 2015; 8:27. https://doi.org/10.1186/s12920-015-0099-8 [PubMed]
-
13.
Tilley SK, Joseph RM, Kuban KC, Dammann OU, O’Shea TM, Fry RC. Genomic biomarkers of prenatal intrauterine inflammation in umbilical cord tissue predict later life neurological outcomes. PLoS One. 2017; 12:e0176953. https://doi.org/10.1371/journal.pone.0176953 [PubMed]
-
14.
Prizer LP, Zimmerman S. Progressive support for activities of daily living for persons living with dementia. Gerontologist. 2018 (suppl_1); 58:S74–87. https://doi.org/10.1093/geront/gnx103 [PubMed]
-
15.
Katzman R, Terry R, DeTeresa R, Brown T, Davies P, Fuld P, Renbing X, Peck A. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol. 1988; 23:138–44. https://doi.org/10.1002/ana.410230206 [PubMed]
-
16.
Crystal H, Dickson D, Fuld P, Masur D, Scott R, Mehler M, Masdeu J, Kawas C, Aronson M, Wolfson L. Clinico-pathologic studies in dementia: nondemented subjects with pathologically confirmed Alzheimer’s disease. Neurology. 1988; 38:1682–87. https://doi.org/10.1212/WNL.38.11.1682 [PubMed]
-
17.
Troncoso JC, Martin LJ, Dal Forno G, Kawas CH. Neuropathology in controls and demented subjects from the Baltimore Longitudinal Study of Aging. Neurobiol Aging. 1996; 17:365–71. https://doi.org/10.1016/0197-4580(96)00028-0 [PubMed]
-
18.
Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999; 45:358–68. https://doi.org/10.1002/1531-8249(199903)45:3<358::AID-ANA12>3.0.CO;2-X [PubMed]
-
19.
Knopman DS, Parisi JE, Salviati A, Floriach-Robert M, Boeve BF, Ivnik RJ, Smith GE, Dickson DW, Johnson KA, Petersen LE, McDonald WC, Braak H, Petersen RC. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol. 2003; 62:1087–95. https://doi.org/10.1093/jnen/62.11.1087 [PubMed]
-
20.
Iacono D, O’Brien R, Resnick SM, Zonderman AB, Pletnikova O, Rudow G, An Y, West MJ, Crain B, Troncoso JC. Neuronal hypertrophy in asymptomatic Alzheimer disease. J Neuropathol Exp Neurol. 2008; 67:578–89. https://doi.org/10.1097/NEN.0b013e3181772794 [PubMed]
-
21.
Monsell SE, Mock C, Fardo DW, Bertelsen S, Cairns NJ, Roe CM, Ellingson SR, Morris JC, Goate AM, Kukull WA. Genetic comparison of symptomatic and asymptomatic persons with Alzheimer Disease neuropathology. Alzheimer Dis Assoc Disord. 2017; 31:232–38. https://doi.org/10.1097/WAD.0000000000000179 [PubMed]
-
22.
Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002; 8:448–60. https://doi.org/10.1017/S1355617702813248 [PubMed]
-
23.
Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012; 11:1006–12. https://doi.org/10.1016/S1474-4422(12)70191-6 [PubMed]
-
24.
Honer WG, Barr AM, Sawada K, Thornton AE, Morris MC, Leurgans SE, Schneider JA, Bennett DA. Cognitive reserve, presynaptic proteins and dementia in the elderly. Transl Psychiatry. 2012; 2:e114. https://doi.org/10.1038/tp.2012.38 [PubMed]
-
25.
Neltner JH, Abner EL, Jicha GA, Schmitt FA, Patel E, Poon LW, Marla G, Green RC, Davey A, Johnson MA, Jazwinski SM, Kim S, Davis D, et al. Brain pathologies in extreme old age. Neurobiol Aging. 2016; 37:1–11. https://doi.org/10.1016/j.neurobiolaging.2015.10.009 [PubMed]
-
26.
Descamps OS, Bruniaux M, Guilmot PF, Tonglet R, Heller FR. Lipoprotein metabolism of pregnant women is associated with both their genetic polymorphisms and those of their newborn children. J Lipid Res. 2005; 46:2405–14. https://doi.org/10.1194/jlr.M500223-JLR200 [PubMed]
-
27.
Steffen KM, Cooper ME, Shi M, Caprau D, Simhan HN, Dagle JM, Marazita ML, Murray JC. Maternal and fetal variation in genes of cholesterol metabolism is associated with preterm delivery. J Perinatol. 2007; 27:672–80. https://doi.org/10.1038/sj.jp.7211806 [PubMed]
-
28.
Procopciuc LM, Caracostea G, Zaharie G, Stamatian F. Newborn APOE genotype influences maternal lipid profile and the severity of high-risk pregnancy - preeclampsia: interaction with maternal genotypes as a modulating risk factor in preeclampsia. Hypertens Pregnancy. 2015; 34:271–83. https://doi.org/10.3109/10641955.2015.1009541 [PubMed]
-
29.
Mauch DH, Nägler K, Schumacher S, Göritz C, Müller EC, Otto A, Pfrieger FW. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001; 294:1354–57. https://doi.org/10.1126/science.294.5545.1354 [PubMed]
-
30.
Tamnes CK, Walhovd KB, Dale AM, Østby Y, Grydeland H, Richardson G, Westlye LT, Roddey JC, Hagler DJJr, Due-Tønnessen P, Holland D, Fjell AM, and Alzheimer’s Disease Neuroimaging Initiative. Brain development and aging: overlapping and unique patterns of change. Neuroimage. 2013; 68:63–74. https://doi.org/10.1016/j.neuroimage.2012.11.039 [PubMed]
-
31.
Lancaster C, Tabet N, Rusted J. The APOE paradox: do attentional control differences in mid-adulthood reflect risk of late-life cognitive decline. Neurobiol Aging. 2016; 48:114–21. https://doi.org/10.1016/j.neurobiolaging.2016.08.015 [PubMed]
-
32.
Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988; 240:622–30. https://doi.org/10.1126/science.3283935 [PubMed]
-
33.
Mahley RW, Rall SCJr. Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet. 2000; 1:507–37. https://doi.org/10.1146/annurev.genom.1.1.507 [PubMed]
-
34.
Mahley RW. Central nervous system lipoproteins: ApoE and regulation of cholesterol metabolism. Arterioscler Thromb Vasc Biol. 2016; 36:1305–15. https://doi.org/10.1161/ATVBAHA.116.307023 [PubMed]
-
35.
Huang Y, Mahley RW. Apolipoprotein E: structure and function in lipid metabolism, neurobiology, and Alzheimer’s diseases. Neurobiol Dis. 2014; 72:3–12. https://doi.org/10.1016/j.nbd.2014.08.025 [PubMed]
-
36.
Hauser PS, Narayanaswami V, Ryan RO. Apolipoprotein E: from lipid transport to neurobiology. Prog Lipid Res. 2011; 50:62–74. https://doi.org/10.1016/j.plipres.2010.09.001 [PubMed]
-
37.
Holtzman DM, Herz J, Bu G. Apolipoprotein E and apolipoprotein E receptors: normal biology and roles in Alzheimer disease. Cold Spring Harb Perspect Med. 2012; 2:a006312. https://doi.org/10.1101/cshperspect.a006312 [PubMed]
-
38.
Herz J. Apolipoprotein E receptors in the nervous system. Curr Opin Lipidol. 2009; 20:190–96. https://doi.org/10.1097/MOL.0b013e32832d3a10 [PubMed]
-
39.
Lane-Donovan C, Philips GT, Herz J. More than cholesterol transporters: lipoprotein receptors in CNS function and neurodegeneration. Neuron. 2014; 83:771–87. https://doi.org/10.1016/j.neuron.2014.08.005 [PubMed]
-
40.
Jones PB, Adams KW, Rozkalne A, Spires-Jones TL, Hshieh TT, Hashimoto T, von Armin CA, Mielke M, Bacskai BJ, Hyman BT. Apolipoprotein E: isoform specific differences in tertiary structure and interaction with amyloid-β in human Alzheimer brain. PLoS One. 2011; 6:e14586. https://doi.org/10.1371/journal.pone.0014586 [PubMed]
-
41.
Simon R, Girod M, Fonbonne C, Salvador A, Clément Y, Lantéri P, Amouyel P, Lambert JC, Lemoine J. Total ApoE and ApoE4 isoform assays in an Alzheimer’s disease case-control study by targeted mass spectrometry (n=669): a pilot assay for methionine-containing proteotypic peptides. Mol Cell Proteomics. 2012; 11:1389–403. https://doi.org/10.1074/mcp.M112.018861 [PubMed]
-
42.
Frieden C, Garai K. Concerning the structure of apoE. Protein Sci. 2013; 22:1820–25. https://doi.org/10.1002/pro.2379 [PubMed]
-
43.
Weisgraber KH, Rall SCJr, Mahley RW. Human E apoprotein heterogeneity. Cysteine-arginine interchanges in the amino acid sequence of the apo-E isoforms. J Biol Chem. 1981; 256:9077–83. [PubMed]
-
44.
Weisgraber KH, Rall SCJr, Innerarity TL, Mahley RW, Kuusi T, Ehnholm C. A novel electrophoretic variant of human apolipoprotein E. Identification and characterization of apolipoprotein E1. J Clin Invest. 1984; 73:1024–33. https://doi.org/10.1172/JCI111287 [PubMed]
-
45.
Lee PY, Costumbrado J, Hsu CY, Kim YH. Agarose gel electrophoresis for the separation of DNA fragments. J Vis Exp. 2012; 62;3923. https://doi.org/10.3791/3923 [PubMed]
-
46.
Weisgraber KH, Newhouse YM, Taylor JM, Tuan B, Nestruck AC, Davignon J, Mahley RW. Apolipoprotein E2(Arg158----Cys) frequency in a hyperlipidemic French-Canadian population of apolipoprotein E2/2 subjects. Determination by synthetic oligonucleotide probes. Arteriosclerosis. 1989; 9:50–57. https://doi.org/10.1161/01.ATV.9.1.50 [PubMed]
-
47.
Rall SCJr, Weisgraber KH, Innerarity TL, Bersot TP, Mahley RW, Blum CB. Identification of a new structural variant of human apolipoprotein E, E2(Lys146 leads to Gln), in a type III hyperlipoproteinemic subject with the E3/2 phenotype. J Clin Invest. 1983; 72:1288–97. https://doi.org/10.1172/JCI111085 [PubMed]
-
48.
Wardell MR, Brennan SO, Janus ED, Fraser R, Carrell RW. Apolipoprotein E2-Christchurch (136 Arg----Ser). New variant of human apolipoprotein E in a patient with type III hyperlipoproteinemia. J Clin Invest. 1987; 80:483–90. https://doi.org/10.1172/JCI113096 [PubMed]
-
49.
Utermann G, Weisgraber KH, Weber W, Mahley RW. Genetic polymorphism of apolipoprotein E: a variant form of apolipoprotein E2 distinguished by sodium dodecyl sulfate--polyacrylamide gel electrophoresis. J Lipid Res. 1984; 25:378–82. [PubMed]
-
50.
Kara E, Marks JD, Fan Z, Klickstein JA, Roe AD, Krogh KA, Wegmann S, Maesako M, Luo CC, Mylvaganam R, Berezovska O, Hudry E, Hyman BT. Isoform- and cell type-specific structure of apolipoprotein E lipoparticles as revealed by a novel Forster resonance energy transfer assay. J Biol Chem. 2017; 292:14720–29. https://doi.org/10.1074/jbc.M117.784264 [PubMed]
-
51.
Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM, and APOE and Alzheimer Disease Meta Analysis Consortium. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. JAMA. 1997; 278:1349–56. https://doi.org/10.1001/jama.1997.03550160069041 [PubMed]
-
52.
Gerdes LU. The common polymorphism of apolipoprotein E: geographical aspects and new pathophysiological relations. Clin Chem Lab Med. 2003; 41:628–31. https://doi.org/10.1515/CCLM.2003.094 [PubMed]
-
53.
Eisenberg DT, Kuzawa CW, Hayes MG. Worldwide allele frequencies of the human apolipoprotein E gene: climate, local adaptations, and evolutionary history. Am J Phys Anthropol. 2010; 143:100–11. https://doi.org/10.1002/ajpa.21298 [PubMed]
-
54.
Beall CM. Two routes to functional adaptation: tibetan and Andean high-altitude natives. Proc Natl Acad Sci USA. 2007 (Suppl 1); 104:8655–60. https://doi.org/10.1073/pnas.0701985104 [PubMed]
-
55.
Egert S, Rimbach G, Huebbe P. ApoE genotype: from geographic distribution to function and responsiveness to dietary factors. Proc Nutr Soc. 2012; 71:410–24. https://doi.org/10.1017/S0029665112000249 [PubMed]
-
56.
Trumble BC, Stieglitz J, Blackwell AD, Allayee H, Beheim B, Finch CE, Gurven M, Kaplan H. Apolipoprotein E4 is associated with improved cognitive function in Amazonian forager-horticulturalists with a high parasite burden. FASEB J. 2017; 31:1508–15. https://doi.org/10.1096/fj.201601084R [PubMed]
-
57.
Eisenberg DT, Kuzawa CW, Hayes MG. Worldwide allele frequencies of the human apolipoprotein E gene: climate, local adaptations, and evolutionary history. Am J Phys Anthropol. 2010; 143:100–11. https://doi.org/10.1002/ajpa.21298 [PubMed]
-
58.
Kern S, Mehlig K, Kern J, Zetterberg H, Thelle D, Skoog I, Lissner L, Blennow K, Börjesson-Hanson A. The distribution of apolipoprotein E genotype over the adult lifespan and in relation to country of birth. Am J Epidemiol. 2015; 181:214–17. https://doi.org/10.1093/aje/kwu442 [PubMed]
-
59.
Heffernan AL, Chidgey C, Peng P, Masters CL, Roberts BR. The neurobiology and age-related prevalence of the ε4 allele of Apolipoprotein E in Alzheimer’s Disease cohorts. J Mol Neurosci. 2016; 60:316–24. https://doi.org/10.1007/s12031-016-0804-x [PubMed]
-
60.
Sawyer RP, Sekar P, Osborne J, Kittner SJ, Moomaw CJ, Flaherty ML, Langefeld CD, Anderson CD, Rosand J, Woo D. Racial/ethnic variation of APOE alleles for lobar intracerebral hemorrhage. Neurology. 2018; 91:e410–20. https://doi.org/10.1212/WNL.0000000000005908 [PubMed]
-
61.
McIntosh AM, Bennett C, Dickson D, Anestis SF, Watts DP, Webster TH, Fontenot MB, Bradley BJ. The apolipoprotein E (APOE) gene appears functionally monomorphic in chimpanzees (Pan troglodytes). PLoS One. 2012; 7:e47760. https://doi.org/10.1371/journal.pone.0047760 [PubMed]
-
62.
Fullerton SM, Clark AG, Weiss KM, Nickerson DA, Taylor SL, Stengârd JH, Salomaa V, Vartiainen E, Perola M, Boerwinkle E, Sing CF. Apolipoprotein E variation at the sequence haplotype level: implications for the origin and maintenance of a major human polymorphism. Am J Hum Genet. 2000; 67:881–900. https://doi.org/10.1086/303070 [PubMed]
-
63.
Hanlon CS, Rubinsztein DC. Arginine residues at codons 112 and 158 in the apolipoprotein E gene correspond to the ancestral state in humans. Atherosclerosis. 1995; 112:85–90. https://doi.org/10.1016/0021-9150(94)05402-5 [PubMed]
-
64.
Huebbe P, Rimbach G. Evolution of human apolipoprotein E (APOE) isoforms: gene structure, protein function and interaction with dietary factors. Ageing Res Rev. 2017; 37:146–61. https://doi.org/10.1016/j.arr.2017.06.002 [PubMed]
-
65.
Conejero-Goldberg C, Gomar JJ, Bobes-Bascaran T, Hyde TM, Kleinman JE, Herman MM, Chen S, Davies P, Goldberg TE. APOE2 enhances neuroprotection against Alzheimer’s disease through multiple molecular mechanisms. Mol Psychiatry. 2014; 19:1243–50. https://doi.org/10.1038/mp.2013.194 [PubMed]
-
66.
Sebastiani P, Gurinovich A, Nygaard M, Sasaki T, Sweigart B, Bae H, Andersen SL, Villa F, Atzmon G, Christensen K, Arai Y, Barzilai N, Puca A, et al. APOE alleles and extreme human longevity. J Gerontol A Biol Sci Med Sci. 2019; 74:E44-51. https://doi.org/10.1093/gerona/gly174 [PubMed]
-
67.
Rodríguez JA, Marigorta UM, Hughes DA, Spataro N, Bosch E, Navarro A. Antagonistic pleiotropy and mutation accumulation influence human senescence and disease. Nat Ecol Evol. 2017; 1:55. https://doi.org/10.1038/s41559-016-0055 [PubMed]
-
68.
Bennet AM, Reynolds CA, Gatz M, Blennow K, Pedersen NL, Prince JA. Pleiotropy in the presence of allelic heterogeneity: alternative genetic models for the influence of APOE on serum LDL, CSF amyloid-β42, and dementia. J Alzheimers Dis. 2010; 22:129–34. https://doi.org/10.3233/JAD-2010-100864 [PubMed]
-
69.
Chang L, Andres M, Sadino J, Jiang CS, Nakama H, Miller E, Ernst T. Impact of apolipoprotein E ε4 and HIV on cognition and brain atrophy: antagonistic pleiotropy and premature brain aging. Neuroimage. 2011; 58:1017–27. https://doi.org/10.1016/j.neuroimage.2011.07.010 [PubMed]
-
70.
Tuminello ER, Han SD. The apolipoprotein e antagonistic pleiotropy hypothesis: review and recommendations. Int J Alzheimers Dis. 2011; 2011:726197. https://doi.org/10.4061/2011/726197 [PubMed]
-
71.
Maxwell TJ, Ballantyne CM, Cheverud JM, Guild CS, Ndumele CE, Boerwinkle E. APOE modulates the correlation between triglycerides, cholesterol, and CHD through pleiotropy, and gene-by-gene interactions. Genetics. 2013; 195:1397–405. https://doi.org/10.1534/genetics.113.157719 [PubMed]
-
72.
Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957; 11:398–411. https://doi.org/10.1111/j.1558-5646.1957.tb02911.x
-
73.
Wang Z, Liao BY, Zhang J. Genomic patterns of pleiotropy and the evolution of complexity. Proc Natl Acad Sci USA. 2010; 107:18034–39. https://doi.org/10.1073/pnas.1004666107 [PubMed]
-
74.
Carter AJ, Nguyen AQ. Antagonistic pleiotropy as a widespread mechanism for the maintenance of polymorphic disease alleles. BMC Med Genet. 2011; 12:160. https://doi.org/10.1186/1471-2350-12-160 [PubMed]
-
75.
Joshi PK, Fischer K, Schraut KE, Campbell H, Esko T, Wilson JF. Variants near CHRNA3/5 and APOE have age- and sex-related effects on human lifespan. Nat Commun. 2016; 7:11174. https://doi.org/10.1038/ncomms11174 [PubMed]
-
76.
Drenos F, Kirkwood TB. Selection on alleles affecting human longevity and late-life disease: the example of apolipoprotein E. PLoS One. 2010; 5:e10022. https://doi.org/10.1371/journal.pone.0010022 [PubMed]
-
77.
Garatachea N, Marín PJ, Santos-Lozano A, Sanchis-Gomar F, Emanuele E, Lucia A. The ApoE gene is related with exceptional longevity: a systematic review and meta-analysis. Rejuvenation Res. 2015; 18:3–13. https://doi.org/10.1089/rej.2014.1605 [PubMed]
-
78.
Vila-Rodriguez F, Lang DJ, Baitz H, Gicas K, Thorton AE, Ehmann TS, Smith GN, Barr AM, Torres IJ, Kopala LC, MacEwan GW, Müller DJ, Kennedy JL, Honer WG. Verbal memory improvement in first-episode psychosis APOE-ε4 carriers: a pleiotropic effect? Neuropsychiatr Dis Treat. 2017; 13:2945–53. https://doi.org/10.2147/NDT.S150488 [PubMed]
-
79.
Jochemsen HM, Muller M, van der Graaf Y, Geerlings MI. APOE ε4 differentially influences change in memory performance depending on age. The SMART-MR study. Neurobiol Aging. 2012; 33:832.e15–22. https://doi.org/10.1016/j.neurobiolaging.2011.07.016 [PubMed]
-
80.
Rusted JM, Evans SL, King SL, Dowell N, Tabet N, Tofts PS. APOE e4 polymorphism in young adults is associated with improved attention and indexed by distinct neural signatures. Neuroimage. 2013; 65:364–73. https://doi.org/10.1016/j.neuroimage.2012.10.010 [PubMed]
-
81.
van Exel E, Koopman JJ, Bodegom DV, Meij JJ, Knijff P, Ziem JB, Finch CE, Westendorp RG. Effect of APOE ε4 allele on survival and fertility in an adverse environment. PLoS One. 2017; 12:e0179497. https://doi.org/10.1371/journal.pone.0179497 [PubMed]
-
82.
Jasienska G, Ellison PT, Galbarczyk A, Jasienski M, Kalemba-Drozdz M, Kapiszewska M, Nenko I, Thune I, Ziomkiewicz A. Apolipoprotein E (ApoE) polymorphism is related to differences in potential fertility in women: a case of antagonistic pleiotropy? Proc Biol Sci. 2015; 282:20142395. https://doi.org/10.1098/rspb.2014.2395 [PubMed]
-
83.
Dowell NG, Evans SL, Tofts PS, King SL, Tabet N, Rusted JM. Structural and resting-state MRI detects regional brain differences in young and mid-age healthy APOE-e4 carriers compared with non-APOE-e4 carriers. NMR Biomed. 2016; 29:614–24. https://doi.org/10.1002/nbm.3502 [PubMed]
-
84.
Falahati F, Ferreira D, Muehlboeck JS, Eriksdotter M, Simmons A, Wahlund LO, Westman E. Monitoring disease progression in mild cognitive impairment: associations between atrophy patterns, cognition, APOE and amyloid. Neuroimage Clin. 2017; 16:418–28. https://doi.org/10.1016/j.nicl.2017.08.014 [PubMed]
-
85.
Jefferson AL, Beiser AS, Seshadri S, Wolf PA, Au R. APOE and mild cognitive impairment: the Framingham Heart Study. Age Ageing. 2015; 44:307–11. https://doi.org/10.1093/ageing/afu183 [PubMed]
-
86.
Sabbagh MN, Malek-Ahmadi M, Dugger BN, Lee K, Sue LI, Serrano G, Walker DG, Davis K, Jacobson SA, Beach TG. The influence of Apolipoprotein E genotype on regional pathology in Alzheimer’s disease. BMC Neurol. 2013; 13:44. https://doi.org/10.1186/1471-2377-13-44 [PubMed]
-
87.
Lim YY, Williamson R, Laws SM, Villemagne VL, Bourgeat P, Fowler C, Rainey-Smith S, Salvado O, Martins RN, Rowe CC, Masters CL, Maruff P, and AIBL Research Group. Effect of APOE genotype on amyloid deposition, brain volume, and memory in cognitively normal older individuals. J Alzheimers Dis. 2017; 58:1293–302. https://doi.org/10.3233/JAD-170072 [PubMed]
-
88.
Liu Y, Yu JT, Wang HF, Han PR, Tan CC, Wang C, Meng XF, Risacher SL, Saykin AJ, Tan L. APOE genotype and neuroimaging markers of Alzheimer’s disease: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2015; 86:127–34. https://doi.org/10.1136/jnnp-2014-307719 [PubMed]
-
89.
Goveas JS, Xie C, Chen G, Li W, Ward BD, Franczak MB, Jones JL, Antuono PG, Li SJ. Functional network endophenotypes unravel the effects of apolipoprotein E epsilon 4 in middle-aged adults. PLoS One. 2013; 8:e55902. https://doi.org/10.1371/journal.pone.0055902 [PubMed]
-
90.
Wang L, Day J, Roe CM, Brier MR, Thomas JB, Benzinger TL, Morris JC, Ances BM. The effect of APOE ε4 allele on cholinesterase inhibitors in patients with Alzheimer disease: evaluation of the feasibility of resting state functional connectivity magnetic resonance imaging. Alzheimer Dis Assoc Disord. 2014; 28:122–27. https://doi.org/10.1097/WAD.0b013e318299d096 [PubMed]
-
91.
Liang Y, Li Z, Wei J, Li C, Zhang X. Neuroimaging Initiative AD. Frequency specific effects of ApoE ε4 allele on resting-state networks in nondemented elders. BioMed Res Int. 2017; 2017:9823501. https://doi.org/10.1155/2017/9823501
-
92.
de Waal H, Stam CJ, Blankenstein MA, Pijnenburg YA, Scheltens P, van der Flier WM. EEG abnormalities in early and late onset Alzheimer’s disease: understanding heterogeneity. J Neurol Neurosurg Psychiatry. 2011; 82:67–71. https://doi.org/10.1136/jnnp.2010.216432 [PubMed]
-
93.
Galluzzi S, Marizzoni M, Babiloni C, Albani D, Antelmi L, Bagnoli C, Bartres-Faz D, Cordone S, Didic M, Farotti L, Fiedler U, Forloni G, Girtler N, et al, and PharmaCog Consortium. Clinical and biomarker profiling of prodromal Alzheimer’s disease in workpackage 5 of the Innovative Medicines Initiative PharmaCog project: a ‘European ADNI study’. J Intern Med. 2016; 279:576–91. https://doi.org/10.1111/joim.12482 [PubMed]
-
94.
Cuesta P, Garcés P, Castellanos NP, López ME, Aurtenetxe S, Bajo R, Pineda-Pardo JA, Bruña R, Marín AG, Delgado M, Barabash A, Ancín I, Cabranes JA, et al. Influence of the APOE ε4 allele and mild cognitive impairment diagnosis in the disruption of the MEG resting state functional connectivity in sources space. J Alzheimers Dis. 2015; 44:493–505. https://doi.org/10.3233/JAD-141872 [PubMed]
-
95.
Kowalewski J, Murphy C. Olfactory ERPs in an odor/visual congruency task differentiate ApoE ε4 carriers from non-carriers. Brain Res. 2012; 1442:55–65. https://doi.org/10.1016/j.brainres.2011.12.030 [PubMed]
-
96.
Corby K, Morgan CD, Murphy C. Abnormal event-related potentials in young and middle-aged adults with the ApoE ε4 allele. Int J Psychophysiol. 2012; 83:276–81. https://doi.org/10.1016/j.ijpsycho.2011.11.001 [PubMed]
-
97.
Tensil M, Hessler JB, Gutsmiedl M, Riedl L, Grimmer T, Diehl-Schmid J. Sex Differences in Neuropsychological Test Performance in Alzheimer’s Disease and the Influence of the ApoE Genotype. Alzheimer Dis Assoc Disord. 2018; 32:145–49. https://doi.org/10.1097/WAD.0000000000000229 [PubMed]
-
98.
Chen J, Shu H, Wang Z, Liu D, Shi Y, Xu L, Zhang Z. Protective effect of APOE epsilon 2 on intrinsic functional connectivity of the entorhinal cortex is associated with better episodic memory in elderly individuals with risk factors for Alzheimer’s disease. Oncotarget. 2016; 7:58789–801. https://doi.org/10.18632/oncotarget.11289 [PubMed]
-
99.
Shinohara M, Kanekiyo T, Yang L, Linthicum D, Shinohara M, Fu Y, Price L, Frisch-Daiello JL, Han X, Fryer JD, Bu G. APOE2 eases cognitive decline during Aging: clinical and preclinical evaluations. Ann Neurol. 2016; 79:758–74. https://doi.org/10.1002/ana.24628 [PubMed]
-
100.
Konishi K, Bhat V, Banner H, Poirier J, Joober R, Bohbot VD. APOE2 Is associated with spatial navigational strategies and increased gray matter in the hippocampus. Front Hum Neurosci. 2016; 10:349. https://doi.org/10.3389/fnhum.2016.00349 [PubMed]
-
101.
Chang L, Douet V, Bloss C, Lee K, Pritchett A, Jernigan TL, Akshoomoff N, Murray SS, Frazier J, Kennedy DN, Amaral DG, Gruen J, Kaufmann WE, et al, and Pediatric Imaging, Neurocognition, and Genetics (PING) Study Consortium. Gray matter maturation and cognition in children with different APOE ε genotypes. Neurology. 2016; 87:585–94. https://doi.org/10.1212/WNL.0000000000002939 [PubMed]
-
102.
Herrmann W, Hanf S, Kaffarnik H, Motzny S, Reissner J, Steinmetz A. The influence of apolipoprotein E polymorphism on plasma concentrations of apolipoprotein B and A-I during the first year of life. Pediatrics. 1994; 93:296–302. [PubMed]
-
103.
Kallio MJ, Salmenperä L, Siimes MA, Perheentupa J, Gylling H, Miettinen TA. Apoprotein E phenotype determines serum cholesterol in infants during both high-cholesterol breast feeding and low-cholesterol formula feeding. J Lipid Res. 1997; 38:759–64. [PubMed]
-
104.
Jacobs MB, Harville EW, Kelly TN, Bazzano LA, Chen W. Maternal apolipoprotein E genotype as a potential risk factor for poor birth outcomes: The Bogalusa Heart Study. J Perinatol. 2016; 36:432–38. https://doi.org/10.1038/jp.2016.4 [PubMed]
-
105.
Atkinson KR, Blumenstein M, Black MA, Wu SH, Kasabov N, Taylor RS, Cooper GJ, North RA, and SCOPE Consortium. An altered pattern of circulating apolipoprotein E3 isoforms is implicated in preeclampsia. J Lipid Res. 2009; 50:71–80. https://doi.org/10.1194/jlr.M800296-JLR200 [PubMed]
-
106.
Alkemade FE, van Vliet P, Henneman P, van Dijk KW, Hierck BP, van Munsteren JC, Scheerman JA, Goeman JJ, Havekes LM, Gittenberger-de Groot AC, van den Elsen PJ, DeRuiter MC. Prenatal exposure to apoE deficiency and postnatal hypercholesterolemia are associated with altered cell-specific lysine methyltransferase and histone methylation patterns in the vasculature. Am J Pathol. 2010; 176:542–48. https://doi.org/10.2353/ajpath.2010.090031 [PubMed]
-
107.
Steinmetz A, Thiemann E, Czekelius P, Kaffarnik H. Polymorphism of apolipoprotein E influences levels of serum apolipoproteins E and B in the human neonate. Eur J Clin Invest. 1989; 19:390–94. https://doi.org/10.1111/j.1365-2362.1989.tb00247.x [PubMed]
-
108.
Sinclair LI, Pleydell-Pearce CW, Day IN. Possible positive effect of the APOE ε2 allele on cognition in early to mid-adult life. Neurobiol Learn Mem. 2017; 146:37–46. https://doi.org/10.1016/j.nlm.2017.10.008 [PubMed]
-
109.
Comley LH, Fuller HR, Wishart TM, Mutsaers CA, Thomson D, Wright AK, Ribchester RR, Morris GE, Parson SH, Horsburgh K, Gillingwater TH. ApoE isoform-specific regulation of regeneration in the peripheral nervous system. Hum Mol Genet. 2011; 20:2406–21. https://doi.org/10.1093/hmg/ddr147 [PubMed]
-
110.
Lorber B, Chew DJ, Hauck SM, Chong RS, Fawcett JW, Martin KR. Retinal glia promote dorsal root ganglion axon regeneration. PLoS One. 2015; 10:e0115996. https://doi.org/10.1371/journal.pone.0115996 [PubMed]
-
111.
Rozenbaum M, Rajman M, Rishal I, Koppel I, Koley S, Medzihradszky KF, Oses-Prieto JA, Kawaguchi R, Amieux PS, Burlingame AL, Coppola G, Fainzilber M. Translatome regulation in neuronal injury and axon regrowth. eNeuro. 2018; 5:ENEURO.0276-17.2018. https://doi.org/10.1523/ENEURO.0276-17.2018 [PubMed]
-
112.
Stoknes M, Lien E, Andersen GL, Bao Y, Blackman JA, Lie RT, Vik T. Child apolipoprotein E gene variants and risk of cerebral palsy: estimation from case-parent triads. Eur J Paediatr Neurol. 2015; 19:286–91. https://doi.org/10.1016/j.ejpn.2014.12.017 [PubMed]
-
113.
Lien E, Andersen G, Bao Y, Gordish-Dressman H, Skranes JS, Blackman JA, Vik T. Genes determining the severity of cerebral palsy: the role of single nucleotide polymorphisms on the amount and structure of apolipoprotein E. Acta Paediatr. 2015; 104:701–06. https://doi.org/10.1111/apa.12983 [PubMed]
-
114.
Norda S, Rausch TK, Orlikowsky T, Hütten M, Schulz S, Göpel W, Pecks U, Apolipoprotein E. Apolipoprotein E genotype in very preterm neonates with intrauterine growth restriction: an analysis of the German Neonatal Network Cohort. BioMed Res Int. 2017; 2017:2837027. https://doi.org/10.1155/2017/2837027 [PubMed]
-
115.
Kypreos KE, Karagiannides I, Fotiadou EH, Karavia EA, Brinkmeier MS, Giakoumi SM, Tsompanidi EM. Mechanisms of obesity and related pathologies: role of apolipoprotein E in the development of obesity. FEBS J. 2009; 276:5720–28. https://doi.org/10.1111/j.1742-4658.2009.07301.x [PubMed]
-
116.
Cotten CM, Goldstein RF, McDonald SA, Goldberg RN, Salhab WA, Carlo WA, Tyson JE, Finer NN, Walsh MC, Ehrenkranz RA, Laptook AR, Guillet R, Schibler K, et al, and Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Apolipoprotein E genotype and outcome in infants with hypoxic-ischemic encephalopathy. Pediatr Res. 2014; 75:424–30. https://doi.org/10.1038/pr.2013.235 [PubMed]
-
117.
Ng S, Lin CC, Hwang YH, Hsieh WS, Liao HF, Chen PC. Mercury, APOE, and children’s neurodevelopment. Neurotoxicology. 2013; 37:85–92. https://doi.org/10.1016/j.neuro.2013.03.012 [PubMed]
-
118.
Snoj Tratnik J, Falnoga I, Trdin A, Mazej D, Fajon V, Miklavčič A, Kobal AB, Osredkar J, Sešek Briški A, Krsnik M, Neubauer D, Kodrič J, Stropnik S, et al. Prenatal mercury exposure, neurodevelopment and apolipoprotein E genetic polymorphism. Environ Res. 2017; 152:375–85. https://doi.org/10.1016/j.envres.2016.08.035 [PubMed]
-
119.
Dean DC3rd, Jerskey BA, Chen K, Protas H, Thiyyagura P, Roontiva A, O’Muircheartaigh J, Dirks H, Waskiewicz N, Lehman K, Siniard AL, Turk MN, Hua X, et al. Brain differences in infants at differential genetic risk for late-onset Alzheimer disease: a cross-sectional imaging study. JAMA Neurol. 2014; 71:11–22. https://doi.org/10.1001/jamaneurol.2013.4544 [PubMed]
-
120.
Knickmeyer RC, Wang J, Zhu H, Geng X, Woolson S, Hamer RM, Konneker T, Lin W, Styner M, Gilmore JH. Common variants in psychiatric risk genes predict brain structure at birth. Cereb Cortex. 2014; 24:1230–46. https://doi.org/10.1093/cercor/bhs401 [PubMed]
-
121.
Korja M, Ylijoki M, Lapinleimu H, Pohjola P, Matomäki J, Kuśmierek H, Mahlman M, Rikalainen H, Parkkola R, Kaukola T, Lehtonen L, Hallman M, Haataja L. Apolipoprotein E, brain injury and neurodevelopmental outcome of children. Genes Brain Behav. 2013; 12:348–52. https://doi.org/10.1111/gbb.12024 [PubMed]
-
122.
Becher JC, Bell JE, McIntosh N, Keeling JW. Distribution of apolipoprotein E alleles in a Scottish healthy newborn population. Biol Neonate. 2005; 88:164–67. https://doi.org/10.1159/000086205 [PubMed]
-
123.
Becher JC, Keeling JW, McIntosh N, Wyatt B, Bell J. The distribution of apolipoprotein E alleles in Scottish perinatal deaths. J Med Genet. 2006; 43:414–18. https://doi.org/10.1136/jmg.2005.033936 [PubMed]
-
124.
Everman ER, Morgan TJ. Antagonistic pleiotropy and mutation accumulation contribute to age-related decline in stress response. Evolution. 2018; 72:303–17. https://doi.org/10.1111/evo.13408 [PubMed]
-
125.
Maklakov AA, Rowe L, Friberg U. Why organisms age: evolution of senescence under positive pleiotropy? BioEssays. 2015; 37:802–07. https://doi.org/10.1002/bies.201500025 [PubMed]
-
126.
Blackman JA, Gordish-Dressman H, Bao Y, Matsumoto JA, Sinkin RA. The apolipoprotein gene and recovery from brain injury among extremely preterm infants. Neonatology. 2014; 105:227–29. https://doi.org/10.1159/000357700 [PubMed]
-
127.
Bell JE, Becher JC, Keeling JW, McIntosh N. The neuropathology of stillbirth - correlation with apolipoprotein genotype in a Scottish population based study. Early Hum Dev. 2015; 91:139–48. https://doi.org/10.1016/j.earlhumdev.2014.12.008 [PubMed]
-
128.
Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006; 355:685–94. https://doi.org/10.1056/NEJMoa053792 [PubMed]
-
129.
Shaw P, Lerch JP, Pruessner JC, Taylor KN, Rose AB, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN. Cortical morphology in children and adolescents with different apolipoprotein E gene polymorphisms: an observational study. Lancet Neurol. 2007; 6:494–500. https://doi.org/10.1016/S1474-4422(07)70106-0 [PubMed]
-
130.
Gaynor JW, Kim DS, Arrington CB, Atz AM, Bellinger DC, Burt AA, Ghanayem NS, Jacobs JP, Lee TM, Lewis AB, Mahle WT, Marino BS, Miller SG, et al. Validation of association of the apolipoprotein E ε2 allele with neurodevelopmental dysfunction after cardiac surgery in neonates and infants. J Thorac Cardiovasc Surg. 2014; 148:2560–66. https://doi.org/10.1016/j.jtcvs.2014.07.052 [PubMed]
-
131.
Zeltser I, Jarvik GP, Bernbaum J, Wernovsky G, Nord AS, Gerdes M, Zackai E, Clancy R, Nicolson SC, Spray TL, Gaynor JW. Genetic factors are important determinants of neurodevelopmental outcome after repair of tetralogy of Fallot. J Thorac Cardiovasc Surg. 2008; 135:91–97. https://doi.org/10.1016/j.jtcvs.2007.04.074 [PubMed]
-
132.
Becher JC, Keeling JW, Bell J, Wyatt B, McIntosh N. Apolipoprotein E e4 and its prevalence in early childhood death due to sudden infant death syndrome or to recognised causes. Early Hum Dev. 2008; 84:549–54. https://doi.org/10.1016/j.earlhumdev.2008.01.002 [PubMed]
-
133.
Li L, Hua J, Jian-Ping H, Yan L. Association between the lipid levels and single nucleotide polymorphisms of ABCA1, APOE and HMGCR genes in subjects with spontaneous preterm delivery. PLoS One. 2015; 10:e0135785. https://doi.org/10.1371/journal.pone.0135785 [PubMed]
-
134.
Sarnat HB, Philippart M, Flores-Sarnat L, Wei XC. Timing in neural maturation: arrest, delay, precociousness, and temporal determination of malformations. Pediatr Neurol. 2015; 52:473–86. https://doi.org/10.1016/j.pediatrneurol.2015.01.020 [PubMed]
-
135.
Ben-Ari Y. Neuro-archaeology: pre-symptomatic architecture and signature of neurological disorders. Trends Neurosci. 2008; 31:626–36. https://doi.org/10.1016/j.tins.2008.09.002 [PubMed]
-
136.
Avelar AT, Perfeito L, Gordo I, Ferreira MG. Genome architecture is a selectable trait that can be maintained by antagonistic pleiotropy. Nat Commun. 2013; 4:2235. https://doi.org/10.1038/ncomms3235 [PubMed]
-
137.
Maharjan R, McKenzie C, Yeung A, Ferenci T. The basis of antagonistic pleiotropy in hfq mutations that have opposite effects on fitness at slow and fast growth rates. Heredity (Edinb). 2013; 110:10–18. https://doi.org/10.1038/hdy.2012.46 [PubMed]
-
138.
Kim SY, Metcalfe NB, Velando A. A benign juvenile environment reduces the strength of antagonistic pleiotropy and genetic variation in the rate of senescence. J Anim Ecol. 2016; 85:705–14. https://doi.org/10.1111/1365-2656.12468 [PubMed]
-
139.
Ruiz JR, Labayen I, Ortega FB, Moreno LA, González-Lamuño D, Martí A, Nova E, Fuentes MG, Redondo-Figuero C, Martínez JA, Sjöström M, Castillo MJ, and AVENA Study Group. Birth weight and blood lipid levels in Spanish adolescents: influence of selected APOE, APOC3 and PPARgamma2 gene polymorphisms. The AVENA Study. BMC Med Genet. 2008; 9:98. https://doi.org/10.1186/1471-2350-9-98 [PubMed]
-
140.
Li YJ, Hauser MA, Scott WK, Martin ER, Booze MW, Qin XJ, Walter JW, Nance MA, Hubble JP, Koller WC, Pahwa R, Stern MB, Hiner BC, et al. Apolipoprotein E controls the risk and age at onset of Parkinson disease. Neurology. 2004; 62:2005–09. https://doi.org/10.1212/01.WNL.0000128089.53030.AC [PubMed]
-
141.
Chiò A, Brunetti M, Barberis M, Iazzolino B, Montuschi A, Ilardi A, Cammarosano S, Canosa A, Moglia C, Calvo A. The role of APOE in the occurrence of frontotemporal dementia in amyotrophic lateral sclerosis. JAMA Neurol. 2016; 73:425–30. https://doi.org/10.1001/jamaneurol.2015.4773 [PubMed]
-
142.
Pecks U, Mohaupt MG, Hütten MC, Maass N, Rath W, Escher G. Cholesterol acceptor capacity is preserved by different mechanisms in preterm and term fetuses. Biochim Biophys Acta. 2014; 1841:251–58. https://doi.org/10.1016/j.bbalip.2013.11.008 [PubMed]
-
143.
Wright RO, Hu H, Silverman EK, Tsaih SW, Schwartz J, Bellinger D, Palazuelos E, Weiss ST, Hernandez-Avila M. Apolipoprotein E genotype predicts 24-month bayley scales infant development score. Pediatr Res. 2003; 54:819–25. https://doi.org/10.1203/01.PDR.0000090927.53818.DE [PubMed]
-
144.
Oriá RB, Patrick PD, Zhang H, Lorntz B, de Castro Costa CM, Brito GA, Barrett LJ, Lima AA, Guerrant RL. APOE4 protects the cognitive development in children with heavy diarrhea burdens in Northeast Brazil. Pediatr Res. 2005; 57:310–16. https://doi.org/10.1203/01.PDR.0000148719.82468.CA [PubMed]
-
145.
Suri S, Heise V, Trachtenberg AJ, Mackay CE. The forgotten APOE allele: a review of the evidence and suggested mechanisms for the protective effect of APOE ɛ2. Neurosci Biobehav Rev. 2013; 37:2878–86. https://doi.org/10.1016/j.neubiorev.2013.10.010 [PubMed]
-
146.
Gesierich B, Opherk C, Rosand J, Gonik M, Malik R, Jouvent E, Hervé D, Adib-Samii P, Bevan S, Pianese L, Silvestri S, Dotti MT, De Stefano N, et al. APOE ɛ2 is associated with white matter hyperintensity volume in CADASIL. J Cereb Blood Flow Metab. 2016; 36:199–203. https://doi.org/10.1038/jcbfm.2015.85 [PubMed]
-
147.
Mueller T, Fischer J, Gessner R, Rosendahl J, Böhm S, van Bömmel F, Knop V, Sarrazin C, Witt H, Marques AM, Kovacs P, Schleinitz D, Stumvoll M, et al. Apolipoprotein E allele frequencies in chronic and self-limited hepatitis C suggest a protective effect of APOE4 in the course of hepatitis C virus infection. Liver Int. 2016; 36:1267–74. https://doi.org/10.1111/liv.13094 [PubMed]
-
148.
Wirth M, Villeneuve S, La Joie R, Marks SM, Jagust WJ. Gene-environment interactions: lifetime cognitive activity, APOE genotype, and β-amyloid burden. J Neurosci. 2014; 34:8612–17. https://doi.org/10.1523/JNEUROSCI.4612-13.2014 [PubMed]
-
149.
Filippini N, Ebmeier KP, MacIntosh BJ, Trachtenberg AJ, Frisoni GB, Wilcock GK, Beckmann CF, Smith SM, Matthews PM, Mackay CE. Differential effects of the APOE genotype on brain function across the lifespan. Neuroimage. 2011; 54:602–10. https://doi.org/10.1016/j.neuroimage.2010.08.009 [PubMed]
-
150.
Woo M, Kim Y. Cortical functional connections and fluid intelligence in adolescent APOE ε4 carriers. Dement Geriatr Cogn Disord. 2017; 44:153–59. https://doi.org/10.1159/000479276 [PubMed]
-
151.
Shen J, Qin W, Xu Q, Xu L, Xu J, Zhang P, Liu H, Liu B, Jiang T, Yu C. Modulation of APOE and SORL1 genes on hippocampal functional connectivity in healthy young adults. Brain Struct Funct. 2017; 222:2877–89. https://doi.org/10.1007/s00429-017-1377-3 [PubMed]
-
152.
Treble-Barna A, Zang H, Zhang N, Martin LJ, Yeates KO, Taylor HG, Wade SL, Kurowski BG. Does Apolipoprotein e4 status moderate the association of family environment with long-term child functioning following early moderate to severe traumatic brain injury? a preliminary study. J Int Neuropsychol Soc. 2016; 22:859–64. https://doi.org/10.1017/S1355617716000631 [PubMed]
-
153.
Kassam I, Gagnon F, Cusimano MD. Association of the APOE-ε4 allele with outcome of traumatic brain injury in children and youth: a meta-analysis and meta-regression. J Neurol Neurosurg Psychiatry. 2016; 87:433–40. https://doi.org/10.1136/jnnp-2015-310500 [PubMed]
-
154.
Moran LM, Taylor HG, Ganesalingam K, Gastier-Foster JM, Frick J, Bangert B, Dietrich A, Nuss KE, Rusin J, Wright M, Yeates KO. Apolipoprotein E4 as a predictor of outcomes in pediatric mild traumatic brain injury. J Neurotrauma. 2009; 26:1489–95. https://doi.org/10.1089/neu.2008.0767 [PubMed]
-
155.
Aboud O, Mrak RE, Boop FA, Griffin WS. Epilepsy: neuroinflammation, neurodegeneration, and APOE genotype. Acta Neuropathol Commun. 2013; 1:41. https://doi.org/10.1186/2051-5960-1-41 [PubMed]
-
156.
Busch RM, Floden D, Lineweaver TT, Chapin JS, Unnwongse K, Wehner T, Diaz-Arrastia R, Najm IM. Effect of apolipoprotein ε4 allele on hippocampal and brain volume in intractable temporal lobe epilepsy. Epilepsy Behav. 2011; 21:88–90. https://doi.org/10.1016/j.yebeh.2011.01.007 [PubMed]
-
157.
Kauffman MA, Consalvo D, Moron DG, Lereis VP, Kochen S. ApoE epsilon4 genotype and the age at onset of temporal lobe epilepsy: a case-control study and meta-analysis. Epilepsy Res. 2010; 90:234–39. https://doi.org/10.1016/j.eplepsyres.2010.05.007 [PubMed]
-
158.
Braga LW, Borigato EV, Speck-Martins CE, Imamura EU, Gorges AM, Izumi AP, Dantas RC, Nunes LG. Apolipoprotein E genotype and cerebral palsy. Dev Med Child Neurol. 2010; 52:666–71. https://doi.org/10.1111/j.1469-8749.2009.03465.x [PubMed]
-
159.
O’Callaghan ME, MacLennan AH, Haan EA, Dekker G, and South Australian Cerebral Palsy Research Group. The genomic basis of cerebral palsy: a HuGE systematic literature review. Hum Genet. 2009; 126:149–72. https://doi.org/10.1007/s00439-009-0638-5 [PubMed]
-
160.
Kantarci K, Lowe V, Przybelski SA, Weigand SD, Senjem ML, Ivnik RJ, Preboske GM, Roberts R, Geda YE, Boeve BF, Knopman DS, Petersen RC, Jack CRJr. APOE modifies the association between Aβ load and cognition in cognitively normal older adults. Neurology. 2012; 78:232–40. https://doi.org/10.1212/WNL.0b013e31824365ab [PubMed]
-
161.
Fennema-Notestine C, Panizzon MS, Thompson WR, Chen CH, Eyler LT, Fischl B, Franz CE, Grant MD, Jak AJ, Jernigan TL, Lyons MJ, Neale MC, Seidman LJ, et al. Presence of ApoE ε4 allele associated with thinner frontal cortex in middle age. J Alzheimers Dis. 2011 (Suppl 3); 26:49–60. https://doi.org/10.3233/JAD-2011-0002 [PubMed]
-
162.
Wishart HA, Saykin AJ, McAllister TW, Rabin LA, McDonald BC, Flashman LA, Roth RM, Mamourian AC, Tsongalis GJ, Rhodes CH. Regional brain atrophy in cognitively intact adults with a single APOE epsilon4 allele. Neurology. 2006; 67:1221–24. https://doi.org/10.1212/01.wnl.0000238079.00472.3a [PubMed]
-
163.
Westlye ET, Hodneland E, Haász J, Espeseth T, Lundervold A, Lundervold AJ. Episodic memory of APOE ε4 carriers is correlated with fractional anisotropy, but not cortical thickness, in the medial temporal lobe. Neuroimage. 2012; 63:507–16. https://doi.org/10.1016/j.neuroimage.2012.06.072 [PubMed]
-
164.
Chiang GC, Zhan W, Schuff N, Weiner MW. White matter alterations in cognitively normal apoE ε2 carriers: insight into Alzheimer resistance? AJNR Am J Neuroradiol. 2012; 33:1392–97. https://doi.org/10.3174/ajnr.A2984 [PubMed]
-
165.
Machulda MM, Jones DT, Vemuri P, McDade E, Avula R, Przybelski S, Boeve BF, Knopman DS, Petersen RC, Jack CRJr. Effect of APOE ε4 status on intrinsic network connectivity in cognitively normal elderly subjects. Arch Neurol. 2011; 68:1131–36. https://doi.org/10.1001/archneurol.2011.108 [PubMed]
-
166.
Trachtenberg AJ, Filippini N, Ebmeier KP, Smith SM, Karpe F, Mackay CE. The effects of APOE on the functional architecture of the resting brain. Neuroimage. 2012; 59:565–72. https://doi.org/10.1016/j.neuroimage.2011.07.059 [PubMed]
-
167.
Evans S, Dowell NG, Tabet N, King SL, Hutton SB, Rusted JM. Disrupted neural activity patterns to novelty and effort in young adult APOE-e4 carriers performing a subsequent memory task. Brain Behav. 2017; 7:e00612. https://doi.org/10.1002/brb3.612 [PubMed]
-
168.
Su YY, Liang X, Schoepf UJ, Varga-Szemes A, West HC, Qi R, Kong X, Chen HJ, Lu GM, Zhang LJ. APOE Polymorphism affects brain default mode network in healthy young adults: a STROBE Article. Medicine (Baltimore). 2015; 94:e1734. https://doi.org/10.1097/MD.0000000000001734 [PubMed]
-
169.
Perkins M, Wolf AB, Chavira B, Shonebarger D, Meckel JP, Leung L, Ballina L, Ly S, Saini A, Jones TB, Vallejo J, Jentarra G, Valla J. Altered energy metabolism pathways in the posterior cingulate in young adult Apolipoprotein E ɛ4 carriers. J Alzheimers Dis. 2016; 53:95–106. https://doi.org/10.3233/JAD-151205 [PubMed]
-
170.
Suri S, Mackay CE, Kelly ME, Germuska M, Tunbridge EM, Frisoni GB, Matthews PM, Ebmeier KP, Bulte DP, Filippini N. Reduced cerebrovascular reactivity in young adults carrying the APOE ε4 allele. Alzheimers Dement. 2015; 11:648–57.e1. https://doi.org/10.1016/j.jalz.2014.05.1755 [PubMed]
-
171.
Love S, Siew LK, Dawbarn D, Wilcock GK, Ben-Shlomo Y, Allen SJ. Premorbid effects of APOE on synaptic proteins in human temporal neocortex. Neurobiol Aging. 2006; 27:797–803. https://doi.org/10.1016/j.neurobiolaging.2005.04.008 [PubMed]
-
172.
Kok E, Haikonen S, Luoto T, Huhtala H, Goebeler S, Haapasalo H, Karhunen PJ. Apolipoprotein E-dependent accumulation of Alzheimer disease-related lesions begins in middle age. Ann Neurol. 2009; 65:650–57. https://doi.org/10.1002/ana.21696 [PubMed]
-
173.
Teasdale GM, Murray GD, Nicoll JA. The association between APOE epsilon4, age and outcome after head injury: a prospective cohort study. Brain. 2005; 128:2556–61. https://doi.org/10.1093/brain/awh595 [PubMed]
-
174.
Merritt VC, Clark AL, Sorg SF, Evangelista ND, Werhane ML, Bondi MW, Schiehser DM, Delano-Wood L. Apolipoprotein E (APOE) ε4 genotype is associated with reduced neuropsychological performance in military veterans with a history of mild traumatic brain injury. J Clin Exp Neuropsychol. 2018; 40:1050–61. https://doi.org/10.1080/13803395.2018.1508555 [PubMed]
-
175.
Main BS, Villapol S, Sloley SS, Barton DJ, Parsadanian M, Agbaegbu C, Stefos K, McCann MS, Washington PM, Rodriguez OC, Burns MP. Apolipoprotein E4 impairs spontaneous blood brain barrier repair following traumatic brain injury. Mol Neurodegener. 2018; 13:17. https://doi.org/10.1186/s13024-018-0249-5 [PubMed]
-
176.
Kadotani H, Kadotani T, Young T, Peppard PE, Finn L, Colrain IM, Murphy GMJr, Mignot E. Association between apolipoprotein E epsilon4 and sleep-disordered breathing in adults. JAMA. 2001; 285:2888–90. https://doi.org/10.1001/jama.285.22.2888 [PubMed]
-
177.
Tranah GJ, Yaffe K, Nievergelt CM, Parimi N, Glymour MM, Ensrud KE, Cauley JA, Ancoli-Israel S, Mariani S, Redline S, Stone KL, and Osteoporotic Fractures in Men Study (MrOS) Research Group. APOEε4 and slow wave sleep in older adults. PLoS One. 2018; 13:e0191281. https://doi.org/10.1371/journal.pone.0191281 [PubMed]
-
178.
Hwang JY, Byun MS, Choe YM, Lee JH, Yi D, Choi JW, Hwang SH, Lee YJ, Lee DY, and KBASE Research Group. Moderating effect of APOE ε4 on the relationship between sleep-wake cycle and brain β-amyloid. Neurology. 2018; 90:e1167–73. https://doi.org/10.1212/WNL.0000000000005193 [PubMed]
-
179.
Pawlikowska L, Poon KY, Achrol AS, McCulloch CE, Ha C, Lum K, Zaroff JG, Ko NU, Johnston SC, Sidney S, Marchuk DA, Lawton MT, Kwok PY, Young WL. Apolipoprotein E epsilon 2 is associated with new hemorrhage risk in brain arteriovenous malformations. Neurosurgery. 2006; 58:838–43. https://doi.org/10.1227/01.NEU.0000209605.18358.E5 [PubMed]
-
180.
St George-Hyslop PH, Tanzi RE, Polinsky RJ, Haines JL, Nee L, Watkins PC, Myers RH, Feldman RG, Pollen D, Drachman D, al. The genetic defect causing familial Alzheimer’s disease maps on chromosome 21. Science. 1987; 235:885–90. https://doi.org/10.1126/science.2880399 [PubMed]
-
181.
Michaelson DM. APOE ε4: the most prevalent yet understudied risk factor for Alzheimer’s disease. Alzheimers Dement. 2014; 10:861–68. https://doi.org/10.1016/j.jalz.2014.06.015 [PubMed]
-
182.
Berge G, Sando SB, Rongve A, Aarsland D, White LR. Apolipoprotein E ε2 genotype delays onset of dementia with Lewy bodies in a Norwegian cohort. J Neurol Neurosurg Psychiatry. 2014; 85:1227–31. https://doi.org/10.1136/jnnp-2013-307228 [PubMed]
-
183.
Srinivasan R, Davidson Y, Gibbons L, Payton A, Richardson AM, Varma A, Julien C, Stopford C, Thompson J, Horan MA, Pendleton N, Pickering-Brown SM, Neary D, et al. The apolipoprotein E epsilon4 allele selectively increases the risk of frontotemporal lobar degeneration in males. J Neurol Neurosurg Psychiatry. 2006; 77:154–58. https://doi.org/10.1136/jnnp.2005.063966 [PubMed]
-
184.
Davidson Y, Gibbons L, Pritchard A, Hardicre J, Wren J, Stopford C, Julien C, Thompson J, Payton A, Pickering-Brown SM, Pendleton N, Horan MA, Burns A, et al. Apolipoprotein E epsilon4 allele frequency and age at onset of Alzheimer’s disease. Dement Geriatr Cogn Disord. 2007; 23:60–66. https://doi.org/10.1159/000097038 [PubMed]
-
185.
Geschwind D, Karrim J, Nelson SF, Miller B. The apolipoprotein E epsilon4 allele is not a significant risk factor for frontotemporal dementia. Ann Neurol. 1998; 44:134–38. https://doi.org/10.1002/ana.410440122 [PubMed]
-
186.
Lovati C, Galimberti D, Albani D, Bertora P, Venturelli E, Cislaghi G, Guidi I, Fenoglio C, Cortini F, Clerici F, Finazzi D, Forloni G, Scarpini E, Mariani C. APOE ε2 and ε4 influence the susceptibility for Alzheimer’s disease but not other dementias. Int J Mol Epidemiol Genet. 2010; 1:193–200. [PubMed]
-
187.
Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993; 261:921–23. https://doi.org/10.1126/science.8346443 [PubMed]
-
188.
Frisoni GB, Louhija J, Geroldi C, Trabucchi M. Longevity and the epsilon2 allele of apolipoprotein E: the Finnish Centenarians Study. J Gerontol A Biol Sci Med Sci. 2001; 56:M75–78. https://doi.org/10.1093/gerona/56.2.M75 [PubMed]
-
189.
Tindale LC, Leach S, Ushey K, Daley D, Brooks-Wilson AR. Rare and common variants in the Apolipoprotein E gene in healthy oldest old. Neurobiol Aging. 2014; 35:727.e1–3. https://doi.org/10.1016/j.neurobiolaging.2013.09.010 [PubMed]
-
190.
Packard CJ, Westendorp RG, Stott DJ, Caslake MJ, Murray HM, Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, Ford I, Gaw A, et al, and Prospective Study of Pravastatin in the Elderly at Risk Group. Association between apolipoprotein E4 and cognitive decline in elderly adults. J Am Geriatr Soc. 2007; 55:1777–85. https://doi.org/10.1111/j.1532-5415.2007.01415.x [PubMed]
-
191.
Scheller E, Peter J, Schumacher LV, Lahr J, Mader I, Kaller CP, Klöppel S. APOE moderates compensatory recruitment of neuronal resources during working memory processing in healthy older adults. Neurobiol Aging. 2017; 56:127–37. https://doi.org/10.1016/j.neurobiolaging.2017.04.015 [PubMed]
-
192.
Reiter K, Nielson KA, Durgerian S, Woodard JL, Smith JC, Seidenberg M, Kelly DA, Rao SM. Five-year longitudinal brain volume change in healthy elders at genetic risk for Alzheimer’s Disease. J Alzheimers Dis. 2017; 55:1363–77. https://doi.org/10.3233/JAD-160504 [PubMed]
-
193.
Nagy Z, Esiri MM, Jobst KA, Johnston C, Litchfield S, Sim E, Smith AD. Influence of the apolipoprotein E genotype on amyloid deposition and neurofibrillary tangle formation in Alzheimer’s disease. Neuroscience. 1995; 69:757–61. https://doi.org/10.1016/0306-4522(95)00331-C [PubMed]
-
194.
Boudreau DA, Scheer WD, Malcom GT, Mulvad G, Pedersen HS, Jul E. Apolipoprotein E and atherosclerosis in Greenland Inuit. Atherosclerosis. 1999; 145:207–19. https://doi.org/10.1016/S0021-9150(99)00067-2 [PubMed]
-
195.
Crawford FC, Vanderploeg RD, Freeman MJ, Singh S, Waisman M, Michaels L, Abdullah L, Warden D, Lipsky R, Salazar A, Mullan MJ. APOE genotype influences acquisition and recall following traumatic brain injury. Neurology. 2002; 58:1115–18. https://doi.org/10.1212/WNL.58.7.1115 [PubMed]
-
196.
Li YJ, Hauser MA, Scott WK, Martin ER, Booze MW, Qin XJ, Walter JW, Nance MA, Hubble JP, Koller WC, Pahwa R, Stern MB, Hiner BC, et al. Apolipoprotein E controls the risk and age at onset of Parkinson disease. Neurology. 2004; 62:2005–09. https://doi.org/10.1212/01.WNL.0000128089.53030.AC [PubMed]
-
197.
Konialis C, Spengos K, Iliopoulos P, Karapanou S, Gialafos E, Hagnefelt B, Vemmos K, Zakopoulos N, Pangalos C. The APOE E4 allele confers increased risk of ischemic stroke among greek carriers. Adv Clin Exp Med. 2016; 25:471–78. https://doi.org/10.17219/acem/38841 [PubMed]
-
198.
Holmes SE, Esterlis I, Mazure CM, Lim YY, Ames D, Rainey-Smith S, Martins RN, Salvado O, Dore V, Villemagne VL, Rowe CC, Laws SM, Masters CL, et al, and Australian Imaging, Biomarkers, Lifestyle Research Group. β-Amyloid, APOE and BDNF genotype, and depressive and anxiety symptoms in cognitively normal older women and men. Am J Geriatr Psychiatry. 2016; 24:1191–95. https://doi.org/10.1016/j.jagp.2016.08.007 [PubMed]
-
199.
Kypreos KE, Karagiannides I, Fotiadou EH, Karavia EA, Brinkmeier MS, Giakoumi SM, Tsompanidi EM. Mechanisms of obesity and related pathologies: role of apolipoprotein E in the development of obesity. FEBS J. 2009; 276:5720–28. https://doi.org/10.1111/j.1742-4658.2009.07301.x [PubMed]
-
200.
Yao X, Gordon EM, Figueroa DM, Barochia AV, Levine SJ. Emerging Roles of Apolipoprotein E and Apolipoprotein A-I in the Pathogenesis and Treatment of Lung Disease. Am J Respir Cell Mol Biol. 2016; 55:159–69. https://doi.org/10.1165/rcmb.2016-0060TR [PubMed]
-
201.
Coto-Segura P, Coto E, Alvarez V, Morales B, Soto-Sánchez J, Corao AI, Santos-Juanes J. Apolipoprotein epsilon4 allele is associated with psoriasis severity. Arch Dermatol Res. 2010; 302:145–49. https://doi.org/10.1007/s00403-009-1002-2 [PubMed]
-
202.
Yang Z, Wang X, Chi X, Zhao F, Guo J, Ma P, Zhong J, Niu J, Pan X, Long G. Neglected but important role of Apolipoprotein E exchange in Hepatitis C virus infection. J Virol. 2016; 90:9632–43. https://doi.org/10.1128/JVI.01353-16 [PubMed]
-
203.
Masuda T, Shimazawa M, Hashimoto Y, Kojima A, Nakamura S, Suemori S, Mochizuki K, Kawakami H, Kawase K, Hara H. Apolipoprotein E2 and E3, but Not E4, promote retinal pathologic neovascularization. Invest Ophthalmol Vis Sci. 2017; 58:1208–17. https://doi.org/10.1167/iovs.16-20539 [PubMed]
-
204.
Zokaei N, Giehl K, Sillence A, Neville MJ, Karpe F, Nobre AC, Husain M. Sex and APOE: A memory advantage in male APOE ε4 carriers in midlife. Cortex. 2017; 88:98–105. https://doi.org/10.1016/j.cortex.2016.12.016 [PubMed]
-
205.
Lancaster C, Tabet N, Rusted J. The APOE paradox: do attentional control differences in mid-adulthood reflect risk of late-life cognitive decline. Neurobiol Aging. 2016; 48:114–21. https://doi.org/10.1016/j.neurobiolaging.2016.08.015 [PubMed]
-
206.
Osuntokun BO, Sahota A, Ogunniyi AO, Gureje O, Baiyewu O, Adeyinka A, Oluwole SO, Komolafe O, Hall KS, Unverzagt FW, Hui SL, Yang M, Hendrie HC. Lack of an association between apolipoprotein E epsilon 4 and Alzheimer’s disease in elderly Nigerians. Ann Neurol. 1995; 38:463–65. https://doi.org/10.1002/ana.410380319 [PubMed]
-
207.
Gureje O, Ogunniyi A, Baiyewu O, Price B, Unverzagt FW, Evans RM, Smith-Gamble V, Lane KA, Gao S, Hall KS, Hendrie HC, Murrell JRJr. APOE epsilon4 is not associated with Alzheimer’s disease in elderly Nigerians. Ann Neurol. 2006; 59:182–85. https://doi.org/10.1002/ana.20694 [PubMed]
-
208.
Tang MX, Stern Y, Marder K, Bell K, Gurland B, Lantigua R, Andrews H, Feng L, Tycko B, Mayeux R. The APOE-epsilon4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA. 1998; 279:751–55. https://doi.org/10.1001/jama.279.10.751 [PubMed]
-
209.
Lipnicki DM, Crawford JD, Dutta R, Thalamuthu A, Kochan NA, Andrews G, Lima-Costa MF, Castro-Costa E, Brayne C, Matthews FE, Stephan BC, Lipton RB, Katz MJ, et al, and Cohort Studies of Memory in an International Consortium (COSMIC). Age-related cognitive decline and associations with sex, education and apolipoprotein E genotype across ethnocultural groups and geographic regions: a collaborative cohort study. PLoS Med. 2017; 14:e1002261. https://doi.org/10.1371/journal.pmed.1002261 [PubMed]
-
210.
Li C, Loewenstein DA, Duara R, Cabrerizo M, Barker W, Adjouadi M, and Alzheimer’s Disease Neuroimaging Initiative. The relationship of brain amyloid load and APOE status to regional cortical thinning and cognition in the ADNI Cohort. J Alzheimers Dis. 2017; 59:1269–82. https://doi.org/10.3233/JAD-170286 [PubMed]
-
211.
Fouquet M, Besson FL, Gonneaud J, La Joie R, Chételat G. Imaging brain effects of APOE4 in cognitively normal individuals across the lifespan. Neuropsychol Rev. 2014; 24:290–99. https://doi.org/10.1007/s11065-014-9263-8 [PubMed]
-
212.
Goryawala M, Zhou Q, Duara R, Loewenstein D, Cabrerizo M, Barker W, Adjouadi M. Altered small-world anatomical networks in Apolipoprotein-E4 (ApoE4) carriers using MRI. Conf Proc IEEE Eng Med Biol Soc. 2014; 2014:2468–71. https://doi.org/10.1109/EMBC.2014.6944122 [PubMed]
-
213.
Herrmann W, Hanf S, Kaffarnik H, Motzny S, Reissner J, Steinmetz A. The influence of apolipoprotein E polymorphism on plasma concentrations of apolipoprotein B and A-I during the first year of life. Pediatrics. 1994; 93:296–302. [PubMed]
-
214.
Nadkarni NK, Perera S, Snitz BE, Mathis CA, Price J, Williamson JD, DeKosky ST, Klunk WE, Lopez OL. Association of brain Amyloid-β with slow gait in elderly individuals without dementia: influence of cognition and Apolipoprotein E ε4 genotype. JAMA Neurol. 2017; 74:82–90. https://doi.org/10.1001/jamaneurol.2016.3474 [PubMed]
-
215.
Wirth M, Villeneuve S, La Joie R, Marks SM, Jagust WJ. Gene-environment interactions: lifetime cognitive activity, APOE genotype, and β-amyloid burden. J Neurosci. 2014; 34:8612–17. https://doi.org/10.1523/JNEUROSCI.4612-13.2014 [PubMed]
-
216.
Martin P, Jazwinski SM, Davey A, Green RC, Macdonald M, Margrett JA, Siegler IC, Arnold J, Woodard JL, Johnson MA, Kim S, Dai J, Li L, et al, and For The Georgia Centenarian Study. APOE ϵ4, rated life experiences, and affect among centenarians. Aging Ment Health. 2014; 18:240–47. https://doi.org/10.1080/13607863.2013.827624 [PubMed]
-
217.
Asada T, Yamagata Z, Kinoshita T, Kinoshita A, Kariya T, Asaka A, Kakuma T. Prevalence of dementia and distribution of ApoE alleles in Japanese centenarians: an almost-complete survey in Yamanashi Prefecture, Japan. J Am Geriatr Soc. 1996; 44:151–55. https://doi.org/10.1111/j.1532-5415.1996.tb02431.x [PubMed]
-
218.
Ossenkoppele R, Pijnenburg YA, Perry DC, Cohn-Sheehy BI, Scheltens NM, Vogel JW, Kramer JH, van der Vlies AE, La Joie R, Rosen HJ, van der Flier WM, Grinberg LT, Rozemuller AJ, et al. The behavioural/dysexecutive variant of Alzheimer’s disease: clinical, neuroimaging and pathological features. Brain. 2015; 138:2732–49. https://doi.org/10.1093/brain/awv191 [PubMed]
-
219.
Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW. Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol. 2011; 10:785–96. https://doi.org/10.1016/S1474-4422(11)70156-9 [PubMed]
-
220.
Gordon BA, Blazey T, Su Y, Fagan AM, Holtzman DM, Morris JC, Benzinger TL. Longitudinal β-Amyloid deposition and hippocampal volume in preclinical Alzheimer Disease and suspected non-alzheimer disease pathophysiology. JAMA Neurol. 2016; 73:1192–200. https://doi.org/10.1001/jamaneurol.2016.2642 [PubMed]
-
221.
Schreiber S, Schreiber F, Lockhart SN, Horng A, Bejanin A, Landau SM, Jagust WJ, and Alzheimer’s Disease Neuroimaging Initiative. Alzheimer Disease signature neurodegeneration and APOE genotype in mild cognitive impairment with suspected non-alzheimer disease pathophysiology. JAMA Neurol. 2017; 74:650–59. https://doi.org/10.1001/jamaneurol.2016.5349 [PubMed]
-
222.
Mehta SG, Watts GD, Adamson JL, Hutton M, Umberger G, Xiong S, Ramdeen S, Lovell MA, Kimonis VE, Smith CD. APOE is a potential modifier gene in an autosomal dominant form of frontotemporal dementia (IBMPFD). Genet Med. 2007; 9:9–13. https://doi.org/10.1097/GIM.0b013e31802d830d [PubMed]
-
223.
Chiò A, Brunetti M, Barberis M, Iazzolino B, Montuschi A, Ilardi A, Cammarosano S, Canosa A, Moglia C, Calvo A. The Role of APOE in the occurrence of frontotemporal dementia in amyotrophic lateral sclerosis. JAMA Neurol. 2016; 73:425–30. https://doi.org/10.1001/jamaneurol.2015.4773 [PubMed]
-
224.
Bras J, Guerreiro R, Darwent L, Parkkinen L, Ansorge O, Escott-Price V, Hernandez DG, Nalls MA, Clark LN, Honig LS, Marder K, Van Der Flier WM, Lemstra A, et al. Genetic analysis implicates APOE, SNCA and suggests lysosomal dysfunction in the etiology of dementia with Lewy bodies. Hum Mol Genet. 2014; 23:6139–46. https://doi.org/10.1093/hmg/ddu334 [PubMed]
-
225.
Berge G, Sando SB, Rongve A, Aarsland D, White LR. Apolipoprotein E ε2 genotype delays onset of dementia with Lewy bodies in a Norwegian cohort. J Neurol Neurosurg Psychiatry. 2014; 85:1227–31. https://doi.org/10.1136/jnnp-2013-307228 [PubMed]
-
226.
Anttila V, Bulik-Sullivan B, Finucane HK, Walters RK, Bras J, Duncan L, Escott-Price V, Falcone GJ, Gormley P, Malik R, Patsopoulos NA, Ripke S, Wei Z, et al, and Brainstorm Consortium. Analysis of shared heritability in common disorders of the brain. Science. 2018; 360:8757. https://doi.org/10.1126/science.aap8757 [PubMed]
-
227.
Uhl GR, Drgon T, Johnson C, Li CY, Contoreggi C, Hess J, Naiman D, Liu QR. Molecular genetics of addiction and related heritable phenotypes: genome-wide association approaches identify “connectivity constellation” and drug target genes with pleiotropic effects. Ann N Y Acad Sci. 2008; 1141:318–81. https://doi.org/10.1196/annals.1441.018 [PubMed]
-
228.
Wu L, Zhang X, Zhao L. Human ApoE isoforms differentially modulate brain glucose and ketone body metabolism: implications for Alzheimer’s Disease risk reduction and early intervention. J Neurosci. 2018; 38:6665–81. https://doi.org/10.1523/JNEUROSCI.2262-17.2018 [PubMed]
-
229.
Huang Y, Mahley RW. Apolipoprotein E: structure and function in lipid metabolism, neurobiology, and Alzheimer’s diseases. Neurobiol Dis. 2014; 72:3–12. https://doi.org/10.1016/j.nbd.2014.08.025 [PubMed]
-
230.
Keeney JT, Ibrahimi S, Zhao L. Human ApoE isoforms differentially modulate glucose and amyloid metabolic pathways in female brain: evidence of the mechanism of neuroprotection by ApoE2 and implications for Alzheimer’s prevention and early intervention. .JAD. 2015; 48:411–24. https://doi.org/10.3233/JAD-150348 [PubMed]
-
231.
Berlau DJ, Corrada MM, Head E, Kawas CH. APOE ε2 is associated with intact cognition but increased Alzheimer pathology in the oldest old. Neurology. 2009; 72:829–34. https://doi.org/10.1212/01.wnl.0000343853.00346.a4 [PubMed]
-
232.
Shinohara M, Kanekiyo T, Yang L, Linthicum D, Shinohara M, Fu Y, Price L, Frisch-Daiello JL, Han X, Fryer JD, Bu G. APOE2 eases cognitive decline during Aging: clinical and preclinical evaluations. Ann Neurol. 2016; 79:758–74. https://doi.org/10.1002/ana.24628 [PubMed]
-
233.
Suri S, Heise V, Trachtenberg AJ, Mackay CE. The forgotten APOE allele: a review of the evidence and suggested mechanisms for the protective effect of APOE ɛ2. Neurosci Biobehav Rev. 2013; 37:2878–86. https://doi.org/10.1016/j.neubiorev.2013.10.010 [PubMed]
-
234.
Kulminski AM, Raghavachari N, Arbeev KG, Culminskaya I, Arbeeva L, Wu D, Ukraintseva SV, Christensen K, Yashin AI. Protective role of the apolipoprotein E2 allele in age-related disease traits and survival: evidence from the Long Life Family Study. Biogerontology. 2016; 17:893–905. https://doi.org/10.1007/s10522-016-9659-3 [PubMed]
-
235.
Li X, Montine KS, Keene CD, Montine TJ. Different mechanisms of apolipoprotein E isoform-dependent modulation of prostaglandin E2 production and triggering receptor expressed on myeloid cells 2 (TREM2) expression after innate immune activation of microglia. FASEB J. 2015; 29:1754–62. https://doi.org/10.1096/fj.14-262683 [PubMed]
-
236.
Chung WS, Verghese PB, Chakraborty C, Joung J, Hyman BT, Ulrich JD, Holtzman DM, Barres BA. Novel allele-dependent role for APOE in controlling the rate of synapse pruning by astrocytes. Proc Natl Acad Sci USA. 2016; 113:10186–91. https://doi.org/10.1073/pnas.1609896113 [PubMed]
-
237.
Woody SK, Zhou H, Ibrahimi S, Dong Y, Zhao L. Human ApoE ε2 promotes regulatory mechanisms of bioenergetic and synaptic function in female brain: a focus on V-type H+-ATPase. .JAD. 2016; 53:1015–31. https://doi.org/10.3233/JAD-160307 [PubMed]
-
238.
Alata W, Ye Y, St-Amour I, Vandal M, Calon F. Human apolipoprotein E ɛ4 expression impairs cerebral vascularization and blood-brain barrier function in mice. J Cereb Blood Flow Metab. 2015; 35:86–94. https://doi.org/10.1038/jcbfm.2014.172 [PubMed]
-
239.
Sen A, Nelson TJ, Alkon DL. ApoE isoforms differentially regulates cleavage and secretion of BDNF. Mol Brain. 2017; 10:19. https://doi.org/10.1186/s13041-017-0301-3 [PubMed]
-
240.
Cooper DN, Krawczak M, Polychronakos C, Tyler-Smith C, Kehrer-Sawatzki H. Where genotype is not predictive of phenotype: towards an understanding of the molecular basis of reduced penetrance in human inherited disease. Hum Genet. 2013; 132:1077–130. https://doi.org/10.1007/s00439-013-1331-2 [PubMed]
-
241.
Lew AR, Kellermayer TR, Sule BP, Szigeti K. Copy number variations in adult-onset neuropsychiatric diseases. Curr Genomics. 2018; 19:420–30. https://doi.org/10.2174/1389202919666180330153842 [PubMed]
-
242.
Mahley RW. Central nervous system lipoproteins: ApoE and regulation of cholesterol metabolism. Arterioscler Thromb Vasc Biol. 2016; 36:1305–15. https://doi.org/10.1161/ATVBAHA.116.307023 [PubMed]
-
243.
Tisato V, Zuliani G, Vigliano M, Longo G, Franchini E, Secchiero P, Zauli G, Paraboschi EM, Vikram Singh A, Serino ML, Ortolani B, Zurlo A, Bosi C, et al. Gene-gene interactions among coding genes of iron-homeostasis proteins and APOE-alleles in cognitive impairment diseases. PLoS One. 2018; 13:e0193867. https://doi.org/10.1371/journal.pone.0193867 [PubMed]
-
244.
Vélez JI, Lopera F, Sepulveda-Falla D, Patel HR, Johar AS, Chuah A, Tobón C, Rivera D, Villegas A, Cai Y, Peng K, Arkell R, Castellanos FX, et al. APOE*E2 allele delays age of onset in PSEN1 E280A Alzheimer’s disease. Mol Psychiatry. 2016; 21:916–24. https://doi.org/10.1038/mp.2015.177 [PubMed]
-
245.
Gomar JJ, Conejero-Goldberg C, Huey ED, Davies P, Goldberg TE, and Alzheimer’s Disease Neuroimaging Initiative. Lack of neural compensatory mechanisms of BDNF val66met met carriers and APOE E4 carriers in healthy aging, mild cognitive impairment, and Alzheimer’s disease. Neurobiol Aging. 2016; 39:165–73. https://doi.org/10.1016/j.neurobiolaging.2015.12.004 [PubMed]
-
246.
Blanchard V, Ramin-Mangata S, Billon-Crossouard S, Aguesse A, Durand M, Chemello K, Nativel B, Flet L, Chétiveaux M, Jacobi D, Bard JM, Ouguerram K, Lambert G, et al. Kinetics of plasma apolipoprotein E isoforms by LC-MS/MS: a pilot study. J Lipid Res. 2018; 59:892–900. https://doi.org/10.1194/jlr.P083576 [PubMed]
-
247.
Martínez-Morillo E, Nielsen HM, Batruch I, Drabovich AP, Begcevic I, Lopez MF, Minthon L, Bu G, Mattsson N, Portelius E, Hansson O, Diamandis EP. Assessment of peptide chemical modifications on the development of an accurate and precise multiplex selected reaction monitoring assay for apolipoprotein e isoforms. J Proteome Res. 2014; 13:1077–87. https://doi.org/10.1021/pr401060x [PubMed]
-
248.
Nishimura M, Satoh M, Nishimura S, Kakinuma S, Sato K, Sawai S, Tsuchida S, Kazama T, Matsushita K, Kado S, Kodera Y, Nomura F. Human apolipoprotein e resequencing by proteomic analysis and its application to serotyping. PLoS One. 2014; 9:e85356. https://doi.org/10.1371/journal.pone.0085356 [PubMed]
-
249.
Iacono D, Markesbery WR, Gross M, Pletnikova O, Rudow G, Zandi P, Troncoso JC. The Nun study: clinically silent AD, neuronal hypertrophy, and linguistic skills in early life. Neurology. 2009; 73:665–73. https://doi.org/10.1212/WNL.0b013e3181b01077 [PubMed]
-
250.
Bapat R, Narayana PA, Zhou Y, Parikh NA. Magnetic resonance spectroscopy at term-equivalent age in extremely preterm infants: association with cognitive and language development. Pediatr Neurol. 2014; 51:53–59. https://doi.org/10.1016/j.pediatrneurol.2014.03.011 [PubMed]
-
251.
Simões RV, Muñoz-Moreno E, Cruz-Lemini M, Eixarch E, Bargalló N, Sanz-Cortés M, Gratacós E. Brain metabolite alterations in infants born preterm with intrauterine growth restriction: association with structural changes and neurodevelopmental outcome. Am J Obstet Gynecol. 2017; 216:62.e1–14. https://doi.org/10.1016/j.ajog.2016.09.089 [PubMed]
-
252.
Pogribna U, Burson K, Lasky RE, Narayana PA, Evans PW, Parikh NA. Role of diffusion tensor imaging as an independent predictor of cognitive and language development in extremely low-birth-weight infants. AJNR Am J Neuroradiol. 2014; 35:790–96. https://doi.org/10.3174/ajnr.A3725 [PubMed]
-
253.
Wright RO, Hu H, Silverman EK, Tsaih SW, Schwartz J, Bellinger D, Palazuelos E, Weiss ST, Hernandez-Avila M. Apolipoprotein E genotype predicts 24-month bayley scales infant development score. Pediatr Res. 2003; 54:819–25. https://doi.org/10.1203/01.PDR.0000090927.53818.DE [PubMed]
-
254.
Chapman BP, Benedict RH, Lin F, Roy S, Porteinsson A, Szigeti K, Federoff H, Mapstone M. Apolipoprotein E genotype impact on memory and attention in older persons: the moderating role of personality phenotype. Int J Geriatr Psychiatry. 2018; 33:332–39. https://doi.org/10.1002/gps.4748 [PubMed]
-
255.
Dar-Nimrod I, Chapman BP, Robbins JA, Porsteinsson A, Mapstone M, Duberstein PR. Gene by neuroticism interaction and cognitive function among older adults. Int J Geriatr Psychiatry. 2012; 27:1147–54. https://doi.org/10.1002/gps.3759 [PubMed]
-
256.
Caselli RJ, Dueck AC, Locke DE, Henslin BR, Johnson TA, Woodruff BK, Hoffman-Snyder C, Geda YE. Impact of personality on cognitive aging: a prospective cohort study. J Int Neuropsychol Soc. 2016; 22:765–76. https://doi.org/10.1017/S1355617716000527 [PubMed]
-
257.
Altschul DM, Starr JM, Deary IJ. Cognitive function in early and later life is associated with blood glucose in older individuals: analysis of the Lothian Birth Cohort of 1936. Diabetologia. 2018; 61:1946–55. https://doi.org/10.1007/s00125-018-4645-8 [PubMed]
-
258.
Sinclair LI, Pleydell-Pearce CW, Day IN. Possible positive effect of the APOE ε2 allele on cognition in early to mid-adult life. Neurobiol Learn Mem. 2017; 146:37–46. https://doi.org/10.1016/j.nlm.2017.10.008 [PubMed]
-
259.
Smith JD. Apolipoprotein E4: an allele associated with many diseases. Ann Med. 2000; 32:118–27. https://doi.org/10.3109/07853890009011761 [PubMed]
-
260.
Mueller T, Fischer J, Gessner R, Rosendahl J, Böhm S, van Bömmel F, Knop V, Sarrazin C, Witt H, Marques AM, Kovacs P, Schleinitz D, Stumvoll M, et al. Apolipoprotein E allele frequencies in chronic and self-limited hepatitis C suggest a protective effect of APOE4 in the course of hepatitis C virus infection. Liver Int. 2016; 36:1267–74. https://doi.org/10.1111/liv.13094 [PubMed]
-
261.
Sapkota S, Wiebe SA, Small BJ, Dixon RA. Apolipoprotein E and Clusterin can magnify effects of personality vulnerability on declarative memory performance in non-demented older adults. Int J Geriatr Psychiatry. 2016; 31:502–09. https://doi.org/10.1002/gps.4355 [PubMed]
-
262.
Yamazaki Y, Painter MM, Bu G, Kanekiyo T. Apolipoprotein E as a therapeutic target in Alzheimer’s Disease: a review of basic research and clinical evidence. CNS Drugs. 2016; 30:773–89. https://doi.org/10.1007/s40263-016-0361-4 [PubMed]
-
263.
Liu S, Park S, Allington G, Prelli F, Sun Y, Martá-Ariza M, Scholtzova H, Biswas G, Brown B, Verghese PB, Mehta PD, Kwon YU, Wisniewski T. Targeting Apolipoprotein E/Amyloid β binding by peptoid CPO_Aβ17-21 P ameliorates Alzheimer’s Disease related pathology and cognitive decline. Sci Rep. 2017; 7:8009. https://doi.org/10.1038/s41598-017-08604-8 [PubMed]
-
264.
Harper AR, Nayee S, Topol EJ. Protective alleles and modifier variants in human health and disease. Nat Rev Genet. 2015; 16:689–701. https://doi.org/10.1038/nrg4017 [PubMed]
-
265.
Mattsson N, Eriksson O, Lindberg O, Schöll M, Lampinen B, Nilsson M, Insel PS, Lautner R, Strandberg O, van Westen D, Zetterberg H, Blennow K, Palmqvist S, et al. Effects of APOE ε4 on neuroimaging, cerebrospinal fluid biomarkers, and cognition in prodromal Alzheimer’s disease. Neurobiol Aging. 2018; 71:81–90. https://doi.org/10.1016/j.neurobiolaging.2018.07.003 [PubMed]
-
266.
Kim DH, Iijima H, Goto K, Sakai J, Ishii H, Kim HJ, Suzuki H, Kondo H, Saeki S, Yamamoto T. Human apolipoprotein E receptor 2. A novel lipoprotein receptor of the low density lipoprotein receptor family predominantly expressed in brain. J Biol Chem. 1996; 271:8373–80. https://doi.org/10.1074/jbc.271.14.8373 [PubMed]
-
267.
Herz J. The LDL receptor gene family: (un)expected signal transducers in the brain. Neuron. 2001; 29:571–81. https://doi.org/10.1016/S0896-6273(01)00234-3 [PubMed]
-
268.
Gotthardt M, Trommsdorff M, Nevitt MF, Shelton J, Richardson JA, Stockinger W, Nimpf J, Herz J. Interactions of the low density lipoprotein receptor gene family with cytosolic adaptor and scaffold proteins suggest diverse biological functions in cellular communication and signal transduction. J Biol Chem. 2000; 275:25616–24. https://doi.org/10.1074/jbc.M000955200 [PubMed]
-
269.
D’Arcangelo G, Homayouni R, Keshvara L, Rice DS, Sheldon M, Curran T. Reelin is a ligand for lipoprotein receptors. Neuron. 1999; 24:471–79. https://doi.org/10.1016/S0896-6273(00)80860-0 [PubMed]
-
270.
Jossin Y, Ignatova N, Hiesberger T, Herz J, Lambert de Rouvroit C, Goffinet AM. The central fragment of Reelin, generated by proteolytic processing in vivo, is critical to its function during cortical plate development. J Neurosci. 2004; 24:514–21. https://doi.org/10.1523/JNEUROSCI.3408-03.2004 [PubMed]
-
271.
Bosch C, Masachs N, Exposito-Alonso D, Martínez A, Teixeira CM, Fernaud I, Pujadas L, Ulloa F, Comella JX, DeFelipe J, Merchán-Pérez A, Soriano E. Reelin regulates the maturation of dendritic spines, synaptogenesis and glial ensheathment of newborn granule cells. Cereb Cortex. 2016; 26:4282–98. https://doi.org/10.1093/cercor/bhw216 [PubMed]
-
272.
Krstic D, Rodriguez M, Knuesel I. Regulated proteolytic processing of Reelin through interplay of tissue plasminogen activator (tPA), ADAMTS-4, ADAMTS-5, and their modulators. PLoS One. 2012; 7:e47793. https://doi.org/10.1371/journal.pone.0047793 [PubMed]
-
273.
Mata-Balaguer T, Cuchillo-Ibañez I, Calero M, Ferrer I, Sáez-Valero J. Decreased generation of C-terminal fragments of ApoER2 and increased reelin expression in Alzheimer’s disease. FASEB J. 2018; 32:3536–46. https://doi.org/10.1096/fj.201700736RR [PubMed]
-
274.
Benveniste H, Liu X, Koundal S, Sanggaard S, Lee H, Wardlaw J. The glymphatic system and waste clearance with brain aging: a review. Gerontology. 2018;:1–14; Epub ahead of print. https://doi.org/10.1159/000490349 [PubMed]
-
275.
Louveau A, Herz J, Alme MN, Salvador AF, Dong MQ, Viar KE, Herod SG, Knopp J, Setliff JC, Lupi AL, Da Mesquita S, Frost EL, Gaultier A, et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat Neurosci. 2018; 21:1380–91. https://doi.org/10.1038/s41593-018-0227-9 [PubMed]
-
276.
Belinson H, Kariv-Inbal Z, Kayed R, Masliah E, Michaelson DM. Following activation of the amyloid cascade, apolipoprotein E4 drives the in vivo oligomerization of amyloid-β resulting in neurodegeneration. J Alzheimers Dis. 2010; 22:959–70. https://doi.org/10.3233/JAD-2010-101008 [PubMed]
-
277.
Liu CC, Zhao N, Fu Y, Wang N, Linares C, Tsai CW, Bu G. ApoE4 accelerates early seeding of amyloid pathology. Neuron. 2017; 96:1024–1032.e3. https://doi.org/10.1016/j.neuron.2017.11.013 [PubMed]
-
278.
Robert J, Button EB, Yuen B, Gilmour M, Kang K, Bahrabadi A, Stukas S, Zhao W, Kulic I, Wellington CL. Clearance of beta-amyloid is facilitated by apolipoprotein E and circulating high-density lipoproteins in bioengineered human vessels. eLife. 2017; 6:e29595. https://doi.org/10.7554/eLife.29595 [PubMed]
-
279.
Shi Y, Yamada K, Liddelow SA, Smith ST, Zhao L, Luo W, Tsai RM, Spina S, Grinberg LT, Rojas JC, Gallardo G, Wang K, Roh J, et al, and Alzheimer’s Disease Neuroimaging Initiative. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature. 2017; 549:523–27. https://doi.org/10.1038/nature24016 [PubMed]
-
280.
Dafnis I, Argyri L, Sagnou M, Tzinia A, Tsilibary EC, Stratikos E, Chroni A. The ability of apolipoprotein E fragments to promote intraneuronal accumulation of amyloid beta peptide 42 is both isoform and size-specific. Sci Rep. 2016; 6:30654. https://doi.org/10.1038/srep30654 [PubMed]
-
281.
Kakuda N, Miyasaka T, Iwasaki N, Nirasawa T, Wada-Kakuda S, Takahashi-Fujigasaki J, Murayama S, Ihara Y, Ikegawa M. Distinct deposition of amyloid-β species in brains with Alzheimer’s disease pathology visualized with MALDI imaging mass spectrometry. Acta Neuropathol Commun. 2017; 5:73. https://doi.org/10.1186/s40478-017-0477-x [PubMed]
-
282.
Mori E, Lee K, Yasuda M, Hashimoto M, Kazui H, Hirono N, Matsui M. Accelerated hippocampal atrophy in Alzheimer’s disease with apolipoprotein E epsilon4 allele. Ann Neurol. 2002; 51:209–14. https://doi.org/10.1002/ana.10093 [PubMed]
-
283.
Basso M, Gelernter J, Yang J, MacAvoy MG, Varma P, Bronen RA, van Dyck CH. Apolipoprotein E epsilon4 is associated with atrophy of the amygdala in Alzheimer’s disease. Neurobiol Aging. 2006; 27:1416–24. https://doi.org/10.1016/j.neurobiolaging.2005.08.002 [PubMed]
-
284.
Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci USA. 2009; 106:7209–14. https://doi.org/10.1073/pnas.0811879106 [PubMed]
-
285.
Persico AM, D’Agruma L, Zelante L, Militerni R, Bravaccio C, Schneider C, Melmed R, Trillo S, Montecchi F, Elia M, Palermo M, Rabinowitz D, Pascucci T, et al. Enhanced APOE2 transmission rates in families with autistic probands. Psychiatr Genet. 2004; 14:73–82. https://doi.org/10.1097/01.ypg.0000128768.37838.17 [PubMed]
-
286.
Burnham N, Ittenbach RF, Stallings VA, Gerdes M, Zackai E, Bernbaum J, Clancy RR, Gaynor JW. Genetic factors are important determinants of impaired growth after infant cardiac surgery. J Thorac Cardiovasc Surg. 2010; 140:144–49. https://doi.org/10.1016/j.jtcvs.2010.01.003 [PubMed]
-
287.
Zhao N, Liu CC, Van Ingelgom AJ, Linares C, Kurti A, Knight JA, Heckman MG, Diehl NN, Shinohara M, Martens YA, Attrebi ON, Petrucelli L, Fryer JD, et al. APOE ε2 is associated with increased tau pathology in primary tauopathy. Nat Commun. 2018; 9:4388. https://doi.org/10.1038/s41467-018-06783-0 [PubMed]
-
288.
White F, Nicoll JA, Roses AD, Horsburgh K. Impaired neuronal plasticity in transgenic mice expressing human apolipoprotein E4 compared to E3 in a model of entorhinal cortex lesion. Neurobiol Dis. 2001; 8:611–25. https://doi.org/10.1006/nbdi.2001.0401 [PubMed]
-
289.
Ji Y, Gong Y, Gan W, Beach T, Holtzman DM, Wisniewski T. Apolipoprotein E isoform-specific regulation of dendritic spine morphology in apolipoprotein E transgenic mice and Alzheimer’s disease patients. Neuroscience. 2003; 122:305–15. https://doi.org/10.1016/j.neuroscience.2003.08.007 [PubMed]
-
290.
Nwabuisi-Heath E, Rebeck GW, Ladu MJ, Yu C. ApoE4 delays dendritic spine formation during neuron development and accelerates loss of mature spines in vitro. ASN Neuro. 2014; 6:e00134. https://doi.org/10.1042/AN20130043 [PubMed]
-
291.
Bloss CS, Delis DC, Salmon DP, Bondi MW. APOE genotype is associated with left-handedness and visuospatial skills in children. Neurobiol Aging. 2010; 31:787–95. https://doi.org/10.1016/j.neurobiolaging.2008.05.021 [PubMed]
-
292.
Marchant NL, King SL, Tabet N, Rusted JM. Positive effects of cholinergic stimulation favor young APOE epsilon4 carriers. Neuropsychopharmacology. 2010; 35:1090–96. https://doi.org/10.1038/npp.2009.214 [PubMed]
-
293.
Filippini N, Rao A, Wetten S, Gibson RA, Borrie M, Guzman D, Kertesz A, Loy-English I, Williams J, Nichols T, Whitcher B, Matthews PM. Anatomically-distinct genetic associations of APOE epsilon4 allele load with regional cortical atrophy in Alzheimer’s disease. Neuroimage. 2009; 44:724–28. https://doi.org/10.1016/j.neuroimage.2008.10.003 [PubMed]
-
294.
Boros BD, Greathouse KM, Gearing M, Herskowitz JH. Dendritic spine remodeling accompanies Alzheimer’s disease pathology and genetic susceptibility in cognitively normal aging. Neurobiol Aging. 2019; 73:92–103; Epub ahead of print. https://doi.org/10.1016/j.neurobiolaging.2018.09.003 [PubMed]
-
295.
Trull TJ, Widiger TA. Dimensional models of personality: the five-factor model and the DSM-5. Dialogues Clin Neurosci. 2013; 15:135–46. [PubMed]
-
296.
Servaas MN, Geerligs L, Renken RJ, Marsman JB, Ormel J, Riese H, Aleman A. Connectomics and neuroticism: an altered functional network organization. Neuropsychopharmacology. 2015; 40:296–304. https://doi.org/10.1038/npp.2014.169 [PubMed]
-
297.
Mulders P, Llera A, Tendolkar I, van Eijndhoven P, Beckmann C. Personality profiles are associated with functional brain networks related to cognition and emotion. Sci Rep. 2018; 8:13874. https://doi.org/10.1038/s41598-018-32248-x [PubMed]
-
298.
Hsu WT, Rosenberg MD, Scheinost D, Constable RT, Chun MM. Resting-state functional connectivity predicts neuroticism and extraversion in novel individuals. Soc Cogn Affect Neurosci. 2018; 13:224–32. https://doi.org/10.1093/scan/nsy002 [PubMed]
-
299.
Chang P, Li X, Ma C, Zhang S, Liu Z, Chen K, Ai L, Chang J, Zhang Z. The Effects of an APOE promoter polymorphism on human white matter connectivity during non-demented aging. J Alzheimers Dis. 2017; 55:77–87. https://doi.org/10.3233/JAD-160447 [PubMed]
-
300.
Korthauer LE, Zhan L, Ajilore O, Leow A, Driscoll I. Disrupted topology of the resting state structural connectome in middle-aged APOE ε4 carriers. Neuroimage. 2018; 178:295–305. https://doi.org/10.1016/j.neuroimage.2018.05.052 [PubMed]
-
301.
Tautvydaitė D, Kukreja D, Antonietti JP, Henry H, von Gunten A, Popp J. Interaction between personality traits and cerebrospinal fluid biomarkers of Alzheimer’s disease pathology modulates cognitive performance. Alzheimers Res Ther. 2017; 9:6. https://doi.org/10.1186/s13195-017-0235-0 [PubMed]
-
302.
Bessi V, Mazzeo S, Padiglioni S, Piccini C, Nacmias B, Sorbi S, Bracco L. From Subjective Cognitive Decline to Alzheimer’s Disease: The Predictive Role of Neuropsychological Assessment, Personality Traits, and Cognitive Reserve. A 7-Year Follow-Up Study. J Alzheimers Dis. 2018; 63:1523–35. https://doi.org/10.3233/JAD-171180 [PubMed]
-
303.
Tangwongchai S, Supasitthumrong T, Hemrunroj S, Tunvirachaisakul C, Chuchuen P, Houngngam N, Snabboon T, Tawankanjanachot I, Likitchareon Y, Phanthumchindad K, Maes M. In Thai Nationals, the ApoE4 Allele Affects Multiple Domains of Neuropsychological, Biobehavioral, and Social Functioning Thereby Contributing to Alzheimer’s Disorder, while the ApoE3 Allele Protects Against Neuropsychiatric Symptoms and Psychosocial Deficits. Mol Neurobiol. 2018; 55:6449–62. https://doi.org/10.1007/s12035-017-0848-0 [PubMed]