Evaluation of the geroprotective effects of withaferin A in Drosophila melanogaster
Abstract
Withanolides are a class of compounds usually found in plant extracts which are an attractive geroprotective drug design starting point. We evaluated the geroprotective properties of Withaferin A (WA) in vivo using the Drosophila model. Flies were supplemented by nutrient medium with WA (at a concentration of 1, 10, or 100 μM dissolved in ethanol) for the experiment group and 30 μM of ethanol for the control group. WA treatment at 10 and 100 μM concentrations prolong the median life span of D. melanogaster’s male by 7.7, 9.6% (respectively) and the maximum life span (the age of death 90% of individuals) by 11.1% both. Also WA treatment at 1, 10 and 100 μM improved the intestinal barrier permeability in older flies and affected an expression of genes involved in antioxidant defense (PrxV), recognition of DNA damage (Gadd45), heat shock proteins (Hsp68, Hsp83), and repair of double-strand breaks (Ku80). WA was also shown to have a multidirectional effect on the resistance of flies to the prooxidant paraquat (oxidative stress) and 33° C hyperthermia (heat shock). WA treatment increased the resistance to oxidative stress in males at 4 and 7 week old and decreased it at 6 weeks old. It increased the male’s resistance to hyperthermia at 2, 4 and 7 weeks old and decreased it at 3, 5 and 8 weeks old. WA treatment decreased the resistance to hyperthermia in females at 1, 2 and 3 weeks old and not affected on their resistance to oxidative stress.
Introduction
One of the key challenges within life sciences is the search for the substances that can increase the resistance of living systems to various stress factors and contribute to their active longevity. The most promising direction of research in this aspect is the identification of such substances among the plant metabolites. Therefore, the properties of plant extracts are currently actively studied to find the optimal approach to include them in new pharmacological preparations. Among these compounds withanolides are considered as a promising class of candidates for the design of new drugs. Indeed, withanolides display a wide range of relevant pharmacological activities, good bio accessibility and a low risk of side effects. Currently, the preparations containing withanolides from Withania somnifera are used in the Ashwagandha composition as a sedative, hypnotic and antiseptic drug [1].
Withanolides are widely studied worldwide. For instance, PubMed contains more than 300 publications with the keyword “withanolides”. Withanolides attract a lot of interest for their potential use as inhibitors of apoptosis. They are also considered as therapeutic candidates for the treatment of neurodegenerative, autoimmune and inflammatory diseases. Their antitumor properties have also attracted a lot of interest for the development of novel cancer therapies. It is common knowledge that Drosophila melanogaster has notable advantages as a model system for studying the effects of pharmacological interventions on aging [2]. In our study we hypothesized that the addition of a Withaferin A (WA) supplement to the diet of Drosophila melanogaster wild type Canton-S (CS) could have a beneficial effect on their health status, especially when they get older.
The first withanolide, “withaferin,” was found in the leaves of the Withania somnifera (Solanaceae) in 1962 [3]. This metabolite was a new type of steroid containing alpha, beta-unsaturated lactone linked to the C-17 of the sterane skeleton [4, 5]. However, this “withaferin” turned out to be 2,3-dihydro-3-methoxywithaferin A, which occurs in mixture with WA [5]. Independently, in 1965 Kupchan et al. found WA in the leaves of Acnistus arborescens (Solanaceae) [6]. Later, other representatives of this class of compounds were discovered in the plants of the Solanaceae family. Withanolides have been found in some Tacca species from the Dioscoreaceae family (taccanolides) and Ajuga sp. from the Lamiaceae family, as well as in some marine organisms [7, 8].
Today, the class of withanolides contains more than 400 chemical compounds. This includes closely related congeners that are found in the plants of Solanaceae [9–14]. They consist of C-28 steroidal lactones built on a sometimes modified framework of ergostane, which can form a six-membered lactone ring formed by the oxidation of C-22 - C-26 (Figure 1) [15].
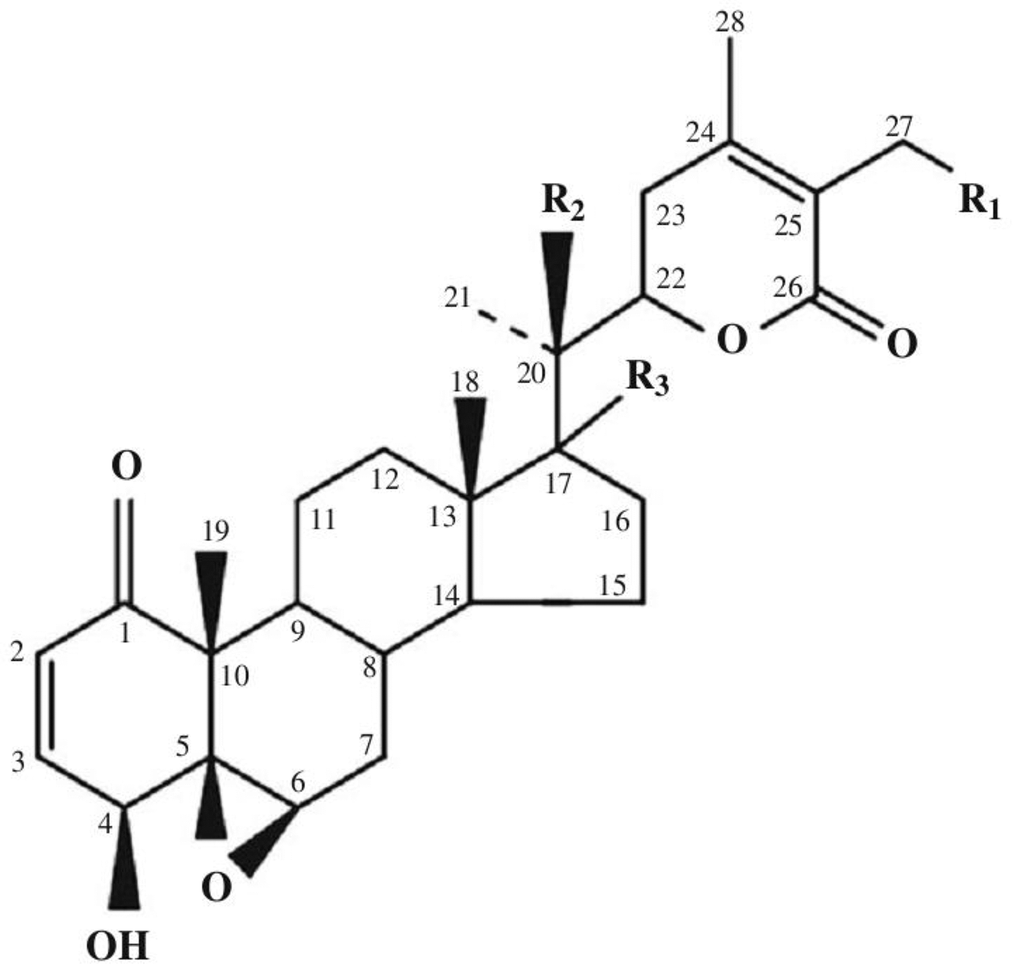
Figure 1. Basic skeleton of withanolides. Withaferin A R1 = OH, R2 = H, R3 = H; Withaferin D R1 = H, R2 = OH, R3 = H; 27-Deoxywithaferin A R1 = H, R2 = H, R3 = H; 27-hydroxywithanolide D R1 = OH, R2 = OH, R3 = H; Dihydrodeoxywithaferin A R1 = H, R2 = H, R3 = H; Dihydrowithaferin A R1 = H, R2 = OH, 17-hydroxywithaferin A, R1 = R3 = OH, R2 = H.
The term “withanolide” is commonly used for 22-hydroxyergostane-26-acid-22,26-olide [4]. Its structural diversity is due to the modifications of the carbocyclic backbone or side chain. The other typical substitutions and modifications of the naturally occurring metabolites are as follows [16]: oxo group at C-1; instead, less commonly a hydroxyl group; double bond C-2 -> C-3; instead, less often the hydroxyl group at C-3; delta-lactone (26 -> 22O), often unsaturated (24, 25); a fragment of gamma-lactone (26 -> 23O) instead of delta-lactone, often also unsaturated; lactol part instead of lactone residue; high oxidation state in many positions of the entire molecule (for example, oxo groups, hydroxyl groups, epoxy substructures, hemicetals); oxidative degradation and or new cyclization of the molecule.
Biosynthesis of withanolides in plants is well studied and proceeds with isoprenoids as precursors [17–19].
WA (4β, 27-dihydroxy-1-oxo-5β, 6β-epoxywitha-2,24-dienolide) (Figure 1) was first isolated from the Withania somnifera plant [4]. WA was also found in plants such as Withania artistata, Ajuga bracteosa, Vassobia breviflora, and Dunalia spinosa [20–23]. In plants, pure WA is found in relatively small quantities ranging around 0.2-0.3% of dry weight [24].
The stereochemistry of WA was determined in 1966 [25]. Its structure has five functional groups: an unsaturated ketone ring A, 2 hydroxyl groups, an epoxide in ring B, a 6-carbon lactone ring, and an unsaturated carbonyl group (Figure 1). The double bond in ring A and the epoxy ring are responsible for the cytotoxicity of the compound. NMR spectral analysis identified C3 as a major nucleophilic target site for ethyl mercaptan, thiophenol, and ethyl L-cysteine in vitro [26]. These five functional groups allow WA to interact with multiple molecular targets leading to a wide range of biological activities.
Previous in vitro and in vivo studies showed that WA displays anti-tumor activity. It is well established that WA induces apoptosis in cancer cells via different mechanisms [27–30]. In most cancer cell lines, WA inhibits tumor cell proliferation by stopping the cell cycle during the G2/M checkpoint [31, 32] and inhibits nuclear factor kappa B (NF-κB) activation by interacting with the IKKγ subunit, which prevents IκB phosphorylation [33, 34]. A decrease in NF-κB activity leads to a decrease in the production of pro-inflammatory and stress response mediators [35]. Anti-tumor activity is also linked to ability of WA to promote oxidative stress. WA decreases the mitochondrial membrane potential and activates various caspases and proteases, which trigger the degradation of various substrates, such as cytoskeletal proteins and poly (ADP-ribose) polymerase [36, 37]. Also WA regulates the activity of antioxidant enzymes (such as superoxide dismutase) [38] and mRNA expression of antioxidant genes: erythroid 2-like 2 (NFE2L2), heme oxygenase 1 (HMOX1), glutathione-disulfide reductase (GSR), and NAD(P)H quinone dehydrogenase 1 (NQO1)) in cancer cells [39]. Also tumor activity of WA involves induction of heat shock response via Akt / mTOR and MAPK signaling pathways [40].
The anti-inflammatory and anti-fibrotic effects of WA have been demonstrated in an in vivo model of bleomycin-induced scleroderma. Daily intraperitoneal injections of WA over the span of 28 days cause reduced dorsal skin thickness in this model. The study has shown that WA suppresses the pro-inflammatory phase of fibrosis regulated by the TGF-β/Smad signaling cascade, and also significantly reduces the proportion of fibroblasts that turn into myofibroblasts. The authors have associated the antifibrotic effect with the inhibition of the FoxO3a-Akt-dependent NF-κβ/IKK-mediated cascade, which is involved in the process of the fibrotic tissue transformation [41].
Due to its wide positive properties and availability, WA can be considered as a promising substance for improving health span and life span. In the present study, our hypothesis is that the addition of WA to the Drosophila’s feed would have a beneficial effect on its vitality, especially with age.
Results
Effect of WA on the life span of Drosophila melanogaster wild type Canton-S
The effect of WA at concentrations of 1, 10, 100 μM on the life span of male and female Drosophila melanogaster of the wild type Canton-S was studied. WA at 10 and 100 μM concentrations increased the median life span in male by 7.7, 9.6% (respectively) (p<0.0001) and the maximum life span (the age of death 90% of individuals) by 11.1% both (Figure 2B). While we sighted significant shift of these group curves to the right relative to the control curve (Figure 2A). The 1 μM of WA treatment not affected on studied life span parameters. Also WA treatment not affected on studied lifespan parameters in Drosophila’s females (Figure 2C, 2D).
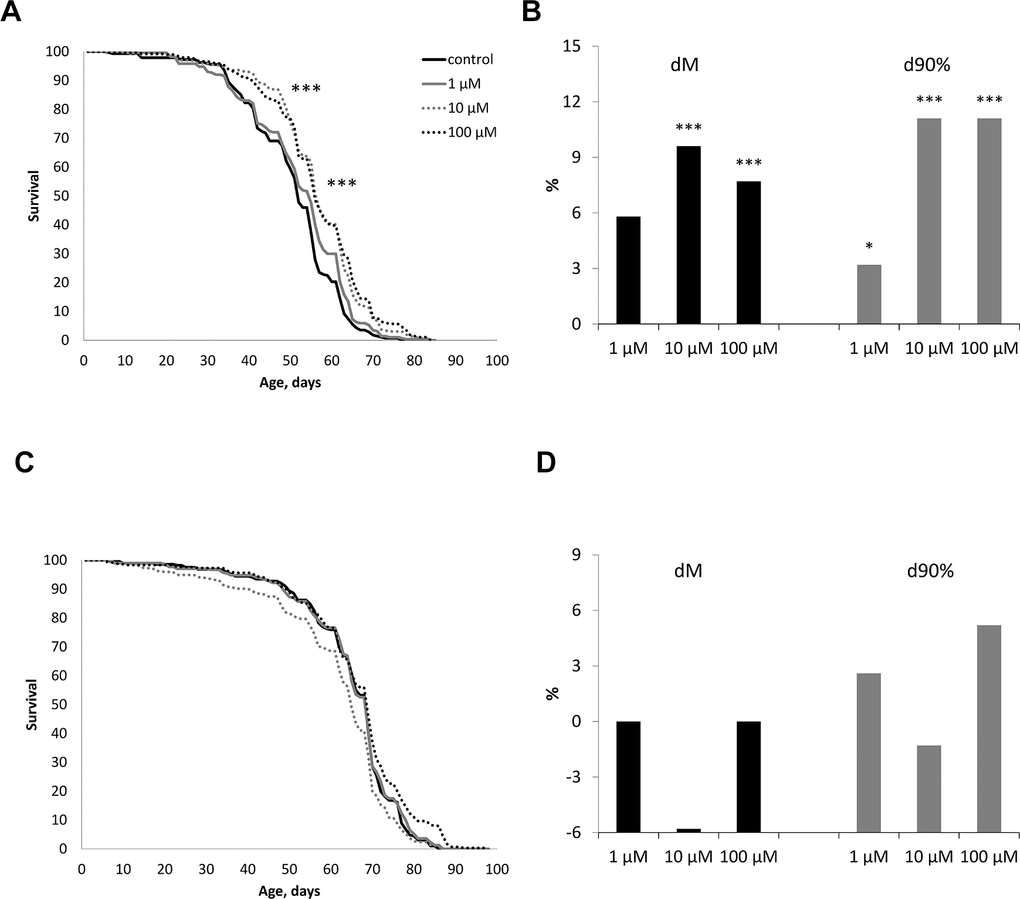
Figure 2. Effect of Withaferin A on the life span in males (A, B) and females (C, D) of Drosophila melanogaster wild type Canton-S. Results of two independent repeats are combined. dM and d90% are the percentage of change in median life span and age of death of 90% of individuals (respectively); * p <0.01, ** p <0.001, *** p <0.0001.
Effect of WA on stress resistance of Drosophila melanogaster wild type Canton-S
The effect of WA at 1, 10, 100 μM concentrations on the resistance of Drosophila to the action of paraquat (20 mM solution in 5% sucrose, oxidative stress) and hyperthermia (33° C, heat shock) at the age of 1 to 8 weeks (from young to old individuals) demonstrated in Tables 1, 2 and Supplementary Figures 1–4.
Table 1. Effect of Withaferin A at concentrations of 1, 10, 100 μM on the resistance of Drosophila melanogaster wild type Canton-S males to paraquat (20 mM, oxidative stress) and hyperthermia (33° C, heat shock) at the age of 1 to 8 weeks.
| Age | Experimental group | Oxidative stress | Hyperthermia |
| Survival, hours |
| 25% | 50% | 75% | 90% | 25% | 50% | 75% | 90% |
| 1 week | control | 64 | 69 | 86 | 92 | 66 | 71 | 81 | 89 |
| 1 μM | 52 | 67 | 82 | 86 | 63 | 73 | 79 | 83 |
| 10 μM | 61 | 69 | 79 | 91 | 70 | 81 | 85 | 90 |
| 100 μM | 48 | 56 | 74 | 86 | 63 | 72 | 81 | 90 |
| 2 weeks | control | 41 | 51 | 60 | 73 | 26 | 31 | 33 | 36 |
| 1 μM | 39 | 52 | 59 | 71 | 28 | 33 | 35 | 41 |
| 10 μM | 42 | 52 | 62 | 75 | 28 | 32 | 36 | 37 |
| 100 μM | 42 | 47 | 54 | 71 | 30 | 34** | 37 | 40 |
| 3 weeks | control | 35 | 40 | 46 | 54 | 18 | 25 | 26 | 27 |
| 1 μM | 33 | 40 | 47 | 57 | 10* | 12 *** | 14 ** | 15 |
| 10 μM | 32 | 40 | 48 | 56 | 6 * | 7 * | 10 * | 13 |
| 100 μM | 36 | 42 | 48 | 56 | 21 | 24 | 25 | 27 |
| 4 weeks | control | 24 | 30 | 38 | 45 | 20 | 28 | 41 | 45 |
| 1 μM | 28 ** | 34 | 39 | 46 | 29 | 36 | 45 | 50 * |
| 10 μM | 27 | 32 | 37 | 42 | 27 | 33 | 42 | 47 |
| 100 μM | 24 | 30 | 37 | 44 | 28 | 33 | 38 | 42 |
| 5 weeks | control | 21 | 25 | 31 | 34 | 14 | 18 | 25 | 29 |
| 1 μM | 19 | 24 | 28 | 35 | 8 * | 11 | 22 | 29 |
| 10 μM | 21 | 25 | 27 | 31 | 7 | 14 | 20 | 27 * |
| 100 μM | 23 | 28 | 33 | 37 | 8 * | 12 | 22 | 27 |
| 6 weeks | control | 21 | 25 | 30 | 34 | 27 | 32 | 35 | 38 |
| 1 μM | 19 | 24 | 29 | 33 | 23 | 31 | 39 | 41 |
| 10 μM | 19 | 23 | 27 | 29 * | 24 | 31 | 40 | 44 |
| 100 μM | 19 | 25 | 29 | 31 | 27 | 31 | 35 | 38 |
| 7 weeks | control | 14 | 20 | 26 | 31 | 10 | 16 | 22 | 26 |
| 1 μM | 20 *** | 23 | 29 | 32 | 10 | 14 | 22 | 29 |
| 10 μM | 16 | 21 | 26 | 30 | 12 | 15 | 23 | 27 |
| 100 μM | 17 | 20 | 25 | 31 | 9 | 19 | 25 | 33 * |
| 8 weeks | control | 13 | 18 | 23 | 27 | 11 | 15 | 20 | 23 |
| 1 μM | 15 | 20 | 26 | 31 | 10 | 13 * | 17 *** | 18 * |
| 10 μM | 14 | 18 | 23 | 28 | 10 | 14 | 17 | 21 |
| 100 μM | 13 | 17 | 23 | 26 | 9 | 13 | 17 | 20 |
| Note: * p <0.01; ** p <0.001, *** p <0.0001. Three biological repeats were combined (32 flies in each). 25%, 50%, 75%, 90% - percentiles. |
Table 2. Effect of Withaferin A at concentrations of 1, 10, 100 μM on the resistance of Drosophila melanogaster wild type Canton-S females to the action of paraquat (20 mM, oxidative stress) and hyperthermia (33° C, heat shock) at the age of 1 to 8 weeks.
| Age | Experimental group | Oxidative stress | Hyperthermia |
| Survival, hours |
| 25% | 50% | 75% | 90% | 25% | 50% | 75% | 90% |
| 1 week | control | 64 | 69 | 86 | 92 | 64 | 73 | 77 | 81 |
| 1 μM | 52 | 67 | 82 | 86 | 60 | 73 | 81 | 88 |
| 10 μM | 61 | 69 | 79 | 91 | 52* | 61* | 71 | 76 |
| 100 μM | 48 | 56 | 74 | 86 | 63 | 72 | 88 | 89 |
| 2 weeks | control | 48 | 72 | 103 | 116 | 28 | 38 | 42 | 47 |
| 1 μM | 47 | 70 | 95 | 111 | 25 | 34 | 42 | 51 |
| 10 μM | 55 | 77 | 95 | 111 | 22 | 34 | 37 | 42 |
| 100 μM | 48 | 78 | 97 | 108 | 19 | 28** | 34 | 42 |
| 3 weeks | control | 42 | 64 | 79 | 99 | 25 | 30 | 35 | 38 |
| 1 μM | 32 | 50 | 75 | 93 | 15 *** | 17 *** | 20 *** | 23 |
| 10 μM | 48 | 66 | 82 | 104 | 11 *** | 13 *** | 16 *** | 18 |
| 100 μM | 40 | 62 | 76 | 95 | 24 | 29 | 33 | 38 |
| 4 weeks | control | 28 | 49 | 60 | 78 | 24 | 31 | 36 | 41 |
| 1 μM | 30 | 48 | 58 | 82 | 26 | 33 | 37 | 43 |
| 10 μM | 31 | 45 | 67 | 85 | 26 | 31 | 38 | 40 |
| 100 μM | 30 | 47 | 68 | 82 | 28 | 34 | 41 | 44 |
| 5 weeks | control | 28 | 31 | 43 | 50 | 15 | 21 | 24 | 29 |
| 1 μM | 27 | 42 | 48 | 61 | 15 | 19 | 23 | 26 |
| 10 μM | 29 | 36 | 45 | 63 | 18 | 22 | 25 | 28 |
| 100 μM | 27 | 31 | 41 | 48 | 14 | 18 | 22 | 24 |
| 6 weeks | control | 21 | 27 | 34 | 57 | 20 | 25 | 31 | 35 |
| 1 μM | 20 | 26 | 39 | 55 | 22 | 26 | 30 | 33 |
| 10 μM | 19 | 26 | 36 | 61 | 21 | 23 | 27 | 31 |
| 100 μM | 21 | 26 | 37 | 51 | 22 | 27 | 30 | 36 |
| 7 weeks | control | 19 | 25 | 34 | 44 | 15 | 21 | 26 | 29 |
| 1 μM | 22 | 25 | 36 | 48 | 13 | 18 | 24 | 27 |
| 10 μM | 17 | 23 | 30 | 45 | 15 | 19 | 24 | 29 |
| 100 μM | 19 | 23 | 33 | 42 | 14 | 18 | 23 | 29 |
| 8 weeks | control | 17 | 23 | 28 | 37 | 8 | 14 | 19 | 26 |
| 1 μM | 14 | 22 | 30 | 48 | 9 | 14 | 21 | 25 |
| 10 μM | 17 | 22 | 30 | 41 | 9 | 14 | 20 | 24 |
| 100 μM | 18 | 23 | 31 | 40 | 10 | 15 | 20 | 24 |
| Note: * p <0.01; ** p <0.001, *** p <0.0001. Three biological repeats were combined (32 flies in each). 25%, 50%, 75%, 90% - percentiles. |
WA treatment had a different effect in response to studied stressors in males. It increased the resistance to paraquat only at the age of 4 and 7 week at 1 μM (by 16.7, 42.9 %% increased 25 percentiles respectively). At 6 weeks decreased 90 percentiles by 14.7% at 10 μM of WA. Also we observed shift in mortality distribution curves at 1 μM of WA in 1 and 8 weeks relative to the control curve. WA treatment increased the resistance of male’s to hyperthermia at 2 weeks after 100 μM of WA (by 9.7% increased 50 percentiles), at 4 weeks after 1 μM of WA (by 11.1% – 90 percentiles) and at 7 weeks after 100 μM of WA (by 26.9% – 90 percentiles). While it decreased the resistance to hyperthermia at 3 weeks after 1 and 10 μM (by 44.4, 52.0, 46.2%%; 66.7, 72.0, 61.5 %% decreased 25, 50, 75 percentiles respectively). At the age 5 weeks we found decreased by 42.9% in 25 percentiles after 1, 100 μM and by 6.9% in 90 percentiles after 10 μM WA treatments. And at the age 8 weeks decreased by 13.3, 15.0, 21.7 %% in 50, 75, 90 percentiles after 1 μM of WA. These data confirm by mortality distribution. We observed shift in mortality curves at 1 μM of WA in 3, 4 and 8 weeks, at 10 μM in 3 and 5 weeks, and at 100 μM at 7 and 8 weeks relative to the control curve. In other variants of the experiment WA not affected on studied survival parameters (Table 2 and Supplementary Figures 1, 2).
WA treatment not affected on female’s resistance to paraquat and reduced resistance to hyperthermia. At the age of 1 week at 10 μM (by 18.8, 16.4 %% decreased 25 and 50 percentiles respectively), of 2 weeks at 100 μM (by 26.3 %– 50 percentiles) and of 3 weeks for 1 and 10 μM (40.0, 43.3,42.9; 56.0, 56.7, 54.3%% - 25, 50 and 75 percentiles respectively). Also these data confirm mortality distribution. We observed shift 100 μM at 2 weeks and 1 μM and 10 μM at 3 weeks curves to the right relative to the control curve. In other variants of the experiment WA not affected on studied survival parameters (Table 2 and Supplementary Figures 3, 4).
Effect of WA on the intestinal barrier permeability of Drosophila melanogaster wild type Canton-S
Changes in the permeability of the intestinal barrier in Drosophila melanogaster wild type Canton-S at the age of 4, 6, 8 weeks were studied against the background of treatment of WA at concentrations of 1, 10, 100 μM.
In males at the age of 8 weeks, a decrease in the proportion of flies with the «smurfs» phenotype by 67, 61 and 89% was observed relative to the control when taking WA at concentrations of 1, 10, 100 μM, respectively (Figure 3C). Females also showed a decrease in the «smurfs» rate only at the age of 8 weeks. The proportion of flies with the «smurfs» phenotype was lower by 73, 42 and 61 %% relative to the control after WA at 1, 10, 100 μM concentrations, respectively (Figure 3F). Other group of the experiment showed no significant differences (Figure 3A, 3B, 3D, 3E).
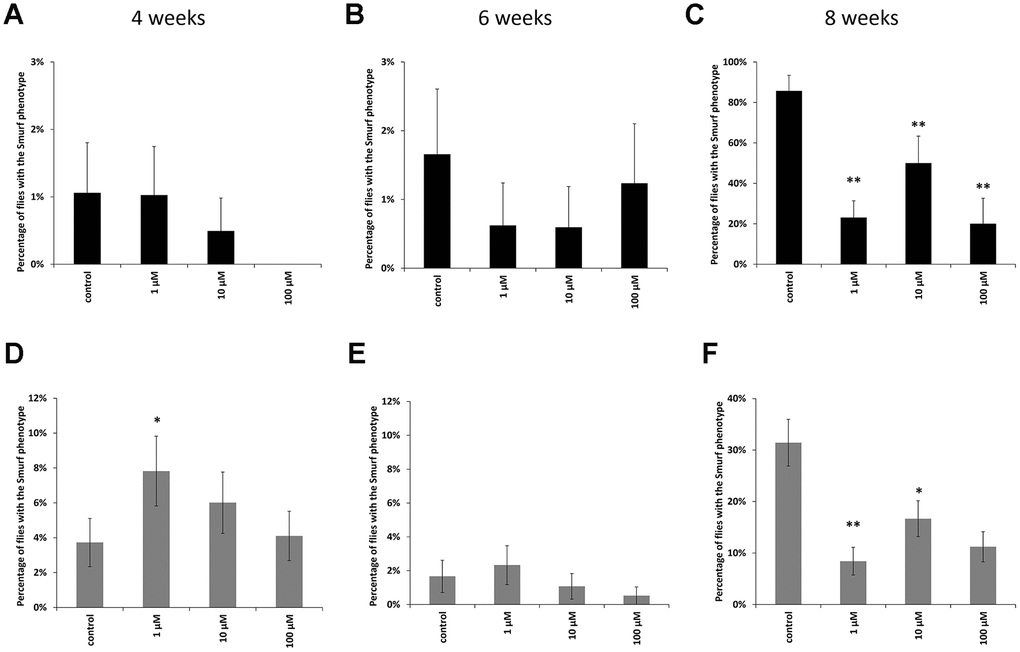
Figure 3. Results of the intestinal barrier permeability test (smurf test) in males (A–C) and females (D–F) of Drosophila melanogaster wild type Canton-S at the age of 4, 6 and 8 weeks. * p <0.01, ** p <0.001.
Effect of WA on the expression of stress response genes in Drosophila melanogaster wild type Canton-S
Changes in the expression of genes involved in antioxidant defense (catalase, Peroxiredoxin V), metal detoxification (frataxin), recognition of DNA damage (Gadd45), heat shock proteins (Hsp68, Hsp83), and repair of double-strand breaks (Ku80) studied. The combined results are presented in Figure 4 and considered. In males 1 μM WA effected on expression of Gadd45 (3.9-fold-increase), Hsp83 (1.5-fold-decrease) and PrxV (3.1-fold-increase) genes. The10 μM and 100 μM of WA decreased the expression of only Hsp68 (2.3-, 1.4-fold respectively) gene (Figure 4). In females the 1 μM of WA treatment decreased the expression of Gadd45 (4.9-fold), Hsp68 (3.6-fold), Ku80 (3.9-fold) genes. The100 μM WA treatment decreased the expression of only Gadd45 (2.7-fold) gene (Figure 4). The expression of other studied genes was not significantly changed.
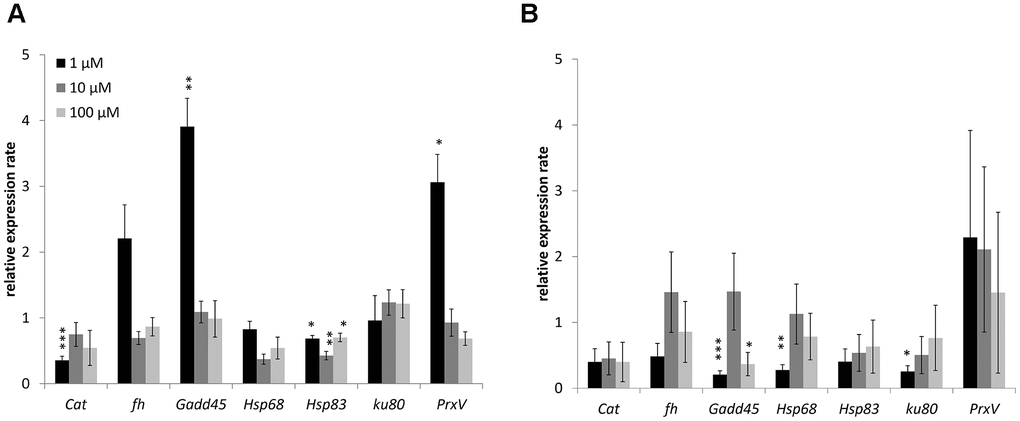
Figure 4. Changing of stress response genes expression in males (A) and females (B) of Drosophila melanogaster wild type Canton-S after WA treatment. Results were normalized to control. * p <0.01, ** p <0.001, *** p <0.0001.
Discussion
Currently, the identification of geroprotectors which could be used as a cure against aging constitutes an important area of research. Although there are around 400 compounds known to extend the life span of model organisms, only few of them meet the criteria to be used as potential geroprotectors [42]. Moreover, there is a lack of clinical studies which have been conducted to analyze the effects of these potential geroprotectors on humans. It should be emphasized that an ideal geroprotector should not only increase the average but also the maximum life span. Furthermore geroprotectors should contribute to shifting the entire mortality curve to the right so that extended life span would be associated with the extension of the active period of life.
Balanced nutrition is one of the most important factors promoting increased life span. Currently, rapamycin, metformin, Skulachev ions (SkQ), and some other compounds are known to be promising geroprotective substances [43–46]. The data available indicate that these geroprotective substances prolong the life in model organisms (Caenorhabditis elegans, D. melanogaster, Mus musculus, Rattus norvegicus, etc.). In many cases they also reduce the likelihood of aging-associated diseases. Metformin and rapamycin are two FDA-approved mTOR inhibitors. However, the use of metformin and rapamycin has various side effects. A. Aliper et al. [47] applied several bioinformatic approaches and deep learning techniques to a dataset from the Library of Integrated Network – based Cellular Signatures (LINCS) to find the substances that could emulate the genetic response to metformin and rapamycin. Using this approach, the authors predicted the safety of each selected compound. As a result of the analysis, many new candidate mimetics of metformin and rapamycin were identified, WA being one of them.
Effect of WA on the life span of Drosophila melanogaster wild type Canton-S
Life span is regulated by multiple interrelated phenotypic and genotypic factors and is a temporal characteristic of the damage-restoration process in the body, leading to old age and death [48–50]. In gerontology, it is important to make a distinction between chronological and biological age. Chronological age is measured as the period passed since the time of birth. In humans, chronological age is not a sufficient metric to evaluate the health and performance of an aging person. A more appropriate measure to this end is the biological age which aims to estimate how the progressive degradation occurring within the aging organism affect a combination of metabolic, structural, functional, regulatory features, and adaptive capabilities. These alterations affect vital functions of the organism, leading to the onset of age related diseases, an increase in the probability of death, or a decrease in life span [51–53]. It is well known that it is possible to delay aging and the onset of age-related diseases to prolong the period of active life. In our study, we have shown that WA at concentrations of 10, 100 μM increases the median and maximum life span of male CSs (Figure 2A, 2B). While WA treatment not affected on life span parameters in Drosophila’s females (Figure 2C, 2D).
Effect of WA on stress resistance of Drosophila melanogaster wild type Canton-S
There are a lot of articles in which plant materials have a positive influence on stress resistance of model organisms. For example it was shown in Drosophila melanogaster that the apple phlorizin [54], cloudberry extract [55], oil from Caryocar coriaceum (Pequi) [56] increase resistance to oxidative stress. Blueberry extract [57], Lonicera japonica extract [58] promote stress tolerance in Caenorhabditis elegans. Styphnolobium japonicum fruits [59], Rhodiola rosea root extract [60], Cotinus coggygria extract [61] increase stress resistance and exert antioxidant properties in mouse models. Therefore, we decided to check the effect of WA on the resistance of Drosophila’s flies to oxidative stress and hyperthermia.
Oxidative stress is caused by elevated intracellular levels of reactive oxygen species (ROS), which damage lipids in cell membranes, oxidize proteins, and damage DNA [62]. In our experiment we use paraquat as inductor of ROS [63]. It is known that WA can suppress oxidative stress [64–67]. In most variants of our experiment, WA not led to significant changes on studied survival parameters. WA treatment at 1 μM increased the resistance of males only at the age of 4 and 7 week. Negative effect was found after 10 μM treatment at the age 6 weeks. Also we observed shift in male’s survival curves at 1 μM of WA in 1 and 8 weeks relative to the control curve (Table 1 and Supplementary Figure 1). While WA treatment not affected on female’s resistance to paraquat (Table 2 and Supplementary Figure 3).
Prolonged or intense heat shock causes numerous changes in cell metabolism and disrupts the state of its structural units [68, 69]. Protein damage is the main type of damage during heat shock. Its downstream effects, higher metabolic rate and free radical production, lead to consequent DNA damage [70–72]. In our experiment, WA treatment had different effect in response to hyperthermia. Thus, it increased the male’s resistance to hyperthermia at 2 and 7 weeks after 100 μM of WA and at 4 weeks after 1 μM of WA. It decreased its resistance to hyperthermia at 3 weeks after 1 and 10 μM, at 5 weeks after 1, 10 μM, 100 μM and at 8 weeks after 1 μM of WA. Also we observed shift in male’s mortality curves at 1 μM of WA in 3, 4 and 8 weeks, at 10 μM in 3 and 5 weeks, and at 100 μM at 7 and 8 weeks relative to the control (Table 1 and Supplementary Figure 2). WA treatment reduced female’s resistance to hyperthermia at the age of 1 week after 10 μM, of 2 weeks after 100 μM and of 3 weeks after 1 and 10 μM. Also we observed shift 100 μM at 2 weeks and 1 μM and 10 μM at 3 weeks curves to the left relative to the control curve (Table 2 and Supplementary Figure 4). Thus, it has been shown that WA has a multidirectional effect on the resistance of CSs to the stress factors under study.
Effect of WA on the intestinal barrier permeability of Drosophila melanogaster wild type Canton-S
The gastrointestinal tract has a barrier function that prevents the penetration of food antigens, bacterial toxins, viruses and microorganisms into circulation [73, 74]. There are a lot of articles that have been written about positive role of plant extracts on intestinal microflora and intestinal epithelial barrier [75–79]. The disadvantage of these studies is that they were performed in vitro and do not take into account the effect of aging. The deregulation of the barrier function, which typically occurs in the elderly, can cause the development of pathological conditions [65, 80–82]. In order to prevent the development of such conditions, methods for diagnosing the violations of the permeability of the intestinal barrier are being intensively developed. Therefore, an analysis of the permeability of the intestinal barrier was performed. We have shown that WA at all studied concentration increases the strength of the intestinal barrier in old CSs (Figure 3C, 3F). The rate of flies with the «smurfs» phenotype was lower by 67, 61 and 89% relative to the control after WA at 1, 10, 100 μM concentrations, respectively in males, and by 73, 42 and 61 %% respectively in females. WA’s effects on the strength of the intestinal barrier have not been found in literature. But there is study in which authors did not succeed to increase the strength of intestinal barter using plant materials in aging aspect: ursolic acid (triterpenoid) does not affect gut integrity in male D. melanogaster at the age 30 days [83]. And pectin supplementation was not affected by the intestinal barrier function in healthy young adults and in healthy elderly [83].
Effect of WA on the expression of stress response genes in Drosophila melanogaster wild type Canton-S
Genetic and epigenetic mechanisms and genes that are involved in the regulation of life span are highly interconnected and related to stress response [50]. Moreover, the overexpression of longevity genes listed in [84] as stress response genes almost exclusively resulted in life span extension. A wide-scale comparative analysis of the 1805 known longevity-associated genes across 205 species disclosed that these genes are consistently overrepresented across diverse taxa, compared with the orthologs of other genes, and this conservation is highly. Also in that study it was shown that longevity-associated genes were enriched in translational processes, energy metabolism and DNA repair genes [84]. The genes analyzed in our study play important roles in the following molecular and biological processes: antioxidant defense (Cat, PrxV), metal detoxification (fh), heat shock response (Hsp68, Hsp83), DNA damage recognition (Gadd45) and double-strand break repair (Ku80). More detailed information of these genes can be found in Supplementary Table 1.
Here in Drosophila’s male WA decreased heat shock proteins (Hsp68 or Hsp83) genes expression at all concentration and increased Gadd45 and PrxV genes expression at 1 μM of treatment. In Drosophila’s female found decreased expression of Gadd45, Hsp68 and Hsp83 genes after 1 μM of WA. The 100 μM of WA treatment decreased expression of only Gadd45 gene.
The effects of plant materials on gene regulation have been shown in numerous experiments on model organisms and cancer cell lines. It was shown that licorice and orange extract provoke enhancement of catalase activity and also extend Caenorhabditis elegans life span [85, 86]. Citrus and apple pectin’s have induced the expression of genes involved in DNA repair (D-Gadd, mei-9, spn-B), apoptosis (wrinkled/hid) and heat shock response (hsp70Aa) in Drosophila [87]. Overexpression of PrxV gene can abrogate shikonin-induced cell apoptosis in HT29 colon cancer cells [88]. Modulation of HSP 90 and HSP 70 genes expressions is a possible mechanism by which the Flueggea leucopyrus (Willd) decoction mediates cytotoxic effects in breast cell lines [89]. Anticancer property has also been studied in Glycyrrhiza glabra which inhibited proliferation in HT-29 cell line due to down-regulation of HSP90 gene expression which implied an ability to induce apoptosis [90]. Crude phenolic extracts from extra virgin olive oil directly up-regulated the expression of the Gadd45 gene family in JIMT-1 human breast cancer cell line that circumvent breast cancer resistance to HER1/HER2-targeting drug [91]. Protective role of Podophyllum hexandrum rhizomes and Myrtus communis leaves against DNA damage proved. Shown significant up-regulation of DNA-PKcs and Ku80 and downregulation of ATM and 53BP1 gene expressions in cell lines which were pre-treated with mixture of three active derivatives isolated from the rhizomes of Podophyllum hexandrum, and then irradiated [92]. Myricetin-3-o-galactoside and myricetin-3-o-rhamnoside, isolated from the leaves of Myrtus communis, modulated the expression patterns of cellular genes involved in DNA damaging repair (XPC, LIG4, RPA3, PCNA, DDIT3, POLD1, XRCC5, MPG) [93].
We have repeatedly observed gender specific reactions to WA treatment. Individuals of different genders can response differently to dietary restriction and distorted activity of nutrient-sensing pathways [94]. The main pathways and interventions that lead to sex-specific life span responses, include the growth-hormone/insulin-like growth factor 1 (GH-IGF1) axis, mechanistic target of rapamycin (mTOR) signaling, and nutritional and pharmacological interventions [95].
Thus, WA at concentrations of 10, 100 μM increases the median and maximum life span and shifts the curve to the right side in Drosophila’s male. Together with WA at all concentration decreased expression of genes involved in heat shock response (Hsp68 or Hsp83). The 1 μM of WA increased expression of DNA damage recognition (Gadd45) and antioxidant (PrxV) genes. WA treatment had no effect on life span parameters in Drosophila’s females. While 1 μM and 100 μM of WA decreased the expression of Gadd45 gene. And 1 μM of WA also decreased the expression of Hsp68 and Ku80 (double-strand breaks repair) genes. WA has also a multidirectional effect on the stress resistance of flies. The 1 μM of WA treatment increased the male’s resistance to oxidative stress only at 4 and 7 week old. Negative effects were found after 10 μM treatment in males at the age 6 weeks, while WA treatment did not affect the female’s resistance to oxidative stress. WA increased the male’s resistance to hyperthermia at 2 and 7 weeks after 100 μM of WA and at 4 weeks after 1 μM of WA. The 1 μM of WA decreased male’s resistance at 3, 5 and 8 weeks old. The 10 μM of WA decreased it resistance at 3 and 5 weeks old. Also 100 μM of WA decreased male’s resistance at the age 5 weeks. WA treatment reduced female’s resistance to hyperthermia at the age of 1 week after 10 μM, of 2 weeks after 100 μM and of 3 weeks after 1 and 10 μM. In contrast to this WA increases the permeability of the intestinal barrier of old flies both sexes.
Materials and Methods
In our study, we used the wild type strain Canton-S (Bloomington, USA) Drosophila melanogaster (CS). All CSs were kept in Binder climate chambers (KBF720-ICH, 720l, Binder, Germany) at 25° C and a 12-hour illumination regime in 40ml tubes with 5ml of nutrient medium [96, 97].
To obtain the experimental CS flies, their parents were pre-planted in jars of nutrient medium in the amount of 10 pairs per tube and left for 24 hours to lay eggs. After the appearance of imago, flies were anesthetized using CO2 anesthesia (Genese Scientific, USA), were separated by sex and were placed in test tubes with nutrient with WA and without for further experiments. Non-virgin females were used. Males and females lived separately with 30 animals per tube. From day 1 of life, 30 μl of of 1, 10 or 100 μM WA ethanol solution on top of the flies’ nutrient medium instilled. As a control, we used flies fed with a medium supplemented with 30 μl of ethanol. The final concentration of the drug in the media was 1, 10 or 100 μM. This concentration has shown its ability to increase life span in human fibroblast (internal preliminary tests) and represents a suitable concentration range for pro-longevity effects in invertebrates. To maintain these concentrations flies were transferred to a fresh nutrient medium twice a week [98–102].
Life span assay
To assess life span, 150 flies were selected for each experiment in a single repetition. Two biological repeats were made. Combined data are presented. Flies were placed in test tubes with nutrient medium (30 animals per tube). The counting of the number of dead flies was performed daily. The data were used to compute survival curves and the median, maximum life span, 90% death time were calculated. The Kolmogorov-Smirnov test was used to compare the distribution of mortality in survival curves and the Gehan-Breslow-Wilcoxon test was used to compare the differences in median life span. The significance of the differences in maximum life span was evaluated using the Wang-Allison test. In order to apply this method, animals in each variant of the experiment were divided into two groups: with a life span above the age of 90% mortality, or below the age of 90% mortality. Data were recorded in a 2x2 contingency table and compared using the chi-squared test. According to Bonferroni correction were considered significant differences at p less than 0.017. Analyses were performed using Statistica 6.1 (Stat Soft), and online application for survival analysis «Oasis2» (Structural Bioinformatics Lab).
Stress resistance analysis
The stress resistance of the flies was assessed every week up to 8 weeks of age. The DAM (Drosophila Activity Monitor) system (TriKinetics, USA) was used to look into stress resistance. For analyzing the resistance to oxidative stress, the flies were placed in glass tubes 5 mm in diameter with 20 mM paraquat (Methyl Viologen, Sigma) in 5% sucrose and kept at 25° C until the complete death of flies’ cohort. To assess the resistance to hyperthermia, the flies were seated in glass tubes 5 mm in diameter with a standard medium and kept at 33° C until the complete death of flies’ cohort. The data were used to compute differences in survival distribution with age and in percentiles (25, 50, 75, 90) of death. Fisher’s exact test was used to calculate the statistical differences in percentiles of death. Log-rank criteria were used to assess the statistical significance in survival function distribution. Data were computed using «Oasis2» (Structural Bioinformatics Lab). According to Bonferroni correction were considered significant differences at p less than 0.017. The experiment was performed in three biological repetitions (32 flies in each).
Smurf test
We used 100 flies per variant of experiment. The smurf test was performed at 4, 6 and 8 weeks of age. For this, the test tubes were prepared with a nutrient medium stained with 2.5% (w / v) blue dye No. 1 (Sigma Aldrich, USA). The flies were moved to this medium for 9 hours. Then the number of «smurfs» and «non-smurfs» was counted. Flies were considered «smurfs» if they were blue outside the digestive system [103]. The data obtained were used to construct histograms of the distribution of «smurf» proportion in samples. Fisher’s exact test was used to assess the statistical significance of differences at p less than 0.017 using «Oasis2» (Structural Bioinformatics Lab).
Analysis of stress response gene expression
For each variant of the experiment, 60 flies were selected, separated into two groups of 30 and kept under standard conditions, (Genesee Scientific, USA). For each point of the experiment, 10 females and 20 males were used. Expression analysis was carried out 10 days after the emergence of adults. The experiment was carried out in two biological and three analytical replicates.
Gene expression was measured by “real-time” quantitative PCR with a reverse transcription step (RT-qPCR). RNA was isolated using an Aurum Total RNA mini kit (Bio-Rad, USA) according to the manufacturer’s instructions. The concentration of the resulting RNA was measured using a Quant-iT RNA Assay Kit (Invitrogen, USA). Next, cDNA was synthesized according to the iScript cDNA Synthesis Kit (Bio-Rad, USA). The reaction mixture for carrying out the PCR reaction was prepared according to the manufacturer’s instructions iTaq Universal SYBR Green Supermix (Bio-Rad, USA) and primers (Lumiprobe, USA) (Table 3). The polymerase chain reaction was carried out in a CFX96 amplifier (Bio-Rad), with a DNA melting step using the following program: 1) 95° C for 30 s, 2) 95° C for 10 s, 3) 60° C for 30 s, 4) steps 2-3 were repeated 40 times, 5) DNA melting cycles.
Table 3. List of studied genes and their nucleotide sequence primers.
| Target gene | Abbreviation | 5’- 3 ‘sequences of forward / reverse primers |
| eukaryotic translation elongation factor 1 alpha 2 | eEF1α2 | AGGGCAAGAAGTAGCTGGTTTGC/
GCTGCTACTACTGCGTGTTGTTG |
| β-Tubulin at 56D | Tubulin | GCAACTCCACTGCCATCC/
CCTGCTCCTCCTCGAACT |
| Ribosomal protein L32 | RpL32 | GAAGCGCACCAAGCACTTCATC/
CGCCATTTGTGCGACAGCTTAG |
| Catalase | Cat | CCCAAGAACTACTTTGCTGAGGTG/
AGGAGAACAGACGACCATGCAG |
| frataxin | fh | TTACAGCGATGGCGTGCTAACC/
AGTGCCGACGAAATCGTATCGC |
| Growth arrest and DNA damage-inducible 45 | Gadd45 | GCAAACGCACAACCAAAC/
GGCCATCAGGCAGAAGAG |
| Heat shock protein 68 | Hsp68 | TGGGCACATTCGATCTCACTGG/
TAACGTCGATCTTGGGCACTCC |
| Heat shock protein 83 | Hsp83 | AAGATGCCAGAAGAAGCAGAGACC/
ATCTTGTCCAGGGCATCGGAAG |
| Ku80 | Ku80 | GAGCTTCAGAATGTCGCAACTACC/
GGAAAGTCGTTGAAATCGAAGAGC |
| Peroxiredoxin V | PrxV | CCGATGAGCTGAAGTCCAAG/
TTGCCGTTCTCCACCACCAG |
The expression of studied genes calculated relatively to the expression of the housekeeping genes Tubulin, eEF1α2, RpL32 using the CFX Manager 3.1 software (Bio-Rad, USA).
Author Contributions
Conceived the study: A.M., A. A., A. Z. Planned experiments: A.M., L.K., N.Z. Performed experiments and analyzed data: L.K. and N.Z. Writing - original draft preparation: L.K. Writing – review &editing, L.K., A.M., A. A., A. Z.
Acknowledgments
We are grateful to the Bloomington stock center (Indiana University, USA) for providing the Drosophila melanogaster strains. We thank the Institute of Biology of the Komi Science Center for assistance in the experiments with Drosophila melanogaster and Insilico Medicine for the help with data analysis.
Conflicts of Interest
Alex Aliper and Alex Zhavoronkov are employed by Insilico Medicine, Inc, a longevity biotechnology and artificial intelligence company. All other authors declare no conflicts of interest.
Funding
L.A. carried out the work within the Russian Science Foundation grant No. 19-75-00043 “Study of the geroprotective properties of the Krebs cycle metabolites using Drosophila melanogaster model”. N.Z., A.M. carried out the work within the framework of the state task on the theme “Molecular-genetic mechanisms of aging, life span, and stress resistance of Drosophila melanogaster”, state registration No. AAAA-A18-118011120004-5.
References
-
1.
Singh N, Bhalla M, de Jager P, Gilca M. An overview on ashwagandha: a rasayana (rejuvenator) of ayurveda. Afr J Tradit Complement Altern Med. 2011; 8:208–13. https://doi.org/10.4314/ajtcam.v8i5S.9 [PubMed]
-
2.
Lee SH, Min KJ. Drosophila melanogaster as a model system in the study of pharmacological interventions in aging. Transl Med Aging. 2019; 3:98–103.
-
3.
Yarden A, Lavie D. Constituents of Withania somnifera. Part I. The functional groups of withaferin. J Chem Soc. 1962; 2925–7. https://doi.org/10.1039/jr9620002925
-
4.
Lavie D, Glotter E, Shvo Y. Constituents of Withania somnifera Dun. III. The side chain of withaferin A. J Org Chem. 1965; 30:1774–8. https://doi.org/10.1021/jo01017a015
-
5.
Lavie D, Glotter E, Shvo Y. Constituents of Withania somnifera Dun. Part IV. The structure of withaferin A. J Chem Soc. 1965; 7517–31. https://doi.org/10.1039/jr9650007517
-
6.
Kupchan SM, Doskotch RW, Bollinger P, Mcphail AT, Sim GA, Renauld JA. The isolation and structural elucidation of a novel steroidal tumor inhibitor from acnistus arborescens. J Am Chem Soc. 1965; 87:5805–06. https://doi.org/10.1021/ja00952a061 [PubMed]
-
7.
Khan PM, Malik A, Ahmad S, Nawaz HR. Withanolides from ajuga parviflora. J Nat Prod. 1999; 62:1290–92. https://doi.org/10.1021/np990029k [PubMed]
-
8.
Huang Y, Liu JK, Mühlbauer A, Henkel T. Three novel taccalonolides from the tropical plant Tacca subflaellata. Helvetica chimica acta. 2002; 85:2553–8. https://doi.org/10.1002/1522-2675(200208)85:8<2553::AID-HLCA2553>3.0.CO;2-8
-
9.
Stein A, Compera D, Karge B, Brönstrup M, Franke J. Isolation and characterisation of irinans, androstane-type withanolides from Physalis Peruviana L. Beilstein J Org Chem. 2019; 15:2003–12. https://doi.org/10.3762/bjoc.15.196 [PubMed]
-
10.
Petreanu M, Maia P, da Rocha Pittarello JL, Loch LC, Monache FD, Perez AL, Solano-Arias G, Filho VC, de Souza MM, Niero R. Antidepressant-like effect and toxicological parameters of extract and withanolides isolated from aerial parts of Solanum capsicoides all. (Solanaceae). Naunyn Schmiedebergs Arch Pharmacol. 2019; 392:979–90. https://doi.org/10.1007/s00210-019-01648-9 [PubMed]
-
11.
Niero R, Da Silva IT, Tonial GC, Santos Camacho BD, Gacs-Baitz E, Monache GD, Monache FD. Cilistepoxide and cilistadiol, two new withanolides from Solanum sisymbiifolium. Nat Prod Res. 2006; 20:1164–68. https://doi.org/10.1080/14786410600888459 [PubMed]
-
12.
Zhu XH, Ando J, Takagi M, Ikeda T, Yoshimitsu A, Nohara T. Four novel withanolide-type steroids from the leaves of Solanum cilistum. Chem Pharm Bull (Tokyo). 2001; 49:1440–43. https://doi.org/10.1248/cpb.49.1440 [PubMed]
-
13.
Zhu XH, Takagi M, Ikeda T, Midzuki K, Nohara T. Withanolide-type steroids from Solanum cilistum. Phytochemistry. 2001; 56:741–45. https://doi.org/10.1016/s0031-9422(00)00487-8 [PubMed]
-
14.
Zhu XH, Ando J, Takagi M, Ikeda T, Nohara T. Six new withanolide-type steroids from the leaves of Solanum cilistum. Chem Pharm Bull (Tokyo). 2001; 49:161–64. https://doi.org/10.1248/cpb.49.161 [PubMed]
-
15.
Alfonso D, Kapetanidis I. Withanolides from Iochroma gesnerioides. Phytochemistry. 1994; 36:179–83. https://doi.org/10.1016/S0031-9422(00)97035-3
-
16.
Eich E. (2008). Solanaceae and Convolvulaceae: Secondary Metabolites. Biosynthesis, Chemotaxonomy, Biological and Economic Significance (A Handbook). Springer-Verlag Berlin Heidelberg. https://doi.org/10.1007/978-3-540-74541-9
-
17.
Tripathi S, Sangwan RS, Mishra B, Jadaun JS, Sangwan NS. Berry transcriptome: insights into a novel resource to understand development dependent secondary metabolism in Withania somnifera (Ashwagandha). Physiol Plant. 2020; 168:148–73. https://doi.org/10.1111/ppl.12943 [PubMed]
-
18.
Tripathi S, Sangwan RS, Narnoliya LK, Srivastava Y, Mishra B, Sangwan NS. Transcription factor repertoire in Ashwagandha (Withania somnifera) through analytics of transcriptomic resources: insights into regulation of development and withanolide metabolism. Sci Rep. 2017; 7:16649. https://doi.org/10.1038/s41598-017-14657-6 [PubMed]
-
19.
Kuzuyama T, Seto H. Two distinct pathways for essential metabolic precursors for isoprenoid biosynthesis. Proc Jpn Acad Ser B Phys Biol Sci. 2012; 88:41–52. https://doi.org/10.2183/pjab.88.41 [PubMed]
-
20.
Samadi AK, Tong X, Mukerji R, Zhang H, Timmermann BN, Cohen MS. Withaferin A, a cytotoxic steroid from vassobia breviflora, induces apoptosis in human head and neck squamous cell carcinoma. J Nat Prod. 2010; 73:1476–81. https://doi.org/10.1021/np100112p [PubMed]
-
21.
Gautam R, Jachak SM, Saklani A. Anti-inflammatory effect of ajuga bracteosa wall ex benth. Mediated through cyclooxygenase (COX) inhibition. J Ethnopharmacol. 2011; 133:928–30. https://doi.org/10.1016/j.jep.2010.11.003 [PubMed]
-
22.
Llanos GG, Araujo LM, Jiménez IA, Moujir LM, Bazzocchi IL. Withaferin a-related steroids from withania aristata exhibit potent antiproliferative activity by inducing apoptosis in human tumor cells. Eur J Med Chem. 2012; 54:499–511. https://doi.org/10.1016/j.ejmech.2012.05.032 [PubMed]
-
23.
Erazo S, Rocco G, Zaldivar M, Delporte C, Backhouse N, Castro C, Belmonte E, Delle Monache F, García R. Active metabolites from dunalia spinosa resinous exudates. Z Naturforsch C J Biosci. 2008; 63:492–96. https://doi.org/10.1515/znc-2008-7-804 [PubMed]
-
24.
Abraham A, Kirson I, Glotter E, Lavie D. A chemotaxonomic study of Withania somnifera (L.) dun. Phytochemistry. 1968; 7:957–62. https://doi.org/10.1016/S0031-9422(00)82182-2
-
25.
Lavie D, Greenfield S, Glotter E. Constituents of Withania somnifera Dun. Part VI. The stereochemistry of withaferin A. J Chem Soc C. 1966; 1753–6. https://doi.org/10.1039/j39660001753
-
26.
Vanden Berghe W, Sabbe L, Kaileh M, Haegeman G, Heyninck K. Molecular insight in the multifunctional activities of Withaferin A. Biochem Pharmacol. 2012; 84:1282–91. https://doi.org/10.1016/j.bcp.2012.08.027 [PubMed]
-
27.
Vyas AR, Singh SV. Molecular targets and mechanisms of cancer prevention and treatment by withaferin a, a naturally occurring steroidal lactone. AAPS J. 2014; 16:1–10. https://doi.org/10.1208/s12248-013-9531-1 [PubMed]
-
28.
Yan Z, Guo R, Gan L, Lau WB, Cao X, Zhao J, Ma X, Christopher TA, Lopez BL, Wang Y. Withaferin a inhibits apoptosis via activated Akt-mediated inhibition of oxidative stress. Life Sci. 2018; 211:91–101. https://doi.org/10.1016/j.lfs.2018.09.020 [PubMed]
-
29.
Xia S, Miao Y, Liu S. Withaferin A induces apoptosis by ROS-dependent mitochondrial dysfunction in human colorectal cancer cells. Biochem Biophys Res Commun. 2018; 503:2363–69. https://doi.org/10.1016/j.bbrc.2018.06.162 [PubMed]
-
30.
Cui ZG, Piao JL, Rehman MU, Ogawa R, Li P, Zhao QL, Kondo T, Inadera H. Molecular mechanisms of hyperthermia-induced apoptosis enhanced by withaferin a. Eur J Pharmacol. 2014; 723:99–107. https://doi.org/10.1016/j.ejphar.2013.11.031 [PubMed]
-
31.
Stan SD, Zeng Y, Singh SV. Ayurvedic medicine constituent withaferin a causes G2 and M phase cell cycle arrest in human breast cancer cells. Nutr Cancer. 2008 (Suppl 1); 60:51–60. https://doi.org/10.1080/01635580802381477 [PubMed]
-
32.
McKenna MK, Gachuki BW, Alhakeem SS, Oben KN, Rangnekar VM, Gupta RC, Bondada S. Anti-cancer activity of withaferin a in b-cell lymphoma. Cancer Biol Ther. 2015; 16:1088–98. https://doi.org/10.1080/15384047.2015.1046651 [PubMed]
-
33.
Kaileh M, Vanden Berghe W, Heyerick A, Horion J, Piette J, Libert C, De Keukeleire D, Essawi T, Haegeman G. Withaferin a strongly elicits IkappaB kinase beta hyperphosphorylation concomitant with potent inhibition of its kinase activity. J Biol Chem. 2007; 282:4253–64. https://doi.org/10.1074/jbc.M606728200 [PubMed]
-
34.
Grover A, Shandilya A, Punetha A, Bisaria VS, Sundar D. Inhibition of the NEMO/IKKβ association complex formation, a novel mechanism associated with the NF-κB activation suppression by Withania somniferasomnifera’s key metabolite withaferin A. BMC Genomics. 2010 (Suppl 4); 11:S25. https://doi.org/10.1186/1471-2164-11-S4-S25 [PubMed]
-
35.
Martorana F, Guidotti G, Brambilla L, Rossi D. Withaferin a inhibits nuclear factor-κB-dependent pro-inflammatory and stress response pathways in the astrocytes. Neural Plast. 2015; 2015:381964. https://doi.org/10.1155/2015/381964 [PubMed]
-
36.
Bhattacharya SK, Satyan KS, Ghosal S. Antioxidant activity of glycowithanolides from Withania somnifera. Indian J Exp Biol. 1997; 35:236–39. [PubMed]
-
37.
Aqil F, Munagala R, Agrawal AK, Gupta R. (2019). Chapter 10 - Anticancer Photocopied: Experimental and Clinical Updates. In: Khan MSA, Ahmad I, Chattopadhyay D, eds. New Look to Phytomedicine, Advancements in Herbal Products as Novel Drug Leads. Academic Press. https://doi.org/10.1016/B978-0-12-814619-4.00010-0
-
38.
Patel P, Julien JP, Kriz J. Early-stage treatment with withaferin a reduces levels of misfolded superoxide dismutase 1 and extends lifespan in a mouse model of amyotrophic lateral sclerosis. Neurotherapeutics. 2015; 12:217–33. https://doi.org/10.1007/s13311-014-0311-0 [PubMed]
-
39.
Yu TJ, Tang JY, Ou-Yang F, Wang YY, Yuan SF, Tseng K, Lin LC, Chang HW. Low concentration of withaferin a inhibits oxidative stress-mediated migration and invasion in oral cancer cells. Biomolecules. 2020; 10:777. https://doi.org/10.3390/biom10050777 [PubMed]
-
40.
Grogan PT, Sleder KD, Samadi AK, Zhang H, Timmermann BN, Cohen MS. Cytotoxicity of withaferin a in glioblastomas involves induction of an oxidative stress-mediated heat shock response while altering Akt/mTOR and MAPK signaling pathways. Invest New Drugs. 2013; 31:545–57. https://doi.org/10.1007/s10637-012-9888-5 [PubMed]
-
41.
Bale S, Pulivendala G, Godugu C. Withaferin a attenuates bleomycin-induced scleroderma by targeting FoxO3a and NF-κβ signaling: connecting fibrosis and inflammation. Biofactors. 2018; 44:507–17. https://doi.org/10.1002/biof.1446 [PubMed]
-
42.
Moskalev A, Chernyagina E, de Magalhães JP, Barardo D, Thoppil H, Shaposhnikov M, Budovsky A, Fraifeld VE, Garazha A, Tsvetkov V, Bronovitsky E, Bogomolov V, Scerbacov A, et al. Geroprotectors.org: a new, structured and curated database of current therapeutic interventions in aging and age-related disease. Aging (Albany NY). 2015; 7:616–28. https://doi.org/10.18632/aging.100799 [PubMed]
-
43.
Anisimov VN, Egorov MV, Krasilshchikova MS, Lyamzaev KG, Manskikh VN, Moshkin MP, Novikov EA, Popovich IG, Rogovin KA, Shabalina IG, Shekarova ON, Skulachev MV, Titova TV, et al. Effects of the mitochondria-targeted antioxidant SkQ1 on lifespan of rodents. Aging (Albany NY). 2011; 3:1110–19. https://doi.org/10.18632/aging.100404 [PubMed]
-
44.
Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin MJ, Schwab M, Pollak M, Zhang Y, et al. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013; 4:2192. https://doi.org/10.1038/ncomms3192 [PubMed]
-
45.
Song J, Jiang G, Zhang J, Guo J, Li Z, Hao K, Liu L, Cheng Z, Tong X, Dai F. Metformin prolongs lifespan through remodeling the energy distribution strategy in silkworm, Bombyx mori. Aging (Albany NY). 2019; 11:240–48. https://doi.org/10.18632/aging.101746 [PubMed]
-
46.
Blagosklonny MV. Rapamycin for longevity: opinion article. Aging (Albany NY). 2019; 11:8048–67. https://doi.org/10.18632/aging.102355 [PubMed]
-
47.
Aliper A, Jellen L, Cortese F, Artemov A, Karpinsky-Semper D, Moskalev A, Swick AG, Zhavoronkov A. Towards natural mimetics of metformin and rapamycin. Aging (Albany NY). 2017; 9:2245–68. https://doi.org/10.18632/aging.101319 [PubMed]
-
48.
Pal S, Tyler JK. Epigenetics and aging. Sci Adv. 2016; 2:e1600584. https://doi.org/10.1126/sciadv.1600584 [PubMed]
-
49.
Brunet A, Berger SL. Epigenetics of aging and aging-related disease. J Gerontol A Biol Sci Med Sci. 2014 (Suppl 1); 69:S17–20. https://doi.org/10.1093/gerona/glu042 [PubMed]
-
50.
Moskalev AA, Aliper AM, Smit-McBride Z, Buzdin A, Zhavoronkov A. Genetics and epigenetics of aging and longevity. Cell Cycle. 2014; 13:1063–77. https://doi.org/10.4161/cc.28433 [PubMed]
-
51.
Hamczyk MR, Nevado RM, Barettino A, Fuster V, Andrés V. Biological versus chronological aging: JACC focus seminar. J Am Coll Cardiol. 2020; 75:919–30. https://doi.org/10.1016/j.jacc.2019.11.062 [PubMed]
-
52.
Ridda I, Macintyre CR, Lindley R, Gao Z, Sullivan JS, Yuan FF, McIntyre PB. Immunological responses to pneumococcal vaccine in frail older people. Vaccine. 2009; 27:1628–36. https://doi.org/10.1016/j.vaccine.2008.11.098 [PubMed]
-
53.
Jazwinski SM, Kim S. Examination of the dimensions of biological age. Front Genet. 2019; 10:263. https://doi.org/10.3389/fgene.2019.00263 [PubMed]
-
54.
Wang H, Sun Z, Liu D, Li X, Rehman RU, Wang H, Wu Z. Apple phlorizin attenuates oxidative stress in drosophila melanogaster. J Food Biochem. 2019; 43:e12744. https://doi.org/10.1111/jfbc.12744 [PubMed]
-
55.
Lashmanova EA, Kuzivanova OA, Dymova OV, Moskalev AA. [The effects of cloudberry extract on drosophila melanogaster lifespan and stress resistance]. Adv Gerontol. 2018; 31:958–65. [PubMed]
-
56.
Duavy SM, Ecker A, Salazar GT, Loreto J, Costa JG, Vargas Barbosa N. Pequi enriched diets protect Drosophila melanogaster against paraquat-induced locomotor deficits and oxidative stress. J Toxicol Environ Health A. 2019; 82:664–77. https://doi.org/10.1080/15287394.2019.1642277 [PubMed]
-
57.
Wang H, Liu J, Li T, Liu RH. Blueberry extract promotes longevity and stress tolerance via DAF-16 in caenorhabditis elegans. Food Funct. 2018; 9:5273–82. https://doi.org/10.1039/c8fo01680a [PubMed]
-
58.
Yang ZZ, Yu YT, Lin HR, Liao DC, Cui XH, Wang HB. Lonicera japonica extends lifespan and healthspan in caenorhabditis elegans. Free Radic Biol Med. 2018; 129:310–22. https://doi.org/10.1016/j.freeradbiomed.2018.09.035 [PubMed]
-
59.
Thabit S, Handoussa H, Roxo M, Cestari de Azevedo B, El Sayed NS, Wink M. Styphnolobium japonicum (L.) Schott Fruits Increase Stress Resistance and Exert Antioxidant Properties in Caenorhabditis elegans and Mouse Models. Molecules. 2019; 24:2633. https://doi.org/10.3390/molecules24142633 [PubMed]
-
60.
Dinel AL, Guinobert I, Lucas C, Blondeau C, Bardot V, Ripoche I, Berthomier L, Pallet V, Layé S, Joffre C. Reduction of acute mild stress corticosterone response and changes in stress-responsive gene expression in male Balb/c mice after repeated administration of a Rhodiola rosea L. Root extract. Food Sci Nutr. 2019; 7:3827–41. https://doi.org/10.1002/fsn3.1249 [PubMed]
-
61.
Matić S, Stanić S, Bogojević D, Vidaković M, Grdović N, Arambašić J, Dinić S, Uskoković A, Poznanović G, Solujić S, Mladenović M, Marković J, Mihailović M. Extract of the plant cotinus coggygria scop. Attenuates pyrogallol-induced hepatic oxidative stress in wistar rats. Can J Physiol Pharmacol. 2011; 89:401–11. https://doi.org/10.1139/y11-043 [PubMed]
-
62.
Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014; 24:R453–62. https://doi.org/10.1016/j.cub.2014.03.034 [PubMed]
-
63.
Dinis-Oliveira RJ, Sarmento A, Reis P, Amaro A, Remião F, Bastos ML, Carvalho F. Acute paraquat poisoning: report of a survival case following intake of a potential lethal dose. Pediatr Emerg Care. 2006; 22:537–40. https://doi.org/10.1097/01.pec.0000223179.07633.8a [PubMed]
-
64.
Batumalaie K, Amin MA, Murugan DD, Sattar MZ, Abdullah NA. Withaferin a protects against palmitic acid-induced endothelial insulin resistance and dysfunction through suppression of oxidative stress and inflammation. Sci Rep. 2016; 6:27236. https://doi.org/10.1038/srep27236 [PubMed]
-
65.
Abu Bakar MH, Azmi MN, Shariff KA, Tan JS. Withaferin a protects against high-fat diet-induced obesity via attenuation of oxidative stress, inflammation, and insulin resistance. Appl Biochem Biotechnol. 2019; 188:241–59. https://doi.org/10.1007/s12010-018-2920-2 [PubMed]
-
66.
Su LJ, Zhang JH, Gomez H, Murugan R, Hong X, Xu D, Jiang F, Peng ZY. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid Med Cell Longev. 2019; 2019:5080843. https://doi.org/10.1155/2019/5080843 [PubMed]
-
67.
Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, Griendling KK. Reactive oxygen species in metabolic and inflammatory signaling. Circ Res. 2018; 122:877–902. https://doi.org/10.1161/CIRCRESAHA.117.311401 [PubMed]
-
68.
Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999; 61:243–82. https://doi.org/10.1146/annurev.physiol.61.1.243 [PubMed]
-
69.
Kedersha N, Anderson P. Stress granules: sites of mRNA triage that regulate mRNA stability and translatability. Biochem Soc Trans. 2002; 30:963–69. https://doi.org/10.1042/bst0300963 [PubMed]
-
70.
Sottile ML, Nadin SB. Heat shock proteins and DNA repair mechanisms: an updated overview. Cell Stress Chaperones. 2018; 23:303–15. https://doi.org/10.1007/s12192-017-0843-4 [PubMed]
-
71.
Kantidze OL, Velichko AK, Luzhin AV, Razin SV. Heat stress-induced DNA damage. Acta Naturae. 2016; 8:75–78. [PubMed]
-
72.
Richter K, Haslbeck M, Buchner J. The heat shock response: life on the verge of death. Mol Cell. 2010; 40:253–66. https://doi.org/10.1016/j.molcel.2010.10.006 [PubMed]
-
73.
Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014; 157:121–41. https://doi.org/10.1016/j.cell.2014.03.011 [PubMed]
-
74.
König J, Wells J, Cani PD, García-Ródenas CL, MacDonald T, Mercenier A, Whyte J, Troost F, Brummer RJ. Human intestinal barrier function in health and disease. Clin Transl Gastroenterol. 2016; 7:e196. https://doi.org/10.1038/ctg.2016.54 [PubMed]
-
75.
Yan Z, Yang F, Hong Z, Wang S, Jinjuan Z, Han B, Xie R, Leng F, Yang Q. Blueberry attenuates liver fibrosis, protects intestinal epithelial barrier, and maintains gut microbiota homeostasis. Can J Gastroenterol Hepatol. 2019; 2019:5236149. https://doi.org/10.1155/2019/5236149 [PubMed]
-
76.
Chi JH, Kim YH, Sohn DH, Seo GS, Lee SH. Ameliorative effect of alnus japonica ethanol extract on colitis through the inhibition of inflammatory responses and attenuation of intestinal barrier disruption in vivo and in vitro. Biomed Pharmacother. 2018; 108:1767–74. https://doi.org/10.1016/j.biopha.2018.10.050 [PubMed]
-
77.
Wang T, Yao W, Li J, Shao Y, He Q, Xia J, Huang F. Dietary garcinol supplementation improves diarrhea and intestinal barrier function associated with its modulation of gut microbiota in weaned piglets. J Anim Sci Biotechnol. 2020; 11:12. https://doi.org/10.1186/s40104-020-0426-6 [PubMed]
-
78.
Patra AK. Influence of plant bioactive compounds on intestinal epithelial barrier in poultry. Mini Rev Med Chem. 2020; 20:566–77. https://doi.org/10.2174/1389557520666191226111405 [PubMed]
-
79.
Valero MS, González M, Ramón-Gimenez M, Andrade PB, Moreo E, Les F, Fernandes F, Gómez-Rincón C, Berzosa C, García de Jalón JA, Arruebo MP, Plaza MÁ, Köhler R, et al. Jasonia glutinosa (L.) DC., a traditional herbal medicine, reduces inflammation, oxidative stress and protects the intestinal barrier in a murine model of colitis. Inflammopharmacology. 2020; 28:1717–34. https://doi.org/10.1007/s10787-019-00626-0 [PubMed]
-
80.
Dambroise E, Monnier L, Ruisheng L, Aguilaniu H, Joly JS, Tricoire H, Rera M. Two phases of aging separated by the smurf transition as a public path to death. Sci Rep. 2016; 6:23523. https://doi.org/10.1038/srep23523 [PubMed]
-
81.
Buford TW. (Dis)Trust your gut: the gut microbiome in age-related inflammation, health, and disease. Microbiome. 2017; 5:80. https://doi.org/10.1186/s40168-017-0296-0 [PubMed]
-
82.
Nagpal R, Mainali R, Ahmadi S, Wang S, Singh R, Kavanagh K, Kitzman DW, Kushugulova A, Marotta F, Yadav H. Gut microbiome and aging: physiological and mechanistic insights. Nutr Healthy Aging. 2018; 4:267–85. https://doi.org/10.3233/NHA-170030 [PubMed]
-
83.
Staats S, Wagner AE, Lüersen K, Künstner A, Meyer T, Kahns AK, Derer S, Graspeuntner S, Rupp J, Busch H, Sina C, Ipharraguerre IR, Rimbach G. Dietary ursolic acid improves health span and life span in male drosophila melanogaster. Biofactors. 2019; 45:169–86. https://doi.org/10.1002/biof.1467 [PubMed]
-
84.
Yanai H, Budovsky A, Barzilay T, Tacutu R, Fraifeld VE. Wide-scale comparative analysis of longevity genes and interventions. Aging Cell. 2017; 16:1267–75. https://doi.org/10.1111/acel.12659 [PubMed]
-
85.
Reigada I, Moliner C, Valero MS, Weinkove D, Langa E, Gómez Rincón C. Antioxidant and antiaging effects of licorice on the Caenorhabditis elegans model. J Med Food. 2020; 23:72–78. https://doi.org/10.1089/jmf.2019.0081 [PubMed]
-
86.
Wang J, Deng N, Wang H, Li T, Chen L, Zheng B, Liu RH. Effects of orange extracts on longevity, healthspan, and stress resistance in Caenorhabditis elegans. Molecules. 2020; 25:351. https://doi.org/10.3390/molecules25020351 [PubMed]
-
87.
Shaposhnikov M, Latkin D, Plyusnina E, Shilova L, Danilov A, Popov S, Zhavoronkov A, Ovodov Y, Moskalev A. The effects of pectins on life span and stress resistance in drosophila melanogaster. Biogerontology. 2014; 15:113–27. https://doi.org/10.1007/s10522-013-9484-x [PubMed]
-
88.
Chandimali N, Sun HN, Kong LZ, Zhen X, Liu R, Kwon T, Lee DS. Shikonin-induced apoptosis of colon cancer cells is reduced by peroxiredoxin V expression. Anticancer Res. 2019; 39:6115–23. https://doi.org/10.21873/anticanres.13819 [PubMed]
-
89.
Mendis AS, Thabrew I, Samarakoon SR, Tennekoon KH. Modulation of expression of heat shock proteins and apoptosis by flueggea leucopyrus (willd) decoction in three breast cancer phenotypes. BMC Complement Altern Med. 2015; 15:404. https://doi.org/10.1186/s12906-015-0927-6 [PubMed]
-
90.
Nourazarian SM, Nourazarian A, Majidinia M, Roshaniasl E. Effect of root extracts of medicinal herb glycyrrhiza glabra on HSP90 gene expression and apoptosis in the HT-29 colon cancer cell line. Asian Pac J Cancer Prev. 2015; 16:8563–66. https://doi.org/10.7314/apjcp.2015.16.18.8563 [PubMed]
-
91.
Oliveras-Ferraros C, Fernández-Arroyo S, Vazquez-Martin A, Lozano-Sánchez J, Cufí S, Joven J, Micol V, Fernández-Gutiérrez A, Segura-Carretero A, Menendez JA. Crude phenolic extracts from extra virgin olive oil circumvent de novo breast cancer resistance to HER1/HER2-targeting drugs by inducing GADD45-sensed cellular stress, G2/M arrest and hyperacetylation of histone H3. Int J Oncol. 2011; 38:1533–47. https://doi.org/10.3892/ijo.2011.993 [PubMed]
-
92.
Srivastava NN, Shukla SK, Yashavarddhan MH, Devi M, Tripathi RP, Gupta ML. Modification of radiation-induced DNA double strand break repair pathways by chemicals extracted from podophyllum hexandrum: an in vitro study in human blood leukocytes. Environ Mol Mutagen. 2014; 55:436–48. https://doi.org/10.1002/em.21853 [PubMed]
-
93.
Hayder N, Bouhlel I, Skandrani I, Kadri M, Steiman R, Guiraud P, Mariotte AM, Ghedira K, Dijoux-Franca MG, Chekir-Ghedira L. In vitro antioxidant and antigenotoxic potentials of myricetin-3-o-galactoside and myricetin-3-o-rhamnoside from myrtus communis: modulation of expression of genes involved in cell defence system using cDNA microarray. Toxicol In Vitro. 2008; 22:567–81. https://doi.org/10.1016/j.tiv.2007.11.015 [PubMed]
-
94.
Regan JC, Partridge L. Gender and longevity: why do men die earlier than women? comparative and experimental evidence. Best Pract Res Clin Endocrinol Metab. 2013; 27:467–79. https://doi.org/10.1016/j.beem.2013.05.016 [PubMed]
-
95.
Garratt M. Why do sexes differ in lifespan extension? Sex-specific pathways of aging and underlying mechanisms for dimorphic responses. Nutrition and Healthy Aging. 2020; 5: 247–59. https://doi.org/10.3233/NHA-190067
-
96.
He Y, Jasper H. Studying aging in drosophila. Methods. 2014; 68:129–33. https://doi.org/10.1016/j.ymeth.2014.04.008 [PubMed]
-
97.
Xia B, de Belle JS. Transgenerational programming of longevity and reproduction by post-eclosion dietary manipulation in drosophila. Aging (Albany NY). 2016; 8:1115–34. https://doi.org/10.18632/aging.100932 [PubMed]
-
98.
Shaposhnikov MV, Zemskaya NV, Koval LA, Schegoleva EV, Zhavoronkov A, Moskalev AA. Effects of N-acetyl-L-cysteine on lifespan, locomotor activity and stress-resistance of 3 Drosophila species with different lifespans. Aging (Albany NY). 2018; 10:2428–58. https://doi.org/10.18632/aging.101561 [PubMed]
-
99.
Shaposhnikov MV, Zemskaya NV, Koval LA, Minnikhanova NR, Kechko OI, Mitkevich VA, Makarov AA, Moskalev AA. Amyloid-β peptides slightly affect lifespan or antimicrobial peptide gene expression in drosophila melanogaster. BMC Genet. 2020 (Suppl 1); 21:65. https://doi.org/10.1186/s12863-020-00866-y [PubMed]
-
100.
Moskalev A, Shaposhnikov M, Zemskaya N, Belyi A, Dobrovolskaya E, Patova A, Guvatova Z, Lukyanova E, Snezhkina A, Kudryavtseva A. Transcriptome analysis reveals mechanisms of geroprotective effects of fucoxanthin in drosophila. BMC Genomics. 2018 (Suppl 3); 19:77. https://doi.org/10.1186/s12864-018-4471-x [PubMed]
-
101.
Danilov A, Shaposhnikov M, Shevchenko O, Zemskaya N, Zhavoronkov A, Moskalev A. Influence of non-steroidal anti-inflammatory drugs on drosophila melanogaster longevity. Oncotarget. 2015; 6:19428–44. https://doi.org/10.18632/oncotarget.5118 [PubMed]
-
102.
Proshkina E, Lashmanova E, Dobrovolskaya E, Zemskaya N, Kudryavtseva A, Shaposhnikov M, Moskalev A. Geroprotective and radioprotective activity of quercetin, (-)-epicatechin, and ibuprofen in Drosophila melanogaster. Front Pharmacol. 2016; 7:505. https://doi.org/10.3389/fphar.2016.00505 [PubMed]
-
103.
Martins RR, McCracken AW, Simons MJ, Henriques CM, Rera M. How to catch a smurf? - ageing and beyond… In vivo assessment of intestinal permeability in multiple model organisms. Bio Protoc. 2018; 8:e2722. https://doi.org/10.21769/BioProtoc.2722 [PubMed]