Abstract
Nutrient oversupply and mitochondrial dysfunction play central roles in nonalcoholic fatty liver disease (NAFLD). The mitochondria are the major sites of β-oxidation, a catabolic process by which fatty acids are broken down. The mitochondrial quality control (MQC) system includes mitochondrial fission, fusion, mitophagy and mitochondrial redox regulation, and is essential for the maintenance of the functionality and structural integrity of the mitochondria. Excessive and uncontrolled production of reactive oxygen species (ROS) in the mitochondria damages mitochondrial components, including membranes, proteins and mitochondrial DNA (mtDNA), and triggers the mitochondrial pathway of apoptosis. The functionality of some damaged mitochondria can be restored by fusion with normally functioning mitochondria, but when severely damaged, mitochondria are segregated from the remaining functional mitochondrial network through fission and are eventually degraded via mitochondrial autophagy, also called as mitophagy. In this review, we describe the functions and mechanisms of mitochondrial fission, fusion, oxidative stress and mitophagy in the development and progression of NAFLD.
Introduction
The continuous intake of excess dietary fat without consumption of excessive alcohol is one of the main causes of non-alcoholic fatty liver disease (NAFLD), which includes a spectrum of liver pathologies such as steatosis, steatohepatitis, fibrosis and cirrhosis. Non-alcoholic steatohepatitis (NASH) is a liver disease that resembles the histology of alcoholic hepatitis, but occurs without the consumption of excessive alcohol. It represents one of the stages of NAFLD. More than 83.1 million people in the United States are diagnosed with NAFLD and 27% of these patients exhibit symptoms of NASH [1]. About 2%–3% subset of patients with NAFLD develop NASH, and 5%–8% of NASH patients develop liver cirrhosis within five years [2].
Hepatic steatosis occurs because of imbalance between hepatic lipid uptake, de novo lipogenesis, and lipid clearance [3]. As shown in Figure 1, lipid metabolism is primarily regulated by the mitochondria, which are enriched in the liver parenchyma cells [4]. Mitochondria are the powerhouse of eukaryotic cells, producing 90% of the cellular energy through oxidative phosphorylation (OXPHOS) in the form of adenosine triphosphate (ATP). Mitochondria are also major sites of reactive oxygen species (ROS) and regulate oxidative programmed cell death or apoptosis. Mitochondrial dysfunction in the hepatocytes is frequently associated with metabolic disturbances observed in fatty liver diseases. For example, chronic lipid consumption alters the status of mitochondrial oxidative phosphorylation in the hepatocytes by suppressing the activity and expression of OXPHOS complex proteins in the mitochondria. In NAFLD, the function and structure of the hepatocyte mitochondria is significantly altered.
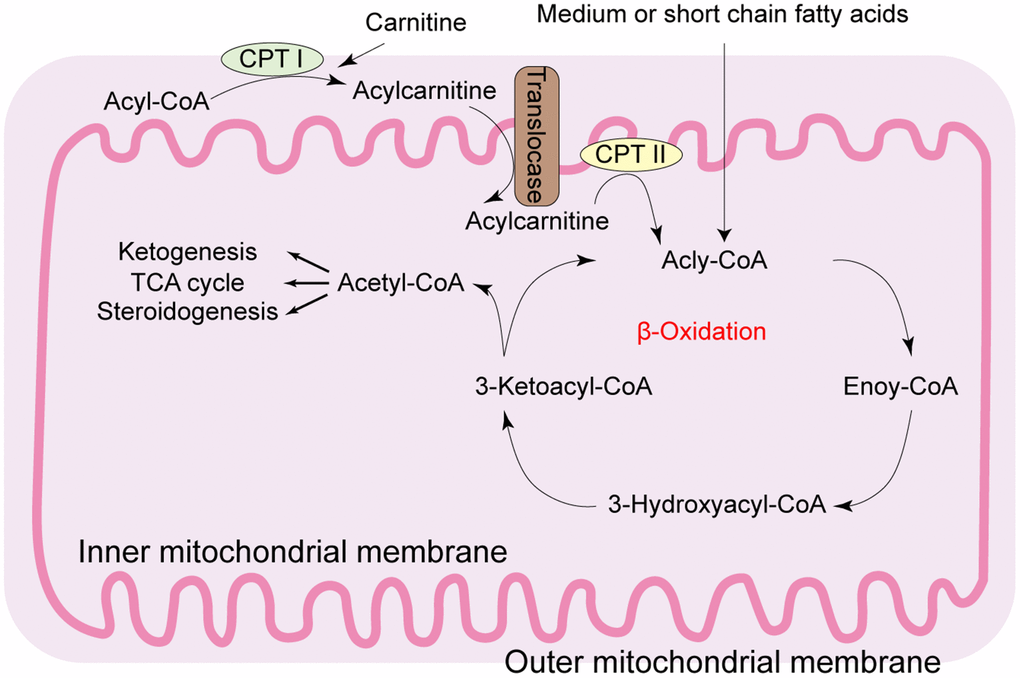
Figure 1. Diagrammatic representation of the mitochondrial fatty acid β-oxidation. During β-oxidation in the mitochondria, free fatty acids (FFAs) undergo step-wise enzymatic dehydrogenation, hydration, a second dehydrogenation, and thiolysis to generate a single 2-carbon acetyl-CoA molecule and a shortened fatty acid. The cycle is repeated until the fatty acid is completely broken down into its constituent acetyl-CoA subunits. The acetyl-CoA molecules enter the citric acid cycle to produce energy-rich NADH and FADH2 molecules that are then converted to ATP in the electron transport chain. Under fasting conditions, acetyl-CoA molecules are converted into ketone bodies (acetoacetate and β-hydroxybutyrate), which are released by the liver to be oxidized in peripheral tissues by the tricarboxylic acid cycle. CPT: carnitine palmitoyl transferase; TCA: tricarboxylic acid.
The mitochondrial quality control (MQC) mechanism involves intricate regulation of several processes such as proteostasis, biogenesis, dynamics, and mitophagy, all of which are integral to maintaining cellular homeostasis as shown in Figure 2 [5–7]. The failure of the quality control processes results in mitochondrial dysfunction and is one of the underlying causes for NAFLD [8]. MQC involves coordinated regulation of a hierarchical network of pathways that act sequentially from an individual protein molecule to the whole organelle [9–11]. Antioxidant systems are the primary line of defense to maintain a functional mitochondrial redox environment and prevent oxidative damage [12, 13]. Mitochondria are highly dynamic organelles that constantly undergo fusion and fission, which are critical for mitochondrial homeostasis, mtDNA inheritance and intracellular distribution of the mitochondria [14–17]. Tight regulation of mitochondrial fission and fusion is required to constantly adapt to altering physiological needs [18–20]. When individual mitochondria or their constituents such as OXPHOS protein complexes and lipids are irreversibly damaged, they are degraded through mitophagy, a lysosome-dependent proteolytic system in order to maintain cellular homeostasis [21–24]. When sustained oxidative insults overwhelm the MQC mechanisms, it will result in significant mitochondrial injury that will detrimental to the function and survival of the hepatocytes. Preclinical evidence suggests that modulation of MQC is therapeutically beneficial against NAFLD/NASH [25–27]. In this review, we discuss recent studies regarding the involvement of MQC processes including mitochondrial fission, mitochondrial fusion, mitophagy, and mitochondrial oxidative stress in fatty liver diseases such as NAFLD and NASH. Elucidation of the molecular mechanisms underlying the defective MQC mechanisms is critical for the design of effective therapeutic strategies to prevent or cure fatty liver diseases.
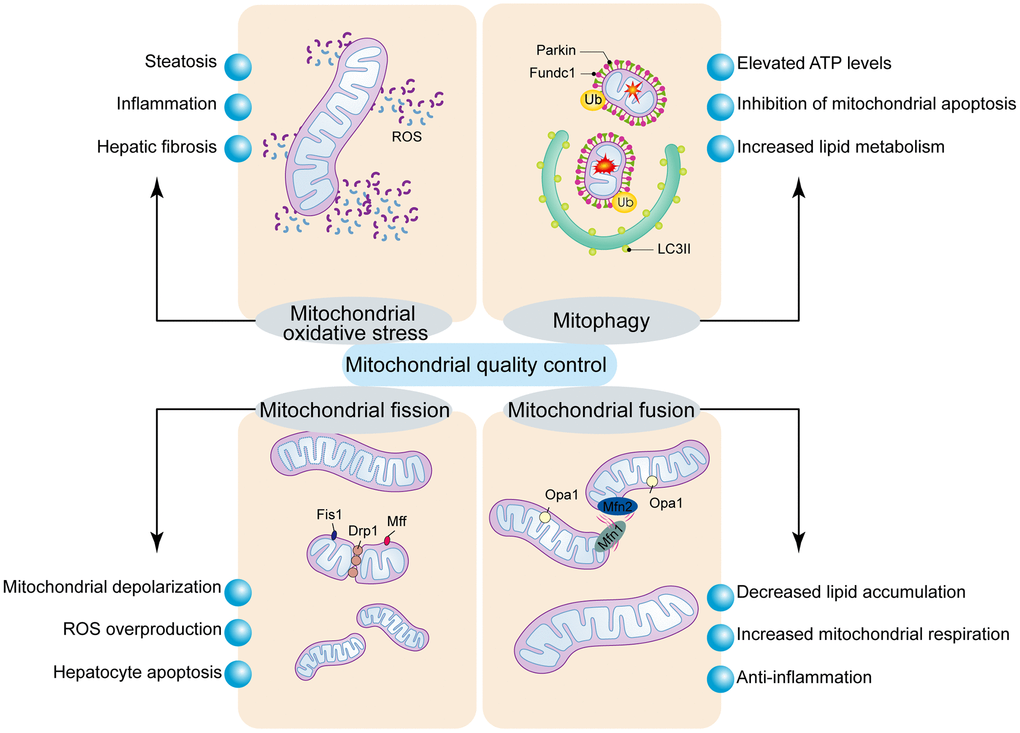
Figure 2. Regulatory mechanism of mitochondrial quality control. Mitochondrial oxidative stress induces mitochondrial dysfunction and hepatocyte apoptosis. Mitochondrial fission is modulated by Drp1 and its receptors, Fis1 and Mff. Excessive mitochondrial fission induces mPTP opening and mitochondrial dysfunction, which results in the activation of mitochondrial pathway of cellular apoptosis. Mitochondrial fusion is regulated by Mfn1/2 and Opa1, and stabilizes the mitochondrial membrane potential and blocks the mitochondrial pathway of apoptosis. Mitophagy is a process that breaks down damaged mitochondria and is controlled by Parkin or Fundc1.
Oxidative Stress
Several anti-oxidative factors such as glutathione (GSH), superoxide dismutase (SOD) and glutathione peroxidase (GPx) regulate the levels of ROS that are mainly generated by the mitochondrial respiratory chain [28–31]. In the cytosol, enzymes such as amino acid oxidases, cyclooxygenases, lipoxygenases, nitric oxide (NO) synthase, and xanthine oxidase also generate ROS such as superoxide anions, peroxides and others [32–34]. ROS modulate several specific signaling pathways that regulate several cellular functions as well as pathological mechanisms in several human diseases [35–38]. The cyclooxygenases and lipoxygenases generate superoxide anions that drive arachidonic acid metabolism and inflammation, which play an important role in several cancers, whereas, the xanthine oxidase is implicated in oxidative stress during ischemia/reperfusion injury [39–41]. The oxidants generated by the sulfhydryl oxidases in the endoplasmic reticulum (ER) are required for disulfide bond formation, protein folding, and the assembly and secretion of proteins through the secretory pathway. Several peroxisomal oxidases generate hydrogen peroxide during the oxidative activities in the peroxisomes [42–44]. NADPH oxidases, the main source of ROS in several liver diseases, generate superoxide anions in the mitochondria by transferring a single electron from NADPH to molecular oxygen [45–47].
Emerging evidence suggests that the main site of superoxide generation in the mitochondria is the flavin mononucleotide group of complex I through reverse electron transfer, consistent with data that shows inhibition of succinate-related ROS generation by diphenyleneiodonium without affecting the flavin group of complex II [48–50]. Moreover, complex III of the mitochondrial respiratory chain generates ROS species through the ubiquinone-reactive sites, Q0 and Qi [51, 52]. The redox activity of 66-kDa Src homology 2 domain-containing protein (p66Shc) within mitochondria has been shown to directly generate hydrogen peroxide through oxidation of cytochrome c without intermediate formation of superoxide anion [53, 54]. The apoptosis-inducing factor (AIF) demonstrates NADH oxidase activity and generates superoxide and hydrogen peroxide [40, 55].
The high-fat diet-induced NAFLD is characterized by increased intracellular lipid accumulation, high mitochondrial ROS and hepatocyte apoptosis as shown in Figure 2 [56]. Mitochondrial oxidative stress also promotes lipid peroxidation in hepatocytes and enhances expression of hepatic CC-chemokines, which promote infiltration of CCR5-positive cells and activation of myofibroblasts resulting in extensive liver fibrosis [57]. The mitochondria in NAFLD/NASH patients exhibit increased rate of proton leakage because of upregulation and activity of the uncoupling protein-2 or UCP2 [58]. Moreover, altered activity of the mitochondrial ATP-sensitive potassium channels (mitoKATP) is associated with higher respiration rate and increased ROS generation [59]. Furthermore, in NAFLD patients, elevated mitochondrial permeability transition pore (mPTP) opening contributes to mitochondrial oxidative stress and apoptosis of hepatocytes [60]. These data suggest that mitochondrial oxidative stress plays an important role in hepatocyte apoptosis, steatosis, liver fibrosis, hepatic inflammation, and other pathophysiological changes that are involved in the development and progression of NAFLD.
Attenuation of mitochondrial oxidative stress is a promising strategy to reduce mitochondrial damage and slow the progression of NAFLD. Administration of mitoquinone mesylate (MitoQ), a mitochondrial-targeted antioxidant with high bioavailability, restores hepatic mitochondrial functions and ameliorates glucose intolerance and hepatic steatosis [61–63]. Antioxidant foods including blueberry juice and probiotics significantly reduce NAFLD-induced mitochondrial swelling and hepatic necrosis by restoring the mitochondrial respiratory chain function and suppressing the production of ROS [64]. Pretreatment with methionine alleviates the pathological changes associated with NAFLD by increasing GSH levels [60].
Several signaling pathways are related to mitochondrial oxidative stress and are potential targets to restoring mitochondrial respiratory functions [65–67]. Pharmacological activation of AMP-activated protein kinase α2 (AMPKα2) attenuates mitochondrial oxidative stress in NAFLD by increasing mitochondrial biogenesis and lipid oxidation [68]. Activation of the c-Jun N-terminal kinase-1 (JNK1) contributes to increased mitochondrial oxidative stress (as assessed by the GSSG:GSH ratio) and decreased ATP levels, which trigger mPTP opening and hepatocyte apoptosis [69]. Aberrant regulation of the Sirt1/STAT3 signaling pathway promotes mitochondrial damage and elevated production of ROS in NAFLD [70]. The noncanonical KEAP1-NFE2L2 pathway is a potential therapeutic target in NAFLD because it promotes antioxidative response against lipotoxicity in the hepatocytes and the mouse liver tissues [71]. Mitochondrial ROS production is also regulated by the Ras/Erk signaling pathway in NAFLD [72]. Thus, results from mouse models of mutant mitochondrial OXPHOS-related genes and pharmacological targeting of β-oxidation reveal that modulation of mitochondrial ROS and fatty acid oxidation can prevent metabolic dysfunction and hepatic pathology in NAFLD.
Mitochondrial Fission
Mitochondria are dynamic organelles that are constantly changing their structure and shape through fusion and fission processes in response to changes in energy demand and supply [73, 74]. As shown in Figure 2, the changes in mitochondrial dynamics are associated with cell viability, apoptosis, and bioenergetic adaptations [75, 76]. Mitochondrial fission is observed when mitochondria are subjected to oxidative stress-induced damage and segregates damaged mitochondria from the normal ones [77–80]. The mitochondrial fusion- fission balance is disrupted by intracellular and extracellular stress, and the fragmented mitochondria form small spheres or short rods in comparison to extensive elongated network of the normal mitochondria [20, 81]. During the last decade, we have gained significant insights into the molecular basis of mitochondrial dynamics in relation to several biological processes such as apoptosis, autophagy, metabolism, development, and aging [82–84]. The molecular machinery governing mitochondrial dynamics was initially discovered in budding yeasts and Drosophila [85]. Subsequently, mammalian orthologs of key proteins involved in mitochondrial fission such as mitochondrial fission 1 (Fis1), dynamin-related protein 1 (Drp1), mitochondrial fission factor (Mff), mitochondrial dynamics proteins of 49 kDa and 51 kDa (MiD49 and MiD51) were discovered [86–89].
Activated Drp1 forms spiral-like structures upon oligomerization at the fission sites of the outer mitochondrial membrane (OMM) after being recruited from the cytosol and returns back to the cytosol upon completion of the fission process [20, 90–93]. Drp1 lacks the lipid-binding pleckstrin homology domain and interacts with other mitochondrial-resident proteins to drive the fission process [94]. Recent reports suggest that proteins such as Mff, Fis1, Mid49, and Mid51 recruit Drp1 to the outer mitochondrial membrane [95–97]. Fis1 is distributed throughout the outer membrane, whereas, Mff and MiDs demonstrate punctate organization along the mitochondrial tubules and exhibit stronger interactions with Drp1 compared to Fis1 [98, 99]. Fis1 and Mff independently contribute to Drp1 recruitment and oligomerization at the outer mitochondrial membrane, with Mff playing a more predominant role [100]. Furthermore, MiD49 and MiD51 can recruit Drp1 to the mitochondria in the absence of Mff and Fis1 [101, 102]. Dysregulation of the proteins involved in mitochondrial fission significantly alter the mitochondrial morphology and impair mitochondrial function [103, 104].
The levels of Fis1 and Drp1 proteins are reduced in the NASH model mice fed with Western diet for more than 2 months and accompanied by hepatic inflammation and liver fibrosis [105]. Overexpression of isocitrate dehydrogenase 2 (IDH2) reduces Drp1 and Fis1 levels in the hepatocytes and prevents NASH progression [106]. High-fat diet induces Drp1-related mitochondrial fission and reduces the levels of anti-inflammatory cytokines such as interleukin-10 (IL-10) and IL-13, thereby suggesting a role for mitochondrial fission in hepatic inflammation [107]. Decreasing mitochondrial fission alters the expression of genes involved in lipid metabolism and alleviates hepatic steatosis in a murine model of NAFLD [108]. Moreover, uncontrolled mitochondrial fission triggers hepatic fibrosis and liver inflammation resulting in increased hepatocyte death through the caspase-9-related apoptotic pathway [109]. Therefore, decreasing mitochondrial fission represents a novel therapeutic target for NAFLD.
Drp1-related mitochondrial fission is regulated by multiple mechanisms [110–113]. In NAFLD, p53 induces Drp1-related mitochondrial fission and mitophagy arrest, which results in mitochondrial dysfunction through mPTP opening, reduced mitochondrial potential, oxidative stress, calcium overload, and ATP depletion [109]. Besides, Drp1 transcript levels are regulated by the SIRT1/SIRT3-FOXO3a signaling pathway [114]. Moreover, nutrient stimuli promote post-transcriptional phosphorylation of Drp1 at Ser616 in the hepatocytes [115].
Several drugs regulate the activity of Drp1-related mitochondrial fission, but, their efficacy for the treatment of NAFLD remains to be established. Pharmacological doses of a first-line diabetic drug, metformin, activates the AMPK signaling pathway and promotes mitochondrial fission in the HFD-fed mice resulting in increased mitochondrial respiration, normalized mitochondrial membrane potential and upregulated ATP levels [116]. Mdivi-1, an inhibitor of Drp1-related mitochondrial fission, promotes apoptosis of hepatocytes by inhibiting mitochondrial depolarization and increasing ROS levels [117, 118]. Irisin is a newly discovered hormone secreted by muscle tissue that blocks mitochondrial fission and alleviates liver ischemia-reperfusion injury [119]. Resolvin D1 (RvD1), a specialized pro-resolving lipid mediator with anti-inflammatory and antioxidant activities, protects the liver against ischemia-reperfusion injury by suppressing Drp1-related mitochondrial fission [120].
Mitochondrial Fusion
Mitochondrial fusion in mammalian cells is mediated primarily by mitofusin-1 or Mfn1 and mitofusin-2 or Mfn2 [16, 121, 122]. Mfn1 proteins tether two opposing mitochondria through their individual HR2 domains [123, 124]. Mfn2 proteins oligomerize with either other Mfn2 proteins or with Mfn1 proteins to promote mitochondrial fusion. Moreover, Mfn2 promotes physical interactions between the ER and the mitochondria, which is essential for Ca2+ signaling [18, 125, 126]. The fusion of the inner mitochondrial membrane (IMM) is regulated by optic atrophy 1 (Opa1), which plays a critical role in maintaining the balance between mitochondrial fusion and fission [121, 127]. Opa1 is processed by the mitochondrial-processing peptidase (MPP) to generate a membrane bound long isoform (L-Opa1), which is further cleaved into the short isoform (S-Opa1) by the IMM peptidases such as the yeast mitochondrial DNA escape 1-like (YME1L) protease and the m-AAA protease, OMA1 [128, 129]. The IMM-bound L-Opa1 is required for fusion, whereas, the stress-activated soluble S-Opa1 limits fusion and promotes fission [130]. The levels of L-Opa1 and S-Opa1 are regulated by YME1L and OMA1 concentrations under basal conditions [131, 132]. The inhibition of mitochondrial fusion in the mouse embryonic fibroblasts through the suppression of Mfn1 and Mfn2 gene expression induces severe growth defects accompanied by changes in the mitochondrial membrane potential and decreased respiration [133, 134].
Fusion allows damaged mitochondria with oxidized lipids, proteins, and mutant mitochondrial DNA, and aberrant mitochondrial membrane potential to mix with healthy ones. This helps restore mitochondrial functions and maintain cellular homeostasis. However, mitochondria that suffer significant loss of membrane potential do not fuse and are subsequently degraded by mitophagy [135–138]. Moderate fusion prevents autophagy of mitochondria during nutrient starvation and excessive fusion of mitochondria inhibits mitophagy [139, 140]. Therefore, fusion of mitochondria is a compensatory and protective mechanism to fix the functional defects in some portions of the mitochondria in the mammalian cells. Metabolic changes and the status of energy supply determine the rate of mitochondrial fusion in the mouse embryonic fibroblasts [141].
Mfn1 expression is decreased in the hepatocytes in response to a high-fat diet and correlates with steatohepatitis [106]. The pro-inflammatory CXCR3 protein induces mitochondrial dysfunction in the NASH model mice by decreasing Mfn1 protein levels [142]. Moreover, reduced Mfn2 levels are observed in the liver biopsies from patients with NASH and in the mouse models of steatosis and NASH; Mfn2 re-expression ameliorates the disease in the NASH model mice, whereas, liver-specific deletion of Mfn2 induces inflammation, triglyceride accumulation, fibrosis, and liver cancer [143]. Moreover, reduced Mfn2 levels are also detected in mouse models of steatosis or NASH, and its re-expression in a NASH mouse model ameliorates the disease progression [143–145]. The inverse correlation between Mfn2 and fatty liver disease progression suggests that mitochondrial fusion is involved in the pathophysiology of fatty liver disorders. Mechanistically, Mfn2 specifically binds and extracts phosphatidylserine (PS) into the mitochondrial membrane domains and promotes mitochondrial phosphatidylethanolamine (PE) synthesis [143]. Consequently, hepatic Mfn2 deficiency reduces PS transfer and phospholipid biosynthesis, and promotes endoplasmic reticulum (ER) stress resulting in liver disease such as NASH and liver cancer; ablation of Mfn2 in the liver disrupts of ER-mitochondrial PS transfer [143]. The mice fed with a high-fat diet also show reduced expression of Opa1, but, this can be reversed by treatment with liraglutide, an anti-diabetic drug [114] Hepatic-specific ablation of Opa1 increases the risk of HFD-induced NAFLD [146]. Mechanistically, the proteolytic cleavage of L-Opa1 is increased in the liver by prohibitin-2 deficiency [146]. These molecular alterations are associated with lipid accumulation, decreased gluconeogenesis, and extensive liver damage. However, re-expression of L-Opa1 through adenovirus delivery restores the function of mitochondria in the hepatocytes and protects against NAFLD [146].
Re-activation of mitochondrial fusion protects against fatty liver disease. Exercise counteracts NASH by improving Mfn1 and Mfn2 expression, which promotes mitochondrial fusion and helps maintain mitochondrial homeostasis in the high-fat diet fed Sprague-Dawley rats [147]. This suggests that normalized mitochondrial fusion may preserve liver function under high-fat stress. Bitter gourd (BG) is a popular fruit in Asia with several medicinal properties. Bitter gourd intake increases Mfn1 expression, superoxide dismutase activity and mitochondrial respiratory function, and decreases sterol regulatory element binding protein/fatty acid synthase (SREBP-1/FAS) pathway activation [148]. Besides, salvianolic acid B is an anti-oxidant derived from Salvia miltiorrhiza that lowers ALT, AST, TG, and TC levels in high-fat diet fed mice [149]. The histological characteristics of inflammation and steatosis are also attenuated by salvianolic acid B by enhancing Mfn2-related mitochondrial fusion and reducing mitochondria-related hepatocyte death [149].
Mitophagy
Autophagy is a mechanism through which eukaryotic cells recycle misfolded or aggregated proteins and damaged organelles such as the mitochondria through the lysosomes to generate amino acids, glucose, and fatty acids [150–154]. The macroautophagy process by which the mitochondria undergo degradation is called mitophagy [155, 156]. As shown in Figure 2, mitophagy plays a significant role in maintaining mitochondrial and cellular homeostasis during hepatic stress, and reduced mitophagy is associated with mitochondrial dysfunction and liver failure [157]. The best studied mechanism of mitophagy is the one mediated by PINK1 and Parkin proteins [74, 158–160]. In healthy mitochondria, PINK1 is degraded by the matrix processing peptidase (MPP) and the presenilin-associated rhomboid-like (PARL) protease [161]. When mitochondria are depolarized, stabilized PINK1 accumulates on the outer mitochondrial membrane, phosphorylates Mfn2 at Thr 11 and Ser 442, and recruits Parkin onto the OMM [162]. Alternatively, PINK1 promotes recruitment of Parkin to the OMM by phosphorylating ubiquitin and Parkin [20]. Cytosolic Parkin translocates to the OMM when deubiquitinated by USP8 [163]. BAG3 translocates to the OMM with Parkin, but, this process is prevented by physical interaction between Parkin and p53 [164]. Parkin ubiquitinates proteins on the OMM of depolarized mitochondria and promote the interaction of ubiquitinated OMM proteins with the mitophagy adaptor proteins such as p62, NBR1, and HDAC6, all of which contain an ubiquitin binding domain and a LC3-interacting region [165]. Finally, LC3 facilitates mitophagy when recruited by the adaptor proteins. Parkin-mediated ubiquitination of OMM proteins can be reversed by USP15 and USP30. PINK1 also promotes localization of LC3 receptors such as optineurin, NDP52, and TAXBP1, which recruit LC3 and facilitate mitophagy [166].
Several LC3 receptors are located on the mitochondria that can directly bind to LC3 and recruit damaged mitochondria to the autophagosomes. Proteins such as Nip3-like protein X (NIX) and BCL2/Adenovirus E1B 19 kDa Interacting Protein 3 (Bnip3) contain a BH3 domain that interacts with LC3 [167, 168]. NIX is required for the removal of mitochondria from reticulocytes during the process of maturation and form functional red blood cells [169]. Moreover, under hypoxic conditions, hypoxia-inducible factor-1α (HIF1α) increases NIX mRNA levels and the NIX protein is phosphorylated at Ser81 to mediate mitophagy [140]. Furthermore, NIX participates in Parkin-dependent mitophagy by recruiting NBR1 to the mitochondria and the knockdown of both Parkin and NIX synergistically reduces the levels of mitophagy [170, 171]. Homodimerization of Bnip3 is necessary for its interaction with LC3, which is further regulated by Ser17 and Ser24 phosphorylation near the LIR motif [172]. FUN14 domain-containing protein 1 (Fundc1) is a mitochondrial outer membrane protein that mediates hypoxia-induced Parkin-independent mitophagy in mammalian cells by binding to LC3; moreover, LC3 is regulated through the phosphorylation of Fundc1 at Ser17 by Unc-51 like autophagy activating kinase-1 (Ulk1) and Ser13 by casein kinase II or CK2 [14, 159, 173, 174].
Bnip3 expression is highly induced upon fasting in the liver of high-fat diet fed adult mice [175, 176]. Moreover, Bnip3 silencing increases lipid biosynthesis in the liver and is accompanied by elevated ATP levels, reduced AMP-regulated kinase (AMPK) activity, and increased expression of lipogenic enzymes [175, 176]. Conversely, in the liver of fasting Bnip3 null mice, β-oxidation of fatty acids and hepatic glucose output is reduced, and mechanistically linked to increased mitochondrial mass and hepatocellular respiration in the presence of glucose [177, 178]. The Bnip3 null mice liver mitochondria exhibit lower mitochondrial membrane potential, abnormal structure, and reduced oxygen consumption, and are associated with increased ROS, inflammation, and steatohepatitis-like features [177]. These results demonstrate that Bnip3-related mitophagy maintains mitochondrial integrity in the liver by decreasing mitochondrial mass, and plays a significant role in the regulation of lipid metabolism and liver disease. The role of Parkin-related mitophagy was first reported in alcoholic liver disease. Alcohol intake significantly elevated mitochondrial damage and oxidative stress in the livers of Parkin-knockout (KO) mice compared to the wild type [179]. Besides, the liver mitochondria of Parkin KO mice were swollen and lacked cristae compared to normal mitochondrial structure observed in the livers of WT mice [179]. These findings were subsequently confirmed in the high-fat diet fed NAFLD model mice, wherein, the expression of PINK1 and Parkin was significantly downregulated and associated with activation of the mitochondria-related apoptotic pathway and mPTP opening [147]. Metformin suppressed p53-mediated hepatocyte apoptosis in response to HFD through Parkin-induced mitophagy [180]. Acyl-CoA lysocardiolipin acyltransferase-1 (ALCAT1) catalyzes cardiolipin remodeling, which is involved in the pathology of several aging-related diseases [181]. Mitophagy arrest and mitochondrial dysfunction in NAFLD is reversed by the targeted deletion of ALCAT1 [181]. ALCAT1 deficiency also improves mitochondrial architecture, mitochondrial DNA (mtDNA) fidelity, oxidative phosphorylation, and other pathological changes induced by NAFLD [181]. These results suggest that mitophagy protects against NAFLD/NASH. Furthermore, mitochondrial fission is important for mitophagy induction in the liver hepatocytes and other cell types [135, 182]. However, excessive mitochondrial fission triggers hepatocyte death by inducing mitochondrial damage. Therefore, the relationship between mitochondrial fission and mitophagy in NAFLD needs to be further investigated. Recent study shows that exposure to alcohol increases mitochondrial fission and inhibits mitophagy, thereby suggesting that mitophagy is independent of mitochondrial fission in the alcoholic liver disease [183]. Moreover, in the renal ischemia reperfusion injury model, mitophagy is repressed and mitochondrial fission is activated, resulting in mitochondrial fragmentation [159, 184], Interestingly, induction of mitophagy removes damaged mitochondria and blocks mitochondrial fission [109]. This suggests that mitophagy corrects excessive mitochondrial fission, but, this concept needs to be further validated in the NAFLD or NASH model.
Conclusion and Future Perspectives
Oxidation of fatty acids in the mitochondria is one of the main sources of energy for eukaryotic cells. Dysregulated fatty acid oxidation (FAO) in the mitochondria contributes to fat accumulation and hepatic steatosis. Mitochondria are dynamic organelles that continuously undergo fusion and fission. Fusion involves joining of the inner and outer mitochondrial membranes of two mitochondria, whereas, fission generates two metabolically distinct daughter mitochondria that may either maintain or lose their membrane potential. Excessive mitochondrial fission is an early event that triggers hepatocyte death by inducing mitochondrial dysfunction. In response to mitochondrial damage, depolarized mitochondria are targeted for degradation by autophagy (mitophagy). If mitochondrial damage is too severe to be fixed by the intracellular protective mechanisms, the dysfunctional organelles break open and release several activated apoptotic factors that trigger programmed cell death. Therefore, the dynamic nature of the double-membrane-bound organelles as well as mitophagy is critical for maintaining their function, structure, and the inheritance of mitochondrial DNA. Taken together, reduced levels of β-oxidation and increased lipogenesis results in lipid accumulation in the hepatocytes that generates excessive ROS and hepatocyte injury that further drives hepatic inflammation and fibrosis. The mitochondrial quality control mechanisms restore mitochondrial function and prevent the progression of NAFLD.
The mitochondrial unfolded protein response (UPRmt) pathway represents a retrograde mitochondria-to-nucleus signaling pathway induced by mitochondrial proteotoxic stress [185]. This pathway was first identified in the late 1990s, and is an area of considerable scientific research using the worm and mammalian models of human aging-related degenerative diseases and metabolic disorders [186, 187]. The molecular mechanisms underlying UPRmt in the progression of NAFLD are not well understood and needs further investigation.
Conflicts of Interest
All the authors declare that they have no conflicts of interest.
Funding
This work was supported by funds from the China Postdoctoral Science Foundation (Grant No. 2019TQ0128) and the National Science Foundation of China (Grant Nos. 81900252, 81770261, 81900254 and 91749128).
References
-
1.
Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018; 67:123–33. https://doi.org/10.1002/hep.29466 [PubMed]
-
2.
Younossi ZM. Non-alcoholic fatty liver disease - A global public health perspective. J Hepatol. 2019; 70:531–44. https://doi.org/10.1016/j.jhep.2018.10.033 [PubMed]
-
3.
Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, Wai-Sun Wong V, Yilmaz Y, George J, Fan J, Vos MB. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology. 2019; 69:2672–82. https://doi.org/10.1002/hep.30251 [PubMed]
-
4.
Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019; 70:151–71. https://doi.org/10.1016/j.jhep.2018.09.014 [PubMed]
-
5.
Larson-Casey JL, He C, Carter AB. Mitochondrial quality control in pulmonary fibrosis. Redox Biol. 2020. [Epub ahead of print]. https://doi.org/10.1016/j.redox.2020.101426 [PubMed]
-
6.
Vazquez-Calvo C, Suhm T, Büttner S, Ott M. The basic machineries for mitochondrial protein quality control. Mitochondrion. 2020; 50:121–31. https://doi.org/10.1016/j.mito.2019.10.003 [PubMed]
-
7.
Tang C, Han H, Liu Z, Liu Y, Yin L, Cai J, He L, Liu Y, Chen G, Zhang Z, Yin XM, Dong Z. Activation of BNIP3-mediated mitophagy protects against renal ischemia-reperfusion injury. Cell Death Dis. 2019; 10:677. https://doi.org/10.1038/s41419-019-1899-0 [PubMed]
-
8.
Sheldon RD, Meers GM, Morris EM, Linden MA, Cunningham RP, Ibdah JA, Thyfault JP, Laughlin MH, Rector RS. eNOS deletion impairs mitochondrial quality control and exacerbates Western diet-induced NASH. Am J Physiol Endocrinol Metab. 2019; 317:E605–16. https://doi.org/10.1152/ajpendo.00096.2019 [PubMed]
-
9.
Breda CN, Davanzo GG, Basso PJ, Saraiva Câmara NO, Moraes-Vieira PM. Mitochondria as central hub of the immune system. Redox Biol. 2019; 26:101255. https://doi.org/10.1016/j.redox.2019.101255 [PubMed]
-
10.
Mitchell T, Chacko B, Ballinger SW, Bailey SM, Zhang J, Darley-Usmar V. Convergent mechanisms for dysregulation of mitochondrial quality control in metabolic disease: implications for mitochondrial therapeutics. Biochem Soc Trans. 2013; 41:127–33. https://doi.org/10.1042/BST20120231 [PubMed]
-
11.
Zhou XL, Wu X, Xu QR, Zhu RR, Xu H, Li YY, Liu S, Huang H, Xu X, Wan L, Wu QC, Liu JC. Notch1 provides myocardial protection by improving mitochondrial quality control. J Cell Physiol. 2019; 234:11835–41. https://doi.org/10.1002/jcp.27892 [PubMed]
-
12.
Kowaltowski AJ. Strategies to detect mitochondrial oxidants. Redox Biol. 2019; 21:101065. https://doi.org/10.1016/j.redox.2018.101065 [PubMed]
-
13.
Aanhane E, Schulkens IA, Heusschen R, Castricum K, Leffler H, Griffioen AW, Thijssen VL. Different angioregulatory activity of monovalent galectin-9 isoforms. Angiogenesis. 2018; 21:545–55. https://doi.org/10.1007/s10456-018-9607-8 [PubMed]
-
14.
Zhou H, Toan S. Pathological Roles of Mitochondrial Oxidative Stress and Mitochondrial Dynamics in Cardiac Microvascular Ischemia/Reperfusion Injury. Biomolecules. 2020; 10:10. https://doi.org/10.3390/biom10010085 [PubMed]
-
15.
Qiu Z, Wei Y, Song Q, Du B, Wang H, Chu Y, Hu Y. The Role of Myocardial Mitochondrial Quality Control in Heart Failure. Front Pharmacol. 2019; 10:1404. https://doi.org/10.3389/fphar.2019.01404 [PubMed]
-
16.
Ding M, Liu C, Shi R, Yu M, Zeng K, Kang J, Fu F, Mi M. Mitochondrial fusion promoter restores mitochondrial dynamics balance and ameliorates diabetic cardiomyopathy in an optic atrophy 1-dependent way. Acta Physiol (Oxf). 2019. [Epub ahead of print]. https://doi.org/10.1111/apha.13428 [PubMed]
-
17.
An F, Wang X, Yang M, Luo J, Kong L. Bioactive A-ring rearranged limonoids from the root barks of Walsura robusta.. Acta Pharm Sin B. 2019; 9:545–56. https://doi.org/10.1016/j.apsb.2019.02.009 [PubMed]
-
18.
Yu F, Abdelwahid E, Xu T, Hu L, Wang M, Li Y, Mogharbel BF, de Carvalho KA, Guarita-Souza LC, An Y, Li P. The role of mitochondrial fusion and fission in the process of cardiac oxidative stress. Histol Histopathol. 2019. [Epub ahead of print]. https://doi.org/10.14670/HH-18-191 [PubMed]
-
19.
Alicka M, Kornicka-Garbowska K, Roecken M, Marycz K. Inhibition of the Low Molecular Weight Protein Tyrosine Phosphatase (LMPTP) as a Potential Therapeutic Strategy for Hepatic Progenitor Cells Lipotoxicity-Short Communication. Int J Mol Sci. 2019; 20:20. https://doi.org/10.3390/ijms20235873 [PubMed]
-
20.
Feng ST, Wang ZZ, Yuan YH, Wang XL, Sun HM, Chen NH, Zhang Y. Dynamin-related protein 1: A protein critical for mitochondrial fission, mitophagy, and neuronal death in Parkinson’s disease. Pharmacol Res. 2020; 151:104553. https://doi.org/10.1016/j.phrs.2019.104553 [PubMed]
-
21.
Zhang M, Shi R, Zhang Y, Shan H, Zhang Q, Yang X, Li Y, Zhang J. Nix/BNIP3L-dependent mitophagy accounts for airway epithelial cell injury induced by cigarette smoke. J Cell Physiol. 2019; 234:14210–20. https://doi.org/10.1002/jcp.28117 [PubMed]
-
22.
Whang YM, Kim MJ, Cho MJ, Yoon H, Choi YW, Kim TH, Chang IH. Rapamycin enhances growth inhibition on urothelial carcinoma cells through LKB1 deficiency-mediated mitochondrial dysregulation. J Cell Physiol. 2019; 234:13083–96. https://doi.org/10.1002/jcp.27979 [PubMed]
-
23.
Zhou H, Ma Q, Zhu P, Ren J, Reiter RJ, Chen Y. Protective role of melatonin in cardiac ischemia-reperfusion injury: from pathogenesis to targeted therapy. J Pineal Res. 2018; 64:64. https://doi.org/10.1111/jpi.12471 [PubMed]
-
24.
Kalyanaraman B, Cheng G, Hardy M, Ouari O, Bennett B, Zielonka J. Teaching the basics of reactive oxygen species and their relevance to cancer biology: mitochondrial reactive oxygen species detection, redox signaling, and targeted therapies. Redox Biol. 2018; 15:347–62. https://doi.org/10.1016/j.redox.2017.12.012 [PubMed]
-
25.
Malik AN, Simões IC, Rosa HS, Khan S, Karkucinska-Wieckowska A, Wieckowski MR. A Diet Induced Maladaptive Increase in Hepatic Mitochondrial DNA Precedes OXPHOS Defects and May Contribute to Non-Alcoholic Fatty Liver Disease. Cells. 2019; 8:8. https://doi.org/10.3390/cells8101222 [PubMed]
-
26.
Lee J, Park JS, Roh YS. Molecular insights into the role of mitochondria in non-alcoholic fatty liver disease. Arch Pharm Res. 2019; 42:935–46. https://doi.org/10.1007/s12272-019-01178-1 [PubMed]
-
27.
Salic K, Gart E, Seidel F, Verschuren L, Caspers M, van Duyvenvoorde W, Wong KE, Keijer J, Bobeldijk-Pastorova I, Wielinga PY, Kleemann R. Combined Treatment with L-Carnitine and Nicotinamide Riboside Improves Hepatic Metabolism and Attenuates Obesity and Liver Steatosis. Int J Mol Sci. 2019; 20:20. https://doi.org/10.3390/ijms20184359 [PubMed]
-
28.
Jové M, Pradas I, Dominguez-Gonzalez M, Ferrer I, Pamplona R. Lipids and lipoxidation in human brain aging. Mitochondrial ATP-synthase as a key lipoxidation target. Redox Biol. 2019; 23:101082. https://doi.org/10.1016/j.redox.2018.101082 [PubMed]
-
29.
Borrelli A, Bonelli P, Tuccillo FM, Goldfine ID, Evans JL, Buonaguro FM, Mancini A. Role of gut microbiota and oxidative stress in the progression of non-alcoholic fatty liver disease to hepatocarcinoma: current and innovative therapeutic approaches. Redox Biol. 2018; 15:467–79. https://doi.org/10.1016/j.redox.2018.01.009 [PubMed]
-
30.
Dridi H, Yehya M, Barsotti R, Reiken S, Angebault C, Jung B, Jaber S, Marks AR, Lacampagne A, Matecki S. Mitochondrial oxidative stress induces leaky ryanodine receptor during mechanical ventilation. Free Radic Biol Med. 2020; 146:383–91. https://doi.org/10.1016/j.freeradbiomed.2019.11.019 [PubMed]
-
31.
Byrne FL, Olzomer EM, Marriott GR, Quek LE, Katen A, Su J, Nelson ME, Hart-Smith G, Larance M, Sebesfi VF, Cuff J, Martyn GE, Childress E, et al. Phenotypic screen for oxygen consumption rate identifies an anti-cancer naphthoquinone that induces mitochondrial oxidative stress. Redox Biol. 2020; 28:101374. https://doi.org/10.1016/j.redox.2019.101374 [PubMed]
-
32.
Tabassum R, Jeong NY. Potential for therapeutic use of hydrogen sulfide in oxidative stress-induced neurodegenerative diseases. Int J Med Sci. 2019; 16:1386–96. https://doi.org/10.7150/ijms.36516 [PubMed]
-
33.
Song X, Li T. Ripk3 mediates cardiomyocyte necrosis through targeting mitochondria and the JNK-Bnip3 pathway under hypoxia-reoxygenation injury. J Recept Signal Transduct Res. 2019; 39:331–40. https://doi.org/10.1080/10799893.2019.1676259 [PubMed]
-
34.
Upadhyay KK, Jadeja RN, Vyas HS, Pandya B, Joshi A, Vohra A, Thounaojam MC, Martin PM, Bartoli M, Devkar RV. Carbon monoxide releasing molecule-A1 improves nonalcoholic steatohepatitis via Nrf2 activation mediated improvement in oxidative stress and mitochondrial function. Redox Biol. 2020; 28:101314. https://doi.org/10.1016/j.redox.2019.101314 [PubMed]
-
35.
Abukar Y, Ramchandra R, Hood SG, McKinley MJ, Booth LC, Yao ST, May CN. Increased cardiac sympathetic nerve activity in ovine heart failure is reduced by lesion of the area postrema, but not lamina terminalis. Basic Res Cardiol. 2018; 113:35. https://doi.org/10.1007/s00395-018-0695-9 [PubMed]
-
36.
Auriau J, Roujeau C, Belaid Choucair Z, Oishi A, Derviaux C, Roux T, Trinquet E, Hermine O, Jockers R, Dam J. Gain of affinity for VEGF165 binding within the VEGFR2/NRP1 cellular complex detected by an HTRF-based binding assay. Biochem Pharmacol. 2018; 158:45–59. https://doi.org/10.1016/j.bcp.2018.09.014 [PubMed]
-
37.
Chen Y, Chen H, Wang S, Liu C, Qian F. A Single Hydrogen to Fluorine Substitution Reverses the Trend of Surface Composition Enrichment of Sorafenib Amorphous Solid Dispersion upon Moisture Exposure. Pharm Res. 2019; 36:105. https://doi.org/10.1007/s11095-019-2632-5 [PubMed]
-
38.
Araki M, Hisamitsu T, Kinugasa-Katayama Y, Tanaka T, Harada Y, Nakao S, Hanada S, Ishii S, Fujita M, Kawamura T, Saito Y, Nishiyama K, Watanabe Y, Nakagawa O. Serum/glucocorticoid-regulated kinase 1 as a novel transcriptional target of bone morphogenetic protein-ALK1 receptor signaling in vascular endothelial cells. Angiogenesis. 2018; 21:415–23. https://doi.org/10.1007/s10456-018-9605-x [PubMed]
-
39.
Zhang Y, Zhou H, Wu W, Shi C, Hu S, Yin T, Ma Q, Han T, Zhang Y, Tian F, Chen Y. Liraglutide protects cardiac microvascular endothelial cells against hypoxia/reoxygenation injury through the suppression of the SR-Ca(2+)-XO-ROS axis via activation of the GLP-1R/PI3K/Akt/survivin pathways. Free Radic Biol Med. 2016; 95:278–92. https://doi.org/10.1016/j.freeradbiomed.2016.03.035 [PubMed]
-
40.
Yan M, Huo Y, Yin S, Hu H. Mechanisms of acetaminophen-induced liver injury and its implications for therapeutic interventions. Redox Biol. 2018; 17:274–83. https://doi.org/10.1016/j.redox.2018.04.019 [PubMed]
-
41.
Bartesaghi S, Radi R. Fundamentals on the biochemistry of peroxynitrite and protein tyrosine nitration. Redox Biol. 2018; 14:618–25. https://doi.org/10.1016/j.redox.2017.09.009 [PubMed]
-
42.
Schmidt HM, Kelley EE, Straub AC. The impact of xanthine oxidase (XO) on hemolytic diseases. Redox Biol. 2019; 21:101072. https://doi.org/10.1016/j.redox.2018.101072 [PubMed]
-
43.
Zhang Z, Zhang L, Zhou L, Lei Y, Zhang Y, Huang C. Redox signaling and unfolded protein response coordinate cell fate decisions under ER stress. Redox Biol. 2019; 25:101047. https://doi.org/10.1016/j.redox.2018.11.005 [PubMed]
-
44.
Battistelli C, Sabarese G, Santangelo L, Montaldo C, Gonzalez FJ, Tripodi M, Cicchini C. The lncRNA HOTAIR transcription is controlled by HNF4α-induced chromatin topology modulation. Cell Death Differ. 2019; 26:890–901. https://doi.org/10.1038/s41418-018-0170-z [PubMed]
-
45.
Xu J, Peng Y, Zeng Y, Hua YQ, Xu XL. 2, 3, 4′, 5-tetrahydroxystilbene-2-0-β-d Glycoside Attenuates Age- and Diet-Associated Non-Alcoholic Steatohepatitis and Atherosclerosis in LDL Receptor Knockout Mice and Its Possible Mechanisms. Int J Mol Sci. 2019; 20:20. https://doi.org/10.3390/ijms20071617 [PubMed]
-
46.
Knebel B, Fahlbusch P, Dille M, Wahlers N, Hartwig S, Jacob S, Kettel U, Schiller M, Herebian D, Koellmer C, Lehr S, Müller-Wieland D, Kotzka J. Fatty Liver Due to Increased de novo Lipogenesis: Alterations in the Hepatic Peroxisomal Proteome. Front Cell Dev Biol. 2019; 7:248. https://doi.org/10.3389/fcell.2019.00248 [PubMed]
-
47.
Erland LA, Shukla MR, Singh AS, Murch SJ, Saxena PK. Melatonin and serotonin: mediators in the symphony of plant morphogenesis. J Pineal Res. 2018; 64:64. https://doi.org/10.1111/jpi.12452 [PubMed]
-
48.
Zhang L, Wang X, Cueto R, Effi C, Zhang Y, Tan H, Qin X, Ji Y, Yang X, Wang H. Biochemical basis and metabolic interplay of redox regulation. Redox Biol. 2019; 26:101284. https://doi.org/10.1016/j.redox.2019.101284 [PubMed]
-
49.
Liu J, Lu W, Shi B, Klein S, Su X. Peroxisomal regulation of redox homeostasis and adipocyte metabolism. Redox Biol. 2019; 24:101167. https://doi.org/10.1016/j.redox.2019.101167 [PubMed]
-
50.
Mao S, Fang L, Liu F, Jiang S, Wu L, Zhang J. Leptin and chronic kidney diseases. J Recept Signal Transduct Res. 2018; 38:89–94. https://doi.org/10.1080/10799893.2018.1431278 [PubMed]
-
51.
Mohammed F, Gorla M, Bisoyi V, Tammineni P, Sepuri NB. Rotenone-induced reactive oxygen species signal the recruitment of STAT3 to mitochondria. FEBS Lett. 2020. [Epub ahead of print]. https://doi.org/10.1002/1873-3468.13741 [PubMed]
-
52.
van Opbergen CJ, den Braven L, Delmar M, van Veen TA. Mitochondrial Dysfunction as Substrate for Arrhythmogenic Cardiomyopathy: A Search for New Disease Mechanisms. Front Physiol. 2019; 10:1496. https://doi.org/10.3389/fphys.2019.01496 [PubMed]
-
53.
Boengler K, Bornbaum J, Schlüter KD, Schulz R. P66shc and its role in ischemic cardiovascular diseases. Basic Res Cardiol. 2019; 114:29. https://doi.org/10.1007/s00395-019-0738-x [PubMed]
-
54.
Zhao Y, Wang Z, Feng D, Zhao H, Lin M, Hu Y, Zhang N, Lv L, Gao Z, Zhai X, Tian X, Yao J. p66Shc Contributes to Liver Fibrosis through the Regulation of Mitochondrial Reactive Oxygen Species. Theranostics. 2019; 9:1510–22. https://doi.org/10.7150/thno.29620 [PubMed]
-
55.
Robinson N, Ganesan R, Hegedűs C, Kovács K, Kufer TA, Virág L. Programmed necrotic cell death of macrophages: focus on pyroptosis, necroptosis, and parthanatos. Redox Biol. 2019; 26:101239. https://doi.org/10.1016/j.redox.2019.101239 [PubMed]
-
56.
Jeong HS, Kim KH, Lee IS, Park JY, Kim Y, Kim KS, Jang HJ. Ginkgolide A ameliorates non-alcoholic fatty liver diseases on high fat diet mice. Biomed Pharmacother. 2017; 88:625–34. https://doi.org/10.1016/j.biopha.2017.01.114 [PubMed]
-
57.
Moro T, Nakao S, Sumiyoshi H, Ishii T, Miyazawa M, Ishii N, Sato T, Iida Y, Okada Y, Tanaka M, Hayashi H, Ueha S, Matsushima K, Inagaki Y. A Combination of Mitochondrial Oxidative Stress and Excess Fat/Calorie Intake Accelerates Steatohepatitis by Enhancing Hepatic CC Chemokine Production in Mice. PLoS One. 2016; 11:e0146592. https://doi.org/10.1371/journal.pone.0146592 [PubMed]
-
58.
Serviddio G, Bellanti F, Tamborra R, Rollo T, Capitanio N, Romano AD, Sastre J, Vendemiale G, Altomare E. Uncoupling protein-2 (UCP2) induces mitochondrial proton leak and increases susceptibility of non-alcoholic steatohepatitis (NASH) liver to ischaemia-reperfusion injury. Gut. 2008; 57:957–65. https://doi.org/10.1136/gut.2007.147496 [PubMed]
-
59.
Alberici LC, Vercesi AE, Oliveira HC. Mitochondrial energy metabolism and redox responses to hypertriglyceridemia. J Bioenerg Biomembr. 2011; 43:19–23. https://doi.org/10.1007/s10863-011-9326-y [PubMed]
-
60.
Chowdhury A, Santra A, Bhattacharjee K, Ghatak S, Saha DR, Dhali GK. Mitochondrial oxidative stress and permeability transition in isoniazid and rifampicin induced liver injury in mice. J Hepatol. 2006; 45:117–26. https://doi.org/10.1016/j.jhep.2006.01.027 [PubMed]
-
61.
Li G, Chan YL, Sukjamnong S, Anwer AG, Vindin H, Padula M, Zakarya R, George J, Oliver BG, Saad S, Chen H. A Mitochondrial Specific Antioxidant Reverses Metabolic Dysfunction and Fatty Liver Induced by Maternal Cigarette Smoke in Mice. Nutrients. 2019; 11:11. https://doi.org/10.3390/nu11071669 [PubMed]
-
62.
Yang DK, Jo DG. Mulberry Fruit Extract Ameliorates Nonalcoholic Fatty Liver Disease (NAFLD) through Inhibition of Mitochondrial Oxidative Stress in Rats. Evid Based Complement Alternat Med. 2018; 2018:8165716. https://doi.org/10.1155/2018/8165716 [PubMed]
-
63.
Misiakiewicz-Has K, Maciejewska D, Kolasa-Wołosiuk A, Pilutin A, Rzeszotek S, Wilk A, Szypulska-Koziarska D, Stachowska E, Łukomska A, Wiszniewska B. Modulatory effect of inulin with soya isoflavones on plasma lipid profile and liver SCD-18 index in rats with induced type-2 diabetes mellitus. Histol Histopathol. 2019; 34:1131–40. https://doi.org/10.14670/HH-18-113 [PubMed]
-
64.
Ren T, Zhu L, Shen Y, Mou Q, Lin T, Feng H. Protection of hepatocyte mitochondrial function by blueberry juice and probiotics via SIRT1 regulation in non-alcoholic fatty liver disease. Food Funct. 2019; 10:1540–51. https://doi.org/10.1039/C8FO02298D [PubMed]
-
65.
Jin F, Hagemann N, Sun L, Wu J, Doeppner TR, Dai Y, Hermann DM. High-density lipoprotein (HDL) promotes angiogenesis via S1P3-dependent VEGFR2 activation. Angiogenesis. 2018; 21:381–94. https://doi.org/10.1007/s10456-018-9603-z [PubMed]
-
66.
Hadebe N, Cour M, Lecour S. The SAFE pathway for cardioprotection: is this a promising target? Basic Res Cardiol. 2018; 113:9. https://doi.org/10.1007/s00395-018-0670-5 [PubMed]
-
67.
Jost PJ, Höckendorf U. Necroinflammation emerges as a key regulator of hematopoiesis in health and disease. Cell Death Differ. 2019; 26:53–67. https://doi.org/10.1038/s41418-018-0194-4 [PubMed]
-
68.
Lin D, He H, Ji H, Willis J, Willard L, Jiang Y, Medeiros DM, Wark L, Han J, Liu Y, Lu B. Wolfberries potentiate mitophagy and enhance mitochondrial biogenesis leading to prevention of hepatic steatosis in obese mice: the role of AMP-activated protein kinase α2 subunit. Mol Nutr Food Res. 2014; 58:1005–15. https://doi.org/10.1002/mnfr.201300186 [PubMed]
-
69.
Gan LT, Van Rooyen DM, Koina ME, McCuskey RS, Teoh NC, Farrell GC. Hepatocyte free cholesterol lipotoxicity results from JNK1-mediated mitochondrial injury and is HMGB1 and TLR4-dependent. J Hepatol. 2014; 61:1376–84. https://doi.org/10.1016/j.jhep.2014.07.024 [PubMed]
-
70.
Zhang T, Gu J, Guo J, Chen K, Li H, Wang J. Renalase Attenuates Mouse Fatty Liver Ischemia/Reperfusion Injury through Mitigating Oxidative Stress and Mitochondrial Damage via Activating SIRT1. Oxid Med Cell Longev. 2019; 2019:7534285. https://doi.org/10.1155/2019/7534285 [PubMed]
-
71.
Lee DH, Park JS, Lee YS, Han J, Lee DK, Kwon SW, Han DH, Lee YH, Bae SH. SQSTM1/p62 activates NFE2L2/NRF2 via ULK1-mediated autophagic KEAP1 degradation and protects mouse liver from lipotoxicity. Autophagy. 2020; 1–25. [Epub ahead of print]. https://doi.org/10.1080/15548627.2020.1712108 [PubMed]
-
72.
Foglia B, Sutti S, Pedicini D, Cannito S, Bocca C, Maggiora M, Bevacqua MR, Rosso C, Bugianesi E, Albano E, Novo E, Parola M, Oncostatin M. Oncostatin M, A Profibrogenic Mediator Overexpressed in Non-Alcoholic Fatty Liver Disease, Stimulates Migration of Hepatic Myofibroblasts. Cells. 2019; 9:9. https://doi.org/10.3390/cells9010028 [PubMed]
-
73.
Zhou H, Shi C, Hu S, Zhu H, Ren J, Chen Y. BI1 is associated with microvascular protection in cardiac ischemia reperfusion injury via repressing Syk-Nox2-Drp1-mitochondrial fission pathways. Angiogenesis. 2018; 21:599–615. https://doi.org/10.1007/s10456-018-9611-z [PubMed]
-
74.
Jin Q, Li R, Hu N, Xin T, Zhu P, Hu S, Ma S, Zhu H, Ren J, Zhou H. DUSP1 alleviates cardiac ischemia/reperfusion injury by suppressing the Mff-required mitochondrial fission and Bnip3-related mitophagy via the JNK pathways. Redox Biol. 2018; 14:576–87. https://doi.org/10.1016/j.redox.2017.11.004 [PubMed]
-
75.
Kim YY, Um JH, Yoon JH, Lee DY, Lee YJ, Kim DH, Park JI, Yun J. p53 regulates mitochondrial dynamics by inhibiting Drp1 translocation into mitochondria during cellular senescence. FASEB J. 2020; 34:2451–64. https://doi.org/10.1096/fj.201901747RR [PubMed]
-
76.
Yoo SM, Yamashita SI, Kim H, Na D, Lee H, Kim SJ, Cho DH, Kanki T, Jung YK. FKBP8 LIRL-dependent mitochondrial fragmentation facilitates mitophagy under stress conditions. FASEB J. 2020; 34:2944–57. https://doi.org/10.1096/fj.201901735R [PubMed]
-
77.
Sumneang N, Siri-Angkul N, Kumfu S, Chattipakorn SC, Chattipakorn N. The effects of iron overload on mitochondrial function, mitochondrial dynamics, and ferroptosis in cardiomyocytes. Arch Biochem Biophys. 2020; 680:108241. https://doi.org/10.1016/j.abb.2019.108241 [PubMed]
-
78.
Vogel E, Pittman PB, Naylor K. The FtsZ Homolog, FszB, Inhibits Mitochondrial Dynamics in Dictyostelium discoideum.. Cells. 2019; 9:9. https://doi.org/10.3390/cells9010064 [PubMed]
-
79.
Kleinbongard P, Skyschally A, Gent S, Pesch M, Heusch G. STAT3 as a common signal of ischemic conditioning: a lesson on “rigor and reproducibility” in preclinical studies on cardioprotection. Basic Res Cardiol. 2017; 113:3. https://doi.org/10.1007/s00395-017-0660-z [PubMed]
-
80.
Ding M, Ning J, Feng N, Li Z, Liu Z, Wang Y, Wang Y, Li X, Huo C, Jia X, Xu R, Fu F, Wang X, Pei J. Dynamin-related protein 1-mediated mitochondrial fission contributes to post-traumatic cardiac dysfunction in rats and the protective effect of melatonin. J Pineal Res. 2018; 64:64. https://doi.org/10.1111/jpi.12447 [PubMed]
-
81.
He Z, Ning N, Zhou Q, Khoshnam SE, Farzaneh M. Mitochondria as a therapeutic target for ischemic stroke. Free Radic Biol Med. 2020; 146:45–58. https://doi.org/10.1016/j.freeradbiomed.2019.11.005 [PubMed]
-
82.
Zhou H, Wang J, Zhu P, Zhu H, Toan S, Hu S, Ren J, Chen Y. NR4A1 aggravates the cardiac microvascular ischemia reperfusion injury through suppressing FUNDC1-mediated mitophagy and promoting Mff-required mitochondrial fission by CK2α. Basic Res Cardiol. 2018; 113:23. https://doi.org/10.1007/s00395-018-0682-1 [PubMed]
-
83.
Zhou H, Wang J, Zhu P, Hu S, Ren J. Ripk3 regulates cardiac microvascular reperfusion injury: the role of IP3R-dependent calcium overload, XO-mediated oxidative stress and F-action/filopodia-based cellular migration. Cell Signal. 2018; 45:12–22. https://doi.org/10.1016/j.cellsig.2018.01.020 [PubMed]
-
84.
Shi C, Cai Y, Li Y, Li Y, Hu N, Ma S, Hu S, Zhu P, Wang W, Zhou H. Yap promotes hepatocellular carcinoma metastasis and mobilization via governing cofilin/F-actin/lamellipodium axis by regulation of JNK/Bnip3/SERCA/CaMKII pathways. Redox Biol. 2018; 14:59–71. https://doi.org/10.1016/j.redox.2017.08.013 [PubMed]
-
85.
Morales PE, Arias-Durán C, Ávalos-Guajardo Y, Aedo G, Verdejo HE, Parra V, Lavandero S. Emerging role of mitophagy in cardiovascular physiology and pathology. Mol Aspects Med. 2020; 71:100822. https://doi.org/10.1016/j.mam.2019.09.006 [PubMed]
-
86.
Wang B, Xiao X, Huang F, Liu R. Syntaxin-17-Dependent Mitochondrial Dynamics is Essential for Protection Against Oxidative-Stress-Induced Apoptosis. Antioxidants. 2019; 8:8. https://doi.org/10.3390/antiox8110522 [PubMed]
-
87.
Zhou L, Zhang L, Zhang Y, Yu X, Sun X, Zhu T, Li X, Liang W, Han Y, Qin C. PINK1 Deficiency Ameliorates Cisplatin-Induced Acute Kidney Injury in Rats. Front Physiol. 2019; 10:1225. https://doi.org/10.3389/fphys.2019.01225 [PubMed]
-
88.
Kuznetsov AV, Javadov S, Margreiter R, Grimm M, Hagenbuchner J, Ausserlechner MJ. The Role of Mitochondria in the Mechanisms of Cardiac Ischemia-Reperfusion Injury. Antioxidants. 2019; 8:8. https://doi.org/10.3390/antiox8100454 [PubMed]
-
89.
Guo Q, Zheng X, Yang P, Pang X, Qian K, Wang P, Xu S, Sheng D, Wang L, Cao J, Lu W, Zhang Q, Jiang X. Small interfering RNA delivery to the neurons near the amyloid plaques for improved treatment of Alzheimer’s disease. Acta Pharm Sin B. 2019; 9:590–603. https://doi.org/10.1016/j.apsb.2018.12.010 [PubMed]
-
90.
Dai W, Jiang L. Dysregulated Mitochondrial Dynamics and Metabolism in Obesity, Diabetes, and Cancer. Front Endocrinol (Lausanne). 2019; 10:570. https://doi.org/10.3389/fendo.2019.00570 [PubMed]
-
91.
Lee KH, Kang TB. The Molecular Links between Cell Death and Inflammasome. Cells. 2019; 8:8. https://doi.org/10.3390/cells8091057 [PubMed]
-
92.
Simula L, Campanella M, Campello S. Targeting Drp1 and mitochondrial fission for therapeutic immune modulation. Pharmacol Res. 2019; 146:104317. https://doi.org/10.1016/j.phrs.2019.104317 [PubMed]
-
93.
DeLeon-Pennell KY, Mouton AJ, Ero OK, Ma Y, Padmanabhan Iyer R, Flynn ER, Espinoza I, Musani SK, Vasan RS, Hall ME, Fox ER, Lindsey ML. LXR/RXR signaling and neutrophil phenotype following myocardial infarction classify sex differences in remodeling. Basic Res Cardiol. 2018; 113:40. https://doi.org/10.1007/s00395-018-0699-5 [PubMed]
-
94.
Qi Z, Huang Z, Xie F, Chen L. Dynamin-related protein 1: A critical protein in the pathogenesis of neural system dysfunctions and neurodegenerative diseases. J Cell Physiol. 2019; 234:10032–46. https://doi.org/10.1002/jcp.27866 [PubMed]
-
95.
Zhou H, Hu S, Jin Q, Shi C, Zhang Y, Zhu P, Ma Q, Tian F, Chen Y. Mff-Dependent Mitochondrial Fission Contributes to the Pathogenesis of Cardiac Microvasculature Ischemia/Reperfusion Injury via Induction of mROS-Mediated Cardiolipin Oxidation and HK2/VDAC1 Disassociation-Involved mPTP Opening. J Am Heart Assoc. 2017; 6:6. https://doi.org/10.1161/JAHA.116.005328 [PubMed]
-
96.
Serasinghe MN, Chipuk JE. Mitochondrial Fission in Human Diseases. Handb Exp Pharmacol. 2017; 240:159–88. https://doi.org/10.1007/164_2016_38 [PubMed]
-
97.
Cherok E, Xu S, Li S, Das S, Meltzer WA, Zalzman M, Wang C, Karbowski M. Novel regulatory roles of Mff and Drp1 in E3 ubiquitin ligase MARCH5-dependent degradation of MiD49 and Mcl1 and control of mitochondrial dynamics. Mol Biol Cell. 2017; 28:396–410. https://doi.org/10.1091/mbc.e16-04-0208 [PubMed]
-
98.
Osellame LD, Singh AP, Stroud DA, Palmer CS, Stojanovski D, Ramachandran R, Ryan MT. Cooperative and independent roles of the Drp1 adaptors Mff, MiD49 and MiD51 in mitochondrial fission. J Cell Sci. 2016; 129:2170–81. https://doi.org/10.1242/jcs.185165 [PubMed]
-
99.
Otera H, Miyata N, Kuge O, Mihara K. Drp1-dependent mitochondrial fission via MiD49/51 is essential for apoptotic cristae remodeling. J Cell Biol. 2016; 212:531–44. https://doi.org/10.1083/jcb.201508099 [PubMed]
-
100.
Losón OC, Song Z, Chen H, Chan DC. Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol Biol Cell. 2013; 24:659–67. https://doi.org/10.1091/mbc.e12-10-0721 [PubMed]
-
101.
Atkins K, Dasgupta A, Chen KH, Mewburn J, Archer SL. The role of Drp1 adaptor proteins MiD49 and MiD51 in mitochondrial fission: implications for human disease. Clin Sci (Lond). 2016; 130:1861–74. https://doi.org/10.1042/CS20160030 [PubMed]
-
102.
López-Lluch G. Mitochondrial activity and dynamics changes regarding metabolism in ageing and obesity. Mech Ageing Dev. 2017; 162:108–21. https://doi.org/10.1016/j.mad.2016.12.005 [PubMed]
-
103.
Horbay R, Bilyy R. Mitochondrial dynamics during cell cycling. Apoptosis. 2016; 21:1327–35. https://doi.org/10.1007/s10495-016-1295-5 [PubMed]
-
104.
Kong B, Tsuyoshi H, Orisaka M, Shieh DB, Yoshida Y, Tsang BK. Mitochondrial dynamics regulating chemoresistance in gynecological cancers. Ann N Y Acad Sci. 2015; 1350:1–16. https://doi.org/10.1111/nyas.12883 [PubMed]
-
105.
Krishnasamy Y, Gooz M, Li L, Lemasters JJ, Zhong Z. Role of mitochondrial depolarization and disrupted mitochondrial homeostasis in non-alcoholic steatohepatitis and fibrosis in mice. Int J Physiol Pathophysiol Pharmacol. 2019; 11:190–204. https://doi.org/10.1016/s0016-5085(18)33646-1 [PubMed]
-
106.
Gong F, Gao L, Ding T. IDH2 protects against nonalcoholic steatohepatitis by alleviating dyslipidemia regulated by oxidative stress. Biochem Biophys Res Commun. 2019; 514:593–600. https://doi.org/10.1016/j.bbrc.2019.04.069 [PubMed]
-
107.
Liu W, Ye C, Cheng Q, Zhang X, Yao L, Li Q, Huang J, Liu Y, Zou Z, Wang H, Yan J, Zhu Y, Wang C, Ai D. Macrophage Raptor Deficiency-Induced Lysosome Dysfunction Exacerbates Nonalcoholic Steatohepatitis. Cell Mol Gastroenterol Hepatol. 2019; 7:211–31. https://doi.org/10.1016/j.jcmgh.2018.09.011 [PubMed]
-
108.
Galloway CA, Lee H, Brookes PS, Yoon Y. Decreasing mitochondrial fission alleviates hepatic steatosis in a murine model of nonalcoholic fatty liver disease. Am J Physiol Gastrointest Liver Physiol. 2014; 307:G632–41. https://doi.org/10.1152/ajpgi.00182.2014 [PubMed]
-
109.
Zhou H, Du W, Li Y, Shi C, Hu N, Ma S, Wang W, Ren J. Effects of melatonin on fatty liver disease: the role of NR4A1/DNA-PKcs/p53 pathway, mitochondrial fission, and mitophagy. J Pineal Res. 2018; 64:64. https://doi.org/10.1111/jpi.12450 [PubMed]
-
110.
Nowak-Sliwinska P, Alitalo K, Allen E, Anisimov A, Aplin AC, Auerbach R, Augustin HG, Bates DO, van Beijnum JR, Bender RH, Bergers G, Bikfalvi A, Bischoff J, et al. Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenesis. 2018; 21:425–532. https://doi.org/10.1007/s10456-018-9613-x [PubMed]
-
111.
Hofmann F. A concise discussion of the regulatory role of cGMP kinase I in cardiac physiology and pathology. Basic Res Cardiol. 2018; 113:31. https://doi.org/10.1007/s00395-018-0690-1 [PubMed]
-
112.
Lim J, Murthy A. Controlling inflammation by selective autophagy. Cell Death Differ. 2018; 25:825–27. https://doi.org/10.1038/s41418-018-0096-5 [PubMed]
-
113.
Liu L, Wang T, Yang X, Xu C, Liao Z, Wang X, Su D, Li Y, Zhou H, Qiu X, Chen Y, Huang D, Lian C, Su P. MTNR1B loss promotes chordoma recurrence by abrogating melatonin-mediated β-catenin signaling repression. J Pineal Res. 2019; 67:e12588. https://doi.org/10.1111/jpi.12588 [PubMed]
-
114.
Tong W, Ju L, Qiu M, Xie Q, Chen Y, Shen W, Sun W, Wang W, Tian J. Liraglutide ameliorates non-alcoholic fatty liver disease by enhancing mitochondrial architecture and promoting autophagy through the SIRT1/SIRT3-FOXO3a pathway. Hepatol Res. 2016; 46:933–43. https://doi.org/10.1111/hepr.12634 [PubMed]
-
115.
Kim HJ, Han YH, Na H, Kim JY, Kim T, Kim HJ, Shin C, Lee JW, Lee MO. Liver-specific deletion of RORα aggravates diet-induced nonalcoholic steatohepatitis by inducing mitochondrial dysfunction. Sci Rep. 2017; 7:16041. https://doi.org/10.1038/s41598-017-16077-y [PubMed]
-
116.
Wang Y, An H, Liu T, Qin C, Sesaki H, Guo S, Radovick S, Hussain M, Maheshwari A, Wondisford FE, O'Rourke B, He L. Metformin Improves Mitochondrial Respiratory Activity through Activation of AMPK. Cell Rep. 2019; 29:1511–23.e5. https://doi.org/10.1016/j.celrep.2019.09.070 [PubMed]
-
117.
Hasnat M, Yuan Z, Ullah A, Naveed M, Raza F, Baig MM, Khan A, Xu D, Su Y, Sun L, Zhang L, Jiang Z. Mitochondria-dependent apoptosis in triptolide-induced hepatotoxicity is associated with the Drp1 activation. Toxicol Mech Methods. 2020; 30:124–33. https://doi.org/10.1080/15376516.2019.1669247 [PubMed]
-
118.
Basalay MV, Davidson SM, Gourine AV, Yellon DM. Neural mechanisms in remote ischaemic conditioning in the heart and brain: mechanistic and translational aspects. Basic Res Cardiol. 2018; 113:25. https://doi.org/10.1007/s00395-018-0684-z [PubMed]
-
119.
Bi J, Zhang J, Ren Y, Du Z, Li Q, Wang Y, Wei S, Yang L, Zhang J, Liu C, Lv Y, Wu R. Irisin alleviates liver ischemia-reperfusion injury by inhibiting excessive mitochondrial fission, promoting mitochondrial biogenesis and decreasing oxidative stress. Redox Biol. 2019; 20:296–306. https://doi.org/10.1016/j.redox.2018.10.019 [PubMed]
-
120.
Kang JW, Choi HS, Lee SM. Resolvin D1 attenuates liver ischaemia/reperfusion injury through modulating thioredoxin 2-mediated mitochondrial quality control. Br J Pharmacol. 2018; 175:2441–53. https://doi.org/10.1111/bph.14212 [PubMed]
-
121.
De Rasmo D, Signorile A, De Leo E, Polishchuk EV, Ferretta A, Raso R, Russo S, Polishchuk R, Emma F, Bellomo F. Mitochondrial Dynamics of Proximal Tubular Epithelial Cells in Nephropathic Cystinosis. Int J Mol Sci. 2019; 21:21. https://doi.org/10.3390/ijms21010192 [PubMed]
-
122.
Jang S, Javadov S. OPA1 regulates respiratory supercomplexes assembly: the role of mitochondrial swelling. Mitochondrion. 2020; 51:30–39. https://doi.org/10.1016/j.mito.2019.11.006 [PubMed]
-
123.
Zhang M, Bener MB, Jiang Z, Wang T, Esencan E, Scott Iii R, Horvath T, Seli E. Mitofusin 1 is required for female fertility and to maintain ovarian follicular reserve. Cell Death Dis. 2019; 10:560. https://doi.org/10.1038/s41419-019-1799-3 [PubMed]
-
124.
Hu L, Ding M, Tang D, Gao E, Li C, Wang K, Qi B, Qiu J, Zhao H, Chang P, Fu F, Li Y. Targeting mitochondrial dynamics by regulating Mfn2 for therapeutic intervention in diabetic cardiomyopathy. Theranostics. 2019; 9:3687–706. https://doi.org/10.7150/thno.33684 [PubMed]
-
125.
Lee J, Choi JA, Cho SN, Son SH, Song CH. Mitofusin 2-Deficiency Suppresses Mycobacterium tuberculosis Survival in Macrophages. Cells. 2019; 8:8. https://doi.org/10.3390/cells8111355 [PubMed]
-
126.
Li P, Wang J, Zhao X, Ru J, Tian T, An Y, Tang L, Bai Y. PTEN inhibition attenuates endothelial cell apoptosis in coronary heart disease via modulating the AMPK-CREB-Mfn2-mitophagy signaling pathway. J Cell Physiol. 2020; 235:4878–89. https://doi.org/10.1002/jcp.29366 [PubMed]
-
127.
Romanello V, Scalabrin M, Albiero M, Blaauw B, Scorrano L, Sandri M. Inhibition of the Fission Machinery Mitigates OPA1 Impairment in Adult Skeletal Muscles. Cells. 2019; 8:8. https://doi.org/10.3390/cells8060597 [PubMed]
-
128.
Kasahara A, Scorrano L. Mitochondria: from cell death executioners to regulators of cell differentiation. Trends Cell Biol. 2014; 24:761–70. https://doi.org/10.1016/j.tcb.2014.08.005 [PubMed]
-
129.
Cerveny KL, Tamura Y, Zhang Z, Jensen RE, Sesaki H. Regulation of mitochondrial fusion and division. Trends Cell Biol. 2007; 17:563–69. https://doi.org/10.1016/j.tcb.2007.08.006 [PubMed]
-
130.
Olichon A, Guillou E, Delettre C, Landes T, Arnauné-Pelloquin L, Emorine LJ, Mils V, Daloyau M, Hamel C, Amati-Bonneau P, Bonneau D, Reynier P, Lenaers G, Belenguer P. Mitochondrial dynamics and disease, OPA1. Biochim Biophys Acta. 2006; 1763:500–09. https://doi.org/10.1016/j.bbamcr.2006.04.003 [PubMed]
-
131.
MacVicar T, Ohba Y, Nolte H, Mayer FC, Tatsuta T, Sprenger HG, Lindner B, Zhao Y, Li J, Bruns C, Krüger M, Habich M, Riemer J, et al. Lipid signalling drives proteolytic rewiring of mitochondria by YME1L. Nature. 2019; 575:361–65. https://doi.org/10.1038/s41586-019-1738-6 [PubMed]
-
132.
MacVicar T, Langer T. OPA1 processing in cell death and disease - the long and short of it. J Cell Sci. 2016; 129:2297–306. https://doi.org/10.1242/jcs.159186 [PubMed]
-
133.
Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003; 160:189–200. https://doi.org/10.1083/jcb.200211046 [PubMed]
-
134.
Wang Q, Xu J, Li X, Liu Z, Han Y, Xu X, Li X, Tang Y, Liu Y, Yu T, Li X. Sirt3 modulate renal ischemia-reperfusion injury through enhancing mitochondrial fusion and activating the ERK-OPA1 signaling pathway. J Cell Physiol. 2019; 234:23495–506. https://doi.org/10.1002/jcp.28918 [PubMed]
-
135.
Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008; 27:433–46. https://doi.org/10.1038/sj.emboj.7601963 [PubMed]
-
136.
Lok R, van Koningsveld MJ, Gordijn MC, Beersma DG, Hut RA. Daytime melatonin and light independently affect human alertness and body temperature. J Pineal Res. 2019; 67:e12583. https://doi.org/10.1111/jpi.12583 [PubMed]
-
137.
Yellon DM, He Z, Khambata R, Ahluwalia A, Davidson SM. The GTN patch: a simple and effective new approach to cardioprotection? Basic Res Cardiol. 2018; 113:20. https://doi.org/10.1007/s00395-018-0681-2 [PubMed]
-
138.
Souza LE, Beckenkamp LR, Sobral LM, Fantacini DM, Melo FU, Borges JS, Leopoldino AM, Kashima S, Covas DT. Pre-culture in endothelial growth medium enhances the angiogenic properties of adipose-derived stem/stromal cells. Angiogenesis. 2018; 21:15–22. https://doi.org/10.1007/s10456-017-9579-0 [PubMed]
-
139.
Twig G, Shirihai OS. The interplay between mitochondrial dynamics and mitophagy. Antioxid Redox Signal. 2011; 14:1939–51. https://doi.org/10.1089/ars.2010.3779 [PubMed]
-
140.
Zhou H, Li N, Yuan Y, Jin YG, Guo H, Deng W, Tang QZ. Activating transcription factor 3 in cardiovascular diseases: a potential therapeutic target. Basic Res Cardiol. 2018; 113:37. https://doi.org/10.1007/s00395-018-0698-6 [PubMed]
-
141.
Gomes LC, Di Benedetto G, Scorrano L. Essential amino acids and glutamine regulate induction of mitochondrial elongation during autophagy. Cell Cycle. 2011; 10:2635–39. https://doi.org/10.4161/cc.10.16.17002 [PubMed]
-
142.
Du J, Zhang X, Han J, Man K, Zhang Y, Chu ES, Nan Y, Yu J. Pro-Inflammatory CXCR3 Impairs Mitochondrial Function in Experimental Non-Alcoholic Steatohepatitis. Theranostics. 2017; 7:4192–203. https://doi.org/10.7150/thno.21400 [PubMed]
-
143.
Hernández-Alvarez MI, Sebastián D, Vives S, Ivanova S, Bartoccioni P, Kakimoto P, Plana N, Veiga SR, Hernández V, Vasconcelos N, Peddinti G, Adrover A, Jové M, et al. Deficient Endoplasmic Reticulum-Mitochondrial Phosphatidylserine Transfer Causes Liver Disease. Cell. 2019; 177:881–895.e17. https://doi.org/10.1016/j.cell.2019.04.010 [PubMed]
-
144.
Bach D, Pich S, Soriano FX, Vega N, Baumgartner B, Oriola J, Daugaard JR, Lloberas J, Camps M, Zierath JR, Rabasa-Lhoret R, Wallberg-Henriksson H, Laville M, et al. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J Biol Chem. 2003; 278:17190–97. https://doi.org/10.1074/jbc.M212754200 [PubMed]
-
145.
Hernández-Alvarez MI, Thabit H, Burns N, Shah S, Brema I, Hatunic M, Finucane F, Liesa M, Chiellini C, Naon D, Zorzano A, Nolan JJ. Subjects with early-onset type 2 diabetes show defective activation of the skeletal muscle PGC-1{alpha}/Mitofusin-2 regulatory pathway in response to physical activity. Diabetes Care. 2010; 33:645–51. https://doi.org/10.2337/dc09-1305 [PubMed]
-
146.
Li L, Martin-Levilain J, Jiménez-Sánchez C, Karaca M, Foti M, Martinou JC, Maechler P. In vivo stabilization of OPA1 in hepatocytes potentiates mitochondrial respiration and gluconeogenesis in a prohibitin-dependent way. J Biol Chem. 2019; 294:12581–98. https://doi.org/10.1074/jbc.RA119.007601 [PubMed]
-
147.
Gonçalves IO, Passos E, Diogo CV, Rocha-Rodrigues S, Santos-Alves E, Oliveira PJ, Ascensão A, Magalhães J. Exercise mitigates mitochondrial permeability transition pore and quality control mechanisms alterations in nonalcoholic steatohepatitis. Appl Physiol Nutr Metab. 2016; 41:298–306. https://doi.org/10.1139/apnm-2015-0470 [PubMed]
-
148.
Xu J, Cao K, Li Y, Zou X, Chen C, Szeto IM, Dong Z, Zhao Y, Shi Y, Wang J, Liu J, Feng Z. Bitter gourd inhibits the development of obesity-associated fatty liver in C57BL/6 mice fed a high-fat diet. J Nutr. 2014; 144:475–83. https://doi.org/10.3945/jn.113.187450 [PubMed]
-
149.
Wang YC, Kong WZ, Jin QM, Chen J, Dong L. Effects of salvianolic acid B on liver mitochondria of rats with nonalcoholic steatohepatitis. World J Gastroenterol. 2015; 21:10104–12. https://doi.org/10.3748/wjg.v21.i35.10104 [PubMed]
-
150.
Cadete VJ, Vasam G, Menzies KJ, Burelle Y. Mitochondrial quality control in the cardiac system: an integrative view. Biochim Biophys Acta Mol Basis Dis. 2019; 1865:782–96. https://doi.org/10.1016/j.bbadis.2018.11.018 [PubMed]
-
151.
Ma ZG, Dai J, Yuan YP, Bian ZY, Xu SC, Jin YG, Zhang X, Tang QZ. T-bet deficiency attenuates cardiac remodelling in rats. Basic Res Cardiol. 2018; 113:19. https://doi.org/10.1007/s00395-018-0678-x [PubMed]
-
152.
Venugopal S, Kao C, Chandna R, Sulochana KN, Subramanian V, Chen M, Kini RM, Ge R. Angio-3, a 10-residue peptide derived from human plasminogen kringle 3, suppresses tumor growth in mice via impeding both angiogenesis and vascular permeability. Angiogenesis. 2018; 21:653–65. https://doi.org/10.1007/s10456-018-9616-7 [PubMed]
-
153.
Lochner A, Marais E, Huisamen B. Melatonin and cardioprotection against ischaemia/reperfusion injury: what’s new? A review. J Pineal Res. 2018; 65:e12490. https://doi.org/10.1111/jpi.12490 [PubMed]
-
154.
Tatullo M, Makeeva I, Rengo S, Rengo C, Spagnuolo G, Codispoti B. Small molecule GSK-3 antagonists play a pivotal role in reducing the local inflammatory response, in promoting resident stem cell activation and in improving tissue repairing in regenerative dentistry. Histol Histopathol. 2019; 34:1195–203. https://doi.org/10.14670/HH-18-133 [PubMed]
-
155.
Skoda J, Borankova K, Jansson PJ, Huang ML, Veselska R, Richardson DR. Pharmacological targeting of mitochondria in cancer stem cells: an ancient organelle at the crossroad of novel anti-cancer therapies. Pharmacol Res. 2019; 139:298–313. https://doi.org/10.1016/j.phrs.2018.11.020 [PubMed]
-
156.
Tahrir FG, Langford D, Amini S, Mohseni Ahooyi T, Khalili K. Mitochondrial quality control in cardiac cells: mechanisms and role in cardiac cell injury and disease. J Cell Physiol. 2019; 234:8122–33. https://doi.org/10.1002/jcp.27597 [PubMed]
-
157.
Blasiak J, Pawlowska E, Szczepanska J, Kaarniranta K. Interplay between Autophagy and the Ubiquitin-Proteasome System and Its Role in the Pathogenesis of Age-Related Macular Degeneration. Int J Mol Sci. 2019; 20:20. https://doi.org/10.3390/ijms20010210 [PubMed]
-
158.
Lin Q, Li S, Jiang N, Shao X, Zhang M, Jin H, Zhang Z, Shen J, Zhou Y, Zhou W, Gu L, Lu R, Ni Z. PINK1-parkin pathway of mitophagy protects against contrast-induced acute kidney injury via decreasing mitochondrial ROS and NLRP3 inflammasome activation. Redox Biol. 2019; 26:101254. https://doi.org/10.1016/j.redox.2019.101254 [PubMed]
-
159.
Wang J, Zhu P, Li R, Ren J, Zhou H. Fundc1-dependent mitophagy is obligatory to ischemic preconditioning-conferred renoprotection in ischemic AKI via suppression of Drp1-mediated mitochondrial fission. Redox Biol. 2020; 30:101415. https://doi.org/10.1016/j.redox.2019.101415 [PubMed]
-
160.
Li R, Xin T, Li D, Wang C, Zhu H, Zhou H. Therapeutic effect of Sirtuin 3 on ameliorating nonalcoholic fatty liver disease: the role of the ERK-CREB pathway and Bnip3-mediated mitophagy. Redox Biol. 2018; 18:229–43. https://doi.org/10.1016/j.redox.2018.07.011 [PubMed]
-
161.
Saxena S, Mathur A, Kakkar P. Critical role of mitochondrial dysfunction and impaired mitophagy in diabetic nephropathy. J Cell Physiol. 2019; 234:19223–36. https://doi.org/10.1002/jcp.28712 [PubMed]
-
162.
Bayne AN, Trempe JF. Mechanisms of PINK1, ubiquitin and Parkin interactions in mitochondrial quality control and beyond. Cell Mol Life Sci. 2019; 76:4589–611. https://doi.org/10.1007/s00018-019-03203-4 [PubMed]
-
163.
Sekine S, Youle RJ. PINK1 import regulation; a fine system to convey mitochondrial stress to the cytosol. BMC Biol. 2018; 16:2. https://doi.org/10.1186/s12915-017-0470-7 [PubMed]
-
164.
McWilliams TG, Muqit MM. PINK1 and Parkin: emerging themes in mitochondrial homeostasis. Curr Opin Cell Biol. 2017; 45:83–91. https://doi.org/10.1016/j.ceb.2017.03.013 [PubMed]
-
165.
Bernardini JP, Lazarou M, Dewson G. Parkin and mitophagy in cancer. Oncogene. 2017; 36:1315–27. https://doi.org/10.1038/onc.2016.302 [PubMed]
-
166.
Rüb C, Wilkening A, Voos W. Mitochondrial quality control by the Pink1/Parkin system. Cell Tissue Res. 2017; 367:111–23. https://doi.org/10.1007/s00441-016-2485-8 [PubMed]
-
167.
Zhang J, Ney PA. Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death Differ. 2009; 16:939–46. https://doi.org/10.1038/cdd.2009.16 [PubMed]
-
168.
Hamacher-Brady A, Brady NR. Mitophagy programs: mechanisms and physiological implications of mitochondrial targeting by autophagy. Cell Mol Life Sci. 2016; 73:775–95. https://doi.org/10.1007/s00018-015-2087-8 [PubMed]
-
169.
Wei H, Liu L, Chen Q. Selective removal of mitochondria via mitophagy: distinct pathways for different mitochondrial stresses. Biochim Biophys Acta. 2015; 1853:2784–90. https://doi.org/10.1016/j.bbamcr.2015.03.013 [PubMed]
-
170.
Liu L, Sakakibara K, Chen Q, Okamoto K. Receptor-mediated mitophagy in yeast and mammalian systems. Cell Res. 2014; 24:787–95. https://doi.org/10.1038/cr.2014.75 [PubMed]
-
171.
Zhang W, Guo T, Wang C, He Y, Zhang X, Li G, Chen Y, Li J, Lin Y, Xu X, Wu L, Zhang S, Zhang J. MOF Capacitates Cyclodextrin to Mega-Load Mode for High-Efficient Delivery of Valsartan. Pharm Res. 2019; 36:117. https://doi.org/10.1007/s11095-019-2650-3 [PubMed]
-
172.
Feng D, Liu L, Zhu Y, Chen Q. Molecular signaling toward mitophagy and its physiological significance. Exp Cell Res. 2013; 319:1697–705. https://doi.org/10.1016/j.yexcr.2013.03.034 [PubMed]
-
173.
Zhou H, Zhu P, Guo J, Hu N, Wang S, Li D, Hu S, Ren J, Cao F, Chen Y. Ripk3 induces mitochondrial apoptosis via inhibition of FUNDC1 mitophagy in cardiac IR injury. Redox Biol. 2017; 13:498–507. https://doi.org/10.1016/j.redox.2017.07.007 [PubMed]
-
174.
Zhou H, Zhu P, Wang J, Zhu H, Ren J, Chen Y. Pathogenesis of cardiac ischemia reperfusion injury is associated with CK2α-disturbed mitochondrial homeostasis via suppression of FUNDC1-related mitophagy. Cell Death Differ. 2018; 25:1080–93. https://doi.org/10.1038/s41418-018-0086-7 [PubMed]
-
175.
Dethlefsen MM, Kristensen CM, Tøndering AS, Lassen SB, Ringholm S, Pilegaard H. Impact of liver PGC-1α on exercise and exercise training-induced regulation of hepatic autophagy and mitophagy in mice on HFF. Physiol Rep. 2018; 6:e13731. https://doi.org/10.14814/phy2.13731 [PubMed]
-
176.
Xu JL, Li LY, Wang YQ, Li YQ, Shan M, Sun SZ, Yu Y, Wang B. Hepatocyte-specific deletion of BAP31 promotes SREBP1C activation, promotes hepatic lipid accumulation, and worsens IR in mice. J Lipid Res. 2018; 59:35–47. https://doi.org/10.1194/jlr.M077016 [PubMed]
-
177.
Glick D, Zhang W, Beaton M, Marsboom G, Gruber M, Simon MC, Hart J, Dorn GW 2nd, Brady MJ, Macleod KF. BNip3 regulates mitochondrial function and lipid metabolism in the liver. Mol Cell Biol. 2012; 32:2570–84. https://doi.org/10.1128/MCB.00167-12 [PubMed]
-
178.
Nosaka K, Makishima K, Sakabe T, Yurugi Y, Wakahara M, Kubouchi Y, Horie Y, Umekita Y. Upregulation of glucose and amino acid transporters in micropapillary carcinoma. Histol Histopathol. 2019; 34:1009–14. https://doi.org/10.14670/HH-18-099 [PubMed]
-
179.
Williams JA, Ni HM, Ding Y, Ding WX. Parkin regulates mitophagy and mitochondrial function to protect against alcohol-induced liver injury and steatosis in mice. Am J Physiol Gastrointest Liver Physiol. 2015; 309:G324–40. https://doi.org/10.1152/ajpgi.00108.2015 [PubMed]
-
180.
Song YM, Lee WK, Lee YH, Kang ES, Cha BS, Lee BW. Metformin Restores Parkin-Mediated Mitophagy, Suppressed by Cytosolic p53. Int J Mol Sci. 2016; 17:17. https://doi.org/10.3390/ijms17010122 [PubMed]
-
181.
Wang L, Liu X, Nie J, Zhang J, Kimball SR, Zhang H, Zhang WJ, Jefferson LS, Cheng Z, Ji Q, Shi Y. ALCAT1 controls mitochondrial etiology of fatty liver diseases, linking defective mitophagy to steatosis. Hepatology. 2015; 61:486–96. https://doi.org/10.1002/hep.27420 [PubMed]
-
182.
Kim I, Lemasters JJ. Mitochondrial degradation by autophagy (mitophagy) in GFP-LC3 transgenic hepatocytes during nutrient deprivation. Am J Physiol Cell Physiol. 2011; 300:C308–17. https://doi.org/10.1152/ajpcell.00056.2010 [PubMed]
-
183.
Zhou H, Zhu P, Wang J, Toan S, Ren J. DNA-PKcs promotes alcohol-related liver disease by activating Drp1-related mitochondrial fission and repressing FUNDC1-required mitophagy. Signal Transduct Target Ther. 2019; 4:56. https://doi.org/10.1038/s41392-019-0094-1 [PubMed]
-
184.
Wang J, Zhu P, Li R, Ren J, Zhang Y, Zhou H. Bax inhibitor 1 preserves mitochondrial homeostasis in acute kidney injury through promoting mitochondrial retention of PHB2. Theranostics. 2020; 10:384–97. https://doi.org/10.7150/thno.40098 [PubMed]
-
185.
Weng H, Ma Y, Chen L, Cai G, Chen Z, Zhang S, Ye Q. A New Vision of Mitochondrial Unfolded Protein Response to the Sirtuin Family. Curr Neuropharmacol. 2020. [Epub ahead of print]. https://doi.org/10.2174/1570159X18666200123165002 [PubMed]
-
186.
Shen Y, Ding M, Xie Z, Liu X, Yang H, Jin S, Xu S, Zhu Z, Wang Y, Wang D, Xu L, Zhou X, Wang P, Bi J. Activation of Mitochondrial Unfolded Protein Response in SHSY5Y Expressing APP Cells and APP/PS1 Mice. Front Cell Neurosci. 2020; 13:568. https://doi.org/10.3389/fncel.2019.00568 [PubMed]
-
187.
Roque W, Cuevas-Mora K, Romero F. Mitochondrial Quality Control in Age-Related Pulmonary Fibrosis. Int J Mol Sci. 2020; 21:21. https://doi.org/10.3390/ijms21020643 [PubMed]