A novel lncRNA-miRNA-mRNA triple network identifies lncRNA XIST as a biomarker for acute myocardial infarction
Abstract
Despite the well-established role of long non-coding RNAs (lncRNAs) across various biological processes, their mechanisms in acute myocardial infarction (AMI) are not fully elucidated. The GSE34198 dataset from the Gene Expression Omnibus (GEO) database, which comprised 49 specimens from individuals with AMI and 47 specimens from controls, was extracted and analysed using the weighted gene co-expression network analysis (WGCNA) package. Twenty-seven key genes were identified through a combination of the degree and gene significance (GS) values, and the CDC42 (degree = 64), JAK2 (degree = 41), and CHUK (degree = 30) genes were identified as having the top three-degree values among the 27 genes. Potential interactions between lncRNA, miRNAs and mRNAs were predicted using the starBase V3.0 database, and a lncRNA-miRNA-mRNA triple network containing the lncRNA XIST, twenty-one miRNAs and three hub genes (CDC42, JAK2 and CHUK) was identified. RT–qPCR validation showed that the expression of the JAK2 and CDC42 genes and the lncRNA XIST was noticeably increased in samples from patients with AMI compared to normal samples. Pearson’s correlation analysis also proved that JAK2 and CDC42 expression levels correlated positively with lncRNA XIST expression levels. The area under ROC curve (AUC) of lncRNA XIST was 0.886, and the diagnostic efficacy of the lncRNA XIST was significantly better than that of JAK2 and CDC42. The results suggested that the lncRNA XIST appears to be a risk factor for AMI likely through its ability to regulate JAK2 and CDC42 gene expressions, and it is expected to be a novel and reliable biomarker for the diagnosis of AMI.
Introduction
Coronary artery disease (CAD) is a ubiquitous chronic heart disease involving the deposition and build-up of atherosclerotic plaques in the coronary artery, leading to a gradual constriction of the vascular lumen and compromised myocardial perfusion [1]. CAD manifests in several different ways, including acute myocardial infarction (AMI), unstable and stable angina, ischemic cardiomyopathy and even sudden cardiac death [2]. The advent of emergency percutaneous coronary intervention (PCI), which rapidly restores cardiac perfusion, has resulted in a tremendously improved prognosis for patients with AMI. Nevertheless, AMI is still the main cause of death in patients with CAD worldwide. AMI is responsible for significant patient morbidity and mortality, especially in China, where these rates have been increasing annually [3, 4]. Epidemiological studies related to AMI have shown that male sex, hyperlipidaemia, smoking, stress, diabetes, hypertension, age, obesity, family history and a sedentary lifestyle all culminate in the development of AMI [5]. Recent research has focused on identifying gene-based prognostic and therapeutic markers for AMI to circumvent the daunting challenge of managing patients with AMI. Interest in gene sequencing technology has increased, because it allows clinicians to obtain deeper insights into the relationship between genes and diseases [6, 7].
Several compelling studies have proposed that several noncoding RNAs (ncRNAs) are intricately involved in multiple biological functions and possess critical functions in the occurrence and development of diseases [8, 9]. Based on transcript length, noncoding RNAs are functionally grouped into two specific subtypes: (1) long ncRNAs (lncRNAs) (> 200 nt), which comprise long intergenic ncRNAs (lincRNAs), natural antisense transcripts, transcribed ultra-conserved regions, and enhancer-like ncRNAs; and (2) short ncRNAs (< 200 nt), which comprise PIWI-interacting RNAs, microRNAs, and transcription initiation RNAs [10, 11]. Unlike highly conserved short ncRNAs, whose function is essentially to participate in posttranscriptional modification, lncRNAs are less well conserved but are implicated in several physiological processes [12, 13].
Recent studies have proposed the essential role of lncRNAs in cardiovascular diseases [14]. Wang et al. confirmed that a lncRNA cardiac and apoptosis-related RNA (CARL), which has previously been associated with cardiomyocyte apoptosis, functions as a miR-539 molecular sponge, leading to inhibited mitochondrial division and apoptosis by stimulating PHB2 gene function [15]. In addition, the lncRNA wisp2 super enhancer related RNA (Wisper) stimulates myocardial fibrosis after myocardial infarction [16]. More importantly, several regulatory networks between lncRNAs and microRNAs (miRNAs) have recently been documented, providing insights into the exact functions of noncoding RNAs and their potential as molecular targets when developing therapeutic modalities for certain illnesses [17, 18]. Therefore, in this study, a scale-free network was constructed using weighted gene co-expression network analysis (WGCNA) [19], followed by a modularized analysis on the scale-free network and in-depth scrutinization of the correlations between modules, phenotypes and clinical data. Finally, hub genes requiring further study were identified among the genes that were significantly associated with phenotypes present in the meaningful modules. Furthermore, using data extracted from the starBase V3.0 database, we examined several miRNAs and lncRNAs that may target the identified hub genes and constructed a lncRNA-miRNA-mRNA triple network to identify specific lncRNAs that may have potential as sensitive and specific AMI biomarkers.
Results
Data preprocessing
Data were first processed by standardizing data formats, adding missing values and removing outliers. A total of 24580 gene symbols were detected in 97 samples. The co-expression network was constructed by selecting the top 25% of genes (6145) with a large variance in expression levels. Gene expression profiles of 6145 genes and clinical characteristics of the samples are described in detail in Supplementary Tables 2, 3.
Weighted gene co-expression networks
After calculation, we discovered that a correlation coefficient greater than 0.8 (the soft threshold of β is 10) was highly correlated and suitable for the construction of various gene modules (Figure 1A). A topological overlap matrix (TOM) was constructed by calculating the correlation and adjacency matrices of gene expression profiles. The gene cluster tree is depicted in Figure 1B. We then sought to identify the gene modules of each gene network using the hierarchical average linkage clustering method combined with TOM. Figure 1C depicts the heatmap. The dynamic tree cut algorithm identified ten gene modules (Figure 1D).
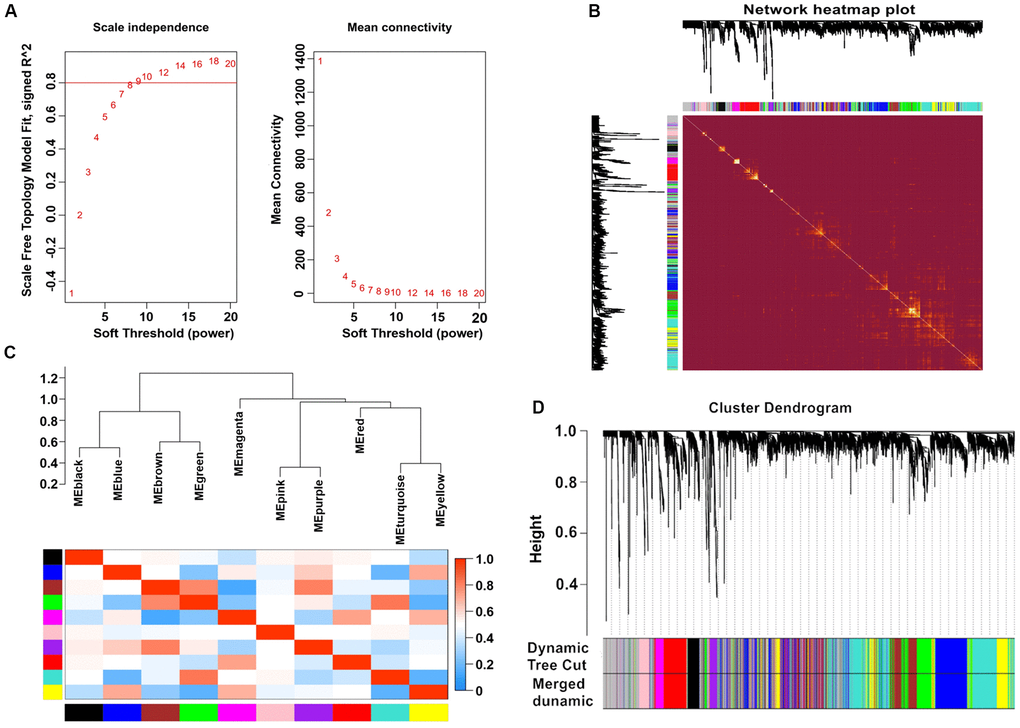
Figure 1. Weighted gene co-expression network analysis. (A) Analysis of network topology for various soft-thresholding powers. (B) Heatmap of the topological overlap in the gene network. (C) Relationship among all the modules. (D) Clustering dendrogram of genes. Gene clustering tree (dendrogram) obtained by hierarchical clustering of adjacency-based dissimilarity.
Determining modules of interest
Modules closely related to clinical features often have important and specific biological significance. As shown in Figure 2, the turquoise module appeared to be highly correlated with BMI (r 2 = 0.33, P = 9e-04). An in-depth calculation was performed to discern the association between the colour module and gene significance (GS). Figure 3A shows that the association between the turquoise module and gene significance was 0.45 (P = 9.1E-51). All gene symbols in the turquoise module and their GS values and corresponding P values are described in detail in Supplementary Table 4.
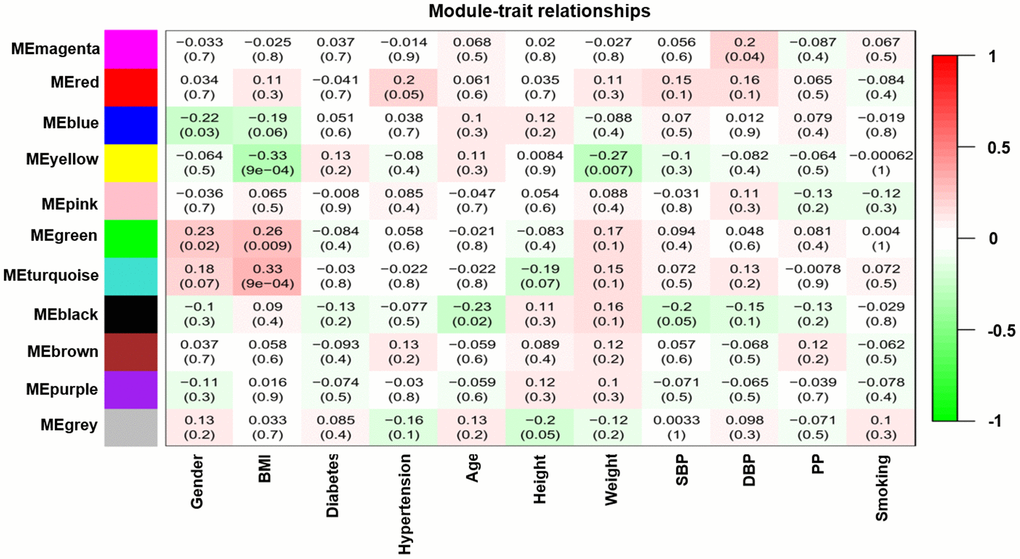
Figure 2. Module-feature associations. Each row corresponds to a modulEigengene and the column to the clinical phenotype. Each cell contains the corresponding correlation in the first line and the P-value in the second line. The table is color-coded by correlation according to the color legend.
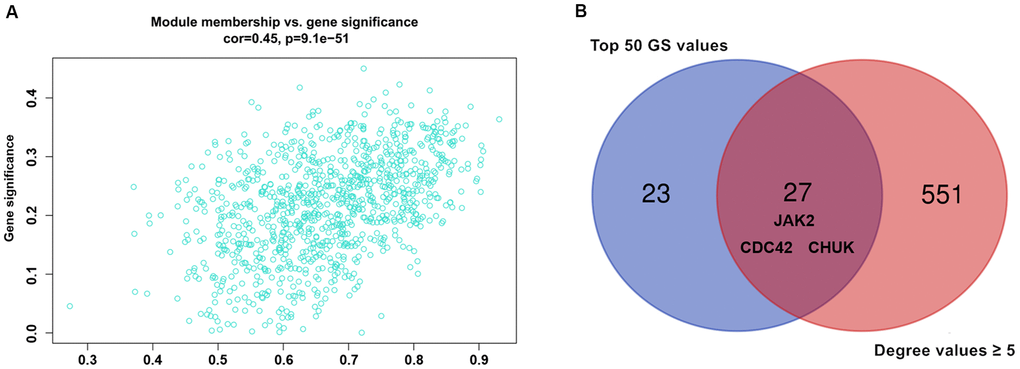
Figure 3. (A) Association between gene significance and module membership. Scatterplot shows a highly significant correlation between gene significant (GS) versus module membership (MM) with AMI in the turquoise module. (B) key genes with top 50 GS values and they degree values ≥ 5 were defined by Venn diagram.
Module preservation test
Gene expression profiles of patients with AMI were subjected to a preservation analysis. We identified one strong module and two moderately preserved modules between patients with AMI and control subjects (Supplementary Figure 1). Both Zsummary and medianRank statistical results were consistent, suggesting that the preservation analysis was not affected by the size of the module. The turquoise module was noted to be highly preserved, while the yellow and blue modules were moderately preserved. These interesting findings suggested the presence of significant variabilities in the gene expression patterns between the AMI and control groups.
Enrichment analysis of interesting modules
KEGG pathway and GO enrichment analyses of genes in the turquoise module were performed to dissect their physiological purposes functions. Figure 4A depicts the KEGG signalling pathways, and the biological process, cytological component and molecular function are shown in Figure 4B–4D. Nine hundred ninety-five genes in the turquoise module were primarily enriched in the following potentially AMI-related inflammatory pathways: hsa05152: tuberculosis; hsa04668: TNF signalling pathway; hsa05161: hepatitis B; hsa04068: FoxO signalling pathway; hsa04660: T cell receptor signalling pathway; hsa04620: Toll-like receptor signalling pathway; hsa04064: NF-kappa B signalling pathway; hsa04662: B cell receptor signalling pathway; hsa05132: Salmonella infection; hsa04666: Fc gamma R-mediated phagocytosis; hsa04932: nonalcoholic fatty liver disease; hsa05164: influenza A; and hsa04621: NOD-like receptor signalling pathway. The details of these analyses are also presented in Supplementary Tables 5, 6.
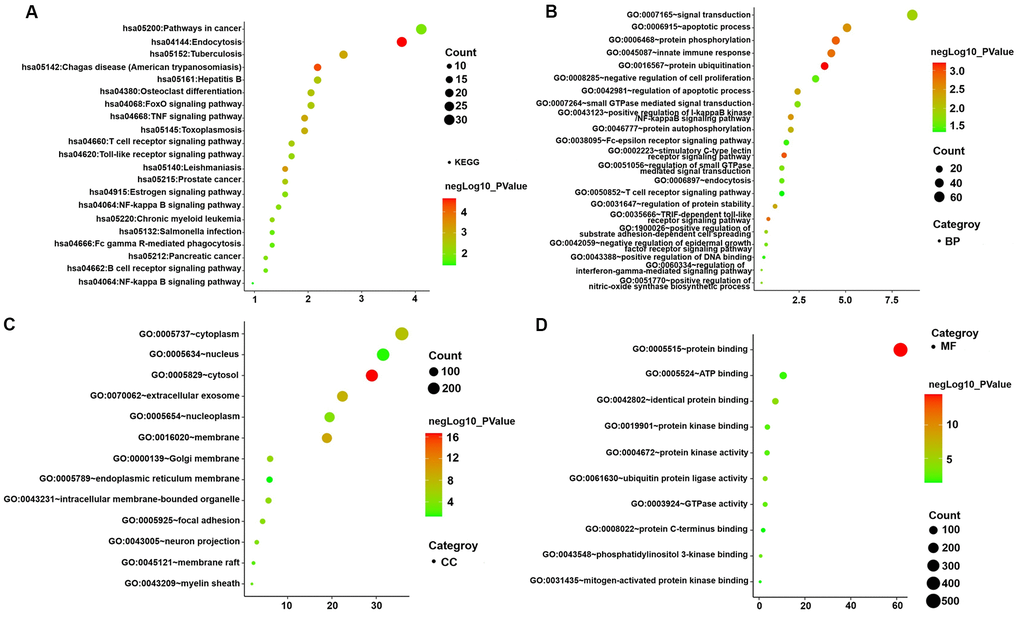
Figure 4. GO functional and KEGG pathway enrichment analyses for genes in the object module. The x-axis shows the number of genes and the y-axis shows the GO and KEGG pathway terms. The -log10 (P-value) of each term is colored according to the legend. (A) KEGG pathway. (B) Biological process. (C) Cytological component. (D) Molecular function.
Construction of a PPI network and identification of hub genes
Nine hundred ninety-five genes in the turquoise module were used to build the PPI network. As shown in Figure 5A, a PPI network consisting of 936 nodes and 4107 edges was built using the STRING tool. In addition, a PPI network of genes with the top 100-degree values is depicted in Figure 5B to better show the interaction between key genes and other genes. We noticed that the JAK2, CDC42 and CHUK genes are widely associated with other genes. The gene degree values were calculated using the Cytohubba plug-in in Cytoscape software, and gene degree values ≥ 5 are also shown in Supplementary Table 7. As shown in Figure 3B and Supplementary Table 8, 27 genes with the top 50 GS values and degree values ≥ 5 were defined as key genes, and the CDC42 (degree = 64), JAK2 (degree = 41), and CHUK (degree = 30) genes were identified as having the top three-degree values among the 27 genes. Then, combined with GO and KEGG analysis results, we noticed that the JAK2, CDC42 and CHUK genes are involved in the signalling pathways and biological processes related to AMI; therefore, the JAK2, CDC42 and CHUK genes were selected as hub genes for further research.
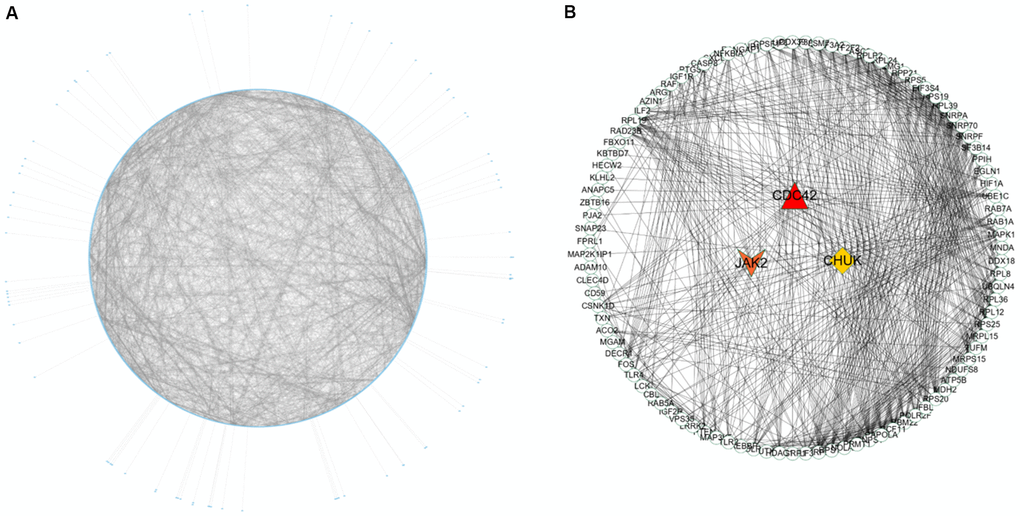
Figure 5. PPI network construction and identification of hub genes. (A) PPI network of genes in turquoise module. The edge shows the interaction between two genes. (B) PPI network of genes with top 100-degree values.
Construction of the lncRNA-miRNA-mRNA regulatory network
First, we identified lncRNAs that may potentially target the JAK2, CDC42 and CHUK genes based on information obtained from the starBase database. As shown in Supplementary Table 9, only one lncRNA, XIST was identified that might represent a common lncRNA forming ceRNA networks with both the JAK2 and CDC42 genes. As shown in Supplementary Tables 10, 11, we predicted miRNAs that potentially bind to the JAK2, CDC42, and CHUK genes and the lncRNA XIST. One hundred miRNAs bound to JAK2; 178 miRNAs bound to CDC42; 150 miRNAs bound to CHUK; and 454 miRNAs bound to the lncRNA XIST. As shown in Figure 6A, 21 common miRNAs (Supplementary Table 12) bound to the JAK2, CDC42, and CHUK genes and the lncRNA XIST simultaneously. Using this information, we built a lncRNA-miRNA-mRNA network that was visualized using Cytoscape software (version 3.71) (Figure 6B).
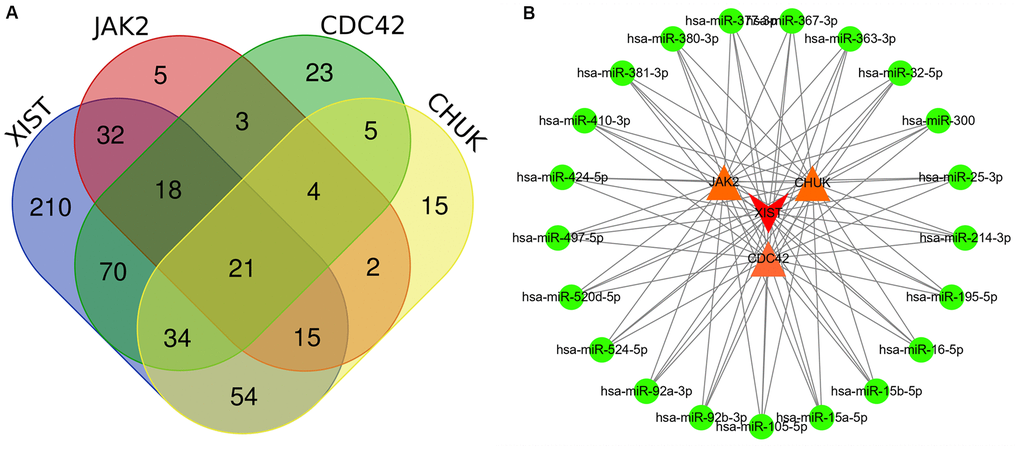
Figure 6. (A) Several common miRNAs that target JAK2, CDC42, and CHUK genes and lncRNA XIST were identified by Venn diagram. (B) A lncRNA-miRNA-mRNA ceRNA network that contained one lncRNA XIST, 21 miRNAs and 3 mRNAs (JAK2, CDC42 and CHUK). Edge stands for the interaction between two items.
Validation analysis using RT–qPCR and Pearson’s correlation analysis
Figure 7A depicts the RT–qPCR results of the relative expression levels of the JAK2 and CDC42 genes and the lncRNA XIST, which were significantly increased in patients with AMI compared to healthy controls. Meanwhile, a Pearson correlation analysis found that the expression levels of JAK2 (Figure 7B, R = 0.83, P < 2.2E-16) and CDC42 (Figure 7C, R = 0.80, P < 2.2E-16) were positively correlated with the lncRNA XIST levels. Conversely, little association was observed between the expression levels of the CHUK gene (Figure 7D, R = 0.32, P = 1.7E-07) and that of the lncRNA XIST.
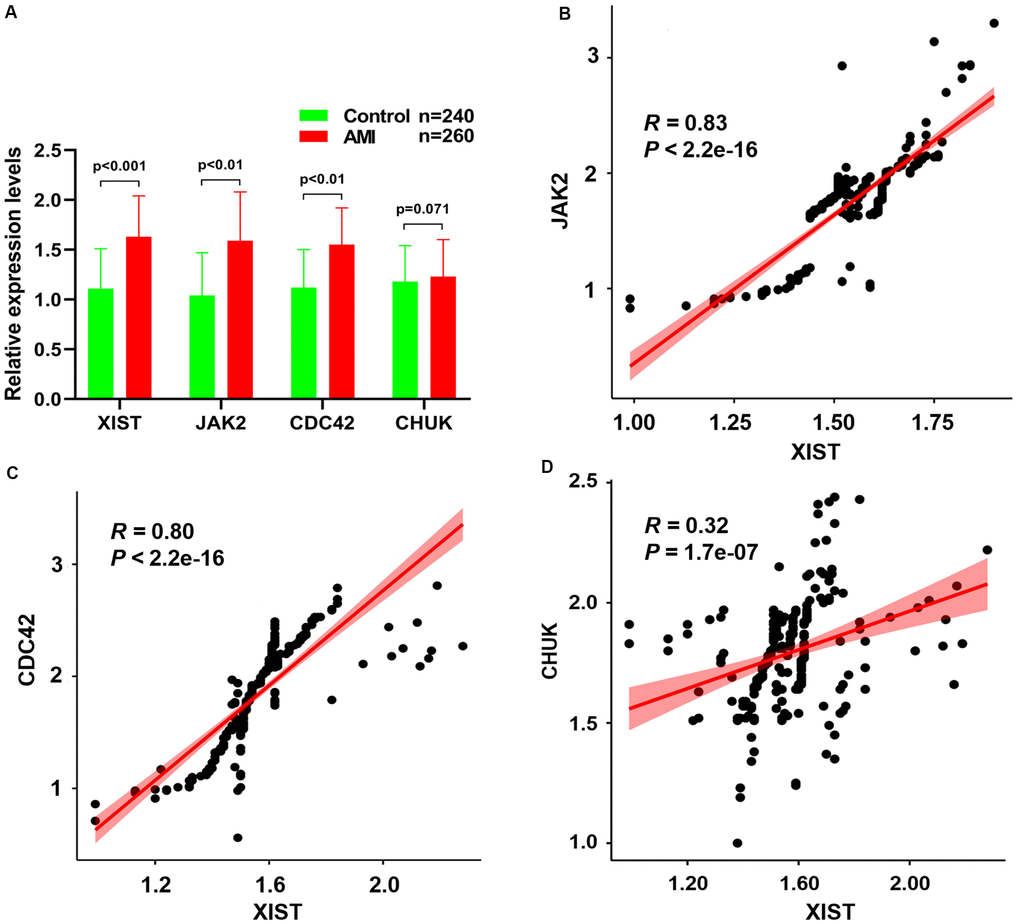
Figure 7. (A) The relative expression levels of JAK2, CDC42, CHUK genes and lncRNA XIST between healthy controls and AMI samples. The correlation between the expression levels of lncRNA XIST and JAK2 (B), CDC42 (C) and CHUK (D) genes that were analyzed by Pearson correlation analysis.
ROC curve for patients with AMI
The predictive values of the lncRNA XIST, JAK2, CDC42 and CHUK for the diagnosis of AMI were investigated using a ROC curve analysis. The AUC values for the lncRNA XIST (Figure 8A), JAK2 (Figure 8B), CDC42 (Figure 8C) and CHUK (Figure 8D) were 0.886 (95% CI 0.885–0.913; P = 0.0163) with a cut-off value of 0.772, a sensitivity of 94.8% and a specificity of 82.4%, 0.706 (95% CI 0.664-0.746; P < 0.001) with a cut-off value of 0.560, a sensitivity of 78.7% and a specificity of 77.3%, 0.692 (95% CI 0.649–0.732; P < 0.001) with a cut-off value of 0.548, a sensitivity of 74.5% and a specificity of 80.3%, and 0.542 (95% CI 0.492-0.593; P = 0.157) with a cut-off value of 0.163, a sensitivity of 43.8% and a specificity of 72.5%, respectively. As shown in Figure 8E, 8F, we noticed that the diagnostic efficacy of the lncRNA XIST was significantly better than that of JAK2, CDC42 and CHUK, and the diagnostic efficacy of JAK2 and CDC42 was significantly better than that of CHUK. However, neither JAK2 nor CDC42 differed significantly in terms of diagnostic efficacy.
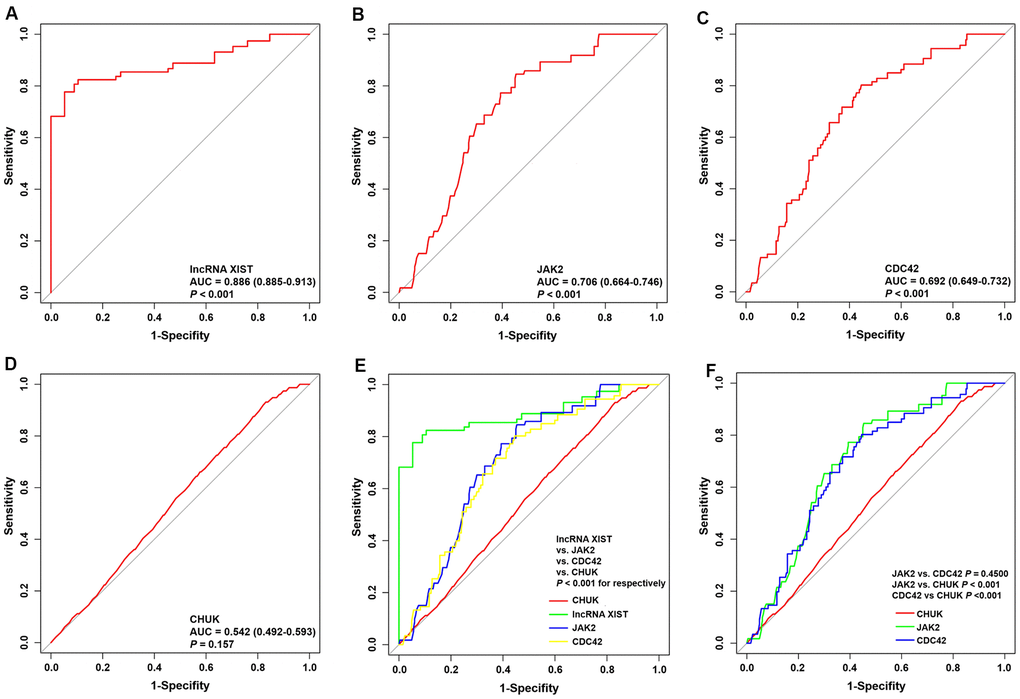
Figure 8. ROC curve analyses of one lncRNA and three genes for the diagnosis of AMI. (A–D) ROC curve analysis of lncRNA XIST, JAK2, CDC42 and CHUK genes. (E, F) The pairwise P-value comparison.
Demographic and biochemical characteristics
Neither patients with AMI nor individuals in the control group differed significantly in terms of weight, age, heart rate, pulse pressure, uric acid level, height, sex ratio, BMI or proportion of alcohol consumers (Table 1). Patients with AMI were more likely to smoke and have higher glucose levels, systolic and diastolic blood pressures, weight, serum low-density lipoprotein cholesterol (LDL-C), apolipoprotein (Apo) B, triglyceride (TG) and total cholesterol (TC) levels, creatinine levels, cardiac troponin T (cTnT) levels, body mass index (BMI) and creatine kinase (CK) and CK-MB levels than healthy participants. Patients in the control group had markedly increased ApoA1/ApoB ratios, ApoA1 levels and serum high-density lipoprotein cholesterol (HDL-C) levels.
Table 1. Comparison of demographic, lifestyle characteristics and serum lipid levels of the participants.
| Characteristic | Control (n=240) | AMI
(n=260) | Test-statistic | P |
| Male/female c | 176/64 | 187/73 | 0.125 | 0.724 |
| Age (years) a | 53.7±11.72 | 53.45±9.15 | 1.931 | 0.486 |
| Height (cm) a | 164.28±7.5 | 164.99±6.81 | 1.795 | 0.690 |
| Weight (kg) a | 59.90±9.10 | 60.26±10.11 | 4.882 | 0.229 |
| BMI (kg/m2) a | 22.31±3.84 | 22.09±3.34 | 3.498 | 0.352 |
| Smoking [n (%)] c | 79(32.9) | 108 (41.5) | 3.962 | 0.047 |
| Alcohol [n (%)] c | 64(26.7) | 68(26.2) | 0.017 | 0.897 |
| SBP (mmHg) a | 131.05±19.01 | 136.67±22.16 | 9.069 | 0.002 |
| DBP (mmHg) a | 80.01±11.99 | 82.90±13.35 | 7.193 | 0.018 |
| PP (mmHg) a | 51.06±14.2 | 53.77±19.62 | 3.881 | 0.057 |
| Glu (mmol/L) a | 6.05±1.57 | 6.45±1.76 | 8.646 | 0.009 |
| TC (mmol/L) a | 4.49±1.00 | 4.82±1.11 | 11.884 | 2.37E-4 |
| TG (mmol/L) b | 1.04(0.75) | 1.43(0.64) | -2.076 | 0.038 |
| HDL-C (mmol/L) a | 1.65±0.47 | 1.13±0.30 | 20.739 | 1.2035E-40 |
| LDL-C (mmol/L) a | 2.78±0.98 | 3.06±1.01 | 9.033 | 0.004 |
| ApoA1 (g/L) a | 1.42±0.33 | 0.98±0.29 | 22.261 | 5.7606E-43 |
| ApoB (g/L) a | 0.88±0.20 | 0.96±0.26 | 17.308 | 1.1219E-31 |
| ApoA1/ApoB a | 1.67±0.51 | 1.11±0.47 | 10.151 | 0.001 |
| Heart rate (beats/minutes) a | 72.90±9.58 | 73.67±7.54 | 3.807 | 0.322 |
| Creatinine, (μmol/L) a | 70.54±12.54 | 76.67±13.77 | 12.923 | 2.99E-7 |
| Uric acid, (μmol/L) a | 270.50±74.82 | 280.40±78.24 | 5.191 | 0.149 |
| Troponin T, (μg/L) a | 0.05±0.03 | 3.46±1.86 | 142.39 | 1.67E-76 |
| CK, (U/L) a | 77.95±41.61 | 1094.10±561.61 | 289.76 | 3.23E-139 |
| CK-MB, (U/L) a | 12.39±2.42 | 125.46±49.06 | 202.91 | 4.04E-104 |
| SBP, Systolic blood pressure; DBP, Diastolic blood pressure; PP, Pulse pressure; Glu, Glucose; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Apo, Apolipoprotein; TC, Total cholesterol; TG, Triglyceride; CK, creatine kinase, CK-MB, creatine kinase-myocardial band. |
| aMean ± SD determined by t-test. |
| bMedian (interquartile range) tested by the Wilcoxon-Mann-Whitney test. |
| cThe rate or constituent ratio between the different groups was analyzed by the chi-square test. |
Discussion
Ischemic cardiovascular disease is the primary contributor to global disability and death, despite the vast number of innovative therapeutic and diagnostic methods that have been developed in the last 10 years. In fact, studies predict a steady increase in the number of patients who will experience ischemic cardiovascular diseases, particularly AMI [20]. A reliable method of identifying early stages of AMI is needed to ensure that patients receive the best and most appropriate treatment, as well as a better prognosis. Although the measurement of traditionally available biomarkers associated with myocardial injury, such as CK-MB and cTnI/T, has significantly improved the rates and speed of AMI diagnosis, these markers are not sufficiently specific. Interestingly, AMI is characterized by a heterogeneous genetic profile, suggesting that AMI development and occurrence may rely heavily on the expression of various genes [21]. Thus, further exploration of AMI-related genetic markers may provide opportunities for the creation of more effective preventive, diagnostic and therapeutic methods for treating AMI.
A number of recent studies have shown that the occurrence of CAD results from the interaction of multiple factors, such as alterations in blood lipid levels, an unhealthy lifestyle, environmental factors and genetic background [22, 23]. As the pathological basis of CAD, atherosclerosis results from abnormal lipid metabolism and chronic inflammation [24, 25]. According to a recent study, inflammatory or immune-related genes such as intercellular adhesion molecule 1 (ICAM1), transmembrane immune signaling adaptor TYROBP (TYROBP), integrin subunit alpha M (ITGAM) and cathelicidin antimicrobial peptide (CAMP) are strongly associated with CAD [26]. Similarly, immune- and inflammation-related genes and biological processes have been reported to play crucial roles in cardiac injury and repair, and together with the activation of innate and adaptive immune responses, have been suggested to be the hallmarks of myocardial infarction (MI). As reported in previous studies, the Janus kinase/signal transducer and activator of transcription (JAK/STAT) signal pathway is required for the development of atherosclerosis [27–29]. Yang et al. reported that ruxolitinib, a JAK2-specific inhibitor, reduces the size of atherosclerotic plaques by inhibiting the JAK2/STAT3/SOCS3 signal pathway [30]. As shown in the study by Zhang et al. oncostatin M receptor β (OSMR-β) deficiency effectively delays the development of atherosclerosis and improves the stability of vulnerable plaques by suppressing the JAK2/STAT3 signal pathway, thereby reducing the incidence of AMI and ischemic stroke [31]. Moreover, Desai HR et al. also described a crucial role for JAK2 in the pathogenesis of obesity-related inflammatory reactions and insulin resistance, and JAK2 deficiency reduces inflammation in the liver and visceral adipose tissue in response to metabolic stress, increases insulin sensitivity and attenuates insulin resistance [32]. Geng et al. suggested that fibronectin type III domain containing 5 (FNDC5) alleviates obesity-induced cardiac hypertrophy by inhibiting JAK2/STAT3-related cardiac inflammation and oxidative stress [33]. Similarly, in the current study, we noticed that the turquoise module was strongly correlated with BMI (r2 = 0.33, P = 9e-04). Meanwhile, as a key gene in the module, the expression levels of JAK2 were significantly higher in patients suffering from AMI than in control subjects, however, whether JAK2 could affect the pathogenesis of AMI by mediating insulin resistance and obesity remains unclear, and further experiments are needed to clarify this mechanism.
Vascular inflammation promotes the occurrence and development of atherosclerosis by accelerating the senescence of vascular endothelial cells. CDC42, as a member of the Rho GTPase family, plays a key role in response to pathological and physiological stimulation [34, 35]. Takashi K. Ito et al. revealed that the CDC42 pathway contributes significantly to chronic inflammation related to endothelial cell senescence, and endothelial-specific deletion of CDC42 markedly relieves the endothelial inflammatory response and the progression of atherosclerosis in atherosclerotic mice [36]. Raut SK et al. revealed that the miR-30c-induced increase in CDC42 levels promotes diabetes-related myocardial injury and hypertrophy [37]. Inhibition of CDC42 expression effectively alleviates myocardial fibrosis and hypertrophy in patients with salt-sensitive hypertension [38]. Moreover, Liu et al. revealed that CDC42 levels are noticeably increased in the myocardium near areas of myocardial infarction, and inhibition of the expression and activity of the CDC42 protein effectively reduces myocardial fibrosis after myocardial infarction in mice [39]. Recent studies have identified a crucial role for CDC42 in the progression of diabetes and diabetes-associated diseases, such as insulin resistance and diabetic nephropathy [35]. Similarly, in the current study, we also noticed significantly higher CDC42 expression in patients suffering from AMI than in control subjects. However, additional studies are needed to confirm whether CDC42 affects the pathogenesis of AMI by mediating insulin resistance, diabetes, obesity and other processes.
Recently, lncRNAs have been repeatedly reported to be crucial modulators of the genome regulatory network and exert a significant effect on disease development [40, 41]. Transcriptome studies have noted that lncRNA expression patterns are highly cell- and tissue-specific, indicating that the disease prognosis may be predicted by assessing these patterns [42]. Previous research has described the involvement of lncRNAs in fundamental biological phenomena, including gene transcription, RNA processing, chromatin modification, gene regulation and posttranscriptional gene regulation [43]. Several meaningful lncRNAs that may exert regulatory effects on the progression of cardiovascular diseases (CVDs), such as cardiac hypertrophy, myocardial infarction and cardiovascular ageing, have been characterized [44, 45]. Zangrando et al. found that up to 30 lncRNAs are differentially expressed in AMI mouse models exhibiting left ventricular remodelling [46]. Another compelling study based on a microarray analysis identified 545 deregulated lncRNAs involved in myocardial fibrosis induced after MI [47]. Recently, lncRNA-miRNA-mRNA ceRNA networks have been utilized to explore the functional roles of lncRNAs in AMI and were successful in identifying key lncRNAs involved in AMI [48–50], further emphasizing the potential of lncRNAs as biomarkers for the early diagnosis of AMI. However, the exact lncRNAs that hold this potential remain to be clarified.
Previous research suggested that the lncRNA XIST (X-inactive specific transcript, encoded by the XIST gene) is the cornerstone of mammalian X inactivation [51]. Based on accumulating evidence, the lncRNA XIST is important in genome maintenance, cell differentiation and proliferation [52, 53]. Liang et al. found that the downregulation of the lncRNA XIST and miR-7a-5P attenuate LPS-induced myocardial apoptosis in a mouse model of sepsis [54]. Similarly, Wang et al. described the involvement of the lncRNA XIST/miR-150-5p/c-Fos axis in LPS-induced myocardial injury, and the knockdown of the lncRNA XIST alleviates LPS-induced myocardial injury [55]. Peng et al. also found that the lncRNA XIST attenuates hypoxia-induced H9c2 cardiomyocyte injury by targeting the miR-122-5p/FOXP2 axis [56]. Furthermore, Zhang et al. proved that the silencing of the lncRNA XIST alleviates myocardial cell apoptosis in rats with AMI by targeting miR-449 [57]. According to Zhou et al., the lncRNA XIST is overexpressed in myocardial cells after MI and promotes MI by targeting miR-130a-3p [58]. In addition, Lin et al. also observed the overexpression of the lncRNA XIST in the infarct area and showed that the lncRNA XIST accelerated myocardial apoptosis after MI by targeting miR-101A-3p to upregulate FOS and apoptosis-related protein expression in a mouse model of MI [59]. Furthermore, Chen et al. found that lncRNA XIST can activate JAK2/STAT3 signal pathway by mediating mir-494/CDK6 regulatory axis, so as to promote the progression of esophageal cancer [60]. Zheng et al. found that silencing lncRNA XIST can effectively reduce the expression of JAK2 by up-regulating Mir-337, thereby inhibiting the proliferation and migration of gastric cancer cells [61]. Although the above evidences suggest that JAK2, CDC42 and lncRNA XIST are closely related to AMI, and lncRNA XIST can target and regulate the expression of JAK2. However, the aforementioned studies on the JAK2, CDC42 and lncRNA XIST were all based on animal or cell models, and it is unclear whether lncRNA XIST is involved in AMI by regulating the expression of JAK2 and CDC42. In order to illustrate this mechanism, our constructed a lncRNA-miRNA-mRNA triple network contained one lncRNA XIST, 21 miRNAs and 3 mRNAs (JAK2, CDC42 and CHUK). We noticed that the expression of the JAK2 and CDC42 genes and the lncRNA XIST were all noticeably increased in patients with AMI compared to healthy subjects, and Pearson correlation analysis confirmed that the expression levels of JAK2 and CDC42 were positively correlated with the lncRNA XIST levels. Results from the ROC curve analysis further proved that the diagnostic efficacy of the lncRNA XIST was significantly better than that of JAK2 and CDC42. Thus, we suggest that the lncRNA XIST may participate in the occurrence of AMI by targeting the expression of JAK2 and CDC42, and lncRNA XIST may function as a reliable biomarker for the diagnosis of AMI.
This research has several limitations. First, the validation cohort included in the current study was recruited from only a single center and included small sample sizes. We did not clearly determine if variabilities exist variabilities among individuals from various regions and ethnicities. Therefore, the validity of the results in the current study must be further tested in multicenter and larger samples. Second, the miRNAs that participated in the lncRNA-miRNA-mRNA triple network not been verified in our validation samples. Follow-up experiments are needed to identify specific miRNAs that may be targeted by the lncRNA XIST to regulate JAK2 and CDC42 expression. Last, the specific mechanism by which the lncRNA-miRNA-mRNA network regulates the pathogenesis of AMI has not been verified in vivo and in vitro.
In conclusion, based on the ceRNA hypothesis, a triple regulatory network consisting of a lncRNA-miRNAs-mRNAs was constructed to explore its biological functions in AMI. We achieved this goal by analysing the gene expression profile of patients with AMI (GSE34198) using the WGCNA method. JAK2, CDC42 and CHUK were identified as hub genes based on their combined GS and degree values. The identified regulatory network contained one lncRNA XIST, 21 miRNAs and 3 mRNAs (JAK2, CDC42 and CHUK). We further identified the primary regulatory factors in this network by performing RT–qPCR combined with a ROC curve analysis and confirmed that the lncRNA XIST was involved in AMI, likely by modulating the expression of JAK2 and CDC42, and lncRNA XIST may function as a reliable biomarker for the diagnosis of AMI.
Materials and Methods
CAD microarray datasets
Gene expression data were extracted from the CAD dataset GSE34198 (including 49 AMI and 48 normal samples) in the public database Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo, which is hosted on the GPL6102 Illumina human-6 v2.0 expression BeadChip platform). Gene expression profiles were normalized using the normalize Between Arrays function in the limma package [62]. Probes that detected more than one gene were excluded from this study. The average expression level of the same gene detected with multiple different probes was calculated as the true expression level of the gene.
Construction of the weighted gene co-expression network
A critical tool in the study of systems biology is WGCNA, which constructs a gene expression data profile-based scale-free network [63]. The reliability of the constructed scale-free network is ensured by removing outlying samples. A standard-scale free network was constructed to approximate the appropriate soft threshold power (soft power = 10) before the power function was used to calculate adjacency values among all differentially expressed genes. The adjacency values were converted into a topological overlap matrix (TOM), and the corresponding dissimilarity (1-TOM) values were also derived. The dynamic tree cut method was used to identify modules by hierarchically clustering genes with the 1-TOM as the distance measure, a minimum size cut-off of 100 and a deep split value of 2 for the resulting dendrogram. In addition, the module preservation function in the WGCNA package allowed us to determine the degree of module preservation and quality statistics [64]. These methods allowed us to verify the conservation of the selected modules.
Preservation analysis of five network modules
Based on published analytical methods [65], the degree of conservativeness of 10 modules was assessed using a composite preservation statistics method based on the WGCNA R package modulePreservation function. Intramodular connectivity metrics and module density in each module were calculated using the Z-summary statistic. In the corresponding network, Zdensity (function 1) was used to calculate the 4 density preservation statistics, Zconnectivity (function 2) was used to calculate the 3 connectivity-based statistics, and the combined intramodular connectivity metrics and module density were measured using Zsummary (function 3) and defined as follows: Z density = median (ZmeanCor, ZmeanAdj, ZpropVarExpl, ZmeanKME) (function 1); Zconnectivity = median (Zcor.kIM, Zcor.kME, Zcor.cor) (function 2); and Zsummary = (Zdensity + Zconnectivity)/2 (function 3). In addition, the module was not determined to be preserved if the Z summary < 2; modules were weakly to moderately preserved if the 2 < Z summary < 10; and modules were highly preserved if the Z summary > 10. Z statistics strongly depended on the size of the modules. Therefore, the medianRank for preservation analysis was used to assess preservation statistics between modules of various sizes. This analysis revealed that preservation statistics were more favorable in modules with a lower median rank in contrast to those with a higher median rank.
Identification of the module of interest and functional annotation
The relationships between modules and clinical parameters were evaluated using Pearson’s correlation analysis to discern modules of biological significance. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses of genes in biologically significant modules were performed using the Database for Annotation, Visualization and Integrated Discovery (DAVID) online tool (version 6.8; https://david.ncifcrf.gov/). The threshold was set to P < 0.05.
Hub gene analysis
The association between module eigengenes (Mes) and the gene expression profile was determined as the degree of module membership (MM). The absolute values of correlations between genes and external traits were defined as the degree of gene significance (GS). A further analysis of modules with increased GS and MM values was performed to determine biological functions [66]. Based on the meaningful modules, a protein–protein interaction (PPI) network was constructed using the Search Tool for the Retrieval of Interacting Genes (STRING) online tool (version 11.0; http://www.string-db.org) [67]. Cytoscape software was then used to visualize this PPI network [68]. Cytohubba, one of the most commonly used plug-ins in Cytoscape software, is often used to determine key genes in PPI networks [69]. This program is able to explore important nodes across various biological networks using 11 different methods, with the method of degree known to exhibit better performance. As reported in previous studies [65], genes with the top 50 GS values that also have degree values ≥ 5 were selected as key genes. Hub genes that required further research were also identified using information available from GO and KEGG analyses.
The prediction of miRNAs and lincRNAs and ceRNA network construction
After GO, KEGG and PPI analyses, meaningful hub genes were used to predict lncRNAs that might regulate selected hub genes using the starBase database [70]. After obtaining the specific lncRNAs that may regulate the hub genes, lncRNA-miRNA interactions and miRNA-mRNA interactions were subsequently predicted based on the starBase database. A Venn diagram was constructed to identify several common miRNAs that potentially regulate both lncRNAs and mRNAs, and then the lncRNA-miRNA-mRNA ceRNA network was established and visualized using Cytoscape software.
Study population
Five hundred inpatients (260 patients with AMI and 240 controls) with chest pain were recruited from the Cardiovascular Department of Hunan Provincial People's Hospital. All patients diagnosed with AMI underwent percutaneous coronary intervention (PCI) within 12 hours of the onset of chest pain. AMI was diagnosed based on 2018 guidelines for the diagnosis of patients with AMI [71] as follows: an electrocardiogram showing new ischemic changes, echocardiogram showing new localized ventricular wall dysplasia or a loss of viable myocardium, and cardiac biomarker (cTnT) levels above the upper limit of the reference value of the 99 percentile. Age- and sex-matched healthy subjects with no history of cardiovascular or other systemic diseases based on a physical examination, blood and electrocardiogram (ECG) tests were also recruited for this study. In addition, coronary angiography of all healthy subjects showed no significant abnormalities. The following exclusion criteria were applied: (i) active inflammation; (ii) patients who received thrombolytic therapy and those who had other underlying heart diseases (such as severe valvular abnormalities, cardiomyopathy, or congenital heart disease); and (iii) patients who had hepatic and/or renal dysfunction, tumours and autoimmune diseases. Baseline clinical characteristics, angiography results and laboratory test results were determined for all patients. Blood samples were collected from patients with AMI upon admission prior to the administration of any antiplatelet or anticoagulants, as well as before PCI treatment and within a few hours after the onset of chest pain. The collection of these samples was timed appropriately to capture the presence of any potential early diagnostic biomarkers. Study protocols were developed according to guidelines from the Ethics Committee of Hunan Provincial People's Hospital and the 2008 revision of the Declaration of Helsinki of 1975 (http://www.wma.net/en/30publications/10policies/b3/). All subjects provided written informed consent.
RNA isolation and RT- quantitative PCR (qPCR)
TRIzol reagent (Invitrogen, CA, USA) was used to extract total RNA from all samples. The cDNA templates were then produced from 1 μg of RNA with the TransScript-Uni cDNA Synthesis SuperMix kit (AU311-03, Transgen, Beijing, China) with the GeneAmp PCR System 9700 HT Fast (Applied Biosystems, USA) for 60 min at 37° C. Real-time PCR was conducted with a LightCycler 480 II Real-time PCR instrument (Roche, Switzerland) using TransStart Top Green qPCR SuperMix (AQ131-03, Transgen, Beijing, China). At the end of amplification, the product quality was verified by constructing a melting curve. Samples were analysed in triplicate and included no-template controls. The quantitative RT-PCR analysis included 1 lncRNA X-inactive specific transcript (XIST) and 3 genes such as Janus kinase 2 (JAK2), cell division cycle 42 (CDC42) and component of inhibitor of nuclear factor kappa B kinase complex (CHUK). All gene expression levels were normalized to GAPDH. The proprietary qPCR primers used in the experiment are shown in Supplementary Table 1, and which were designed and validated by Songon Biotech (Songon Biotech, Shanghai, China). Relative gene expression was assessed with the 2−ΔΔCt method.
Statistical analyses
SPSS (Version 22.0) software was used to analyse all data collected in the current study. An independent sample t test was used to evaluate continuous data (means ± SD) that were normally distributed between control subjects and patients with AMI. Triglyceride levels that were not normally distributed were reported as medians and interquartile ranges and were evaluated using the Wilcoxon-Mann–Whitney test. Data such as the sex ratio and numbers of smokers and drinkers were analysed using the chi-square test. The nonparametric ROC curve analysis was performed using MedCalc software (MedCalc Software, Mariakerke, Belgium, version 19.7.4), which produced an empirical ROC curve and nonparametric estimate of the area under the empirical ROC curve along with its 95% CI, based on the method developed by Hanley et al. [72]. The difference between the areas under the two empirical ROC curves was assessed using the Z-test [73]. Bioinformatics analysis and Pearson’s correlation analysis were performed using R software (version 4.1.0).
Abbreviations
WGCNA: weighted gene co-expression network analysis;
XIST: X inactive specific transcript;
CAD: coronary artery disease;
GS: gene significance;
AMI: acute myocardial infarction;
DAVID: Database for Annotation, Visualization and Integrated Discovery;
T2DM: Type 2 diabetes mellitus;
GO: Gene Ontology;
HDL-C: High-density lipoprotein cholesterol;
IS: Ischemic stroke;
KEGG: Kyoto Encyclopedia of Genes and genomes;
LDL-C: Low-density lipoprotein cholesterol;
MCODE: Molecular Complex Detection;
Apo: Apolipoprotein;
PPI: Protein-protein interaction;
GEO: Gene Expression Omnibus;
BMI: Body mass index;
TG: Triglyceride;
RT-qPCR: Quantitative real time polymerase chain reaction;
TC: Total cholesterol;
PCI: percutaneous coronary intervention;
ncRNAs: non-coding RNAs;
lncRNAs: long ncRNAs;
lincRNAs: long intergenic ncRNAs;
WISPER: wisp2 super enhancer related RNA;
TOM: topological overlap matrix;
Mes: module eigengenes;
MM: module membership;
STRING: Search Tool for the Retrieval of Interacting Genes;
CK-MB: creatine kinase-MB;
cTns: cardiac troponins;
CVD: cardiovascular diseases;
MM: module membership;
OSMR-β: oncostatin M receptor β;
FNDC5: Fibronectin type III domain containing 5;
TLR2: toll-like receptor 2.
Author Contributions
P.-F.Z., P.-L. and H.W.P. conceived the study, participated in the design, performed the statistical analyses, and drafted the manuscript. H.W.P. conceived the study, participated in the design and helped to draft the manuscript. P.-F.Z. carried out the epidemiological survey and collected the samples. L.-Z.C. performed the statistical analyses. All authors read and approved the final manuscript.
Acknowledgments
We thank all the participants of this study.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Funding
This study was supported by the Key Research and Development Program of Hunan Provincial Science and Technology Department (No: 2019SK2021). There was no role of the funding body in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
References
-
1.
Kishi S, Magalhães TA, Cerci RJ, Matheson MB, Vavere A, Tanami Y, Kitslaar PH, George RT, Brinker J, Miller JM, Clouse ME, Lemos PA, Niinuma H, et al. Total coronary atherosclerotic plaque burden assessment by CT angiography for detecting obstructive coronary artery disease associated with myocardial perfusion abnormalities. J Cardiovasc Comput Tomogr. 2016; 10:121–7. https://doi.org/10.1016/j.jcct.2016.01.005 [PubMed]
-
2.
Wong ND. Epidemiological studies of CHD and the evolution of preventive cardiology. Nat Rev Cardiol. 2014; 11:276–89. https://doi.org/10.1038/nrcardio.2014.26 [PubMed]
-
3.
Xu H, Li W, Yang J, Wiviott SD, Sabatine MS, Peterson ED, Xian Y, Roe MT, Zhao W, Wang Y, Tang X, Jia X, Wu Y, et al, and CAMI Registry study group. The China Acute Myocardial Infarction (CAMI) Registry: A national long-term registry-research-education integrated platform for exploring acute myocardial infarction in China. Am Heart J. 2016; 175:193–201.e3. https://doi.org/10.1016/j.ahj.2015.04.014 [PubMed]
-
4.
Chang J, Deng Q, Guo M, Ezzati M, Baumgartner J, Bixby H, Chan Q, Zhao D, Lu F, Hu P, Su Y, Sun J, Long Y, Liu J. Trends and Inequalities in the Incidence of Acute Myocardial Infarction among Beijing Townships, 2007-2018. Int J Environ Res Public Health. 2021; 18:12276. https://doi.org/10.3390/ijerph182312276 [PubMed]
-
5.
Khera AV, Kathiresan S. Genetics of coronary artery disease: discovery, biology and clinical translation. Nat Rev Genet. 2017; 18:331–44. https://doi.org/10.1038/nrg.2016.160 [PubMed]
-
6.
Haemmig S, Simion V, Yang D, Deng Y, Feinberg MW. Long noncoding RNAs in cardiovascular disease, diagnosis, and therapy. Curr Opin Cardiol. 2017; 32:776–83. https://doi.org/10.1097/HCO.0000000000000454 [PubMed]
-
7.
Osmak G, Baulina N, Koshkin P, Favorova O. Collapsing the list of myocardial infarction-related differentially expressed genes into a diagnostic signature. J Transl Med. 2020; 18:231. https://doi.org/10.1186/s12967-020-02400-1 [PubMed]
-
8.
Aryal B, Rotllan N, Fernández-Hernando C. Noncoding RNAs and atherosclerosis. Curr Atheroscler Rep. 2014; 16:407. https://doi.org/10.1007/s11883-014-0407-3 [PubMed]
-
9.
Fu XD. Non-coding RNA: a new frontier in regulatory biology. Natl Sci Rev. 2014; 1:190–204. https://doi.org/10.1093/nsr/nwu008 [PubMed]
-
10.
Boon RA, Jaé N, Holdt L, Dimmeler S. Long Noncoding RNAs: From Clinical Genetics to Therapeutic Targets? J Am Coll Cardiol. 2016; 67:1214–26. https://doi.org/10.1016/j.jacc.2015.12.051 [PubMed]
-
11.
Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011; 12:861–74. https://doi.org/10.1038/nrg3074 [PubMed]
-
12.
Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011; 43:904–14. https://doi.org/10.1016/j.molcel.2011.08.018 [PubMed]
-
13.
Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet. 2016; 17:47–62. https://doi.org/10.1038/nrg.2015.10 [PubMed]
-
14.
Correia CCM, Rodrigues LF, de Avila Pelozin BR, Oliveira EM, Fernandes T. Long Non-Coding RNAs in Cardiovascular Diseases: Potential Function as Biomarkers and Therapeutic Targets of Exercise Training. Noncoding RNA. 2021; 7:65. https://doi.org/10.3390/ncrna7040065 [PubMed]
-
15.
Wang K, Long B, Zhou LY, Liu F, Zhou QY, Liu CY, Fan YY, Li PF. CARL lncRNA inhibits anoxia-induced mitochondrial fission and apoptosis in cardiomyocytes by impairing miR-539-dependent PHB2 downregulation. Nat Commun. 2014; 5:3596. https://doi.org/10.1038/ncomms4596 [PubMed]
-
16.
Micheletti R, Plaisance I, Abraham BJ, Sarre A, Ting CC, Alexanian M, Maric D, Maison D, Nemir M, Young RA, Schroen B, González A, Ounzain S, Pedrazzini T. The long noncoding RNA Wisper controls cardiac fibrosis and remodeling. Sci Transl Med. 2017; 9:eaai9118. https://doi.org/10.1126/scitranslmed.aai9118 [PubMed]
-
17.
Huang Y. The novel regulatory role of lncRNA-miRNA-mRNA axis in cardiovascular diseases. J Cell Mol Med. 2018; 22:5768–75. https://doi.org/10.1111/jcmm.13866 [PubMed]
-
18.
Ratti M, Lampis A, Ghidini M, Salati M, Mirchev MB, Valeri N, Hahne JC. MicroRNAs (miRNAs) and Long Non-Coding RNAs (lncRNAs) as New Tools for Cancer Therapy: First Steps from Bench to Bedside. Target Oncol. 2020; 15:261–78. https://doi.org/10.1007/s11523-020-00717-x [PubMed]
-
19.
Miao L, Yin RX, Pan SL, Yang S, Yang DZ, Lin WX. Weighted Gene Co-Expression Network Analysis Identifies Specific Modules and Hub Genes Related to Hyperlipidemia. Cell Physiol Biochem. 2018; 48:1151–63. https://doi.org/10.1159/000491982 [PubMed]
-
20.
Chen ZH, Zhang M, Li YC, Zhao ZP, Zhang X, Huang ZJ, Li C, Wang LM. [Study on relationship between prevalence or co-prevalence of risk factors for cardiovascular disease and blood pressure level in adults in China]. Zhonghua Liu Xing Bing Xue Za Zhi. 2018; 39:640–5. https://doi.org/10.3760/cma.j.issn.0254-6450.2018.05.019 [PubMed]
-
21.
Xiao SJ, Zhou YF, Wu Q, Ma WR, Chen ML, Pan DF. Uncovering the differentially expressed genes and pathways involved in the progression of stable coronary artery disease to acute myocardial infarction using bioinformatics analysis. Eur Rev Med Pharmacol Sci. 2021; 25:301–12. https://doi.org/10.26355/eurrev_202101_24396 [PubMed]
-
22.
Zhang QH, Yin RX, Chen WX, Cao XL, Wu JZ. TRIB1 and TRPS1 variants, G × G and G × E interactions on serum lipid levels, the risk of coronary heart disease and ischemic stroke. Sci Rep. 2019; 9:2376. https://doi.org/10.1038/s41598-019-38765-7 [PubMed]
-
23.
Zheng PF, Yin RX, Deng GX, Guan YZ, Wei BL, Liu CX. Association between the XKR6 rs7819412 SNP and serum lipid levels and the risk of coronary artery disease and ischemic stroke. BMC Cardiovasc Disord. 2019; 19:202. https://doi.org/10.1186/s12872-019-1179-z [PubMed]
-
24.
Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005; 111:3481–8. https://doi.org/10.1161/CIRCULATIONAHA.105.537878 [PubMed]
-
25.
Li B, Li W, Li X, Zhou H. Inflammation: A Novel Therapeutic Target/Direction in Atherosclerosis. Curr Pharm Des. 2017; 23:1216–27. https://doi.org/10.2174/1381612822666161230142931 [PubMed]
-
26.
Zheng PF, Chen LZ, Guan YZ, Liu P. Weighted gene co-expression network analysis identifies specific modules and hub genes related to coronary artery disease. Sci Rep. 2021; 11:6711. https://doi.org/10.1038/s41598-021-86207-0 [PubMed]
-
27.
Liao M, Xu J, Clair AJ, Ehrman B, Graham LM, Eagleton MJ. Local and systemic alterations in signal transducers and activators of transcription (STAT) associated with human abdominal aortic aneurysms. J Surg Res. 2012; 176:321–8. https://doi.org/10.1016/j.jss.2011.05.041 [PubMed]
-
28.
Manea A, Tanase LI, Raicu M, Simionescu M. Jak/STAT signaling pathway regulates nox1 and nox4-based NADPH oxidase in human aortic smooth muscle cells. Arterioscler Thromb Vasc Biol. 2010; 30:105–12. https://doi.org/10.1161/ATVBAHA.109.193896 [PubMed]
-
29.
Mo ZC, Xiao J, Liu XH, Hu YW, Li XX, Yi GH, Wang Z, Tang YL, Liao DF, Tang CK. AOPPs inhibits cholesterol efflux by down-regulating ABCA1 expression in a JAK/STAT signaling pathway-dependent manner. J Atheroscler Thromb. 2011; 18:796–807. https://doi.org/10.5551/jat.6569 [PubMed]
-
30.
Yang X, Jia J, Yu Z, Duanmu Z, He H, Chen S, Qu C. Inhibition of JAK2/STAT3/SOCS3 signaling attenuates atherosclerosis in rabbit. BMC Cardiovasc Disord. 2020; 20:133. https://doi.org/10.1186/s12872-020-01391-7 [PubMed]
-
31.
Zhang X, Li J, Qin JJ, Cheng WL, Zhu X, Gong FH, She Z, Huang Z, Xia H, Li H. Oncostatin M receptor β deficiency attenuates atherogenesis by inhibiting JAK2/STAT3 signaling in macrophages. J Lipid Res. 2017; 58:895–906. https://doi.org/10.1194/jlr.M074112 [PubMed]
-
32.
Desai HR, Sivasubramaniyam T, Revelo XS, Schroer SA, Luk CT, Rikkala PR, Metherel AH, Dodington DW, Park YJ, Kim MJ, Rapps JA, Besla R, Robbins CS, et al. Macrophage JAK2 deficiency protects against high-fat diet-induced inflammation. Sci Rep. 2017; 7:7653. https://doi.org/10.1038/s41598-017-07923-0 [PubMed]
-
33.
Geng Z, Fan WY, Zhou B, Ye C, Tong Y, Zhou YB, Xiong XQ. FNDC5 attenuates obesity-induced cardiac hypertrophy by inactivating JAK2/STAT3-associated inflammation and oxidative stress. J Transl Med. 2019; 17:107. https://doi.org/10.1186/s12967-019-1857-8 [PubMed]
-
34.
Zhang Y, Li J, Lai XN, Jiao XQ, Xiong JP, Xiong LX. Focus on Cdc42 in Breast Cancer: New Insights, Target Therapy Development and Non-Coding RNAs. Cells. 2019; 8:146. https://doi.org/10.3390/cells8020146 [PubMed]
-
35.
Huang QY, Lai XN, Qian XL, Lv LC, Li J, Duan J, Xiao XH, Xiong LX. Cdc42: A Novel Regulator of Insulin Secretion and Diabetes-Associated Diseases. Int J Mol Sci. 2019; 20:179. https://doi.org/10.3390/ijms20010179 [PubMed]
-
36.
Ito TK, Yokoyama M, Yoshida Y, Nojima A, Kassai H, Oishi K, Okada S, Kinoshita D, Kobayashi Y, Fruttiger M, Aiba A, Minamino T. A crucial role for CDC42 in senescence-associated inflammation and atherosclerosis. PLoS One. 2014; 9:e102186. https://doi.org/10.1371/journal.pone.0102186 [PubMed]
-
37.
Raut SK, Kumar A, Singh GB, Nahar U, Sharma V, Mittal A, Sharma R, Khullar M. miR-30c Mediates Upregulation of Cdc42 and Pak1 in Diabetic Cardiomyopathy. Cardiovasc Ther. 2015; 33:89–97. https://doi.org/10.1111/1755-5922.12113 [PubMed]
-
38.
Ye H, Ling S, Castillo AC, Thomas B, Long B, Qian J, Perez-Polo JR, Ye Y, Chen X, Birnbaum Y. Nebivolol induces distinct changes in profibrosis microRNA expression compared with atenolol, in salt-sensitive hypertensive rats. Hypertension. 2013; 61:1008–13. https://doi.org/10.1161/HYPERTENSIONAHA.111.00892 [PubMed]
-
39.
Liu D, Tian X, Liu Y, Song H, Cheng X, Zhang X, Yan C, Han Y. CREG ameliorates the phenotypic switching of cardiac fibroblasts after myocardial infarction via modulation of CDC42. Cell Death Dis. 2021; 12:355. https://doi.org/10.1038/s41419-021-03623-w [PubMed]
-
40.
Li X, Wu Z, Fu X, Han W. lncRNAs: insights into their function and mechanics in underlying disorders. Mutat Res Rev Mutat Res. 2014; 762:1–21. https://doi.org/10.1016/j.mrrev.2014.04.002 [PubMed]
-
41.
Li M, Wang YF, Yang XC, Xu L, Li WM, Xia K, Zhang DP, Wu RN, Gan T. Circulating Long Noncoding RNA LIPCAR Acts as a Novel Biomarker in Patients with ST-Segment Elevation Myocardial Infarction. Med Sci Monit. 2018; 24:5064–70. https://doi.org/10.12659/MSM.909348 [PubMed]
-
42.
Iyer MK, Niknafs YS, Malik R, Singhal U, Sahu A, Hosono Y, Barrette TR, Prensner JR, Evans JR, Zhao S, Poliakov A, Cao X, Dhanasekaran SM, et al. The landscape of long noncoding RNAs in the human transcriptome. Nat Genet. 2015; 47:199–208. https://doi.org/10.1038/ng.3192 [PubMed]
-
43.
Ørom UA, Derrien T, Beringer M, Gumireddy K, Gardini A, Bussotti G, Lai F, Zytnicki M, Notredame C, Huang Q, Guigo R, Shiekhattar R. Long noncoding RNAs with enhancer-like function in human cells. Cell. 2010; 143:46–58. https://doi.org/10.1016/j.cell.2010.09.001 [PubMed]
-
44.
Ounzain S, Micheletti R, Beckmann T, Schroen B, Alexanian M, Pezzuto I, Crippa S, Nemir M, Sarre A, Johnson R, Dauvillier J, Burdet F, Ibberson M, et al. Genome-wide profiling of the cardiac transcriptome after myocardial infarction identifies novel heart-specific long non-coding RNAs. Eur Heart J. 2015; 36:353–68a. https://doi.org/10.1093/eurheartj/ehu180 [PubMed]
-
45.
Wang K, Liu F, Zhou LY, Long B, Yuan SM, Wang Y, Liu CY, Sun T, Zhang XJ, Li PF. The long noncoding RNA CHRF regulates cardiac hypertrophy by targeting miR-489. Circ Res. 2014; 114:1377–88. https://doi.org/10.1161/CIRCRESAHA.114.302476 [PubMed]
-
46.
Zangrando J, Zhang L, Vausort M, Maskali F, Marie PY, Wagner DR, Devaux Y. Identification of candidate long non-coding RNAs in response to myocardial infarction. BMC Genomics. 2014; 15:460. https://doi.org/10.1186/1471-2164-15-460 [PubMed]
-
47.
Qu X, Song X, Yuan W, Shu Y, Wang Y, Zhao X, Gao M, Lu R, Luo S, Zhao W, Zhang Y, Sun L, Lu Y. Expression signature of lncRNAs and their potential roles in cardiac fibrosis of post-infarct mice. Biosci Rep. 2016; 36:e00337. https://doi.org/10.1042/BSR20150278 [PubMed]
-
48.
Sun C, Jiang H, Sun Z, Gui Y, Xia H. Identification of long non-coding RNAs biomarkers for early diagnosis of myocardial infarction from the dysregulated coding-non-coding co-expression network. Oncotarget. 2016; 7:73541–51. https://doi.org/10.18632/oncotarget.11999 [PubMed]
-
49.
Wang P, Fu H, Cui J, Chen X. Differential lncRNA-mRNA co-expression network analysis revealing the potential regulatory roles of lncRNAs in myocardial infarction. Mol Med Rep. 2016; 13:1195–203. https://doi.org/10.3892/mmr.2015.4669 [PubMed]
-
50.
Zhuo LA, Wen YT, Wang Y, Liang ZF, Wu G, Nong MD, Miao L. LncRNA SNHG8 is identified as a key regulator of acute myocardial infarction by RNA-seq analysis. Lipids Health Dis. 2019; 18:201. https://doi.org/10.1186/s12944-019-1142-0 [PubMed]
-
51.
Brown CJ, Ballabio A, Rupert JL, Lafreniere RG, Grompe M, Tonlorenzi R, Willard HF. A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature. 1991; 349:38–44. https://doi.org/10.1038/349038a0 [PubMed]
-
52.
Yildirim E, Kirby JE, Brown DE, Mercier FE, Sadreyev RI, Scadden DT, Lee JT. Xist RNA is a potent suppressor of hematologic cancer in mice. Cell. 2013; 152:727–42. https://doi.org/10.1016/j.cell.2013.01.034 [PubMed]
-
53.
Yao Y, Ma J, Xue Y, Wang P, Li Z, Liu J, Chen L, Xi Z, Teng H, Wang Z, Li Z, Liu Y. Knockdown of long non-coding RNA XIST exerts tumor-suppressive functions in human glioblastoma stem cells by up-regulating miR-152. Cancer Lett. 2015; 359:75–86. https://doi.org/10.1016/j.canlet.2014.12.051 [PubMed]
-
54.
Liang D, Jin Y, Lin M, Xia X, Chen X, Huang A. Down-regulation of Xist and Mir-7a-5p improves LPS-induced myocardial injury. Int J Med Sci. 2020; 17:2570–7. https://doi.org/10.7150/ijms.45408 [PubMed]
-
55.
Wang X, Li XL, Qin LJ. The lncRNA XIST/miR-150-5p/c-Fos axis regulates sepsis-induced myocardial injury via TXNIP-modulated pyroptosis. Lab Invest. 2021; 101:1118–29. https://doi.org/10.1038/s41374-021-00607-4 [PubMed]
-
56.
Peng H, Luo Y, Ying Y. lncRNA XIST attenuates hypoxia-induced H9c2 cardiomyocyte injury by targeting the miR-122-5p/FOXP2 axis. Mol Cell Probes. 2020; 50:101500. https://doi.org/10.1016/j.mcp.2019.101500 [PubMed]
-
57.
Zhang M, Liu HY, Han YL, Wang L, Zhai DD, Ma T, Zhang MJ, Liang CZ, Shen Y. Silence of lncRNA XIST represses myocardial cell apoptosis in rats with acute myocardial infarction through regulating miR-449. Eur Rev Med Pharmacol Sci. 2019; 23:8566–72. https://doi.org/10.26355/eurrev_201910_19172 [PubMed]
-
58.
Zhou T, Qin G, Yang L, Xiang D, Li S. LncRNA XIST regulates myocardial infarction by targeting miR-130a-3p. J Cell Physiol. 2019; 234:8659–67. https://doi.org/10.1002/jcp.26327 [PubMed]
-
59.
Lin B, Xu J, Wang F, Wang J, Zhao H, Feng D. LncRNA XIST promotes myocardial infarction by regulating FOS through targeting miR-101a-3p. Aging (Albany NY). 2020; 12:7232–47. https://doi.org/10.18632/aging.103072 [PubMed]
-
60.
Chen Z, Hu X, Wu Y, Cong L, He X, Lu J, Feng J, Liu D. Long non-coding RNA XIST promotes the development of esophageal cancer by sponging miR-494 to regulate CDK6 expression. Biomed Pharmacother. 2019; 109:2228–36. https://doi.org/10.1016/j.biopha.2018.11.049 [PubMed]
-
61.
Zheng W, Li J, Zhou X, Cui L, Wang Y. The lncRNA XIST promotes proliferation, migration and invasion of gastric cancer cells by targeting miR-337. Arab J Gastroenterol. 2020; 21:199–206. https://doi.org/10.1016/j.ajg.2020.07.010 [PubMed]
-
62.
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015; 43:e47. https://doi.org/10.1093/nar/gkv007 [PubMed]
-
63.
Horvath S, Dong J. Geometric interpretation of gene coexpression network analysis. PLoS Comput Biol. 2008; 4:e1000117. https://doi.org/10.1371/journal.pcbi.1000117 [PubMed]
-
64.
Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008; 9:559. https://doi.org/10.1186/1471-2105-9-559 [PubMed]
-
65.
Chen J, Zhao X, Cui L, He G, Wang X, Wang F, Duan S, He L, Li Q, Yu X, Zhang F, Xu M. Genetic regulatory subnetworks and key regulating genes in rat hippocampus perturbed by prenatal malnutrition: implications for major brain disorders. Aging (Albany NY). 2020; 12:8434–58. https://doi.org/10.18632/aging.103150 [PubMed]
-
66.
Fuller TF, Ghazalpour A, Aten JE, Drake TA, Lusis AJ, Horvath S. Weighted gene coexpression network analysis strategies applied to mouse weight. Mamm Genome. 2007; 18:463–72. https://doi.org/10.1007/s00335-007-9043-3 [PubMed]
-
67.
Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M, Bork P, Jensen LJ, von Mering C. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015; 43:D447–52. https://doi.org/10.1093/nar/gku1003 [PubMed]
-
68.
Smoot ME, Ono K, Ruscheinski J, Wang PL, Ideker T. Cytoscape 2.8: new features for data integration and network visualization. Bioinformatics. 2011; 27:431–2. https://doi.org/10.1093/bioinformatics/btq675 [PubMed]
-
69.
Chin CH, Chen SH, Wu HH, Ho CW, Ko MT, Lin CY. cytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst Biol. 2014 (Suppl 4); 8:S11. https://doi.org/10.1186/1752-0509-8-S4-S11 [PubMed]
-
70.
Li JH, Liu S, Zhou H, Qu LH, Yang JH. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014; 42:D92–7. https://doi.org/10.1093/nar/gkt1248 [PubMed]
-
71.
Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD, and Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). Circulation. 2018; 138:e618–51. https://doi.org/10.1161/CIR.0000000000000617 [PubMed]
-
72.
Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982; 143:29–36. https://doi.org/10.1148/radiology.143.1.7063747 [PubMed]
-
73.
Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983; 148:839–43. https://doi.org/10.1148/radiology.148.3.6878708 [PubMed]