Ferulic acid ameliorates the quality of in vitro-aged bovine oocytes by suppressing oxidative stress and apoptosis
Abstract
Ferulic acid (FA) is a well-known natural antioxidant that scavenges oxygen free radicals and alleviates oxidative stress. This study investigated the chemopreventive potential of FA against bovine oocyte quality decline during in vitro aging. The results showed that 5 μM FA supplementation decreased the abnormality rate of in vitro-aged bovine oocytes. In addition, FA supplementation effectively improved antioxidant capacity by removing excessive ROS and maintaining intracellular GSH levels and antioxidant enzyme activity. The mitochondrial activity, mitochondrial membrane potential and intracellular ATP levels in aged bovine oocytes were obviously enhanced by FA supplementation. Furthermore, FA supplementation reduced in vitro aging-induced DNA damage and maintained DNA stability in bovine oocytes. Moreover, sperm binding assay showed the number of sperm that bound to the zona pellucida on aged bovine oocytes was significantly higher in the FA supplemented group than in the Aged group. Therefore, FA is beneficial for maintaining in vitro-aged bovine oocyte quality and could become a potential antioxidant for preventing bovine oocyte in vitro aging during in vitro maturation.
Introduction
In the process of oocyte maturation in vivo or in vitro, oocytes in the metaphase of the second meiosis (MII) phase undergo time-related quality degradation if they are not fertilized in time [1]. In vivo, the inability to accurately predict the optimal fertilization time causes a delay in fertilization. Consequently, the oocytes can be retained in the oviduct after ovulation, which may cause oocyte aging [2]. In vitro, oocytes need to be cultured to maturation before micromanipulation and in vitro fertilization. Nevertheless, variations in individual oocytes result in distinct maturation durations, and extending the culture time is inevitable. This, in turn, contributes to oocyte aging [3].
Oocyte aging substantially diminishes fertilization rates and subsequent embryonic development potential [1], whilst also increasing the risk of miscarriage and fetal malformation [4]. Oocyte aging adversely affects oocyte quality mainly in terms of morphology and organelles as well as biochemical and molecular perspectives [5]. In terms of morphology and organelles, aging oocytes exhibit perivitelline space (PVS) increases, first polar body degradation [6], zona pellucida (ZP) hardening [7], chromosome disorder [8] and spindle morphological abnormalities [9]. From the biochemical and molecular perspectives, aging is often accompanied by excessive intracellular ROS accumulation [10], GSH levels reduction [11] and Ca2+ oscillation signal disorder [12]. There is much evidence revealing the close relationship between aging and ROS [13–15]. Excessive accumulation of ROS causes oxidative damage to DNA, proteins, and lipids, and the accumulation of oxidative damage is a common feature of aging [16–18]. As a matter of fact, aging-induced oxidative damage typically results in the malfunction or deactivation of multiple enzymes, which in turn causes DNA damage. The effects of DNA damage are varied. The blockage of gene transcription and DNA replication can result in various adverse effects, including cellular dysfunction or apoptosis [19]. With the gradual deepening of research on aging, especially oocyte aging, it has been found that supplementation with antioxidants during in vitro aging period can effectively delay oocyte aging, such as melatonin and coenzyme Q10 [20–23].
Ferulic acid (FA; 4-hydroxy-3-methoxycinnamic acid) is a natural antioxidant that is widely present in the cell walls of monocotyledonous plants [24]. It mainly prevents the occurrence of oxidative stress by scavenging excessive intracellular ROS [25]. In addition, FA has been shown to have antiaging effects [26]. Since aging is often accompanied by oxidative stress [10], we hypothesized that FA can delay oocyte aging and improve oocyte quality by resisting oxidative stress.
Here, we investigated the effect of FA on the abnormality rate of aging in bovine oocytes and evaluated the antioxidant capacity, mitochondrial activity and membrane potential (MMP), ATP levels, apoptosis and sperm binding capacity of in vitro-aged bovine oocytes. Our results will help to clarify the molecular mechanism of oocyte quality control and provide some data support and reference value for delaying oocyte aging and improving animal reproduction.
Results
FA palliates aging-induced oocyte morphological anomalies
After 20–22 h of in vitro culture, the oocytes were matured and categorized as the Fresh group. The culture time was prolonged to achieve in vitro aging for 6 h, 12 h, 24 h and 36 h. These oocytes were used and categorized as the Aged group (Figure 1A). Here, we detected the abnormality rate of oocytes. In this study, oocytes with a very granular PVS, large PVS, first polar body degradation, or nonuniform cytoplasm were considered as abnormal oocytes based on the observed oocyte morphology (Figure 1B). As shown in Figure 1C, there was a positive correlation between the abnormality rate of oocytes and the time of in vitro culture. Compared with that of the Fresh group (25.78 ± 2.83%, n = 93), the abnormality rates of oocytes aged for 12 h and above were significantly increased (in vitro aging for 12 h: 59.03 ± 1.46%, n = 109, P < 0.001; 24 h: 69.13 ± 3.77%, n = 114, P < 0.001 and 36 h: 83.78 ± 2.30%, n = 97, P < 0.001). In order to ensure the proper conduct of subsequent experiments, in vitro aging for 12 h was selected for further studies.
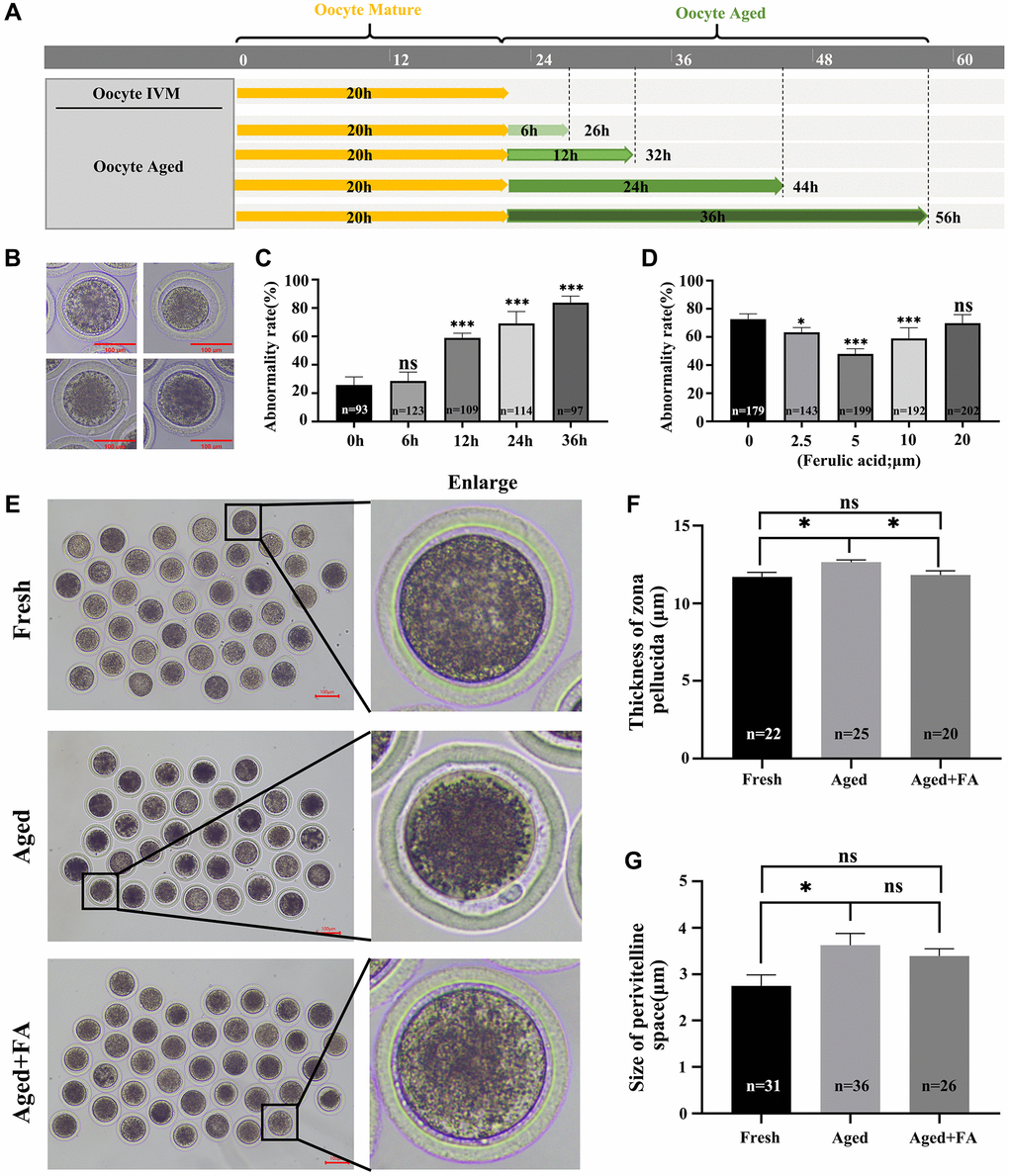
Figure 1. FA palliates aging-induced oocyte morphological anomalies. (A) Timeline diagram of in vitro-aged bovine oocytes. (B) Representative images of oocyte morphological anomalies (a very granular PVS, large PVS, first polar body degradation, and nonuniform cytoplasm). (C) The abnormality rates of in vitro aging for 6 h, 12 h, 24 h and 36 h groups. R = 4. (D) The abnormality rates of oocytes in vitro aged 12 h treated with different concentrations of FA (0, 2.5, 5, 10, or 20 μM). R = 7. (E) Representative images of PVS morphology in the Fresh, Aged, and Aged + FA groups. Scale bars: 100 μm. (F, G) Thickness of ZP and size of PVS of Fresh, Aged, Aged + FA groups. *P < 0.05, **P < 0.01, and ***P < 0.001 indicate significant differences.
To explore the effect of FA on the abnormality rate of oocytes during in vitro aging, different concentrations of FA (0, 2.5, 5, 10 and 20 μM) were supplemented during in vitro aging process. As shown in Figure 1D, compared with the control group (72.74 ± 1.41%, n = 179), the 2.5, 5 and 10 μM FA treatment groups had significantly lower abnormality rates of aging oocytes (2.5 μM: 63.36 ± 1.38%, n = 143, P < 0.05; 5 μM: 47.93 ± 1.30%, n = 199, P < 0.001; 10 μM: 59.08 ± 2.64%, n = 192, P < 0.001). Among them, the abnormality rate of aging oocytes in the 5 μM treatment group was the lowest. Therefore, a concentration of 5 μM was selected for subsequent studies.
The ZP thickness and PVS size are important indicators for evaluating whether oocytes are abnormal and for evaluating subsequent embryonic development. Here, we used existing evaluation methods to calculate the above two indices (Supplementary Figure 1) [27]. The results were shown in Figure 1E–1G. Compared with those in the Aged group (thickness: 12.64 ± 0.81 μm, n = 25; size: 3.83 ± 1.64 μm, n = 36), the thickness of the ZP (11.82 ± 1.22 μm, n = 20, P < 0.05) was significantly reduced, the size of the PVS (3.65 ± 1.00 μm, n = 26) showed no obvious change in the FA treatment group and higher than those in the Fresh group (thickness: 11.70 ± 1.36 μm, n = 22; size: 2.42 ± 1.34 μm, n = 31). The above results showed that FA could effectively alleviate aging-induced bovine oocytes morphological abnormality.
FA relieves aging-induced oocyte oxidation resistance
To explore the effect of FA on the antioxidant capacity of in vitro-aged oocytes, DCFH and CMF2HC were used to detect intracellular ROS and GSH levels, respectively. As shown in Figure 2A–2C, compared with those in Fresh group (ROS: 1.00 ± 0.03, n = 51; GSH: 1.00 ± 0.02, n = 64), the ROS levels in Aged group were significantly increased (3.36 ± 0.21, n = 50, P < 0.001), and the GSH levels were significantly decreased (0.61 ± 0.02, n = 52, P < 0.001). After FA supplementation, the levels of ROS in aged oocytes decreased significantly (1.18 ± 0.05, n = 39, P < 0.001), and the levels of GSH increased significantly (0.76 ± 0.03, n = 69, P < 0.001). To further explore the effect of FA on the antioxidant capacity of aging oocytes, we detected intracellular CAT and SOD activity. As shown in Figure 2D, 2E, the activity of CAT (0.29 ± 0.88, P < 0.001) and SOD (0.90 ± 0.01, P < 0.01) in the oocytes of the Aged group was significantly lower than that in the oocytes of the Fresh group (CAT: 1.00 ± 0.03; SOD: 1.00 ± 0.02), and the activity of the above two enzymes was significantly increased after FA supplementation (CAT:0.63 ± 0.13, P < 0.05; SOD:0.96 ± 0.02, P < 0.05). The above results indicated that FA could enhance the antioxidant capacity of aging oocytes.
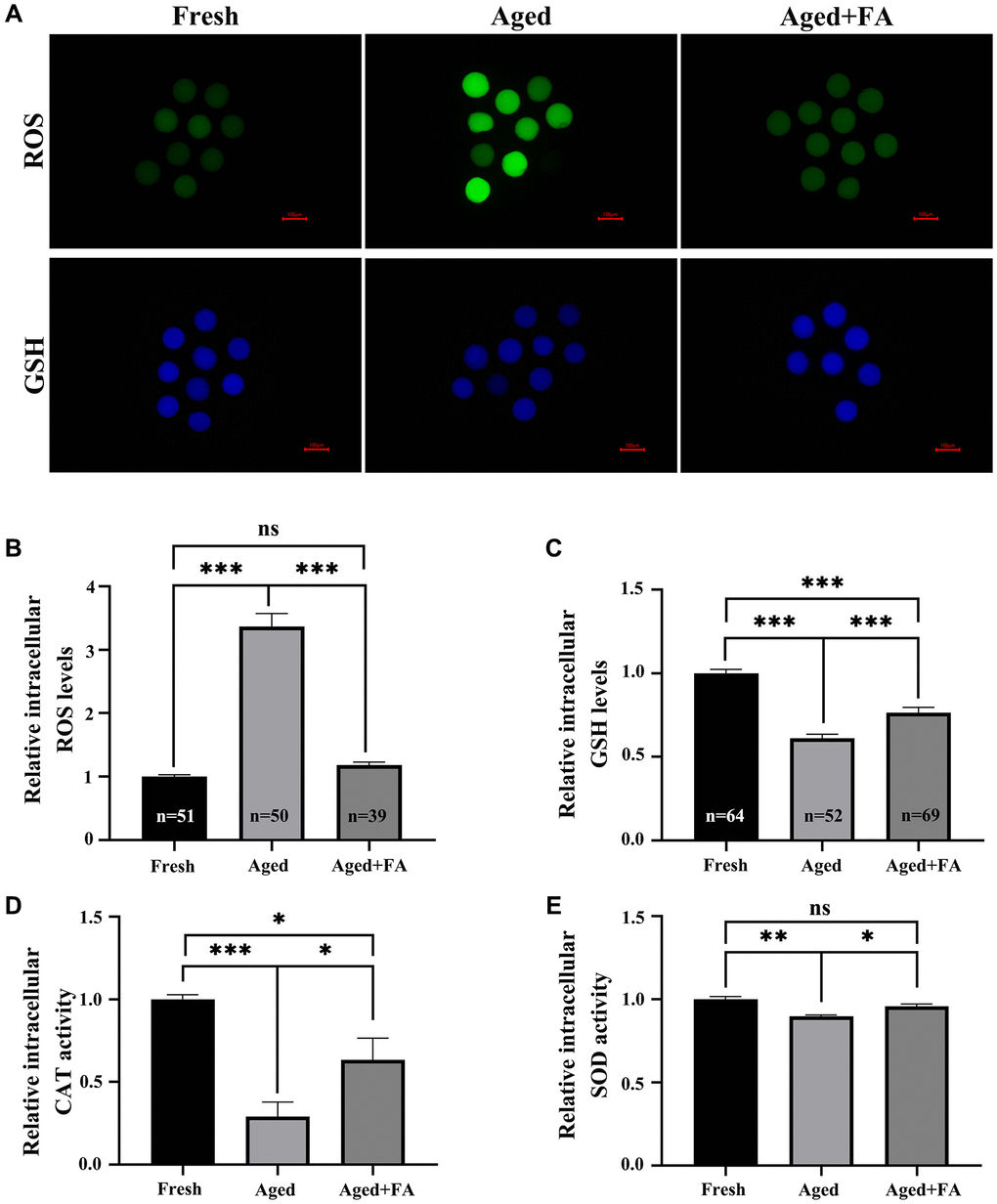
Figure 2. FA relieves aging-induced oocyte oxidation resistance. (A) Oocytes were stained with DCFH and CMF2HC to detect the intracellular ROS and GSH levels. Scale bar: 100 μm. R = 3. (B, C) Relative intracellular levels of ROS and GSH in bovine oocytes of the three groups (Fresh, Aged and Aged + FA group). (D, E) Relative intracellular activity of CAT and SOD in bovine oocytes from the three groups (Fresh, Aged, and Aged + FA). R = 4. *P < 0.05, **P < 0.01, and ***P < 0.001 indicate significant differences.
FA alleviates aging-induced oocyte mitochondrial dysfunction
We used MitoTracker Red CMXRos and JC-1 dyes to stain oocytes to detect mitochondrial activity and MMP levels in oocytes, respectively. It is known to all that JC-1 monomers accumulate in mitochondria and form red fluorescent “J-aggregates” at a high MMP. At mitochondrial transmembrane potentials depolarised at low MMP, JC-1 exists as a green fluorescent monomer (Figure 3A). As shown in Figure 3A–3C, compared with those in the Fresh group (mitochondrial activity: 1.00 ± 0.03, n = 40; MMP: 1.03 ± 0.03, n = 52), the mitochondrial activity and MMP levels of oocytes in the aged group were significantly decreased (mitochondrial activity: 0.63 ± 0.03, n = 44, P < 0.001; MMP: 0.39 ± 0.02, n = 43, P < 0.001). After FA supplementation, the mitochondrial activity and MMP levels of aging oocytes were significantly increased (mitochondrial activity: 0.83 ± 0.03, n = 45, P < 0.001; MMP: 0.60 ± 0.01, n = 42, P < 0.001).
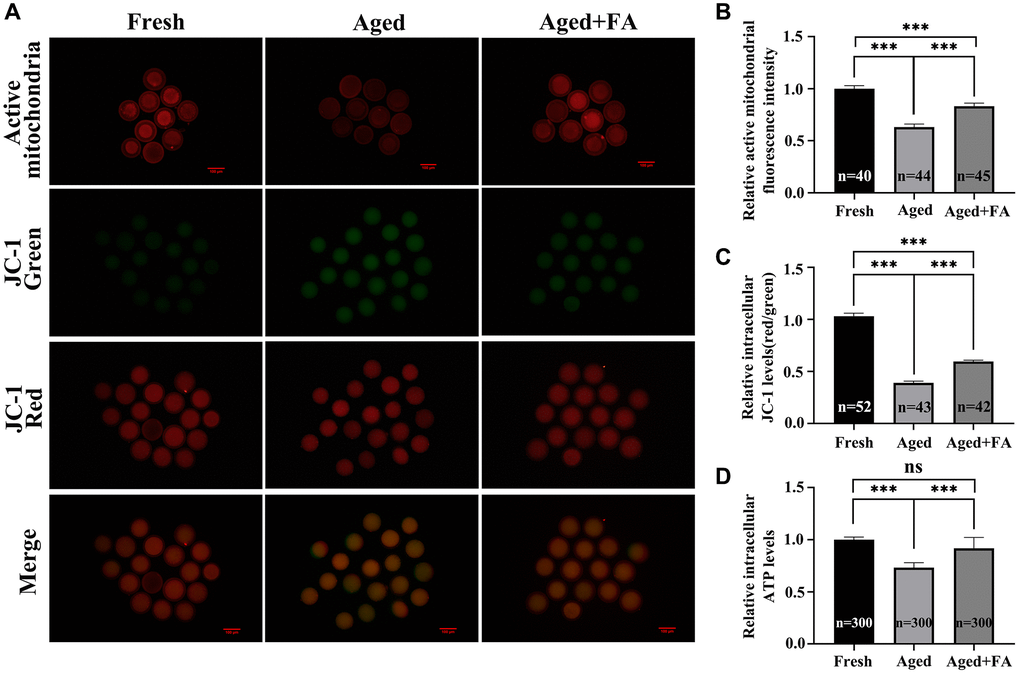
Figure 3. FA alleviates aging-induced oocyte mitochondrial dysfunction. (A) Oocytes were stained with MitoTracker Red CMXRos and JC-1 dyes to detect intracellular mitochondrial activity and MMP levels. Scale bar: 100 μm. R = 3. (B, C) Relative intracellular levels of active mitochondria and MMP in bovine oocytes from the three groups (Fresh, Aged, and Aged + FA group). (D) Relative intracellular ATP levels in bovine oocytes from the three groups (Fresh, Aged, and Aged + FA group). R = 6. *P < 0.05, **P < 0.01, and ***P < 0.001 indicate significant differences.
A decrease in MMP is often accompanied by changes in mitochondrial function, so we analyzed the intracellular ATP levels. As shown in Figure 3D, compared with those in the Fresh group (1.00 ± 0.01, n = 300), the ATP levels of oocytes in the Aged group were significantly decreased (0.73 ± 0.02, n = 300, P < 0.001). After FA supplementation, the ATP levels of aging oocytes were significantly increased (0.92 ± 0.04, n = 300, P < 0.001).
FA mitigates aging-induced cellular senescence and DNA damage
To explore the effect of FA on the cellular senescence of aging oocytes, we detected SA-β-Gal activity in oocytes. As shown in Figure 4A, 4B, the SA-β-Gal activity of oocytes in the Aged group was significantly higher than that of fresh oocytes (Aged group: 8.05 ± 0.26, n = 48; Fresh group: 1.00 ± 0.02, n = 50, P < 0.001). After FA supplementation, the activity of SA-β-Gal in aging oocytes was significantly decreased (2.76 ± 0.09, n = 47, P < 0.001).
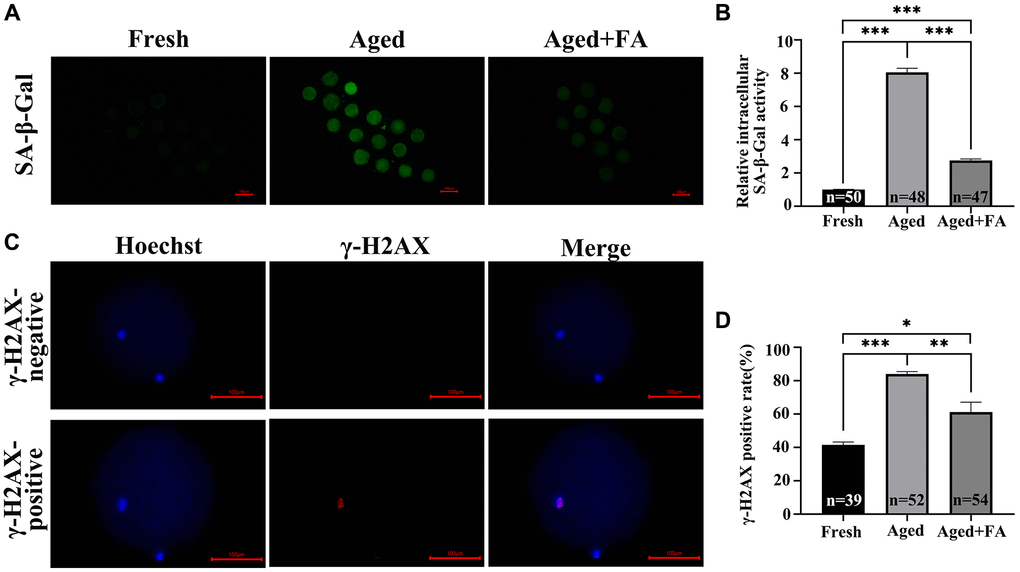
Figure 4. FA mitigates aging-induced cellular senescence and DNA damage. (A) Representative fluorescence images of intracellular SA-β-Gal activity in the three groups (Fresh, Aged, and Aged + FA). Scale bar: 100 μm. R = 3. (B) Relative intracellular levels of SA-β-Gal in bovine oocytes from the three groups (Fresh, Aged, and Aged + FA group). (C) Representative fluorescence images of positive and negative γ-H2AX staining. Scale bar: 100 μm. R = 3. (D) The γ-H2AX positivity rate in bovine oocytes from the three groups (Fresh, Aged, and Aged + FA group). *P < 0.05, **P < 0.01, and ***P < 0.001 indicate significant differences.
As aging occurs, DNA damage accumulates. This will reduce the stability of DNA double strands, which will lead to the decrease of oocyte quality. Therefore, we examined the expression of the DNA double-strand damage repair marker γ-H2AX among the three groups (Figure 4C). As shown in Figure 4D, compared with that in the Fresh group (41.66 ± 1.61%, n = 39), the proportion of γ-H2AX-positive oocytes in the Aged group was significantly higher (84.16 ± 1.40%, n = 52, P < 0.001), while the positive proportion decreased to 61.22 ± 5.98% after FA supplementation (n = 54, P < 0.01).
The above results indicated that FA reduced in vitro aging-induced DNA damage and breakage and maintained DNA stability.
FA inhibits aging-induced oocyte apoptosis
Persistent DNA damage is a trigger for apoptosis. To evaluate whether FA inhibited the apoptosis of aging oocytes, we detected the protein expression levels of cleaved caspase-3, Bax and Bcl2 (Figure 5). Western blot analysis showed that the expression levels of cleaved caspase-3 (1.42 ± 0.07, P < 0.01) and BAX/Bcl2 (1.54 ± 0.13, P < 0.01) in aged oocytes were significantly higher than those in fresh oocytes, while the expression levels of cleaved caspase-3 (1.14 ± 0.08, P < 0.05) and BAX/Bcl2 (1.19 ± 0.10, P < 0.05) in aged oocytes were significantly decreased after FA supplementation. The above results indicated that FA supplementation could inhibit the apoptosis of aging oocytes.
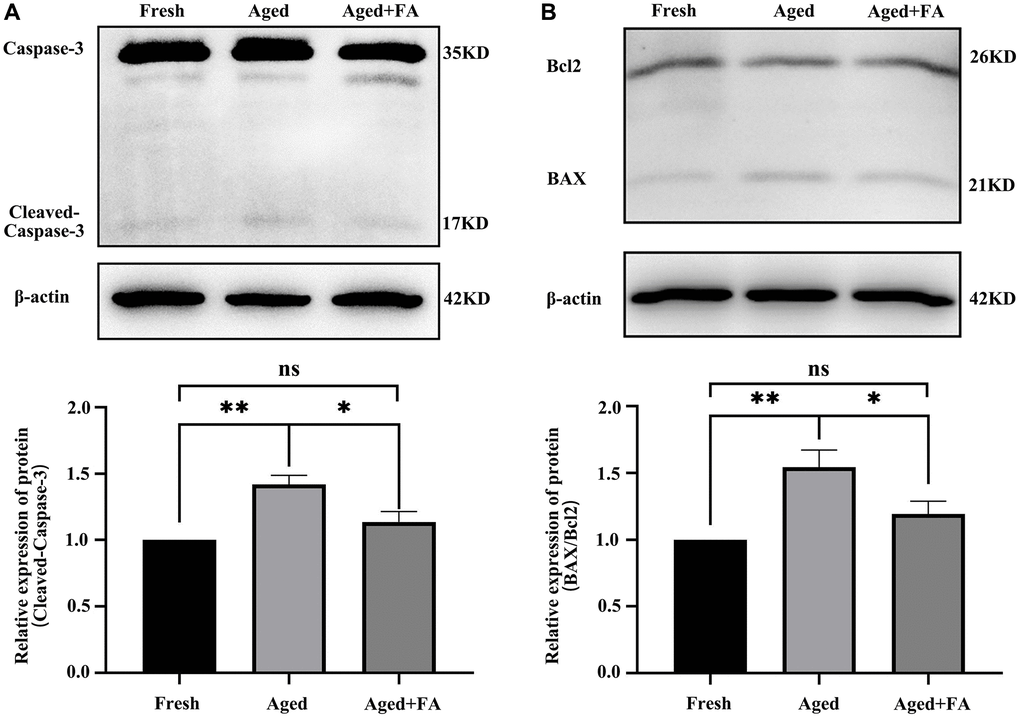
Figure 5. FA inhibits aging-induced oocyte apoptosis. (A, B) Representative Western blot images and relative expression levels of cleaved caspase-3 and BAX/Bcl2 in the three groups (Fresh, Aged, and Aged + FA group). R = 4. *P < 0.05, **P < 0.01, and ***P < 0.001 indicate significant differences.
FA improves the sperm-binding ability of aged oocytes
To explore the effect of FA on the fertilization ability of aging oocytes, we detected the number of sperm bound to oocytes by sperm-oocyte binding analysis (Figure 6). The results showed that compared with that of the Fresh group (256.44 ± 14, n =27), the number of sperm bound to the ZP of oocytes in the aged group was significantly reduced (100.76 ± 6.26, n = 34, P < 0.001). After FA supplementation, the number of ZP-bound sperm in aged oocytes increased significantly (187.70 ± 14.26, n = 23, P < 0.01). The above results showed that FA supplementation could improve the fertilization ability of bovine oocytes.
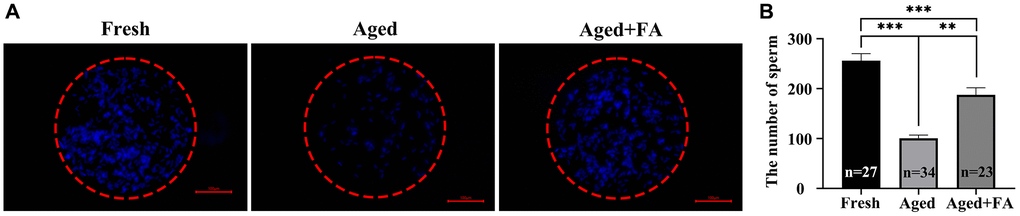
Figure 6. FA improves the sperm binding ability of aged oocytes. (A) Representative fluorescence images of sperm binding to the surface of the zona pellucida surrounding oocytes stained with Hoechst 33342 from the three groups (Fresh, Aged, and Aged + FA group). Scale bar: 100 μm. R = 3. (B) Number of sperm binding to the surface of the zona pellucida surrounding oocytes from the three groups (Fresh, Aged, and Aged + FA group). *P < 0.05, **P < 0.01, and ***P < 0.001 indicate significant differences.
Discussion
IVM is a significant part of assisted reproductive technology (ART), and oocyte quality exerts an important effect on IVM efficiency [28]. When the in vitro culture time is prolonged, the oocyte quality decreases in a time-dependent manner [29]. Many studies have shown that the optimal culture time of bovine oocytes are 20–22 h [30–32]. Here, we extended the culture time to set up an in vitro aging model, which demonstrated the positive effect of FA on in vitro-aged bovine oocytes.
Extensive results have shown that morphological abnormalities occur during IVM, especially after extended IVM period. These include a rise in the size of the PVS, an increase in debris within the PVS [6], fragmentation of the first polar body [33] and thickening of the ZP [34]. In this study, we observed the morphology of aged bovine oocytes at different culture time and treatment concentrations. In line with previous studies, our results suggest that the abnormality rate of oocytes increases in a time-dependent manner [29]. Moreover, FA supplementation has the potential to ameliorate the morphological abnormalities of aged oocytes.
The balance between ROS and GSH is essential for maintaining normal cell function [35]. ROS are widely involved in biological processes such as follicular development, meiosis, ovulation and embryonic development [36]. However, prolonged in vitro culture time can lead to excessive accumulation of ROS in unfertilized oocytes [37], resulting in oxidative stress that compromises oocyte quality [38, 39]. As a nonenzymatic antioxidant, GSH is responsible for cleaning up the excessive ROS to maintain cellular redox balance to alleviate intracellular oxidative stress [40] and improve the antioxidant capacity of aged oocytes [3, 41, 42]. In this study, we found that FA supplementation alleviated the increase in intracellular ROS and the decrease in GSH caused by oocyte aging. In addition, studies have shown that, as important members of antioxidative defense, the activity of CAT and SOD will decrease with the aging process [41, 43]. Meanwhile, FA can reduce intracellular ROS levels by increasing the activity of the above two antioxidant enzymes, thereby alleviating oxidative stress in oocytes [44, 45]. As expected, the activity of CAT and SOD in aged oocytes was increased after FA supplementation. The results of the experiment provide clear support for the statement that FA improves in vitro-aged bovine oocyte quality by resisting oxidative stress.
Mitochondria are the power sources of oocyte. They enable diverse physiological activities of cells by synthesizing ATP and have a fundamental role in oocyte maturation, fertilization and subsequent embryonic development [46–48]. There is mounting evidence indicating that mitochondrial dysfunction, as a cause or consequence of oxidative stress, is intrinsically linked to the process of aging [49]. Studies have shown that significant reductions in mitochondrial activity, MMP level and ATP production in aging oocytes can severely affect oocyte quality, which in turn leads to a decrease in oocyte development potential [50–52]. Our results showed that FA alleviated the decrease in MMP and partially restored mitochondrial function in aged oocytes. These results provide evidence that exogenous antioxidant supplementation can improve mitochondrial function in aged oocytes and thus promote oocyte developmental potential [42, 53, 54].
Aging-induced oocyte mitochondrial dysfunction can easily cause DNA damage in cells [55]. γH2AX, a biomarker of DNA double-strand breaks, recruits DNA repair proteins at the end of broken chromosomes to repair DNA damage [56, 57]. Previous studies have shown that aging can lead to a significant increase in the amount of γH2AX in cells [58, 59], which is consistent with our results. After FA supplementation, we observed a significant decrease in the γH2AX positive proportion. This suggests that FA has the potential to alleviate DNA double-strand breaks induced by aging and maintain the stability of DNA double-strands.
One of the prevalent cell responses to DNA damage is programmed cell death, or apoptosis [60, 61]. Caspase-3 is a crucial zymogen during cellular apoptosis, and is activated by cleavage during this process [62]. The antiapoptotic protein Bcl2 and the proapoptotic protein BAX induce apoptosis by permeabilizing the mitochondrial outer membrane (OMM) and then initiating the caspase cascade [63]. Our study found that the levels of cleaved caspase-3 and BAX/Bcl2 in aged oocytes were significantly increased, which was consistent with the findings of previous studies [64, 65]. After FA supplementation, the levels of the above apoptosis-related proteins were significantly reduced. These results clearly support our hypothesis that FA can protect oocytes against in vitro aging-induced apoptosis.
Sperm binding ability is one of the indicators used to evaluate the quality of oocytes. Since the complex process of fertilization begins with the binding of sperm to the ZP [66], we evaluated the sperm binding ability of oocytes via a sperm-oocyte binding assay. Here, we found that FA can increase the number of sperm bound to aging oocytes. Studies have shown that oocyte aging is usually accompanied by changes in the ZP [4]. It has been confirmed in mouse oocytes that postovulatory aging can lead to abnormal distribution of cortical granules and ovastacin in oocytes, resulting in premature cleavage of ZP2 before fertilization, thus hindering the normal binding of sperm to oocytes [67]. Therefore, we speculated that FA might improve the binding ability of sperm by alleviating premature exocytosis of aged oocytes.
In summary, this study revealed that bovine oocytes in vitro aged may lead to a series of molecular events in oocytes, including oocyte morphological abnormalities, oxidative damage, mitochondrial dysfunction, increasing apoptosis and decreasing sperm-oocyte binding ability. FA supplementation could effectively improve the quality of in vitro-aged bovine oocytes by improving the antioxidant capacity, ameliorating mitochondrial function and inhibiting apoptosis. The above results indicate that FA may be useful for delaying oocyte aging in other mammals and provide new ideas for improving oocyte quality (Figure 7).
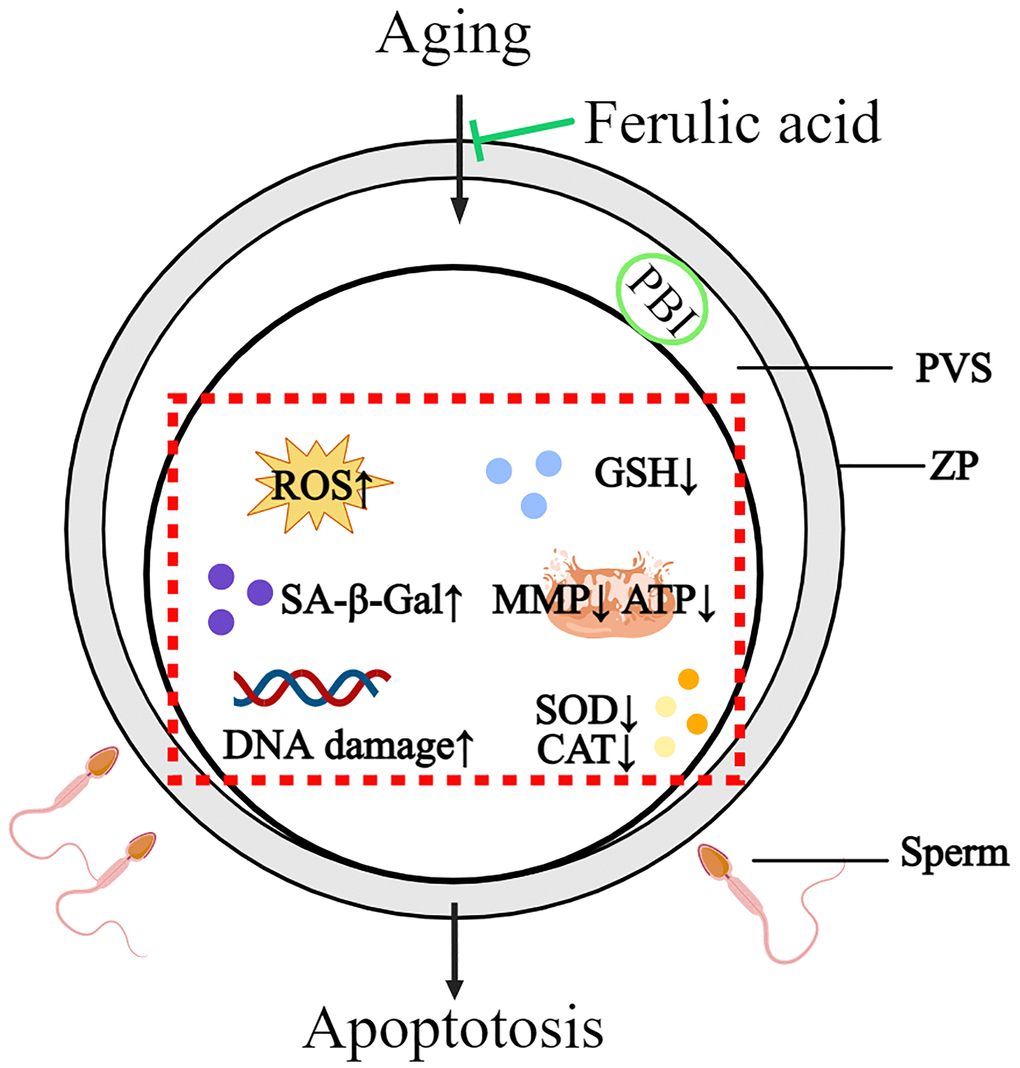
Figure 7. Schematic diagram of the protective action of FA on in vitro-aged bovine oocytes. After FA supplementation, intracellular ROS, SA-β-Gal, and DNA damage were decreased, while intracellular GSH, activity of CAT and SOD as well as mitochondria activity and function (MMP, ATP production) were increased in aged oocyte. These may help oocyte delay aging process and improve oocyte quality.
Materials and Methods
Chemicals and reagents
Unless otherwise specified, all chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA). FA (CymitQuimica, Spain, CAS: 537-98-4) was diluted with DMSO to working concentrations of 2.5 μM, 5 μM, 10 μM and 20 μM. The control group was treated with the same concentration of DMSO.
In vitro maturation and aging of bovine oocytes
Bovine ovaries were collected from the local slaughterhouse and transported to the laboratory within 2 hours at 37.5°C in normal saline supplemented with 1% penicillin G (75 mg/L) and streptomycin sulfate (50 mg/L). Cumulus oocyte complexes (COCs) were extracted from follicles with a diameter of 2–8 mm using 10 mL disposable syringes with an 18-gauge needle. Under a stereomicroscope (Zeiss, Stemi 305), oocytes wrapped in three or more intact cumulus layers were selected, washed three times in HEPES, placed in in vitro maturation (IVM) medium (tissue culture medium 199 supplemented with 100 mM Na pyruvate, 10 ng/mL EGF, 10% fetal bovine serum, 10 IU/mL follicle-stimulating hormone, 10 IU/mL luteinizing hormone and 2 μg/mL β-Estradiol), covered with mineral oil (Sage, ART-4008-5P) and placed in an environment under 38.5°C and 5% CO2 until maturation. For subsequent experiments, all experiments were performed on the basis of using 0.2% hyaluronidase to remove the cumulus cells of naked oocytes.
In vitro aging treatment
Oocytes were cultured to maturity (in vitro culture for 20 h), and the culture time was extended to 26, 32, 44 and 56 h to observe the abnormality rates. Subsequent experiments were carried out on naked oocytes.
Intracellular ROS and GSH level assay
To determine intracellular ROS and GSH levels, oocytes were treated with 2.5 μg/L 2′,7′-dichlorodihydrofluorescein diacetate (DCFH; S0033, Beyotime, China) or 10 μM 4-chloromethyl-6,8-difluoro-7-hydroxycoumarin (CMF2HC; C12881, Invitrogen, USA) and incubated in PBS-PVA medium at 37°C for 30 min. After washing the oocytes in PBS-PVA three times, the fluorescence intensity of each group of oocytes was captured by using a fluorescence microscope (Nikon, S22-LGB) and photographed. The fluorescence intensity was analyzed using ImageJ software (NIH, Stapleton, NY, USA).
Superoxide dismutase and catalase activity assay
The superoxide dismutase (SOD) activity and catalase (CAT) activity were measured using a WST-8 total superoxide dismutase detection kit (Beyotime, S101S) and a catalase detection kit (BC0205, Solarbio, China). After preparing the standard reaction solution and curve, 40 oocytes were dissolved in the relevant lysis buffer and incubated with the reaction buffer for 30 min. Finally, the absorbance values were measured by a microplate reader (SpectraMax i3× Multi-Mode Detection Platform, Molecular devices, China), and the activity of SOD and CAT was calculated based on the absorbance values and standard curves.
Mitochondrial activity assay
To assess mitochondrial activity, oocytes were incubated in the IVM medium accompanying MitoTracker Red CMXRos (Invitrogen, M7512) at 37°C for 30 min. After washing three times in PBS-PVA, the fluorescence intensity of each group of oocytes was captured by fluorescence microscope and photographed, and the fluorescence intensity was analyzed by ImageJ software.
MMP assay
To determine the level of MMP, oocytes were incubated in PBS-PVA containing 2 μM 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide dye (JC-1; Beyotime, C2006) at 37°C for 30 min. After washing the oocytes in PBS-PVA three times, images were captured by using a fluorescence microscope, and the fluorescence intensity was analyzed by using ImageJ software. The average MMP of oocytes was calculated as the ratio of red fluorescence intensity (corresponding to activated mitochondria) to green fluorescence intensity (corresponding to inactive mitochondria).
Intracellular ATP levels assay
Intracellular ATP levels were measured using an ATP assay kit (Beyotime, S0027). Briefly, 50 oocytes were collected from each group into a 1.5 ml centrifuge tube containing 45 μL of lysis buffer. The cells were lysed by ultrasound and centrifuged at 12,000 rpm for 5 min at 4°C. The supernatant was taken for subsequent determination. Then, 100 μL of ATP working solution and 20 μL of supernatant were added to a 96-well opaque plate, and the mixtures were measured by a microplate reader. The intracellular ATP levels were calculated according to the measured value and the standard curve.
Intracellular senescence-associated β-galactosidase (SA-β-gal) activity assay
Intracellular SA-β-gal activity was measured by using a Cellular Senescence Detection Kit - SPiDER-βGal (SG03, Dojindo, Japan). Briefly, oocytes were cultured in an environment under 38.5°C and 5% CO2 for 1 h after adding 1 mL of Bafilomycin A1 working solution. Subsequently, 1 mL of SPiDER-βGal working solution was added, and the oocytes were cultured in an environment under 38.5°C and 5% CO2 for 30 min. After washing three times in PBS-PVA, the fluorescence intensity of each group of oocytes was captured by fluorescence microscope and photographed, and the fluorescence intensity was analyzed by ImageJ software.
Sperm binding assay
Straw frozen semen was removed from liquid nitrogen and thawed. The purified sperm were obtained by density gradient centrifugation on Percoll and resuspended so that the sperm density was 1 × 106/mL. Oocytes and resuspended sperm were co-incubated in IVF drops in an environment under 38.5°C and 5% CO2 for 1 h. Then, they were fixed in PBS-PVA containing 4% paraformaldehyde for 30 min. After fixation, PBS-PVA was used to wash three times, after which the samples were transferred to 10 μg/mL Hoechst 33343 to label the spermatid nuclei. Afterward, the stained sperm-oocyte complexes were mounted onto glass slides, examined and photographed by a microscope under fluorescent light. The sperm number was analyzed by ImageJ software.
Immunofluorescence staining
Oocytes were fixed in PBS-PVA containing 4% paraformaldehyde for 30 min and permeabilized in 0.3% Triton X-100 at room temperature for 15 min. The oocytes were then blocked in PBS-PVA containing 3% BSA at room temperature for 2 hours. Next, the oocytes were incubated with a primary anti-H2AX antibody (9718S; CST, USA) at 4°C overnight. After first antibody incubation, the oocytes were washed three times in PBS-PVA, and the oocytes were incubated with goat anti-rabbit IgG (CST; 4413S for H2AX staining) at room temperature for 2 hours. Afterward, the oocytes were transferred to 10 μg/mL Hoechst 33343 at room temperature for 15 min. Fluorescence microscopy was used to determine the positivity and negativity of γ-H2AX.
Western blot
For Western blotting, 70 oocytes were collected and lysed in SDS lysis buffer (40% ddH2O, 20% glycerol, 20% SDS, 12.5% 0.5 M Tris-HCl, 20 mM β-mercaptoethanol and trace bromophenol blue) and incubated in a 95°C metal bath for 10 min. Next, the total protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene fluoride membrane (Millipore, Billerica, MA, USA). Blocking buffer (WLA066a, Wanleibio, China) was used to block the transferred membrane, and the membrane was incubated overnight with first antibodies against β-actin (CST, 4970T), Bcl2 (Proteintech, 12789-1-AP), BAX (Proteintech, 50599-2-Ig) and Caspase-3 (CST, 9662S) at 4°C. After washing in TBST 3 times for 10 min each time, the membrane was incubated with goat anti-rabbit IgG (CST, 7074S) at room temperature for 1 hour. The images were analyzed with a Tanon 5200 image analyzer (Tanon, China), and ImageJ software was used for visualization and analysis.
Statistical analysis
All the above experiments were repeated at least three times. The statistical results are expressed as the mean ± standard error of the mean (SEM). The total number of oocytes used in each experiment (n) is shown by the bar. The number of independent repetitions (R) is shown in the diagram annotation. Statistical analysis was performed by one-way analysis of variance (ANOVA). All statistical analyses were performed using SPSS version 22.0 (IBM, Chicago, IL, USA) software. Significant differences are expressed as (*P < 0.05), (**P < 0.01) and (***P < 0.001).
Author Contributions
Shuang Liang, Bao Yuan, Yong-Hong Zhang and Yi-Jing Yin conceived the experiment. Yi-Jing Yin performed the experiments. Shuang Liang, Yong-Hong Zhang and Yi-Jing Yin analyzed the data and wrote the article. Yu Wang, Hao Jiang and Jia-Bao Zhang helped with the analysis of the results and revised the figures and article.
Conflicts of Interest
The authors declare no conflicts of interest related to this study.
Ethical Statement
The study was approved by the Institutional Animal Care and Use Committee of Jilin University.
Funding
This study was supported by the National Natural Science Foundation of China (U20A2053 and U22A20509) and the Modern Agricultural Industry Technology System (CARS-37).
References
-
1.
Lord T, Aitken RJ. Oxidative stress and ageing of the post-ovulatory oocyte. Reproduction. 2013; 146:R217–27. https://doi.org/10.1530/REP-13-0111 [PubMed]
-
2.
Di Nisio V, Antonouli S, Damdimopoulou P, Salumets A, Cecconi S, and SIERR. In vivo and in vitro postovulatory aging: when time works against oocyte quality? J Assist Reprod Genet. 2022; 39:905–18. https://doi.org/10.1007/s10815-022-02418-y [PubMed]
-
3.
Niu YJ, Zhou W, Nie ZW, Zhou D, Xu YN, Ock SA, Yan CG, Cui XS. Ubiquinol-10 delays postovulatory oocyte aging by improving mitochondrial renewal in pigs. Aging (Albany NY). 2020; 12:1256–71. https://doi.org/10.18632/aging.102681 [PubMed]
-
4.
Miao YL, Kikuchi K, Sun QY, Schatten H. Oocyte aging: cellular and molecular changes, developmental potential and reversal possibility. Hum Reprod Update. 2009; 15:573–85. https://doi.org/10.1093/humupd/dmp014 [PubMed]
-
5.
Takahashi T, Igarashi H, Amita M, Hara S, Matsuo K, Kurachi H. Molecular mechanism of poor embryo development in postovulatory aged oocytes: mini review. J Obstet Gynaecol Res. 2013; 39:1431–9. https://doi.org/10.1111/jog.12111 [PubMed]
-
6.
Miao Y, Ma S, Liu X, Miao D, Chang Z, Luo M, Tan J. Fate of the first polar bodies in mouse oocytes. Mol Reprod Dev. 2004; 69:66–76. https://doi.org/10.1002/mrd.20148 [PubMed]
-
7.
Goud AP, Goud PT, Diamond MP, Van Oostveldt P, Hughes MR. Microtubule turnover in ooplasm biopsy reflects ageing phenomena in the parent oocyte. Reprod Biomed Online. 2005; 11:43–52. https://doi.org/10.1016/s1472-6483(10)61297-7 [PubMed]
-
8.
Eichenlaub-Ritter U, Stahl A, Luciani JM. The microtubular cytoskeleton and chromosomes of unfertilized human oocytes aged in vitro. Hum Genet. 1988; 80:259–64. https://doi.org/10.1007/BF01790094 [PubMed]
-
9.
Wang WH, Meng L, Hackett RJ, Odenbourg R, Keefe DL. The spindle observation and its relationship with fertilization after intracytoplasmic sperm injection in living human oocytes. Fertil Steril. 2001; 75:348–53. https://doi.org/10.1016/s0015-0282(00)01692-7 [PubMed]
-
10.
Wang L, Tang J, Wang L, Tan F, Song H, Zhou J, Li F. Oxidative stress in oocyte aging and female reproduction. J Cell Physiol. 2021; 236:7966–83. https://doi.org/10.1002/jcp.30468 [PubMed]
-
11.
Takahashi T, Takahashi E, Igarashi H, Tezuka N, Kurachi H. Impact of oxidative stress in aged mouse oocytes on calcium oscillations at fertilization. Mol Reprod Dev. 2003; 66:143–52. https://doi.org/10.1002/mrd.10341 [PubMed]
-
12.
Takahashi T, Saito H, Hiroi M, Doi K, Takahashi E. Effects of aging on inositol 1,4,5-triphosphate-induced Ca(2+) release in unfertilized mouse oocytes. Mol Reprod Dev. 2000; 55:299–306. https://doi.org/10.1002/(SICI)1098-2795(200003)55:3%3c299::AID-MRD8%3e3.0.CO;2-G [PubMed]
-
13.
Ionescu-Tucker A, Cotman CW. Emerging roles of oxidative stress in brain aging and Alzheimer's disease. Neurobiol Aging. 2021; 107:86–95. https://doi.org/10.1016/j.neurobiolaging.2021.07.014 [PubMed]
-
14.
Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Aging-induced proinflammatory shift in cytokine expression profile in coronary arteries. FASEB J. 2003; 17:1183–5. https://doi.org/10.1096/fj.02-1049fje [PubMed]
-
15.
Höhn A, König J, Grune T. Protein oxidation in aging and the removal of oxidized proteins. J Proteomics. 2013; 92:132–59. https://doi.org/10.1016/j.jprot.2013.01.004 [PubMed]
-
16.
Szabó C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007; 6:662–80. https://doi.org/10.1038/nrd2222 [PubMed]
-
17.
Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996; 273:59–63. https://doi.org/10.1126/science.273.5271.59 [PubMed]
-
18.
Su WP, Li CJ, Lin LT, Lin PH, Wen ZH, Sheu JJ, Tsui KH. Boosting mitochondrial function and metabolism in aging female germ cells with dual ROCK/ROS inhibition. Biomed Pharmacother. 2023; 163:114888. https://doi.org/10.1016/j.biopha.2023.114888 [PubMed]
-
19.
Hasty P, Campisi J, Hoeijmakers J, van Steeg H, Vijg J. Aging and genome maintenance: lessons from the mouse? Science. 2003; 299:1355–9. https://doi.org/10.1126/science.1079161 [PubMed]
-
20.
Wang T, Gao YY, Chen L, Nie ZW, Cheng W, Liu X, Schatten H, Zhang X, Miao YL. Melatonin prevents postovulatory oocyte aging and promotes subsequent embryonic development in the pig. Aging (Albany NY). 2017; 9:1552–64. https://doi.org/10.18632/aging.101252 [PubMed]
-
21.
Ben-Meir A, Burstein E, Borrego-Alvarez A, Chong J, Wong E, Yavorska T, Naranian T, Chi M, Wang Y, Bentov Y, Alexis J, Meriano J, Sung HK, et al. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell. 2015; 14:887–95. https://doi.org/10.1111/acel.12368 [PubMed]
-
22.
Lord T, Nixon B, Jones KT, Aitken RJ. Melatonin prevents postovulatory oocyte aging in the mouse and extends the window for optimal fertilization in vitro. Biol Reprod. 2013; 88:67. https://doi.org/10.1095/biolreprod.112.106450 [PubMed]
-
23.
Zhou J, Xue Z, He HN, Liu X, Yin SY, Wu DY, Zhang X, Schatten H, Miao YL. Resveratrol delays postovulatory aging of mouse oocytes through activating mitophagy. Aging (Albany NY). 2019; 11:11504–19. https://doi.org/10.18632/aging.102551 [PubMed]
-
24.
Klepacka J, Fornal Ł. Ferulic acid and its position among the phenolic compounds of wheat. Crit Rev Food Sci Nutr. 2006; 46:639–47. https://doi.org/10.1080/10408390500511821 [PubMed]
-
25.
Zduńska K, Dana A, Kolodziejczak A, Rotsztejn H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Skin Pharmacol Physiol. 2018; 31:332–6. https://doi.org/10.1159/000491755 [PubMed]
-
26.
Neopane D, Ansari VA, Singh A. Ferulic Acid: Signaling Pathways in Aging. Drug Res (Stuttg). 2023; 73:318–24. https://doi.org/10.1055/a-2061-7129 [PubMed]
-
27.
Ueno S, Yoshida N, Niimura S. Amount of hyaluronan produced by mouse oocytes and role of hyaluronan in enlargement of the perivitelline space. J Reprod Dev. 2009; 55:496–501. https://doi.org/10.1262/jrd.20226 [PubMed]
-
28.
Keefe D, Kumar M, Kalmbach K. Oocyte competency is the key to embryo potential. Fertil Steril. 2015; 103:317–22. https://doi.org/10.1016/j.fertnstert.2014.12.115 [PubMed]
-
29.
Wang Y, Li L, Fan LH, Jing Y, Li J, Ouyang YC, Wang ZB, Hou Y, Sun QY. N-acetyl-L-cysteine (NAC) delays post-ovulatory oocyte aging in mouse. Aging (Albany NY). 2019; 11:2020–30. https://doi.org/10.18632/aging.101898 [PubMed]
-
30.
Ward F, Enright B, Rizos D, Boland M, Lonergan P. Optimization of in vitro bovine embryo production: effect of duration of maturation, length of gamete co-incubation, sperm concentration and sire. Theriogenology. 2002; 57:2105–17. https://doi.org/10.1016/s0093-691x(02)00696-9 [PubMed]
-
31.
Demyda-Peyrás S, Dorado J, Hidalgo M, Anter J, De Luca L, Genero E, Moreno-Millán M. Effects of oocyte quality, incubation time and maturation environment on the number of chromosomal abnormalities in IVF-derived early bovine embryos. Reprod Fertil Dev. 2013; 25:1077–84. https://doi.org/10.1071/RD12140 [PubMed]
-
32.
Gliedt DW, Rosenkrans CF Jr, Rorie RW, Rakes JM. Effects of oocyte maturation length, sperm capacitation time, and heparin on bovine embryo development. J Dairy Sci. 1996; 79:532–5. https://doi.org/10.3168/jds.S0022-0302(96)76396-8 [PubMed]
-
33.
Takase K, Ishikawa M, Hoshiai H. Apoptosis in the degeneration process of unfertilized mouse ova. Tohoku J Exp Med. 1995; 175:69–76. https://doi.org/10.1620/tjem.175.69 [PubMed]
-
34.
Miao YL, Liu XY, Qiao TW, Miao DQ, Luo MJ, Tan JH. Cumulus cells accelerate aging of mouse oocytes. Biol Reprod. 2005; 73:1025–31. https://doi.org/10.1095/biolreprod.105.043703 [PubMed]
-
35.
Khan M, Li T, Ahmad Khan MK, Rasul A, Nawaz F, Sun M, Zheng Y, Ma T. Alantolactone induces apoptosis in HepG2 cells through GSH depletion, inhibition of STAT3 activation, and mitochondrial dysfunction. Biomed Res Int. 2013; 2013:719858. https://doi.org/10.1155/2013/719858 [PubMed]
-
36.
Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. 2012; 10:49. https://doi.org/10.1186/1477-7827-10-49 [PubMed]
-
37.
Goud AP, Goud PT, Diamond MP, Gonik B, Abu-Soud HM. Reactive oxygen species and oocyte aging: role of superoxide, hydrogen peroxide, and hypochlorous acid. Free Radic Biol Med. 2008; 44:1295–304. https://doi.org/10.1016/j.freeradbiomed.2007.11.014 [PubMed]
-
38.
Wu Y, Zhang N, Li YH, Zhao L, Yang M, Jin Y, Xu YN, Guo H. Citrinin exposure affects oocyte maturation and embryo development by inducing oxidative stress-mediated apoptosis. Oncotarget. 2017; 8:34525–33. https://doi.org/10.18632/oncotarget.15776 [PubMed]
-
39.
Jeelani R, Khan SN, Shaeib F, Kohan-Ghadr HR, Aldhaheri SR, Najafi T, Thakur M, Morris R, Abu-Soud HM. Cyclophosphamide and acrolein induced oxidative stress leading to deterioration of metaphase II mouse oocyte quality. Free Radic Biol Med. 2017; 110:11–8. https://doi.org/10.1016/j.freeradbiomed.2017.05.006 [PubMed]
-
40.
Liu T, Sun L, Zhang Y, Wang Y, Zheng J. Imbalanced GSH/ROS and sequential cell death. J Biochem Mol Toxicol. 2022; 36:e22942. https://doi.org/10.1002/jbt.22942 [PubMed]
-
41.
Luo D, Zhang JB, Li SP, Liu W, Yao XR, Guo H, Jin ZL, Jin YX, Yuan B, Jiang H, Kim NH. Imperatorin Ameliorates the Aging-Associated Porcine Oocyte Meiotic Spindle Defects by Reducing Oxidative Stress and Protecting Mitochondrial Function. Front Cell Dev Biol. 2020; 8:592433. https://doi.org/10.3389/fcell.2020.592433 [PubMed]
-
42.
Jiang WJ, Yao XR, Zhao YH, Gao QS, Jin QG, Li YH, Yan AG, Xu YN. L-carnitine prevents bovine oocyte aging and promotes subsequent embryonic development. J Reprod Dev. 2019; 65:499–506. https://doi.org/10.1262/jrd.2019-046 [PubMed]
-
43.
Inal ME, Kanbak G, Sunal E. Antioxidant enzyme activities and malondialdehyde levels related to aging. Clin Chim Acta. 2001; 305:75–80. https://doi.org/10.1016/s0009-8981(00)00422-8 [PubMed]
-
44.
Wang JM, Sheng YC, Ji LL, Wang ZT. Ferulic acid prevents liver injury and increases the anti-tumor effect of diosbulbin B in vivo. J Zhejiang Univ Sci B. 2014; 15:540–7. https://doi.org/10.1631/jzus.B1300250 [PubMed]
-
45.
Liu M, Zhang C, Xu X, Zhao X, Han Z, Liu D, Bo R, Li J, Liu Z. Ferulic acid inhibits LPS-induced apoptosis in bovine mammary epithelial cells by regulating the NF-κB and Nrf2 signalling pathways to restore mitochondrial dynamics and ROS generation. Vet Res. 2021; 52:104. https://doi.org/10.1186/s13567-021-00973-3 [PubMed]
-
46.
Dalton CM, Szabadkai G, Carroll J. Measurement of ATP in single oocytes: impact of maturation and cumulus cells on levels and consumption. J Cell Physiol. 2014; 229:353–61. https://doi.org/10.1002/jcp.24457 [PubMed]
-
47.
Van Blerkom J, Caltrider K. Sperm attachment and penetration competence in the human oocyte: a possible aetiology of fertilization failure involving the organization of oolemmal lipid raft microdomains influenced by the ΔΨm of subplasmalemmal mitochondria. Reprod Biomed Online. 2013; 27:690–701. https://doi.org/10.1016/j.rbmo.2013.09.011 [PubMed]
-
48.
Ge H, Tollner TL, Hu Z, Dai M, Li X, Guan H, Shan D, Zhang X, Lv J, Huang C, Dong Q. The importance of mitochondrial metabolic activity and mitochondrial DNA replication during oocyte maturation in vitro on oocyte quality and subsequent embryo developmental competence. Mol Reprod Dev. 2012; 79:392–401. https://doi.org/10.1002/mrd.22042 [PubMed]
-
49.
Kudryavtseva AV, Krasnov GS, Dmitriev AA, Alekseev BY, Kardymon OL, Sadritdinova AF, Fedorova MS, Pokrovsky AV, Melnikova NV, Kaprin AD, Moskalev AA, Snezhkina AV. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget. 2016; 7:44879–905. https://doi.org/10.18632/oncotarget.9821 [PubMed]
-
50.
Koyama K, Kang SS, Huang W, Yanagawa Y, Takahashi Y, Nagano M. Aging-related changes in in vitro-matured bovine oocytes: oxidative stress, mitochondrial activity and ATP content after nuclear maturation. J Reprod Dev. 2014; 60:136–42. https://doi.org/10.1262/jrd.2013-115 [PubMed]
-
51.
Au HK, Yeh TS, Kao SH, Tzeng CR, Hsieh RH. Abnormal mitochondrial structure in human unfertilized oocytes and arrested embryos. Ann N Y Acad Sci. 2005; 1042:177–85. https://doi.org/10.1196/annals.1338.020 [PubMed]
-
52.
Wilding M, Dale B, Marino M, di Matteo L, Alviggi C, Pisaturo ML, Lombardi L, De Placido G. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum Reprod. 2001; 16:909–17. https://doi.org/10.1093/humrep/16.5.909 [PubMed]
-
53.
Hu WY, Li XX, Diao YF, Qi JJ, Wang DL, Zhang JB, Sun BX, Liang S. Asiatic acid protects oocytes against in vitro aging-induced deterioration and improves subsequent embryonic development in pigs. Aging (Albany NY). 2020; 13:3353–67. https://doi.org/10.18632/aging.202184 [PubMed]
-
54.
Zhang X, Liu X, Chen L, Wu DY, Nie ZW, Gao YY, Miao YL. Caffeine delays oocyte aging and maintains the quality of aged oocytes safely in mouse. Oncotarget. 2017; 8:20602–11. https://doi.org/10.18632/oncotarget.15292 [PubMed]
-
55.
Yu Y, Cui Y, Niedernhofer LJ, Wang Y. Occurrence, Biological Consequences, and Human Health Relevance of Oxidative Stress-Induced DNA Damage. Chem Res Toxicol. 2016; 29:2008–39. https://doi.org/10.1021/acs.chemrestox.6b00265 [PubMed]
-
56.
Kuo LJ, Yang LX. Gamma-H2AX - a novel biomarker for DNA double-strand breaks. In Vivo. 2008; 22:305–9. [PubMed]
-
57.
Celeste A, Petersen S, Romanienko PJ, Fernandez-Capetillo O, Chen HT, Sedelnikova OA, Reina-San-Martin B, Coppola V, Meffre E, Difilippantonio MJ, Redon C, Pilch DR, Olaru A, et al. Genomic instability in mice lacking histone H2AX. Science. 2002; 296:922–7. https://doi.org/10.1126/science.1069398 [PubMed]
-
58.
Pospelova TV, Demidenko ZN, Bukreeva EI, Pospelov VA, Gudkov AV, Blagosklonny MV. Pseudo-DNA damage response in senescent cells. Cell Cycle. 2009; 8:4112–8. https://doi.org/10.4161/cc.8.24.10215 [PubMed]
-
59.
Lorda-Diez CI, Solis-Mancilla ME, Sanchez-Fernandez C, Garcia-Porrero JA, Hurle JM, Montero JA. Cell senescence, apoptosis and DNA damage cooperate in the remodeling processes accounting for heart morphogenesis. J Anat. 2019; 234:815–29. https://doi.org/10.1111/joa.12972 [PubMed]
-
60.
Lowndes NF. DNA-damage signaling and apoptosis. Genome Biol. 2001; 2:REPORTS4028. https://doi.org/10.1186/gb-2001-2-11-reports4028 [PubMed]
-
61.
Smith ML, Fornace AJ Jr. Mammalian DNA damage-inducible genes associated with growth arrest and apoptosis. Mutat Res. 1996; 340:109–24. https://doi.org/10.1016/s0165-1110(96)90043-3 [PubMed]
-
62.
Asadi M, Taghizadeh S, Kaviani E, Vakili O, Taheri-Anganeh M, Tahamtan M, Savardashtaki A. Caspase-3: Structure, function, and biotechnological aspects. Biotechnol Appl Biochem. 2022; 69:1633–45. https://doi.org/10.1002/bab.2233 [PubMed]
-
63.
Edlich F. BCL-2 proteins and apoptosis: Recent insights and unknowns. Biochem Biophys Res Commun. 2018; 500:26–34. https://doi.org/10.1016/j.bbrc.2017.06.190 [PubMed]
-
64.
Gupta A, Singh AK, Loka M, Pandey AK, Bishayee A. Ferulic acid-mediated modulation of apoptotic signaling pathways in cancer. Adv Protein Chem Struct Biol. 2021; 125:215–57. https://doi.org/10.1016/bs.apcsb.2020.12.005 [PubMed]
-
65.
Chen Y, Xue F, Han C, Yang H, Han L, Li K, Li J, Xu Q, Li Z, Yuan B, Yu L, Gao X, Yan Y. Ferulic acid ameliorated placental inflammation and apoptosis in rat with preeclampsia. Clin Exp Hypertens. 2019; 41:524–30. https://doi.org/10.1080/10641963.2018.1516773 [PubMed]
-
66.
Georgadaki K, Khoury N, Spandidos DA, Zoumpourlis V. The molecular basis of fertilization (Review). Int J Mol Med. 2016; 38:979–86. https://doi.org/10.3892/ijmm.2016.2723 [PubMed]
-
67.
Zhang M, ShiYang X, Zhang Y, Miao Y, Chen Y, Cui Z, Xiong B. Coenzyme Q10 ameliorates the quality of postovulatory aged oocytes by suppressing DNA damage and apoptosis. Free Radic Biol Med. 2019; 143:84–94. https://doi.org/10.1016/j.freeradbiomed.2019.08.002 [PubMed]