Programmed cell death-ligand 1 expression predicts poor treatment response and prognostic value in esophageal squamous cell carcinoma patients without esophagectomy
Abstract
Research on association between programmed cell death ligand 1 (PD-L1) expression in cancer cells and prognosis of esophageal squamous cell carcinoma (ESCC) has been controversial and has focused on patients with surgical resection. We aimed to investigate impact of PD-L1 on treatment response and prognostic value in ESCC and analyze which subset of patients may benefit from immunotherapy. The PD-L1 expression was evaluated by immunohistochemical analysis in all patients. Stratification analysis was performed according to whether surgery was performed. There were no significant correlations between PD-L1 expression with 3-year overall survival (OS) and progression-free survival (PFS) in 81 ESCC patients. Then stratification analysis was performed. Among these 44 patients without surgery, disease control rate (DCR) in negative PD-L1 expression group (78%) was significantly better than those (42%) in positive PD-L1 expression group (P = 0.032). There were no significant correlations between PD-L1 expression with 3-year OS and PFS in 37 ESCC patients receiving surgery. However, in 44 ESCC patients without surgery, the Kaplan-Meier method showed that 3-year OS and PFS in negative PD-L1 expression group were significantly better than those in positive PD-L1 expression group. In Cox univariate and multivariate model, PD-L1 was an independent prognosticator for inferior OS (p = 0.011; p = 0.017). Our research revealed prognostic role of PD-L1 expression in cancer cells may be variable in different treatment methods. Consequently, PD-L1 may serve as an independent prognostic factor and provide a theoretical basis for combining conventional therapy with immunotherapy targeting PD-L1 to achieve better treatment outcome in ESCC patients without esophagectomy.
Introduction
According to 2018 global cancer statistics, the incidence of esophageal cancer (EC) ranks seventh, and the cancer-related mortality ranks sixth in the world [1]. Every year, almost half of the new cases diagnosed and deaths of EC in the world come from China, which is the country with the largest burden of EC [1]. In China, the mortality rate of EC is the fourth among cancer-related deaths, and esophageal squamous cell carcinoma (ESCC) accounts for more than 90% of cases [2, 3]. With the significant advancement of diagnostic and treatment technologies, the survival rates of EC had increased, but the 5-year Overall survival (OS) was still around 30% in China from 2003 to 2015 [4]. Currently, the immunotherapy had rapidly emerged as a novel treatment option, achieved very significant efficacy, and it was thought that it would turn into one of the main treatment methods of EC. Through the combination of programmed cell death-1 (PD-1) and programmed cell death-ligand 1 (PD-L1), it can promote tumor cells escape from host immune surveillance [5]. A series of clinical trials have confirmed that EC patients with immunotherapy targeting PD-1/PD-L1 can obtain good treatment response and long-lasting effects, but the number of effective patients is only 12% to 30%, indicating that screening the benefiting population is the key [6–9].
PD-L1 is called B7 homologous protein 1 and composed of five domains, which is a type I transmembrane protein. PD-L1 is constitutively expressed in antigen presenting cells, tumor cells, non-hematopoietic cells and non-lymphoid organs such as liver, heart and lung [10]. After PD-L1 in tumor cell binds to PD-1 receptor on T cell, it inhibits the migration and proliferation of T cell and helps tumor cell escape from host immune surveillance [11]. It alters the production and maturation of cytokines, and leads to T cells apoptosis, which contributes to the growth of cancer cells [10, 12].
Some studies have shown that the PD-L1 was a good prognostic factor of patients with ESCC [13, 14]. Conversely, some studies have observed that PD-L1 positive ESCC patients have a worse prognosis [15–17]. Additionally, some studies confirmed that there was no correlation between PD-L1 expression in cancer cells and survival for ESCC patients [18, 19]. All the above evidences about the association between PD-L1 in cancer cells and ESCC survival have concentrated on patients with esophagectomy and been controversial. However, prognostic value of PD-L1 in ESCC patients without surgery remains to be determined. We aimed to observe the impact of PD-L1 expression on treatment response and prognostic value in ESCC and analyze which part of patients are beneficiaries of immunotherapy. Further understanding of the influence of PD-L1 expression on prognosis is important for improving prognosis and screening the benefiting population from immunotherapy for ESCC patients.
Materials and Methods
Patients
From January 2015 to December 2017, 81 patients who were primary ESCC were available for this study. Enrollment criteria include no history of other malignant tumors, pathologically confirmed ESCC, complete case data and follow-up information. The exclusion criteria were as follows: non-squamous cell carcinoma and second primary cancer. Consequently, 81 patients were recruited for the present study. Record clinical data, mainly including pathological data and basic characteristics of patients. According to the seventh edition of the AJCC TNM staging system, the selected patients were staged. We followed up all patients until death or 31 December 2020. OS was defined from the date of diagnosis to death due to any cause or the last follow-up. Progression-free survival (PFS) was defined as the time from start time of treatment to death from any cause or date of first relapse. Disease control rate (DCR) is defined as the percentage of patients whose tumors shrink or remain unchanged within a certain period of time, including complete remission, partial remission and stable cases. The patients who were alive until 31 December 2020 was defined as censored data. In our research, all procedures were carried out according to ethical principles, and the ethics committee of our hospital had approved the study.
Immunohistochemistry
The patient’s tissue was fixed in 4% buffered formalin, embedded with paraffin, and finally cut into 3 μm-thick sections for immunohistochemistry (IHC) examination. Briefly, the section was dewaxed in xylene, rehydrated in descending grades of ethanol, soaked in distilled water for 10 min; and blocked for 30 min with 3% H2O2. After blocking, Antigen retrieval: 0.01M sodium citrate buffer solution (pH6.0), microwave intensity 100, microwave antigen retrieval 10min. After blocking at 37°C, incubate the sections with rabbit anti-PD-L1monoclonal antibody (1:60, AB205921, Abcam) at 4°C overnight. On the second day, add second antibody to the sections and incubate at room temperature for 60 min, then observe through DAB system staining and counterstain the sections with hematoxylin.
Evaluation of immunostaining
Two experienced pathologists independently examined all specimens blindly. Analyze the slides semi-quantitatively, and give reports based on the relative staining percentage as previously described on tumor cells [20]. During the IHC analysis, we use positive controls in accordance with the instructions to ensure quality control. Five visual fields were selected for PD-L1 expression score. Calculate the average percentage of PD-L1 positive cells in the five fields of each sample. If PD-L1 staining of the tumor cell is >5%, it is considered that the expression of PD-L1 is positive.
Statistical analysis
We use SPSS version 20.0 statistical package for data processing (SPSS Inc., Chicago, IL, USA). Fisher's exact test or Chi-square test was applied for categorical data between the two groups. We use Kaplan-Meier curve and log-rank analysis to analyze and compare the survival of patients. We applied the Cox proportional hazard model to conduct univariate and multivariate analysis to determine the hazard ratio (HR) of the variables to the survival. The 95% HR confidence interval (CI) was given. Two-sided test was performed. We considered that P < 0.05 was statistically significant.
Ethical statement
The study was approved by the institutional ethics committee of Yantai Affiliated Hospital of Binzhou Medical University (Application number: 20200104001) and all procedures were conducted in accordance with ethical principles.
Results
Patient's characteristics
In this study, we retrospectively analyzed 81 ESCC patients, including 75 males and 6 females. Based on the time the patient was diagnosed, the age range of the patients was 43 to 92 years, with a median age of 74 years. There were 37 patients receiving surgery and 44 patients without surgery. PD-L1 expression was positive in 36 (44%) of 81 patients, 24 (65%) of 37 patients, and 12 (27%) of 44 patients, respectively (Figure 1). 32 (40%) patients were I and II, and 49 (60%) patients were III and IV. Additionally, 34 (42%) patients had negative status of lymph nodes, and 47 (58%) patients had positive status of lymph nodes. The patient's primary tumor location was 23 cases (28%) in the upper esophagus, 32 cases (40%) in the middle esophagus, and 26 cases (32%) in the lower esophagus. Among the 81 patients, 28 (35%) patients were non-smokers, whereas 53 (65%) patients were smokers. Among the 81 patients, 32 (40%) patients were non-drinkers, whereas 49 (60%) patients were drinkers. Patient characteristics are presented in Table 1.
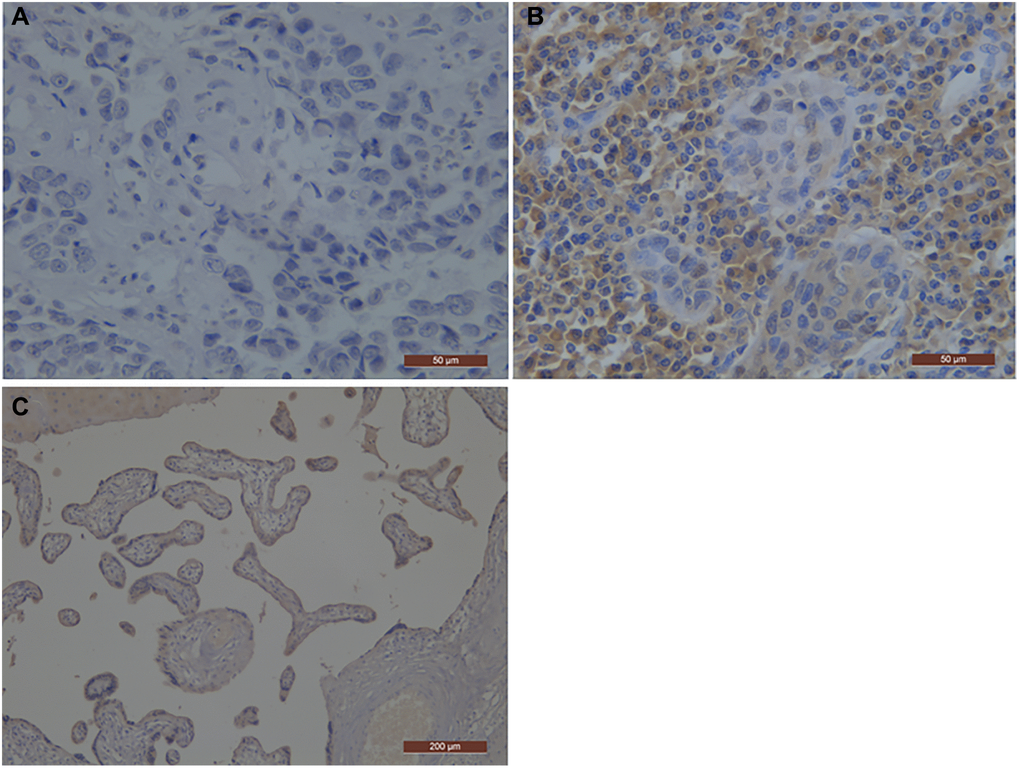
Figure 1. Representative photographs of PD-L1 immunostaining in esophageal squamous cell carcinoma. The positive staining was assessed against the positive control staining (Placental tissue). (A) Negative immunohistochemical staining pattern for PD-L1; (B) Positive immunohistochemical staining pattern for PD-L1; (C) the positive control staining (Placental tissue); PD-L1, programmed death-ligand 1.
Table 1. Clinicopathologic features of 81 patients with esophageal squamous cell carcinoma.
| Parameters | No. of cases (Percentage) |
| Age (years) (median:74, range 43–92) | ≤65
>65 | 44 (54%)
37 (46%) |
| Sex | Male
Female | 75 (93%)
6 (7%) |
| TNM stage | I II
III IV | 32 (40%)
49 (60%) |
| Status of lymph nodes | Negative
Positive | 34 (42%)
47 (58%) |
| Primary tumor location | Upper
Middle
Lower | 23 (28%)
32 (40%)
26 (32%) |
| PD-L1 expression | Negative
Positive | 45 (56%)
36 (44%) |
| Smoking history | No
Yes | 28 (35%)
53 (65%) |
| Alcohol history | No
Yes | 32 (40%)
49 (60%) |
| Surgery | No
Yes | 44 (54%)
37 (46%) |
The correlations between PD-L1 and patient characteristics.
Among all ESCC patients, there were no obvious relationships between PD-L1 and smoking history, alcohol history, age, sex, lymph node status, M stage and TNM stage. Although there were associations between PD-L1 and primary tumor location (P = 0.078) and T stage (P = 0.054), they did not achieve statistical significance (P ≥ 0.05) (Table 2).
Table 2. Correlations between PD-L1 expression and clinicopathologic parameters of 81 ESCC patients.
| Parameters | PD-L1 |
| + | − | P value |
| Age (years) | ≤65
>65 | 21
15 | 23
22 | 0.517 |
| Sex | Male
Female | 32
4 | 43
2 | 0.399 |
| Tumor location | Upper
Middle
Low | 7
19
10 | 16
13
16 | 0.078 |
| TNM stage | I II
III IV | 14
22 | 18
27 | 0.919 |
| T stage | T1/2
T3/4 | 8
28 | 3
42 | 0.054 |
| M stage | M0
M1 | 34
2 | 40
5 | 0.454 |
| Status of lymph nodes | Negative
Positive | 13
23 | 21
24 | 0.339 |
| Smoking history | Yes
No | 23
13 | 30
15 | 0.818 |
| Alcohol history | Yes
No | 22
14 | 27
18 | 0.919 |
Groups were performed according to the operation, one group of 37 ESCC patients receiving surgery and one group of 44 ESCC patients without surgery. Similarly, there were no obvious associations between PD-L1 and smoking history, alcohol history, age, sex, tumor location, T stage, lymph node status, TNM stage and M stage in both groups (Tables 3 and 4).
Table 3. Correlations between PD-L1 expression and clinicopathologic parameters of 37 ESCC patients receiving surgery.
| Parameters | PD-L1 |
| + | − | P value |
| Age (years) | ≤65
>65 | 17
7 | 12
1 | 0.216 |
| Sex | Male
Female | 22
2 | 13
0 | 0.532 |
| Tumor location | Upper
Middle
Low | 3
14
7 | 3
6
4 | 0.664 |
| TNM stage | I II
III IV | 10
14 | 5
8 | 0.850 |
| T stage | T1/2
T3/4 | 7
17 | 2
11 | 0.446 |
| Status of lymph nodes | Negative
Positive | 7
17 | 4
9 | 0.919 |
| Smoking history | Yes
No | 17
7 | 9
4 | 0.602 |
| Alcohol history | Yes
No | 16
8 | 8
5 | 0.755 |
Table 4. Correlations between PD-L1 expression and clinicopathologic parameters of 44 ESCC patients without surgery.
| Parameters | PD-L1 |
| + | − | P value |
| Age (years) | ≤65
>65 | 4
8 | 11
21 | 0.621 |
| Sex | Male
Female | 10
2 | 30
2 | 0.297 |
| Tumor location | Upper
Middle
Low | 4
5
3 | 13
7
12 | 0.412 |
| TNM stage | I II
III IV | 4
8 | 13
19 | 0.739 |
| T stage | T1/2/3
T4 | 7
5 | 26
6 | 0.139 |
| M stage | M0
M1 | 11
1 | 28
4 | 0.583 |
| Status of lymph nodes | Negative
Positive | 6
6 | 17
15 | 0.853 |
| Smoking history | Yes
No | 6
6 | 21
11 | 0.489 |
| Alcohol history | Yes
No | 6
6 | 19
13 | 0.576 |
Associations between DCR with clinicopathological characteristics in 44 ESCC patients without surgery
From Table 5, we found that there were statistical associations between DCR with Tumor location (P = 0.046), TNM stage (P = 0.038) and PD-L1 expression (P = 0.032) in 44 ESCC patients without surgery. DCR (78%) in the PD-L1 negative group was significantly better than those (42%) in the PD-L1 positive group. However, there were no statistical correlations between DCR and smoking history, alcohol history, age, sex, T stage and status of lymph nodes.
Table 5. Associations between disease control rate with clinicopathological characteristics in 44 ESCC patients without surgery.
| Parameters | DCR |
| Present | Absent | P value |
| Age (years) | ≤65
>65 | 8
22 | 7
7 | 0.759 |
| Sex | Male
Female | 27
3 | 13
1 | 0.759 |
| Tumor location | Upper
Middle
Low | 15
6
9 | 2
7
5 | 0.046 |
| TNM stage | I II
III IV | 14
16 | 2
12 | 0.038 |
| T stage | T1/2/3
T4 | 24
6 | 9
5 | 0.287 |
| Status of lymph nodes | Negative
Positive | 18
12 | 5
9 | 0.133 |
| Smoking history | Yes
No | 16
14 | 11
3 | 0.184 |
| Alcohol history | Yes
No | 15
15 | 10
4 | 0.211 |
| PD-L1 | +
− | 5
25 | 7
7 | 0.032 |
Stratification analysis between patient survival with clinicopathological characteristics
Survival analysis
Table 6 revealed the relationship between 3-Year OS and PFS with PD-L1. There were no statistical associations between PD-L1 and 3-year OS and PFS in 81 ESCC patients (P > 0.05). Then stratification analysis was performed according to whether surgery was performed. There were no statistical associations between PD-L1 and 3-year OS and PFS in 37 ESCC patients receiving surgery (P > 0.05). However, the Kaplan-Meier method showed that 3-year OS and PFS in the PD-L1 negative group were obviously better than those in the PD-L1 positive group (P < 0.05; Table 6 and Figure 2) in 44 ESCC patients without surgery.
Table 6. Results of log-rank analysis between 3-Year OS and PFS with PD-L1 expression.
| Factors | No. of patients | 3-Year OS p value | 3-Year PFS p value |
| PD-L1 of 81 ESCC patients | +
− | 36
45 | 0.383 | 0.217 |
| PD-L1 of 37 ESCC patients receiving surgery | +
− | 24
13 | 0.583 | 0.298 |
| PD-L1 of 44 ESCC patients without surgery | +
− | 12
32 | 0.007 | 0.035 |
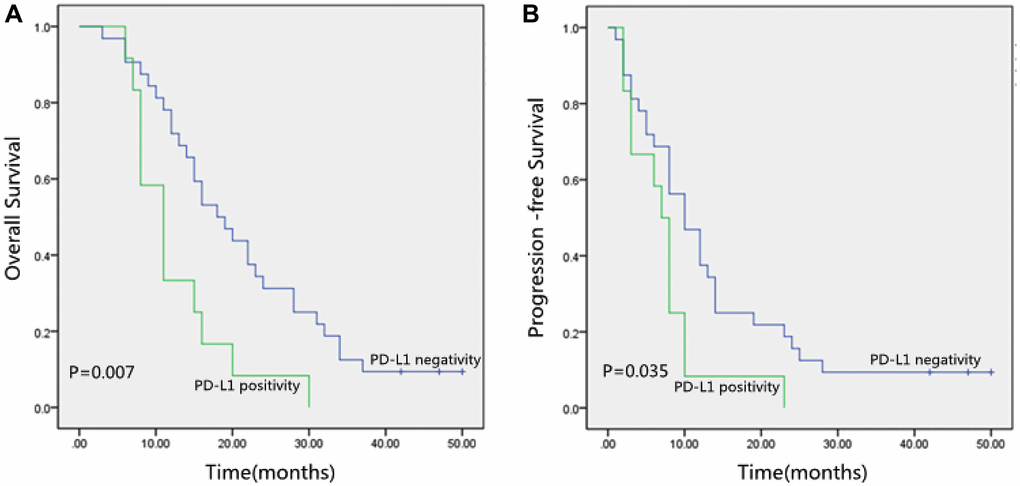
Figure 2. (A) Kaplan-Meier curves of Overall Survival (OS) according to PD-L1 expression. (B) Kaplan-Meier curves of Progression-Free Survival (PFS) according to PD-L1 expression. PD-L1, programmed death-ligand 1.
We observed that PD-L1 positive (p = 0.007; Figure 2A), TNM stage (p = 0.008), and Lymph nodes status (p = 0.001) were correlated with inferior OS significantly by the Kaplan-Meier method and log-rank test (Table 7). Besides, Table 7 also showed that PD-L1 positive (p = 0.035; Figure 2B), Tumor location (p = 0.049), TNM stage (p = 0.018), and Lymph nodes status (p = 0.044) were significantly correlated with poor PFS.
Table 7. Results of log-rank analysis of clinicopathologic parameters for 3-Year OS and PFS in 44 ESCC patients without surgery.
| Factors | No. of patients | 3-Year OS p value | 3-Year PFS p value |
| Age (years) | ≤65
>65 | 15
29 | 0.830 | 0.776 |
| Sex | Male
Female | 40
4 | 0.991 | 0.533 |
| Tumor location | Upper
Middle
Low | 17
12
15 | 0.059 | 0.049 |
| TNM stage | I II
III IV | 17
27 | 0.008 | 0.018 |
| T stage | T1-3
T4 | 33
11 | 0.106 | 0.032 |
| Status of lymph nodes | Negative
Positive | 23
21 | 0.001 | 0.044 |
| Smoking history | Yes
No | 27
17 | 0.730 | 0.483 |
| Alcohol history | Yes
No | 25
19 | 0.536 | 0.624 |
| PD-L1 | +
− | 12
32 | 0.007 | 0.035 |
The cox univariate and multivariate analysis
For 44 ESCC patients without surgery, we performed the Cox Univariate and Multivariate Analysis (Tables 8 and 9). In the Cox univariate model, PD-L1 positive (p = 0.011, HR: 2.516, 95% CI: 1.234–5.127), TNM stage (p = 0.012, HR: 2.373, 95% CI: 1.207–4.663) and Status of lymph nodes (p = 0.002, HR: 2.944, 95% CI: 1.485–5.836) were independently poor prognosticators for inferior OS. Meanwhile, TNM stage (p = 0.029, HR: 2.085, 95% CI: 1.080–4.027) and T stage (p = 0.049, HR: 2.126, 95% CI: 1.004–4.504) were related with inferior PFS. Although they are not statistically significant, there was a trend between the positive PD-L1 expression with 3-Year PFS (P = 0.052). The positive PD-L1 was correlated with worse survival and increased risk of recurrence.
Table 8. Cox univariate analysis for 3-year survival in 44 patients ESCC patients without surgery.
| 3-Year OS | 3-Year PFS |
| HR | 95% CI | P | HR | 95% CI | P |
| Tumor location | 1.246 | 0.881–1.761 | 0.213 | 1.108 | 0.787–1.560 | 0.557 |
| TNM stage | 2.373 | 1.207–4.663 | 0.012 | 2.085 | 1.080–4.027 | 0.029 |
| T stage | 1.745 | 0.863–3.527 | 0.121 | 2.126 | 1.004–4.504 | 0.049 |
| Status of lymph nodes | 2.944 | 1.485–5.836 | 0.002 | 1.815 | 0.973–3.385 | 0.061 |
| PD-L1 | 2.516 | 1.234–5.127 | 0.011 | 2.008 | 0.993–4.058 | 0.052 |
Table 9. Cox multivariate analysis for 3-year survival in 44 patients ESCC patients without surgery.
| 3-Year OS | 3-Year PFS |
| HR | 95% CI | P | HR | 95% CI | P |
| Age | 1.157 | 0.497–2.694 | 0.735 | 0.718 | 0.322–1.598 | 0.417 |
| Sex | 1.190 | 0.350–4.045 | 0.780 | 1.536 | 0.418–5.642 | 0.518 |
| Tumor location | 1.096 | 0.702–1.711 | 0.686 | 1.126 | 0.745–1.701 | 0.575 |
| TNM stage | 1.103 | 0.290–4.199 | 0.885 | 1.500 | 0.397–5.669 | 0.550 |
| T stage | 1.374 | 0.514–3.673 | 0.527 | 1.463 | 0.536–3.987 | 0.458 |
| Status of lymph nodes | 2.762 | 0.820–9.308 | 0.101 | 1.288 | 0.407–4.077 | 0.666 |
| Smoking history | 0.979 | 0.280–3.418 | 0.973 | 1.391 | 0.392–4.941 | 0.610 |
| Alcohol history | 1.251 | 0.376–4.155 | 0.715 | 0.875 | 0.250–3.060 | 0.835 |
| PD-L1 | 2.737 | 1.201–6.240 | 0.017 | 1.914 | 0.849–4.317 | 0.118 |
Discussion
Usually, ESCC patients present with advanced disease or metastatic disease at diagnosis, who can not undergo esophagectomy. In addition, some patients can not undergo surgery because of the tumor location, severe cardiopulmonary dysfunction, or unwillingness of surgery. However, previous research on the association between PD-L1 in cancer cell and ESCC survival has been controversial and has focused on patients with esophagectomy. For patients without surgery, the prognostic value of PD-L1 remains to be determined. We analyzed prognostic significance of PD-L1 in patients without surgery for the first time. At present, immunotherapy has become an important treatment method for tumors with great potential. PD-L1 is the most commonly used predictor of immunotherapy. But in clinical applications, we have observed that not all PD-L1 positive patients can benefit from immunotherapy [21, 22]. Since only a few patients have good results after immunotherapy, screening beneficiary populations to guide treatment decisions has important clinical significance.
In the present study, we firstly analyzed all patients, then all patients were classified into two subgroups according to whether surgery was performed, one group of 37 ESCC patients receiving surgery and one group of 44 ESCC patients without surgery. In our study, PD-L1 proteins in cancer cells were positively expressed in 36 (44%) of 81 ESCC patients, 24 (65%) of 37 ESCC patients receiving esophagectomy, and 12 (27%) of 44 ESCC patients without surgery, respectively. The PD-L1 positive rate in surgical patients was obviously higher than those in non-surgical patients. In ESCC patients, multiple studies [23–25] have described that PD-L1 positive rate was between 18.9% and 79.7%. The differences between these researches may be due to discrepancy in the IHC evaluation methods and PD-L1 antibodies. On the contrary, Hirsch FR et al. [26] thought that although the PD-L1 assessment methods were different, the researchers emphasized that the results by Dako22C3 assay or Dako 28-8 assay were consistent, so this may have little effect on the results. In our study, we considered that the inconsistent expression rate may be due to different sources of specimens. For patients undergoing esophagectomy, we evaluated resection specimens, while for patients without surgery, we evaluated gastroscopic biopsy specimens.
Among all ESCC patients, there were no obvious associations between PD-L1 and alcohol history, smoking history, primary tumor location, age, sex, T stage, status of lymph nodes, M stage and TNM stage. Similarly, stratification analysis found that there were no obvious associations between PD-L1 and clinical and pathological features in both groups, respectively. These results were consistent with some studies [27, 28]. Wan-Ting Huang et al. [27] found that PD-L1 expression has no significant relationship with age, histological grade, primary tumor site, the clinical stages of T staging, N staging, and TNM staging. Sha Zhou et al. [28] showed that PDL1 positivity was not related with tumor location, T staging, alcohol history, age, sex, N staging and TNM staging. These observations indicated that PD-L1 expression was not affected by clinical and pathological characteristics.
For ESCC patients without surgery, associations between DCR with clinicopathological characteristics were also analyzed. In our study, we found that there were statistical associations between DCR with Tumor location (P = 0.046), TNM stage (P = 0.038) and PD-L1 expression (P = 0.032). DCR (78%) in the PD-L1 negative group was significantly better than those (42%) in the PD-L1 positive group. However, there were no apparent correlations between DCR and smoking history, alcohol history, age, sex, T stage and status of lymph nodes. Similarly, Wan-Ting Huang et al. [27] reported that pathologically complete responses (pCR) of PD-L1 negative patients after neoadjuvant chemoradiotherapy was higher than those of PD-L1 positive patients. Likely, Sha Zhou et al. [28] reported that patients with high PD-L1 expression had an obviously lower pCR rate. This was helpful to distinguish between responders and non-responders, thereby providing more appropriate treatment options.
In 81 ESCC patients, we did not find a significant association between PD-L1 and 3-year OS and 3-year PFS. Consistent with previous reports [18, 19], the expression of PD-L1 was not significantly correlated with patient's prognosis in ESCC patients underwent neoadjuvant treatment and esophagectomy. Differently, Qiao Wang et al. [15] reported that positive PD-L1 was statistically correlated with worse OS (P = 0.010) in ESCC patients received radical esophagectomy. And also, Jing-Jing Zhao et al. [16] reported that PD-L1 negative patients have longer OS than PD-L1 positive patients (P = 0.005) in ESCC underwent surgical resection. Furthermore, among the 378 patients with advanced ESCC who did not receive neoadjuvant chemoradiation and directly underwent radical esophagectomy, PD-L1 positive patients had worse disease-free survival (HR = 1.436, P = 0.009) [17]. Interestingly, in patients with ESCC undergoing postoperative adjuvant radiotherapy, Chenxue Jiang et al. [14] indicated that high expression of PD-L1 was correlated with a favorable prognosis. Similarly, Matteo Fassan et al. [29] discovered that PD-L1 positive patients had significantly higher pCR in patients with neoadjuvant chemoradiotherapy and surgery (P = 0.004). These results may show the prognostic role of PD-L1 in tumor cells was variable in different treatment methods. Thus stratification analysis was performed according to whether surgery was performed. The Kaplan-Meier method and log-rank test indicated that PD-L1 positive expression was significantly correlated with inferior 3-Year OS and PFS in 44 ESCC patients without surgery. In the Cox univariate and multivariate model, the PD-L1 positive expression was an independently poor prognosticators for inferior OS. For ESCC patients without surgery, the positive PD-L1 expression was correlated with inferior survival and increased risk of recurrence. However, in 37 ESCC patients receiving surgery, there was no obvious relationship between the positive expression of PD-L1 and 3-year OS and PFS. These findings indicated the impact of the immune predictors on the prognosis may vary with different treatment options, which was of great significance for the formulation of personalized treatment strategies, thereby provide a theoretical basis for provide a theoretical basis for combining conventional therapy with anti-PD-L1 immunotherapy currently recommended.
The present study has several limitations. First, our study was retrospective and the patient number was relatively small. Second, the expression of PD-L1 in immune infiltrating cells had not been assessed and we can not differentiate the central tumor from the invasion front because sample obtained by endoscopic biopsy was too small to have sufficient cells assessed. Besides, the discrepancy of positive PD-L1 expression rate between two groups may have resulted from different sources of specimens.
Conclusions
In conclusion, our research revealed the prognostic role of PD-L1 expression in cancer cells may be variable with different treatment methods, which was of great significance to the development of personalized treatment plans. For ESCC patients without esophagectomy, the positive PD-L1 expression was correlated with inferior DCR and poor prognosis, and increased risk of recurrence. Consequently, PD-L1 may be used as an independent prognostic factor and provide a theoretical basis for combining conventional therapy with immunotherapy targeting PD-L1 to reach better therapeutic effect in ESCC patients without esophagectomy.
Data availability statement
Some or all data during the study are available from the corresponding author by request. (Immunohistochemistry datas, OS and PFS).
Abbreviations
CI: Confidence Interval;
DCR: Disease Control Rate;
EC: Esophageal Cancer;
ESCC: Esophageal Squamous Cell Carcinoma;
HR: Hazard Ratio;
IHC: Immunohistochemical;
OS: Overall Survival;
PD-1: Programmed Cell Death-1;
PD-L1: Programmed Cell Death Ligand 1;
PFS: Progression-Free Survival.
Author Contributions
Conceptualization: F.Z.; Methodology: F.Z.; P.Z. and Q.Z.; Data Curation: F.Z.; P.Z. and Q.Z.; Investigation: F.Z. and X.Z.; Software: L.H.; Resources: X.Z.; P.Z. and Q.Z.; Supervision: F.Z.; Writing, Original Draft Preparation: F.Z.; Writing, Review and Editing: F.Z.; All authors have read and agreed to the published version of the manuscript.
Acknowledgments
The authors would like to thank Xiaohe Tang in the First Hospital of Zibo for collecting ESCC samples.
Conflicts of Interest
The authors declare no conflicts of interest related to this study.
Funding
This work was supported by grants from Binzhou Medical University (BY2019KYQD49).
References
-
1.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424. https://doi.org/10.3322/caac.21492 [PubMed]
-
2.
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016; 66:115–32. https://doi.org/10.3322/caac.21338 [PubMed]
-
3.
Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med. 2014; 371:2499–509. https://doi.org/10.1056/NEJMra1314530 [PubMed]
-
4.
Sun D, Li H, Cao M, He S, Lei L, Peng J, Chen W. Cancer burden in China: trends, risk factors and prevention. Cancer Biol Med. 2020; 17:879–95. https://doi.org/10.20892/j.issn.2095-3941.2020.0387 [PubMed]
-
5.
Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014; 515:568–71. https://doi.org/10.1038/nature13954 [PubMed]
-
6.
Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, Yoshikawa T, Oh SC, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017; 390:2461–71. https://doi.org/10.1016/S0140-6736(17)31827-5 [PubMed]
-
7.
Kudo T, Hamamoto Y, Kato K, Ura T, Kojima T, Tsushima T, Hironaka S, Hara H, Satoh T, Iwasa S, Muro K, Yasui H, Minashi K, et al. Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicentre, phase 2 trial. Lancet Oncol. 2017; 18:631–39. https://doi.org/10.1016/S1470-2045(17)30181-X [PubMed]
-
8.
Janjigian YY, Bendell J, Calvo E, Kim JW, Ascierto PA, Sharma P, Ott PA, Peltola K, Jaeger D, Evans J, de Braud F, Chau I, Harbison CT, et al. CheckMate-032 Study: Efficacy and Safety of Nivolumab and Nivolumab Plus Ipilimumab in Patients With Metastatic Esophagogastric Cancer. J Clin Oncol. 2018; 36:2836–44. https://doi.org/10.1200/JCO.2017.76.6212 [PubMed]
-
9.
Doi T, Piha-Paul SA, Jalal SI, Saraf S, Lunceford J, Koshiji M, Bennouna J. Safety and Antitumor Activity of the Anti-Programmed Death-1 Antibody Pembrolizumab in Patients With Advanced Esophageal Carcinoma. J Clin Oncol. 2018; 36:61–67. https://doi.org/10.1200/JCO.2017.74.9846 [PubMed]
-
10.
Ceeraz S, Nowak EC, Noelle RJ. B7 family checkpoint regulators in immune regulation and disease. Trends Immunol. 2013; 34:556–63. https://doi.org/10.1016/j.it.2013.07.003 [PubMed]
-
11.
Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007; 27:111–22. https://doi.org/10.1016/j.immuni.2007.05.016 [PubMed]
-
12.
Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999; 5:1365–69. https://doi.org/10.1038/70932 [PubMed]
-
13.
Hatogai K, Fujii S, Kojima T, Daiko H, Nomura S, Doi T, Kitano S, Ohtsu A, Takiguchi Y, Yoshino T, Ochiai A. Large-scale comprehensive immunohistochemical biomarker analyses in esophageal squamous cell carcinoma. J Cancer Res Clin Oncol. 2017; 143:2351–61. https://doi.org/10.1007/s00432-017-2482-7 [PubMed]
-
14.
Jiang C, Zhu Y, Tang S, Zhang G, Lin Q, Xu Y, Shang J. High PD-L1 expression is associated with a favorable prognosis in patients with esophageal squamous cell carcinoma undergoing postoperative adjuvant radiotherapy. Oncol Lett. 2019; 17:1626–34. https://doi.org/10.3892/ol.2018.9747 [PubMed]
-
15.
Wang Q, Feng F, Wang F, Liu Z, Liu S, Xu G, Zheng G, Guo M, Lian X, Zhang H. PD-L1 Expression On tumor Cells Was Associated With Unfavorable Prognosis In Esophageal Squamous Cell Carcinoma. J Cancer. 2018; 9:2224–31. https://doi.org/10.7150/jca.24493 [PubMed]
-
16.
Zhao JJ, Zhou ZQ, Wang P, Chen CL, Liu Y, Pan QZ, Zhu Q, Tang Y, Weng DS, Xia JC. Orchestration of immune checkpoints in tumor immune contexture and their prognostic significance in esophageal squamous cell carcinoma. Cancer Manag Res. 2018; 10:6457–68. https://doi.org/10.2147/CMAR.S181949 [PubMed]
-
17.
Rong L, Liu Y, Hui Z, Zhao Z, Zhang Y, Wang B, Yuan Y, Li W, Guo L, Ying J, Song Y, Wang L, Zhou Z, et al. PD-L1 expression and its clinicopathological correlation in advanced esophageal squamous cell carcinoma in a Chinese population. Diagn Pathol. 2019; 14:6. https://doi.org/10.1186/s13000-019-0778-4 [PubMed]
-
18.
Zhou S, Yang H, Zhang J, Wang J, Liang Z, Liu S, Li Y, Pan Y, Zhao L, Xi M. Changes in Indoleamine 2,3-Dioxygenase 1 Expression and CD8+ Tumor-Infiltrating Lymphocytes after Neoadjuvant Chemoradiation Therapy and Prognostic Significance in Esophageal Squamous Cell Carcinoma. Int J Radiat Oncol Biol Phys. 2020; 108:286–94. https://doi.org/10.1016/j.ijrobp.2020.01.020 [PubMed]
-
19.
Fukuoka E, Yamashita K, Tanaka T, Sawada R, Sugita Y, Arimoto A, Fujita M, Takiguchi G, Matsuda T, Oshikiri T, Nakamura T, Suzuki S, Kakeji Y. Neoadjuvant Chemotherapy Increases PD-L1 Expression and CD8+ Tumor-infiltrating Lymphocytes in Esophageal Squamous Cell Carcinoma. Anticancer Res. 2019; 39:4539–48. https://doi.org/10.21873/anticanres.13631 [PubMed]
-
20.
Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014; 515:563–67. https://doi.org/10.1038/nature14011 [PubMed]
-
21.
Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, Yusof MM, Gallardo C, Lipatov O, Barrios CH, Holgado E, Iwata H, Masuda N, Otero MT, et al, and KEYNOTE-355 Investigators. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020; 396:1817–28. https://doi.org/10.1016/S0140-6736(20)32531-9 [PubMed]
-
22.
Halmos B, Burke T, Kalyvas C, Insinga R, Vandormael K, Frederickson A, Piperdi B. A Matching-Adjusted Indirect Comparison of Pembrolizumab + Chemotherapy vs. Nivolumab + Ipilimumab as First-Line Therapies in Patients with PD-L1 TPS ≥1% Metastatic NSCLC. Cancers (Basel). 2020; 12:3648. https://doi.org/10.3390/cancers12123648 [PubMed]
-
23.
Qu HX, Zhao LP, Zhan SH, Geng CX, Xu L, Xin YN, Jiang XJ. Clinicopathological and prognostic significance of programmed cell death ligand 1 (PD-L1) expression in patients with esophageal squamous cell carcinoma: a meta-analysis. J Thorac Dis. 2016; 8:3197–204. https://doi.org/10.21037/jtd.2016.11.01 [PubMed]
-
24.
Jiang Y, Lo AWI, Wong A, Chen W, Wang Y, Lin L, Xu J. Prognostic significance of tumor-infiltrating immune cells and PD-L1 expression in esophageal squamous cell carcinoma. Oncotarget. 2017; 8:30175–89. https://doi.org/10.18632/oncotarget.15621 [PubMed]
-
25.
Ito S, Okano S, Morita M, Saeki H, Tsutsumi S, Tsukihara H, Nakashima Y, Ando K, Imamura Y, Ohgaki K, Oki E, Kitao H, Mimori K, Maehara Y. Expression of PD-L1 and HLA Class I in Esophageal Squamous Cell Carcinoma: Prognostic Factors for Patient Outcome. Ann Surg Oncol. 2016 (Suppl 4); 23:508–15. https://doi.org/10.1245/s10434-016-5376-z [PubMed]
-
26.
Hirsch FR, McElhinny A, Stanforth D, Ranger-Moore J, Jansson M, Kulangara K, Richardson W, Towne P, Hanks D, Vennapusa B, Mistry A, Kalamegham R, Averbuch S, et al. PD-L1 Immunohistochemistry Assays for Lung Cancer: Results from Phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J Thorac Oncol. 2017; 12:208–22. https://doi.org/10.1016/j.jtho.2016.11.2228 [PubMed]
-
27.
Huang WT, Lu HI, Wang YM, Chen YH, Lo CM, Lin WC, Lan YC, Tseng LH, Li SH. Positive Programmed Cell Death-Ligand 1 Expression Predicts Poor Treatment Outcomes in Esophageal Squamous Cell Carcinoma Patients Receiving Neoadjuvant Chemoradiotherapy. J Clin Med. 2019; 8:1864. https://doi.org/10.3390/jcm8111864 [PubMed]
-
28.
Zhou S, Zhao L, Liang Z, Liu S, Li Y, Liu S, Yang H, Liu M, Xi M. Indoleamine 2,3-dioxygenase 1 and Programmed Cell Death-ligand 1 Co-expression Predicts Poor Pathologic Response and Recurrence in Esophageal Squamous Cell Carcinoma after Neoadjuvant Chemoradiotherapy. Cancers (Basel). 2019; 11:169. https://doi.org/10.3390/cancers11020169 [PubMed]
-
29.
Fassan M, Cavallin F, Guzzardo V, Kotsafti A, Scarpa M, Cagol M, Chiarion-Sileni V, Maria Saadeh L, Alfieri R, Castagliuolo I, Rugge M, Castoro C, Scarpa M. PD-L1 expression, CD8+ and CD4+ lymphocyte rate are predictive of pathological complete response after neoadjuvant chemoradiotherapy for squamous cell cancer of the thoracic esophagus. Cancer Med. 2019; 8:6036–48. https://doi.org/10.1002/cam4.2359 [PubMed]