Introduction
Esophageal cancer (EC) is an aggressive and invasive disease and early diagnosis is clinically challenging. It is associated with one of the highest mortality rates (500,000 per year) and incidence rates (570,000 new cases per year) [1] and the global incidence and mortality of EC are predicted to increase in the coming decades [1, 2]. The growing risk from this malignancy presents a heavy burden on health care providers in almost every population, particularly in Eastern Asia, the world leader in tobacco use, which is one of the most important risk factors for EC [2].
EC can be divided into esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC) based on the different cell origins. ESCC originates from squamous cells, while EAC originates predominantly from Barrett mucosa [3]. It is known that the incidences of ESCC and EAC vary geographically. ESCC is predominant in East Asia and parts of Africa and accounts for 90% of the new cases of EC every year [4]. The major causes of ESCC include smoking and excessive drinking. Other risk factors are dietary deficiencies, hot beverage intake, achalasia, history of head and neck squamous cell cancer, and radiation therapy [5, 6]. EAC is found more frequently in Europe and North America and is related to chronic inflammation, intestinal metaplasia (Barrett’s esophagus) in the distal esophageal epithelium and obesity [7–9]. Notably, compared to ESCC, the incidence of EAC has increased persistently in some developed countries in recent years [10]. Although age has not been listed as a risk factor of EC, age may affect patient survival and treatment methods [11, 12]. One study demonstrated that overall survival of patients ≥70 years old was shorter, while length of stay was longer than those <70 years old [13]. In another study, patients ≥70 years old were less likely to be subjected to surgery or/and radiotherapy [14]. Given the great challenge of this disease, it is urgently needed to develop more powerful disease models for a better understanding of the pathogenesis of esophageal cancer and developing new approaches to esophageal cancer prevention, early diagnosis, and treatment.
Patient-derived xenograft (PDX) models are established by the engrafting patients’ tumor tissues into immunodeficient mice to obtain a framework that faithfully simulates human cancer biology in vivo. Particularly, PDX models largely recapitulate the genetic, phenotypic, and functional characteristics of the primary tumors after transplantation. Here we summarize the methods of PDX model construction for EC and elucidate the practical value of the PDX model in EC treatment, including its use in screening predictive markers and therapeutic targets. PDX models are of great value in understanding cancer progression of EC and developing precision medicine methods to combat EC.
Methods for establishing EC PDX models and characteristics of EC-PDX
The engraftment rates of PDX
The engraftment rates of subcutaneous PDX for EC vary from 13.3% to 55.5%, as reported in several studies listed in Table 1. In Table 2, the success rates of PDX for other tumors including neuroblastoma, osteosarcoma, osteosarcoma and so on are listed, which vary from 24% to 100%. The take rate of orthotopic PDX for EC is claimed as 100%, however, the extremely limited sample size (only one case) used in this study may strongly affect the estimation of the take rate [15]. Because of the anatomical location of the esophagus, the establishment of orthotopic model of EC requires advanced surgical techniques, and hardly achieves simplicity or reproducibility [16, 17]. Therefore, orthotopic PDX models for EC are rarely employed. Both resected tumor tissues and endoscopic biopsies are suitable for the engraftment. Resected tumor tissues are often obtained during surgery, while biopsy specimens are obtained during endoscopic examination for pathological confirmation. Tumor tissues or biopsy specimens are then fragmented and these tissue fragments will be directly implanted or blended with Matrigel before implanting into immunocompromised mice for tumor growth and expansion. Esophageal tumor cell populations isolated from ESCC tissues have also been implanted to establish PDX model [18].
Table 1. A summary of PDX models for esophageal cancer.
| Histology | Tissue type | Implantation method | Mouse strain | Xenograft success rate (%) | Refs. |
| ESCC | Resected | SC | SCID mice | 37/96 (38.5) | [19] |
| ESCC/EAC | Resected | SC | NSG mice | ESCC 4/12 (25) EAC 13/49 (33) | [20] |
| ESCC | Resected | SC | SCID mice | 14/26 (53.6) | [21] |
| ESCC | Biopsy | SC | NOD-SCID mice | 25/188 (13.3) | [22] |
| ESCC / EAC | Biopsy | SC | NOD-SCID/NSG mice | ESCC 5/16(31) EAC 8/54(33) | [27] |
| GEJ adenocarcinoma | Biopsy | Ort | SCID mice | 1/1 (100) | [15] |
| GEJ/ESCC/EAC | Resected/biopsy | IM/SC | SCID/NOD-SCID/NSG mice | IM 13/18 (72) SC 1/6 (16) | [33] |
| ESCC | Resected | SC | Athymic nude mice | 61/110 (55.5) | [36] |
| ESCC | Resected | SC | NOD-SCID mice | 23/54 (42.6) | [42] |
| GEJ/ ESCC/EAC | Resected | SC | NOD-SCID mice | 21/55 (38) | [43] |
| ESCC | Resected | SC | SICD mice | 25/54(46.3) | [39] |
| ESCC: esophageal squamous cell carcinoma; GEJ: gastroesophageal junction; EAC: esophageal adenocarcinoma; SC: Subcutaneous; Ort: orthotopic; IM: Intramuscular; SCID: C.B17-Prkdcscid; NOD-SCID: NOD.C.B17-Prkdcscid; NSG: NOD.Cg-PrkdcscidIl2rgtm1Wjl. |
Table 2. The success rate of PDX for other tumor types.
| Tumor types | Methods | Recipient | Success rates% | Refs. |
| neuroblastoma | Ort | NSG/ athymic nude mice | 24 | [44] |
| osteosarcoma | Ort | NSG/ athymic nude mice | 48 | [44] |
| rhabdomyosarcoma | Ort | NSG/ athymic nude mice | 65 | [44] |
| retinoblastoma | Ort | SCID/athymic nude mice | 70 | [44] |
| Wilms tumour | Ort | NSG/ athymic nude mice | 78 | [44] |
| desmoplastic small round-cell tumour | Ort | NSG/ athymic nude mice | 22 | [44] |
| Ewing sarcoma | Ort | NSG/ athymic nude mice | 29 | [44] |
| high-grade sarcoma | Ort | NSG/ athymic nude mice | 83 | [44] |
| Colorectal cancer | SC | athymic nude mice | 52 | [45] |
| Prostate cancer | SC | SCID/NSG/ C57BL/6 pfp/rag2 mice | 100-66 | [46] |
| SC: Subcutaneous; Ort: orthotopic; SCID: C.B17-Prkdcscid; NOD-SCID: NOD.C.B17-Prkdcscid; NSG: NOD.Cg-PrkdcscidIl2rgtm1Wjl. |
Tumor tissues or biopsy specimens from EC patients are termed P0 (Passage zero); established PDX models are termed passage 1 (P1); when P1 tumors reached 500~1500 mm3, fresh tumor fragments are harvested from mice and then subsequently re-implanted into other mice (P2, P3, and so on) [19–22]. In general, PDX models undergoing more than 3 passages (P3) are applicable for drug test experiments. If tumor growth was not detected for at least 6 months or the mass was caused by non-epithelial cell proliferation, the engraftment would be considered as a failure.
The recipients of PDXs
Immunodeficient mice suitable to receive human tumor tissues include athymic nude mice, C.B17-Prkdcscid (SCID) mice, non-obese diabetic.C.B17-Prkdcscid (NOD-SCID) mice, NOD.Cg-PrkdcscidIl2rgtm1Wjl (NSG) mice and NODShi.Cg-Prkdcscid Il2rgtm1Sug (NOG/SCID) (Table 1). A spontaneous mutation of Foxn1 gene in athymic nude mice results in the deteriorated or absent thymus [23]. They are also characterized by the defective differentiation and proliferation of thymic epithelial cells (TECs) and progenitors of T-lymphocytes [23]. However, an intact innate immune system remains and NK cell activity is high, thus engraftment is limited for most primary solid human tumors and impossible for human normal or malignant hematopoietic cells [24]. SCID mice lack both functional T and B lymphocytes because of a Prkdc gene deficiency. The concept of SCID now expands to all severely immunodeficient strains of mice, such as those with Recombination activating gene-1/2 mutation (Rag-1null/Rag-2null). The engraftment takes rates of human tumor cells (including neuroblastoma, colon cancer, and breast cancer cell line) are higher in SCID mice than nude mice [25]. However, moderate NK cell activity remaining in SCID mice restricts the growth of human hematopoietic cells and PDX tumors after implantation. NOD-SCID mice are cultivated by crossbreeding NOD mice and SCID mice. These immunocompromised mice display defective innate immunity, including the dampened activity of NK cell and macrophages, abnormal dendritic cell development and function, and a lack of complement activation [26]. Therefore, NOD-SICD mice are more suitable for the engraftment of human solid tumors and hematopoietic cells that fail in SCID mice. IL-2 receptor subunit gamma (IL-2Rγ) is indispensable for high-affinity signaling for the IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21 receptors. A lack of IL-2Rγ cripples both the adaptive and innate immune system. NSG mice combine the characters of NOD-SICD mice and IL-2Rγnull mice, and are highly receptive to engraftment of human primary tumors. Nevertheless, no significant improvement in primary EC engraftment has been found using NSG mice compared with NOD-SCID mice [27]. Similarly to NSG mice, NOG mice also lack T and B lymphocytes and NK cells and are compatible with human cells and tissues [28]. The engraftment rate of human hematopoietic cells in NOG mice are significantly elevated when compared with NOD-SCID mice [29]. However, there is no evidence indicating NOG mice are superior recipients for EC-PDX.
Most studies that establish a EC-PDX model used animals aged 6-8 weeks for engraftment of patient-derived xenografts, aging mice might not be suitable for xenografts implantation. The reasons may include: (1) The activity of T cell in athymic nude mice tends to increase with the age. Therefore, engraftment rate of tumor cells or tissues could be enhanced in younger mice (5-10 weeks) (reviewed by Szadvari et al [23]); (2) In some aging mice, such as SCID mice, spontaneous thymic and non-thymic tumors may develop and seriously affect their survival, even they are maintained in an SPF, barrier-protected environment [30]; (3) the life spans of immunodeficient mice vary across different species. The median life span of NOD-SCID mice has been reported as 37 weeks, while that of NSG mice was 89 weeks (range, 59–95 weeks) [26, 31]. (4) Inflammatory conditions are also present in aging NSG female mice and contribute to morbidity and mortality in these mice [32].
The engraftment methods
Currently, subcutaneous, orthotopic, and intramuscular implanting are three methods employed by researchers in the establishment of PDX models for EC (Table 1). Subcutaneous engraftment is a well-established technique employed by most researchers in establishing PDX models. Both resected tumor tissues or biopsy derived from human ESCC or EAC could be engrafted subcutaneously into immunodeficient mice. Orthotopic implantation of human primary EC tissues is scarcely reported. Veeranki et al [15] transabdominally implanted a biopsy sample of EAC at the distal esophagus/gastroesophageal junction to mimic tumor growth patterns in patients. The orthotopic mouse model closely mimics tumor growth patterns seen in patients and recapitulated the response to radiation treatment in patients with EAC [15]. A study showed that intramuscular engraftment might improve the success rate of esophageal PDX establishment (intramuscular vs subcutaneous, 72% vs 16%) [33]. They attributed the improvement to a more abundant blood supply in the muscles than cutaneous tissue. This novel method in tumor tissue engraftment may optimize the process of testing therapeutic drugs for EC. However, lymphomatous transformation occurred in some xenografts when using the intramuscular method [33]. Intramuscular engraftment has also been used in establishing xenograft models for canine osteosarcoma and human ovarian tissues [34, 35]. The feasibility of this engraftment approach should be further validated by more studies. The procedures in establishing PDX models of EC are summarized in Figure 1.
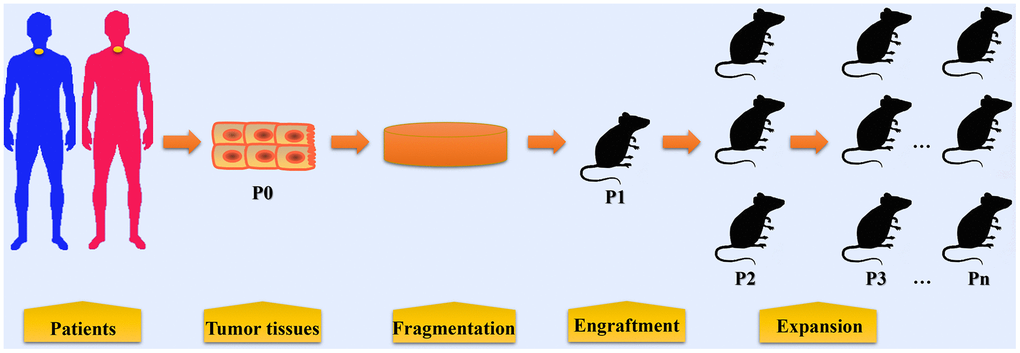
Figure 1. The procedures in establishing patient-derived xenograft models of esophageal cancer. Tumor tissues or biopsy are obtained from patients with EC during surgery or endoscopic examination. These tumor tissues and biopsy are termed P0 and are then fragmented before implantation. In some condition, cell populations are isolated from tumor tissues for PDX model establishment. Fragmented samples or primary tumor cells are then implanted into immunocompromised mice (termed P1), either subcutaneously or orthotopically. When P1 tumors reached 500~1500 mm3, fresh tumor fragments are harvested from mice and then subsequently re-implanted into other mice for expansion (P2, P3, and so on).
Characteristics of EC-PDX
Distinguishing features of EC-PDX models support them become useful tools in translational cancer research. Firstly, the morphology and histology in EC-PDX remained consistent when compared that of the corresponding primary tumor tissues [22]. Through 3-4 passages, the degree of differentiation in tumor xenografts varied slightly [22]. Importantly, drug sensitivity, including paclitaxel and cisplatin, in PDX models correlates well with the clinical response in corresponding patients [22]. Thus, EC-PDX model provided a realistic model for drug sensitivity selection for EC patients [22, 36, 37]. Secondly, EC-PDXs are able to mimic the current clinical genetic setting of EC, including mutations in PIK3CA, EGFR, K-Ras, B-Raf and HER2 amplification [19]. These models may support further investigation of the effect of driver gene mutation on treatment response. For instance, the efficacy of Trastuzumab has been developed for the treatment of HER2 positive breast cancer [38]. Likewise, Trastuzumab caused tumor regression in HER2 positive EC-PDX models [39]. However, when PIK3CA mutation was present in the models, Trastuzumab lost the ability to suppress tumor growth, which suggest PIK3CA mutation may be a mechanism of Trastuzumab resistance [39]. Clinical response to chemotherapy using 5-FU and cisplatin was also compromised in EC-PDX models with PIK3CA mutation [19]. Additionally, cancer associated fibroblasts (CAFs) constitute the majority of the tumor microenvironment (TME) [8]. CAFs may promote tumor growth through their mechanical contributions to the stroma and cytokines secretion [40]. Unlike in cell line xenograft, patient-derived CAFs are preserved well in PDX models and contribute to the therapy resistance of EAC [41]. Therefore, PDX models are superior in studying the interaction between EC and TME.
Application of PDX models in screening predictive biomarkers for chemoradiotherapy
Although multidisciplinary approaches have been developed for the treatment of locally advanced EC, only a small percentage (less than 40%) of patients respond well to these treatments [47]. Many nonresponsive patients may suffer severe adverse effects and even lose the option of surgical resection [48]. Therefore, predictive biomarkers are critical in determining whether chemoradiotherapy solutions are suitable and effective in preventing EC progression in patients. Identification of predictive biomarkers would facilitate accurate risk stratification of patients for therapy and avoid potential morbidity due to ineffective treatment. The employment of PDXs in screening biomarkers has been carried out by many researchers. For instance, CAFs derived from EAC PDXs were shown to play important roles in inducing resistance to chemoradiotherapy [41]. Interleukin-6 (IL-6) produced from/by CAFs drives EMT and enhances cell migration and survival in EAC [41]. Therefore, IL-6 expression from CAFs may provide value in prediction of patient resistance to chemotherapy and radiotherapy [41]. TP53-induced glycolysis and apoptosis regulator (TIGAR) is a downstream regulator of p53 and highly expressed in many hematologic and solid tumors, including leukemia, breast cancer, and EC [49, 50]. TIGAR remodels energy metabolism in ESCC cells and promotes cell proliferation and colony formation [51]. Compared to ESCC-PDXs with low TIGAR expression, those with TIGAR overexpression were more resistant to 5-fluorouracil/Cisplatin, whereas they were sensitized by a glutaminase inhibitor, CB-839 [51]. Therefore, TIGAR expression in EC tissues might be a predictive biomarker in guiding chemotherapeutic strategies [51]. Furthermore, NAD(P)H quinone dehydrogenase 1 (NQO1), an enzyme involved in cellular reactive oxygen species clearance [52], showed enhanced expression in ESCC cells during the treatment of a preparation of curcumin (THC) and was associated with THC resistance [53]. However, the combination of THC and NQO1 inhibitor exerted a superior effect on tumor growth than THC monotherapy in ESCC-PDX, suggesting that NQO1 expression might be a critical biomarker of THC response in ESCC patients [53].
Application of PDX models in evaluating therapeutic targets for chemotherapy
Topoisomerase I
Topoisomerase I binds to the supercoiled DNA and cleaves the phosphate backbone of the DNA to release supercoiled DNA [54]. It functions as a critical nuclear enzyme that facilitates DNA replication, transcription, recombination and repair [55–57]. High expression of topoisomerase I can be found in human ESCC tissues and is related to poor prognosis, while topoisomerase I expression is relatively low in the normal squamous epithelium [58, 59]. Gimatecan is a modified lipophilic analog of camptothecin [60], which exerts anti-tumor activity through specifically inhibiting topoisomerase I activity. Gimatecan can induce DNA damage, S-phage arrest and apoptosis in ESCC cells in cell-line-derived xenograft (CDX) models as well as in PDX models through suppressing the expression and function of topoisomerase I [61]. (S)-10-Hydroxycamptothecin (HCPT) is another topoisomerase I inhibitor isolated from Camptotheca cuminata. HCPT suppresses the enzymatic activity of topoisomerase I, impedes cell proliferation, and induces cell cycle arrest and apoptosis in ESCC cells [59]. The tumor growth of PDX models was also suppressed by HCPT, supporting its anti-tumor activity [59]. Both studies validated the antitumor efficacy of topoisomerase I inhibitors in ESCC cells and PDX models, which may pave the way for the clinical use of these drugs in the treatment of EC.
EGFR and HER2
The HER family of receptor tyrosine kinases contains epidermal growth factor receptor (EGFR/ErbB1/HER1), erb-b2 receptor tyrosine kinase 2 (ERBB2/HER2/Neu), erb-b2 receptor tyrosine kinase 3 (ERBB3/HER3), and erb-b2 receptor tyrosine kinase 4 (ERBB4/HER4) [62, 63]. The aberrant activation of these receptor tyrosine kinases facilitates the tumorigenesis and progression of multiple malignant tumors, such as EC, lung cancer, gastric cancer, and colon cancer [64]. EGFR and HER2 are overexpressed in human primary EC tissues and significantly associated with overall survival in EC [65, 66]. The effect and mechanism of inhibitors targeting EGFR and HER2 have been evaluated using EC-PDX models. Theliatinib is a potent and highly selective EGFR inhibitor currently in Phase I clinical study in China (NCT02601248). Theliatinib was effective in restraining the tumor growth of ESCC-PDX models with EGFR gene amplification [42]. However, PIK3CA mutation or FGFR1 over-expression in PDX attenuated the effect of theliatinib, suggesting care to apply theliatinib to only responsive subsets of patients is required [42]. Cetuximab is a mouse-human chimeric antibody that functions through binding with EGFR, leading to an inhibition of EGFR phosphorylation and activation [67]. Zhu et al [36] tested the response of ESCC to cetuximab via PDX models. It is notable that EGFR amplification, EGFR mRNA levels and EGFR protein expression could be significantly correlated with the ESCC-PDXs response to cetuximab treatment [36].
The anti-HER2 monoclonal antibody, trastuzumab has been shown its efficacy in prolonging overall survival of patients with HER2-positive advanced gastric or gastro-oesophageal junction cancer [68, 69]. Trastuzumab resistance was observed in PDX models of ESCC with a dose-dependent decrease in HER2 expression and a significant increase of HER3 and HER4 expression [70]. The HER3 might be a potential therapeutic targets for trastuzumab resistant cancer, as inhibition of HER3 could reverse trastuzumab resistance in ESCC and EAC cells [70]. Afatinib is a pan-HER inhibitor for clinical treatment of lung cancer and breast cancer [71, 72]. EGFR gene amplification or overexpression was a predictor for afatinib sensitivity of ESCC [73]. Afatinib inhibited the phosphorylation of EGFR, S6, and ERK and induced G1 phase arrest and apoptosis in ESCC cells and PDX models [73]. In the PDX model of esophagogastric cancer, afatinib resistance could be caused by MET amplification, which might be overcome by MET inhibitor [74]. Additionally, lapatinib, a dual tyrosine kinase inhibitor of EGFR2 and HER2, was able to contain tumor growth of PDX in combination with 5-fluorouracil [75].
Aurora-A and -B
Aurora-A and -B are two members of Aurora family kinases that are implicated in the control of mitosis [76, 77]. Aurora-A is required for centrosome maturation and separation and bipolar spindle assembly, while Aurora-B regulates cytokinesis and acts as a member of the chromosome passenger complex [77]. Both Aurora-A and -B can be found overexpressed in human EC tissues [78, 79] and act to enhance cell invasion and malignant phenotypes in ESCC [80, 81]. Treatment of APIO-EE-9, an Aurora kinase inhibitor, resulted in inhibition of cell growth and proliferation, induction of apoptosis, and reduction of Aurora-A and -B activities in ESCC cell lines [82]. In PDX models of ESCC, APIO-EE-9 effectively inhibited tumor growth with minimal toxicity [82]. The inhibition of Aurora-A and -B might be effective in reducing uncontrolled proliferation in ESCC, thus contributing to tumor suppression.
Cyclin-dependent kinase 4/6/9
Cyclin-dependent kinases (CDKs) can integrate many extracellular signaling pathways to drive cell cycle transition [83]. The employment of CDK4/6 inhibitors, such as palbociclib, ribociclib, and abemaciclib for clinical treatment of breast cancer has been approved in the United States [84, 85]. These inhibitors may interrupt the hyperactive cyclin D associated kinases in Rb positive tumor cells, resulting in cell cycle arrest [84]. The effectiveness of CDK4/6 inhibitors for EC therapy has been also investigated in preclinical and clinical trials [86–88]. The results of high-throughput sequencing in EACs showed that more than half of EACs contained biomarkers of response to CDK4/6 inhibitors [89]. SHR6390 is an orally bioavailable inhibitor of CDK4/6. Suppression of proliferation of EC cells and tumor growth of PDX model were observed following SHR6390 treatment [90]. The combination of SHR6390 with paclitaxel or cisplatin synergistically inhibited tumor growth in a PDX model [90]. The effects of another two CDK4/6 inhibitors, palbociclib (PD-0332991) and ribociclib (LEE011) on human ESCC were validated in vitro and using CDX and PDX models [37]. ESCC cells and PDX models with cyclin dependent kinase inhibitor 2A (CDKN2A) or CDKN2B loss were more sensitive to palbociclib and ribociclib treatment than cells with wild-type genes [37]. Intriguingly, through using a mouse avatar model of ESCC, the authors demonstrated that CDKN2A and CDKN2B loss were critical biomarkers for CDK4/6 inhibitor therapy [37]. Lastly, researchers examined the expression of CDK9 in human EAC tissues and Barrett's esophagus and found that CDK9 was overexpressed in EAC [91]. Pharmaceutical inhibition of CDK9 by Flavopiridol and CAN508 diminished cell proliferation and promoted apoptosis in EAC cells [91]. Furthermore, the treatment using a CDK9 inhibitor might enhance the cell-killing effect of radiation on EAC. Synergetic effect of the CDK9 inhibitor, BAY1143572 and radiation were assessed in EAC cell lines and PDX models [92]. By inhibiting CDK9 activation, BAY1143572 could sensitize EAC cells and PDX of EAC to radiation [92]. The precise mechanism by which CDK4/6/9 inhibitors suppress EC progression remains unclear and needs further investigation.
JAK/STAT3 signaling pathway
Janus kinase (JAK)/ signal transducer and activator of transcription 3 (STAT3) signaling activation frequently present in primary ESCC and is associated with poor prognosis in patients [93]. JAK/STAT3 can be recruited by EGFR and contributes to esophageal keratinocyte migration [94]. Aberrant activated STAT3 in cancer cells induces epithelial mesenchymal transition and facilitated metastasis [95]. Suppressor of cytokine signaling 1 (SOCS1) is a multifunction protein that functions as a signal inhibitor and a regulator in the process of ubiquitination [96]. SOCS1 negatively regulates JAK/STAT3 signaling transduction via interaction with JAK proteins [97]. Overexpression of SOCS1 using recombinant adenoviral vectors reduced cell proliferation and inactivated JAK/STAT3 signaling in ESCC cells as well as in ESCC PDX models [98]. By restraining the JAK/STAT3/c-MYC pathway, metformin could inhibit the transition of normal endothelial cells toward tumor endothelial cells induced by tumor conditioned medium [99]. In the human ESCC PDX model, metformin prevented tumor growth and tumor angiogenesis [99]. The phosphorylation of STAT3 in ESCC cells could be blocked by a small molecular STAT3 inhibitor, Stattic [100]. Stattic alone or in combination with 5-fluorouracil markedly suppressed tumor growth of ESCC-PDX, with less cell proliferation and increased apoptosis in xenografts [100].
The MAPK cascades
Mitogen-activated protein kinase kinase (MEK)/ extracellular signal-regulated kinase (ERK) signaling is an essential component of the mitogen-activated protein kinase (MAPK) cascades [101]. Mutations of MEK/ERK signaling are frequently seen in many human tumors, including EC [102], lung cancer [103], and breast cancer [104]. Researchers have taken efforts to develop inhibitors of MEK and ERK as cancer therapeutic agents [105, 106]. Purpurogallin, a phenol distilled from oak nutgalls, inhibits the function of MEK1 and MEK2 by binding within their ATP-binding pocket [107]. Purpurogallin inhibited the malignant phenotypes of ESCC cells and tumor growth of ESCC PDX model by targeting MEK1 and MEK2 [107]. Ethyl gallate (EG) is a natural phenolic compound obtained from herbs like Galla Rhois, Longan and Acacia nilotica Wild [108–110]. EG directly interacts with ERK1/2 and negatively regulated ERK1/2 activities in ESCC cells, leading to the inhibition of cell proliferation, interruption of cell cycle, and increase of cell apoptosis [111]. In ESCC PDX models, EG administration suppressed tumor growth via the inactivation of ERK1/2 [111]. MSK2 acts as a downstream of the ERK1/2 or p38 MAPK pathways and has a regulatory effect on CREB and histone H3 [112, 113]. MSK2 activation as well as downstream CREB-Bcl-2 pathway could be dampened by sulforaphene, leading to the induction of apoptosis and cell cycle arrest and inhibition of cell migration and invasion in EC cells [114] and using EC-PDX models, the anti-tumor effect of sulforaphene was validated [114]. Finally, MKK3/6 acts as an upstream activator of p38 MAPK. A hexahydroxylated flavonoid named gossypetin reduces cell viability and anchorage-independent growth and induces apoptosis in ESCC through binding with MKK3 and MKK6 [115]. Using an ESCC PDX model, the anti-tumor activity of gossypetin was further demonstrated in vivo [115].
Glypican-1
Glypican-1 is a cell surface proteoglycan that presents in a variety of solid tumors and modulates tumor growth, invasion and progression [116]. Glypican-1 overexpression is associated with cisplatin resistance and promotes malignant transition of ESCC via the PTEN/AKT/β-catenin signaling pathway [117, 118]. Glypican-1 expression is relatively weaker in human normal heart, kidney, small intestine, colon and esophageal tissues compared to ESCC tissues [119]. Knockdown of glypican-1 inhibits cell growth and the activation of EGFR, AKT and p44/42-MAPK signaling pathways [119]. Targeting glypican-1 using anti-glypican-1 monoclonal antibody restrained tumor growth and promoted apoptosis in ESCC PDX models [119].
Hedgehog signaling
Hedgehog signaling pathway is critical for tissue development, injury repair and tumorigenesis [120, 121]. The Hedgehog signaling cascade contains 3 ligands, Sonic (SHH), Indian, and Desert Hedgehog, which activate downstream signal transducer protein smoothened (SMO) and subsequently the GLI protein family (GLI1, GLI2, and GLI3) by binding with the transmembrane receptor Patched-1 [122]. Aberrant activation of Hedgehog signaling is linked to cancer progression and chemoresistance. GLI1 activity was elevated in EAC and correlated with EAC differentiation as well as the response to neoadjuvant chemotherapy [123]. In the established EAC PDX models, upregulation of hedgehog ligands (e.g. SHH) was found in tumor epithelium and upregulation was further enhanced by radiation treatment [20, 124]. Unlike EAC and Barrett’s Esophagus, SHH expression in ESCC is relatively rare [125]. Hence, researchers concentrated on developing inhibitors targeting SHH signaling for invasive EAC [126, 127]. Evidence has shown that the Hedgehog signaling pathway is a target for improving chemoradiation therapy in EC [128]. SHH inhibition using a monoclonal antibody 5E1 augmented the growth delay of PDX tumors following radiation [124]. Likewise, SMO inhibition with an SMO inhibitor, LDE225, also increased growth delay induced by radiation [124].
PI3K/AKT signaling pathway
The phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/ serine/threonine kinase 1 (AKT) signaling pathway plays a critical role in modulating cellular processes such as cell proliferation, survival, protein synthesis and glucose homeostasis [129, 130]. The PI3K/AKT signaling pathway can be activated by different receptor tyrosine kinases (RTKs) including the EGFR family, insulin-like growth factor 1 (IGF-1) receptor, and fibroblastic growth factor [131]. The strategies that target the PI3K/AKT pathway help to inhibit cadherin switching, diminish cell proliferation and migration, alleviate inflammation, restore chemosensitivity, and increase radiosensitivity in EC cells. For instance, the combination of a clinical PI3Kα-selective inhibitor CYH33 and radiation promoted DNA damage, cell cycle arrest and apoptosis in ESCC cells [132]. In the PDX model, CYH33 and radiation inhibited tumor growth, lowered Akt phosphorylation and M2-like macrophage infiltration [132]. Oridonin, Xanthohumol, and Scutellarin are natural compounds isolated from herbs. Their activities in targeting AKT activation in ESCC cells and ESCC-PDX have been reported [133–135]. These AKT inhibitors were effective in suppressing cell growth and inducing cell cycle arrest in ESCC cells as well as decreasing PDX tumor growth in vivo. Importantly, the effects of these inhibitors were dependent on AKT protein level in ESCC cells [134, 135].
VEGFR2
The vascular endothelial growth factor (VEGF)/vascular endothelial growth factor receptor 2 (VEGFR2) system plays an important role in tumor angiogenesis. Patients with solid tumors have demonstrated benefit from drugs targeting VEGF and/or VEGFR2 [136, 137]. Ramucirumab is an anti-VEGFR2 monoclonal antibody that may prevent VEGFR2 dimerization and thus suppress downstream signaling transduction [138]. VEGFR2 expression was found to be significantly elevated in EC tissues and correlated with poor efficacy of cytotoxic treatment [139]. Ramucirumab has been approved by FDA for treating gastric and GEJ adenocarcinomas either as a single agent or in combination with paclitaxel [140, 141]. Apatinib, a broad inhibitor of VEGFR2, RET, c-Kit and c-Src, induced cell apoptosis and cell cycle arrest, inhibited malignant transformation and sensitized EC to cisplatin [139]. The efficacy of apatinib monotherapy as second- or further-line treatment for advanced EC has been validated in a Phase II study [142]. Apatinib also exhibited its potential efficacy in patients with metastatic ESCC when combined with docetaxel [143]. Moreover, inhibition of VEGFR2 using DC101, a murine VEGFR2 inhibitor, delayed tumor growth and prolonged survival of animals with EAC xenografts [144]. However, vascular regression induced by DC101 impaired the uptake of intraperitoneally administered nab-paclitaxel [144]. This study suggested the limits of the combination of anti-angiogenesis and cytotoxic agents in EAC therapy [144].
HSP90
A significant correlation between heat shock protein 90 (HSP90) expression and Her2 status has been found in EAC [145]. Serum HSP90a level was a significant predictor for definitive chemoradiotherapy in patients with ESCC [146]. The reduction ratio of HSSP90a could be an independent prognostic factor for ESCC patients [146]. The detailed role of HSP90 as a therapeutic target in EC has been reviewed in a previous study [147]. Drugs targeting HSP90 alone or combined with other chemotherapeutic drugs (i.e. cisplatin) and radiation play inhibitory roles in EC cell survival. For instance, the inhibitor of HSP90 Ganetespib (STA-9090) could inhibit cell proliferation and induce apoptosis in ESCC cells and PDX models [148]. Interestingly, the effect of HSP90 inhibition seemed to be dependent on MYC expression. ESCC cells and xenografted primary tumors overexpressing MYC were more sensitive to STA-9090 [148].
Notch signaling pathway
Dysregulation of notch signaling due to NOTCH1, NOTCH2 or NOTCH3 gene mutation has been shown in ESCC [149, 150]. Nuclear accumulation of notch intracellular domain (NICD) is closely linked to tumor grade and stage in human ESCC [151]. Higher expression of NICD is detected in human EAC tissues compared with the normal esophageal mucosa and the normal gastric cardia [152] and NICD expression correlates with the stage of EAC. Notch signaling regulates EAC cell proliferation and transformation of normal esophageal epithelial cells [152, 153]. DAPT treatment suppressed tumor growth and promoted apoptosis in EAC CDX models and PDX models [152]. Inhibition of Notch signaling also decreased the expression of cancer stem-cell markers in EAC cells [152].
Other targets and utilities
Microtubules are composed of alpha- and beta-tubulin heterodimers, the basic structures that are essential for cell shape and behavior. Microtubules are highly dynamic structures that change during the cell cycle. Clinically, tubulin binding agents (TBA) can suppress microtubule dynamics and induce cell cycle arrest, thus contributing to tumor growth inhibition [154, 155]. PPMP (2-[4-(3,4-dimethoxyphenyl)-3-methyl-1H-pyrazol-5-yl]-5-[(2-methylprop- 2-en-1-yl)oxy]phenol), a novel TBA, reduced cell viability, caused cell cycle arrest and apoptosis in ESCC cell lines [156]. PPMP might occupy the colchicine binding site of tubulin and inhibit tubulin polymerization in ESCC cells [156]. In vivo, PPMP effectively suppressed tumor growth in animals bearing ESCC PDX [156].
With the extensive application of next-generation sequencing to cancer transcriptomes, the role of long non-coding RNAs (LncRNAs) in tumor progression has increasingly drawn people’s attention [157]. LncRNAs act as tumor suppressors or oncogenes by modulating tumor-suppressive or oncogenic pathways [158]. LncRNA AGPG is highly enhanced in human ESCC tissues and cell lines. AGPG interacts with PFKFB3, contributing to metabolism remodeling in ESCC cells. Administration of an AGPG inhibitor to ESCC PDX models markedly reduced tumor growth [159].
Additionally, PDXs are also of great value in establishing chemoresistant cell lines and identity new therapeutic targets. Liu et al [18] established cisplatin-resistant ESCC cell lines through repeatedly treating ESCC-PDX models with cisplatin. With these cisplatin-resistant ESCC cells, they were able to pick out microRNA-455-3p as a potential therapeutic target to overcome drug resistance in EC patients [18].
Conclusions
PDX models of EC are increasingly utilized for studying tumor biology, investigating genetic heterogeneity, and screening predictive biomarkers and therapeutic targets (Table 3). Indeed, investigators have tested various drugs or radiation therapy on mice bearing PDX and screened predictive biomarkers/ therapeutic targets that may guide for EC therapy in patients (Figure 2).
Table 3. Agents and their targets tested in PDX models of esophageal cancer.
| Agent | Target | Histology | Administration method | Mouse strain | Reference |
| Gimatecan | Topoisomerase I | ESCC | Oral gavage | NOD-SCID mice | [61] |
| HCPT | Topoisomerase I | ESCC | Paraneoplastic injection | SCID mice | [59] |
| Cetuximab | EGFR | ESCC | Intraperitoneal injection | Athymic nude mice | [36] |
| Theliatinib | EGFR | ESCC | Oral gavage | NOD-SCID mice | [42] |
| Trastuzumab | HER2 | EAC | Intraperitoneal injection | NSG mice | [70] |
| Trastuzumab/ pertuzumab | HER2/HER3 | EAC | Intraperitoneal injection | NSG mice | [160] |
Afatinib
dasatinib | EGFR /Src family kinase | ESCC | Oral gavage | NOD-SCID mice | [73] |
| Afatinib/AMG 337 | HER2/MET | EG | - | - | [74] |
| Lapatinib | EGFR/HER2 | ESCC | Oral gavage | Athymic nude mice | [75] |
| APIO-EE-9 | Aurora A and B | ESCC | - | SCID mice | [82] |
| SHR6390 | CDK4/6 | ESCC | Oral gavage | NOD-SCID mice | [90] |
| Palbociclib | CDK4/6 | ESCC | Oral gavage | BALB/c nude mice | [37] |
| BAY1143572 | CDK9 | EAC | Intraperitoneal injection | Athymic nude mice | [92] |
| AdSOCS1 | SOCS1 | ESCC | Intratumoral injection | NOD-SCID | [98] |
| Metformin | JAK/STAT3 | ESCC | - | SCID mice | [99] |
| Stattic | STAT3 | ESCC | Intraperitoneal injection | SCID mice | [100] |
| Purpurogallin | MEK1/2 | ESCC | Oral gavage | SCID mice | [107] |
| Ethyl gallate | ERK1/2 | ESCC | Oral gavage | SCID mcie | [111] |
| Sulforaphene | MSK2 | ESCC | Intraperitoneal injection | SCID mice | [114] |
| Gossypetin | MKK3/6 | ESCC | Oral gavage | SCID mice | [115] |
| Anti-Glypican-1 mAb | Glypican-1 | ESCC | Intraperitoneal injection | NOG/SCID mice | [119] |
5E1
LDE225 | SHH
SMO | EAC | Intraperitoneal injection
Oral gavage | NOD-SCID/NSG mice | [124] |
| CYH33 | PI3Kα | ESCC | Oral gavage | BALB/c nude mice | [132] |
| Oridonin | Akt | ESCC | Oral gavage | SCID mice | [133] |
| Xanthohumol | Akt | ESCC | Oral gavage | SCID mice | [134] |
| Scutellarin | Akt1/2 | ESCC | Oral gavage | SCID mice | [135] |
| DC101 | VEGFR2 | EAC | Intraperitoneal injection | Athymic nude mice | [144] |
| Ganetespib | HSP90 | ESCC | Intraperitoneal injection | NSG mice | [148] |
| DAPT | Notch signaling | EAC | Intraperitoneal injection | NSG | [152] |
| PPMP | Tubulin | ESCC | Intraperitoneal injection | SCID mice | [156] |
| Antisense oligonucleotides | LncRNA AGPG | ESCC | Intratumoral injection | Athymic nude mice | [159] |
| HCPT: (S)-10-Hydroxycamptothecin; EGFR: epidermal growth factor receptor; HER2: erb-b2 receptor tyrosine kinase 2; HER3: erb-b2 receptor tyrosine kinase 3; MET: MET proto-oncogene, receptor tyrosine kinase; CDK4/6/9:cyclin dependent kinase 4/6/9; SOCS1:suppressor of cytokine signaling 1; JAK: Janus kinase; STAT3:signal transducer and activator of transcription 3; MEK1/2:mitogen-activated protein kinase kinase 1/2; ERK1/2: extracellular signal-regulated kinase 1/2; MSK2:ribosomal protein S6 kinase A4; MKK3/6:mitogen-activated protein kinase kinase 3/6; SHH: sonic hedgehog; SMO: smoothened, frizzled class receptor; PI3Kα:phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha; Akt: serine/threonine kinase 1; VEGFR2: vascular endothelial growth factor receptor 2; HSP90: heat shock protein 90; EAC: esophageal adenocarcinoma; ESCC: esophageal squamous cell carcinoma; EG: esophagogastric cancer; SCID: C.B17-Prkdcscid; NOD-SCID: NOD.C.B17-Prkdcscid; NOG/SCID: NODShi.Cg-Prkdcscid Il2rgtm1Sug; NSG: NOD.Cg-PrkdcscidIl2rgtm1Wjl. |
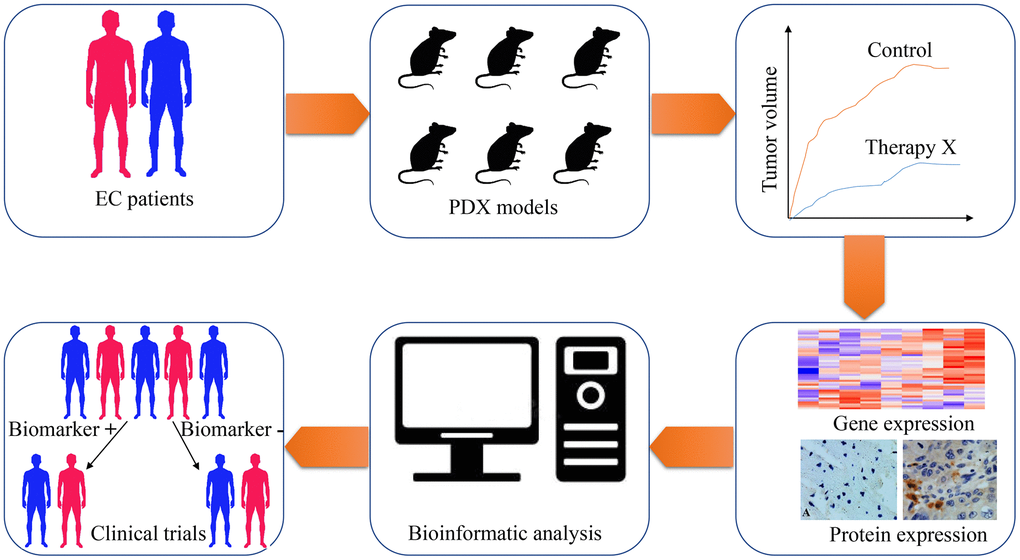
Figure 2. The application of patient-derived xenograft (PDX) in screening predictive biomarkers and therapeutic targets for esophageal cancer therapy. Esophageal cancer tissues are obtained from patients and implanted into immunodeficient mice for PDX models establishment. With the PDX models, the treatment response of chemotherapeutic drugs, radiotherapeutic methods or targeted drugs are tested on these tumor xenografts. Subsequently, genome-wide sequencing techniques and expressional analysis are carried out to screen genes with differential expression, which are related to various therapeutic methods. Through bioinformatic analysis, potential biomarkers are selected from differentially expressed genes. Finally, clinical trials are designed and performed to validate the feasibility of these biomarkers.
However, there are still several problems that need to be solved in the establishment and usage of EC-PDX:
The engraftment rates of EC-PDX remain relatively low with the current methods, and only a minority of tumor tissues derived from patients can be successfully engrafted. As a result, there is a high cost in establishing successful PDX models. To solve this issue, novel immunodeficient animals are needed, such as gene-modified rats and hamsters [161–163];
Although subcutaneous engraftment is commonly employed by most researchers [164], subcutaneous models less accurately reflect tumor progression compared with orthotopic methods and hinder the investigation of tumor metastasis, angiogenesis and tumor microenvironment in EC. The difficulties in establishing and examining orthotopic PDX models have become the roadblocks for the popularization of this tool;
A replacement of human stromal cells by mouse stroma occurs in the initial stage of PDX establishment [20], which blocks the study of the interaction between EC cells and stromal cells due to the loss of human stromal cells in PDX;
A lack of a functional immune system also prevents the analysis of immunotherapeutic approaches to EC therapy.
Although the drawbacks exist in the current EC-PDX models, the development of novel immunodeficient animals may help accelerate their usage in a preclinical study. For instance, tumor cells in immunodeficient Syrian hamster can communicate with host fibroblasts, which may provide growth factors to keep human cancer and stromal cells survive longer [163]. Moreover, humanized animal models with reconstituted human immune cells will be more meaningful, which allow the investigation of the interaction between cancer cells and various human immune cells.
TL, JM, and YW: manuscript concept and design. TL: manuscript writing. XX, JM, LCD, and YW: manuscript revising.
The authors declare that they have no conflicts of interest.
This project is supported by the National Key R&D Program of China (NO. 2016YFE0200800), Natural Science Foundation of Henan Province (No. 202300410259), Postdoctoral Research Startup Project in Henan Province (No.202001043), the Nature Sciences Foundation of China (NO. 81771776 and U1704282) and The MRC (NO. MR/M015696/1).