* The list of ALFA Score Consortium members is provided in Editorial Note.
Abstract
The World Health Organization predicts that by 2050, 2.1 billion people worldwide will be over 60 years old, a drastic increase from only 1 billion in 2019. Considering these numbers, strategies to ensure an extended “healthspan” or healthy longevity are urgently needed.
The present study approaches the promotion of healthspan from an epigenetic perspective. Epigenetic phenomena are modifiable in response to an individual’s environmental exposures, and therefore link an individual’s environment to their gene expression pattern. Epigenetic studies demonstrate that aging is associated with decondensation of the chromatin, leading to an altered heterochromatin structure, which promotes the accumulation of errors.
In this review, we describe how aging impacts epigenetics and how nutrition and physical exercise can positively impact the aging process, from an epigenetic point of view. Canonical histones are replaced by histone variants, concomitant with an increase in histone post-translational modifications. A slight increase in DNA methylation at promoters has been observed, which represses transcription of previously active genes, in parallel with global genome hypomethylation. Aging is also associated with deregulation of gene expression - usually provided by non-coding RNAs - leading to both the repression of previously transcribed genes and to the transcription of previously repressed genes.
Age-associated epigenetic events are less common in individuals with a healthy lifestyle, including balanced nutrition, caloric restriction and physical exercise. Healthy aging is associated with more tightly condensed chromatin, fewer PTMs and greater regulation by ncRNAs.
Introduction
Aging is a complex time-dependent multifactorial biological process, involving a gradual decline in cognitive and physiological functions over time. This results in a reduced capacity to respond to stressors, which in turn increases morbidity and mortality [1–3]. Pathological phenotypes associated with aging include frailty (a condition associated with progressive physical and mental decline [4], chronic medical conditions, such as diabetes and cardiovascular disease [5, 6], visual impairment, for example age-related macular disease [7, 8], cancer [9], and neurodegenerative disorders, such as Alzheimer’s, Parkinson’s and Huntington’s diseases [10, 11], to name some of the most prevalent.
Nonetheless, and despite many studies on aging, this subject remains poorly understood [2, 12]. Over the last 7 decades, human lifespan has steeply increased, which was reflected in the global population – as a result, the population of individuals over age 60 has increased dramatically from 205 million in 1950 to 1 billion in 2019. According to a World Health Organization (WHO) prediction, by 2030, individuals over 60 years old will outnumber children younger than 10 years old, and by 2050 the over-60 population will number 2.1 billion [13]. On the one hand, this extended lifespan is a tribute to the technological advances achieved in the last century, in terms of health, medicine, sanitation, and education. On the other hand, such increase of aged populations sets pressure on societies to develop specialized policies and services for the elderly, to reduce the impact of this trend on our communities [13].
The decade of 2020–2030 was termed by the WHO as the “Decade of Healthy Aging”, with the target of sustainably extending healthspan [14]. WHO has defined “Healthy Aging” as the possibility for everyone to be and do what they value throughout their life. For older adults, this means remaining independent and capable of participating in their daily activities, even if affected by illness [15].
Strategies to improve healthy aging include lifestyle modification (to limit the effects of risk behaviors, namely tobacco consumption, and alcohol abuse), regenerative medicine and tissue/organ engineering, manipulation of genes and pathways associated with longevity, and pharmacological compounds to extend healthy lifespan [12, 16].
Epigenetic alterations and genomic instability are also potential targets for healthy aging interventions [17–19]. The importance of epigenetic alterations for healthy aging was suggested in 1995 by Herskind and colleagues, who reported on a large cohort of Danish twins, demonstrating that 25% of their longevity (26% for males and 23% for females) was related to DNA sequence [20]. The 75% unaccounted for was attributed to the modulation of age-associated genetic factors by non-heritable environmental influences such as diet habits, physical activity, tobacco consumption, as well as social interactions established and education [21, 22]. Notwithstanding, several studies were performed in both animal models and humans, that highlighted the relevance of particular genes such as insulin or insulin-like growth factor 1 (IGF-1), Forkhead box O 3 (FOXO3) and AMP-activated protein kinase (AMPK) [23, 24]. Also, these genes were shown to be drug targetable, therefore promoting healthspan [24].
During the last decades, several studies suggested a key role for epigenetics during the aging process, in contrast to the first hypotheses formulated, which attributed aging to the accumulation of mutations in the genome [25]. Recent studies developed by David Sinclair’s Lab further explored this topic, demonstrating that using a system that induces changes of the epigenome in mice accelerated approximately 50% the DNA methylation epigenetic clock [25, 26].
The present manuscript provides an overview of aging from the (epi)genetic perspective, and summarizes lifestyle strategies that can be adopted to potentiate healthier epigenetic modifications and consequentially slow down biological aging.
Epigenetics
Epigenetics can be defined as de novo or inherited reversible modifications of the genome, which can affect gene expression without altering the DNA sequence. Epigenetic alterations are mediated by multiple mechanisms, of which histone modifications, DNA methylation and changes in non-coding RNAs expression are the best studied [18, 27] and whose alterations with aging are schematized in Figure 1.
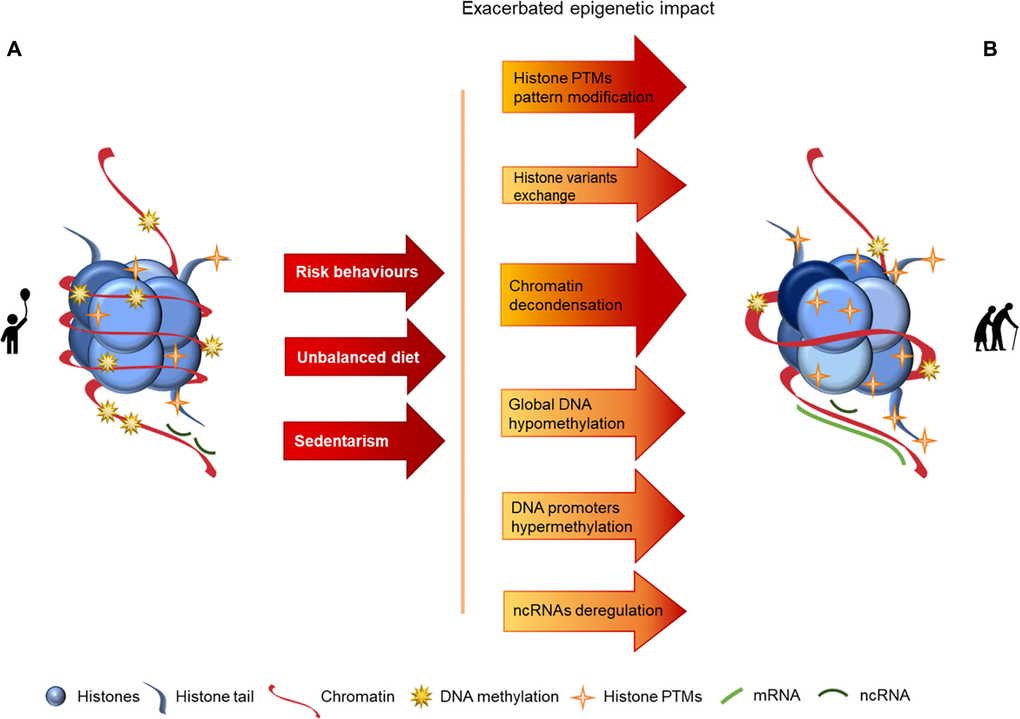
Figure 1. Representation of age-associated epigenetic changes. (A) Representation of a young individual chromatin: tight chromatin compactation, high levels of DNA methylation, few histone PTMs (particularly acetylation), canonical histones and balanced non-coding RNA regulation; (B) Representation of an old aged individual chromatin, we may observe a looser chromatin structure, lower levels of DNA methylation, higher levels of histones PTMs (particularly acetylation), histone variants presence, chromatin remodeling and disturbances in ncRNA regulation, what is reflected in an overall chromatin instability increase, when compared with the structure presented in A. The transition between the structures presented in A and B is represented by arrows. The arrows in red present the causes (here presented as a lifetime of risk behaviors (namely tobacco and alcohol consumption), unbalanced diet and sedentarism) whose effects are reflected by the arrows in orange/red, where darker colors represent more pronounced epigenetic alterations.
Eukaryotic DNA is organized into higher-order chromatin through nucleosomes, which are core particles composed of histone octamers. Each octamer is constituted by two of each core histone proteins (H2, H3A, H3B and H4), around which 147 base pairs of DNA are wrapped [28, 29]. The linker histone H1 is also found in proximity to this core, promoting its stability [30]. Nucleosome core particles are arranged on the DNA like “beads on a string”, with a distance of approximately 200 base pairs between “beads”. This open DNA conformation is termed “euchromatin”. Additionally, the tightly packed or closed conformation that DNA and nucleosome core particles adopt after condensation by higher-order structures was termed “heterochromatin”. Condensation of the DNA into heterochromatin makes it inaccessible and protects it from damage – a benefit that is gradually lost with old age [30, 31].
Histone modifications and heterochromatin decline
Post-translational modifications (PTMs) on histones have multiple effects on gene expression. PTMs regulate chromatin compaction by altering histone-histone or histone-DNA interactions, modulate local gene expression directly and impact the recruitment of effector proteins and transcription factors, thereby controlling gene expression indirectly [31, 32]. There are more than 20 post-translational histone modifications currently described in literature - among these are methylation, acetylation, phosphorylation, ubiquitylation and sumoylation - and their effects are interdependent, making it challenging to study how one specific alteration impacts genetic regulation [33], since it is often a combination of modifications which guides specific regulatory functions [34].
Histone modifications occur in the flexible (and easily accessible) N-terminal histone tails protruding from the nucleosome core or in other histone domains, such as the lateral histone regions or the central globular domains, directly impacting chromatin binding and associated activity [33]. Additionally, histone variants, such as H2A.W and H3.3, gradually replace some of the core histones during aging, promoting chromatin architecture changes, and adding another layer of complexity to an already intricate system [35].
In the last decade, several studies have described how histone modifications work in tandem to regulate genomic expression [36, 37]. The best studied PTMs are acetylation and methylation, which occur primarily on lysine and arginine residues in the histone tails [38, 39]. These residues are likely selected for PTM due to their positive electrostatic charges, which interact with the negatively charged nucleic acid nucleotides forming strong electrostatic bonds [38, 40]. In the case of acetylation, the addition of acetyl groups neutralizes the positive charge of arginine or lysine residues, decreasing the strength with which the modified histones interact with the DNA, resulting in euchromatin formation, increased transcription and increased genomic instability [38, 41]. Nonetheless, a study by Li and colleagues reported that the acetylation of lysine 85 of linker histone 1 resulted in heterochromatin condensation, a clear example of how the same PTM may impact genomic structure differently, depending on which residue is modified and the interactions it establishes [42]. The impact of histone methylation is similarly complex, with the stability of the genome being altered in accordance with the position of the modified residue and its interactions with other PTMs, with some methylated residues favoring genomic transcription in some contexts, while promoting repression in others [43, 44]. For example, methylation of H3K4 (lysine 4 of histone 3) represses transcription in the presence of H3K9 methylation but increases transcription when H3K9 is acetylated [45].
Histone methylation is mediated by lysine or arginine methyltransferases (KMTs and PRMTs, respectively) that add methyl groups to the target histone residues using S-adenosylmethionine (SAM) as a methyl group donor [46]. Demethylation is mediated by histone demethylases (HDMs), that remove methyl groups [41, 47]. Histone acetylation is mediated by histone acetyltransferases (HATs), which add acetyl groups from acetyl-CoA [46]. Deacetylation is mediated by histone deacetylases (HDACs) – which remove those groups [47]. HATs and HDACs have been the target of recent pharmaceutical studies, looking for therapeutic agents that can modulate gene expression in cancer [44, 48] and aging.
Aging is associated with an overall increase in histone acetylation [49]. A group of HDACs known as Sirtuins have been studied as a target for improving healthspan [50]. Sirtuins belong to a family of NAD+ dependent proteins with histone deacetylase function and their activity levels decrease with aging, in tandem with a decrease in NAD+ levels [49, 51]. Members of the Sirtuin family (SIRT), namely SIRT1, SIRT3 and SIRT6, have been shown to promote health and longevity in various model organisms, from yeast to mammals (particularly in situations of caloric restriction and high exercise). As a result, Sirtuins have been considered as potential therapeutic targets to extend healthy aging, either by increasing the availability of NAD+, essential to keeping metabolism at the required rates, or by using pharmacological compounds (such as resveratrol) that directly activate Sirtuins function [49, 52–54].
Several specific PTMs have also been associated with aging including H4K16ac, acetylation of lysine 16 of histone H4 (associated with reduced chromatin condensation), H4K20me2 and H4K20me3, di- and tri-methylation of lysine 20 of histone H4 (which increase chromatin compaction in vitro) and H3K9me3, tri-methylation of lysine 9 of histone 3 (which promotes a stronger binding between the DNA and the histone octamer - a modification that is gradually lost with aging) [29, 31, 55].
In addition to changes in acetylation and other PTMs, aging is associated with a global reduction in core histones proteins accompanied by a decline in protein synthesis [17]. The loss of histones implies a decrease in heterochromatin, an observation which led to the “heterochromatin loss model of aging”. This model suggests that there are conformational changes in heterochromatin structure with aging that result in the activation of genes that were previously silenced. These alterations, in turn, cause aging and cellular senescence along with increased genomic instability - further aggravated by chromatin relaxation, leading to age-associated changes in gene expression and increased genome damage [18, 29].
DNA methylation and aging
DNA methylation (DNAm) is observed in all eukaryotes, from plants and fungi to animals, and it is central to cell differentiation and embryonic development [56]. After replication, a methyl group is added to the fifth carbon of the cytosine base ring (5mC), most commonly in genomic locations that are rich in cytosine-guanine dinucleotides, known as CpG islands [57]. Methylation can also be observed at other nucleotide pairs (adenine-cytosine; thymine-cytosine; cytosine-cytosine), which are known as non-CpG, or CpH sites [39, 58], although it is less frequent than at CpG sites. Non-CpG methylation is particularly common in embryonic stem cells [59] and neurons [60]. DNAm is much more prevalent in heterochromatin than euchromatin [30].
DNAm is mediated by the DNA methyltransferases (DNMTs) 1 and 3, with S-adenosyl methionine (SAM) serving as donor for the methyl group, the same molecule that serves as donor for histone methyltransferases [46, 61]. The function of DNMT1 is to maintain existing methylation sites, by adding methyl groups at CpG sites that are methylated on only one DNA strand, such as after DNA replication. Conversely, DNMT3A, DNMT3B and DNMT3L promote de novo DNA methylation [57, 58, 61].
DNMTs activity is balanced by DNA demethylation, which can be either passive or active. In passive or spontaneous demethylation, the cytosine loses its methyl group during replication and is converted to thymine, which is posteriorly exchanged through repair mechanisms to an unmethylated cytosine. Active demethylation is mediated by Ten-Eleven Translocation (TET) proteins. These catalyze the demethylation process through successive oxidations, from 5mC to 5-hydroxymethyl cytosine (5hmC) and afterwards to 5-formylcytosine and 5-carboxylcytosine, which are recognized by Thymine DNA Glycosylase (TDG) and returned to their unmethylated cytosine state by the base excision repair (BER) pathway [57, 62]. There are several proteins involved in this process. Among them, are the protein GADD45A (“Growth Arrest and DNA Damage Protein 45 A”) and the members of the AID/APOBEC (Activation Induced Cytidine Deaminase (AID)/Apolipoprotein B Editing Complex (APOBEC)) protein family. GADD45A recruits TDG in situations of DNA damage [63], and recognizes promoters’ R-loop conformation, leading to TET recruitment and demethylation at CpG islands [64]. In addition, the members of the protein family AID/APOBEC are described as having a role in demethylation through deamination of 5mC cytosine [65], which generates a G-T mismatch that is posteriorly corrected by TDG [66].
In young stages of life, eukaryotic DNA is hypermethylated in repetitive elements of the genome, in intergenic regions and in gene bodies. Hypermethylation promotes genomic stability and limits transcription of these regions of the genome. With aging, there is global hypomethylation of previously methylated regions, leaving these more available to transcription factors and other effectors and consequently promoting transcription of these genomic sites [59]. This hypomethylation is associated with a decrease in DNMT1 expression [67], which likely contributes to impaired maintenance of DNAm patterns associated with aging.
There is also, contradictorily, some evidence of age-associated DNA hypermethylation in genomic regions that in young individuals’ DNA are usually unmethylated. Methylation of these regions is associated with genomic compactation, restricting gene transcription, which may also lead to genomic instability, if genes that should be transcribed find themselves restricted by this event [29, 56, 68].
DNAm alterations are widely described in cancer studies. Hypermethylation of tumor suppressor genes and hypomethylation of oncogenes are both associated with genomic imbalance and dysregulated proliferation of cancer cells [69]. A similar phenomenon is hypothesized to occur in aging, with age-associated genes as the target - in this case, hypomethylation at the promoters of genes regulating senescence may translate into increased expression of these genes and accelerated aging.
Recently, DNAm has been used as a biomarker to measure aging by analyzing methylation status across a large set of CpG sites [70]. This led to the creation of epigenetic clocks (e.g. Horvath’s, Hannum’s, PhenoAge and GrimAge [71, 72], which were designed to predict chronological age based on epigenetic criteria. Subsequently, epigenetic clocks have been used as a proxy for biological age and have been extensively studied for their ability to predict healthspan, disease risk and mortality [73, 74] Epigenetic clocks are reliable predictors of chronological age, but their utility in predicting mortality and healthspan is less clear. Further studies are required to determine whether specific CpG sites can be used to predict healthy longevity [75–78].
Modulation of aging by non-coding RNAs
Non-coding RNAs (ncRNA) contribute for the control of gene expression as they have key regulatory functions in both physiological and biological processes [79]. As measurable molecules, circulating ncRNAs and particularly microRNAs (miRNAs), are considered promising biomarkers for the study of aging and age-associated processes, as they have been linked to aging (and age-related pathways), cellular senescence and age-associated conditions [80–83].
MiRNAs, the most studied of the ncRNAs, are short molecules of 18 to 25 nucleotides that regulate gene expression by binding to the 3′ untranslated region (3′UTR) of their target genes blocking protein translation or inducing mRNA degradation [84]. There are more than 2800 miRNAs encoded in the human genome, and it is estimated that at least 60% of all human genes are regulated by miRNAs [80, 85, 86]. MiRNAs may also impact other epigenetic events by regulating their associated enzymes. For instance, members of miR-29 family, modulate the expression of DMNTs and TET enzymes in both healthy and pathological conditions, namely brain maturation and cancer [84, 87], leading to the reduction of DNA hypermethylation in tumor suppressor genes [87, 88].
LncRNAs or “long non-coding RNAs” are a diverse class of ncRNAs longer than 200 nucleotides. They can bind to DNA, RNA or proteins and are involved in the post-transcriptional and post-translational regulation of gene expression. It has also been suggested that lncRNAs may interfere with chromatin structure modulation and/or transcription [79, 89].
Both of these ncRNAs have been implicated in senescence and inflammation-related pathways (such as the p23/p21 and the nuclear factor kappa light-chain enhancer of activated B cells (NF-κB)), in the development of neurological disorders (such as Huntington’s and Alzheimer’s disease) [90] and other age associated conditions, such as fibrosis [79], cardiovascular disease [89, 91] and osteoporosis [92]. Thus, ncRNAs are increasingly being targeted for anti-aging therapies [93].
ncRNAs influencing aging include miR-28e3p and miR-126, whose levels are reduced in diabetes mellitus type 2 [94], and miR-146a, that inhibits NF-κB pro-inflammatory activity and shows reduced expression levels associated with aging [6]. Another ncRNA, miR-21, was demonstrated to modulate inflammation and the NF-κB pathway and shows altered levels in cardiovascular diseases and osteoporosis [92, 95]. Another ncRNA that has been linked to aging is nc886 (or pre-miR-886), which was shown to impact senescence by reducing the expression of senescence biomarkers, such as p16INK4A and Cyclin Kinase Inhibitor p21 (p21Waf1/Cip1), and decreasing reactive oxygen species (ROS) levels in fibroblast cell models. This molecule is under investigation as a potential target for anti-aging therapy [96].
In short, the aging process leads to alterations in non-coding RNA levels, which in turn modify the expression levels of age-related genes and increases age-related genomic instability.
Application of nutritional and lifestyle strategies to epigenetically modulate healthspan
Studies have shown that from an economic point of view is more advantageous for society to promote even a slight increase in healthspan, rather than investing in disease-specific adaptions that cater to an aged population [16, 97]. Henceforth, it is important to identify biomarkers and develop biological clocks that can assess the value of novel strategies that may potentially overcome or delay conditions leading to poor health amongst the elderly [98–100].
Over the years, there have been many theories about how to extend healthy lifespan: from eating superfoods and following fad diets, to adopting specific behavioral habits – making it difficult to distinguish what is myth from what is backed up by scientific evidence. While there is broad scientific consensus that daily habits impact aging, there is no magic solution, much less a “one size fits all” approach to achieving a long and healthy life. Nonetheless, some practices have shown promising results in scientific studies [97].
One lifestyle factor that has an impact on healthspan is literacy [101]. Intriguingly, low levels of literacy are associated with epigenetic changes, similar to the effect of alcohol consumption [102, 103]. Other lifestyle factors that impact epigenetic age acceleration include living environment, stress levels [104, 105], the complexity of an individual’s social network and their economic power [99, 102]. Lack of sleep [106, 107], coffee and tea consumption [108, 109], and smoking [110] are also associated with similar epigenetic age acceleration.
In the following sections, we examine the evidence for the impact of lifestyle factors on healthy aging and present suggestions for ways to promote healthy aging through simple changes regarding nutrition and physical activity.
The impact of nutrigenomics on healthspan
Nutrigenomics is the study of nutrients and diet, and of their influence on the epigenome. It aims to describe, characterize, and understand the mechanisms by which our dietary intake influences gene expression. The field of nutrigenomics has emerged with the adoption of better sequencing technologies, such as next-generation sequencing (NGS), and the development of precise techniques for whole-genome chromatin analysis, namely HiC and ATAC-seq (which stand for “High-throughput Chromosome Conformation Capture technique” and “Assay for Transposase-Accessible Chromatin with high-throughput sequencing”, respectively) [111–113]. These technologies have enabled scientists to study the impact of nutrition on gene expression both in the short and long term [111, 112].
Popular wisdom suggests that there are multiple diets and foods that can promote healthy aging. However, few of these have been shown to positively impact aging and aging associated pathologies. Evidence does exist for the Mediterranean and the Okinawan diets, both of which are associated with lower levels of inflammation and oxidative stress, reduced incidence of cancer, as well as a decreased risk of cardiovascular disease [114–116].
Both the Mediterranean and Okinawan diets include low glycemic index foods and favor the consumption of seasonal local foods - particularly fruits, vegetables, and nuts – along with moderate intake of animal products, olive oil, spices and red wine. They ensure a proper intake of valuable nutrients (see Table 1), while preventing overeating [115, 117, 118]. In epigenetic studies, both diets have demonstrated anti-aging benefits in human subjects (Table 1) [114], and they have also been shown to reduce epigenetic age, according to the DNAGrimAge epigenetic clock [72, 102].
Table 1. Food-induced epigenetic alterations.
| Compound | Foods | Overall impact | Epigenetics impact | References |
| Betalains (Indicaxanthin) | Red Beetroot; Cactus Pear (Opuntia Ficus Indica) | Antioxidant, Anti-inflammatory activity, Anti-carcinogenic | DNAm modulator: increases the expression of genes involved in DNA demethylation of tumor suppressor gene promoters | [216, 217] |
| Catechin/Epicatechin (EGCG) | Green Tea, Cocoa, Apple | Antioxidant, Neuroprotective, Anti-inflammatory activity, Anti-carcinogenic | MiRNA modulator: increases expression of oncosuppressor miRNAs (i.e., miR-29) | [88, 133, 218] |
| Curcumin (diferuloyl-methane) | Turmeric | Anti-inflammatory activity | DNAm modulator: DNMTs’ regulating functions through hypomethylation of oncosuppressor genes; Histone modifications: regulates HATs and HDACs; MiRNA modulator: increases expression of oncosuppressor miRNAs | [122, 219, 220] |
| Hydroxytyrosol Oleic acid | Olive oil | Antioxidant, Cholesterol and Low-Density Lipoprotein (LDL) reductor | MiRNA modulator: increases expression of oncosuppressor miRNAs, as well as miRNAs associated with fatty acid biosynthesis (let-7e-5p) and age-associated signalling (miR-17-5p) | [117, 126, 127] |
| Lycopene | Tomato | Antioxidant, Anti-inflammatory activity | DNAm modulator: high lycopene levels coincided with T-cell signalling protein hypermethylation and altered T-cell signalling pathway in “head and neck cancer survivors” | [121, 221] |
| Omega-3 fatty acid | Fish oil (i.e., Sardine, Salmon), Nuts | Anti-inflammatory activity, Antioxidant | Histone modifications: suppresses HDACs, promoting gene transduction; DNAm modulator: alters TET1 expression; MiRNA modulator: upregulates hsa-miR-551a (tumor suppressor miRNA) | [222–224] |
| Quercetin | Tomato, Onion, Capers | Anti-inflammatory activity, Anti-carcinogenic, Neuroprotective | MiRNA modulator: increases expression of oncosuppressor miRNAs | [131, 225, 226] |
| Resveratrol | Grapes, Nuts, Berries, Red Wine | Anti-inflammatory activity, Antioxidant, Vasoprotective properties; Neuroprotective | Histone modifications: inhibits HATs and HDACs; DNAm modulator: regulates DNMTs; MiRNA modulator: upregulates the tumor suppressor miR-let7A | [53, 182] |
| Sulforaphane/isothiocyanates | Broccoli, Cabbage, Kale | Anti-carcinogenic, Anti-inflammatory activity, Antioxidant,
Proteostasis promoter | Histone modifications: inhibits HDACs; DNAm modulator: regulates DNMTs, by decreasing DNMTs’ expression and promoting the activation of tumor suppressor genes | [227–229] |
| Sulfur-containing compounds, i.e., diallyl trisulfide (DATS) and S-allylcysteine (SAC) | Garlic | Anti-carcinogenic | DNAm modulator: DNMTs’ regulating functions, suppressing tumor proliferation; Histone modifications: reduces HATs and HDACs activity | [230, 231] |
| Organization by alphabetical order of compounds. For each compound there exists at least one study performed in human subject. |
“Superfoods”, such as algae [119, 120], curcumin [121, 122], kale [123, 124], and olive oil [125–127] as well as particular nutrients and/or food components, such as anti-oxidants [53, 128, 129], vitamins [113, 130] and polyphenols [131–133] have also been studied to determine their impact on epigenetics. In Table 1, we present food-associated epigenetic alterations reported in the scientific literature with evidence from human studies.
Caloric restriction
Caloric restriction (CR), reducing an individuals’ caloric intake by 10% to 40% without compromising nutritional value, has been shown to have a significant and sustained effects on health and lifespan in several model organisms, from yeasts to mice, as well as in humans [134–137]. In communities with extended longevity, particularly the Okinawa region of Japan, there is evidence of reduced calorie consumption, which several researchers have suggested is a key reason for increased healthspan [1, 138–140]. In addition, there are reports of individuals practicing CR who have achieved remarkable healthspan [141, 142]. A concept associated with caloric restriction is hormesis, which may be defined as the adaptative response of an organism to its exposure to chemical compounds or environmental factors. Dietary restriction is considered an environmental factor on hormetic studies [143].
At a cellular and molecular level, the benefits of CR include an increase in DNA repair - by promoting the maintenance of BER activity [144]-, delayed neurodegeneration in the central nervous system, improvement in glucose metabolism [145], a reduction in the incidence of diabetes and cancer, and a reduction in epigenetic aging-associated events, namely age-associated global hypomethylation [1]. CR is also closely related with autophagy. This is in large cause due to nutrient depletion, which leads to a reduction of intracellular acetyl coenzyme A (AcCoA) and consequentially to protein deacetylation [146].
On a physiological level, CR exerts its influence through nutrient-sensing pathways [147] by generating a cascade that begins with a reduction in blood glucose levels, leading to an increase in insulin sensitivity [148] and to a reduction in insulin/insulin-like signalling and its’ associated pathways such as IGF-1 pathway [149]. These alterations decrease cell growth and proliferation and promote cell maintenance through repair mechanisms [150]. Furthermore, the reduced availability of nutrients also inhibits the serine/threonine protein kinase mechanistic Target Of Rapamycin (mTOR) pathway and FOXO proteins, particularly FOXO3 [151]. FOXO3 and SIRT1 are phosphorylated by AMPK, whose activation leads to a decrease in protein synthesis and to the referred increase of autophagy at the cellular level [151, 152] as well as ketogenesis and fatty-acid oxidation in the liver [141].
One of the results of CR is weight loss, that diminishes the risk of age-associated diseases, namely cardiovascular, as well as an increase in lifespan [1, 139]. The CALERIE 2 study, which studied the impact of CR on longevity in humans, recruited over 200 healthy participants who maintained 25% CR for two years. In this study, CR led to improvements in multiple dimensions including quality of life, sleep and sexual function of the participants [153].
At the epigenetic level, CR has a significant impact on epigenetic events associated with aging. CR delays DNAm age-related alterations [136, 145], such as the increase in DNMT3a immunoreactivity [154], reduces histone modifications, partly through Sirtuin activation [155], and alters microRNA activity [156], namely miR-125 whose target gene - chinmo - impacts fat metabolism and longevity [157]. Interestingly, a study by Maegawa and colleagues, in 2017, reported a significant decrease in age-related methylation progression in Rhesus monkeys who maintained 30% to 40% CR during approximately 10 years. The monkeys also showed epigenetic age deceleration relative to their chronological age [158]. The benefits of CR are further supported by a study from Pifferi and team in 2018 that demonstrated a 50% increase in the lifespan of the grey mouse lemur in response to 30% CR. There was also a significant reduction in age-associated diseases, compared to a control group with a normal diet [159].
Notwithstanding these advantages, CR is not easy to adopt, which led to the development of intermittent fasting approaches in the hopes of reaping some of the benefits of CR [160]. These range from alternating fasting with normal feeding on different days of the week [139, 161] to restricting the time of the day individual eats (time-restricted feeding) [160, 162]. However, the health benefits of these strategies are not as clear as the benefits provided by classic caloric restriction.
The benefits of CR need to be balanced against potential negative effects [141]. A study of grey mouse lemurs reported grey matter reduction in the group that underwent 30% CR [159]. Extreme CR may also result in significant weight loss and decreased body fat, which can lead to health complications such as bone density disorders (osteoporosis) and compromised healing [163], infertility, and increased cognitive impairment and weight gain in offspring [164]. There is also the risk of eating disorders, such as anorexia and bulimia [165].
For these reasons, there is great interest in pharmaceutical agents that provide the beneficial effects of CR without the need to undergo a calorie-restricted diet [166]. Several drugs are already in clinical trials [167], while other compounds are being commercialized by emerging companies [168]. One class of potential CR mimics is the NAD+ precursors [169] including nicotinamide mononucleotide (NMN) [170, 171] and nicotinamide riboside (NR) [172]. These molecules have been shown to have a preventive effect on age-associated conditions. FOXO3 transcription factor is also emerging as a therapeutic-target in age-related diseases and healthspan focused studies, as it has been associated with longevity in human and animal model studies [173–175]. Since proteins from this family group are mainly regulated through PTMs (namely by HATs and HDACs), they become particularly desirable targets to drug-based treatments [173, 174]. Moreover, in the USA it was established a consortium named “Interventions Testing Program (ITP), where drugs are tested to assess their effects in mice’ lifespan [24, 176].
Some polyphenol treatments have also demonstrated to mimic CR effects by interacting with longevity associated signalling pathways and molecules [50, 52, 138, 150, 177]. Among these we can find resveratrol (presented in Table 1) [146], metformin (an anti-diabetic drug used in type 2 diabetes mellitus to control high blood sugar) [178, 179] and rapamycin [180] that demonstrated not only to have anti-inflammatory and anti-oxidant activities, and to promote autophagy, but also to induce positive aging-associated epigenetic alterations:
In an extensive revision work performed by Pyo et al., the mechanisms through which resveratrol impacts aging in humans and in different animal models were thoroughly described. It was considered that the main mechanisms involved were the activation of proteins from Sirtuin family (such as SIR2 and SIRT1, which have deacetylase activity) and peroxisome-proliferator-activated receptor-g coactivator-1α (PGC-1α), and regulation of the AMPK pathway [53, 181, 182]. Furthermore, studies with human old aged subjects demonstrated that resveratrol supplementation decreased inflammation markers such as Interleukin (i.e., IL-6) and Tumor Necrosis Factor-α (TNF-α), and reversed histone PTM markers of aging, particularly through modulation of acetylation [182–184]. In addition, a study performed by Jimenez et al. showed resveratrol is able to induce nuclear translocation of FOXO3 and to activate this protein independently of phosphoinositide 3-kinase (PI3K)/AKT signalling [173].
Studies developed using metformin - both as a therapeutic intervention on aging and age-associated diseases as well as on the hallmarks of aging themselves - further demonstrated the potential of this compound, through modulating IGF-1 and AMPK activation and mTOR pathways [185, 186]. It was demonstrated that this drug impacts the epigenetic events described above by altering the activity of HATs, HDACs and DNMTs enzymes, which are phosphorylated by AMPK [187]. Moreover, a study by Cuyas and colleagues computationally predicted metformin’ targets, including several epigenetic modifiers, among which KDM6A/UTX, a member of the H3K27me3-specific demethylase subfamily [188].
Rapamycin is a drug able to mimic CR by compromising a cell capacity to sense the nutrients in its environment [150]. It targets the mTOR pathway, by binding to FK-506 binding protein 12 (FKBP12) to establish a complex of three different molecules, which inhibits the mTORC1 subunit. This mechanism showed advantages when applied to disease models, in both animal models and humans [150, 180, 189, 190], as well as lifespan, by increasing autophagy rates. Additionally, the impact of rapamycin on epigenetics has also been demonstrated in several studies. It was shown that it not only maintains young markers of DNAm in mice [191], but also that it promotes the recovery of histone methylation markers which are lost with old age in mice brain tissues [189].
Henceforth, it is possible to observe the closeness of the mechanisms described as associated to CR and to the compounds presented, further reiterating the similarities between them. Furthermore, this also suggests the potential of these strategies as being both complementary and extensions of each other.
The impact of physical exercise on epigenetics
Another strategy to achieve extended healthspan is physical exercise. Physical activity may be defined as “any movement executed by skeletal muscles, that requires energy expenditure” [48, 192]. Its opposite, sedentary behavior, is known to increase age-associated conditions including cardiovascular conditions, metabolic syndrome [193], incontinence [194], cancer, cognitive decline, and neurodegenerative disorders [4, 195–197]. Studies have demonstrated that even a slight increase in physical activity positively impacts healthspan and reduces frailty, as well as having neuroprotective effects and improving cognition [198, 199]. It also reduces age-associated conditions and reduces inflammaging (a generalized increase in circulating pro-inflammatory cytokines, such as Interleukin-6 (IL-6) and Tumor necrosis factor α (TNF-α), that is associated with old age) and immunosenescence [48, 200].
Contrasting with the topics of nutrition and caloric restriction, the cellular and molecular mechanisms underlying the health benefits of physical activity in humans remain poorly understood [201, 202]. In a study performed by Hoshi et al., 145 bioactive lipids (cellular signalling molecules which are strongly related with immunity regulation, inflammation and homeostasis) were associated with higher levels of physical activity, 12 of which were shown to be inversely correlated with cardiovascular disease events [202]. Moreover, a genome-wide association study by Wang et al. screened genomic data from approximately 704 000 individuals with differing physical activity habits. They targeted 99 loci, where 104 independent association signals were identified, among which p.Glu635Ala, a missense variant rs2229456 of ACTN3. This variant was further explored using molecular dynamics simulations, and was associated with increased “moderate-to-vigorous intensity physical activity during leisure time” habits, likely “by reducing susceptibility to exercise-induced muscle damage” [203]. O’Reilley and colleagues reviewed how physical activity relates with mitochondrial remodelling and how these, in turn, influence neurodegeneration. They highlighted how mitochondrial function strongly benefits from aerobic exercise in some tissues, and that this may positively impact the brain by delaying the age-associated increase of oxidative stress that leads to mitochondrial decline [204]. Furthermore, a review by Reddy et al. explores the “exercise responsome” and several molecules (exerkines) which are released during physical activity (such as Cathepsin B, Adiponectin, Osteocalcin and FGF21) [205, 206]. In this same study, exercise mimetic-drugs are studied and it is proposed that AMPK-SIRT1-PGC1α-BDNF pathway is the main mediator of the “cognitive benefits associated with exercise” [205].
On an epigenetic level, regular exercise leads to slower progression of the DNA methylation alterations associated with age [207–209], and to beneficial changes in miRNAs that regulate inflammation levels [48]. The epigenetic effects of physical exercise on human subjects reported in the literature are presented in Table 2. These studies demonstrate the many positive impacts of physical exercise on health status, by reducing age-associated and disease-associated epigenetic changes.
Table 2. Exercise-associated epigenetic alterations.
| Exercise | Individuals tested* | Intensity/Frequency during/in study | Epigenetic impact | References |
| Aerobic and resistance training (jogging, cycling, swimming, and others) | Healthy (male marathon runners) | Immediately after and 24h post marathon; | MiRNA modulator: changes in circulatory miRNA levels after racing | [232] |
| Healthy (male cyclists) | Before and after the supplementation in EVOO ** - alongside cycling - 4 weeks | DNAm modulator: levels of DNMT3A and DNMT3B mRNA expression decreased following exercise | [233] |
| Non-Healthy (obese) | 3 months study, 2 times per week, 90 minutes session; | MiRNA modulator: i.e., miR-146a-5p levels were significantly decreased after intervention - positive impact in inflammation | [234] |
| Non-Healthy (hypertensive) | 3 months study, 4 times per week, 40 minutes session | DNAm modulator: repetitive elements methylation (i.e., ALU), potential role in reducing systemic blood pressure | [208] |
| Non-Healthy (colorectal cancer survivors) | 6 weeks - resistance exercise training - samples collected before and after intervention | DNAm modulator: changes in promoters of biologically related genes of processes such as immune response and disease; reduced methylation of disease preventive genes | [235] |
| Aged (women; 68 ± 7.5 years old) | 4 study groups: resistance training, water aerobics, water aerobics and resistance exercise, and control group (non-practitioners) individuals, with 3+ months of practice, 2/3 times per week | DNAm modulator: increased global and gene specific (Interleukin-17 (IL-17A) and Interferon gamma (IFN-γ)) DNA methylation - positively impacting inflammation | [236] |
| Aged (70–75 years old) | 12 weeks, low frequency, moderate intensity, explosive-type resistance training | DNAm modulator: reduction of global DNAm levels, together with better leukocyte telomere length maintenance | [237] |
| Relaxation and stress targeted exercises (Tai Chi, Meditation, Yoga) | Healthy (women – 45 to 88 years old) | Tai Chi practitioners for 3+ years, for at least 1 hour per week) | DNAm modulator: decreased age-associated DNAm levels - trend more evident in older subjects (>55y) | [207] |
| Healthy (individuals younger than 52 years old and individuals aged 52 or older) | Meditation practitioners for 3+ years, for at least 30 minutes daily | DNAm modulator: Intrinsic Epigenetic Age Acceleration (IEAA) similar in practitioners younger and older than 52 years old. In the control group there were significative differences between age groups, with higher IEAA in the ≥ 52 group. | [211] |
| Healthy (30 to 65 years old) | Yoga and Meditation Based Lifestyle Intervention (YMLI) 12 weeks, 5 days per week, 90 minutes per day | Genomic stability: DNA damage and genomic instability reduction. Cellular aging biomarkers improvement: increased balance of inflammation and cellular oxidative stress levels | [238] |
| Non-Healthy (infertile males) | 21 days, daily Yoga practice – 1 hour per day Collection of samples before and after intervention | DNAm modulator: over 400 DNAm changes observed in fertility-associated gene promoters, together with improved sperm parameters | [239] |
| Aged (community dwelling 60 + years old) | 1 month, daily Tai Chi practice - 1 hour per day | DNAm modulator: Brain-derived neurotrophic factor (BDNF) promoter demethylation - marker of depression recovery: marked results in depression symptoms improvement | [240] |
| *The individual tested/participants were discriminated in their respective studies in accordance to medical conditions, gender and age. **EVOO stands for Extra Virgin Olive Oil. |
The World Health Organization has promoted the philosophy that “some (exercise) is better than nothing”, even if it is just part of an individual’s daily routine, associated with everyday tasks [192]. However, studies have demonstrated that consistent physical activity of variable intensity has more impact on biological aging than exercise done in a purely occupational context [210], and research also described that exercises targeting relaxation - such as yoga and meditation -, show a decrease of Intrinsic Epigenetic Age Acceleration (IEAA) [211]. Moreover, the epigenetic benefits that appear with exercise are severely reduced or disappear shortly after physical activity interruption [212], so consistency in physical activity is likely to be important.
It has been shown that DNAm events are influenced by physical exercise and physical fitness. There are emerging DNAm clocks which have presented promising results in estimating healthy aging and its’ progression. One of those is DNAmFitAge, which demonstrated to distinguish between individuals who are highly fit from individuals with low to medium fitness, while also relating verbal short-term memory to decelerated aging [213]. Another DNAm clock is the DNAm GrimAge clock, which shows predictive capacity of lifespan and healthspan and a strong relationship with lifestyle factors, including physical activity [72, 214]. Moreover, several other epigenetic clocks, including the Hannun’s, Horvath’s and PhenoAge [215] associated aging markers with physical activity and disease.
Conclusion and future perspectives
Aging is a complex and natural process in every living organism’s life cycle and it manifests in many ways, including epigenetics. In this article we explore aging and its associated epigenetic changes as well as how these changes may be delayed or reversed through nutrition, caloric restriction and sustained physical activity, as schematized in Figure 2. In this Figure we may observe a looser chromatin structure in the component B than in the component A, as well as lower levels of DNA methylation and increased histone PTMs, but nonetheless these values are closer to the component A than to the component B presented in Figure 1, where lifestyle strategies to promote a healthy aging are not considered, and consequentially imply an overall increase of chromatin instability.
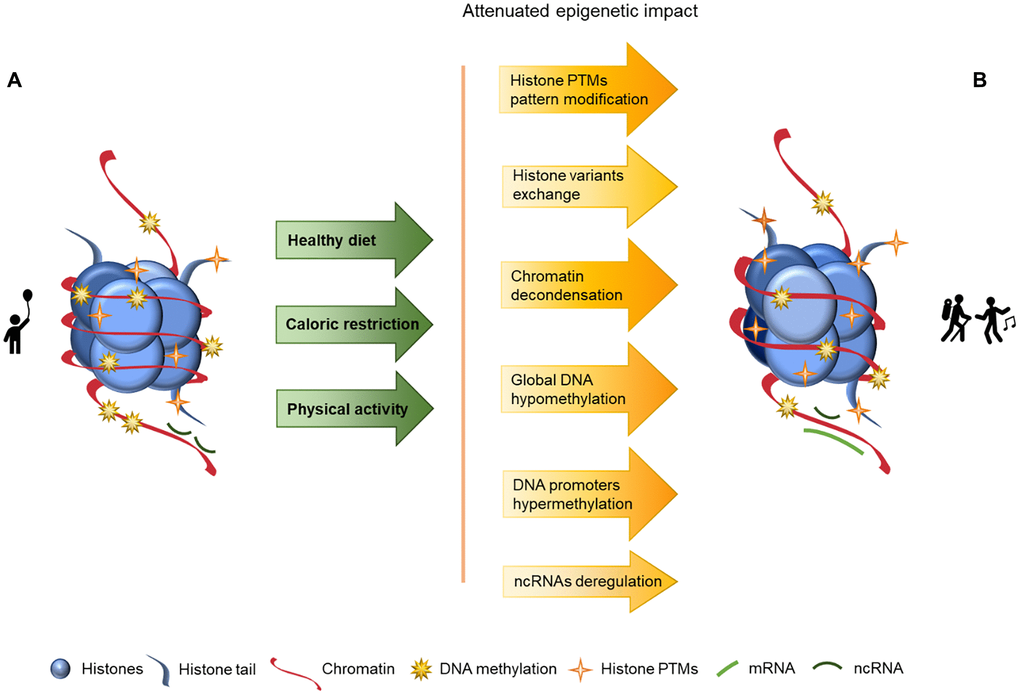
Figure 2. Representation of age-associated epigenetic changes after following a healthy lifestyle. (A) Representation of a young individual chromatin, with tight chromatin compactation, high levels of DNA methylation, decreased histone PTMs (particularly acetylation), canonical histones and balanced non-coding RNA regulation; (B) Representation of a healthy old aged individual chromatin, we may observe a looser chromatin structure and lower levels of DNA methylation than in A, and higher levels of histones PTMs (acetylation) than in A. There are also different histone variants presence (in exchange of the canonical histones) and an increase of ncRNA imbalance, which is reflected in an overall increase of chromatin instability. The alterations between the structures presented in A and B are represented by arrows. The arrows in green present the causes (here presented as a mindful lifestyle - achieved through a healthy diet, caloric restriction and physical activity) whose effects are reflected by the arrows in yellow/orange, with the lighter colors representing less marked epigenetic alterations.
It is important to note, however, that epigenetic events are dynamic and interdependent. Moreover, the relationship between age-associated epigenetic changes and healthspan is not always well-established and one epigenetic modification may lead to folding or unfolding of the chromatin structure depending on other nearby modifications. Future epigenomic studies must adopt a genome-wide perspective, rather than a targeted approach, and should adopt a three-dimensional perspective to give deeper insight into their impact on chromatin structure. Furthermore, it would be relevant to develop strategies that could enable the differentiation of standard and healthy older aging from age-associated pathologies themselves.
The aging global population is placing ever-growing demands on social and health infrastructure. Strategies to promote health aging are important to maximize quality of life for the elderly and minimize pressure on health care systems. The simplest “treatments” are lifestyle changes including healthy eating and regular physical exercise. Pharmacological or biological treatments are also in development, and may, in the future, help reduce the risks associated with unhealthy behaviors, such as smoking and drug abuse. Understanding the relationship between biological aging and healthspan is critical to assessing the value of these interventions and identifying new therapies that can promote healthy aging.
Author Contributions
Ana Teresa Rajado - planned the article, performed the literature search and wrote the manuscript. Nádia Silva, Filipa Esteves, David Brito, the ALFA Score Consortium, Alexandra Binnie, Inês Araújo, Clévio Nóbrega and José Bragança - provided intellectual input and manuscript writing and revision. Pedro Castelo-Branco - planned the article, provided scientific guidance and critical revision of the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The research by the authors is developed at Algarve Biomedical Center. We acknowledge the Algarve Biomedical Center for the structural support.
Conflicts of Interest
The authors declare no conflicts of interest related to this study.
Funding
This work was supported by the Algarve Regional Operational Programme (CRESC – Algarve), Reference: ALG-01-0145-FEDER-072586.
Editorial Note
*
The list of ALFA Score Consortium members:
Raquel P. Andrade1,2,3,5, Joana Apolónio1,2,3, Sofia M. Calado1,2,3, Maria Leonor Faleiro1,2,3,4, Carlos Matos1,2,3, Nuno Marques1,2,3, Ana Marreiros1,2,3, Hipólito Nzwalo1,2,3, Sandra Pais1,2,3, Isabel Palmeirim1,2,3,5, Vânia Palma Roberto1,2,3,6, Sónia Simão1,2,3, Natércia Joaquim7, Rui Miranda7, António Pêgas8 and Ana Sardo9
1Algarve Biomedical Center, Research Institute (ABC-RI), University of Algarve, Campus Gambelas, Bld.2, 8005-139 Faro, Portugal
2Algarve Biomedical Center (ABC), University of Algarve, Campus Gambelas, Bld.2, 8005-139 Faro, Portugal
3Faculty of Medicine and Biomedical Sciences (FMCB), University 28 of Algarve, Gambelas Campus, Bld. 2, 8005-139 Faro, Portugal
4Faculty of Science and Technology (FCT), University of Algarve, Gambelas Campus, Bld.8, Faro Portugal
5Champalimaud Research Program, Champalimaud Centre for the Unknown, Lisbon, Portugal
6ABC Collaborative Laboratory, Association for Integrated Aging and Rejuvenation Solutions (ABC CoLAB), 8100-735 Loulé, Portugal
7USF Balsa, Tavira
8USF Ossónoba, Faro
9USF Mirante, Olhão
References
-
1.
Gensous N, Franceschi C, Santoro A, Milazzo M, Garagnani P, Bacalini MG. The Impact of Caloric Restriction on the Epigenetic Signatures of Aging. Int J Mol Sci. 2019; 20:2022. https://doi.org/10.3390/ijms20082022 [PubMed]
-
2.
MacNee W, Rabinovich RA, Choudhury G. Ageing and the border between health and disease. Eur Respir J. 2014; 44:1332–52. https://doi.org/10.1183/09031936.00134014 [PubMed]
-
3.
Rodríguez-Rodero S, Fernández-Morera JL, Menéndez-Torre E, Calvanese V, Fernández AF, Fraga MF. Aging genetics and aging. Aging Dis. 2011; 2:186–95. [PubMed]
-
4.
Rodriguez-Larrad A, Arrieta H, Rezola C, Kortajarena M, Yanguas JJ, Iturburu M, Susana MG, Irazusta J. Effectiveness of a multicomponent exercise program in the attenuation of frailty in long-term nursing home residents: study protocol for a randomized clinical controlled trial. BMC Geriatr. 2017; 17:60. https://doi.org/10.1186/s12877-017-0453-0 [PubMed]
-
5.
Bommer C, Sagalova V, Heesemann E, Manne-Goehler J, Atun R, Bärnighausen T, Davies J, Vollmer S. Global Economic Burden of Diabetes in Adults: Projections From 2015 to 2030. Diabetes Care. 2018; 41:963–70. https://doi.org/10.2337/dc17-1962 [PubMed]
-
6.
Mensà E, Giuliani A, Matacchione G, Gurău F, Bonfigli AR, Romagnoli F, De Luca M, Sabbatinelli J, Olivieri F. Circulating miR-146a in healthy aging and type 2 diabetes: Age- and gender-specific trajectories. Mech Ageing Dev. 2019; 180:1–10. https://doi.org/10.1016/j.mad.2019.03.001 [PubMed]
-
7.
Wang J, Zibetti C, Shang P, Sripathi SR, Zhang P, Cano M, Hoang T, Xia S, Ji H, Merbs SL, Zack DJ, Handa JT, Sinha D, et al. ATAC-Seq analysis reveals a widespread decrease of chromatin accessibility in age-related macular degeneration. Nat Commun. 2018; 9:1364. https://doi.org/10.1038/s41467-018-03856-y [PubMed]
-
8.
Lu Y, Brommer B, Tian X, Krishnan A, Meer M, Wang C, Vera DL, Zeng Q, Yu D, Bonkowski MS, Yang JH, Zhou S, Hoffmann EM, et al. Reprogramming to recover youthful epigenetic information and restore vision. Nature. 2020; 588:124–9. https://doi.org/10.1038/s41586-020-2975-4 [PubMed]
-
9.
Ong ALC, Ramasamy TS. Role of Sirtuin1-p53 regulatory axis in aging, cancer and cellular reprogramming. Ageing Res Rev. 2018; 43:64–80. https://doi.org/10.1016/j.arr.2018.02.004 [PubMed]
-
10.
Labbé C, Lorenzo-Betancor O, Ross OA. Epigenetic regulation in Parkinson's disease. Acta Neuropathol. 2016; 132:515–30. https://doi.org/10.1007/s00401-016-1590-9 [PubMed]
-
11.
Sujkowski A, Hong L, Wessells RJ, Todi SV. The protective role of exercise against age-related neurodegeneration. Ageing Res Rev. 2022; 74:101543. https://doi.org/10.1016/j.arr.2021.101543 [PubMed]
-
12.
Jin K, Simpkins JW, Ji X, Leis M, Stambler I. The Critical Need to Promote Research of Aging and Aging-related Diseases to Improve Health and Longevity of the Elderly Population. Aging Dis. 2014; 6:1–5. https://doi.org/10.14336/AD.2014.1210 [PubMed]
-
13.
United Nations. World Population Ageing - Highlights. 2017.
-
14.
World Health Organization. UN Decade of Healthy Ageing - 2021-2030. World Health Organization. 2020.
-
15.
World Health Organization. Healthy ageing and functional ability. World Health Organization. 2020.
-
16.
Rivadeneira MF, Mendieta MJ, Villavicencio J, Caicedo-Gallardo J, Buendía P. A multidimensional model of healthy ageing: proposal and evaluation of determinants based on a population survey in Ecuador. BMC Geriatr. 2021; 21:615. https://doi.org/10.1186/s12877-021-02548-5 [PubMed]
-
17.
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013; 153:1194–217. https://doi.org/10.1016/j.cell.2013.05.039 [PubMed]
-
18.
Pal S, Tyler JK. Epigenetics and aging. Sci Adv. 2016; 2:e1600584. https://doi.org/10.1126/sciadv.1600584 [PubMed]
-
19.
Wang K, Liu H, Hu Q, Wang L, Liu J, Zheng Z, Zhang W, Ren J, Zhu F, Liu GH. Epigenetic regulation of aging: implications for interventions of aging and diseases. Signal Transduct Target Ther. 2022; 7:374. https://doi.org/10.1038/s41392-022-01211-8 [PubMed]
-
20.
Herskind AM, McGue M, Holm NV, Sørensen TI, Harvald B, Vaupel JW. The heritability of human longevity: a population-based study of 2872 Danish twin pairs born 1870-1900. Hum Genet. 1996; 97:319–23. https://doi.org/10.1007/BF02185763 [PubMed]
-
21.
Martin SL, Hardy TM, Tollefsbol TO. Medicinal chemistry of the epigenetic diet and caloric restriction. Curr Med Chem. 2013; 20:4050–9. https://doi.org/10.2174/09298673113209990189 [PubMed]
-
22.
Quach A, Levine ME, Tanaka T, Lu AT, Chen BH, Ferrucci L, Ritz B, Bandinelli S, Neuhouser ML, Beasley JM, Snetselaar L, Wallace RB, Tsao PS, et al. Epigenetic clock analysis of diet, exercise, education, and lifestyle factors. Aging (Albany NY). 2017; 9:419–46. https://doi.org/10.18632/aging.101168 [PubMed]
-
23.
Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993; 366:461–4. https://doi.org/10.1038/366461a0 [PubMed]
-
24.
Zhang ZD, Milman S, Lin JR, Wierbowski S, Yu H, Barzilai N, Gorbunova V, Ladiges WC, Niedernhofer LJ, Suh Y, Robbins PD, Vijg J. Genetics of extreme human longevity to guide drug discovery for healthy ageing. Nat Metab. 2020; 2:663–72. https://doi.org/10.1038/s42255-020-0247-0 [PubMed]
-
25.
Yang JH, Griffin PT, Vera DL, Apostolides JK, Hayano M, Meer MV, Salfati EL, Su Q, Munding EM, Blanchette M, Bhakta M, Dou Z, Xu C, et al. Erosion of the Epigenetic Landscape and Loss of Cellular Identity as a Cause of Aging in Mammals. bioRxiv. 2019. https://doi.org/10.1101/808642
-
26.
Yang JH, Hayano M, Griffin PT, Amorim JA, Bonkowski MS, Apostolides JK, Salfati EL, Blanchette M, Munding EM, Bhakta M, Chew YC, Guo W, Yang X, et al. Loss of epigenetic information as a cause of mammalian aging. Cell. 2023; 186:305–26.e27. https://doi.org/10.1016/j.cell.2022.12.027 [PubMed]
-
27.
Rea IM, Dellet M, Mills KI, and ACUME2 Project. Living long and ageing well: is epigenomics the missing link between nature and nurture? Biogerontology. 2016; 17:33–54. https://doi.org/10.1007/s10522-015-9589-5 [PubMed]
-
28.
Richmond TJ, Davey CA. The structure of DNA in the nucleosome core. Nature. 2003; 423:145–50. https://doi.org/10.1038/nature01595 [PubMed]
-
29.
Lee JH, Kim EW, Croteau DL, Bohr VA. Heterochromatin: an epigenetic point of view in aging. Exp Mol Med. 2020; 52:1466–74. https://doi.org/10.1038/s12276-020-00497-4 [PubMed]
-
30.
Bourguet P, Picard CL, Yelagandula R, Pélissier T, Lorković ZJ, Feng S, Pouch-Pélissier MN, Schmücker A, Jacobsen SE, Berger F, Mathieu O. The histone variant H2A.W and linker histone H1 co-regulate heterochromatin accessibility and DNA methylation. Nat Commun. 2021; 12:2683. https://doi.org/10.1038/s41467-021-22993-5 [PubMed]
-
31.
Lawrence M, Daujat S, Schneider R. Lateral Thinking: How Histone Modifications Regulate Gene Expression. Trends Genet. 2016; 32:42–56. https://doi.org/10.1016/j.tig.2015.10.007 [PubMed]
-
32.
Biggs R, Liu PZ, Stephens AD, Marko JF. Effects of altering histone posttranslational modifications on mitotic chromosome structure and mechanics. Mol Biol Cell. 2019; 30:820–7. https://doi.org/10.1091/mbc.E18-09-0592 [PubMed]
-
33.
Villaseñor R, Baubec T. Regulatory mechanisms governing chromatin organization and function. Curr Opin Cell Biol. 2021; 70:10–7. https://doi.org/10.1016/j.ceb.2020.10.015 [PubMed]
-
34.
Azevedo R, Jacquemin C, Villain N, Fenaille F, Lamari F, Becher F. Mass Spectrometry for Neurobiomarker Discovery: The Relevance of Post-Translational Modifications. Cells. 2022; 11:1279. https://doi.org/10.3390/cells11081279 [PubMed]
-
35.
Tvardovskiy A, Schwämmle V, Kempf SJ, Rogowska-Wrzesinska A, Jensen ON. Accumulation of histone variant H3.3 with age is associated with profound changes in the histone methylation landscape. Nucleic Acids Res. 2017; 45:9272–89. https://doi.org/10.1093/nar/gkx696 [PubMed]
-
36.
Jayani RS, Ramanujam PL, Galande S. Studying histone modifications and their genomic functions by employing chromatin immunoprecipitation and immunoblotting. Methods Cell Biol. 2010; 98:35–56. https://doi.org/10.1016/S0091-679X(10)98002-3 [PubMed]
-
37.
Sidoli S, Garcia BA. Characterization of Individual Histone Posttranslational Modifications and Their Combinatorial Patterns by Mass Spectrometry-Based Proteomics Strategies. Methods Mol Biol. 2017; 1528:121–48. https://doi.org/10.1007/978-1-4939-6630-1_8 [PubMed]
-
38.
Di Cerbo V, Mohn F, Ryan DP, Montellier E, Kacem S, Tropberger P, Kallis E, Holzner M, Hoerner L, Feldmann A, Richter FM, Bannister AJ, Mittler G, et al. Acetylation of histone H3 at lysine 64 regulates nucleosome dynamics and facilitates transcription. Elife. 2014; 3:e01632. https://doi.org/10.7554/eLife.01632 [PubMed]
-
39.
Pagiatakis C, Musolino E, Gornati R, Bernardini G, Papait R. Epigenetics of aging and disease: a brief overview. Aging Clin Exp Res. 2021; 33:737–45. https://doi.org/10.1007/s40520-019-01430-0 [PubMed]
-
40.
Otterstrom J, Castells-Garcia A, Vicario C, Gomez-Garcia PA, Cosma MP, Lakadamyali M. Super-resolution microscopy reveals how histone tail acetylation affects DNA compaction within nucleosomes in vivo. Nucleic Acids Res. 2019; 47:8470–84. https://doi.org/10.1093/nar/gkz593 [PubMed]
-
41.
Wesche J, Kühn S, Kessler BM, Salton M, Wolf A. Protein arginine methylation: a prominent modification and its demethylation. Cell Mol Life Sci. 2017; 74:3305–15. https://doi.org/10.1007/s00018-017-2515-z [PubMed]
-
42.
Li Y, Li Z, Dong L, Tang M, Zhang P, Zhang C, Cao Z, Zhu Q, Chen Y, Wang H, Wang T, Lv D, Wang L, et al. Histone H1 acetylation at lysine 85 regulates chromatin condensation and genome stability upon DNA damage. Nucleic Acids Res. 2018; 46:7716–30. https://doi.org/10.1093/nar/gky568 [PubMed]
-
43.
Delcuve GP, Khan DH, Davie JR. Roles of histone deacetylases in epigenetic regulation: emerging paradigms from studies with inhibitors. Clin Epigenetics. 2012; 4:5. https://doi.org/10.1186/1868-7083-4-5 [PubMed]
-
44.
Lillico R, Sobral MG, Stesco N, Lakowski TM. HDAC inhibitors induce global changes in histone lysine and arginine methylation and alter expression of lysine demethylases. J Proteomics. 2016; 133:125–33. https://doi.org/10.1016/j.jprot.2015.12.018 [PubMed]
-
45.
Ghare SS, Joshi-Barve S, Moghe A, Patil M, Barker DF, Gobejishvili L, Brock GN, Cave M, McClain CJ, Barve SS. Coordinated histone H3 methylation and acetylation regulate physiologic and pathologic fas ligand gene expression in human CD4+ T cells. J Immunol. 2014; 193:412–21. https://doi.org/10.4049/jimmunol.1400055 [PubMed]
-
46.
Simithy J, Sidoli S, Garcia BA. Integrating Proteomics and Targeted Metabolomics to Understand Global Changes in Histone Modifications. Proteomics. 2018; 18:e1700309. https://doi.org/10.1002/pmic.201700309 [PubMed]
-
47.
Bilmez Y, Talibova G, Ozturk S. Dynamic changes of histone methylation in mammalian oocytes and early embryos. Histochem Cell Biol. 2022; 157:7–25. https://doi.org/10.1007/s00418-021-02036-2 [PubMed]
-
48.
Ferioli M, Zauli G, Maiorano P, Milani D, Mirandola P, Neri LM. Role of physical exercise in the regulation of epigenetic mechanisms in inflammation, cancer, neurodegenerative diseases, and aging process. J Cell Physiol. 2019; 234:14852–64. https://doi.org/10.1002/jcp.28304 [PubMed]
-
49.
Bradshaw PC. Acetyl-CoA Metabolism and Histone Acetylation in the Regulation of Aging and Lifespan. Antioxidants (Basel). 2021; 10:572. https://doi.org/10.3390/antiox10040572 [PubMed]
-
50.
Bonkowski MS, Sinclair DA. Slowing ageing by design: the rise of NAD+ and sirtuin-activating compounds. Nat Rev Mol Cell Biol. 2016; 17:679–90. https://doi.org/10.1038/nrm.2016.93 [PubMed]
-
51.
Moskalev AA, Aliper AM, Smit-McBride Z, Buzdin A, Zhavoronkov A. Genetics and epigenetics of aging and longevity. Cell Cycle. 2014; 13:1063–77. https://doi.org/10.4161/cc.28433 [PubMed]
-
52.
Dai H, Sinclair DA, Ellis JL, Steegborn C. Sirtuin activators and inhibitors: Promises, achievements, and challenges. Pharmacol Ther. 2018; 188:140–54. https://doi.org/10.1016/j.pharmthera.2018.03.004 [PubMed]
-
53.
Pyo IS, Yun S, Yoon YE, Choi JW, Lee SJ. Mechanisms of Aging and the Preventive Effects of Resveratrol on Age-Related Diseases. Molecules. 2020; 25:4649. https://doi.org/10.3390/molecules25204649 [PubMed]
-
54.
Zullo A, Guida R, Sciarrillo R, Mancini FP. Redox Homeostasis in Cardiovascular Disease: The Role of Mitochondrial Sirtuins. Front Endocrinol (Lausanne). 2022; 13:858330. https://doi.org/10.3389/fendo.2022.858330 [PubMed]
-
55.
Kebede AF, Schneider R, Daujat S. Novel types and sites of histone modifications emerge as players in the transcriptional regulation contest. FEBS J. 2015; 282:1658–74. https://doi.org/10.1111/febs.13047 [PubMed]
-
56.
Phillips T. The Role of Methylation in Gene Expression. Nat Educ. 2008; 1:116.
-
57.
Jang HS, Shin WJ, Lee JE, Do JT. CpG and Non-CpG Methylation in Epigenetic Gene Regulation and Brain Function. Genes (Basel). 2017; 8:148. https://doi.org/10.3390/genes8060148 [PubMed]
-
58.
Unnikrishnan A, Freeman WM, Jackson J, Wren JD, Porter H, Richardson A. The role of DNA methylation in epigenetics of aging. Pharmacol Ther. 2019; 195:172–85. https://doi.org/10.1016/j.pharmthera.2018.11.001 [PubMed]
-
59.
Zampieri M, Ciccarone F, Calabrese R, Franceschi C, Bürkle A, Caiafa P. Reconfiguration of DNA methylation in aging. Mech Ageing Dev. 2015; 151:60–70. https://doi.org/10.1016/j.mad.2015.02.002 [PubMed]
-
60.
Lister R, Mukamel EA, Nery JR, Urich M, Puddifoot CA, Johnson ND, Lucero J, Huang Y, Dwork AJ, Schultz MD, Yu M, Tonti-Filippini J, Heyn H, et al. Global epigenomic reconfiguration during mammalian brain development. Science. 2013; 341:1237905. https://doi.org/10.1126/science.1237905 [PubMed]
-
61.
Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999; 99:247–57. https://doi.org/10.1016/s0092-8674(00)81656-6 [PubMed]
-
62.
Saul D, Kosinsky RL. Epigenetics of Aging and Aging-Associated Diseases. Int J Mol Sci. 2021; 22:401. https://doi.org/10.3390/ijms22010401 [PubMed]
-
63.
Zhou L, Wang W, Yang C, Zeng T, Hu M, Wang X, Li N, Sun K, Wang C, Zhou J, Ren M, Yan L. GADD45a Promotes Active DNA Demethylation of the MMP-9 Promoter via Base Excision Repair Pathway in AGEs-Treated Keratinocytes and in Diabetic Male Rat Skin. Endocrinology. 2018; 159:1172–86. https://doi.org/10.1210/en.2017-00686 [PubMed]
-
64.
Arab K, Karaulanov E, Musheev M, Trnka P, Schäfer A, Grummt I, Niehrs C. GADD45A binds R-loops and recruits TET1 to CpG island promoters. Nat Genet. 2019; 51:217–23. https://doi.org/10.1038/s41588-018-0306-6 [PubMed]
-
65.
Kohli RM, Zhang Y. TET enzymes, TDG and the dynamics of DNA demethylation. Nature. 2013; 502:472–9. https://doi.org/10.1038/nature12750 [PubMed]
-
66.
Hashimoto H, Hong S, Bhagwat AS, Zhang X, Cheng X. Excision of 5-hydroxymethyluracil and 5-carboxylcytosine by the thymine DNA glycosylase domain: its structural basis and implications for active DNA demethylation. Nucleic Acids Res. 2012; 40:10203–14. https://doi.org/10.1093/nar/gks845 [PubMed]
-
67.
Agrawal A, Murphy RF, Agrawal DK. DNA methylation in breast and colorectal cancers. Mod Pathol. 2007; 20:711–21. https://doi.org/10.1038/modpathol.3800822 [PubMed]
-
68.
Sen P, Shah PP, Nativio R, Berger SL. Epigenetic Mechanisms of Longevity and Aging. Cell. 2016; 166:822–39. https://doi.org/10.1016/j.cell.2016.07.050 [PubMed]
-
69.
Bronner C, Alhosin M, Hamiche A, Mousli M. Coordinated Dialogue between UHRF1 and DNMT1 to Ensure Faithful Inheritance of Methylated DNA Patterns. Genes (Basel). 2019; 10:65. https://doi.org/10.3390/genes10010065 [PubMed]
-
70.
Shahal T, Segev E, Konstantinovsky T, Marcus Y, Shefer G, Pasmanik-Chor M, Buch A, Ebenstein Y, Zimmet P, Stern N. Deconvolution of the epigenetic age discloses distinct inter-personal variability in epigenetic aging patterns. Epigenetics Chromatin. 2022; 15:9. https://doi.org/10.1186/s13072-022-00441-y [PubMed]
-
71.
Levine ME, Lu AT, Quach A, Chen BH, Assimes TL, Bandinelli S, Hou L, Baccarelli AA, Stewart JD, Li Y, Whitsel EA, Wilson JG, Reiner AP, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY). 2018; 10:573–91. https://doi.org/10.18632/aging.101414 [PubMed]
-
72.
Lu AT, Quach A, Wilson JG, Reiner AP, Aviv A, Raj K, Hou L, Baccarelli AA, Li Y, Stewart JD, Whitsel EA, Assimes TL, Ferrucci L, Horvath S. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY). 2019; 11:303–27. https://doi.org/10.18632/aging.101684 [PubMed]
-
73.
Hannum G, Guinney J, Zhao L, Zhang L, Hughes G, Sadda S, Klotzle B, Bibikova M, Fan JB, Gao Y, Deconde R, Chen M, Rajapakse I, et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. 2013; 49:359–67. https://doi.org/10.1016/j.molcel.2012.10.016 [PubMed]
-
74.
Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013; 14:R115. https://doi.org/10.1186/gb-2013-14-10-r115 [PubMed]
-
75.
Bergsma T, Rogaeva E. DNA Methylation Clocks and Their Predictive Capacity for Aging Phenotypes and Healthspan. Neurosci Insights. 2020; 15:2633105520942221. https://doi.org/10.1177/2633105520942221 [PubMed]
-
76.
Bryant P, Elofsson A. The relationship between ageing and changes in the human blood and brain methylomes. NAR Genom Bioinform. 2022; 4:lqac001. https://doi.org/10.1093/nargab/lqac001 [PubMed]
-
77.
Salameh Y, Bejaoui Y, El Hajj N. DNA Methylation Biomarkers in Aging and Age-Related Diseases. Front Genet. 2020; 11:171. https://doi.org/10.3389/fgene.2020.00171 [PubMed]
-
78.
Fan H, Xie Q, Zhang Z, Wang J, Chen X, Qiu P. Chronological Age Prediction: Developmental Evaluation of DNA Methylation-Based Machine Learning Models. Front Bioeng Biotechnol. 2022; 9:819991. https://doi.org/10.3389/fbioe.2021.819991 [PubMed]
-
79.
Yang Z, Jiang S, Shang J, Jiang Y, Dai Y, Xu B, Yu Y, Liang Z, Yang Y. LncRNA: Shedding light on mechanisms and opportunities in fibrosis and aging. Ageing Res Rev. 2019; 52:17–31. https://doi.org/10.1016/j.arr.2019.04.001 [PubMed]
-
80.
Kumar S, Vijayan M, Bhatti JS, Reddy PH. MicroRNAs as Peripheral Biomarkers in Aging and Age-Related Diseases. Prog Mol Biol Transl Sci. 2017; 146:47–94. https://doi.org/10.1016/bs.pmbts.2016.12.013 [PubMed]
-
81.
Smith-Vikos T, Liu Z, Parsons C, Gorospe M, Ferrucci L, Gill TM, Slack FJ. A serum miRNA profile of human longevity: findings from the Baltimore Longitudinal Study of Aging (BLSA). Aging (Albany NY). 2016; 8:2971–87. https://doi.org/10.18632/aging.101106 [PubMed]
-
82.
Kumar P, Dezso Z, MacKenzie C, Oestreicher J, Agoulnik S, Byrne M, Bernier F, Yanagimachi M, Aoshima K, Oda Y. Circulating miRNA biomarkers for Alzheimer's disease. PLoS One. 2013; 8:e69807. https://doi.org/10.1371/journal.pone.0069807 [PubMed]
-
83.
Olivieri F, Capri M, Bonafè M, Morsiani C, Jung HJ, Spazzafumo L, Viña J, Suh Y. Circulating miRNAs and miRNA shuttles as biomarkers: Perspective trajectories of healthy and unhealthy aging. Mech Ageing Dev. 2017; 165:162–70. https://doi.org/10.1016/j.mad.2016.12.004 [PubMed]
-
84.
Morita S, Horii T, Kimura M, Ochiya T, Tajima S, Hatada I. miR-29 represses the activities of DNA methyltransferases and DNA demethylases. Int J Mol Sci. 2013; 14:14647–58. https://doi.org/10.3390/ijms140714647 [PubMed]
-
85.
Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Res. 2008; 36:D154–8. https://doi.org/10.1093/nar/gkm952 [PubMed]
-
86.
Griffiths-Jones S. miRBase: the microRNA sequence database. Methods Mol Biol. 2006; 342:129–38. https://doi.org/10.1385/1-59745-123-1:129 [PubMed]
-
87.
Swahari V, Nakamura A, Hollville E, Stroud H, Simon JM, Ptacek TS, Beck MV, Flowers C, Guo J, Plestant C, Liang J, Kurtz CL, Kanke M, et al. MicroRNA-29 is an essential regulator of brain maturation through regulation of CH methylation. Cell Rep. 2021; 35:108946. https://doi.org/10.1016/j.celrep.2021.108946 [PubMed]
-
88.
Kansal V, Agarwal A, Harbour A, Farooqi H, Singh VK, Prasad R. Regular Intake of Green Tea Polyphenols Suppresses the Development of Nonmelanoma Skin Cancer through miR-29-Mediated Epigenetic Modifications. J Clin Med. 2022; 11:398. https://doi.org/10.3390/jcm11020398 [PubMed]
-
89.
Guo Q, Wang J, Sun R, Gu W, He Z, Chen Q, Liu W, Chen Y, Wang J, Zhang Y. Identification of circulating hub long noncoding RNAs associated with hypertrophic cardiomyopathy using weighted correlation network analysis. Mol Med Rep. 2020; 22:4637–44. https://doi.org/10.3892/mmr.2020.11566 [PubMed]
-
90.
Takousis P, Sadlon A, Schulz J, Wohlers I, Dobricic V, Middleton L, Lill CM, Perneczky R, Bertram L. Differential expression of microRNAs in Alzheimer's disease brain, blood, and cerebrospinal fluid. Alzheimers Dement. 2019; 15:1468–77. https://doi.org/10.1016/j.jalz.2019.06.4952 [PubMed]
-
91.
Díez-Ricote L, Ruiz-Valderrey P, Micó V, Blanco-Rojo R, Tomé-Carneiro J, Dávalos A, Ordovás JM, Daimiel L. Trimethylamine n-Oxide (TMAO) Modulates the Expression of Cardiovascular Disease-Related microRNAs and Their Targets. Int J Mol Sci. 2021; 22:11145. https://doi.org/10.3390/ijms222011145 [PubMed]
-
92.
Ko NY, Chen LR, Chen KH. The Role of Micro RNA and Long-Non-Coding RNA in Osteoporosis. Int J Mol Sci. 2020; 21:4886. https://doi.org/10.3390/ijms21144886 [PubMed]
-
93.
Wang YN, Yang CE, Zhang DD, Chen YY, Yu XY, Zhao YY, Miao H. Long non-coding RNAs: A double-edged sword in aging kidney and renal disease. Chem Biol Interact. 2021; 337:109396. https://doi.org/10.1016/j.cbi.2021.109396 [PubMed]
-
94.
Jimenez-Lucena R, Alcala-Diaz JF, Roncero-Ramos I, Lopez-Moreno J, Camargo A, Gomez-Delgado F, Quintana-Navarro GM, Vals-Delgado C, Rodriguez-Cantalejo F, Luque RM, Delgado-Lista J, Ordovas JM, Perez-Martinez P, et al. MiRNAs profile as biomarkers of nutritional therapy for the prevention of type 2 diabetes mellitus: From the CORDIOPREV study. Clin Nutr. 2021; 40:1028–38. https://doi.org/10.1016/j.clnu.2020.06.035 [PubMed]
-
95.
Olivieri F, Prattichizzo F, Giuliani A, Matacchione G, Rippo MR, Sabbatinelli J, Bonafè M. miR-21 and miR-146a: The microRNAs of inflammaging and age-related diseases. Ageing Res Rev. 2021; 70:101374. https://doi.org/10.1016/j.arr.2021.101374 [PubMed]
-
96.
Kim Y, Ji H, Cho E, Park NH, Hwang K, Park W, Lee KS, Park D, Jung E. nc886, a Non-Coding RNA, Is a New Biomarker and Epigenetic Mediator of Cellular Senescence in Fibroblasts. Int J Mol Sci. 2021; 22:13673. https://doi.org/10.3390/ijms222413673 [PubMed]
-
97.
Fitzgerald KN, Hodges R, Hanes D, Stack E, Cheishvili D, Szyf M, Henkel J, Twedt MW, Giannopoulou D, Herdell J, Logan S, Bradley R. Potential reversal of epigenetic age using a diet and lifestyle intervention: a pilot randomized clinical trial. Aging (Albany NY). 2021; 13:9419–32. https://doi.org/10.18632/aging.202913 [PubMed]
-
98.
Jia L, Zhang W, Chen X. Common methods of biological age estimation. Clin Interv Aging. 2017; 12:759–72. https://doi.org/10.2147/CIA.S134921 [PubMed]
-
99.
Declerck K, Vanden Berghe W. Back to the future: Epigenetic clock plasticity towards healthy aging. Mech Ageing Dev. 2018; 174:18–29. https://doi.org/10.1016/j.mad.2018.01.002 [PubMed]
-
100.
Vega Magdaleno GD, Bespalov V, Zheng Y, Freitas AA, de Magalhaes JP. Machine learning-based predictions of dietary restriction associations across ageing-related genes. BMC Bioinformatics. 2022; 23:10. https://doi.org/10.1186/s12859-021-04523-8 [PubMed]
-
101.
Cutler DM, Lleras-Muney A. Understanding differences in health behaviors by education. J Health Econ. 2010; 29:1–28. https://doi.org/10.1016/j.jhealeco.2009.10.003 [PubMed]
-
102.
Fiorito G, McCrory C, Robinson O, Carmeli C, Ochoa-Rosales C, Zhang Y, Colicino E, Dugué PA, Artaud F, McKay GJ, Jeong A, Mishra PP, Nøst TH, et al, and BIOS Consortium, and Lifepath consortium. Socioeconomic position, lifestyle habits and biomarkers of epigenetic aging: a multi-cohort analysis. Aging (Albany NY). 2019; 11:2045–70. https://doi.org/10.18632/aging.101900 [PubMed]
-
103.
Zhao W, Ammous F, Ratliff S, Liu J, Yu M, Mosley TH, Kardia SLR, Smith JA. Education and Lifestyle Factors Are Associated with DNA Methylation Clocks in Older African Americans. Int J Environ Res Public Health. 2019; 16:3141. https://doi.org/10.3390/ijerph16173141 [PubMed]
-
104.
Zannas AS, Arloth J, Carrillo-Roa T, Iurato S, Röh S, Ressler KJ, Nemeroff CB, Smith AK, Bradley B, Heim C, Menke A, Lange JF, Brückl T, et al. Lifetime stress accelerates epigenetic aging in an urban, African American cohort: relevance of glucocorticoid signaling. Genome Biol. 2015; 16:266. https://doi.org/10.1186/s13059-015-0828-5 [PubMed]
-
105.
Park C, Rosenblat JD, Brietzke E, Pan Z, Lee Y, Cao B, Zuckerman H, Kalantarova A, McIntyre RS. Stress, epigenetics and depression: A systematic review. Neurosci Biobehav Rev. 2019; 102:139–52. https://doi.org/10.1016/j.neubiorev.2019.04.010 [PubMed]
-
106.
Qureshi IA, Mehler MF. Epigenetics of sleep and chronobiology. Curr Neurol Neurosci Rep. 2014; 14:432. https://doi.org/10.1007/s11910-013-0432-6 [PubMed]
-
107.
Gaine ME, Chatterjee S, Abel T. Sleep Deprivation and the Epigenome. Front Neural Circuits. 2018; 12:14. https://doi.org/10.3389/fncir.2018.00014 [PubMed]
-
108.
Ek WE, Tobi EW, Ahsan M, Lampa E, Ponzi E, Kyrtopoulos SA, Georgiadis P, Lumey LH, Heijmans BT, Botsivali M, Bergdahl IA, Karlsson T, Rask-Andersen M, et al, and Epigenome-Wide Association Study Consortium. Tea and coffee consumption in relation to DNA methylation in four European cohorts. Hum Mol Genet. 2017; 26:3221–31. https://doi.org/10.1093/hmg/ddx194 [PubMed]
-
109.
Karabegović I, Portilla-Fernandez E, Li Y, Ma J, Maas SCE, Sun D, Hu EA, Kühnel B, Zhang Y, Ambatipudi S, Fiorito G, Huang J, Castillo-Fernandez JE, et al. Epigenome-wide association meta-analysis of DNA methylation with coffee and tea consumption. Nat Commun. 2021; 12:2830. https://doi.org/10.1038/s41467-021-22752-6 [PubMed]
-
110.
Cardenas A, Ecker S, Fadadu RP, Huen K, Orozco A, McEwen LM, Engelbrecht HR, Gladish N, Kobor MS, Rosero-Bixby L, Dow WH, Rehkopf DH. Epigenome-wide association study and epigenetic age acceleration associated with cigarette smoking among Costa Rican adults. Sci Rep. 2022; 12:4277. https://doi.org/10.1038/s41598-022-08160-w [PubMed]
-
111.
Müller M, Kersten S. Nutrigenomics: goals and strategies. Nat Rev Genet. 2003; 4:315–22. https://doi.org/10.1038/nrg1047 [PubMed]
-
112.
Meyer CA, Liu XS. Identifying and mitigating bias in next-generation sequencing methods for chromatin biology. Nat Rev Genet. 2014; 15:709–21. https://doi.org/10.1038/nrg3788 [PubMed]
-
113.
Carlberg C. Nutrigenomics of Vitamin D. Nutrients. 2019; 11:676. https://doi.org/10.3390/nu11030676 [PubMed]
-
114.
Willcox DC, Scapagnini G, Willcox BJ. Healthy aging diets other than the Mediterranean: a focus on the Okinawan diet. Mech Ageing Dev. 2014; 136-137:148–62. https://doi.org/10.1016/j.mad.2014.01.002 [PubMed]
-
115.
Puca AA, Spinelli C, Accardi G, Villa F, Caruso C. Centenarians as a model to discover genetic and epigenetic signatures of healthy ageing. Mech Ageing Dev. 2018; 174:95–102. https://doi.org/10.1016/j.mad.2017.10.004 [PubMed]
-
116.
Meccariello R, D'Angelo S. Impact of Polyphenolic-Food on Longevity: An Elixir of Life. An Overview. Antioxidants (Basel). 2021; 10:507. https://doi.org/10.3390/antiox10040507 [PubMed]
-
117.
Caradonna F, Consiglio O, Luparello C, Gentile C. Science and Healthy Meals in the World: Nutritional Epigenomics and Nutrigenetics of the Mediterranean Diet. Nutrients. 2020; 12:1748. https://doi.org/10.3390/nu12061748 [PubMed]
-
118.
Shannon OM, Ashor AW, Scialo F, Saretzki G, Martin-Ruiz C, Lara J, Matu J, Griffiths A, Robinson N, Lillà L, Stevenson E, Stephan BCM, Minihane AM, et al. Mediterranean diet and the hallmarks of ageing. Eur J Clin Nutr. 2021; 75:1176–92. https://doi.org/10.1038/s41430-020-00841-x [PubMed]
-
119.
Wang L, Lee W, Jayawardena TU, Cha SH, Jeon YJ. Dieckol, an algae-derived phenolic compound, suppresses airborne particulate matter-induced skin aging by inhibiting the expressions of pro-inflammatory cytokines and matrix metalloproteinases through regulating NF-κB, AP-1, and MAPKs signaling pathways. Food Chem Toxicol. 2020; 146:111823. https://doi.org/10.1016/j.fct.2020.111823 [PubMed]
-
120.
Olsthoorn SEM, Wang X, Tillema B, Vanmierlo T, Kraan S, Leenen PJM, Mulder MT. Brown Seaweed Food Supplementation: Effects on Allergy and Inflammation and Its Consequences. Nutrients. 2021; 13:2613. https://doi.org/10.3390/nu13082613 [PubMed]
-
121.
Cheng HM, Koutsidis G, Lodge JK, Ashor AW, Siervo M, Lara J. Lycopene and tomato and risk of cardiovascular diseases: A systematic review and meta-analysis of epidemiological evidence. Crit Rev Food Sci Nutr. 2019; 59:141–58. https://doi.org/10.1080/10408398.2017.1362630 [PubMed]
-
122.
Bahrami A, Montecucco F, Carbone F, Sahebkar A. Effects of Curcumin on Aging: Molecular Mechanisms and Experimental Evidence. Biomed Res Int. 2021; 2021:8972074. https://doi.org/10.1155/2021/8972074 [PubMed]
-
123.
Šamec D, Urlić B, Salopek-Sondi B. Kale (Brassica oleracea var. acephala) as a superfood: Review of the scientific evidence behind the statement. Crit Rev Food Sci Nutr. 2019; 59:2411–22. https://doi.org/10.1080/10408398.2018.1454400 [PubMed]
-
124.
Reda T, Thavarajah P, Polomski R, Bridges W, Shipe E, Thavarajah D. Reaching the highest shelf: A review of organic production, nutritional quality, and shelf life of kale (Brassica oleracea var. acephala). Plants, People, Planet. 2021; 3:308–18. https://doi.org/10.1002/ppp3.10183
-
125.
Fernández del Río L, Gutiérrez-Casado E, Varela-López A, Villalba JM. Olive Oil and the Hallmarks of Aging. Molecules. 2016; 21:163. https://doi.org/10.3390/molecules21020163 [PubMed]
-
126.
De Santis S, Cariello M, Piccinin E, Sabbà C, Moschetta A. Extra Virgin Olive Oil: Lesson from Nutrigenomics. Nutrients. 2019; 11:2085. https://doi.org/10.3390/nu11092085 [PubMed]
-
127.
Daimiel L, Micó V, Valls RM, Pedret A, Motilva MJ, Rubió L, Fitó M, Farrás M, Covas MI, Solá R, Ordovás JM. Impact of Phenol-Enriched Virgin Olive Oils on the Postprandial Levels of Circulating microRNAs Related to Cardiovascular Disease. Mol Nutr Food Res. 2020; 64:e2000049. https://doi.org/10.1002/mnfr.202000049 [PubMed]
-
128.
Ito N, Seki S, Ueda F. The Protective Role of Astaxanthin for UV-Induced Skin Deterioration in Healthy People-A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients. 2018; 10:817. https://doi.org/10.3390/nu10070817 [PubMed]
-
129.
Rizwana N, Agarwal V, Nune M. Antioxidant for Neurological Diseases and Neurotrauma and Bioengineering Approaches. Antioxidants (Basel). 2021; 11:72. https://doi.org/10.3390/antiox11010072 [PubMed]
-
130.
Fantacone ML, Lowry MB, Uesugi SL, Michels AJ, Choi J, Leonard SW, Gombart SK, Gombart JS, Bobe G, Gombart AF. The Effect of a Multivitamin and Mineral Supplement on Immune Function in Healthy Older Adults: A Double-Blind, Randomized, Controlled Trial. Nutrients. 2020; 12:2447. https://doi.org/10.3390/nu12082447 [PubMed]
-
131.
Vaiserman A, Koliada A, Lushchak O. Neuroinflammation in pathogenesis of Alzheimer's disease: Phytochemicals as potential therapeutics. Mech Ageing Dev. 2020; 189:111259. https://doi.org/10.1016/j.mad.2020.111259 [PubMed]
-
132.
Ruskovska T, Budić-Leto I, Corral-Jara KF, Ajdžanović V, Arola-Arnal A, Bravo FI, Deligiannidou GE, Havlik J, Janeva M, Kistanova E, Kontogiorgis C, Krga I, Massaro M, et al. Systematic Bioinformatic Analyses of Nutrigenomic Modifications by Polyphenols Associated with Cardiometabolic Health in Humans-Evidence from Targeted Nutrigenomic Studies. Nutrients. 2021; 13:2326. https://doi.org/10.3390/nu13072326 [PubMed]
-
133.
Payne A, Nahashon S, Taka E, Adinew GM, Soliman KFA. Epigallocatechin-3-Gallate (EGCG): New Therapeutic Perspectives for Neuroprotection, Aging, and Neuroinflammation for the Modern Age. Biomolecules. 2022; 12:371. https://doi.org/10.3390/biom12030371 [PubMed]
-
134.
Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010; 328:321–6. https://doi.org/10.1126/science.1172539 [PubMed]
-
135.
Fontana L, Partridge L. Promoting health and longevity through diet: from model organisms to humans. Cell. 2015; 161:106–18. https://doi.org/10.1016/j.cell.2015.02.020 [PubMed]
-
136.
Hahn O, Grönke S, Stubbs TM, Ficz G, Hendrich O, Krueger F, Andrews S, Zhang Q, Wakelam MJ, Beyer A, Reik W, Partridge L. Dietary restriction protects from age-associated DNA methylation and induces epigenetic reprogramming of lipid metabolism. Genome Biol. 2017; 18:56. https://doi.org/10.1186/s13059-017-1187-1 [PubMed]
-
137.
Hearn J, Pearson M, Blaxter M, Wilson PJ, Little TJ. Genome-wide methylation is modified by caloric restriction in Daphnia magna. BMC Genomics. 2019; 20:197. https://doi.org/10.1186/s12864-019-5578-4 [PubMed]
-
138.
Willcox BJ, Willcox DC. Caloric restriction, caloric restriction mimetics, and healthy aging in Okinawa: controversies and clinical implications. Curr Opin Clin Nutr Metab Care. 2014; 17:51–8. https://doi.org/10.1097/MCO.0000000000000019 [PubMed]
-
139.
Dorling JL, Martin CK, Redman LM. Calorie restriction for enhanced longevity: The role of novel dietary strategies in the present obesogenic environment. Ageing Res Rev. 2020; 64:101038. https://doi.org/10.1016/j.arr.2020.101038 [PubMed]
-
140.
Buettner D. Blue Zones - Lessons for Living Longer From the People Who’ve Lived the Longest. National Geographic Society. 2010.
-
141.
Speakman JR, Mitchell SE. Caloric restriction. Mol Aspects Med. 2011; 32:159–221. https://doi.org/10.1016/j.mam.2011.07.001 [PubMed]
-
142.
CR Society International. Welcome to the CR Society International. CR Society International. 1994.
-
143.
Mattson MP. Hormesis defined. Ageing Res Rev. 2008; 7:1–7. https://doi.org/10.1016/j.arr.2007.08.007 [PubMed]
-
144.
Kisby GE, Kohama SG, Olivas A, Churchwell M, Doerge D, Spangler E, de Cabo R, Ingram DK, Imhof B, Bao G, Kow YW. Effect of caloric restriction on base-excision repair (BER) in the aging rat brain. Exp Gerontol. 2010; 45:208–16. https://doi.org/10.1016/j.exger.2009.12.003 [PubMed]
-
145.
Hadad N, Unnikrishnan A, Jackson JA, Masser DR, Otalora L, Stanford DR, Richardson A, Freeman WM. Caloric restriction mitigates age-associated hippocampal differential CG and non-CG methylation. Neurobiol Aging. 2018; 67:53–66. https://doi.org/10.1016/j.neurobiolaging.2018.03.009 [PubMed]
-
146.
Yessenkyzy A, Saliev T, Zhanaliyeva M, Masoud AR, Umbayev B, Sergazy S, Krivykh E, Gulyayev A, Nurgozhin T. Polyphenols as Caloric-Restriction Mimetics and Autophagy Inducers in Aging Research. Nutrients. 2020; 12:1344. https://doi.org/10.3390/nu12051344 [PubMed]
-
147.
Riera CE, Merkwirth C, De Magalhaes Filho CD, Dillin A. Signaling Networks Determining Life Span. Annu Rev Biochem. 2016; 85:35–64. https://doi.org/10.1146/annurev-biochem-060815-014451 [PubMed]
-
148.
Johnson ML, Distelmaier K, Lanza IR, Irving BA, Robinson MM, Konopka AR, Shulman GI, Nair KS. Mechanism by Which Caloric Restriction Improves Insulin Sensitivity in Sedentary Obese Adults. Diabetes. 2016; 65:74–84. https://doi.org/10.2337/db15-0675 [PubMed]
-
149.
Brown-Borg HM, Bartke A. GH and IGF1: roles in energy metabolism of long-living GH mutant mice. J Gerontol A Biol Sci Med Sci. 2012; 67:652–60. https://doi.org/10.1093/gerona/gls086 [PubMed]
-
150.
Gillespie ZE, Pickering J, Eskiw CH. Better Living through Chemistry: Caloric Restriction (CR) and CR Mimetics Alter Genome Function to Promote Increased Health and Lifespan. Front Genet. 2016; 7:142. https://doi.org/10.3389/fgene.2016.00142 [PubMed]
-
151.
Shimokawa I, Komatsu T, Hayashi N, Kim SE, Kawata T, Park S, Hayashi H, Yamaza H, Chiba T, Mori R. The life-extending effect of dietary restriction requires Foxo3 in mice. Aging Cell. 2015; 14:707–9. https://doi.org/10.1111/acel.12340 [PubMed]
-
152.
Hofer SJ, Carmona-Gutierrez D, Mueller MI, Madeo F. The ups and downs of caloric restriction and fasting: from molecular effects to clinical application. EMBO Mol Med. 2022; 14:e14418. https://doi.org/10.15252/emmm.202114418 [PubMed]
-
153.
Martin CK, Bhapkar M, Pittas AG, Pieper CF, Das SK, Williamson DA, Scott T, Redman LM, Stein R, Gilhooly CH, Stewart T, Robinson L, Roberts SB, and Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy (CALERIE) Phase 2 Study Group. Effect of Calorie Restriction on Mood, Quality of Life, Sleep, and Sexual Function in Healthy Nonobese Adults: The CALERIE 2 Randomized Clinical Trial. JAMA Intern Med. 2016; 176:743–52. https://doi.org/10.1001/jamainternmed.2016.1189 [PubMed]
-
154.
Chouliaras L, van den Hove DL, Kenis G, Keitel S, Hof PR, van Os J, Steinbusch HW, Schmitz C, Rutten BP. Prevention of age-related changes in hippocampal levels of 5-methylcytidine by caloric restriction. Neurobiol Aging. 2012; 33:1672–81. https://doi.org/10.1016/j.neurobiolaging.2011.06.003 [PubMed]
-
155.
Moreno CL, Mobbs CV. Epigenetic mechanisms underlying lifespan and age-related effects of dietary restriction and the ketogenic diet. Mol Cell Endocrinol. 2017; 455:33–40. https://doi.org/10.1016/j.mce.2016.11.013 [PubMed]
-
156.
Zhang R, Wang X, Qu JH, Liu B, Zhang P, Zhang T, Fan PC, Wang XM, Xiao GY, Su Y, Xie Y, Liu Y, Pei JF, et al. Caloric Restriction Induces MicroRNAs to Improve Mitochondrial Proteostasis. iScience. 2019; 17:155–66. https://doi.org/10.1016/j.isci.2019.06.028 [PubMed]
-
157.
Pandey M, Bansal S, Bar S, Yadav AK, Sokol NS, Tennessen JM, Kapahi P, Chawla G. miR-125-chinmo pathway regulates dietary restriction-dependent enhancement of lifespan in Drosophila. Elife. 2021; 10:e62621. https://doi.org/10.7554/eLife.62621 [PubMed]
-
158.
Maegawa S, Lu Y, Tahara T, Lee JT, Madzo J, Liang S, Jelinek J, Colman RJ, Issa JJ. Caloric restriction delays age-related methylation drift. Nat Commun. 2017; 8:539. https://doi.org/10.1038/s41467-017-00607-3 [PubMed]
-
159.
Pifferi F, Terrien J, Marchal J, Dal-Pan A, Djelti F, Hardy I, Chahory S, Cordonnier N, Desquilbet L, Hurion M, Zahariev A, Chery I, Zizzari P, et al. Caloric restriction increases lifespan but affects brain integrity in grey mouse lemur primates. Commun Biol. 2018; 1:30. https://doi.org/10.1038/s42003-018-0024-8 [PubMed]
-
160.
Duregon E, Pomatto-Watson LCD, Bernier M, Price NL, de Cabo R. Intermittent fasting: from calories to time restriction. Geroscience. 2021; 43:1083–92. https://doi.org/10.1007/s11357-021-00335-z [PubMed]
-
161.
Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017; 39:46–58. https://doi.org/10.1016/j.arr.2016.10.005 [PubMed]
-
162.
Liu X, Morris MC, Dhana K, Ventrelle J, Johnson K, Bishop L, Hollings CS, Boulin A, Laranjo N, Stubbs BJ, Reilly X, Carey VJ, Wang Y, et al. Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) study: Rationale, design and baseline characteristics of a randomized control trial of the MIND diet on cognitive decline. Contemp Clin Trials. 2021; 102:106270. https://doi.org/10.1016/j.cct.2021.106270 [PubMed]
-
163.
Dirks AJ, Leeuwenburgh C. Caloric restriction in humans: potential pitfalls and health concerns. Mech Ageing Dev. 2006; 127:1–7. https://doi.org/10.1016/j.mad.2005.09.001 [PubMed]
-
164.
Paul T. One Study, Decades of Discoveries. Columbia - Mailman School of Public Health. 2021.
-
165.
Landi F, Calvani R, Tosato M, Martone AM, Ortolani E, Savera G, Sisto A, Marzetti E. Anorexia of Aging: Risk Factors, Consequences, and Potential Treatments. Nutrients. 2016; 8:69. https://doi.org/10.3390/nu8020069 [PubMed]
-
166.
Belsky DW, Huffman KM, Pieper CF, Shalev I, Kraus WE. Change in the Rate of Biological Aging in Response to Caloric Restriction: CALERIE Biobank Analysis. J Gerontol A Biol Sci Med Sci. 2017; 73:4–10. https://doi.org/10.1093/gerona/glx096 [PubMed]
-
167.
Hofer SJ, Davinelli S, Bergmann M, Scapagnini G, Madeo F. Caloric Restriction Mimetics in Nutrition and Clinical Trials. Front Nutr. 2021; 8:717343. https://doi.org/10.3389/fnut.2021.717343 [PubMed]
-
168.
Calerie Health. Self Care Starts With Cell Care. Calerie Health. 2022.
-
169.
Lautrup S, Sinclair DA, Mattson MP, Fang EF. NAD+ in Brain Aging and Neurodegenerative Disorders. Cell Metab. 2019; 30:630–55. https://doi.org/10.1016/j.cmet.2019.09.001 [PubMed]
-
170.
Kiss T, Giles CB, Tarantini S, Yabluchanskiy A, Balasubramanian P, Gautam T, Csipo T, Nyúl-Tóth Á, Lipecz A, Szabo C, Farkas E, Wren JD, Csiszar A, Ungvari Z. Nicotinamide mononucleotide (NMN) supplementation promotes anti-aging miRNA expression profile in the aorta of aged mice, predicting epigenetic rejuvenation and anti-atherogenic effects. Geroscience. 2019; 41:419–39. https://doi.org/10.1007/s11357-019-00095-x [PubMed]
-
171.
Tarantini S, Valcarcel-Ares MN, Toth P, Yabluchanskiy A, Tucsek Z, Kiss T, Hertelendy P, Kinter M, Ballabh P, Süle Z, Farkas E, Baur JA, Sinclair DA, et al. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol. 2019; 24:101192. https://doi.org/10.1016/j.redox.2019.101192 [PubMed]
-
172.
Yoshino J, Baur JA, Imai SI. NAD+ Intermediates: The Biology and Therapeutic Potential of NMN and NR. Cell Metab. 2018; 27:513–28. https://doi.org/10.1016/j.cmet.2017.11.002 [PubMed]
-
173.
Jimenez L, Silva A, Calissi G, Grenho I, Monteiro R, Mayoral-Varo V, Blanco-Aparicio C, Pastor J, Bustos V, Bracher F, Megías D, Ferreira BI, Link W. Screening Health-Promoting Compounds for Their Capacity to Induce the Activity of FOXO3. J Gerontol A Biol Sci Med Sci. 2022; 77:1485–93. https://doi.org/10.1093/gerona/glab265 [PubMed]
-
174.
Calissi G, Lam EW, Link W. Therapeutic strategies targeting FOXO transcription factors. Nat Rev Drug Discov. 2021; 20:21–38. https://doi.org/10.1038/s41573-020-0088-2 [PubMed]
-
175.
Orea-Soufi A, Paik J, Bragança J, Donlon TA, Willcox BJ, Link W. FOXO transcription factors as therapeutic targets in human diseases. Trends Pharmacol Sci. 2022; 43:1070–84. https://doi.org/10.1016/j.tips.2022.09.010 [PubMed]
-
176.
Miller RA, Harrison DE, Astle CM, Floyd RA, Flurkey K, Hensley KL, Javors MA, Leeuwenburgh C, Nelson JF, Ongini E, Nadon NL, Warner HR, Strong R. An Aging Interventions Testing Program: study design and interim report. Aging Cell. 2007; 6:565–75. https://doi.org/10.1111/j.1474-9726.2007.00311.x [PubMed]
-
177.
Hernández-Saavedra D, Moody L, Xu GB, Chen H, Pan YX. Epigenetic Regulation of Metabolism and Inflammation by Calorie Restriction. Adv Nutr. 2019; 10:520–36. https://doi.org/10.1093/advances/nmy129 [PubMed]
-
178.
Anisimov VN, Bartke A. The key role of growth hormone-insulin-IGF-1 signaling in aging and cancer. Crit Rev Oncol Hematol. 2013; 87:201–23. https://doi.org/10.1016/j.critrevonc.2013.01.005 [PubMed]
-
179.
Mohammed I, Hollenberg MD, Ding H, Triggle CR. A Critical Review of the Evidence That Metformin Is a Putative Anti-Aging Drug That Enhances Healthspan and Extends Lifespan. Front Endocrinol (Lausanne). 2021; 12:718942. https://doi.org/10.3389/fendo.2021.718942 [PubMed]
-
180.
Partridge L, Fuentealba M, Kennedy BK. The quest to slow ageing through drug discovery. Nat Rev Drug Discov. 2020; 19:513–32. https://doi.org/10.1038/s41573-020-0067-7 [PubMed]
-
181.
Fang WJ, Wang CJ, He Y, Zhou YL, Peng XD, Liu SK. Resveratrol alleviates diabetic cardiomyopathy in rats by improving mitochondrial function through PGC-1α deacetylation. Acta Pharmacol Sin. 2018; 39:59–73. https://doi.org/10.1038/aps.2017.50 [PubMed]
-
182.
Griñán-Ferré C, Bellver-Sanchis A, Izquierdo V, Corpas R, Roig-Soriano J, Chillón M, Andres-Lacueva C, Somogyvári M, Sőti C, Sanfeliu C, Pallàs M. The pleiotropic neuroprotective effects of resveratrol in cognitive decline and Alzheimer's disease pathology: From antioxidant to epigenetic therapy. Ageing Res Rev. 2021; 67:101271. https://doi.org/10.1016/j.arr.2021.101271 [PubMed]
-
183.
Dani C, Dias KM, Trevizol L, Bassôa L, Fraga I, Proença ICT, Pochmann D, Elsner VR. The impact of red grape juice (Vitis labrusca) consumption associated with physical training on oxidative stress, inflammatory and epigenetic modulation in healthy elderly women. Physiol Behav. 2021; 229:113215. https://doi.org/10.1016/j.physbeh.2020.113215 [PubMed]
-
184.
Meng X, Zhou J, Zhao CN, Gan RY, Li HB. Health Benefits and Molecular Mechanisms of Resveratrol: A Narrative Review. Foods. 2020; 9:340. https://doi.org/10.3390/foods9030340 [PubMed]
-
185.
Menendez JA. Metformin: Sentinel of the Epigenetic Landscapes That Underlie Cell Fate and Identity. Biomolecules. 2020; 10:780. https://doi.org/10.3390/biom10050780 [PubMed]
-
186.
Kulkarni AS, Gubbi S, Barzilai N. Benefits of Metformin in Attenuating the Hallmarks of Aging. Cell Metab. 2020; 32:15–30. https://doi.org/10.1016/j.cmet.2020.04.001 [PubMed]
-
187.
Bridgeman SC, Ellison GC, Melton PE, Newsholme P, Mamotte CDS. Epigenetic effects of metformin: From molecular mechanisms to clinical implications. Diabetes Obes Metab. 2018; 20:1553–62. https://doi.org/10.1111/dom.13262 [PubMed]
-
188.
Cuyàs E, Verdura S, Llorach-Pares L, Fernández-Arroyo S, Luciano-Mateo F, Cabré N, Stursa J, Werner L, Martin-Castillo B, Viollet B, Neuzil J, Joven J, Nonell-Canals A, et al. Metformin directly targets the H3K27me3 demethylase KDM6A/UTX. Aging Cell. 2018; 17:e12772. https://doi.org/10.1111/acel.12772 [PubMed]
-
189.
Gong H, Qian H, Ertl R, Astle CM, Wang GG, Harrison DE, Xu X. Histone modifications change with age, dietary restriction and rapamycin treatment in mouse brain. Oncotarget. 2015; 6:15882–90. https://doi.org/10.18632/oncotarget.4137 [PubMed]
-
190.
Chung CL, Lawrence I, Hoffman M, Elgindi D, Nadhan K, Potnis M, Jin A, Sershon C, Binnebose R, Lorenzini A, Sell C. Topical rapamycin reduces markers of senescence and aging in human skin: an exploratory, prospective, randomized trial. Geroscience. 2019; 41:861–9. https://doi.org/10.1007/s11357-019-00113-y [PubMed]
-
191.
Yin Z, Guo X, Qi Y, Li P, Liang S, Xu X, Shang X. Dietary Restriction and Rapamycin Affect Brain Aging in Mice by Attenuating Age-Related DNA Methylation Changes. Genes (Basel). 2022; 13:699. https://doi.org/10.3390/genes13040699 [PubMed]
-
192.
Segman Y, Duek A, Goldhechet Y, Maklovsky M, Chipuck M, Leiba M. [PROLONGED POST-COVID-19 ANEMIA]. Harefuah. 2022; 161:732–5. [PubMed]
-
193.
de Rezende LF, Rey-López JP, Matsudo VK, do Carmo Luiz O. Sedentary behavior and health outcomes among older adults: a systematic review. BMC Public Health. 2014; 14:333. https://doi.org/10.1186/1471-2458-14-333 [PubMed]
-
194.
Leirós-Rodríguez R, Romo-Pérez V, García-Soidán JL. Prevalence of urinary incontinence and its relation with sedentarism in Spain. Actas Urol Esp. 2017; 41:624–30. https://doi.org/10.1016/j.acuro.2017.04.002 [PubMed]
-
195.
Wheeler MJ, Dempsey PC, Grace MS, Ellis KA, Gardiner PA, Green DJ, Dunstan DW. Sedentary behavior as a risk factor for cognitive decline? A focus on the influence of glycemic control in brain health. Alzheimers Dement (N Y). 2017; 3:291–300. https://doi.org/10.1016/j.trci.2017.04.001 [PubMed]
-
196.
Yan S, Fu W, Wang C, Mao J, Liu B, Zou L, Lv C. Association between sedentary behavior and the risk of dementia: a systematic review and meta-analysis. Transl Psychiatry. 2020; 10:112. https://doi.org/10.1038/s41398-020-0799-5 [PubMed]
-
197.
Sellami M, Bragazzi N, Prince MS, Denham J, Elrayess M. Regular, Intense Exercise Training as a Healthy Aging Lifestyle Strategy: Preventing DNA Damage, Telomere Shortening and Adverse DNA Methylation Changes Over a Lifetime. Front Genet. 2021; 12:652497. https://doi.org/10.3389/fgene.2021.652497 [PubMed]
-
198.
Lautenschlager NT, Cox KL, Flicker L, Foster JK, van Bockxmeer FM, Xiao J, Greenop KR, Almeida OP. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008; 300:1027–37. https://doi.org/10.1001/jama.300.9.1027 [PubMed]
-
199.
Awick EA, Ehlers DK, Aguiñaga S, Daugherty AM, Kramer AF, McAuley E. Effects of a randomized exercise trial on physical activity, psychological distress and quality of life in older adults. Gen Hosp Psychiatry. 2017; 49:44–50. https://doi.org/10.1016/j.genhosppsych.2017.06.005 [PubMed]
-
200.
Abd El-Kader SM, Al-Shreef FM. Inflammatory cytokines and immune system modulation by aerobic versus resisted exercise training for elderly. Afr Health Sci. 2018; 18:120–31. https://doi.org/10.4314/ahs.v18i1.16 [PubMed]
-
201.
Neufer PD, Bamman MM, Muoio DM, Bouchard C, Cooper DM, Goodpaster BH, Booth FW, Kohrt WM, Gerszten RE, Mattson MP, Hepple RT, Kraus WE, Reid MB, et al. Understanding the Cellular and Molecular Mechanisms of Physical Activity-Induced Health Benefits. Cell Metab. 2015; 22:4–11. https://doi.org/10.1016/j.cmet.2015.05.011 [PubMed]
-
202.
Hoshi RA, Liu Y, Luttmann-Gibson H, Tiwari S, Giulianini F, Andres AM, Watrous JD, Cook NR, Costenbader KH, Okereke OI, Ridker PM, Manson JE, Lee IM, et al. Association of Physical Activity With Bioactive Lipids and Cardiovascular Events. Circ Res. 2022; 131:e84–99. https://doi.org/10.1161/CIRCRESAHA.122.320952 [PubMed]
-
203.
Wang Z, Emmerich A, Pillon NJ, Moore T, Hemerich D, Cornelis MC, Mazzaferro E, Broos S, Ahluwalia TS, Bartz TM, Bentley AR, Bielak LF, Chong M, et al, and Lifelines Cohort Study. Genome-wide association analyses of physical activity and sedentary behavior provide insights into underlying mechanisms and roles in disease prevention. Nat Genet. 2022; 54:1332–44. https://doi.org/10.1038/s41588-022-01165-1 [PubMed]
-
204.
O'Reilly CL, Miller BF, Lewis TL Jr. Exercise and mitochondrial remodeling to prevent age-related neurodegeneration. J Appl Physiol (1985). 2023; 134:181–9. https://doi.org/10.1152/japplphysiol.00611.2022 [PubMed]
-
205.
Reddy I, Yadav Y, Dey CS. Cellular and Molecular Regulation of Exercise-A Neuronal Perspective. Cell Mol Neurobiol. 2023. [Epub ahead of print]. https://doi.org/10.1007/s10571-022-01272-x [PubMed]
-
206.
Safdar A, Saleem A, Tarnopolsky MA. The potential of endurance exercise-derived exosomes to treat metabolic diseases. Nat Rev Endocrinol. 2016; 12:504–17. https://doi.org/10.1038/nrendo.2016.76 [PubMed]
-
207.
Ren H, Collins V, Clarke SJ, Han JS, Lam P, Clay F, Williamson LM, Andy Choo KH. Epigenetic changes in response to tai chi practice: a pilot investigation of DNA methylation marks. Evid Based Complement Alternat Med. 2012; 2012:841810. https://doi.org/10.1155/2012/841810 [PubMed]
-
208.
Ferrari L, Vicenzi M, Tarantini L, Barretta F, Sironi S, Baccarelli AA, Guazzi M, Bollati V. Effects of Physical Exercise on Endothelial Function and DNA Methylation. Int J Environ Res Public Health. 2019; 16:2530. https://doi.org/10.3390/ijerph16142530 [PubMed]
-
209.
Murach KA, Dimet-Wiley AL, Wen Y, Brightwell CR, Latham CM, Dungan CM, Fry CS, Watowich SJ. Late-life exercise mitigates skeletal muscle epigenetic aging. Aging Cell. 2022; 21:e13527. https://doi.org/10.1111/acel.13527 [PubMed]
-
210.
Kankaanpää A, Tolvanen A, Bollepalli S, Leskinen T, Kujala UM, Kaprio J, Ollikainen M, Sillanpää E. Leisure-Time and Occupational Physical Activity Associates Differently with Epigenetic Aging. Med Sci Sports Exerc. 2021; 53:487–95. https://doi.org/10.1249/MSS.0000000000002498 [PubMed]
-
211.
Chaix R, Alvarez-López MJ, Fagny M, Lemee L, Regnault B, Davidson RJ, Lutz A, Kaliman P. Epigenetic clock analysis in long-term meditators. Psychoneuroendocrinology. 2017; 85:210–4. https://doi.org/10.1016/j.psyneuen.2017.08.016 [PubMed]
-
212.
Saghiv MS, Sira DB, Goldhammer E, Sagiv M. The effects of aerobic and anaerobic exercises on circulating soluble-Klotho and IGF-I in young and elderly adults and in CAD patients. J Circ Biomark. 2017; 6:1849454417733388. https://doi.org/10.1177/1849454417733388 [PubMed]
-
213.
Jokai M, Torma F, McGreevy KM, Koltai E, Bori Z, Babszki G, Bakonyi P, Gombos Z, Gyorgy B, Aczel D, Toth L, Osvath P, Fridvalszky M, et al. DNA methylation clock DNAmFitAge shows regular exercise is associated with slower aging and systemic adaptation. medRxiv. 2022. https://doi.org/10.1101/2022.07.22.22277842
-
214.
Föhr T, Waller K, Viljanen A, Sanchez R, Ollikainen M, Rantanen T, Kaprio J, Sillanpää E. Does the epigenetic clock GrimAge predict mortality independent of genetic influences: an 18 year follow-up study in older female twin pairs. Clin Epigenetics. 2021; 13:128. https://doi.org/10.1186/s13148-021-01112-7 [PubMed]
-
215.
Lo YH, Lin WY. Cardiovascular health and four epigenetic clocks. Clin Epigenetics. 2022; 14:73. https://doi.org/10.1186/s13148-022-01295-7 [PubMed]
-
216.
Naselli F, Belshaw NJ, Gentile C, Tutone M, Tesoriere L, Livrea MA, Caradonna F. Phytochemical Indicaxanthin Inhibits Colon Cancer Cell Growth and Affects the DNA Methylation Status by Influencing Epigenetically Modifying Enzyme Expression and Activity. J Nutrigenet Nutrigenomics. 2015; 8:114–27. https://doi.org/10.1159/000439382 [PubMed]
-
217.
Bellafiore M, Pintaudi AM, Thomas E, Tesoriere L, Bianco A, Cataldo A, Cerasola D, Traina M, Livrea MA, Palma A. Redox and autonomic responses to acute exercise-post recovery following Opuntia ficus-indica juice intake in physically active women. J Int Soc Sports Nutr. 2021; 18:43. https://doi.org/10.1186/s12970-021-00444-2 [PubMed]
-
218.
Sebastiani G, Almeida-Toledano L, Serra-Delgado M, Navarro-Tapia E, Sailer S, Valverde O, Garcia-Algar O, Andreu-Fernández V. Therapeutic Effects of Catechins in Less Common Neurological and Neurodegenerative Disorders. Nutrients. 2021; 13:2232. https://doi.org/10.3390/nu13072232 [PubMed]
-
219.
Boyanapalli SS, Kong AT. "Curcumin, the King of Spices": Epigenetic Regulatory Mechanisms in the Prevention of Cancer, Neurological, and Inflammatory Diseases. Curr Pharmacol Rep. 2015; 1:129–39. https://doi.org/10.1007/s40495-015-0018-x [PubMed]
-
220.
Cheng D, Li W, Wang L, Lin T, Poiani G, Wassef A, Hudlikar R, Ondar P, Brunetti L, Kong AN. Pharmacokinetics, Pharmacodynamics, and PKPD Modeling of Curcumin in Regulating Antioxidant and Epigenetic Gene Expression in Healthy Human Volunteers. Mol Pharm. 2019; 16:1881–9. https://doi.org/10.1021/acs.molpharmaceut.8b01246 [PubMed]
-
221.
Moody L, Crowder SL, Fruge AD, Locher JL, Demark-Wahnefried W, Rogers LQ, Delk-Licata A, Carroll WR, Spencer SA, Black M, Erdman JW Jr, Chen H, Pan YX, Arthur AE. Epigenetic stratification of head and neck cancer survivors reveals differences in lycopene levels, alcohol consumption, and methylation of immune regulatory genes. Clin Epigenetics. 2020; 12:138. https://doi.org/10.1186/s13148-020-00930-5 [PubMed]
-
222.
Bottero V, Potashkin JA. A Comparison of Gene Expression Changes in the Blood of Individuals Consuming Diets Supplemented with Olives, Nuts or Long-Chain Omega-3 Fatty Acids. Nutrients. 2020; 12:3765. https://doi.org/10.3390/nu12123765 [PubMed]
-
223.
Minokawa Y, Sawada Y, Nakamura M. The Influences of Omega-3 Polyunsaturated Fatty Acids on the Development of Skin Cancers. Diagnostics (Basel). 2021; 11:2149. https://doi.org/10.3390/diagnostics11112149 [PubMed]
-
224.
Gil-Zamorano J, Cofán M, López de Las Hazas MC, García-Blanco T, García-Ruiz A, Doménech M, Serra-Mir M, Roth I, Valls-Pedret C, Rajaram S, Sabaté J, Ros E, Dávalos A, Sala-Vila A. Interplay of Walnut Consumption, Changes in Circulating miRNAs and Reduction in LDL-Cholesterol in Elders. Nutrients. 2022; 14:1473. https://doi.org/10.3390/nu14071473 [PubMed]
-
225.
Park S, Lim W, Bazer FW, Whang KY, Song G. Quercetin inhibits proliferation of endometriosis regulating cyclin D1 and its target microRNAs in vitro and in vivo. J Nutr Biochem. 2019; 63:87–100. https://doi.org/10.1016/j.jnutbio.2018.09.024 [PubMed]
-
226.
Nishihira J, Nishimura M, Kurimoto M, Kagami-Katsuyama H, Hattori H, Nakagawa T, Muro T, Kobori M. The effect of 24-week continuous intake of quercetin-rich onion on age-related cognitive decline in healthy elderly people: a randomized, double-blind, placebo-controlled, parallel-group comparative clinical trial. J Clin Biochem Nutr. 2021; 69:203–15. https://doi.org/10.3164/jcbn.21-17 [PubMed]
-
227.
Dashwood RH, Ho E. Dietary histone deacetylase inhibitors: from cells to mice to man. Semin Cancer Biol. 2007; 17:363–9. https://doi.org/10.1016/j.semcancer.2007.04.001 [PubMed]
-
228.
Li Y, Buckhaults P, Li S, Tollefsbol T. Temporal Efficacy of a Sulforaphane-Based Broccoli Sprout Diet in Prevention of Breast Cancer through Modulation of Epigenetic Mechanisms. Cancer Prev Res (Phila). 2018; 11:451–64. https://doi.org/10.1158/1940-6207.CAPR-17-0423 [PubMed]
-
229.
Santín-Márquez R, Alarcón-Aguilar A, López-Diazguerrero NE, Chondrogianni N, Königsberg M. Sulforaphane - role in aging and neurodegeneration. Geroscience. 2019; 41:655–70. https://doi.org/10.1007/s11357-019-00061-7 [PubMed]
-
230.
Xu Y, Su D, Zhu L, Zhang S, Ma S, Wu K, Yuan Q, Lin N. S-allylcysteine suppresses ovarian cancer cell proliferation by DNA methylation through DNMT1. J Ovarian Res. 2018; 11:39. https://doi.org/10.1186/s13048-018-0412-1 [PubMed]
-
231.
De Greef D, Barton EM, Sandberg EN, Croley CR, Pumarol J, Wong TL, Das N, Bishayee A. Anticancer potential of garlic and its bioactive constituents: A systematic and comprehensive review. Semin Cancer Biol. 2021; 73:219–64. https://doi.org/10.1016/j.semcancer.2020.11.020 [PubMed]
-
232.
Baggish AL, Park J, Min PK, Isaacs S, Parker BA, Thompson PD, Troyanos C, D'Hemecourt P, Dyer S, Thiel M, Hale A, Chan SY. Rapid upregulation and clearance of distinct circulating microRNAs after prolonged aerobic exercise. J Appl Physiol (1985). 2014; 116:522–31. https://doi.org/10.1152/japplphysiol.01141.2013 [PubMed]
-
233.
Hunter DJ, James L, Hussey B, Wadley AJ, Lindley MR, Mastana SS. Impact of aerobic exercise and fatty acid supplementation on global and gene-specific DNA methylation. Epigenetics. 2019; 14:294–309. https://doi.org/10.1080/15592294.2019.1582276 [PubMed]
-
234.
Russo A, Bartolini D, Mensà E, Torquato P, Albertini MC, Olivieri F, Testa R, Rossi S, Piroddi M, Cruciani G, De Feo P, Galli F. Physical Activity Modulates the Overexpression of the Inflammatory miR-146a-5p in Obese Patients. IUBMB Life. 2018; 70:1012–22. https://doi.org/10.1002/iub.1926 [PubMed]
-
235.
Hwang SH, Kang DW, Lee MK, Byeon JY, Park H, Park DH, Kim KC, Lee ST, Chu SH, Kim NK, Jeon JY. Changes in DNA methylation after 6-week exercise training in colorectal cancer survivors: A preliminary study. Asia Pac J Clin Oncol. 2022; 18:52–60. https://doi.org/10.1111/ajco.13482 [PubMed]
-
236.
Machado OAS, Diniz VLS, Passos MEP, de Oliveira HH, Santos-Oliveira LC, Alecrim AL, Bertola Lobato T, Manoel R, Correa I, Silva EB, de Oliveira Poma S, Mendes de Almeida M, Pithon-Curi TC, et al. Physical exercise increases global and gene-specific (interleukin-17 and interferon-γ) DNA methylation in lymphocytes from aged women. Exp Physiol. 2021; 106:1878–85. https://doi.org/10.1113/EP089673 [PubMed]
-
237.
Dimauro I, Scalabrin M, Fantini C, Grazioli E, Beltran Valls MR, Mercatelli N, Parisi A, Sabatini S, Di Luigi L, Caporossi D. Resistance training and redox homeostasis: Correlation with age-associated genomic changes. Redox Biol. 2016; 10:34–44. https://doi.org/10.1016/j.redox.2016.09.008 [PubMed]
-
238.
Tolahunase M, Sagar R, Dada R. Impact of Yoga and Meditation on Cellular Aging in Apparently Healthy Individuals: A Prospective, Open-Label Single-Arm Exploratory Study. Oxid Med Cell Longev. 2017; 2017:7928981. https://doi.org/10.1155/2017/7928981 [PubMed]
-
239.
Bisht S, Banu S, Srivastava S, Pathak RU, Kumar R, Dada R, Mishra RK. Sperm methylome alterations following yoga-based lifestyle intervention in patients of primary male infertility: A pilot study. Andrologia. 2020; 52:e13551. https://doi.org/10.1111/and.13551 [PubMed]
-
240.
Liao S, Tan M, Li M, Ren J, Wang Y, Zheng R, Luo B, Xu W. Tai chi improves depressive symptoms among community-dwelling older persons by mediating BDNF methylation: A preliminary study. Geriatr Nurs. 2022; 44:137–42. https://doi.org/10.1016/j.gerinurse.2022.01.015 [PubMed]