Cause of death among patients with colorectal cancer: a population-based study in the United States
Abstract
CRC (Colorectal cancer) is one of the most common causes of death worldwide and in the US (United States). In this study, we aim to perform a population-based analysis on the cause of death among patients with CRC in the US. A total of 834,510 CRC patients diagnosed between 1975 and 2016 in the US were selected from the SEER (Surveillance, Epidemiology, and End Results) program. Causes of death among CRC patients were characterized and SMRs (standardized mortality ratios) of death from non-cancer causes were calculated. Among all CRC patients included in this study, a total of 531,507 deaths were recorded, of which 51.3% were due to CRC, 10.3% were due to other cancers, and 38.4% were due to non-cancer causes. Recently, there has been a relative decrease in index-cancer deaths and an increase in non-cancer causes among CRC patients. The mortality risk from non-cancer rises with accumulating age and longer follow-up time. Cardiovascular diseases are the most prevalent non-cancer causes, accounting for 20.3% of all deaths among CRC patients. Compared with the general population, the mortality rate of non-cancer deaths among CRC patients is doubled (SMR, 2.02; 95% confidence interval, 2.01-2.03).
Introduction
CRC (Colorectal cancer) is the second most common cause of cancer-related death worldwide [1] and the third in the US [2]. Worldwide, in 2018, CRC killed 861,663 people [1]. In the US, during 2018, CRC was responsible for approximately 50,630 deaths [2].
In recent years, significant improvements have been made in the prevention, diagnosis, and treatment of CRC [3], resulting in a continuously improved survival. For patients with CRC in the United States, the relative 5-year survival is nearly 65% [4, 5]. As survivorship of CRC continues to increase [6–8], those involved in healthcare should aim to identify factors increasing the risk of death, which would help identify cancer patients at the highest risk of dying.
In this work, we aimed to characterize the causes of death among patients with CRC in the United States during a long-term follow-up time of more than 40 years. We also analyzed the causes of death among CRC patients as a function of calendar year, age at cancer diagnosis and time after diagnosis. Our work provides a contemporary resource for oncologists and PCPs (primary care physicians) as we highlight both major causes of death and basic clinical presentations (e.g., calendar year, age at cancer diagnosis and follow-up time after cancer diagnosis) among CRC patients which together may influence health care, patient-level decisions.
Results
Using data from the SEER (Surveillance, Epidemiology, and End Results) program, a total of 834,510 patients diagnosed with CRC between 1975 and 2016 were included in this study. Median follow-up time was 3.7 years (range: 0 to 41.9 years). The baseline characteristics of CRC patients are shown in Table 1. The mean age at diagnosis for all patients with CRC was 67.2 years. The majority of the patients were elderly (> 60 of age: 71.4%) and White (80.9%). During the entire follow-up, a total of 531,507 deaths were recorded. Among all the deaths, 51.3% were due to the index cancer (i.e. the CRC originally diagnosed in the patient), 10.3% were due to other cancers (i.e. a second primary cancer), and 38.4% were due to non-cancer causes (i.e. deaths from any medical cause other than cancer) (Table 2). Cardiovascular diseases caused the largest number of deaths among all non-cancer causes, accounting for over 20% of all deaths in patients with CRC.
Table 1. Characteristics of patients diagnosed with colorectal cancer between 1975 and 2016 in SEER 18 registries.
| Characteristics | No. of patients (%) | Person-years of follow-up | No. of deaths (%) | Non-cancer deaths |
| No. of observed deaths (%) | SMR1 (95% CI) |
| All | 834,510 (100%) | 5,055,991 | 531,507 (100%) | 203,990 (100%) | 2.02 (2.01-2.03) |
| Age at diagnosis | | | | | |
| 0-19 | 809 (0.1%) | 4,665 | 170 (0.03%) | 15 (0.01%) | 6.18 (3.72-10.3) |
| 20-39 | 22,855 (2.7%) | 177,196 | 8,187 (1.5%) | 744 (0.4%) | 3.38 (3.15-3.64) |
| 40-59 | 215,379 (25.8%) | 1,634,525 | 91,560 (17.2%) | 18,357 (9.0%) | 2.70 (2.67-2.74) |
| 60-79 | 426,178 (51.1%) | 2,694,377 | 285,297 (53.7%) | 115,513 (56.6%) | 2.40 (2.38-2.41) |
| 80+ | 169,289 (20.3%) | 545,228 | 146,293 (27.5%) | 69,361 (34.0%) | 1.52 (1.51-1.53) |
| Sex | | | | | |
| Female | 406,704 (48.7%) | 2,493,868 | 259,344 (48.8%) | 101,566 (49.8%) | 2.10 (2.09-2.11) |
| Male | 427,806 (51.3%) | 2,562,123 | 272,163 (51.2%) | 102,424 (50.2%) | 1.95 (1.94-1.97) |
| Race | | | | | |
| White | 675,249 (80.9%) | 4,143,177 | 441,802 (83.1%) | 174,670 (85.6%) | 2.01 (2.00-2.02) |
| Black | 89,262 (10.7%) | 472,527 | 55,525 (10.4%) | 17,839 (8.7%) | 1.94 (1.91-1.97) |
| Other | 69,999 (8.4%) | 440,287 | 34,180 (6.4%) | 11,481 (5.6%) | 2.49 (2.44-2.53) |
| Year of diagnosis | | | | | |
| 1975-1989 | 151,952 (18.2%) | 1,267,407 | 144,324 (27.2%) | 63,546 (31.2%) | 2.34 (2.32-2.36) |
| 1990-1999 | 141,277 (16.9%) | 1,171,883 | 116,398 (21.9%) | 52,005 (25.5%) | 2.15 (2.14-2.17) |
| 2000-2009 | 326,362 (39.1%) | 2,098,941 | 201,504 (37.9%) | 72,762 (35.7%) | 1.81 (1.79-1.82) |
| 2010-2016 | 214,919 (25.8%) | 517,760 | 69,281 (13.0%) | 15,677 (7.7%) | 1.70 (1.67-1.72) |
| Marital status | | | | | |
| Married | 452,732 (54.3%) | 3,148,549 | 271,835 (51.1%) | 101,673 (49.8%) | 1.95 (1.94-1.96) |
| Unmarried | 339,099 (40.6%) | 1,661,754 | 238,270 (44.8%) | 93,497 (45.8%) | 2.11 (2.10-2.12) |
| Unknown | 42,679 (5.1%) | 245,688 | 21,402 (4.0%) | 8,820 (4.3%) | 2.02 (1.97-2.06) |
| Stage | | | | | |
| In situ | 38,726 (4.6%) | 390,706 | 19,398 (3.6%) | 14,488 (7.1%) | 2.19 (2.15-2.23) |
| Localized | 297,373 (35.6%) | 2,456,175 | 156,479 (29.4%) | 98,197 (48.1%) | 1.97 (1.96-1.98) |
| Regional | 271,069 (32.5%) | 1,783,020 | 177,373 (33.4%) | 68,573 (33.6%) | 1.91 (1.90-1.93) |
| Distant | 155,377 (18.6%) | 266,283 | 141,487 (26.6%) | 11,112 (5.4%) | 2.46 (2.41-2.51) |
| Unstaged | 71,965 (8.6%) | 159,807 | 36,770 (6.9%) | 11,620 (5.7%) | 2.89 (2.84-2.94) |
| Surgery | | | | | |
| Yes | 715,101 (85.7%) | 4,840,935 | 431,581 (81.2%) | 186,280 (91.3%) | 1.95 (1.94-1.96) |
| No | 110,264 (13.2%) | 189,800 | 92,843 (17.5%) | 16,129 (7.9%) | 3.47 (3.42-3.52) |
| Unknown | 9,145 (1.1%) | 25,257 | 7,083 (1.3%) | 1,581 (0.8%) | 2.76 (2.63-2.90) |
| Chemotherapy | | | | | |
| Yes | 238,583 (28.6%) | 1,215,176 | 139,652 (26.3%) | 23,589 (11.6%) | 1.62 (1.60-1.64) |
| No/Unknown | 595,927 (71.4%) | 3,840,815 | 391,855 (73.7%) | 180,401 (88.4%) | 2.09 (2.08-2.10) |
| Radiotherapy | | | | | |
| Yes | 97,217 (11.6%) | 548,483 | 57,892 (10.9%) | 13,042 (6.4%) | 1.84 (1.81-1.88) |
| No/unknown | 737,293 (88.4%) | 4,507,508 | 473,615 (89.1%) | 190,948 (93.6%) | 2.04 (2.03-2.05) |
| Site | | | | | |
| Cecum | 126,941 (15.2%) | 698,483 | 87,405 (16.4%) | 34,823 (17.1%) | 1.88 (1.86-1.90) |
| Appendix | 12,526 (1.5%) | 66,680 | 4,769 (0.9%) | 1,028 (0.5%) | 1.83 (1.72-1.94) |
| Ascending colon | 95,882 (11.5%) | 531,641 | 60,438 (11.4%) | 26,269 (12.9%) | 1.90 (1.88-1.92) |
| Hepatic flexure | 27,550 (3.3%) | 156,177 | 18,758 (3.5%) | 7,638 (3.7%) | 1.96 (1.92-2.01) |
| Transverse colon | 50,104 (6.0%) | 284,944 | 33,377 (6.3%) | 13,862 (6.8%) | 2.08 (2.05-2.12) |
| Splenic flexure | 19,854 (2.4%) | 117,130 | 13,483 (2.5%) | 4,934 (2.4%) | 2.03 (1.98-2.09) |
| Descending colon | 37,296 (4.5%) | 244,856 | 24,022 (4.5%) | 9,980 (4.9%) | 2.19 (2.15-2.23) |
| Sigmoid colon | 185,664 (22.2%) | 1,295,333 | 115,320 (21.7%) | 48,365 (23.7%) | 2.09 (2.07-2.11) |
| Large intestine, NOS | 29,555 (3.5%) | 469,411 | 24,401 (4.6%) | 16,727 (8.2%) | 2.09 (2.06-2.12) |
| Rectosigmoid junction | 71,794 (8.6%) | 90,971 | 46,862 (8.8%) | 4,983 (2.4%) | 2.45 (2.38-2.52) |
| Rectum | 177,344 (21.3%) | 1,100,366 | 102,672 (19.3%) | 35,381 (17.3%) | 2.06 (2.03-2.08) |
| Abbreviations: SMR, standardized mortality ratios; CI, confidence interval. |
| 1 The SMRs were estimated as the ratios of observed to expected number of deaths. The observed values represented the number of deaths in cancer patients, whereas the expected values represented the number of individuals who died of the same causes in the general population, with a similar distribution of age, sex, race, and calendar year. |
The mortality rate of all non-cancer deaths was 2.02 (95% CI [confidence interval], 2.01-2.03) times that of the general cancer-free population with similar demographic distribution. The most frequent distant metastatic site in CRC was liver metastasis (14.8%), followed by lung metastasis (5%). Though less frequent, SMRs (standardized mortality ratios) were elevated for patients who were with distant metastases (Supplementary Tables 1–3). Compared to the cause-specific mortality in the general population, SMRs were the highest for renal diseases and infectious disease among all major causes, with an SMR of 2.47 (95% CI, 2.41-2.54) and 2.32 (95% CI, 2.28-2.35), respectively. Among less frequent causes, elevations in the SMR were remarkable for certain conditions originating in the perinatal period (SMR, 124.5; 95% CI, 59.3-261.1), and complications of pregnancy, childbirth and puerperium (SMR, 20.6; 95% CI, 14.6-29.0). This is because the mortality rates from these causes are particularly low in the general population. Detailed causes of death for CRC patients are presented in Table 2. For most causes, the SMR were higher for patients with advanced diseases, not receiving surgery, chemotherapy or radiotherapy (Table 1 and Supplementary Tables 4–7).
Table 2. Causes of death for patients diagnosed with colorectal cancers between 1975 and 2016 in SEER 18 registries.
| Causes of death | Patients with colorectal cancer | | General population1 | SMR1,2 (95% CI) |
| No. of observed deaths (%) | Mortality rates (per 100,000 person-years) | | No. of expected deaths (%) | Mortality rates (per 100,000 person-years) |
| All causes of death | 531,507 (100.0%) | 10,512.4 | | NA | NA | NA |
| Colorectal cancer | 272,901 (51.3%) | 5,397.6 | | NA | NA | NA |
| Other cancers | 54,616 (10.3%) | 1,080.2 | | NA | NA | NA |
| Non-cancer causes | 203,990 (38.4%) | 4,034.6 | | 100,833.4 | 1,994.3 | 2.02 (2.01 -2.03) |
| Infectious diseases | 14,942 (2.8%) | 295.5 | | 6,453.0 | 127.6 | 2.32 (2.28 -2.35) |
| Pneumonia and influenza | 8,792 (1.7%) | 173.9 | | 3,978.9 | 78.7 | 2.21 (2.16 -2.26) |
| Syphilis | 2 (0.0004%) | 0.04 | | 2.6 | 0.05 | 0.76 (0.19 -3.05) |
| Tuberculosis | 75 (0.01%) | 1.5 | | 79.5 | 1.6 | 0.94 (0.75 -1.18) |
| Septicemia | 4,207 (0.8%) | 83.2 | | 1,505.5 | 29.8 | 2.79 (2.71 -2.88) |
| Other infectious and parasitic diseases including HIV | 1,866 (0.4%) | 36.9 | | 659.8 | 13.0 | 2.83 (2.70 -2.96) |
| Cardiovascular diseases | 108,086 (20.3%) | 2,137.8 | | 57,753.6 | 1,142.3 | 1.87 (1.86 -1.88) |
| Diseases of heart | 82,947 (15.6%) | 1,640.6 | | 44,470.3 | 879.6 | 1.87 (1.85 -1.88) |
| Hypertension without heart disease | 2,820 (0.5%) | 55.8 | | 995.9 | 19.7 | 2.83 (2.73 -2.94) |
| Aortic aneurysm and dissection | 1,478 (0.3%) | 29.2 | | 997.1 | 19.7 | 1.48 (1.41 -1.56) |
| Atherosclerosis | 1,961 (0.4%) | 38.8 | | 1,023.8 | 20.2 | 1.92 (1.83 -2.00) |
| Cerebrovascular diseases | 17,750 (3.3%) | 351.1 | | 9,690.2 | 191.7 | 1.83 (1.81 -1.86) |
| Other diseases of arteries, arterioles, capillaries | 1,130 (0.2%) | 22.3 | | 574.8 | 11.4 | 1.97 (1.85 -2.08) |
| Respiratory diseases | 13,713 (2.6%) | 271.2 | | 7,031.0 | 139.1 | 1.95 (1.92 -1.98) |
| Chronic obstructive pulmonary disease and allied cond | 13,713 (2.6%) | 271.2 | | 7,031.0 | 139.1 | 1.95 (1.92 -1.98) |
| Gastrointestinal diseases | 2,907 (0.5%) | 57.5 | | 1,719.9 | 34.0 | 1.69 (1.63 -1.75) |
| Stomach and duodenal ulcers | 618 (0.1%) | 12.2 | | 314.9 | 6.2 | 1.96 (1.81 -2.12) |
| Chronic liver disease and cirrhosis | 2,289 (0.4%) | 45.3 | | 1,404.5 | 27.8 | 1.63 (1.56 -1.70) |
| Renal diseases | 4,919 (0.9%) | 97.3 | | 1,988.9 | 39.3 | 2.47 (2.41 -2.54) |
| Nephritis, nephrotic syndrome and nephrosis | 4,919 (0.9%) | 97.3 | | 1,988.9 | 39.3 | 2.47 (2.41 -2.54) |
| External injuries | 6,646 (1.3%) | 131.4 | | 4,257.7 | 84.2 | 1.56 (1.52 -1.60) |
| Accidents and adverse effects | 5,118 (1.0%) | 101.2 | | 3,169.6 | 62.7 | 1.61 (1.57 -1.66) |
| Suicide and self-inflicted injury | 1,360 (0.3%) | 26.9 | | 859.1 | 17.0 | 1.58 (1.50 -1.67) |
| Homicide and legal intervention | 168 (0.0%) | 3.3 | | 227.9 | 4.5 | 0.74 (0.63 -0.86) |
| Other non-cancer causes | 52,777 (9.9%) | 1,043.9 | | 21,629.1 | 427.8 | 2.44 (2.42 -2.46) |
| Alzheimer’s disease | 7,533 (1.4%) | 149.0 | | 2,432.7 | 48.1 | 3.10 (3.03 -3.17) |
| Diabetes mellitus | 7,249 (1.4%) | 143.4 | | 3,676.3 | 72.7 | 1.97 (1.93 -2.02) |
| Congenital anomalies | 174 (0.03%) | 3.4 | | 129.7 | 2.6 | 1.34 (1.16 -1.56) |
| Certain conditions originating in perinatal period | 7 (0.001%) | 0.1 | | 0.1 | 0.001 | 124.5 (59.3 -261.1) |
| Complications of pregnancy, childbirth, puerperium | 33 (0.01%) | 0.7 | | 1.6 | 0.03 | 20.58 (14.63 -28.95) |
| Symptoms, signs and ill-defined conditions | 2,582 (0.5%) | 51.1 | | 1,291.2 | 25.5 | 2.00 (1.92 -2.08) |
| Other cause of death | 35,199 (6.6%) | 696.2 | | 14,093.9 | 278.8 | 2.50 (2.47 -2.52) |
| Abbreviations: SMR, standardized mortality ratios; CI, confidence interval. |
| 1The calculating of SMR is based on the hypothesis that the general population is cancer-free, thus the expected deaths and SMR for colorectal cancer and other cancers cannot be calculated. |
| 2The SMRs were estimated as the ratios of observed to expected number of deaths. The observed values represented the number of deaths in cancer patients, whereas the expected values represented the number of individuals who died of the same causes in the general population, with a similar distribution of age, sex, race, and calendar year. |
Objective 1: Cause of death in patients with CRC per calendar year
We analyzed death trends in CRC patients by assessing deaths due to CRC (index cancer), deaths due to other cancers and deaths due to non-cancer causes among all CRC subtypes. Patients who are more likely to die from index cancer are those with cancers of the appendix and large intestine, NOS, cancer types for which the cancer-specific prognosis has been relatively stable for the past few decades (Figure 1). With the improvement in cancer prognosis (cecum, ascending colon, hepatic flexure, transvers colon, splenic flexure, descending colon, segment colon, rectum and rectosigmoid junction), there appears to be a continuous increase in the number of deaths due to non-cancer causes, and a decrease in the number of deaths due to index cancer in cancer survivors (Figure 1). In 2016, non-cancer causes accounted for 48.8% of all deaths among all CRC patients, while the index cancer accounted for 39.4% of all deaths.
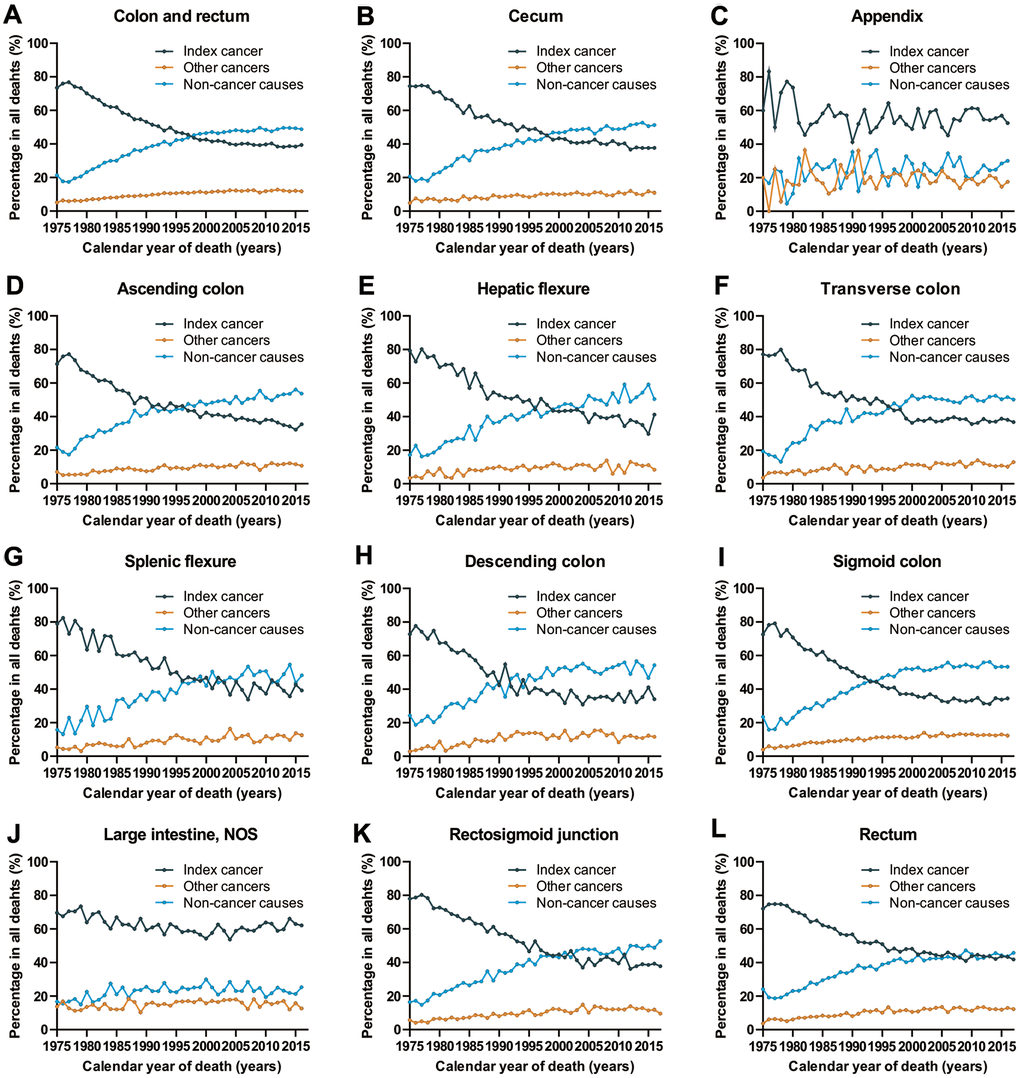
Figure 1. Cause of death among patients diagnosed with CRC in SEER 9 registries by calendar year of death. (A) Cause of death among patients with CRC by year of death; (B) cause of death among patients with cancer of cecum by year of death; (C) cause of death among patients with appendicular cancer by year of death; (D) cause of death among patients with cancer of ascending colon by year of death; (E) cause of death among patients with cancer of hepatic flexure by year of death; (F) cause of death among patients with cancer of transverse colon by year of death; (G) cause of death among patients with cancer of splenic flexure by year of death; (H) cause of death among patients with cancer of descending colon by year of death; (I) cause of death among patients with cancer of sigmoid colon by year of death; (J) cause of death among patients with cancer of large intestine, NOS by year of death; (K) cause of death among patients with cancer of rectosigmoid junction by year of death; (L) cause of death among patients with cancer of rectum by year of death.
Objective 2: Cause of death in patients with CRC by age at cancer diagnosis
Among CRC patients, the number of deaths increased with age, reaching a peak at 70-75 years of age (Figure 2A). Although CRC was the major cause of death among all age groups, the percentage of patients dying from CRC decreased with increasing of age, while the non-cancer causes (specifically cardiovascular diseases) were increasing (Figure 2B).
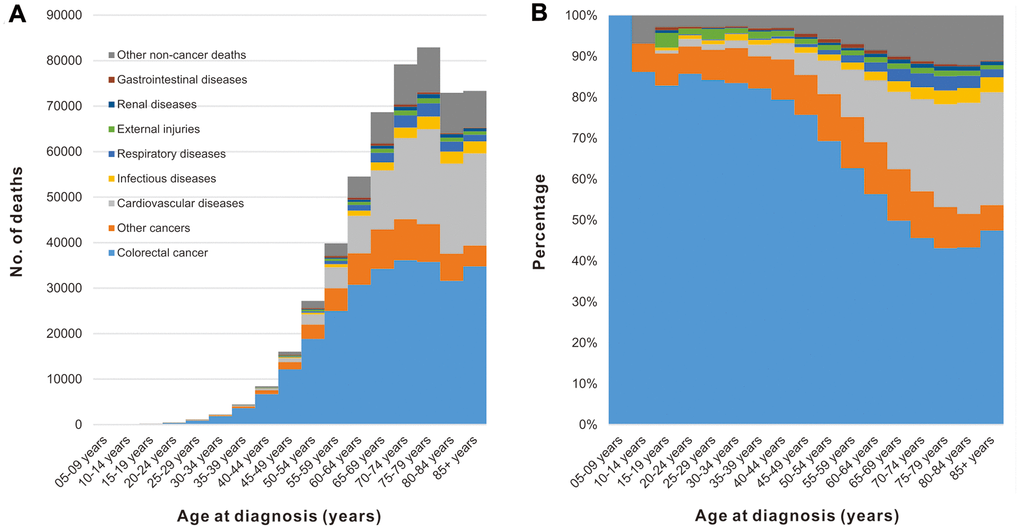
Figure 2. Cause of death among patients diagnosed with CRC in SEER 18 registries by age at diagnosis. (A) Number of deaths from different causes by age at diagnosis. (B) Percentage of deaths from different causes by age at diagnosis.
Compared to individuals of a similar age in the general US population, cancer patients of all ages have an increased risk of non-cancer deaths (Figure 3). For nearly all types of non-cancer causes, the highest SMR of death was observed in cancer patients younger than 40 years old. However, the prevalence of non-cancer deaths in CRC patients was very low with only 759 deaths in CRC patients younger than 40 years of age between 1975 and 2016 (Table 1). The SMR of non-cancer deaths among CRC patients gradually decreases with increasing age at cancer diagnosis. This decreasing trend was not observed in deaths caused by external injuries. In this context, another peak of SMR in CRC patients occurred at ages between 70 and 74 years old (Figure 3).
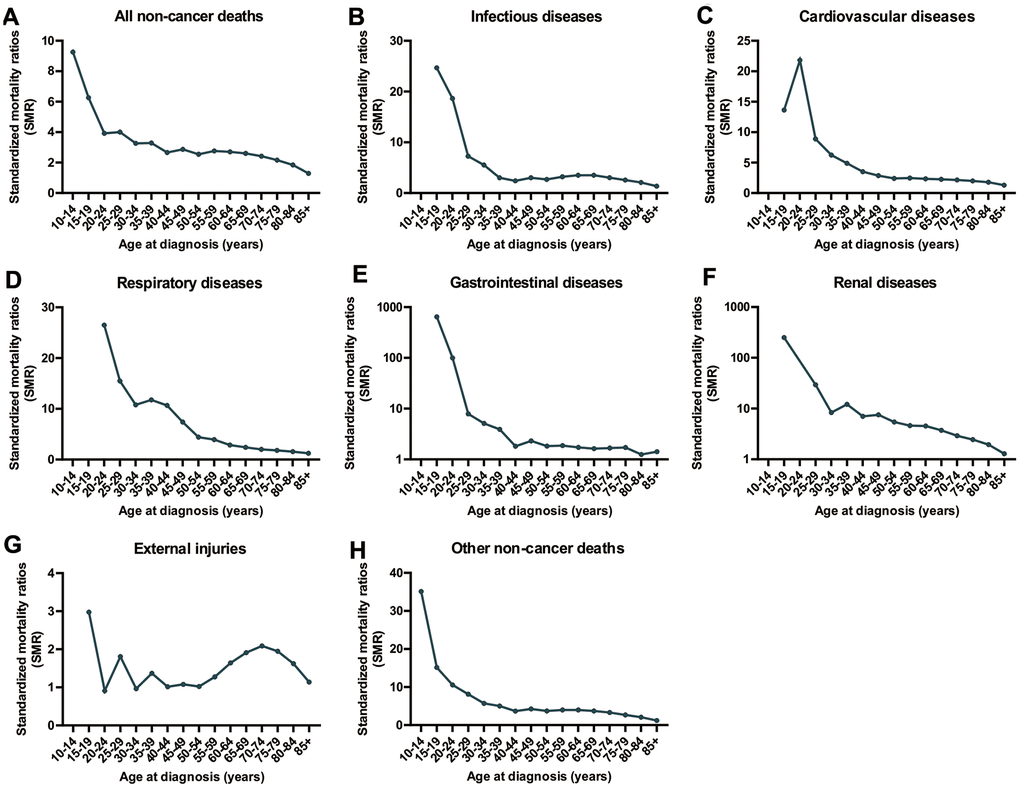
Figure 3. SMRs of non-cancer deaths among patients diagnosed with CRC in SEER 18 registries by age at diagnosis. (A) SMRs of all non-cancer deaths by age; (B) SMRs of infectious diseases by age; (C) SMRs of cardiovascular diseases by age; (D) SMRs of respiratory diseases by age; (E) SMRs of renal diseases by age; (F) SMRs of external injuries by age; (G) SMRs of cardiovascular diseases by age; (H) SMRs of other non-cancer deaths by age.
Objective 3: Cause of death in patients with CRC by time after diagnosis
The time period following a cancer diagnosis (all sites) represents the period with the highest risk of cancer-specific mortality (Figure 4 and Supplementary Table 8). With an increasing period of time from the point of cancer diagnosis, there was an increasing trend in deaths from non-cancer causes. In fact, non-cancer causes became the leading cause of death in cancer survivors with a cancer history of more than 5 years (Figure 4 and Supplementary Table 8).
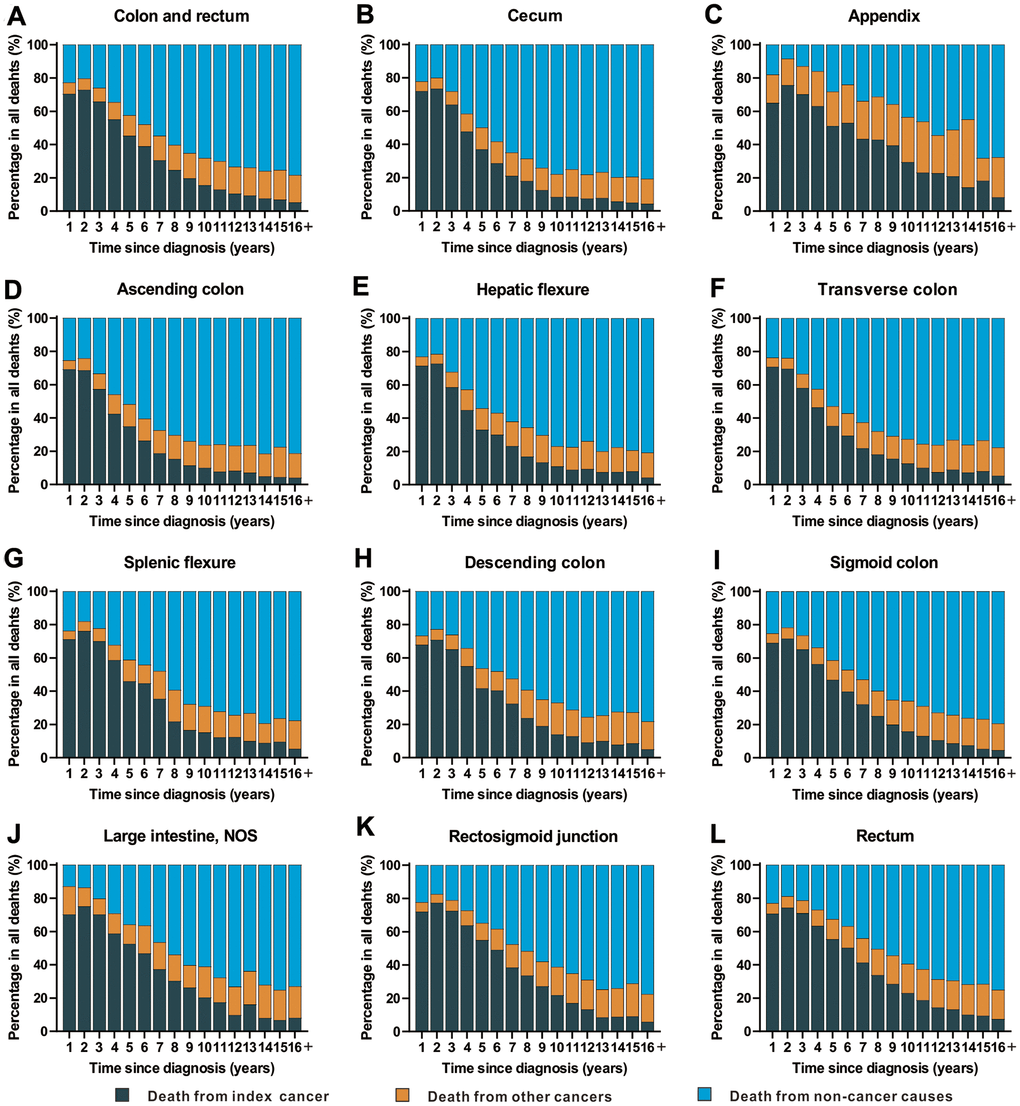
Figure 4. Cause of death among patients diagnosed with CRC in SEER 18 registries by follow-up time from diagnosis. (A) Cause of death among patients with CRC by time from diagnosis; (B) cause of death among patients with cancer of cecum by time from diagnosis; (C) cause of death among patients with appendicular cancer by time from diagnosis; (D) cause of death among patients with cancer of ascending colon by time from diagnosis; (E) cause of death among patients with cancer of hepatic flexure by time from diagnosis; (F) cause of death among patients with cancer of transverse colon by time from diagnosis; (G) cause of death among patients with cancer of splenic flexure by time from diagnosis; (H) cause of death among patients with cancer of descending colon by time from diagnosis; (I) cause of death among patients with cancer of sigmoid colon by time from diagnosis; (J) cause of death among patients with cancer of large intestine, NOS by time from diagnosis; (K) cause of death among patients with cancer of rectosigmoid junction by time from diagnosis; (L) cause of death among patients with cancer of rectum by time from diagnosis.
Compared with the general population, CRC patients displayed SMRs which were higher than the general population throughout the entire follow-up period (Supplementary Table 8). In the first 5-years after diagnosis, SMRs decreased slightly. An increasing trend for the SMR of non-cancer death was observed in cancer survivors with a cancer diagnosis of more than five years, and the highest SMR of non-cancer mortality was observed after a long-term follow-up of more than 15 years (Supplementary Table 8).
Discussion
In this study we present an analysis of cause of death among more than 0.8 million CRC patients. In patients diagnosed with CRC, cause of death varies as a function of calendar year of diagnosis, follow-up time, and other clinical variables, such as age and race. Non-cancer causes are the major health threats for long-term CRC survivors, and the mortality rate of non-cancer death is more than double in cancer patients compared to the general population. In recent years, with the increase in the number of long-term CRC survivors, non-cancer causes (especially cardiovascular diseases) have become the major cause of death in CRC patients, and are still showing an increasing trend. Previous studies have analyzed the causes of death among patients with different types of cancer [9, 10]. Further, several investigations have also reported the causes of death in specific cancer types such as breast cancer, and head and neck cancer [11, 12], or among a subgroup of patients such as adolescents and young adults [13]. Unfortunately, no detailed research has been performed on colorectal cancer. Thus, the work presented here is unique.
We found that deaths from non-cancer causes and second primary cancer were increasing in recent years, and non-cancer diseases are now the main cause of death among CRC patients. An increase in the risk of deaths from non-cancer causes and second cancer was observed when the follow-up time was accumulating. It is known that the advances in cancer treatments, although remarkably reduced mortality from the primary cancer, may increase the future risk of death from the causes other than the primary cancer, including development of a second primary cancer or other non-cancer comorbidities [12, 14, 15]. With better treatment patterns in palliative [16] and adjuvant [17–20] settings, the last 2 decades have seen remarkable improvements in the prognosis among patients with CRC in the US [21]. Systematic therapy for metastatic CRC is tailored with disease-specific and patient-specific predictive markers. With the progress in the research of tumorigenic mechanism, many potential therapy targets have been found [22–24], which contributed greatly to the prognosis of CRC patients. Paralleled with the breakthroughs in surgical and allied areas, the increasing kinds and number of effective drugs for CRC has led to considerable improvements of overall survival. Higher education status and healthy dietary can also improve the prognosis of CRC patients [25–27]. Thus, after a CRC diagnosis, patients may live longer to a stage when non-cancer causes could affect their survival time significantly. The Sweden cohort revealed that after the median follow-up, almost half of the CRC patients who were dead had died from other causes than colon cancer. Our study further demonstrated that non-cancer causes account for the majority of the deaths for the patients with a long CRC history [28]. It’s also reported that long-term CRC survivors are at high risk of developing a second primary malignancy [29]. With an increasing risk of second primary cancer by time from diagnosis, nearly 10% of CRC survivors will have a second primary cancer.
Our work also evaluated the cause of death among cancer patients as a function of age at diagnosis. We found that the proportion of non-cancer deaths, especially the cardiovascular diseases, were increasing with an increased age. Most patients with colorectal cancer are older and may have multiple comorbidities. In a study from Canada, the researchers found that cardiovascular diseases is the major non-cancer cause of death in the elderly CRC patients [30], consistent with our results. Age is one of the major risk factors associated with some non-cancer diseases, such as cardiovascular diseases and chronic obstructive pulmonary disease [31, 32]. Intriguingly, we found that patients younger than 40 posed a very high risk of death from non-cancer causes when comparing to the age-matched general population, which is agree with previous studies [10, 13]. This elevation may be due to the low mortality rate of deaths from non-cancer diseases in young individuals of the general cancer-free population, as these diseases are often related with elderly age.
Throughout all time periods, the most common non-cancer cause of death was cardiovascular diseases, mainly heart disease and cerebrovascular disease. The high risk of heart diseases in CRC patients may due to the cardiac side effects of systemic chemotherapy. Acute cardiotoxicity is recognized as a potentially severe adverse event of 5-FU and capecitabine treatment [33, 34]. This dose-dependent toxicity could lead to acute myocardial infarction and ischemia [35, 36]. A retrospective study of adjuvant chemotherapy revealed that there was no relevance between relative dose intensity and overall survival for stage III colon cancer [37], while another survey found better 5-year overall survival for patients who received >70% relative dose intensities [38]. Thus, the scientific evidence is too rare to guide clinical decisions on whether to treat patients with these drugs, but the risk of severe cardiotoxicity should be evaluated against the expected treatment benefits. In addition, a study of the Dutch Colorectal Cancer Group analyzed cardiotoxicity in 1973 patients who received capecitabine for metastatic colorectal cancer [39]. They reported that a significant number of patients suffered cardiotoxicity following treatment with capecitabine, and a high incidence of cardiac cases was observed in patients who were treated with the combination of capecitabine, oxaliplatin and bevacizumab. A few cases of oxaliplatin-induced arrhythmias have also been reported [40]. Further, the risk of cerebrovascular events was significantly increased among patients with advanced colorectal cancer who received bevacizumab [41], as bevacizumab may cause rare toxic effects such as cerebral hemorrhage, intracranial hemorrhage and subarachnoid hemorrhage [42]. Bevacizumab was also associated with higher risk of hypertension and arterial thrombotic events [43], which in turn increased the risk of cardiac ischemia (myocardial infarction or angina), stroke or transient ischemic attack.
Patients with CRC has a particularly increased mortality rate from infectious diseases. As a predominant cause of death among patients with cancer, fatal infections have been interpreted to be a consequence of the immunosuppression induced by the malignancy itself and by various modern cancer therapies, including chemotherapeutic regimens, invasive procedures, or medical devices and malnutrition [44–46]. Neutropenia-related infection has been shown to be a common side effect of chemotherapy and can result in sepsis [47, 48]. Further, patients undergoing monoclonal antibody therapy have also been shown to be at risk of developing immunosuppression and thus being at equal risk of infections. In cases of rapid tumor growth, insufficient blood supply can lead to a nidus of infection. The risk of infectious disease should be evaluated individually as it is dependent on the patients’ immune status, treatment methods and history of infections. Therefore, health practitioners should use preventive measures to reduce the risk of infections and select appropriate treatments.
Furthermore, our study showed that the SMR of suicide and self-inflicted injury were higher in the first year after diagnosis (Supplementary Table 3). The incidence of suicidal death increased substantially after the diagnosis of a cancer [49]. Depression, anxiety and opioid use have been linked with suicide among cancer patients. In patients with CRC, such issues are often linked with demands related to the care of ostomies [50]. In addition, stress may weaken the immune system, which could lead to poorer response to treatments and mental stress, which in turn add to the risk of a suicide attempt [51]. AD (Alzheimer’s disease) was also another important non-cancer cause of death among CRC patients (Supplementary Table 3). In recent years, some researchers have found that diet and constipation might influence the progression of AD [52]. Constipation is a growing health problem among CRC patients and such patients might have a higher risk of mortality from AD than the general US population.
This study has some limitations. First, there is a risk of reporting bias in death certificates which could lead to misclassification of causes of death [53, 54]. However, the SEER mortality data were provided by the NCHS (National Center for Health Statistics) and NVSS (National Vital Statistics System) and systematic and standardized data collection procedures are used to ensure that the causes of death recorded in SEER are accurate [55]. Further, previous studies have also examined the validity and reliability of the use of death certificates recorded in the SEER and the results suggested that they were acceptable [56, 57]. Second, SEER does not contain information regarding pre-existing comorbidities, performance status, quality of life, as well as detailed and complete information of cancer treatment. Thus, we were not able to analyze the cause and effect relationship between different risk factors and distinct causes of death. Nevertheless, analyzing the extensive amount of data available from the SEER database remains a powerful, useful, and integral tool in medical research for the purpose of exploratory analyses [55]. Third, we were unable to obtain data on genetic information (such as MMR (defective mismatch repair) genes or mutations status of KRAS and BRAF) and dietary habits, which prohibited us from taking these features into account.
In conclusion, the risk of index cancer death in CRC patients decreases with time, while that of non-cancer causes increases. Patients with CRC are at high risk of non-cancer death. In fact, the mortality rate of non-cancer death among CRC patients is twice that of the general population. The SMR of non-cancer deaths increases with longer follow-up time. CRC is the major cause of death in the first few years after a cancer diagnosis. Cardiovascular diseases are also important causes of death in CRC patients. Identifying the major causes of death among cancer patients is important for the formulation of health strategies. Investigating the influence of non-cancer deaths on the health outcomes of CRC patients is an important field for future research.
Materials and Methods
Data source and study population
Using data from the SEER program, we performed a retrospective population-based cohort study. As a system of population-based cancer registries from the National Cancer Institute, the program routinely collects and reports data on cancer demographics, incidence, follow-up data, anatomic site, morphology, stage, therapy and socioeconomic status of cancer patents in the United States [58].
All patients diagnosed with CRC between 1975 and 2016 were extracted from the SEER 18 database (2019 submission) using SEER*Stat software version 8.3.6 [58]. All types of cancers excluding a histology of mesothelial neoplasms (ICD-O-3 [International Classification of Diseases for Oncology third edition] codes: 9050-9055), Kaposi sarcoma (ICD-O-3 codes: 9140) and hematological malignancies (ICD-O-3 codes: 9590-9992) diagnosed in the colon and rectum (ICD-O-3 site codes: C18.0-C18.9, C26.0) were included in this study. Only the first primary diagnosis of colorectal cancer was included. Patients were excluded if their diagnoses were obtained only from death certificates or autopsies. We further excluded patients without active follow-up and those with unknown follow-up time, age at diagnosis or cause of death. (Figure 5). To analyze the causes of death among CRC patients as a function of calendar year of death, we extracted death data of CRC patients from 1975 to 2016 using the SEER-9 incidence-based mortality session, which accurately records the year of death but not the year of diagnosis for cancer survivors [59]. To compare with the general population, mortality data of the general US population collected by the National Center for Health Statistics between 1975 and 2016 were also extracted from the SEER program [60].
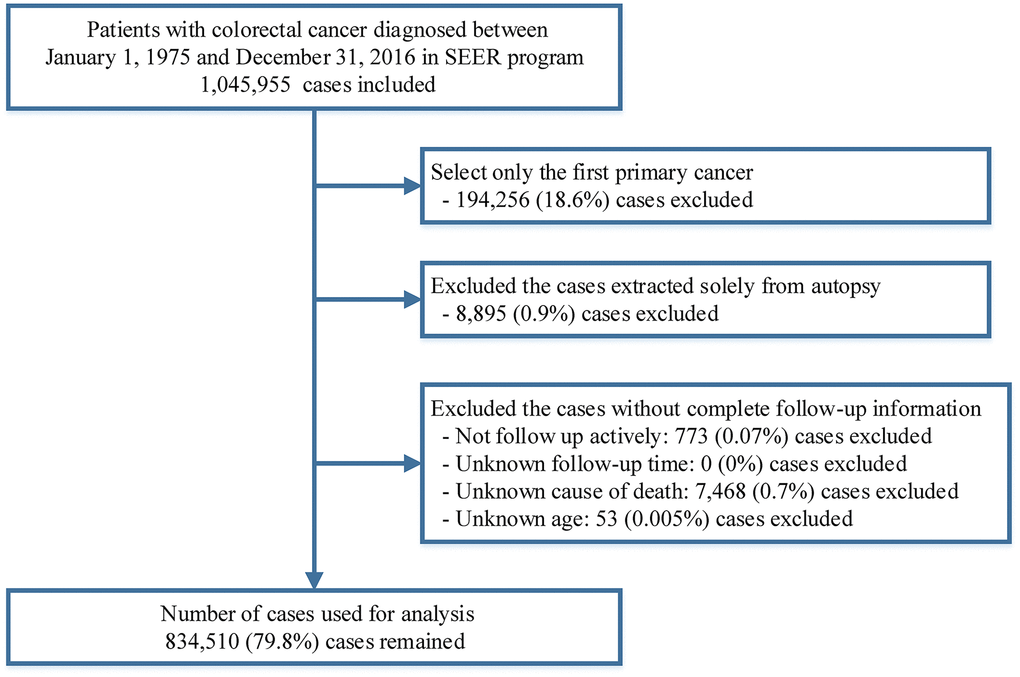
Figure 5. Flow chart of case inclusion and exclusion criteria in this study.
As a publicly available database, the access of SEER data required a signed Research Data Agreement form. As it is considered as a non-human subject research and all information are anonymized, the data derived from SEER program are waived from IRB (Institutional Review Board) approval and informed consents.
Definition of variables
Patients were followed up from the time of cancer diagnosis until death, the date of last follow-up, or exit at the end of the study (December 31, 2016). We examined the following variables for patients included in this study: age at diagnosis, sex, race, year of diagnosis, marital status, survival months, cause of death, anatomic site, cancer stage, and therapies including surgery, radiotherapy and chemotherapy.
Causes of death of patients with CRC were classified into three major groups: death from index cancer, death from other cancers and death from non-cancer causes. Causes of death were defined by SEER cause-specific death classification variable from death certificates [13]. Non-cancer causes were categorized into 26 major groups. These groups were further consolidated into 7 broad categories: infectious diseases, cardiovascular diseases, respiratory diseases, gastrointestinal and liver diseases, renal diseases, external injuries, and other causes. Although deaths from in situ, benign, or unknown behavior neoplasm were also classified to as non-cancer deaths by SEER program [13], these deaths were not considered as non-cancer deaths in our analyses.
In the SEER program, the survival duration of patients in the SEER database was measured in months and a month was the shortest time interval available for analysis. Any patient survival duration shorter than a month was recorded as 0 months. Thus, patients with a survival time coded as zero were converted to a-half month according to standard epidemiologic convention [61].
Statistical analysis
Mortality rates were calculated as the number of deaths divided by the total person-years of follow-up. For non-cancer causes, the SMRs and 95% CIs of non-cancer deaths were calculated to perform a comparison with the general population [55, 61–63]. The SMR is based on the assumptions that the general population is cancer-free, and thus it can be used to compare the mortality rates from non-cancer diseases (e.g. cardiovascular, infectious and respiratory diseases) in the cancer population and the cancer-free population, which may reflect the impact of cancer and its treatments on non-cancer diseases. However, the SMR cannot be used for cancer-related causes. SMRs were estimated as the ratios of observed to expected number of deaths. The observed number of deaths represents the number of deaths from certain causes in cancer patients, and the expected number of deaths represents the number of people who died from the same causes in the general population with a similar distribution of age, sex, race and calendar year. For both age and calendar year, the value at diagnosis was used and were further divided into five-year categories in the course of standardization. 95% CI of SMR were obtained using an approximation from a Poisson distribution [61, 64].
For Objective 1, death data were extracted from SEER 9 registries database, which continually coded death trends from various causes by calendar year of death. For Objectives 2 and 3, we described the risk of death from non-cancer causes as a function of age at cancer diagnosis and follow-up time after cancer diagnosis, respectively. All statistical tests were two-sided, and P values less than 0.05 were considered to be statistically significant. Analyses were performed with SEER*Stat software version 8.3.6 and the R version 3.52 statistical software [58, 65].
Abbreviations
SEER: Surveillance, Epidemiology, and End Results;
SMRs: standardized mortality ratios;
CI: confidence interval;
CRC: colorectal cancer;
PCPs: primary care physicians;
AD: Alzheimer’s disease;
NCHS: National Center for Health Statistics;
NVSS: National Vital Statistics System;
MMR: mismatch repair.
Author Contributions
Research designer: Jiayuan Chen, Zhenyu Lin, Tao Zhang. Collecting, analyzing and interpreting data: Yongqiang Zheng, Haihong Wang, Dejun Zhang, Lei Zhao, Dandan Yu. The main contributors to writing manuscripts: Jiayuan Chen, Zhenyu Lin. The final draft read and approved by all authors.
Acknowledgments
The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER database.
Conflicts of Interest
All authors of this study stated that they have no conflicts of interest.
Funding
This work was supported by the National Natural Science Foundation of China (grant 81874061) and Chinese Society of Clinical Oncology Shiyao Cancer Research Fund (grant Ysy2019009).
References
-
1.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424. https://doi.org/10.3322/caac.21492 [PubMed]
-
2.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018; 68:7–30. https://doi.org/10.3322/caac.21442 [PubMed]
-
3.
Edwards BK, Noone AM, Mariotto AB, Simard EP, Boscoe FP, Henley SJ, Jemal A, Cho H, Anderson RN, Kohler BA, Eheman CR, Ward EM. Annual report to the nation on the status of cancer, 1975-2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014; 120:1290–314. https://doi.org/10.1002/cncr.28509 [PubMed]
-
4.
White A, Joseph D, Rim SH, Johnson CJ, Coleman MP, Allemani C. Colon cancer survival in the United States by race and stage (2001-2009): findings from the CONCORD-2 study. Cancer. 2017 (Suppl 24); 123:5014–36. https://doi.org/10.1002/cncr.31076 [PubMed]
-
5.
Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL, Siegel RL. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019; 69:363–85. https://doi.org/10.3322/caac.21565 [PubMed]
-
6.
Hashim D, Boffetta P, La Vecchia C, Rota M, Bertuccio P, Malvezzi M, Negri E. The global decrease in cancer mortality: trends and disparities. Ann Oncol. 2016; 27:926–33. https://doi.org/10.1093/annonc/mdw027 [PubMed]
-
7.
Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017; 66:683–91. https://doi.org/10.1136/gutjnl-2015-310912 [PubMed]
-
8.
Markham MJ, Wachter K, Agarwal N, Bertagnolli MM, Chang SM, Dale W, Diefenbach CS, Rodriguez-Galindo C, George DJ, Gilligan TD, Harvey RD, Johnson ML, Kimple RJ, et al. Clinical cancer advances 2020: annual report on progress against cancer from the American society of clinical oncology. J Clin Oncol. 2020; 38:1081. https://doi.org/10.1200/JCO.19.03141 [PubMed]
-
9.
Ye Y, Otahal P, Marwick TH, Wills KE, Neil AL, Venn AJ. Cardiovascular and other competing causes of death among patients with cancer from 2006 to 2015: an Australian population-based study. Cancer. 2019; 125:442–52. https://doi.org/10.1002/cncr.31806 [PubMed]
-
10.
Zaorsky NG, Churilla TM, Egleston BL, Fisher SG, Ridge JA, Horwitz EM, Meyer JE. Causes of death among cancer patients. Ann Oncol. 2017; 28:400–07. https://doi.org/10.1093/annonc/mdw604 [PubMed]
-
11.
Afifi AM, Saad AM, Al-Husseini MJ, Elmehrath AO, Northfelt DW, Sonbol MB. Causes of death after breast cancer diagnosis: a US population-based analysis. Cancer. 2020; 126:1559–67. https://doi.org/10.1002/cncr.32648 [PubMed]
-
12.
Baxi SS, Pinheiro LC, Patil SM, Pfister DG, Oeffinger KC, Elkin EB. Causes of death in long-term survivors of head and neck cancer. Cancer. 2014; 120:1507–13. https://doi.org/10.1002/cncr.28588 [PubMed]
-
13.
Anderson C, Lund JL, Weaver MA, Wood WA, Olshan AF, Nichols HB. Noncancer mortality among adolescents and young adults with cancer. Cancer. 2019; 125:2107–14. https://doi.org/10.1002/cncr.32063 [PubMed]
-
14.
Micheli A, Mugno E, Krogh V, Quinn MJ, Coleman M, Hakulinen T, Gatta G, Berrino F, Capocaccia R, and EUROPREVAL Working Group. Cancer prevalence in European registry areas. Ann Oncol. 2002; 13:840–65. https://doi.org/10.1093/annonc/mdf127 [PubMed]
-
15.
Shin DW, Ahn E, Kim H, Park S, Kim YA, Yun YH. Non-cancer mortality among long-term survivors of adult cancer in Korea: national cancer registry study. Cancer Causes Control. 2010; 21:919–29. https://doi.org/10.1007/s10552-010-9521-x [PubMed]
-
16.
Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, Barbara C, Crinò L, Benedetti G, Evangelista W, Fanchini L, Cortesi E, Picone V, Vitello S, et al, and Gruppo Oncologico Nord Ovest. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007; 25:1670–6. https://doi.org/10.1200/JCO.2006.09.0928 [PubMed]
-
17.
Haller DG, Tabernero J, Maroun J, de Braud F, Price T, Van Cutsem E, Hill M, Gilberg F, Rittweger K, Schmoll HJ. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011; 29:1465–71. https://doi.org/10.1200/JCO.2010.33.6297 [PubMed]
-
18.
Yothers G, O’Connell MJ, Allegra CJ, Kuebler JP, Colangelo LH, Petrelli NJ, Wolmark N. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol. 2011; 29:3768–74. https://doi.org/10.1200/JCO.2011.36.4539 [PubMed]
-
19.
van der Valk MJ, Hilling DE, Bastiaannet E, Meershoek-Klein Kranenbarg E, Beets GL, Figueiredo NL, Habr-Gama A, Perez RO, Renehan AG, van de Velde CJ, Ahlberg M, Appelt A, Asoglu O, et al, and IWWD Consortium. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet. 2018; 391:2537–45. https://doi.org/10.1016/S0140-6736(18)31078-X [PubMed]
-
20.
Lefevre JH, Mineur L, Kotti S, Rullier E, Rouanet P, de Chaisemartin C, Meunier B, Mehrdad J, Cotte E, Desrame J, Karoui M, Benoist S, Kirzin S, et al. Effect of Interval (7 or 11 weeks) Between Neoadjuvant Radiochemotherapy and Surgery on Complete Pathologic Response in Rectal Cancer: A Multicenter, Randomized, Controlled Trial (GRECCAR-6). J Clin Oncol. 2016; 34:3773–3780. https://doi.org/10.1200/JCO.2016.67.6049 [PubMed]
-
21.
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RG, Barzi A, Jemal A. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017; 67:177–93. https://doi.org/10.3322/caac.21395 [PubMed]
-
22.
Kou Y, Koag MC, Lee S. N7 methylation alters hydrogen-bonding patterns of guanine in duplex DNA. J Am Chem Soc. 2015; 137:14067–70. https://doi.org/10.1021/jacs.5b10172 [PubMed]
-
23.
Koag MC, Kou Y, Ouzon-Shubeita H, Lee S. Transition-state destabilization reveals how human DNA polymerase β proceeds across the chemically unstable lesion N7-methylguanine. Nucleic Acids Res. 2014; 42:8755–66. https://doi.org/10.1093/nar/gku554 [PubMed]
-
24.
Kou Y, Koag MC, Lee S. Promutagenicity of 8-chloroguanine, a major inflammation-induced halogenated DNA lesion. Molecules. 2019; 24:3507. https://doi.org/10.3390/molecules24193507 [PubMed]
-
25.
Liang PS, Mayer JD, Wakefield J, Trinh-Shevrin C, Kwon SC, Sherman SE, Ko CW. Trends in sociodemographic disparities in colorectal cancer staging and survival: a SEER-medicare analysis. Clin Transl Gastroenterol. 2020; 11:e00155. https://doi.org/10.14309/ctg.0000000000000155 [PubMed]
-
26.
Zhu Y, Wu H, Wang PP, Savas S, Woodrow J, Wish T, Jin R, Green R, Woods M, Roebothan B, Buehler S, Dicks E, McLaughlin JR, et al. Dietary patterns and colorectal cancer recurrence and survival: a cohort study. BMJ Open. 2013; 3:e002270. https://doi.org/10.1136/bmjopen-2012-002270 [PubMed]
-
27.
Meyerhardt JA, Niedzwiecki D, Hollis D, Saltz LB, Hu FB, Mayer RJ, Nelson H, Whittom R, Hantel A, Thomas J, Fuchs CS. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA. 2007; 298:754–64. https://doi.org/10.1001/jama.298.7.754 [PubMed]
-
28.
Sjödahl R, Rosell J, Starkhammar H. Causes of death after surgery for colon cancer-impact of other diseases, urgent admittance, and gender. Scand J Gastroenterol. 2013; 48:1160–65. https://doi.org/10.3109/00365521.2013.828771 [PubMed]
-
29.
Jia H, Li Q, Yuan J, Sun X, Wu Z. Second primary Malignancies in patients with colorectal cancer: a population-based analysis. Oncologist. 2020; 25:e644–50. https://doi.org/10.1634/theoncologist.2019-0266 [PubMed]
-
30.
Raycraft T, Cheung WY, Yin Y, Speers C, Ko JJ, Mariano C. Causes of mortality in older patients with stage 3 colon cancer. J Geriatr Oncol. 2019; 10:138–42. https://doi.org/10.1016/j.jgo.2018.06.002 [PubMed]
-
31.
Costantino S, Paneni F, Cosentino F. Ageing, metabolism and cardiovascular disease. J Physiol. 2016; 594:2061–73. https://doi.org/10.1113/JP270538 [PubMed]
-
32.
Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, Zielinski J, and Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007; 176:532–55. https://doi.org/10.1164/rccm.200703-456SO [PubMed]
-
33.
Dyhl-Polk A, Vaage-Nilsen M, Schou M, Vistisen KK, Lund CM, Kümler T, Appel JM, Nielsen DL. Incidence and risk markers of 5-fluorouracil and capecitabine cardiotoxicity in patients with colorectal cancer. Acta Oncol. 2020; 59:475–83. https://doi.org/10.1080/0284186X.2019.1711164 [PubMed]
-
34.
Abdel-Rahman O. 5-fluorouracil-related cardiotoxicity; findings from five randomized studies of 5-fluorouracil-based regimens in metastatic colorectal cancer. Clin Colorectal Cancer. 2019; 18:58–63. https://doi.org/10.1016/j.clcc.2018.10.006 [PubMed]
-
35.
Kosmas C, Kallistratos MS, Kopterides P, Syrios J, Skopelitis H, Mylonakis N, Karabelis A, Tsavaris N. Cardiotoxicity of fluoropyrimidines in different schedules of administration: a prospective study. J Cancer Res Clin Oncol. 2008; 134:75–82. https://doi.org/10.1007/s00432-007-0250-9 [PubMed]
-
36.
Jensen SA, Sørensen JB. Risk factors and prevention of cardiotoxicity induced by 5-fluorouracil or capecitabine. Cancer Chemother Pharmacol. 2006; 58:487–93. https://doi.org/10.1007/s00280-005-0178-1 [PubMed]
-
37.
Smoragiewicz M, Javaheri KR, Yin Y, Gill S. Neutropenia and relative dose intensity on adjuvant FOLFOX chemotherapy are not associated with survival for resected colon cancer. J Gastrointest Cancer. 2014; 45:460–65. https://doi.org/10.1007/s12029-014-9639-2 [PubMed]
-
38.
Aspinall SL, Good CB, Zhao X, Cunningham FE, Heron BB, Geraci M, Passero V, Stone RA, Smith KJ, Rogers R, Shields J, Sartore M, Boyle DP, et al. Adjuvant chemotherapy for stage III colon cancer: relative dose intensity and survival among veterans. BMC Cancer. 2015; 15:62. https://doi.org/10.1186/s12885-015-1038-y [PubMed]
-
39.
Kwakman JJ, Simkens LH, Mol L, Kok WE, Koopman M, Punt CJ. Incidence of capecitabine-related cardiotoxicity in different treatment schedules of metastatic colorectal cancer: a retrospective analysis of the CAIRO studies of the Dutch Colorectal Cancer Group. Eur J Cancer. 2017; 76:93–99. https://doi.org/10.1016/j.ejca.2017.02.009 [PubMed]
-
40.
Chang RY, Lee MY, Kan CB, Hsu WP, Hsiao PC. Oxaliplatin-induced acquired long QT syndrome with torsades de pointes and myocardial injury in a patient with dilated cardiomyopathy and rectal cancer. J Chin Med Assoc. 2013; 76:466–69. https://doi.org/10.1016/j.jcma.2013.05.001 [PubMed]
-
41.
Allegra CJ, Yothers G, O'Connell MJ, Sharif S, Colangelo LH, Lopa SH, Petrelli NJ, Goldberg RM, Atkins JN, Seay TE, Fehrenbacher L, O'Reilly S, Chu L, et al. Initial safety report of NSABP C-08: A randomized phase III study of modified FOLFOX6 with or without bevacizumab for the adjuvant treatment of patients with stage II or III colon cancer. J Clin Oncol. 2009; 27:3385–90. https://doi.org/10.1200/JCO.2009.21.9220 [PubMed]
-
42.
Letarte N, Bressler LR, Villano JL. Bevacizumab and central nervous system (CNS) hemorrhage. Cancer Chemother Pharmacol. 2013; 71:1561–65. https://doi.org/10.1007/s00280-013-2155-4 [PubMed]
-
43.
Hurwitz HI, Tebbutt NC, Kabbinavar F, Giantonio BJ, Guan ZZ, Mitchell L, Waterkamp D, Tabernero J. Efficacy and safety of bevacizumab in metastatic colorectal cancer: pooled analysis from seven randomized controlled trials. Oncologist. 2013; 18:1004–12. https://doi.org/10.1634/theoncologist.2013-0107 [PubMed]
-
44.
Chanock S. Evolving risk factors for infectious complications of cancer therapy. Hematol Oncol Clin North Am. 1993; 7:771–93. [PubMed]
-
45.
Zembower TR. Epidemiology of infections in cancer patients. Cancer Treat Res. 2014; 161:43–89. https://doi.org/10.1007/978-3-319-04220-6_2 [PubMed]
-
46.
Donnelly JP, Blijlevens NM, van der Velden WJ. Host impairments in patients with neoplastic diseases. Cancer Treat Res. 2014; 161:1–41. https://doi.org/10.1007/978-3-319-04220-6_1 [PubMed]
-
47.
Dikken C, Sitzia J. Patients’ experiences of chemotherapy: side-effects associated with 5-fluorouracil + folinic acid in the treatment of colorectal cancer. J Clin Nurs. 1998; 7:371–79. https://doi.org/10.1046/j.1365-2702.1998.00159.x [PubMed]
-
48.
Schuell B, Gruenberger T, Kornek GV, Dworan N, Depisch D, Lang F, Schneeweiss B, Scheithauer W. Side effects during chemotherapy predict tumour response in advanced colorectal cancer. Br J Cancer. 2005; 93:744–48. https://doi.org/10.1038/sj.bjc.6602783 [PubMed]
-
49.
Saad AM, Gad MM, Al-Husseini MJ, AlKhayat MA, Rachid A, Alfaar AS, Hamoda HM. Suicidal death within a year of a cancer diagnosis: a population-based study. Cancer. 2019; 125:972–79. https://doi.org/10.1002/cncr.31876 [PubMed]
-
50.
Spoletini I, Gianni W, Caltagirone C, Madaio R, Repetto L, Spalletta G. Suicide and cancer: where do we go from here? Crit Rev Oncol Hematol. 2011; 78:206–19. https://doi.org/10.1016/j.critrevonc.2010.05.005 [PubMed]
-
51.
Mitchell AJ. Why doesn’t depression treatment improve cancer survival? Lancet Psychiatry. 2018; 5:289–91. https://doi.org/10.1016/S2215-0366(18)30086-5 [PubMed]
-
52.
Zhang T, Han Y, Wang J, Hou D, Deng H, Deng YL, Song Z. Comparative epidemiological investigation of Alzheimer’s disease and colorectal cancer: the possible role of gastrointestinal conditions in the pathogenesis of AD. Front Aging Neurosci. 2018; 10:176. https://doi.org/10.3389/fnagi.2018.00176 [PubMed]
-
53.
Park HS, Lloyd S, Decker RH, Wilson LD, Yu JB. Limitations and biases of the surveillance, epidemiology, and end results database. Curr Probl Cancer. 2012; 36:216–24. https://doi.org/10.1016/j.currproblcancer.2012.03.011 [PubMed]
-
54.
Sun M, Trinh QD. A surveillance, epidemiology and end results (SEER) database malfunction: perceptions, pitfalls and verities. BJU Int. 2016; 117:551–52. https://doi.org/10.1111/bju.13226 [PubMed]
-
55.
Yang K, Zheng Y, Peng J, Chen J, Feng H, Yu K, Chen Y, Luo W, Yang P, Yang Y, Wu B. Incidence of death from unintentional injury among patients with cancer in the United States. JAMA Netw Open. 2020; 3:e1921647. https://doi.org/10.1001/jamanetworkopen.2019.21647 [PubMed]
-
56.
Lund JL, Harlan LC, Yabroff KR, Warren JL. Should cause of death from the death certificate be used to examine cancer-specific survival? a study of patients with distant stage disease. Cancer Invest. 2010; 28:758–64. https://doi.org/10.3109/07357901003630959 [PubMed]
-
57.
Hu CY, Xing Y, Cormier JN, Chang GJ. Assessing the utility of cancer-registry-processed cause of death in calculating cancer-specific survival. Cancer. 2013; 119:1900–07. https://doi.org/10.1002/cncr.27968 [PubMed]
-
58.
Baghestani AR, Daneshva T, Pourhoseingholi MA, Asadzadeh H. Survival of colorectal cancer in the presence of competing- risks - modeling by weibull distribution. Asian Pac J Cancer Prev. 2016; 17:1193–96. [PubMed]
-
59.
Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence-Based Mortality - SEER 9 Regs Research Data, Nov 2018 Sub (1975-2016) - Linked To County Attributes - Total U.S., 1969-2017 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2019, based on the November 2018 submission. http://www.seer.cancer.gov.
-
60.
Enewold L, Horner MJ, Shriver CD, Zhu K. Socioeconomic disparities in colorectal cancer mortality in the United States, 1990-2007. J Community Health. 2014; 39:760–66. https://doi.org/10.1007/s10900-014-9824-z [PubMed]
-
61.
Koepsell TD, Weiss NS. Epidemiologic Methods: Studying the Occurrence of Illness. New York: Oxford University Press; 2003.
-
62.
Misono S, Weiss NS, Fann JR, Redman M, Yueh B. Incidence of suicide in persons with cancer. J Clin Oncol. 2008; 26:4731–38. https://doi.org/10.1200/JCO.2007.13.8941 [PubMed]
-
63.
Breslow NE, Day NE. Statistical methods in cancer research. Volume II—the design and analysis of cohort studies. IARC Sci Publ. 1987; 1–406. [PubMed]
-
64.
Ury HK, Wiggins AD. Another shortcut method for calculating the confidence interval of a poisson variable (or of a standardized mortality ratio). Am J Epidemiol. 1985; 122:197–98. https://doi.org/10.1093/oxfordjournals.aje.a114083 [PubMed]
-
65.
Xu J, Murphy SL, Kochanek KD, Bastian B, Arias E and Statistics DoV. Deaths: Final Data for 2016. Natl Vital Stat Rep. 2016; 67:1–76. https://www.cdc.gov/nchs/data/nvsr/nvsr67/nvsr67_05.pdf.