HCMMD: systematic evaluation of metabolites in body fluids as liquid biopsy biomarker for human cancers
Abstract
Metabolomics is a rapidly expanding field in systems biology used to measure alterations of metabolites and identify metabolic biomarkers in response to disease processes. The discovery of metabolic biomarkers can improve early diagnosis, prognostic prediction, and therapeutic intervention for cancers. However, there are currently no databases that provide a comprehensive evaluation of the relationship between metabolites and cancer processes. In this review, we summarize reported metabolites in body fluids across pan-cancers and characterize their clinical applications in liquid biopsy. We conducted a search for metabolic biomarkers using the keywords (“metabolomics” OR “metabolite”) AND “cancer” in PubMed. Of the 22,254 articles retrieved, 792 were deemed potentially relevant for further review. Ultimately, we included data from 573,300 samples and 17,083 metabolic biomarkers. We collected information on cancer types, sample size, the human metabolome database (HMDB) ID, metabolic pathway, area under the curve (AUC), sensitivity and specificity of metabolites, sample source, detection method, and clinical features were collected. Finally, we developed a user-friendly online database, the Human Cancer Metabolic Markers Database (HCMMD), which allows users to query, browse, and download metabolite information. In conclusion, HCMMD provides an important resource to assist researchers in reviewing metabolic biomarkers for diagnosis and progression of cancers.
Introduction
Cancer is a major global public health problem, with a significant impact on mortality worldwide. In 2020, an estimated 10 million people died of cancer globally [1]. Early diagnosis is crucial for effective cancer management and better prognosis. However, approximately 50% of cancers are diagnosed at advanced stages [2, 3]. Effective treatment of advanced cancer often involves the use of modern systemic and targeted drugs, which can be costly and may have limited efficacy [4]. Early cancer detection has been shown to provide substantial health benefits, including increased survival rates and reduced morbidity [2]. Although several blood-based biomarkers, such as carcinoembryonic antigen (CEA) and prostate-specific antigen (PSA), have been used for cancer screening in the past few decades, their sensitivity and specificity have been found to be unsatisfactory, limiting their effectiveness [5]. Therefore, there is a pressing need to identify biomarkers that exhibit high sensitivity and specificity for the early detection of cancer.
Metabolomics, which involves the comprehensive analysis of small molecule metabolites in cells, tissues, or whole organisms, has undergone rapid technological evolution in the past two decades [6–8]. By measuring downstream chemical phenotypes of genomic, transcriptomic, and proteomic variability, metabolomics can provide a more comprehensive understanding of the biological system [6, 9, 10]. Research has shown that metabolites play a crucial role in various diseases such as obesity, diabetes, cardiovascular disease, respiratory conditions, and cancer [6, 11]. Metabolomics has emerged as an accurate and non-invasive diagnostic tool, accompanied by the development of novel and sensitive measurement techniques [12, 13]. The uncontrolled proliferation of tumor cells requires metabolic regulation [14–16], and metabolic reprogramming is a hallmark of malignancy [17]. In recent years, several highly sensitive and specific metabolic biomarkers have been identified in liquid biopsy studies. For instance, Sreekumar et al. reported that sarcosine had a diagnostic value with an AUC of 0.69 (95% CI: 0.55, 0.84) for prostate cancer [18]. Soga et al. discovered that serum γ-glutamyl dipeptides had an AUC of 0.76 for hepatocellular carcinoma [19]. Tyrosine and glutamine-leucine in serum had an AUC of 0.98 for the diagnosis of colorectal cancer [20]. N1, N12-diacetylspermine in serum had an AUC of 0.65 (95% CI, 0.59 to 0.72) for the diagnosis of non–small-cell lung cancer [21]. The AUC value of creatine nucleoside in urine was 0.79 for differential diagnosis between adrenocortical carcinoma and benign adrenal tumors [22].
This study aimed to comprehensively evaluate the role of metabolites in cancers. Cancer-related metabolites were searched from the PubMed database. The collected information includes cancer types, sample size, HMDB ID, metabolic pathway, area under the curve (AUC), sensitivity, specificity, sample source, detection method, and clinical features. Importantly, a user-friendly online database was developed, named Human Cancer Metabolic Markers Database (HCMMD), to assist users in querying, browsing, and downloading information about the cancer-related metabolites.
Metabolism-related biomarkers for cancers
Database establishment
To search for cancer-related metabolites from PubMed, we used the keywords “metabolomics” OR “metabolite” AND “cancer”. The eligible data included 22,254 articles published before July 30, 2022. We selected literature that focused on human tumors, samples from liquid biopsies, and individual biomarkers with diagnostic, prognostic, and predictive value. After filtering the results, we identified 792 studies with a total of 573,300 samples and 17,083 metabolic biomarkers. The included studies covered 24 types of cancer derived from 92 subtypes. We recorded information such as cancer type, sample size, HMDB ID, metabolic pathway, AUC, sensitivity, specificity, sample source, detection method, and clinical features. Figure 1 summarizes the basic information and diagnostic value of metabolic biomarkers in different cancers. Importantly, we created a new online database, called the HCMMD, which allows users to explore and analyze cancer-related metabolic biomarkers (Figure 2).
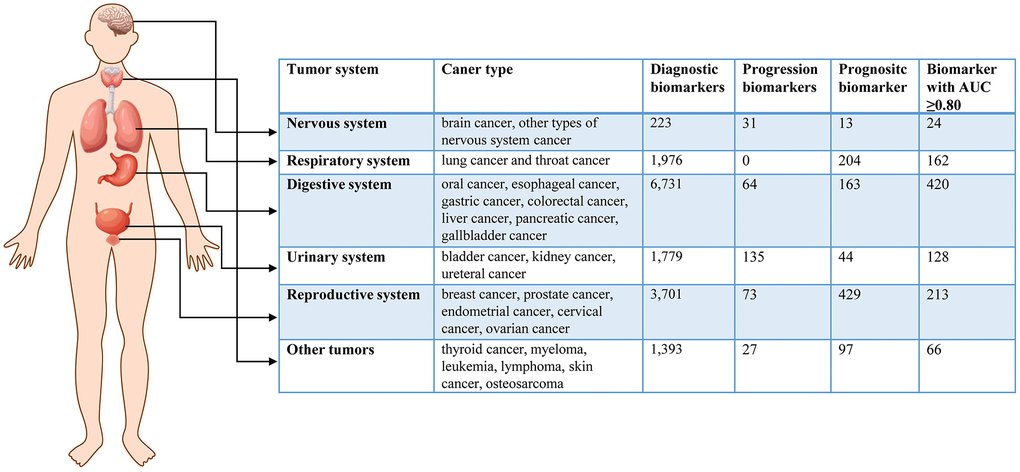
Figure 1. The basic information of metabolic biomarkers in different system cancers.
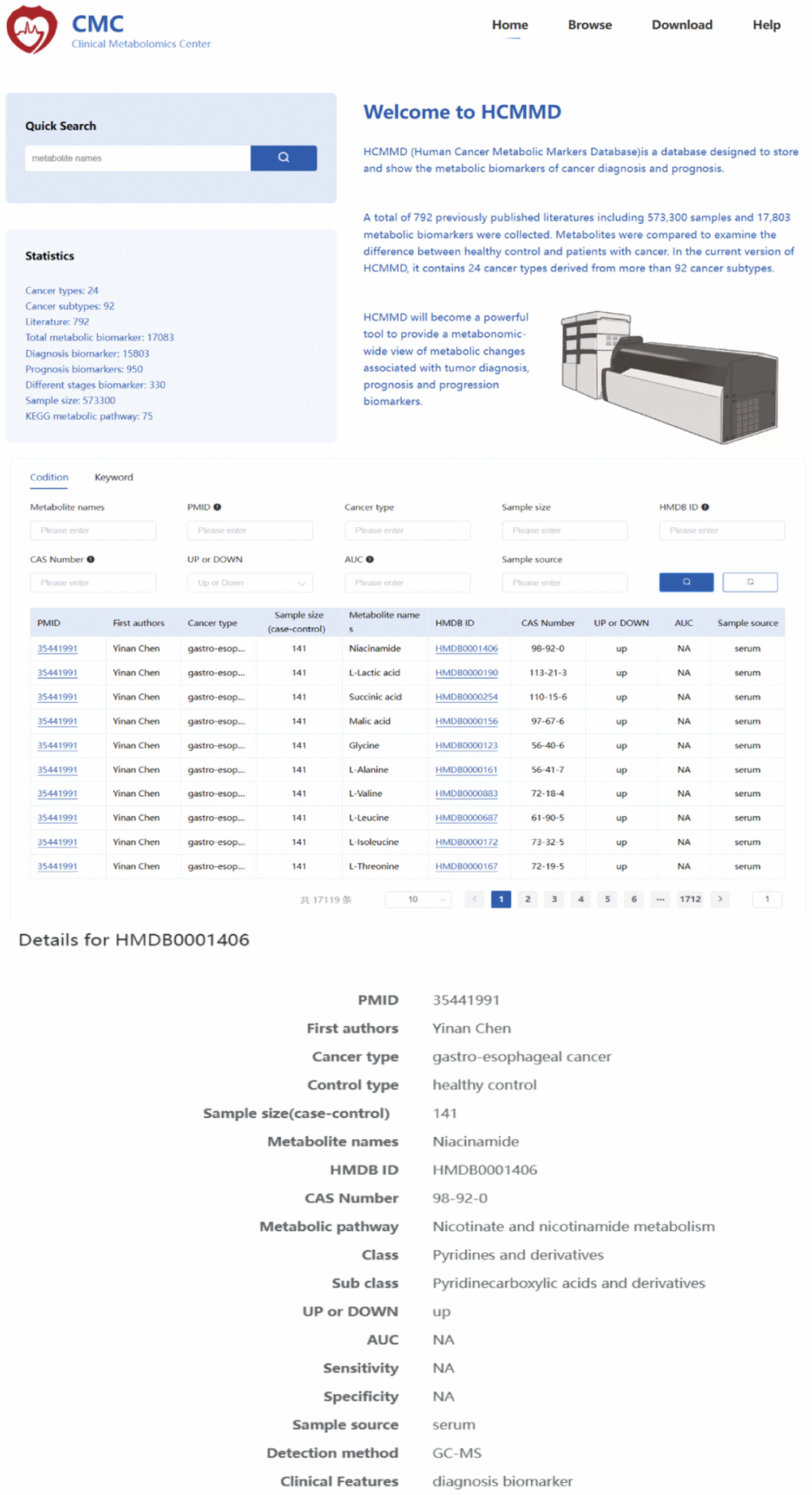
Figure 2. The web interface of the HCMMD database.
Digestive system tumors
Digestive system cancer encompasses oral cancer, esophageal cancer, gastric cancer, colorectal cancer, liver cancer, pancreatic cancer, and gallbladder cancer. The five-year survival rates for liver cancer and pancreatic cancer are only 20% and 11%, respectively [40]. A total of 315 articles were collected, covering 7 cancer types and 23 subtypes, with sample sizes ranging from 9 to 3,109 [20, 41]. The reports included 6,731 diagnostic biomarkers, 64 progressive biomarkers, and 163 prognostic biomarkers. Of these biomarkers, 3,609 were from serum samples, 1,619 from plasma samples, 974 from urine samples, and 460 from saliva samples. Among the biomarkers, 1,763 belonged to amino acids and 246 belonged to bile acids. Several studies have shown that bile acids are closely related to digestive system tumors [23, 42, 43]. Of the biomarkers, 79 had an AUC ≥0.95, and 420 had an AUC ≥0.80.
To explore the underlying pathogenesis of digestive system cancer, metabolite pathway enrichment analysis was conducted (Supplementary Figure 1). As shown in Figure 3, the four most closely related pathways for digestive system cancer were glycine serine and threonine metabolism, arginine biosynthesis, alanine aspartate and glutamate metabolism, and valine leucine and isoleucine biosynthesis.
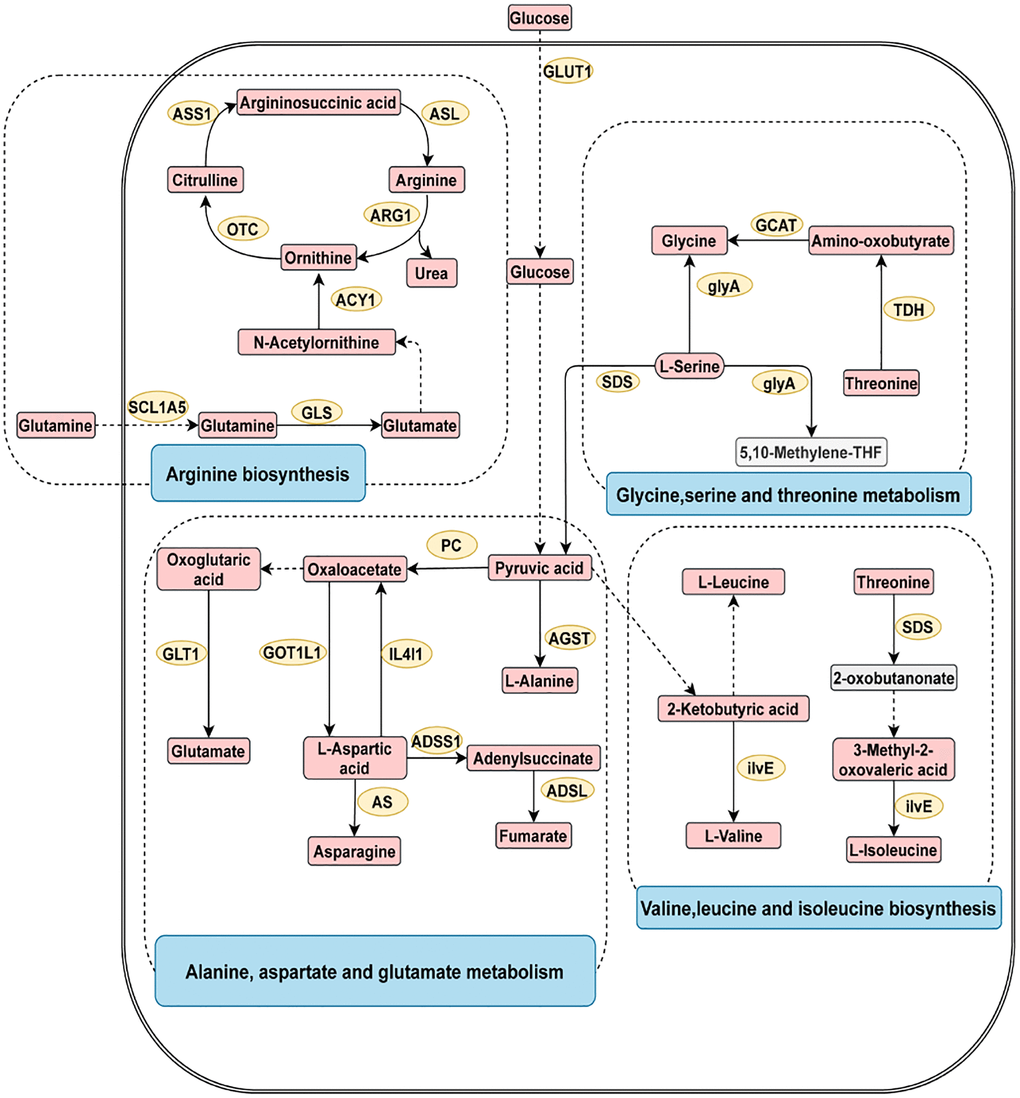
Figure 3. The four most related metabolic pathways in digestive system tumors. The metabolic process of metabolites and the proteins involved in their regulation. Differentially expressed metabolites (red), unchanged metabolites (grey), regulated proteins (yellow), metabolic pathway names (blue). Abbreviations: ACY1: aminoacylase; ADSL: adenylosuccinate lyase; ADSS1: adenylosuccinate synthase 1; AGST: alanine-glyoxylate transaminase; ARG1: arginase 1; AS: asparagine synthase; ASL: argininosuccinate lyase; ASS1: argininosuccinate synthase 1; GCAT: glycine C-acetyltransferase; GLS: glutaminase; GLT1: glutamate synthase; GLUT1: glucose transporter 1; glyA: glycine hydroxymethyltransferase; GOT1L1: glutamic-oxaloacetic transaminase 1 like 1; ilvE: branched-chain amino acid aminotransferase; IL4I1: interleukin 4 induced 1; OTC: ornithine transcarbamylase; PC: pyruvate carboxylase; SDS: threonine ammonia-lyase; SCL1A5: solute carrier family 1; TDH: threonine 3-dehydrogenase.
Reproductive system tumors
Reproductive system cancer comprises breast cancer, prostate cancer, endometrial cancer, cervical cancer, and ovarian cancer. Among all cancers, new cases of reproductive system cancer rank first [40]. In this review, we collected 238 articles covering 5 cancer types and 14 subtypes. The minimum sample size was 12 [44], and the maximum sample size was 6,114 [45]. These studies included 3,701 diagnostic biomarkers, 73 progression biomarkers, and 429 prognostic biomarkers. Of these metabolic markers, 1,462 biomarkers were from serum samples, 1,715 were from plasma samples, and 750 were from urine samples. Among the metabolic markers, 1,044 belonged to amino acids. A total of 213 diagnostic markers had an AUC ≥0.80.
Metabolite pathway enrichment analysis of diagnostic metabolites was performed for reproductive system cancers (Supplementary Figure 2). The four most closely related pathways for reproductive system cancer were glycine serine and threonine metabolism, alanine aspartate and glutamate metabolism, arginine and proline metabolism, and valine leucine and isoleucine biosynthesis. Figure 4 shows the three most significant pathways involved in reproductive system cancer.
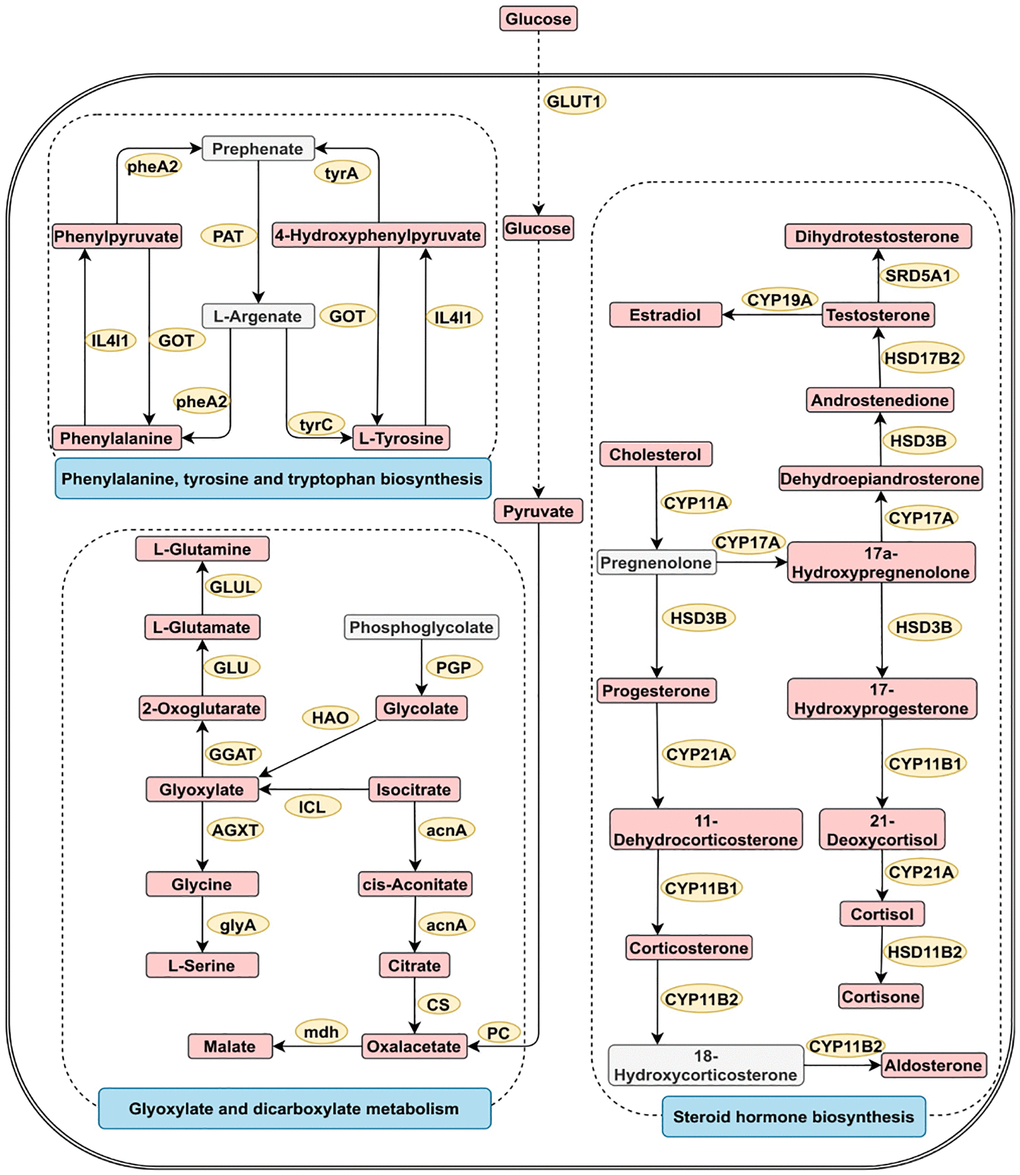
Figure 4. Three greatly important metabolic pathways in digestive system tumors. The metabolic process of metabolites and the proteins involved in their regulation. Differentially expressed metabolites (red), unchanged metabolites (grey) regulated proteins (yellow), metabolic pathway names (blue). Abbreviations: acnA: aconitate hydratase; AGXT: alanine-glyoxylate transaminase/serine-glyoxylate transaminase and serine-pyruvate transaminase; CS: citrate synthase; CYP11A: cholesterol monooxygenase; CYP17A: steroid 17alpha-monooxygenase/17alpha-hydroxyprogesterone deacetylase; CYP19A: aromatase; CYP11B1: steroid 11beta-monooxygenase; CYP11B2: steroid 11beta-monooxygenase/corticosterone 18-monooxygenase; CYP21A: steroid 21-monooxygenase; GGAT: glutamate--glyoxylate aminotransferase; GLU: glutamate synthase; GLUL: glutamine synthetase; GLUT1: glucose transporter 1; glyA: glycine hydroxymethyltransferase; GOT: aspartate aminotransferase; HAO: (S)-2-hydroxy-acid oxidase; HSD17B2: corticosteroid 11-beta-dehydrogenase isozyme 2; HSD17B2: 17beta-estradiol 17-dehydrogenase/3alpha(17beta)-hydroxysteroid dehydrogenase; HSD3B: 3beta-hydroxy-Delta5-steroid dehydrogenase; IL4I1: L-amino-acid oxidase; ICL: isocitrate lyase; mdh: malate dehydrogenase; PAT: bifunctional aspartate aminotransferase and glutamate/aspartate-prephenate aminotransferase; PC: pyruvate carboxylase; PGP: phosphoglycolate phosphatase; pheA2: prephenate dehydratase; SRD5A1: 3-oxo-5-alpha-steroid 4-dehydrogenase 1; tyrA2: prephenate dehydrogenase; tyrC: cyclohexadieny/prephenate dehydrogenase.
Respiratory system tumors
Respiratory system cancer includes lung cancer and throat cancer. Lung cancer is the leading cause of cancer-related morbidity and mortality worldwide, with a five-year relative survival rate of only 22% [1, 40]. In this review, we collected 102 publications on respiratory system tumors, including 2 cancer types and 8 cancer subtypes. The sample size ranged from 14 to 1,196 [46, 47]. These publications included 1,976 diagnostic biomarkers and 204 prognostic biomarkers. Among these biomarkers, 1,335 biomarkers came from serum samples, and 467 biomarkers came from plasma samples. In addition, metabolites from breath samples were used to diagnose lung cancer in seven studies [46, 48–53]. These studies identified 162 diagnostic markers with an AUC greater than or equal to 0.80. We also enriched the metabolic pathways of diagnostic metabolites in respiratory system cancers (Supplementary Figure 3). The main relevant pathways were aminoacyl-tRNA biosynthesis, arginine biosynthesis, glycine serine and threonine metabolism, and glyoxylate and dicarboxylate metabolism.
Urinary system tumors
Urinary system cancer includes bladder cancer, kidney cancer, and ureteral cancer. Bladder cancer is known for its high recurrence rate worldwide [1, 40]. In this review, we collected 77 publications on urinary system tumors, encompassing 3 cancer types and 17 cancer subtypes. The sample size varied from 12 to 2,610 [54, 55]. The publications included 1,779 diagnostic biomarkers, 135 progression biomarkers, and 44 prognostic biomarkers. Urine samples were the primary diagnostic tool for urinary system cancers, with 51 articles including 1,483 biomarkers from urine used to detect these cancers. Additionally, there were 382 biomarkers from serum samples and 93 biomarkers from plasma samples. We identified 128 diagnostic markers with an AUC of 0.80 or higher. The enrichment analysis of metabolic pathways of diagnostic metabolites in urinary system cancer is presented in Supplementary Figure 4, showing that these metabolites were enriched in aminoacyl-tRNA biosynthesis, steroid hormone biosynthesis, glycine serine and threonine metabolism, and arginine biosynthesis.
Nervous system tumors
Nervous system cancer, including brain cancer and other types of nervous system cancer, is one of the deadliest cancers, with 18,600 people in the United States dying from the disease in 2021 [40, 56]. Six cancer subtypes were reviewed in 16 articles, with sample sizes ranging from 17 to 220 [57, 58]. The studies included 223 diagnostic biomarkers, 31 progression biomarkers, and 13 prognostic biomarkers. Biomarkers were derived from serum samples (92 biomarkers), plasma samples (118 biomarkers), and cerebrospinal fluid samples (38 biomarkers). Of these studies, 24 diagnostic markers had an AUC of 0.80 or higher. A metabolic pathway enrichment analysis of diagnostic metabolites in nervous system cancers was conducted, and the four most relevant pathways were aminoacyl-tRNA biosynthesis, arginine biosynthesis, alanine aspartate and glutamate metabolism, and glyoxylate and dicarboxylate metabolism (Supplementary Figure 5).
Other tumors
In addition to the aforementioned common cancers, abnormal metabolites have also been observed in other types of cancer. We collected 65 articles for the diagnosis of other cancers, including six cancer types (thyroid cancer, myeloma, leukemia, lymphoma, skin cancer, and osteosarcoma) and 24 cancer subtypes. The sample sizes ranged from 10 to 846 [59, 60]. Those studies included 1,393 diagnostic biomarkers, 27 progression biomarkers, and 97 prognostic biomarkers. Biomarkers were mainly derived from three types of samples: 725 biomarkers in serum samples, 586 biomarkers in plasma samples, and 105 biomarkers in urine samples. Those publications contained a total of 66 diagnostic markers with an AUC of 0.80 or higher.
Discussion
Cancer is one of the major threats to human health because of its high morbidity and mortality rates [61]. Highly specific and sensitive diagnostic or prognostic biomarkers can improve the efficiency of treatment and prolong the survival of patients [2]. Metabolomics has many exciting opportunities to promote the treatment of cancer [62]. For example, metabolomics combined with other “omics” can uncover valuable drug targets [63–65]. Metabolomics also has the potential to influence cancer screening and diagnosis. Since many studies have identified biomarkers in body fluids with high diagnostic value for human cancers [66, 67]. Zhou et al. reported that 4-Dodecylbenzenesulfonic acid, PC (30:1) and PC (44:5) were downregulated in the serum of colorectal adenoma patients compared to healthy subjects, with an AUC of 1.00 [68]. Plasma levels of beta-sitosterol were upregulated in pancreatic cancer patients compared to healthy individuals with an AUC value of 0.99 [69]. Plasma of hexadecasphinganine with an AUC value of 0.99 in the diagnosis of gastric cancer [70]. Serum levels of hypoxanthine were upregulated in patients with lung adenocarcinoma compared to normal controls with an AUC value of 0.99 [71]. Jové et al. found that hexanoic acid in the plasma had an AUC value of 1.00 for breast cancer diagnosis [72]. Metabolite pathway enrichment analysis is a good method to discover potential pathogenesis of different systemic cancers. Glycine serine and threonine metabolism and arginine biosynthesis were enriched in each system of cancer. These two metabolic pathways may provide inspiration for future cancer research.
Although many liquid biopsy biomarkers with high diagnostic value in human cancers have been reported, there are still difficulties and challenges in the clinical application of these metabolites. First, there is a lack of multi-center, large-scale studies to validate the clinical feasibility and reproducibility of metabolic markers [73]. Second, in order to incorporate biomarker assays into the clinical workflow, supporting assay resources, staff logistics, and technical education are needed, which can be costly in the clinic [73, 74]. Third, there are huge fluctuations in the concentration of metabolites in vivo, as well as a fragmented distribution of specialized small molecules in the body [75]. In addition, metabolomics is diverse and chemically complex, and varies in different tumor lesions. For example, L-alanine is significantly downregulated in pancreatic cancer but significantly upregulated in colorectal cancer, which adds great difficulty for tumor screening [7, 47, 76]. This article has some innovations that need to be clarified. First, previous studies mainly focused on biopsy markers of a single tumor and lacked a summary of diagnostic data on multiple tumor biopsies. This article summarizes diagnostic markers covering 24 tumor types. Second, our database contains more detailed information, such as AUC, accuracy, specificity, HMDB ID, metabolic pathway, sample source and so on. In addition, pathway analysis demonstrated that glycine, serine and threonine metabolism and arginine biosynthesis metabolic pathways were enriched in multiple cancer systems, suggesting that these two metabolic pathways play an important role in cancer diagnosis and treatment.
Conclusion
With the development of standardized protocols, the measurement of metabolomics has become cheaper and more convenient. Metabolomics plays an increasingly important role in cancers, alongside other diagnostic and prognostic tests in the clinic. To provide an important resource for users to query, browse, and download information on cancer-related metabolites, we have established a user-friendly website.
Abbreviations
HMDB: human metabolome database;
AUC: area under curve;
HCMMD: Human Cancer Metabolic Markers Database;
CEA: carcinoembryonic antigen;
PSA: prostate-specific antigen;
GC-MS: gas chromatography mass spectrometry;
LC-MS: liquid chromatography mass spectrometry;
CE-MS: capillary electrophoresis mass spectrometry;
NMR: nuclear magnetic resonance spectroscopy.
Author Contributions
XD drafted the manuscript and collected the literatures. XD and YMF prepared Figures, Tables and website. GXM and YQG designed the study and revised the manuscript. All authors approved the final version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest related to this study.
Funding
This work was partly supported by the National Natural Science Foundation of China (82204146).
References
-
1.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021; 71:209–49. https://doi.org/10.3322/caac.21660 [PubMed]
-
2.
Crosby D, Bhatia S, Brindle KM, Coussens LM, Dive C, Emberton M, Esener S, Fitzgerald RC, Gambhir SS, Kuhn P, Rebbeck TR, Balasubramanian S. Early detection of cancer. Science. 2022; 375:eaay9040. https://doi.org/10.1126/science.aay9040 [PubMed]
-
3.
Wender RC, Brawley OW, Fedewa SA, Gansler T, Smith RA. A blueprint for cancer screening and early detection: Advancing screening's contribution to cancer control. CA Cancer J Clin. 2019; 69:50–79. https://doi.org/10.3322/caac.21550 [PubMed]
-
4.
Hackshaw A, Clarke CA, Hartman AR. New genomic technologies for multi-cancer early detection: Rethinking the scope of cancer screening. Cancer Cell. 2022; 40:109–13. https://doi.org/10.1016/j.ccell.2022.01.012 [PubMed]
-
5.
Schiffman JD, Fisher PG, Gibbs P. Early detection of cancer: past, present, and future. Am Soc Clin Oncol Educ Book. 2015; 57–65. https://doi.org/10.14694/EdBook_AM.2015.35.57 [PubMed]
-
6.
Newgard CB. Metabolomics and Metabolic Diseases: Where Do We Stand? Cell Metab. 2017; 25:43–56. https://doi.org/10.1016/j.cmet.2016.09.018 [PubMed]
-
7.
Schmidt DR, Patel R, Kirsch DG, Lewis CA, Vander Heiden MG, Locasale JW. Metabolomics in cancer research and emerging applications in clinical oncology. CA Cancer J Clin. 2021; 71:333–58. https://doi.org/10.3322/caac.21670 [PubMed]
-
8.
Rinschen MM, Ivanisevic J, Giera M, Siuzdak G. Identification of bioactive metabolites using activity metabolomics. Nat Rev Mol Cell Biol. 2019; 20:353–67. https://doi.org/10.1038/s41580-019-0108-4 [PubMed]
-
9.
Zamboni N, Saghatelian A, Patti GJ. Defining the metabolome: size, flux, and regulation. Mol Cell. 2015; 58:699–706. https://doi.org/10.1016/j.molcel.2015.04.021 [PubMed]
-
10.
Mader S, Pantel K. Liquid Biopsy: Current Status and Future Perspectives. Oncol Res Treat. 2017; 40:404–8. https://doi.org/10.1159/000478018 [PubMed]
-
11.
Metallo CM, Vander Heiden MG. Understanding metabolic regulation and its influence on cell physiology. Mol Cell. 2013; 49:388–98. https://doi.org/10.1016/j.molcel.2013.01.018 [PubMed]
-
12.
González-Riano C, Dudzik D, Garcia A, Gil-de-la-Fuente A, Gradillas A, Godzien J, López-Gonzálvez Á, Rey-Stolle F, Rojo D, Ruperez FJ, Saiz J, Barbas C. Recent Developments along the Analytical Process for Metabolomics Workflows. Anal Chem. 2020; 92:203–26. https://doi.org/10.1021/acs.analchem.9b04553 [PubMed]
-
13.
Rizzo C, Camarda F, Donzella D, La Barbera L, Guggino G. Metabolomics: An Emerging Approach to Understand Pathogenesis and to Assess Diagnosis and Response to Treatment in Spondyloarthritis. Cells. 2022; 11:549. https://doi.org/10.3390/cells11030549 [PubMed]
-
14.
Vander Heiden MG, DeBerardinis RJ. Understanding the Intersections between Metabolism and Cancer Biology. Cell. 2017; 168:657–69. https://doi.org/10.1016/j.cell.2016.12.039 [PubMed]
-
15.
Teicher BA, Linehan WM, Helman LJ. Targeting cancer metabolism. Clin Cancer Res. 2012; 18:5537–45. https://doi.org/10.1158/1078-0432.CCR-12-2587 [PubMed]
-
16.
Pavlova NN, Thompson CB. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016; 23:27–47. https://doi.org/10.1016/j.cmet.2015.12.006 [PubMed]
-
17.
Faubert B, Solmonson A, DeBerardinis RJ. Metabolic reprogramming and cancer progression. Science. 2020; 368:eaaw5473. https://doi.org/10.1126/science.aaw5473 [PubMed]
-
18.
Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009; 457:910–4. https://doi.org/10.1038/nature07762 [PubMed]
-
19.
Soga T, Sugimoto M, Honma M, Mori M, Igarashi K, Kashikura K, Ikeda S, Hirayama A, Yamamoto T, Yoshida H, Otsuka M, Tsuji S, Yatomi Y, et al. Serum metabolomics reveals γ-glutamyl dipeptides as biomarkers for discrimination among different forms of liver disease. J Hepatol. 2011; 55:896–905. https://doi.org/10.1016/j.jhep.2011.01.031 [PubMed]
-
20.
Li J, Li J, Wang H, Qi LW, Zhu Y, Lai M. Tyrosine and Glutamine-Leucine Are Metabolic Markers of Early-Stage Colorectal Cancers. Gastroenterology. 2019; 157:257–9.e5. https://doi.org/10.1053/j.gastro.2019.03.020 [PubMed]
-
21.
Wikoff WR, Hanash S, DeFelice B, Miyamoto S, Barnett M, Zhao Y, Goodman G, Feng Z, Gandara D, Fiehn O, Taguchi A. Diacetylspermine Is a Novel Prediagnostic Serum Biomarker for Non-Small-Cell Lung Cancer and Has Additive Performance With Pro-Surfactant Protein B. J Clin Oncol. 2015; 33:3880–6. https://doi.org/10.1200/JCO.2015.61.7779 [PubMed]
-
22.
Patel D, Thompson MD, Manna SK, Krausz KW, Zhang L, Nilubol N, Gonzalez FJ, Kebebew E. Unique and Novel Urinary Metabolomic Features in Malignant versus Benign Adrenal Neoplasms. Clin Cancer Res. 2017; 23:5302–10. https://doi.org/10.1158/1078-0432.CCR-16-3156 [PubMed]
-
23.
Wishart DS, Guo A, Oler E, Wang F, Anjum A, Peters H, Dizon R, Sayeeda Z, Tian S, Lee BL, Berjanskii M, Mah R, Yamamoto M, et al. HMDB 5.0: the Human Metabolome Database for 2022. Nucleic Acids Res. 2022; 50:D622–31. https://doi.org/10.1093/nar/gkab1062 [PubMed]
-
24.
Goodman RP, Markhard AL, Shah H, Sharma R, Skinner OS, Clish CB, Deik A, Patgiri A, Hsu YH, Masia R, Noh HL, Suk S, Goldberger O, et al. Hepatic NADH reductive stress underlies common variation in metabolic traits. Nature. 2020; 583:122–6. https://doi.org/10.1038/s41586-020-2337-2 [PubMed]
-
25.
Zhu G, Wang S, Huang Z, Zhang S, Liao Q, Zhang C, Lin T, Qin M, Peng M, Yang C, Cao X, Han X, Wang X, et al. Rewiring of the Fruit Metabolome in Tomato Breeding. Cell. 2018; 172:249–61.e12. https://doi.org/10.1016/j.cell.2017.12.019 [PubMed]
-
26.
Medina CB, Mehrotra P, Arandjelovic S, Perry JSA, Guo Y, Morioka S, Barron B, Walk SF, Ghesquière B, Krupnick AS, Lorenz U, Ravichandran KS. Metabolites released from apoptotic cells act as tissue messengers. Nature. 2020; 580:130–5. https://doi.org/10.1038/s41586-020-2121-3 [PubMed]
-
27.
Zhang Z, Du C, de Barsy F, Liem M, Liakopoulos A, van Wezel GP, Choi YH, Claessen D, Rozen DE. Antibiotic production in Streptomyces is organized by a division of labor through terminal genomic differentiation. Sci Adv. 2020; 6:eaay5781. https://doi.org/10.1126/sciadv.aay5781 [PubMed]
-
28.
Wishart DS. Metabolomics for Investigating Physiological and Pathophysiological Processes. Physiol Rev. 2019; 99:1819–75. https://doi.org/10.1152/physrev.00035.2018 [PubMed]
-
29.
Ducker GS, Ghergurovich JM, Mainolfi N, Suri V, Jeong SK, Hsin-Jung Li S, Friedman A, Manfredi MG, Gitai Z, Kim H, Rabinowitz JD. Human SHMT inhibitors reveal defective glycine import as a targetable metabolic vulnerability of diffuse large B-cell lymphoma. Proc Natl Acad Sci U S A. 2017; 114:11404–9. https://doi.org/10.1073/pnas.1706617114 [PubMed]
-
30.
Mills EL, Pierce KA, Jedrychowski MP, Garrity R, Winther S, Vidoni S, Yoneshiro T, Spinelli JB, Lu GZ, Kazak L, Banks AS, Haigis MC, Kajimura S, et al. Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature. 2018; 560:102–6. https://doi.org/10.1038/s41586-018-0353-2 [PubMed]
-
31.
Griffin JL, Shockcor JP. Metabolic profiles of cancer cells. Nat Rev Cancer. 2004; 4:551–61. https://doi.org/10.1038/nrc1390 [PubMed]
-
32.
Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011; 11:85–95. https://doi.org/10.1038/nrc2981 [PubMed]
-
33.
Hu J, Locasale JW, Bielas JH, O'Sullivan J, Sheahan K, Cantley LC, Vander Heiden MG, Vitkup D. Heterogeneity of tumor-induced gene expression changes in the human metabolic network. Nat Biotechnol. 2013; 31:522–9. https://doi.org/10.1038/nbt.2530 [PubMed]
-
34.
Bamji-Stocke S, van Berkel V, Miller DM, Frieboes HB. A review of metabolism-associated biomarkers in lung cancer diagnosis and treatment. Metabolomics. 2018; 14:81. https://doi.org/10.1007/s11306-018-1376-2 [PubMed]
-
35.
Günther UL. Metabolomics Biomarkers for Breast Cancer. Pathobiology. 2015; 82:153–65. https://doi.org/10.1159/000430844 [PubMed]
-
36.
Kdadra M, Höckner S, Leung H, Kremer W, Schiffer E. Metabolomics Biomarkers of Prostate Cancer: A Systematic Review. Diagnostics (Basel). 2019; 9:21. https://doi.org/10.3390/diagnostics9010021 [PubMed]
-
37.
Erben V, Bhardwaj M, Schrotz-King P, Brenner H. Metabolomics Biomarkers for Detection of Colorectal Neoplasms: A Systematic Review. Cancers (Basel). 2018; 10:246. https://doi.org/10.3390/cancers10080246 [PubMed]
-
38.
Wishart DS. Emerging applications of metabolomics in drug discovery and precision medicine. Nat Rev Drug Discov. 2016; 15:473–84. https://doi.org/10.1038/nrd.2016.32 [PubMed]
-
39.
Bi J, Chowdhry S, Wu S, Zhang W, Masui K, Mischel PS. Altered cellular metabolism in gliomas - an emerging landscape of actionable co-dependency targets. Nat Rev Cancer. 2020; 20:57–70. https://doi.org/10.1038/s41568-019-0226-5 [PubMed]
-
40.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022; 72:7–33. https://doi.org/10.3322/caac.21708 [PubMed]
-
41.
Winter H, Kaisaki PJ, Harvey J, Giacopuzzi E, Ferla MP, Pentony MM, Knight SJL, Sharma RA, Taylor JC, McCullagh JSO. Identification of Circulating Genomic and Metabolic Biomarkers in Intrahepatic Cholangiocarcinoma. Cancers (Basel). 2019; 11:1895. https://doi.org/10.3390/cancers11121895 [PubMed]
-
42.
Jia W, Xie G. Probiotics, bile acids and gastrointestinal carcinogenesis. Nat Rev Gastroenterol Hepatol. 2018; 15:205. https://doi.org/10.1038/nrgastro.2018.24 [PubMed]
-
43.
Jia W, Xie G, Jia W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol. 2018; 15:111–28. https://doi.org/10.1038/nrgastro.2017.119 [PubMed]
-
44.
Audet-Delage Y, Villeneuve L, Grégoire J, Plante M, Guillemette C. Identification of Metabolomic Biomarkers for Endometrial Cancer and Its Recurrence after Surgery in Postmenopausal Women. Front Endocrinol (Lausanne). 2018; 9:87. https://doi.org/10.3389/fendo.2018.00087 [PubMed]
-
45.
Schmidt JA, Fensom GK, Rinaldi S, Scalbert A, Appleby PN, Achaintre D, Gicquiau A, Gunter MJ, Ferrari P, Kaaks R, Kühn T, Boeing H, Trichopoulou A, et al. Patterns in metabolite profile are associated with risk of more aggressive prostate cancer: A prospective study of 3,057 matched case-control sets from EPIC. Int J Cancer. 2020; 146:720–30. https://doi.org/10.1002/ijc.32314 [PubMed]
-
46.
Xu H, Wei Y, Zhu L, Huang J, Li Y, Liu F, Wang S, Liu S. Bifunctional magnetic nanoparticles for analysis of aldehyde metabolites in exhaled breath of lung cancer patients. J Chromatogr A. 2014; 1324:29–35. https://doi.org/10.1016/j.chroma.2013.11.041 [PubMed]
-
47.
Miyagi Y, Higashiyama M, Gochi A, Akaike M, Ishikawa T, Miura T, Saruki N, Bando E, Kimura H, Imamura F, Moriyama M, Ikeda I, Chiba A, et al. Plasma free amino acid profiling of five types of cancer patients and its application for early detection. PLoS One. 2011; 6:e24143. https://doi.org/10.1371/journal.pone.0024143 [PubMed]
-
48.
Koureas M, Kirgou P, Amoutzias G, Hadjichristodoulou C, Gourgoulianis K, Tsakalof A. Target Analysis of Volatile Organic Compounds in Exhaled Breath for Lung Cancer Discrimination from Other Pulmonary Diseases and Healthy Persons. Metabolites. 2020; 10:317. https://doi.org/10.3390/metabo10080317 [PubMed]
-
49.
Peralbo-Molina A, Calderón-Santiago M, Priego-Capote F, Jurado-Gámez B, Luque de Castro MD. Identification of metabolomics panels for potential lung cancer screening by analysis of exhaled breath condensate. J Breath Res. 2016; 10:026002. https://doi.org/10.1088/1752-7155/10/2/026002 [PubMed]
-
50.
Peralbo-Molina A, Calderón-Santiago M, Priego-Capote F, Jurado-Gámez B, Luque de Castro MD. Metabolomics analysis of exhaled breath condensate for discrimination between lung cancer patients and risk factor individuals. J Breath Res. 2016; 10:016011. https://doi.org/10.1088/1752-7155/10/1/016011 [PubMed]
-
51.
Huang J, Deng H, Song D, Xu H. Electrospun polystyrene/graphene nanofiber film as a novel adsorbent of thin film microextraction for extraction of aldehydes in human exhaled breath condensates. Anal Chim Acta. 2015; 878:102–8. https://doi.org/10.1016/j.aca.2015.03.053 [PubMed]
-
52.
Wang C, Dong R, Wang X, Lian A, Chi C, Ke C, Guo L, Liu S, Zhao W, Xu G, Li E. Exhaled volatile organic compounds as lung cancer biomarkers during one-lung ventilation. Sci Rep. 2014; 4:7312. https://doi.org/10.1038/srep07312 [PubMed]
-
53.
Filipiak W, Filipiak A, Sponring A, Schmid T, Zelger B, Ager C, Klodzinska E, Denz H, Pizzini A, Lucciarini P, Jamnig H, Troppmair J, Amann A. Comparative analyses of volatile organic compounds (VOCs) from patients, tumors and transformed cell lines for the validation of lung cancer-derived breath markers. J Breath Res. 2014; 8:027111. https://doi.org/10.1088/1752-7155/8/2/027111 [PubMed]
-
54.
Ganti S, Taylor SL, Kim K, Hoppel CL, Guo L, Yang J, Evans C, Weiss RH. Urinary acylcarnitines are altered in human kidney cancer. Int J Cancer. 2012; 130:2791–800. https://doi.org/10.1002/ijc.26274 [PubMed]
-
55.
Guida F, Tan VY, Corbin LJ, Smith-Byrne K, Alcala K, Langenberg C, Stewart ID, Butterworth AS, Surendran P, Achaintre D, Adamski J, Amiano P, Bergmann MM, et al. The blood metabolome of incident kidney cancer: A case-control study nested within the MetKid consortium. PLoS Med. 2021; 18:e1003786. https://doi.org/10.1371/journal.pmed.1003786 [PubMed]
-
56.
Miller KD, Ostrom QT, Kruchko C, Patil N, Tihan T, Cioffi G, Fuchs HE, Waite KA, Jemal A, Siegel RL, Barnholtz-Sloan JS. Brain and other central nervous system tumor statistics, 2021. CA Cancer J Clin. 2021; 71:381–406. https://doi.org/10.3322/caac.21693 [PubMed]
-
57.
Locasale JW, Melman T, Song S, Yang X, Swanson KD, Cantley LC, Wong ET, Asara JM. Metabolomics of human cerebrospinal fluid identifies signatures of malignant glioma. Mol Cell Proteomics. 2012; 11:M111.014688. https://doi.org/10.1074/mcp.M111.014688 [PubMed]
-
58.
Björkblom B, Wibom C, Jonsson P, Mörén L, Andersson U, Johannesen TB, Langseth H, Antti H, Melin B. Metabolomic screening of pre-diagnostic serum samples identifies association between α- and γ-tocopherols and glioblastoma risk. Oncotarget. 2016; 7:37043–53. https://doi.org/10.18632/oncotarget.9242 [PubMed]
-
59.
Rad Pour S, Morikawa H, Kiani NA, Gomez-Cabrero D, Hayes A, Zheng X, Pernemalm M, Lehtiö J, Mole DJ, Hansson J, Eriksson H, Tegnér J. Immunometabolic Network Interactions of the Kynurenine Pathway in Cutaneous Malignant Melanoma. Front Oncol. 2020; 10:51. https://doi.org/10.3389/fonc.2020.00051 [PubMed]
-
60.
Chen WL, Wang JH, Zhao AH, Xu X, Wang YH, Chen TL, Li JM, Mi JQ, Zhu YM, Liu YF, Wang YY, Jin J, Huang H, et al. A distinct glucose metabolism signature of acute myeloid leukemia with prognostic value. Blood. 2014; 124:1645–54. https://doi.org/10.1182/blood-2014-02-554204 [PubMed]
-
61.
Bradner JE, Hnisz D, Young RA. Transcriptional Addiction in Cancer. Cell. 2017; 168:629–43. https://doi.org/10.1016/j.cell.2016.12.013 [PubMed]
-
62.
DeBerardinis RJ, Keshari KR. Metabolic analysis as a driver for discovery, diagnosis, and therapy. Cell. 2022; 185:2678–89. https://doi.org/10.1016/j.cell.2022.06.029 [PubMed]
-
63.
Fathi AT, Sadrzadeh H, Borger DR, Ballen KK, Amrein PC, Attar EC, Foster J, Burke M, Lopez HU, Matulis CR, Edmonds KM, Iafrate AJ, Straley KS, et al. Prospective serial evaluation of 2-hydroxyglutarate, during treatment of newly diagnosed acute myeloid leukemia, to assess disease activity and therapeutic response. Blood. 2012; 120:4649–52. https://doi.org/10.1182/blood-2012-06-438267 [PubMed]
-
64.
Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, Fantin VR, Jang HG, Jin S, Keenan MC, Marks KM, Prins RM, Ward PS, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009; 462:739–44. https://doi.org/10.1038/nature08617 [PubMed]
-
65.
Ward PS, Patel J, Wise DR, Abdel-Wahab O, Bennett BD, Coller HA, Cross JR, Fantin VR, Hedvat CV, Perl AE, Rabinowitz JD, Carroll M, Su SM, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010; 17:225–34. https://doi.org/10.1016/j.ccr.2010.01.020 [PubMed]
-
66.
Mayers JR, Wu C, Clish CB, Kraft P, Torrence ME, Fiske BP, Yuan C, Bao Y, Townsend MK, Tworoger SS, Davidson SM, Papagiannakopoulos T, Yang A, et al. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat Med. 2014; 20:1193–8. https://doi.org/10.1038/nm.3686 [PubMed]
-
67.
Liu X, Romero IL, Litchfield LM, Lengyel E, Locasale JW. Metformin Targets Central Carbon Metabolism and Reveals Mitochondrial Requirements in Human Cancers. Cell Metab. 2016; 24:728–39. https://doi.org/10.1016/j.cmet.2016.09.005 [PubMed]
-
68.
Zhou H, Nong Y, Zhu Y, Liang Y, Zhang J, Chen H, Zhu P, Zhang Q. Serum untargeted lipidomics by UHPLC-ESI-HRMS aids the biomarker discovery of colorectal adenoma. BMC Cancer. 2022; 22:314. https://doi.org/10.1186/s12885-022-09427-1 [PubMed]
-
69.
Luo X, Liu J, Wang H, Lu H. Metabolomics identified new biomarkers for the precise diagnosis of pancreatic cancer and associated tissue metastasis. Pharmacol Res. 2020; 156:104805. https://doi.org/10.1016/j.phrs.2020.104805 [PubMed]
-
70.
Yu L, Lai Q, Feng Q, Li Y, Feng J, Xu B. Serum Metabolic Profiling Analysis of Chronic Gastritis and Gastric Cancer by Untargeted Metabolomics. Front Oncol. 2021; 11:636917. https://doi.org/10.3389/fonc.2021.636917 [PubMed]
-
71.
Yu M, Sun R, Zhao Y, Shao F, Zhu W, Aa J. Detection and verification of coexisting diagnostic markers in plasma and serum of patients with non-small-cell lung cancer. Future Oncol. 2021; 17:4355–69. https://doi.org/10.2217/fon-2021-0025 [PubMed]
-
72.
Jové M, Collado R, Quiles JL, Ramírez-Tortosa MC, Sol J, Ruiz-Sanjuan M, Fernandez M, de la Torre Cabrera C, Ramírez-Tortosa C, Granados-Principal S, Sánchez-Rovira P, Pamplona R. A plasma metabolomic signature discloses human breast cancer. Oncotarget. 2017; 8:19522–33. https://doi.org/10.18632/oncotarget.14521 [PubMed]
-
73.
Ignatiadis M, Sledge GW, Jeffrey SS. Liquid biopsy enters the clinic - implementation issues and future challenges. Nat Rev Clin Oncol. 2021; 18:297–312. https://doi.org/10.1038/s41571-020-00457-x [PubMed]
-
74.
Dinges SS, Hohm A, Vandergrift LA, Nowak J, Habbel P, Kaltashov IA, Cheng LL. Cancer metabolomic markers in urine: evidence, techniques and recommendations. Nat Rev Urol. 2019; 16:339–62. https://doi.org/10.1038/s41585-019-0185-3 [PubMed]
-
75.
Böttcher T. From Molecules to Life: Quantifying the Complexity of Chemical and Biological Systems in the Universe. J Mol Evol. 2018; 86:1–10. https://doi.org/10.1007/s00239-017-9824-6 [PubMed]
-
76.
Michálková L, Horník Š, Sýkora J, Habartová L, Setnička V, Bunganič B. Early Detection of Pancreatic Cancer in Type 2 Diabetes Mellitus Patients Based on 1H NMR Metabolomics. J Proteome Res. 2021; 20:1744–53. https://doi.org/10.1021/acs.jproteome.0c00990 [PubMed]