Introduction
Amyloid-β pathology is the starting event in the serial biomarker cascade models of Alzheimer’s disease (AD), which precedes abnormality in clinical symptoms for almost two decades and subsequently contributes to clinical progression [1, 2]. Approximately one-fourth of cognitively normal populations and half of individuals with mild cognitive impairment (MCI) have elevated levels of cerebral amyloidosis [3, 4]. The 2018 National Institute on Aging and Alzheimer’s Association (NIA-AA) research framework updated the biomarker classification system consisting of β-amyloidosis, tauopathy and neurodegeneration or neuronal injury (the ATN system) and emphasized the presence of amyloid pathology to identify the first stage of the disease [5, 6]. Abnormality of amyloid-β precedes abnormality in tau, which leads to AD–related brain atrophy, [18F]-fluorodeoxyglucose (FDG) decline and accelerated cognitive decline [7].
AD is a defined biological entity with a progressive continuum in clinical abnormalities. The molecular pathology of AD is accomplished by biological entity-related continuous measures, and highlighted by three disease components, including β-amyloidosis, tauopathy and neurodegeneration. Importantly, quantifications of amyloid-β have been incorporated into standard diagnostic guidelines and clinical trials, and abnormal amyloid-β can be measured by decreased cerebrospinal fluid (CSF) amyloid-β42 peptide (Aβ42) levels or increased ligand retention of amyloid positron emission tomography (PET) imaging in the preclinical phase [8–11]. Previous cross-sectional and longitudinal studies have indicated that abnormally low levels of CSF Aβ42 can be detected before the identification of abnormal Aβ42 by PET in preclinical AD [12, 13]. Consequently, the reduction in CSF Aβ42 levels over time has stronger predictive value for identifying population with the disease or condition and monitoring disease trajectories at early stage.
Genetic risk factors play a key role in disease pathology with a high heritability of 70% to 80% [14]. The apolipoprotein E (APOE) genotype is regarded as the strongest genetic regulator for amyloid pathology in late-onset AD. However, at present, the genetic risk factors that are related to longitudinal changes of CSF Aβ42 levels remain poorly understood. Use of quantitative traits in genome-wide association studies (GWAS) provides novel and important insights into broader trends in associations with genes and related pathways [15–17]. Therefore, we performed a quantitative trait GWAS with longitudinal measures of CSF Aβ42 levels from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database in order to identify genetic regulators of CSF Aβ42 change rate in non-demented elders.
Results
Characteristics of included subjects
The final participants were consolidated for 321 non-Hispanic Caucasian subjects from the ADNI-1, 2 and Grand Opportunities (GO) databases. Participants included a mixture of 195 MCI and 126 cognitively normal subjects. The mean duration from the first measurement of CSF Aβ42 to the last was 31.3 months. Table 1 shows the distribution of baseline demographic information. No significant differences in gender (p = 0.11) and education level (p = 0.11) were found between the two diagnostic groups, while age, cognitive score, APOE genotype and CSF Aβ42 level differed between groups (p < 0.001).
Table 1. Demographic information and clinical characteristics of participants at baseline.
| Baseline diagnosis | CN | MCI | P value |
| Participants (n) | 126 | 195 | |
| Baseline age, y, mean (SD) | 75.06 (5.41) | 72.05 (7.19) | <0.001 |
| Gender (male/female) | 66/60 | 120/75 | 0.11 |
| Education, y, mean (SD) | 16.68 (2.75) | 16.19 (2.78) | 0.11 |
| APOE (ε4−/ε4+) | 102/24 | 101/94 | <0.001 |
| CSF Aβ42, mean (SD) | 203.52 (57.00) | 171.86 (53.09) | <0.001 |
| MMSE scores, mean (SD) | 29.17 (1.13) | 27.79 (1.75) | <0.001 |
| ADAS-cog11 scores, mean (SD) | 5.69 (2.72) | 9.83 (4.22) | <0.001 |
| Hippocampus, mm³, mean (SD) | 7081.30 (1080.34) | 7078.58 (1083.55) | <0.001 |
| Abbreviations: Aβ42 = amyloid-β42 peptide; CSF = cerebrospinal fluid; CN = cognitively normal; MCI = mild cognitive impairment; APOE ε4 = apolipoprotein E ε4 allele; MMSE = Mini-Mental State Examination; ADAS-cog = Alzheimer’s disease assessment scale-cognitive subscale. |
| P values are from the Wilcoxon rank-sum test |
GWAS of longitudinal changes in CSF Aβ42
To discover novel genetic modifiers of annualized percent change in CSF Aβ42, we conducted a GWAS adjusting for age, gender, education level, disease status (MCI or CN), APOE ε4 status, follow-up duration and two principal component factors. The strongest association of the longitudinal changes in CSF Aβ42 was identified with rs13333659, an intronic variant located in the gene CBFA2T3 (CBFA2/RUNX1 translocation partner 3; OMIM*603870, 16q 24.3; Figure 1A). Figure 2A shows the mean concentration of CSF Aβ42 during the follow-up period for the minor allele (T) carriers vs. non-carriers in the discovery cohort and baseline amyloid-positive group. There are 214 individuals who were homozygous wild-type (G/G), 98 individuals who were heterozygous (G/T) and 9 individuals who were homozygous recessive (T/T) at rs13333659. The minor allele T (MAF = 0.1) was associated with a more rapid decline in CSF Aβ42 levels over time (β = −0.034, p = 2.24×10−9). This novel statistically significant association was shown in both baseline amyloid-positive (β = −0.034, p = 1.95×10−6Figure 2B) and amyloid-negative (β = −0.027, p = 2.74×10−3) groups.
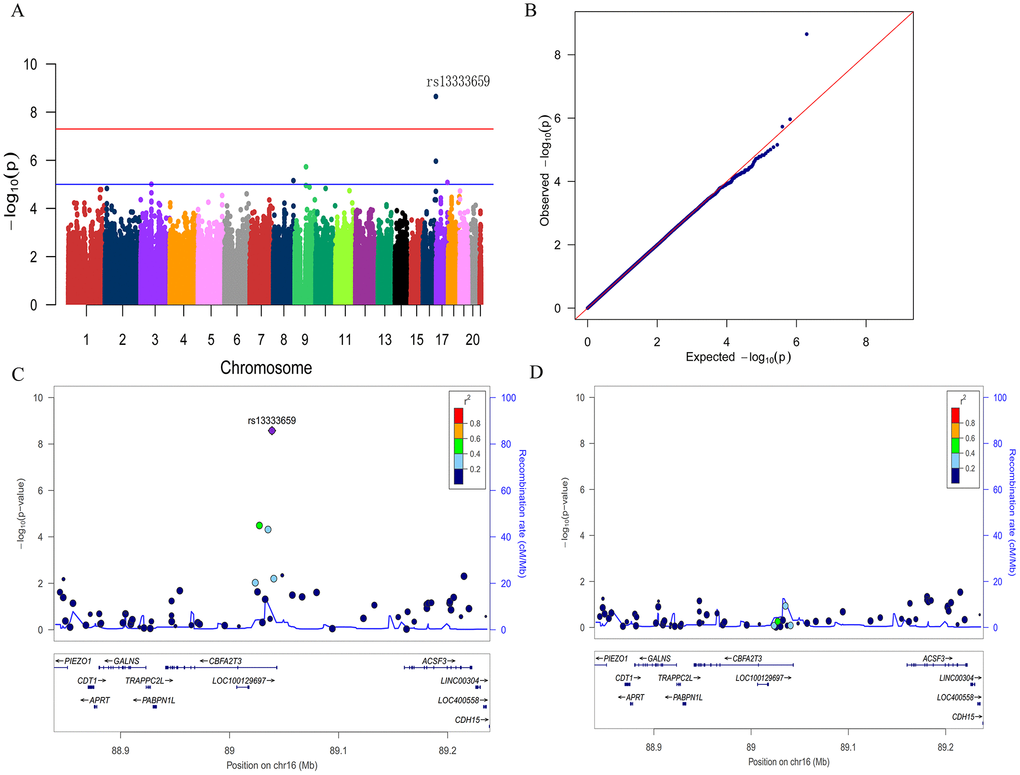
Figure 1. Manhattan plot (A), quantile-quantile plot (B) and regional plots (C and D) for the GWAS of longitudinal changes of CSF Aβ42. (A) A genome-wide significant association (P < 5×10−8; red line) with longitudinal change of CSF Aβ42 was identified on chromosome 16 within CBFA2T3. Suggestive associations are at the threshold of P < 1×10−5 (blue line). (B) Quantile-quantile plot. (C) Regional association results for the 88.9 Mb to 89.2 Mb region of chromosome 16. (D) Association results for the 88.9 Mb to 89.2 Mb region of chromosome 16 controlling for rs13333659.
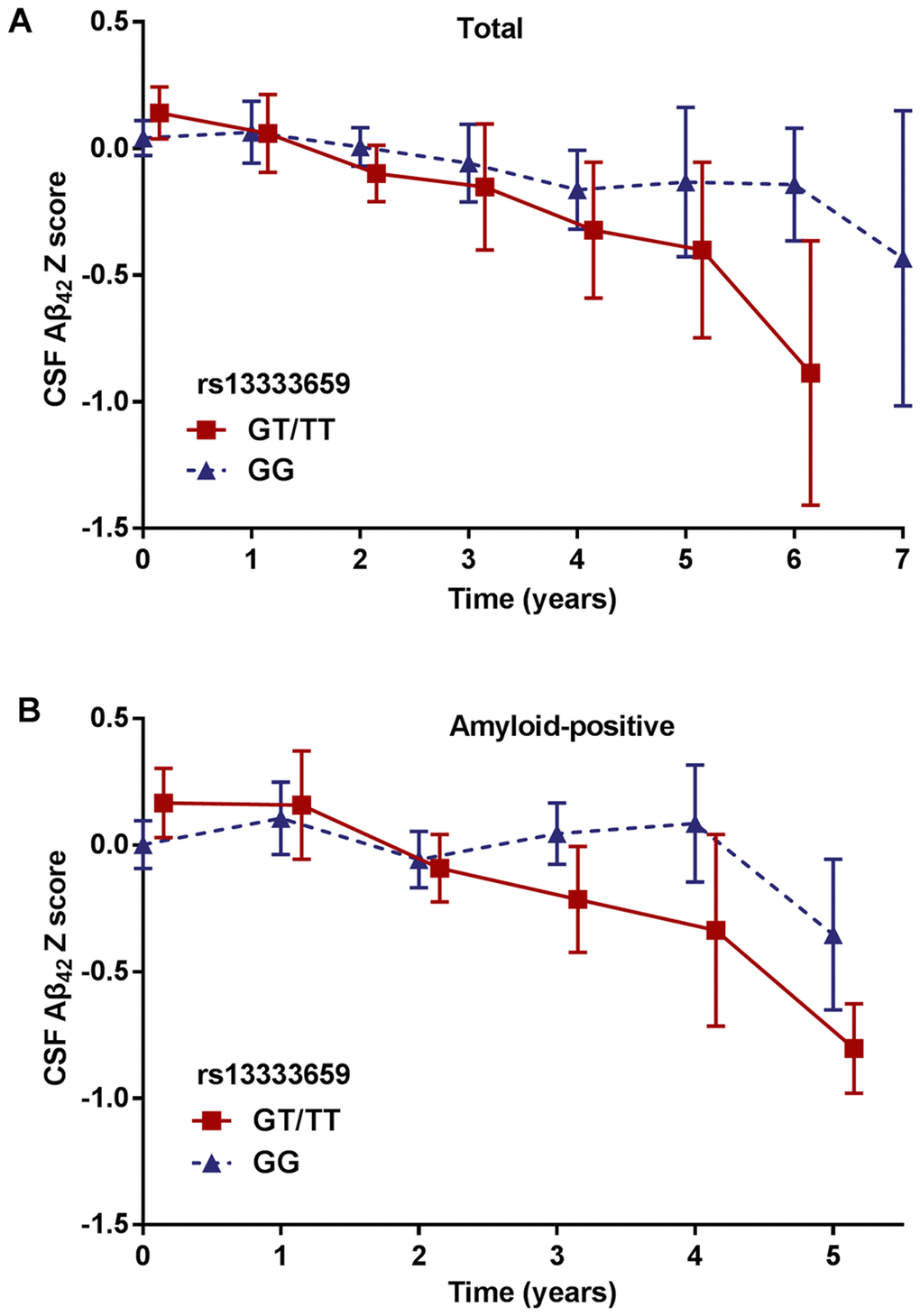
Figure 2. Mean changes of CSF Aβ42 over time in rs13333659 minor allele carriers vs. non-carriers. (A) Mean concentrations of CSF Aβ42 ± SE (standard error) change for rs13333659 minor allele carriers vs. non-carriers in total GWAS cohort during follow-up period. (B) Mean concentrations of CSF Aβ42 ± SE change for rs13333659 minor allele carriers vs. non-carriers in baseline amyloid-positive group during follow-up period.
We then utilized the Q-Q plot to compare the observed distribution and the expected distribution for the GWAS (Figure 1B). There was a deviation from expected p-values in the upper tail distribution, suggesting the presence of a significant genetic association. The genomic inflation factor λ was 1.007, indicating no evidence of significant confounding due to population stratification. The Figure 1C shows the linkage disequilibrium (LD) between rs13333659 in CBFA2T3 and other nearby SNPs, several of which showed strong associations with the longitudinal changes of CSF Aβ42 (p < 0.01). No significant associations were observed after controlling for rs13333659 genotype, indicating that these associations for nearby SNPs were driven by rs13333659 (Figure 1D). Five suggestive associations (p < 1 × 10−5) with longitudinal changes of CSF Aβ42 were demonstrated in this GWAS (Figure 1A and Table 2), which included an additional SNP in CBFA2T3 (rs57706252), TRPM6 (rs35878400, OMIM*607009), RBFOX3 (rs113685315, OMIM*616999), PDZRN3 (rs55732227, OMIM*609729) and an intergenic locus on 8q21.1 (rs6987191).
Table 2. Peak SNPs associated with annualized percent change of CSF Aβ42.
| CHR | SNP | Gene | Position | Function | A1/2 | MAF | βa | P value |
| 16 | rs13333659 | CBFA2T3 | 89038880 | Intron | T/G | 0.177 | −0.034 | 2.24×10−9 |
| 16 | rs57706252 | CBFA2T3 | 89030609 | Intron | C/T | 0.167 | −0.029 | 1.09×10−6 |
| 9 | rs35878400 | TRPM6 | 77482795 | Intron | T/C | 0.273 | 0.024 | 1.87×10−6 |
| 8 | rs6987191 | / | 138246221 | / | C/T | 0.131 | −0.031 | 6.99×10−6 |
| 17 | rs113685315 | RBFOX3 | 77390248 | Intron | A/G | 0.162 | 0.025 | 8.31×10−6 |
| 3 | rs55732227 | PDZRN3 | 73611629 | Intron | T/A | 0.309 | −0.020 | 9.86×10−6 |
| Abbreviations: CHR = chromosome; SNP = single nucleotide polymorphism; MAF = minor allele frequency; A1/2 = minor allele/major allele. |
| aβ effect size from GWAS means the annualized percent change of CSF Aβ42 conferred by one copy of the minor allele. |
Impact of CBFA2T3 on longitudinal cognitive performance
Amyloid aggregation confers the greatest risk for steeper long-term cognitive decline [18]. Several recent studies have confirmed that the negative effects of amyloidosis on cognitive performance in cognitively normal individuals, which are distinct from the influence of aging [18–21]. Taken together, we hypothesized that carriers with rs13333659-T would display a higher rate of cognitive decline. In order to assess the relationship between rs13333659 variants and cognitive decline over time, we performed a linear mixed effects analysis on 687 non-demented elderly subjects (MCI = 433; CN = 254) from the ADNI cohort with a 6-year follow-up. The cognitive assessments were based on ADAS-cog 11 scale. After controlling for genotype, age, gender, education level, disease status, APOE genotype and follow-up duration, we found rs13333659-T carriers were associated with accelerated decline in cognitive performance compared to non-carriers in all non-demented samples (p = 0.029, β = 0.097, n = 687; Figure 3A). The effects were more significant in baseline amyloid-positive group (p = 0.005, β = 0.196, n = 297; Figure 3B) but not in baseline amyloid-negative group (p = 0.980, β= 0.003, n = 240; Figure 3C). These data supported the association between the minor allele (T) of rs13333659 and a more rapid cognitive decline, related to amyloid status.
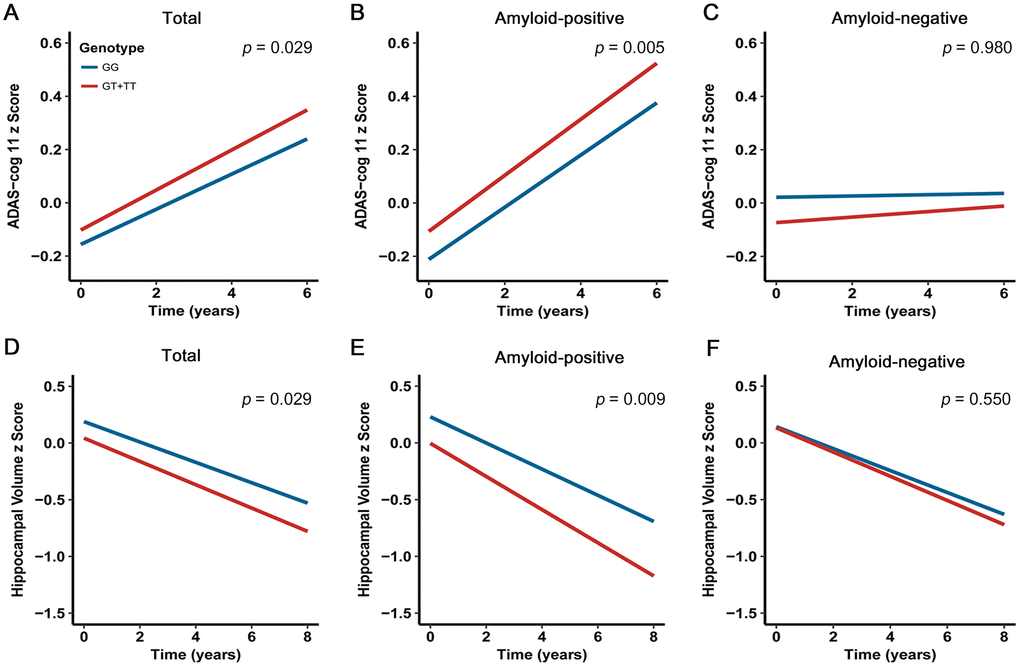
Figure 3. Effects of CBFA2T3 rs13333659-T on cognitive performance and hippocampal volume over time. Data from linear mixed-effects models adjusted for age, gender, educational level, APOE ε4 genotype, disease status, follow-up duration, as well as intracranial volume for hippocampal volume. ADAS-cog 11 indicates Alzheimer Disease Assessment Scale-cognitive subscale.
Impact of CBFA2T3 on longitudinal brain structure
The emerging amyloid pathology has been demonstrated to result in atrophy rates of brain structures during the development of AD [22–24]. We hypothesized that rs13333659-T carriers in CBFA2T3 would show faster rates of atrophy in AD-related brain regions (such as the hippocampus region). In order to assess the relationship between rs13333659 variants and hippocampal atrophy over time, we examined longitudinal changes of hippocampal volume over 8 years using linear mixed models. Five hundred and eighty-six non-demented elders (MCI = 361, CN = 225) with structural MRI data from ADNI were examined in this longitudinal analysis. Our analyses were conducted when genotype, age, gender, education level, disease status, APOE ε4 genotype, follow-up duration and intracranial volume were included as covariates. The results revealed that rs13333659-T carriers have increased hippocampal atrophy rates compared to non-carriers across all subjects (p = 0.029, β= −0.160, n = 586; Figure 3D). In particular, the higher rate of hippocampal atrophy was observed in baseline amyloid-positive group (p = 0.009, β = −0.285, n = 260; Figure 3E), while no significant differences were observed between rs13333659-T carriers and non-carriers in baseline amyloid-negative group (p = 0.550, β = −0.083, n = 207; Figure 3F).
DISCUSSION
In a series of longitudinal measurements of CSF Aβ42, we have identified a genome-wide significant association of CBFA2T3 rs13333659-T with a more rapid decline rate of CSF Aβ42 in non-demented elders. The novel association for the SNP may reflect a relative specificity for amyloid versus the more heterogeneous case-control status, increasing power obtained via endophenotype analysis. This novel association was further validated by converging evidence from cognitive performance and brain MRI structure analyses with consistent downtrends in a longitudinal framework. Our findings provide evidence that CBFA2T3 as a novel candidate gene may promote the application of CSF Aβ42 as an early biomarker to the prediction of the conversion from cognitive health to AD and the detection of disease trajectories.
CBFA2T3 encodes a myeloid translocation gene (MTG) family protein with highly conserved sequences across species, which interacts with transcription factors of DNA-bounding sites and recruits multiple co-repressors to facilitate transcription repression [25]. The gene expresses during the process of neurogenesis and neuronal differentiation, which may function to promote the transition from precursor to neuron and the expression of neuronal genes in differentiated cells [26]. The putative dominant-negative mutant of the gene causes a large number of differentiated neurons markedly reduced [26]. Prior to this study, the definite association mechanism of CSF Aβ42 and CBFA2T3 has not been previously been discovered. The expression of CBFA2T3, NEUROG2 (OMIM*606624) and ASCL1 (OMIM*100790) genes overlapped, and transcription assay showed that NEUROG2 and ASCL1 are inhibited by the MTGprotein family, in which the CBFA2T3 gene was the most efficient to inhibit the transcription activity [27]. The proneural factor NEUROG2 mediates APP-stimulated neuronal differentiation of neural stem/progenitor cells (NSPCs) to neurons, and levels of NEUROG2 are correlated with amyloid-precursor protein (APP) expression [28], which means that rare mutations of CBFA2T3 might influence the APP expression. APP is a single-pass transmembrane protein expressed at high levels in the brain, and the generation and accumulation of Aβ42 from sequential APP proteolysis is the crucial step in the development of AD [29]. However, the specific mechanism through which NEUROG2 regulates APP is not yet clear. Furthermore, NEUROG2 expression increased with rising relative Aβ42 levels [30]. The recent findings from Alzheimer’s disease mouse models revealed that lentiviral vector-mediated overexpression of NEUROG2 and ASCL1 can ameliorate learning and memory impairment, and the ASCL1gene treatment could be linked to inhibition of the neuroinflammatory response and enhancement of neuroprotection and neurogenesis [31]. Taken together, we reasoned that CBFA2T3 might affect the neurogenesis process and AD-related cognitive decline.
The Aβ is a product of the metabolism of the amyloid precursor protein, a type 1 transmembrane protein [32]. APP can mediate cell adhesion and stimulate neurite outgrowth. Moreover, a secreted form of APP stimulates cell proliferation and is neuroprotective against a variety of toxic insults, including oxidative stress, glucose deprivation and excitotoxicity [33]. An imbalance between the production and clearance of Aβ has a crucial role in the development and progression of AD. The toxic soluble Aβ oligomers (AβOs) accumulate in AD brain and constitute long-lived alternatives to the disease-defining Aβ fibrils deposited in amyloid plaques [34]. The β-amyloidosis triggers a redistribution of critical synaptic proteins and induces hyperactivity in metabotropic and ionotropic glutamate receptors. This leads to Ca2+ overload and instigates major facets of AD neuropathology, including tau hyperphosphorylation, insulin resistance, oxidative stress, synapse loss and so on [35]. The AβO hypothesis has been described as a small conceptual revolution and is widely regarded as accounting for the onset of neuron damage leading to AD.
Suggestive associations were identified through GWAS and might reach genome-wide significance in larger cohorts of non-demented older adults. The suggestive associations included a SNP in RBFOX3, which is exclusively expressed in neurons. RBFOX3 promotes neuronal differentiation through alternative splicing of Numb pre-mRNA during brain development and maturation [36]. RBFOX3 mutations have been observed to be linked to cognitive impairments. Moreover, RBFOX3 is critical for normal hippocampal function. Especially in hippocampal CA1 and dentate gyrus regions, the dysfunctional RBFOX3 affects synaptic transmission and short-time or long-time plasticity and results in impairment of learning, memory and cognition [37, 38]. The suggestive findings argue for further investigation into RBFOX3 to clarify the potential functional variants related to amyloid pathology.
Following this GWAS scan, we assessed the associations of CBFA2T3 rs13333659-T with other AD-related endophenotypes in the longitudinal framework. In particular, it was observed that rs13333659-T carriers had more rapid rates of cognitive decline and hippocampal atrophy among the non-demented elders with detectable amyloid pathology at baseline. It has been validated by previous studies that amyloid status can be present in the brain and has a minor impact on cognition over the long preclinical period [20, 39, 40]. Our results also suggest that baseline amyloidosis as an enrolling criterion in many prevention trials might be useful for risk enrichment and risk stratification [41]. The CSF Aβ42 (or the Aβ42/Aβ40 ratio) and amyloid-PET were widely accepted as measurable indicators of abnormal pathological states associated with cerebral amyloidosis levels [42]. The updated 2018 NIA-AA research framework indicates that focusing on longitudinal cohort studies and randomized placebo-controlled trials facilitate the biological definition of AD [5]. In the absence of successful anti-amyloid trials, the clearly identifiable stages and mechanisms behind the efficacy of the anti-amyloid treatment remain unknown. Probably the most effective approach would be to find preventive targets to inhibit the initial accumulation of Aβ42. Thus, understanding the genetic mechanisms underlying the rate of amyloid aggregation in individuals without dementia may be the best window on a better understanding of the pathological changes in AD and the design of prevention trials [43]. This study discovered a novel risk locus which can influence the decline rate of CSF Aβ42 decline preceding the onset of clinical symptoms, providing reliable and valid estimates for further investigations.
Our findings must be interpreted in the light of the limitations. Although we leveraged publicly available ANDI genetics and longitudinal CSF Aβ42 data to perform this original study, the sample size for analysis was relatively small, leading to the limited power to identify variants which have small effects. Given that the buildup of brain Aβ42 is postulated to take decades, the follow-up of this study is not sufficiently long to monitor longitudinal changes. Replication testing is necessary in larger samples with long duration of follow-up to confirm our findings and additional discovery. The study was limited to non-Hispanic Caucasian participants to avoid population stratification across ethnicities, but the rs13333659 in CBFA2T3 has various frequencies in different races. The contradiction determines the racial limitation of our research and the necessity of replication analysis in other races. Furthermore, this study provided evidence for association between rs13333659 in CBFA2T3 genetic variants with the decline rate of CSF Aβ42 as well as that of rs13333659 and cognitive performance traits, but the specific mechanisms of influencing expression level or protein structure and affecting phenotypes were not illustrated. Functional genomics experiments have not yet been performed, which included immunohistochemistry and analyses of CBFA2T3 knockout models.
Methods
Participants
In this study, the final participants (n = 321) included cognitively normal subjects (n = 126) and patients with MCI (n = 195) after all quality control. All participants were enrolled from the ADNI 1, ADNI 2 and ADNI GO phases. The ADNI was launched in 2003 as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), PET, other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD. For up-to-date information see www.adni-info.org.
A total of 370 participants with longitudinal CSF Aβ42 data and genotype data were available before quality control. Participants were restricted to non-demented elders and we removed 13 patients diagnosed with AD. To address confounding due to population stratification, we restricted the participants to non-Hispanic Caucasians and this step removed 33 subjects. To reduce potential bias due to due to family structure and cryptic relatedness, we checked the preliminary results with genomic identity-by-descent (IBD) and multidimensional scaling (MDS) components using PLINK v1.9 software. Two samples were outliers based on the second MDS component and the identity-by-descent fraction (π) of these two was equal to 0.43, suggesting they are probably first-degree relatives (Figure 4A). This step removed these two individuals. Finally, using the HapMap cohort, they showed tight clustering with individuals of European ancestry (Figure 4B).
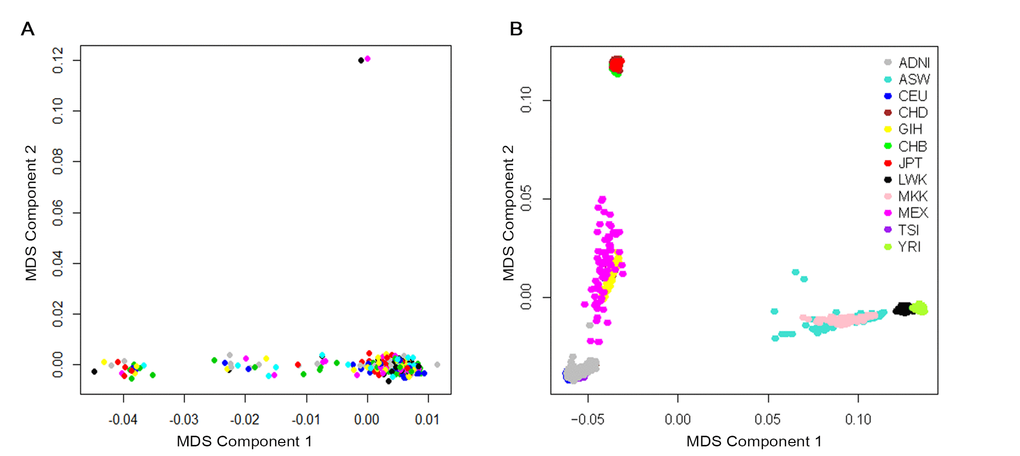
Figure 4. Cryptic relatedness and population stratification checked with genomic identity-by-descent (IBD) and multidimensional scaling (MDS) components. (A) MDS plot of ADNI non-Hispanic White samples. Two samples were outliers based on the second MDS component (at above of plot; 024_S_2239 and 024_S_4084). (B) MDS plot of ADNI samples overlaid on HapMap samples. The ancestry of the HapMap participants is shown by the point color. Abbreviations: ADNI: Alzheimer’s Disease Neuroimaging Initiative; ASW: African ancestry in Southwest USA; CEU: Utah residents with Northern and Western European ancestry from the CEPH collection; CHB: Han Chinese individuals from Beijing, China; CHD: Chinese in Metropolitan Denver, Colorado; GIH: Gujarati Indians in Houston, Texas; JPT: Tokyo, Japan; LWK: Luhya in Webuye, Kenya; MEX: Mexican ancestry in Los Angeles, California; MKK: Maasai in Kinyawa, Kenya; TSI: Tuscans in Italy; YRI: Yoruba in Ibadan, Nigeria.
CSF measurements
Cerebrospinal fluid was acquired by lumbar puncture and stored at −80°C in the ADNI Biomarker Core laboratory at the University of Pennsylvania Medical Center. CSF Aβ42 was measured using the xMAP Luminex platform and Innogenetics/Fujirebio AlzBio3 immunoassay kits. Additional information can be found at http://adni.loni.usc.edu/methods/biomarker-analysis. Longitudinal CSF Aβ42 information was collected in this GWAS with at least one year follow-up measurements. The annualized percent changes in CSF Aβ42 were based on the measurements in each follow-up. The annualized percent changes in CSF Aβ42 were calculated as follows:

Where Y= the annualized percent changes in CSF Aβ42; n = the number of measurements in CSF Aβ42 (when n = 1 indicates the baseline measurement); ak= the CSF Aβ42 level at the kth measurement (a1 indicates the baseline level). Extreme outliers (annualized percentage change > four standard deviations from the sample mean) were excluded to limit the potential bias for spurious associations. This step removed one additional individual, resulting 321 available subjects. Participants were categorized into amyloid-positive and amyloid-negative groups using the cut-off values that were ± 5% from the original cut-offs to avoid borderline effects [44, 45]. The cut-offs used in this study were: CSF amyloid-positive < 182.4ng/L, CSF amyloid-negative > 201.6ng/L; PET amyloid-positive > 1.1655 standardized uptake value ratio (SUVR), PET amyloid-negative < 1.0545 SUVR.
Genotyping and quality control
Genotyping was performed according to the manufacturer’s protocol using blood DNA samples with the Illumina Human610-Quad BeadChip (Illumina Inc) or theIllumina HumanOmniExpressBeadChip (Illumina Inc). Stringent quality control assessment was performed on samples using the PLINK v1.9 software with the following criteria: minimum call rate for SNPs and subjects > 95%, minimum allele frequencies (MAF) > 0.1, Hardy-Weinberg equilibrium (HWE) test p > 0.001. The final data set included 1040042 SNPs and 321 participants. APOE alleles which were defined by rs7412 and rs429358 were genotyped separately by an APOE genotyping kit [17].
Statistical analysis
GWAS was performed using liner regression analysis under the assumption of the additive genetic model in PLINK v1.9 software. Covariates included baseline age, genders, education level, baseline diagnosis (MCI or CN), follow-up duration and APOE ε4 status and two principal component factors. We included APOE ε4 as a covariate in the GWAS for limiting the effects on APOE ε4 genotype. The suggestive association threshold was p < 1×10−5and the conservative significance threshold for genome-wide significance was p < 1×10−8 [46]. The Manhattan plot and Q-Q plot were generated with the qqman package in R software (Version 3.4.3). Regional association plots were obtained using LocusZoom web tool (http://locuszoom.org/).
Additional analyses were performed to test the association between genotypes of the top SNP and longitudinal changes in other AD-related phenotypes (included cognitive performance and hippocampal structure) for ADNI participants using linear mixed effects model implemented in R software (version 3.4.3). The linear mixed model interprets the correlation structure with in each time sequence of measurements in each participant, and it permits different follow-up periods and varying rates among individuals. Longitudinal changes in cognitive performance were assessed by Alzheimer Disease Assessment Scale-cognitive subscale (ADAS-cog 11). This assessment scale is known to be sensitive measures of cognitive decline and it was acquired longitudinally from the ADNI dataset. Longitudinal changes of hippocampus volume were acquired using T1-weighted structural MRI scans with a sagittal volumetric magnetization-prepared rapid acquisition gradient echo sequence. All outcome variables in linear mixed model were standardized to z scores to facilitate comparisons between modalities, and statistical significance was set at the threshold of p < 0.05.
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
The authors have no conflicts of interest to disclose.
The work was funded by the National Key R&D Program of China (2016YFC1305803,2018YFC1314700), the National Natural Science Foundation of China (91849126, 81471309, 81571245, and 81771148), Shanghai Municipal Science and Technology Major Project (No.2018SHZDZX01) and ZHANGJIANG LAB, Tianqiao and Chrissy Chen Institute, and the State Key Laboratory of Neurobiology and Frontiers Center for Brain Science of Ministry of Education, Fudan University.