Pretreatment neutrophil-to-lymphocyte ratio predicts the benefit of gastric cancer patients with systemic therapy
Abstract
Pretreatment neutrophil-to-lymphocyte ratio (NLR) has been reported to be associated with the prognosis of inoperable gastric cancer patients with systemic therapy. However, no consensus on the association has been reached. In this study, we mainly evaluated whether pretreatment NLR predicted the benefit of inoperable gastric cancer patients with systemic therapy, including chemotherapy, targeted therapy and immunotherapy. PubMed, Embase and Cochrane Library databases were systematically searched from inception up to September 16th, 2020. A total of 36 studies including 8614 patients were involved in the meta-analysis. Pooled data revealed that high pretreatment NLR was significantly associated with poor outcomes of OS (HR = 1.78, 95% CI = [1.59, 1.99]) and PFS (HR = 1.63, 95% CI = [1.39, 1.91]) in gastric cancer. Subgroup analyses stratified by country, study type, case load, analysis of HR, cutoff of pretreatment NLR, or treatment types arrived at the same conclusion. Pooled data based on different effect models and sensitivity analyses did not change the conclusion. Overall, high pretreatment NLR predicts the poor prognosis of inoperable gastric cancer patients with systemic therapy. Measurement of pretreatment NLR will assist clinicians with patient counseling and clinical treatment guiding accordingly.
Introduction
Gastric cancer is one of the most common malignant tumors, ranking the third highest mortality worldwide [1]. Although its morbidity is declining in most countries, the increase of incidence in the under-50 population could reverse the overall decline in gastric cancer [2, 3]. Moreover, though advances in diagnosis and surgical treatment have reduced mortality for early-stage gastric cancer [4], many patients were often initially diagnosed at advanced stages [5], which highlights the importance of effective systemic therapy.
Systemic therapy consists of chemotherapy, targeted therapy, and immunotherapy [6]. Chemotherapy is a relatively traditional therapy mainly based on fluoropyrimidine and platinum agents. It is the first-line treatment for metastatic gastric patients with human epidermal growth factor receptor-2 (HER-2)-negative expression in accordance with the latest international guidelines [7–9]. While, targeted therapy with trastuzumab is recommended to treat gastric cancer patients with HER2 overexpression [10, 11]. Usually, those patients are treated with trastuzumab as well as the first-line chemotherapy [7, 8, 12]. Immunotherapy has emerged as a powerful treatment for chemo-refractory gastric cancer [2]. Chemotherapy combined with immunotherapy might achieve better therapeutic efficacy compared with chemotherapy alone [2]. Overall, systemic therapy has revolutionized the treatment and improved the prognosis of patients with inoperable gastric cancer. Therefore, identifying novel biomarkers is of great significance to predict the outcome of inoperable gastric cancer patients with systemic therapy.
Neutrophil-to-lymphocyte ratio (NLR) is well-known as a systemic inflammation biomarker, which could be accessed from blood routine easily. Previous meta-analyses showed that NLR was a prognostic biomarker of gastric cancer, especially after gastrectomy [13–15]. Moreover, increasing studies demonstrated the correlation between pretreatment NLR and the gastric cancer prognosis after systemic therapy [16–51]. However, there is a lack of meta-analysis to comprehensively evaluate the association between pretreatment NLR and the outcomes of systemic therapy for inoperable gastric cancer.
Therefore, from the above and with the introduction of systemic therapy for advanced inoperable gastric cancer patients, it is timely to systematically review the association between pretreatment NLR and therapeutic efficacy of gastric cancer patients with systemic therapy, including chemotherapy, targeted therapy and immunotherapy.
Results
Literature search and studies characteristics
A flow chart of study selection was presented in Figure 1. The initial searching retrieved 947 relevant studies. After the removal of duplicated studies, 682 studies remained, of which 532 studies were ruled out after a scanning of the titles and abstracts. Full-test article evaluation for eligibility were implemented in 150 studies, among which 124 studies were removed owing to 97 studies with no relevant outcomes, six with unavailable outcomes, 14 without clarifying treatment types, three being review or meta-analyses, and four duplicates. Eventually, a total of 36 studies were of eligibility and enrolled into the meta-analysis [16–51].
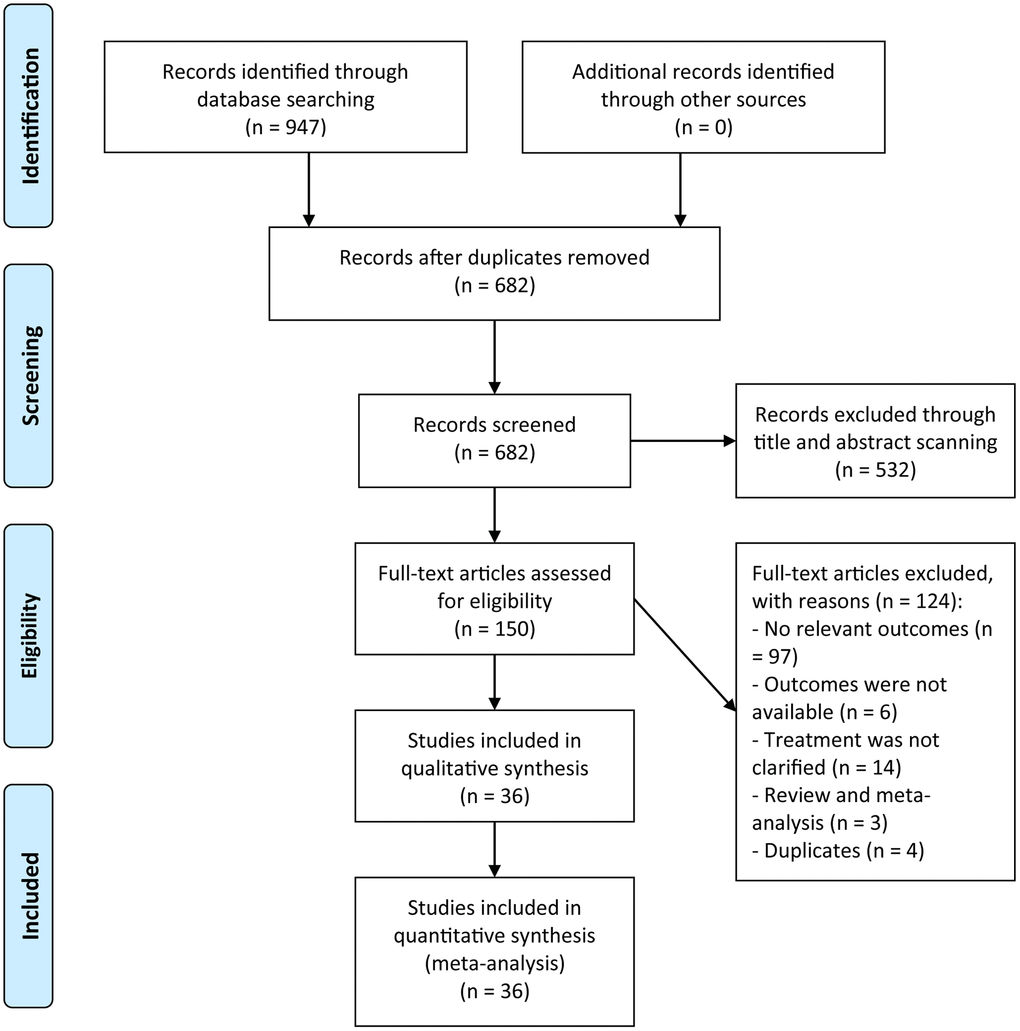
Figure 1. A flowchart of the study selection.
Table 1 summarized the characteristics of eligible studies published between 2007 and 2020, and from four different regions including Japan, China, Korea, or Europe. Among them, there were five multi-center studies and 30 single-center studies. The cutoffs of NLR were not consistent in these studies, ten of which used three as the cutoff of high versus low pretreatment NLR. In terms of systemic treatment, 20 studies assessed the prognostic significance of pretreatment NLR in chemotherapy, 13 studies in chemo/targeted therapy, and 3 studies in immunotherapy. All studies reported on overall survival (OS) and 17 studies reported on progression-free survival (PFS). The Newcastle-Ottawa Scale (NOS) was adopted to evaluate the methodological quality of eligible studies for observational studies [52]. All studies were identified as high quality with stars above six on the basis of quality assessment. The response of each individual study to NOS was exhibited in Table 2.
Table 1. Characteristics of eligible studies.
| First author | Year | Country | Study type | Cases | Age (years) | Sex (male %) | Cutoff | Treatment | Variables | NOS scores | References |
| Yamanaka | 2007 | Japan | Multi-center | 1220 | – | 869 (71.2%) | 2.5 | Chemotherapy | OS* | 8 | [16] |
| Jeong | 2012 | Korea | Single-center | 104 | 52.8 ± 10.7 | 69 (66.3%) | 3 | Chemotherapy | OS*, PFS | 8 | [17] |
| Lee | 2013 | Korea | Single-center | 174 | – | 114 (65.5%) | 3 | Chemotherapy | OS*, PFS* | 8 | [18] |
| Cho | 2014 | Korea | Single-center | 268 | 55.4 ± 12.5 | 175 (65.3 %) | 3.06 | Chemotherapy | OS*, PFS* | 9 | [19] |
| Dogan | 2015 | Turkey | Single-center | 109 | 53.9 ± 9.1 | 80 (73.4%) | 2.5 | Chemotherapy | OS, PFS | 7 | [20] |
| Liu | 2015 | China | Single-center | 135 | 61.1 ± 12.1 | 79 (58.5%) | 4 | Chemotherapy | OS | 7 | [21] |
| Wang | 2015 | China | Single-center | 120 | 66.9 ± 9.75 | 75 (62.5%) | 4.62 | Chemotherapy | OS, PFS | 7 | [22] |
| Zhang | 2015 | China | Multi-center | 99 | – | 76 (76.8%) | 4.558 | Chemotherapy | OS | 7 | [23] |
| Hsieh | 2016 | China | Single-center | 256 | 59.7 ± 10.5 | 176 (68.8%) | 3 | Chemotherapy | OS* | 9 | [24] |
| Musri | 2016 | Turkey | Single-center | 143 | 59.0 ± 12.0 | 103 (72.0%) | 3.34 | Chemotherapy | OS, PFS | 7 | [25] |
| Wang | 2016 | China | Single-center | 310 | 57.7 ± 9.6 | 213 (68.7%) | median | Chemotherapy | OS* | 9 | [26] |
| Giampieri | 2017 | Italy | Single-center | 103 | – | 71 (68.9%) | 0.4 | Chemotherapy | OS, PFS | 7 | [27] |
| Gonda | 2017 | Japan | Single-center | 100 | 65.2 ± 9.0 | 56 (56.0%) | 3 | Chemotherapy | OS* | 9 | [28] |
| Manikhas | 2017 | Russia | Single-center | 32 | 60.5 | – | 3 | Chemotherapy | OS* | 8 | [29] |
| Marshall | 2017 | Japan | Single-center | 143 | – | – | 3.11 | Chemo/targeted therapy | OS* | 7 | [30] |
| Ock | 2017 | Korea | Single-center | 745 | 59.8 ± 11.0 | 534 (71.7 %) | 2.42 | Chemo/targeted therapy | OS* | 9 | [31] |
| Huang | 2018 | China | Single-center | 136 | 55.1 ± 10.9 | 82 (60.3%) | 3.04 | Chemotherapy | PFS* | 9 | [32] |
| Hwang | 2018 | Korea | Single-center | 73 | 61.7 ± 14.0 | 61 (83.6%) | 3 | Chemo/targeted therapy | OS*, PFS* | 9 | [33] |
| Kim | 2018 | Korea | Single-center | 502 | 57.7 ± 10.1 | 300 (59.8%) | 3 | Chemotherapy | OS*, PFS* | 9 | [34] |
| Kondoh | 2018 | Japan | Single-center | 50 | 65.2 ± 9.4 | 29 (58.0%) | 3.5 | Chemo/targeted therapy | OS* | 9 | [35] |
| Migita | 2018 | Japan | Single-center | 177 | 67.6 ± 11.3 | 124 (70.1%) | 2.2 | Chemo/targeted therapy | OS* | 9 | [36] |
| Ogata | 2018 | Japan | Multi-center | 26 | 64.3 ± 10.6 | 19 (73.1%) | 5 | Immunotherapy | OS, PFS | 7 | [37] |
| Ryu | 2018 | Korea | Multi-center | 236 | 58.8 ± 10.0 | 185 (78.4%) | 2.08 | Chemotherapy | OS*, PFS* | 9 | [38] |
| Bozkurt | 2019 | Turkey | Single-center | 194 | 58.7 ± 9.4 | 129 (66.5%) | 2.6 | Chemo/targeted therapy | OS*, PFS | 8 | [39] |
| Mitani | 2019 | Japan | Multi-center | 112 | 61.4 ± 10.6 | 84 (75.0%) | 3 | Chemo/targeted therapy | OS | 7 | [40] |
| Murakami | 2019 | Japan | Single-center | 92 | – | 73 (79.3%) | 2.83 | Chemo/targeted therapy | OS* | 8 | [41] |
| Namikawa | 2019 | Japan | Single-center | 262 | 68.1 ± 12.4 | 171 (65.3%) | 3.9 | Chemo/targeted therapy | OS* | 9 | [42] |
| Sugimoto | 2018 | Japan | Single-center | 141 | 71.9 ± 10.6 | 98 (69.5%) | 4 | Chemo/targeted therapy | OS* | 9 | [43] |
| Cipriano | 2020 | Portugal | Single-center | 55 | 62.0 ± 9.2 | 43 (78.2%) | 5 | Chemo/targeted therapy | OS* | 9 | [44] |
| Kim | 2020 | Korea | Single-center | 1156 | 57.3 ± 12.3 | 738 (63.8%) | 3 | Chemo/targeted therapy | OS* | 9 | [45] |
| Namikawa | 2020 | Japan | Single-center | 21 | 70.2 ± 9.1 | 19 (65.5%) | 2.5 | Immunotherapy | OS, PFS | 7 | [46] |
| Ota | 2020 | Japan | Single-center | 98 | 65.1 ± 10.2 | 68 (69.4%) | 3 | Immunotherapy | OS*, PFS | 8 | [47] |
| Shigeto | 2020 | Japan | Single-center | 109 | 69.1 ± 5.9 | 85 (78.0%) | 3.15 | Chemo/targeted therapy | OS | 7 | [48] |
| Wang | 2020 | China | Single-center | 466 | 59.8 ± 11.3 | 327 (70.2%) | 2.8 | Chemotherapy | OS*, PFS | 8 | [49] |
| Zhao | 2020 | China | Single-center | 110 | – | 84 (76.4%) | 2.48 | Chemotherapy | OS* | 8 | [50] |
| Zhou | 2020 | China | Single-center | 537 | 55.0 ± 9.5 | 321 (59.8%) | 2.610 | Chemotherapy | OS*, PFS* | 9 | [51] |
| *Variables are calculated by multivariable analysis. Abbreviations: OS, overall survival; PFS, progression-free survival; NOS, Newcastle-Ottawa Scale. |
Table 2. Methodological quality of studies included in the meta-analysis based on Newcastle-Ottawa Scale.
| First author | Year | Selection | Comparison | Exposure/Outcome | Total score | References |
| Yamanaka | 2007 | **** | * | *** | 8 | [16] |
| Jeong | 2012 | **** | * | *** | 8 | [17] |
| Lee | 2013 | **** | * | *** | 8 | [18] |
| Cho | 2014 | **** | ** | *** | 9 | [19] |
| Dogan | 2015 | **** | – | *** | 7 | [20] |
| Liu | 2015 | **** | – | *** | 7 | [21] |
| Wang | 2015 | **** | – | *** | 7 | [22] |
| Zhang | 2015 | **** | – | *** | 7 | [23] |
| Hsieh | 2016 | **** | ** | *** | 9 | [24] |
| Musri | 2016 | **** | – | *** | 7 | [25] |
| Wang | 2016 | **** | ** | *** | 9 | [26] |
| Giampieri | 2017 | **** | – | *** | 7 | [27] |
| Gonda | 2017 | **** | ** | *** | 9 | [28] |
| Manikhas | 2017 | **** | * | *** | 8 | [29] |
| Marshall | 2017 | **** | – | *** | 7 | [30] |
| Ock | 2017 | **** | ** | *** | 9 | [31] |
| Huang | 2018 | **** | ** | *** | 9 | [32] |
| Hwang | 2018 | **** | ** | *** | 9 | [33] |
| Kim | 2018 | **** | ** | *** | 9 | [34] |
| Kondoh | 2018 | **** | ** | *** | 9 | [35] |
| Migita | 2018 | **** | ** | *** | 9 | [36] |
| Ogata | 2018 | **** | – | *** | 7 | [37] |
| Ryu | 2018 | **** | ** | *** | 9 | [38] |
| Bozkurt | 2019 | **** | * | *** | 8 | [39] |
| Mitani | 2019 | **** | – | *** | 7 | [40] |
| Murakami | 2019 | **** | * | *** | 8 | [41] |
| Namikawa | 2019 | **** | ** | *** | 9 | [42] |
| Sugimoto | 2018 | **** | ** | *** | 9 | [43] |
| Cipriano | 2020 | **** | ** | *** | 9 | [44] |
| Kim | 2020 | **** | ** | *** | 9 | [45] |
| Namikawa | 2020 | **** | – | *** | 7 | [46] |
| Ota | 2020 | **** | * | *** | 8 | [47] |
| Shigeto | 2020 | **** | – | *** | 7 | [48] |
| Wang | 2020 | **** | * | *** | 8 | [49] |
| Zhao | 2020 | **** | * | *** | 8 | [50] |
| Zhou | 2020 | **** | ** | *** | 9 | [51] |
Correlation between pretreatment NLR and OS
Thirty-six studies comprising of 8614 gastric cancer patients reported the association between pretreatment NLR and OS. With great heterogeneity (I2 = 80.3%, P < 0.001), we utilized random effect model to analyze the pooled hazard ratio (HRs) and results showed that higher pretreatment NLR was correlated with a poorer OS (HR = 1.78, 95% confidential interval (CI) = [1.59, 1.99]) (Figure 2). Analysis with fixed effect model showed a consistent conclusion (Figure 2). The conclusion also remained unchanged with sensitivity analysis (Supplementary Figure 1A).
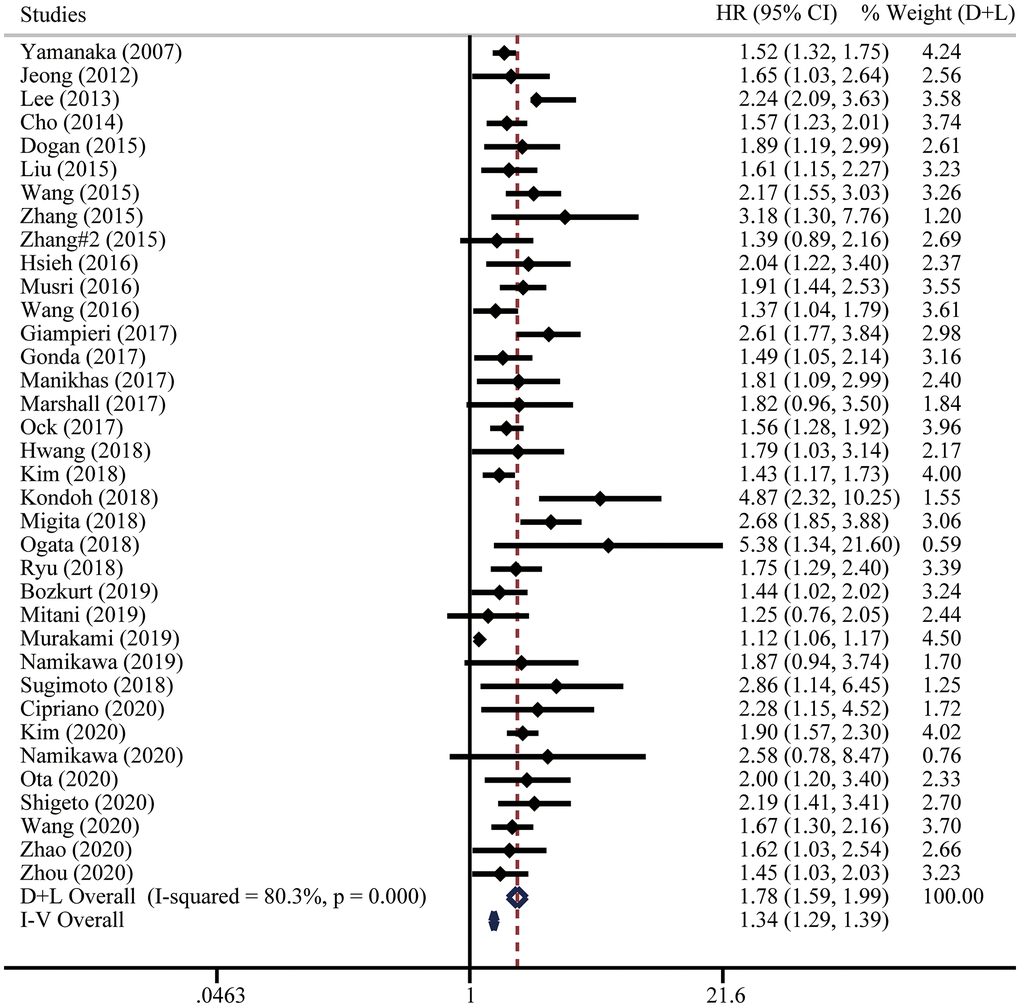
Figure 2. Forest plot for the hazard ratios (HRs) of overall survival (OS) in gastric cancer patients with systemic therapy between low and high pretreatment NLR. “D+L” means DerSimonian and Laird method. “I-V” means generic inverse variance method.
Considering the existence of heterogeneity, univariate meta-regression analysis was performed and indicated that case load and cutoff of pretreatment NLR could be the possible significant moderators (Table 3). Then we adopted subgroup analyses following these clinical parameters. Notably, higher pretreatment NLR was correlated with poorer OS from multi-center studies (HR = 1.60, 95% CI = [1.32, 1.94]) and multivariate analysis (HR = 1.72, 95% CI = [1.51, 1.95]). Moreover, subgroup analysis on the basis of the NLR cutoff values demonstrated that the prognostic value of pretreatment NLR consisted in all the NLR groups (<3 HR = 1.67, 95% CI = [1.39, 2.01]; = 3 HR = 1.73, 95% CI = [1.53, 1.95]; >3 HR = 1.96, 95% CI = [1.67, 2.29]). Subgroup analysis by treatment types suggested the same conclusions in chemotherapy (HR = 1.68, 95% CI = [1.55, 1.82]), chemo/targeted therapy (HR = 1.85, 95% CI = [1.47, 2.34]), and immunotherapy (HR = 2.30, 95% CI = [1.47, 3.61]). The results of subgroup analyses were summarized in Figure 3, highlighting that elevated pretreatment NLR was correlated with poor OS.
Table 3. Univariate meta regression of hazard ratios (HRs) of overall survival (OS) in inoperable gastric cancer patients with systemic therapy.
| Variables | β | 95% LCI | 95% UCI | P |
| Country | | | | |
| Japan | 1.00 | 0.82 | 1.23 | 0.991 |
| China | 0.94 | 0.76 | 1.17 | 0.589 |
| Korea | 0.98 | 0.80 | 1.20 | 0.817 |
| Europe | 1.13 | 0.88 | 1.44 | 0.341 |
| Study type | 0.92 | 0.71 | 1.20 | 0.545 |
| Cases | | | | |
| <100 | 0.99 | 0.78 | 1.26 | 0.952 |
| 100–200 | 1.17 | 0.99 | 1.39 | 0.072 |
| >200 | 0.87 | 0.72 | 1.05 | 0.141 |
| Analysis of HR | 0.86 | 0.70 | 1.06 | 0.153 |
| Cut-off | | | | |
| <3 | 0.90 | 0.75 | 1.08 | 0.235 |
| =3 | 0.99 | 0.81 | 1.22 | 0.98 |
| >3 | 1.20 | 0.98 | 1.47 | 0.08 |
| Treatment | | | | |
| Chemotherapy | 0.96 | 0.79 | 1.16 | 0.665 |
| Chemo/targeted therapy | 1.00 | 0.82 | 1.22 | 0.994 |
| Immunotherapy | 1.39 | 0.80 | 2.40 | 0.234 |
| Abbreviations: LCI, Lower confidence interval; UCI, Upper confidence interval. |
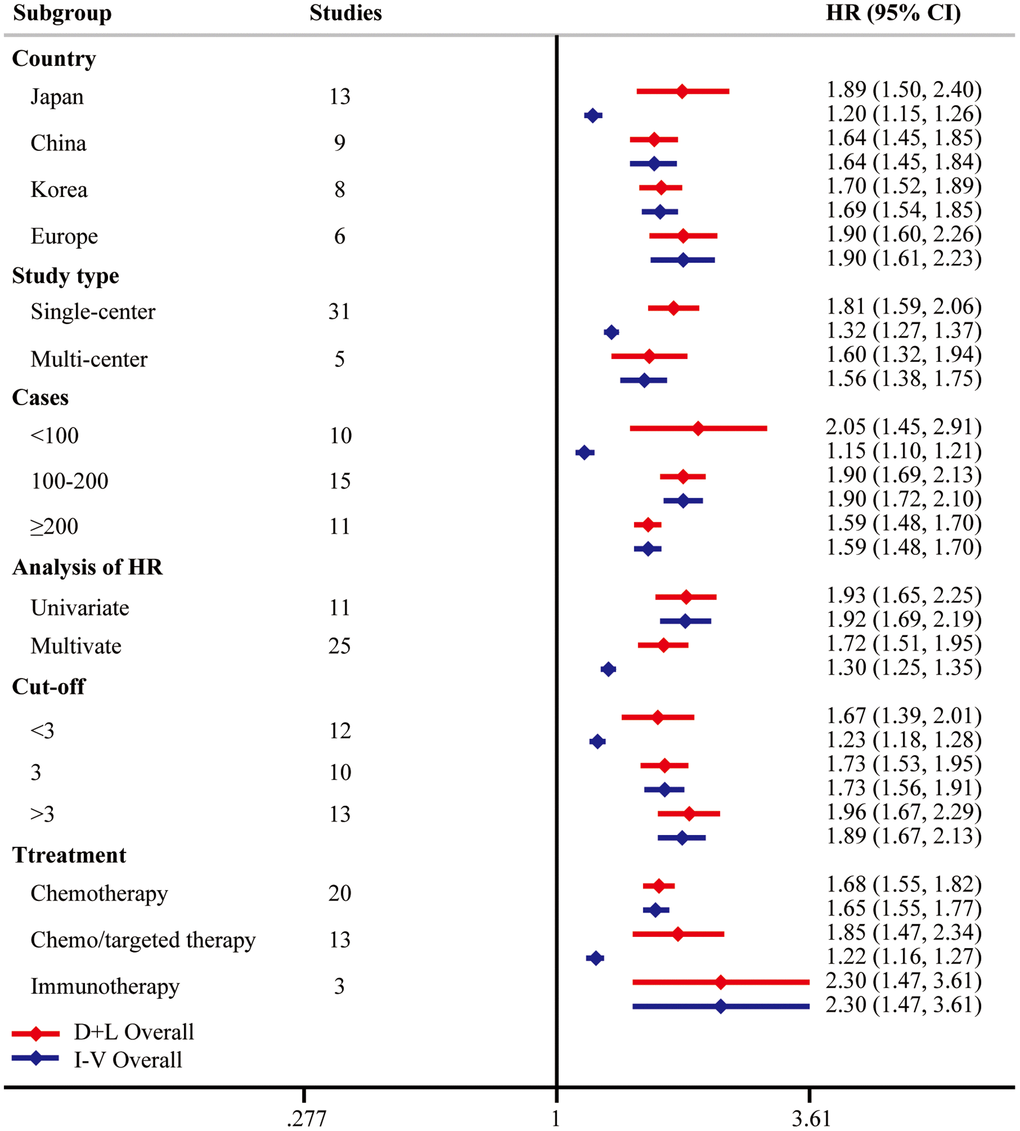
Figure 3. Subgroup analysis of OS. “D+L” means DerSimonian and Laird method. “I-V” means generic inverse variance method.
For OS subset, the asymmetry of funnel plot indicated that there existed publication bias (Supplementary Figure 1B). Egger’s test was used for further validation (Supplementary Figure 1C). The Duval and Tweedie trim-and-fill method was then conducted and twelve studies were filled, without changing the conclusion in both fixed effect model (HR = 1.32, 95% CI = [1.27, 1.36]) and random effect model (HR = 1.58, 95% CI = [1.43, 1.74]).
Correlation between pretreatment NLR and PFS
Seventeen studies including 3318 patients were included to analyze the relationship between pretreatment NLR and PFS. Due to significant heterogeneity, we applied a random effect model and the results suggested that higher pretreatment NLR was related to inferior PFS (HR = 1.63, 95% CI = [1.39, 1.91]) (Figure 4). The fixed effect model (Figure 4) and sensitivity analysis (Supplementary Figure 2A) did not change the conclusion.
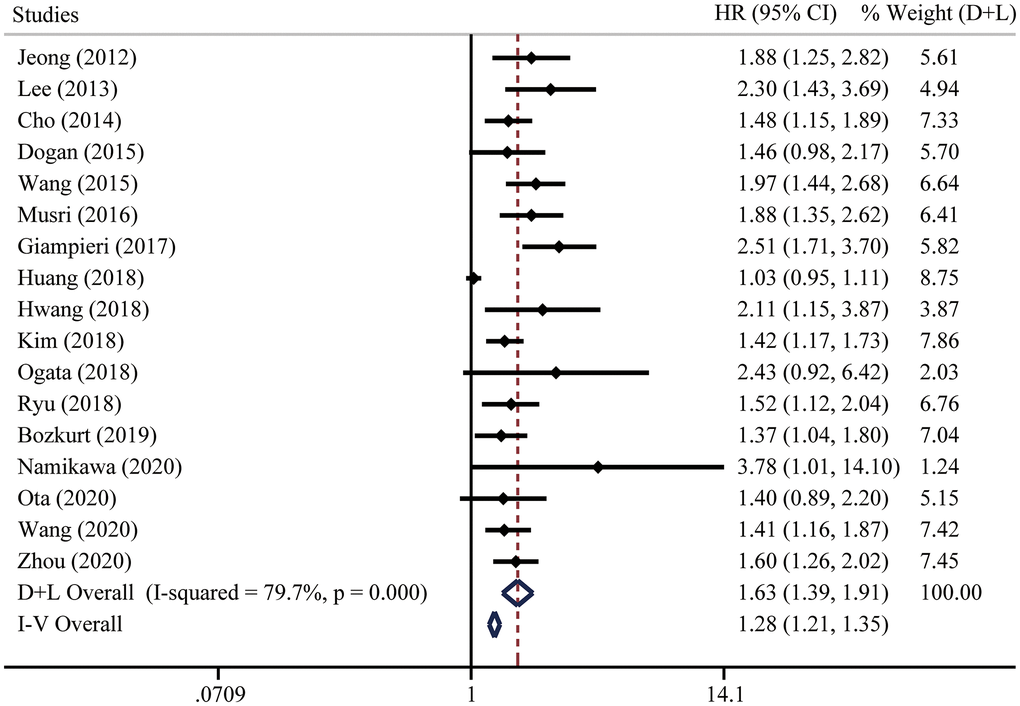
Figure 4. Forest plot for the HRs of progression-free survival (PFS) in gastric cancer patients with systemic therapy between low and high pretreatment NLR. “D+L” means DerSimonian and Laird method. “I-V” means generic inverse variance method.
To investigate the origin of heterogeneity, univariate meta-regression was performed. We did not find the possible significant moderator (Table 4). To validate the robustness of the results, subgroup analyses were performed based on country, study type, case load, analysis of HR, cutoff of pretreatment NLR, or treatment types. The conclusions were consistent in all the subgroup analyses (Figure 5). Noteworthily, stratified analysis demonstrated that higher pretreatment NLR was correlated with poorer OS from multi-center studies (HR = 1.58, 95% CI = [1.19, 2.11]) and multivariate analysis (HR = 1.50, 95% CI = [1.20, 1.88]). When three was determined as the cutoff of NLR, significant differences were found in all these subgroups (<3 HR = 1.58, 95% CI = [1.36, 1.85]; =3 HR = 1.66, 95% CI = [1.36, 2.02]; >3 HR = 1.57, 95% CI = [1.11, 2.23]). Subgroup analysis based on treatment types suggested that the prognostic significance of pretreatment NLR existed in all kinds of systemic therapy, including chemotherapy (HR = 1.61, 95% CI = [1.35, 1.94]), chemo/targeted therapy (HR = 1.56, 95% CI = [1.06, 2.30]), and immunotherapy (HR = 1.83, 95% CI = [1.08, 3.10]).
Table 4. Univariate meta regression of hazard ratios (HRs) of progression-free survival (PFS) in inoperable gastric cancer patients with systemic therapy.
| Variables | β | 95% LCI | 95% UCI | P |
| Country | | | | |
| Japan | 1.13 | 0.65 | 1.96 | 0.647 |
| China | 0.83 | 0.62 | 1.10 | 0.176 |
| Korea | 1.06 | 0.79 | 1.43 | 0.668 |
| Europe | 1.11 | 0.80 | 1.54 | 0.497 |
| Study type | 1.10 | 0.73 | 1.67 | 0.618 |
| Cases | | | | |
| <100 | 1.20 | 0.76 | 1.91 | 0.411 |
| 100-200 | 1.05 | 0.79 | 1.40 | 0.719 |
| >200 | 0.88 | 0.66 | 1.19 | 0.381 |
| Analysis of HR | 0.85 | 0.65 | 1.11 | 0.204 |
| Cut-off | | | | |
| <3 | 1.01 | 0.76 | 1.35 | 0.919 |
| =3 | 1.09 | 0.79 | 1.51 | 0.561 |
| >3 | 0.91 | 0.67 | 1.22 | 0.498 |
| Treatment | | | | |
| Chemotherapy | 0.96 | 0.66 | 1.39 | 0.819 |
| Chemo/targeted therapy | 0.98 | 0.61 | 1.56 | 0.919 |
| Immunotherapy | 1.13 | 0.65 | 1.96 | 0.647 |
| Abbreviations: LCI: Lower confidence interval; UCI: Upper confidence interval. |
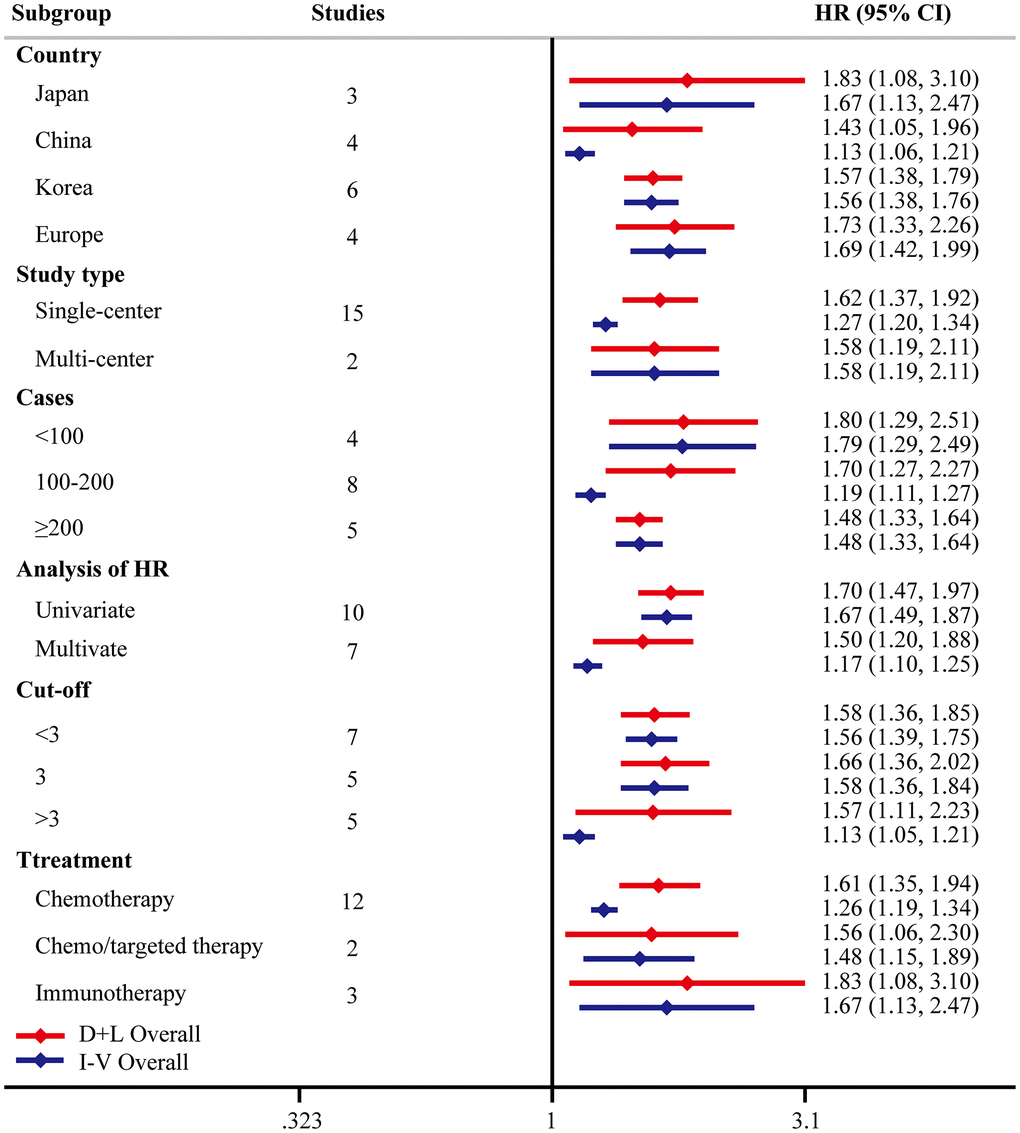
Figure 5. Subgroup analysis of PFS. “D+L” means DerSimonian and Laird method. “I-V” means generic inverse variance method.
For PFS subset, the funnel plot was not symmetrical and five studies were over the pseudo 95% CI (pseudo 95% CI was defined as 95% CI assuming these included studies did not have heterogeneity [53]) (Supplementary Figure 2B). We then performed the Egger’s test, which detected the existence of publication bias (P < 0.001) (Supplementary Figure 2C). Twelve studies were filled after the Duval and Tweedie trim-and-fill method. The conclusion remained consistent in both fixed effect model (HR = 1.27, 95% CI = [1.20, 1.34]) and random effect model (HR = 1.55, 95% CI = [1.34, 1.80]).
Discussion
Gastric cancer has become a threat worldwide with over one million estimated new cases and about 784000 deaths globally annually [1]. Even worse, many cases were diagnosed at advanced stages and lost the chance of gastrectomy. Systemic therapy has been recommended to treat those patients with inoperable gastric cancer, but the prognostic biomarkers have not been well clarified.
Increasing studies demonstrated the association of systemic inflammation and the prognosis of gastric cancer with systemic therapy [54–57]. As the representative of systemic inflammation, NLR is easily calculated from regular blood tests. There were accumulating studies on the topic of the prognostic effect of pretreatment NLR on the gastric cancer patients with systemic therapy [16–51]. Therefore, an extensive analysis on the topic is essential to clarify the association.
In this study, a total of 36 studies including 8, 614 patients were finally enrolled through searching all the relevant articles. We found that higher pretreatment NLR was associated with an inferior OS and PFS. Considering the huge heterogeneity in these comparisons, univariate meta-regression analyses were conducted to investigate the origin of heterogeneity. Case load and cutoff of pretreatment NLR could be the possible significant moderators for OS. Moreover, six studies used the Engauge Digitizer software to estimate the univariate HRs, which were grouped into subgroup with univariate analysis. However, the conclusions were not changed by fixed effect model, sensitivity analyses and subgroup analyses, which highlights the prognostic value of pretreatment NLR in inoperable gastric cancer patients with systemic therapy.
Currently, quite a few meta-analyses with regard to the prognostic effect of NLR on gastric cancer were published. In 2015, Chen et al. performed a meta-analysis based on nine studies including 3709 gastric cancer patients, and suggested that higher pretreatment NLR was associated with poorer OS and PFS in gastric cancer patients undergoing resection and palliative chemotherapy [15]. However, this study did not describe the prognostic effect of pretreatment NLR on targeted therapy or immunotherapy. Moreover, Sun and his colleagues included 19 studies in the meta-analysis and validated Chen et al.’s conclusion [14]. Furthermore, Kim et al. comprehensively assessed the association between the OS of gastric cancer patients and NLR. They included 24 studies to analyze the pooled HRs of OS but did not report the prognostic effect of NLR on the prognosis of inoperable gastric cancer patients with systemic therapy [13]. Overall, these studies mainly focused on the gastric cancer patients with gastrectomy, while we concentrated on inoperable gastric cancer patients with systemic therapy. We highlighted the prognostic effect of pretreatment NLR not only on the gastric cancer patients with chemotherapy, but also on gastric cancer patients with chemo/targeted therapy and immunotherapy.
The mechanisms underlying the relationship between pretreatment NLR and the prognosis of inoperable gastric cancer patients with systemic therapy were poorly known, but many studies provided the potential mechanisms [13–15, 58]. In summary, most neutrophils promote the progression of tumors through inhibiting immune activity, while lymphocytes are regarded as the primary effector cells in the immunotherapy. NLR is calculated by circulating neutrophil to lymphocyte counts, which reflects a balance between the detrimental roles of neutrophilia and the beneficial roles of lymphocyte-mediated immunity [59]. Even so, more studies are still needed to investigate the underlying mechanism in the association. There are other predictive biomarkers with prognostic value in gastric cancer patients with systemic therapy. For example, a recent study showed that an immune checkpoint score system could be used for the evaluation of prognosis and the selection for adjuvant chemotherapy in gastric cancer [60]. Moreover, a deep learning computed tomography (CT) signature was developed to predict the prognosis and benefit from adjuvant chemotherapy in gastric cancer [61]. Many single biomarkers such as MTA1 [62], TFF3 [63], and CA72-4 [64] were also reported to be related to the prognosis of gastric cancer with systemic therapy. As a simple and feasible biomarker, NLR is easily obtained from the regular blood tests, which highlights its practicability in clinical practice.
Admittedly, some limitations existed within our meta-analysis. First, two eligible studies were meeting abstracts providing limited data and this could be improved by updating with the latest data. Second, considerable heterogeneity existed in the meta-analysis, though sensitivity analyses and subgroup analyses did not change the conclusion. Third, publication bias existed in both OS and PFS, though the Duval and Tweedie trim-and-fill method indicated the same trend of the results. Finally, NLR is a non-specific biomarker and could be affected by the concurrent disease, such as infections and drug therapy. Most of the studies did not include these descriptions.
In conclusion, as a simple, inexpensive and readily available biomarker, NLR could be used to predict the benefit of inoperable gastric cancer patients with systemic therapy. Measurement of this biomarker before treatment will assist clinicians with patient counseling and clinical treatment guiding accordingly.
Materials and Methods
Search strategy
We implemented the meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [65, 66]. The databases of the PubMed, Embase and Cochrane libraries were retrieved from inception to September 16th, 2020. The search terms were indicated as below: “Stomach neoplasms” OR “gastric and (cancer or carcinoma? or adenocarcinoma? or neoplasm? or neoplasia)” OR “stomach adj3 (cancer or carcinoma? or adenocarcinoma? or neoplasm? or neoplasia)” AND (“Neutrophil-Lymphocyte ratio" OR "Neutrophil Lymphocyte ratio" OR "Neutrophil-to-Lymphocyte ratio" OR "Neutrophil to Lymphocyte ratio" OR "Neutrophil/Lymphocyte ratio" OR NLR). There was no limitation of language and study type. References lists of eligible articles and main reviews were explored manually to guarantee a thorough literature search. We have registered our systematic review in PROSPRO website (https://www.crd.york.ac.uk/PROSPERO/). The identifier of systematic review registration was PROSPERO CRD42021224114.
Selection criteria
NLR was defined as absolute neutrophil counts divided by absolute lymphocyte counts. Studies eligible for inclusion should satisfy the following inclusion criteria: (1) the patients with metastatic or inoperable gastric cancer; (2) receiving systemic therapy, including chemotherapy, targeted therapy and immunotherapy; (3) accessible HR and their corresponding 95% CI for OS and PFS between high and low pretreatment NLR group; (4) nonrandomized studies with or without the use of randomized samples. Exclusion criteria were as follows: (1) studies receiving gastrectomy or not specifying therapy types; (2) studies including patients with other types of tumors without performing of subgroup analysis about gastric cancer; (3) duplicated studies with small sample size in the same institutes or hospitals; (4) studies with insufficient usable data; (5) review, case reports or meta-analyses.
Data extraction and quality assessment
Two authors (FZ, ZF) autonomously selected eligible studies, and discordance was resolved by a third author (GD). The following information were collected from eligible studies: first authors, published year, country, type of study, case load, age, gender, cutoff of pretreatment NLR, treatment types, HR and their corresponding 95% CI for OS and PFS. HRs were extracted from multivariable analyses preferentially where available; otherwise, HRs were retrieved from univariate analyses. If studies did not report specified HRs, Engauge Digitizer software was adopted to digitize and estimate HRs from Kaplan-Meier curves between high and low NLR groups [67, 68]. Six studies in the meta-analysis used the Engauge Digitizer software to estimate the univariate HRs [20–23, 25, 48]. Newcastle-Ottawa Scale (NOS) was used for quality evaluation in three aspects: selection, comparability and outcome [52]. Studies with stars above six were regarded as high-quality.
Statistical analysis
STATA software (Version 12.0; STATA Corporation) was applied for all the statistical analyses. HRs with their corresponding 95% CI were pooled to evaluate the survival values. Statistical heterogeneity was assessed with I2 and P-value. Considering the existence of heterogeneity in the comparisons, random effect model was preferentially performed for all the analyses. To ensure the robustness of the results, fixed effect model was also performed in all the analyses. Univariate meta-regression analysis was conducted to investigate the origin of heterogeneity. Moreover, sensitivity analysis was executed by omitting one study each time as previously described [69]. Subgroup analyses were used to test the consistency of the results based on country, study type, case load, analysis of HR, cutoff of pretreatment NLR, or treatment types. Funnel plots and Egger’s tests were performed to assess publication bias. Duval and Tweedie trim-and-fill method was used for the adjustment of the publication bias. P value less than 0.05 was regarded statistically significant.
Abbreviations
NLR: neutrophil-to-lymphocyte ratio;
OS: overall survival;
PFS: progression-free survival;
HR: hazard ratio;
CI: confidence interval;
NOS: Newcastle-Ottawa Scale.
Author Contributions
Songtao Du: Data curation; Resources; Roles/Writing – original draft. Zhenhao Fang: Data curation; Resources; Writing - review & editing; Lin Ye: Writing – review & editing; Huiyan Sun: Writing – review & editing; Guangtong Deng: Conceptualization; Funding acquisition; Supervision; Roles/Writing – original draft. Wei Wu: Supervision; Funding acquisition. Furong Zeng: Conceptualization; Data curation; Formal analysis; Funding acquisition; Methodology; Software; Writing – review & editing.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This research was supported by the fellowship of China postdoctoral Science Foundation (No. 2020M682594), National Natural Science Foundation of China (No. 81873581) and The Youth Science Foundation of Xiangya Hospital (2020Q10).
References
-
1.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424. https://doi.org/10.3322/caac.21492 [PubMed]
-
2.
Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020; 396:635–48. https://doi.org/10.1016/S0140-6736(20)31288-5 [PubMed]
-
3.
GBD 2017 Stomach Cancer Collaborators. The global, regional, and national burden of stomach cancer in 195 countries, 1990-2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol Hepatol. 2020; 5:42–54. https://doi.org/10.1016/S2468-1253(19)30328-0 [PubMed]
-
4.
Katai H, Ishikawa T, Akazawa K, Isobe Y, Miyashiro I, Oda I, Tsujitani S, Ono H, Tanabe S, Fukagawa T, Nunobe S, Kakeji Y, Nashimoto A, and Registration Committee of the Japanese Gastric Cancer Association. Five-year survival analysis of surgically resected gastric cancer cases in Japan: a retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese Gastric Cancer Association (2001–2007). Gastric Cancer. 2018; 21:144–54. https://doi.org/10.1007/s10120-017-0716-7 [PubMed]
-
5.
Zhu AL, Sonnenberg A. Is gastric cancer again rising? J Clin Gastroenterol. 2012; 46:804–06. https://doi.org/10.1097/MCG.0b013e3182604254 [PubMed]
-
6.
Wang Z, Zhan P, Lv Y, Shen K, Wei Y, Liu H, Song Y. Prognostic role of pretreatment neutrophil-to-lymphocyte ratio in non-small cell lung cancer patients treated with systemic therapy: a meta-analysis. Transl Lung Cancer Res. 2019; 8:214–26. https://doi.org/10.21037/tlcr.2019.06.10 [PubMed]
-
7.
Muro K, Van Cutsem E, Narita Y, Pentheroudakis G, Baba E, Li J, Ryu MH, Zamaniah WIW, Yong WP, Yeh KH, Kato K, Lu Z, Cho BC, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with metastatic gastric cancer: a JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann Oncol. 2019; 30:19–33. https://doi.org/10.1093/annonc/mdy502 [PubMed]
-
8.
Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D, and ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016 (Suppl 5); 27:v38–49. https://doi.org/10.1093/annonc/mdw350 [PubMed]
-
9.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) version 2.2021. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1428.
-
10.
Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, Pignon JP, Rougier P, Sakamoto J, Sargent D, Sasako M, Van Cutsem E, Buyse M, and GASTRIC (Global Advanced/Adjuvant Stomach Tumor Research International Collaboration) Group. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010; 303:1729–37. https://doi.org/10.1001/jama.2010.534 [PubMed]
-
11.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, et al, and ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010; 376:687–97. https://doi.org/10.1016/S0140-6736(10)61121-X [PubMed]
-
12.
Ajani JA, D'Amico TA, Almhanna K, Bentrem DJ, Chao J, Das P, Denlinger CS, Fanta P, Farjah F, Fuchs CS, Gerdes H, Gibson M, Glasgow RE, et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016; 14:1286–312. https://doi.org/10.6004/jnccn.2016.0137 [PubMed]
-
13.
Kim MR, Kim AS, Choi HI, Jung JH, Park JY, Ko HJ. Inflammatory markers for predicting overall survival in gastric cancer patients: A systematic review and meta-analysis. PLoS One. 2020; 15:e0236445. https://doi.org/10.1371/journal.pone.0236445 [PubMed]
-
14.
Sun J, Chen X, Gao P, Song Y, Huang X, Yang Y, Zhao J, Ma B, Gao X, Wang Z. Can the Neutrophil to Lymphocyte Ratio Be Used to Determine Gastric Cancer Treatment Outcomes? A Systematic Review and Meta-Analysis. Dis Markers. 2016; 2016:7862469. https://doi.org/10.1155/2016/7862469 [PubMed]
-
15.
Chen J, Hong D, Zhai Y, Shen P. Meta-analysis of associations between neutrophil-to-lymphocyte ratio and prognosis of gastric cancer. World J Surg Oncol. 2015; 13:122. https://doi.org/10.1186/s12957-015-0530-9 [PubMed]
-
16.
Yamanaka T, Matsumoto S, Teramukai S, Ishiwata R, Nagai Y, Fukushima M. The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancer. Oncology. 2007; 73:215–20. https://doi.org/10.1159/000127412 [PubMed]
-
17.
Jeong JH, Lim SM, Yun JY, Rhee GW, Lim JY, Cho JY, Kim YR. Comparison of two inflammation-based prognostic scores in patients with unresectable advanced gastric cancer. Oncology. 2012; 83:292–99. https://doi.org/10.1159/000342376 [PubMed]
-
18.
Lee S, Oh SY, Kim SH, Lee JH, Kim MC, Kim KH, Kim HJ. Prognostic significance of neutrophil lymphocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with FOLFOX chemotherapy. BMC Cancer. 2013; 13:350. https://doi.org/10.1186/1471-2407-13-350 [PubMed]
-
19.
Cho IR, Park JC, Park CH, Jo JH, Lee HJ, Kim S, Shim CN, Lee H, Shin SK, Lee SK, Lee YC. Pre-treatment neutrophil to lymphocyte ratio as a prognostic marker to predict chemotherapeutic response and survival outcomes in metastatic advanced gastric cancer. Gastric Cancer. 2014; 17:703–10. https://doi.org/10.1007/s10120-013-0330-2 [PubMed]
-
20.
Dogan M, Eren T, Ozdemir N, Cigirgan CL, Zengin N. The relationship between platelet-lymphocyte ratio, neutrophil-lymphocyte ratio, and survival in metastatic gastric cancer on firstline modified docetaxel and cisplatinum plus 5 Fluorourasil Regimen: A single institute experience. Saudi J Gastroenterol. 2015; 21:320–24. https://doi.org/10.4103/1319-3767.166207 [PubMed]
-
21.
Liu H, Song M, Fang F, Gao X, Zhang Z, Wang S. Prediction of chemotherapeutic efficacy using the ratio of neutrophils to lymphocytes in patients with unresectable or recurrent gastric cancer. Oncol Lett. 2015; 10:2244–48. https://doi.org/10.3892/ol.2015.3575 [PubMed]
-
22.
Wang F, Liu ZY, Xia YY, Zhou C, Shen XM, Li XL, Han SG, Zheng Y, Mao ZQ, Gong FR, Tao M, Lian L, Li W. Changes in neutrophil/lymphocyte and platelet/ lymphocyte ratios after chemotherapy correlate with chemotherapy response and prediction of prognosis in patients with unresectable gastric cancer. Oncol Lett. 2015; 10:3411–18. https://doi.org/10.3892/ol.2015.3783 [PubMed]
-
23.
Zhang ZY, Xu HY, Chen B, Yang YJ, Zhang L, Wang M, Xu YC, Zhang FC. Influence of deep hyperthermia combined with systemic chemotherapy on overall survival of recurrent gastric cancer patients: Predictors of response to treatment. World Chin J Digestology. 2015; 23:438–44. https://doi.org/10.11569/wcjd.v23.i3.438
-
24.
Hsieh MC, Wang SH, Chuah SK, Lin YH, Lan J, Rau KM. A Prognostic Model Using Inflammation- and Nutrition-Based Scores in Patients With Metastatic Gastric Adenocarcinoma Treated With Chemotherapy. Medicine (Baltimore). 2016; 95:e3504. https://doi.org/10.1097/MD.0000000000003504 [PubMed]
-
25.
Musri FY, Mutlu H, Eryilmaz MK, Salim DK, Gunduz S, Coskun HS. The Neutrophil to Lymphocyte Ratio is an Independent Prognostic Factor in Patients with Metastatic Gastric Cancer. Asian Pac J Cancer Prev. 2016; 17:1309–12. https://doi.org/10.7314/apjcp.2016.17.3.1309 [PubMed]
-
26.
Wang J, Qu J, Li Z, Che X, Zhang J, Liu J, Teng Y, Jin B, Zhao M, Liu Y, Qu X. A Prognostic Model in Metastatic or Recurrent Gastric Cancer Patients with Good Performance Status Who Received First-Line Chemotherapy. Transl Oncol. 2016; 9:256–61. https://doi.org/10.1016/j.tranon.2016.04.004 [PubMed]
-
27.
Giampieri R, Maccaroni E, Mandolesi A, Del Prete M, Andrikou K, Faloppi L, Bittoni A, Bianconi M, Scarpelli M, Bracci R, Scartozzi M, Cascinu S. Mismatch repair deficiency may affect clinical outcome through immune response activation in metastatic gastric cancer patients receiving first-line chemotherapy. Gastric Cancer. 2017; 20:156–63. https://doi.org/10.1007/s10120-016-0594-4 [PubMed]
-
28.
Gonda K, Shibata M, Sato Y, Washio M, Takeshita H, Shigeta H, Ogura M, Oka S, Sakuramoto S. Elevated neutrophil-to-lymphocyte ratio is associated with nutritional impairment, immune suppression, resistance to S-1 plus cisplatin, and poor prognosis in patients with stage IV gastric cancer. Mol Clin Oncol. 2017; 7:1073–78. https://doi.org/10.3892/mco.2017.1438 [PubMed]
-
29.
Manikhas GM, Beliak NP, Kutukova SI, Zhukova NV, Popova NV, Erdniev S. The prognostic value of systemic inflammatory factors in patient with metastatic gastric cancer. J Clin Oncol. 2017; 35:e15505. https://doi.org/10.1200/JCO.2017.35.15_suppl.e15505
-
30.
Marshall S, Wakatsuki T, Matsushima T, Osumi H, Ogura M, Ichimura T, Takahari D, Shinozaki E, Chin K, Yamaguchi K. Prognostic factors of trastuzumab-based chemotherapy in patients with advanced HER2 positive gastric cancer. J Clin Oncol. 2017; 35:41. https://doi.org/10.1200/JCO.2017.35.4_suppl.41
-
31.
Ock CY, Nam AR, Lee J, Bang JH, Lee KH, Han SW, Kim TY, Im SA, Kim TY, Bang YJ, Oh DY. Prognostic implication of antitumor immunity measured by the neutrophil-lymphocyte ratio and serum cytokines and angiogenic factors in gastric cancer. Gastric Cancer. 2017; 20:254–62. https://doi.org/10.1007/s10120-016-0613-5 [PubMed]
-
32.
Huang Z, Liu Y, Yang C, Li X, Pan C, Rao J, Li N, Liao W, Lin L. Combined neutrophil/platelet/lymphocyte/ differentiation score predicts chemosensitivity in advanced gastric cancer. BMC Cancer. 2018; 18:515. https://doi.org/10.1186/s12885-018-4414-6 [PubMed]
-
33.
Hwang GY, Baek DW, Cho HJ, Lee SJ, Chae YS, Kang BW, Lee IH, Kim JG, Seo AN, Bae HI, Park KB, Park JY, Kwon OK, et al. Elevated Neutrophil-to-Lymphocyte Ratio Predicts Survival in Patients with Advanced Gastric Cancer Treated with Trastuzumab Combination Chemotherapy. Anticancer Res. 2018; 38:3151–56. https://doi.org/10.21873/anticanres.12578 [PubMed]
-
34.
Kim H, Ro SM, Yang JH, Jeong JW, Lee JE, Roh SY, Kim IH. The neutrophil-to-lymphocyte ratio prechemotherapy and postchemotherapy as a prognostic marker in metastatic gastric cancer. Korean J Intern Med. 2018; 33:990–99. https://doi.org/10.3904/kjim.2016.293 [PubMed]
-
35.
Kondoh C, Kadowaki S, Komori A, Narita Y, Taniguchi H, Ura T, Ando M, Muro K. Salvage chemotherapy with the combination of oxaliplatin, leucovorin, and 5-fluorouracil in advanced gastric cancer refractory or intolerant to fluoropyrimidines, platinum, taxanes, and irinotecan. Gastric Cancer. 2018; 21:1050–57. https://doi.org/10.1007/s10120-018-0825-y [PubMed]
-
36.
Migita K, Matsumoto S, Wakatsuki K, Ito M, Kunishige T, Nakade H, Kitano M, Nakatani M, Sho M. The prognostic significance of inflammation-based markers in patients with recurrent gastric cancer. Surg Today. 2018; 48:282–91. https://doi.org/10.1007/s00595-017-1582-y [PubMed]
-
37.
Ogata T, Satake H, Ogata M, Hatachi Y, Inoue K, Hamada M, Yasui H. Neutrophil-to-lymphocyte ratio as a predictive or prognostic factor for gastric cancer treated with nivolumab: a multicenter retrospective study. Oncotarget. 2018; 9:34520–27. https://doi.org/10.18632/oncotarget.26145 [PubMed]
-
38.
Ryu MH, Kim JH, Oh SC, Park S, Kim JG, Kim JW, Cho SH, Yoon KE, Kang YK. Neutrophil-lymphocyte ratio (NLR) as an important prognostic factor for paclitaxel as a second line chemotherapy in advanced gastric cancer (AGC): Results from phase III DREAM study. Ann Oncol. 2018; 29:viii232. https://doi.org/10.1093/annonc/mdy282.073
-
39.
Bozkurt O, Firat ST, Dogan E, Cosar R, Inanc M, Ozkan M. The prognostic value of the change in neutrophil-to-lymphocyte ratio during first-line palliative chemotherapy in patients with metastatic gastric cancer: A retrospective study. J BUON. 2019; 24:1992–99. [PubMed]
-
40.
Mitani S, Kadowaki S, Hasegawa H, Wakatsuki T, Hara H, Tajika M, Nishikawa K, Hirao M, Takahari D, Chin K, Muro K. Systemic chemotherapy for gastric cancer with early recurrence after adjuvant S-1 monotherapy: a multicenter retrospective study. Int J Clin Oncol. 2019; 24:1197–203. https://doi.org/10.1007/s10147-019-01477-z [PubMed]
-
41.
Murakami Y, Saito H, Shimizu S, Kono Y, Shishido Y, Miyatani K, Matsunaga T, Fukumoto Y, Fujiwara Y. Neutrophil-to-Lymphocyte Ratio as a Prognostic Indicator in Patients With Unresectable Gastric Cancer. Anticancer Res. 2019; 39:2583–89. https://doi.org/10.21873/anticanres.13381 [PubMed]
-
42.
Namikawa T, Ishida N, Tsuda S, Fujisawa K, Munekage E, Iwabu J, Munekage M, Uemura S, Tsujii S, Tamura T, Yatabe T, Maeda H, Kitagawa H, et al. Prognostic significance of serum alkaline phosphatase and lactate dehydrogenase levels in patients with unresectable advanced gastric cancer. Gastric Cancer. 2019; 22:684–91. https://doi.org/10.1007/s10120-018-0897-8 [PubMed]
-
43.
Sugimoto A, Nishida T, Osugi N, Takahashi K, Mukai K, Nakamatsu D, Matsubara T, Hayashi S, Yamamoto M, Nakajima S, Fukui K, Inada M. Prediction of survival benefit when deciding between chemotherapy and best supportive therapy in elderly patients with advanced gastric cancer: A retrospective cohort study. Mol Clin Oncol. 2019; 10:83–91. https://doi.org/10.3892/mco.2018.1772 [PubMed]
-
44.
Cipriano E, Estevinho F, Magalhães H. P-172 Prognostic factors in metastatic gastric cancer patients. Ann Oncol. 2020; 31:S146. https://doi.org/10.1016/j.annonc.2020.04.254
-
45.
Kim J, Hong JY, Kim ST, Park SH, Jekal SY, Choi JS, Chang DK, Kang WK, Seo SW, Lee J. Clinical scoring system for the prediction of survival of patients with advanced gastric cancer. ESMO Open. 2020; 5:e000670. https://doi.org/10.1136/esmoopen-2020-000670 [PubMed]
-
46.
Namikawa T, Maeda M, Yokota K, Tanioka N, Fukudome I, Iwabu J, Munekage M, Uemura S, Maeda H, Kitagawa H, Kobayashi M, Hanazaki K. Assessment of Systemic Inflammatory Response and Nutritional Markers in Patients With Trastuzumab-treated Unresectable Advanced Gastric Cancer. In Vivo. 2020; 34:2851–57. https://doi.org/10.21873/invivo.12112 [PubMed]
-
47.
Ota Y, Takahari D, Suzuki T, Osumi H, Nakayama I, Oki A, Wakatsuki T, Ichimura T, Ogura M, Shinozaki E, Suenaga M, Chin K, Yamaguchi K. Changes in the neutrophil-to-lymphocyte ratio during nivolumab monotherapy are associated with gastric cancer survival. Cancer Chemother Pharmacol. 2020; 85:265–72. https://doi.org/10.1007/s00280-019-04023-w [PubMed]
-
48.
Shigeto K, Kawaguchi T, Koya S, Hirota K, Tanaka T, Nagasu S, Fukahori M, Ushijima T, Matsuse H, Miwa K, Nagafuji K, Torimura T. Profiles Combining Muscle Atrophy and Neutrophil-to-Lymphocyte Ratio Are Associated with Prognosis of Patients with Stage IV Gastric Cancer. Nutrients. 2020; 12:1884. https://doi.org/10.3390/nu12061884 [PubMed]
-
49.
Wang H, Ding Y, Li N, Wu L, Gao Y, Xiao C, Jiang H, Zheng Y, Mao C, Deng J, Wang H, Xu N. Prognostic Value of Neutrophil-Lymphocyte Ratio, Platelet-Lymphocyte Ratio, and Combined Neutrophil-Lymphocyte Ratio and Platelet-Lymphocyte Ratio in Stage IV Advanced Gastric Cancer. Front Oncol. 2020; 10:841. https://doi.org/10.3389/fonc.2020.00841 [PubMed]
-
50.
Zhao G, Liu N, Wang S, Guo J, Song X, Qi Y, Qiu W, Lv J. Prognostic significance of the neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio in patients with metastatic gastric cancer. Medicine (Baltimore). 2020; 99:e19405. https://doi.org/10.1097/MD.0000000000019405 [PubMed]
-
51.
Zhou D, Wu Y, Zhu Y, Lin Z, Yu D, Zhang T. The Prognostic Value of Neutrophil-to-lymphocyte Ratio and Monocyte-to-lymphocyte Ratio in Metastatic Gastric Cancer Treated with Systemic Chemotherapy. J Cancer. 2020; 11:4205–12. https://doi.org/10.7150/jca.39575 [PubMed]
-
52.
Wells G, Shea B, O'Connell D, Peterson J, Welch, Losos M, Tugwell P, Ga SW, Zello G, Petersen J. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2014. http://www.evidencebasedpublichealth.de/download/Newcastle_Ottowa_Scale_Pope_Bruce.pdf.
-
53.
Altman MEDSG. Systematic Reviews in Health Care: Meta-Analysis in Context, Second Edition. 2001. https://onlinelibrary.wiley.com/doi/pdf/10.1002/9780470693926.fmatter.
-
54.
Gao Y, Huang D. The value of the systematic inflammation-based Glasgow Prognostic Score in patients with gastric cancer: a literature review. J Cancer Res Ther. 2014; 10:799–804. https://doi.org/10.4103/0973-1482.146054 [PubMed]
-
55.
Li QQ, Lu ZH, Yang L, Lu M, Zhang XT, Li J, Zhou J, Wang XC, Gong JF, Gao J, Li J, Li Y, Shen L. Neutrophil count and the inflammation-based glasgow prognostic score predict survival in patients with advanced gastric cancer receiving first-line chemotherapy. Asian Pac J Cancer Prev. 2014; 15:945–50. https://doi.org/10.7314/apjcp.2014.15.2.945 [PubMed]
-
56.
Kubota T, Hiki N, Nunobe S, Kumagai K, Aikou S, Watanabe R, Sano T, Yamaguchi T. Significance of the inflammation-based Glasgow prognostic score for short- and long-term outcomes after curative resection of gastric cancer. J Gastrointest Surg. 2012; 16:2037–44. https://doi.org/10.1007/s11605-012-2036-x [PubMed]
-
57.
Gu L, Wang M, Cui X, Mo J, Yuan L, Mao F, Zhang K, Ng DM, Chen P, Wang D. Clinical significance of peripheral blood-derived inflammation markers in advanced gastric cancer after radical resection. BMC Surg. 2020; 20:219. https://doi.org/10.1186/s12893-020-00884-8 [PubMed]
-
58.
Qi Y, Liao D, Mei D, Zhang Y, Liu Y. Elevated Neutrophil-to-Lymphocyte Ratio Is Associated With Poor Outcomes for Melanoma Patients Treated With PD-1 Inhibitor or Chemotherapy in a Chinese Population. Front Oncol. 2020; 10:1752. https://doi.org/10.3389/fonc.2020.01752 [PubMed]
-
59.
Howard R, Kanetsky PA, Egan KM. Exploring the prognostic value of the neutrophil-to-lymphocyte ratio in cancer. Sci Rep. 2019; 9:19673. https://doi.org/10.1038/s41598-019-56218-z [PubMed]
-
60.
Wang JB, Li P, Liu XL, Zheng QL, Ma YB, Zhao YJ, Xie JW, Lin JX, Lu J, Chen QY, Cao LL, Lin M, Liu LC, et al. An immune checkpoint score system for prognostic evaluation and adjuvant chemotherapy selection in gastric cancer. Nat Commun. 2020; 11:6352. https://doi.org/10.1038/s41467-020-20260-7 [PubMed]
-
61.
Jiang Y, Jin C, Yu H, Wu J, Chen C, Yuan Q, Huang W, Hu Y, Xu Y, Zhou Z, Fisher GA Jr, Li G, Li R. Development and Validation of a Deep Learning CT Signature to Predict Survival and Chemotherapy Benefit in Gastric Cancer: A Multicenter, Retrospective Study. Ann Surg. 2020. [Ahead of Print]. https://doi.org/10.1097/SLA.0000000000003778 [PubMed]
-
62.
Li P, Cao W, Ding R, Cheng M, Xu X, Chen S, Chen B, Cao G, Xiong M. Expression and Prognostic Significance of Metastasis-Associated Protein 1 in Gastrointestinal Cancer. Front Oncol. 2020; 10:542330. https://doi.org/10.3389/fonc.2020.542330 [PubMed]
-
63.
Zhang CX, Wu CT, Xiao L, Tang SH. The diagnostic and clinicopathological value of trefoil factor 3 in patients with gastric cancer: a systematic review and meta-analysis. Biomarkers. 2021; 26:95–102. https://doi.org/10.1080/1354750X.2020.1871411 [PubMed]
-
64.
Li M, Xue F, Yang J, Pan X. Correlation between tumor marker CA72-4 and prognosis of patients with gastric cancer: A protocol for systematic review and meta-analysis. Medicine (Baltimore). 2020; 99:e23723. https://doi.org/10.1097/MD.0000000000023723 [PubMed]
-
65.
Moher D, Liberati A, Tetzlaff J, Altman DG, and PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009; 339:b2535. https://doi.org/10.1136/bmj.b2535 [PubMed]
-
66.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009; 339:b2700. https://doi.org/10.1136/bmj.b2700 [PubMed]
-
67.
Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998; 17:2815–34. https://doi.org/10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8 [PubMed]
-
68.
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007; 8:16. https://doi.org/10.1186/1745-6215-8-16 [PubMed]
-
69.
Zeng F, Li L, Zeng J, Deng Y, Huang H, Chen B, Deng G. Can we predict the severity of coronavirus disease 2019 with a routine blood test? Pol Arch Intern Med. 2020; 130:400–06. https://doi.org/10.20452/pamw.15331 [PubMed]