A real-world study was conducted to develop a nomogram that predicts the occurrence of anastomotic leakage in patients with esophageal cancer following esophagectomy
Abstract
Background: The incidence of anastomotic leakage (AL) following esophagectomy is regarded as a noteworthy complication. There is a need for biomarkers to facilitate early diagnosis of AL in high-risk esophageal cancer (EC) patients, thereby minimizing its morbidity and mortality. We assessed the predictive abilities of inflammatory biomarkers for AL in patients after esophagectomy.
Methods: In order to ascertain the predictive efficacy of biomarkers for AL, Receiver Operating Characteristic (ROC) curves were generated. Furthermore, univariate, LASSO, and multivariate logistic regression analyses were conducted to discern the risk factors associated with AL. Based on these identified risk factors, a diagnostic nomogram model was formulated and subsequently assessed for its predictive performance.
Results: Among the 438 patients diagnosed with EC, a total of 25 patients encountered AL. Notably, elevated levels of interleukin-6 (IL-6), IL-10, C-reactive protein (CRP), and procalcitonin (PCT) were observed in the AL group as compared to the non-AL group, demonstrating statistical significance. Particularly, IL-6 exhibited the highest predictive capacity for early postoperative AL, exhibiting a sensitivity of 92.00% and specificity of 61.02% at a cut-off value of 132.13 pg/ml. Univariate, LASSO, and multivariate logistic regression analyses revealed that fasting blood glucose ≥7.0mmol/L and heightened levels of IL-10, IL-6, CRP, and PCT were associated with an augmented risk of AL. Consequently, a nomogram model was formulated based on the results of multivariate logistic analyses. The diagnostic nomogram model displayed a robust discriminatory ability in predicting AL, as indicated by a C-Index value of 0.940. Moreover, the decision curve analysis provided further evidence supporting the clinical utility of this diagnostic nomogram model.
Conclusions: This predictive instrument can serve as a valuable resource for clinicians, empowering them to make informed clinical judgments aimed at averting the onset of AL.
Introduction
Esophageal cancer (EC) represents a widespread malignant neoplasm on a global scale, with China bearing a particularly high burden of mortality and morbidity associated with this disease [1, 2]. The 5-year overall survival rate for individuals undergoing curative treatment for EC varies between 40% and 50% [3, 4]. The management of EC typically encompasses a multimodal approach, incorporating various strategies such as surgical resection, chemotherapy, radiotherapy, immunotherapy, and targeted therapy [2, 5–7]. Esophagectomy is widely recognized as the foremost therapeutic modality for EC, given its efficacy. Nevertheless, it is an invasive intervention that entails the potential for postoperative complications [8]. Anastomotic leakage (AL) represents a grave and potentially life-threatening complication that may arise following esophagectomy. Its occurrence is linked to heightened mortality and morbidity rates, prolonged hospital stays, and elevated financial burdens on both patients and healthcare systems [9–12]. In addition, the recurrence of EC is affected by AL [13]. The incidence of AL among patients with EC can vary, ranging from 11.4% to 21.2% [14–17]. The precise etiology of AL remains elusive. Nevertheless, various risk factors have been identified, including cardiac comorbidity, male gender, advanced age, diabetes mellitus, renal disease, higher American Society of Anesthesiologists (ASA) score, pulmonary comorbidity, vascular comorbidity, higher body mass index (BMI), and hypertension. These factors have been correlated with an elevated susceptibility to developing AL subsequent to esophagectomy [18–21]. Consequently, there exists a necessity to identify biomarkers that can facilitate the timely detection of AL in individuals with high-risk EC [22]. However, the clinical manifestations of AL often manifest at a later stage or display nonspecific features [11]; thus, it is difficult to predict AL in EC patients at an early stage.
The timely prediction of AL has the potential to considerably enhance the quality of life for individuals diagnosed with EC. Moreover, it can exert a positive influence on survival rates and recurrence, thereby contributing to improved patient outcomes [23]. A plethora of biomarkers have been extensively investigated to prognosticate the incidence of AL subsequent to esophagectomy [24–30], including C-reactive protein (CRP) or procalcitonin (PCT) [31–35]. Plasma cytokine levels have shown promise as potential predictors of AL [36–39] and reportedly have greater diagnostic abilities for AL, compared with CRP or PCT [37].
There exists a critical need for reliable preoperative predictive models to identify patients at risk of anastomotic leakage, enabling early intervention and personalized treatment strategies. Addressing this gap in the literature, this study aims to develop and validate a novel predictive model for anastomotic leakage in esophageal cancer patients, with the ultimate goal of improving clinical outcomes and enhancing patient care.
Materials and Methods
Patients
For this particular investigation, a total of 438 patients diagnosed with EC were enrolled, and they were admitted to the Affiliated Huaian No. 1 People’s Hospital of Nanjing Medical University during the period spanning September 2020 to March 2022. Inclusion criteria encompassed an age exceeding 18 years, confirmed diagnosis of EC supported by pathological evidence, and having undergone esophagectomy. Exclusion criteria entailed the presence of concurrent malignancies, infectious diseases, autoimmune disorders, or inadequate availability of data. The diagnosis of AL was established through the utilization of various criteria, including the identification of contrast extravasation on postoperative computed tomography, leakage of gastrointestinal tract contents via a wound or drainage tube, appearance of blue liquid in the incision or pleural drainage following oral administration of methylene blue, and endoscopic observations indicative of AL. The diagnosis of AL among EC patients occurred within the time frame ranging from the 3rd to the 10th day postoperatively. The severity of AL was classified into three grades: grade A, necessitating no active therapeutic intervention; grade B, requiring active therapeutic interventions excluding surgery; and grade C, necessitating surgical intervention. Informed consent was obtained from all participants, and the study protocol obtained approval from the Ethics Committee of Huaian No. 1 People’s Hospital, adhering to the principles outlined in the Helsinki Declaration.
Measurement of cytokine levels
Cytometric bead array analysis was conducted using the Human Th1/Th2 CBA kit from JIANGXI CELLCENE BIOTECH Co., Ltd., following the manufacturer’s instructions. Flow cytometry was employed to quantify the cytokine levels in individuals diagnosed with EC following esophagectomy. All cytokine levels were assessed on the first day postoperatively.
Statistical analysis
The collected data were subjected to various statistical analyses. Continuous variables were evaluated using Student’s t-test, while the chi-squared test was employed for categorical variables. The Mann-Whitney U-test was applied for nonparametric variables. Receiver Operating Characteristic (ROC) curves were constructed to assess the diagnostic capabilities of the biomarkers for AL. Univariate, LASSO, and multivariate logistic regression analyses were performed to identify the risk factors associated with AL. Variables that yielded a p-value of less than 0.05 in the univariate analyses were included in the multivariate regression analysis. Odds ratios (OR) and their corresponding 95% confidence intervals (CI) were computed. A significance level of p < 0.05 was considered statistically significant. The statistical analyses were conducted using GraphPad Prism (version 8.0, La Jolla, CA, USA), SPSS (version 21.0, Chicago, IL, USA), R software (version 4.1.3), and MedCalc software.
Results
Clinicopathological variables in EC patients
The study enrolled a total of 438 patients diagnosed with EC, as illustrated in Figure 1. Among them, 25 patients experienced AL. The clinicopathological characteristics of the EC patients are presented in Table 1. Notably, the AL group displayed a considerably prolonged hospitalization duration in comparison to the non-AL group, as depicted in Figure 2 (29.28 ± 5.73 days vs. 12.78 ± 1.58 days, p < 0.001). Age (62.00 ± 9.77 years vs. 57.56 ± 9.84 years), smoking status, and the presence of fasting blood glucose (FBG) exhibited significant disparities between the two groups (Table 2). No other variables demonstrated significant differences between the AL and non-AL groups. The severity of AL was categorized into grades A, B, and C, encompassing 3, 21, and 1 patient(s) in each respective category (Supplementary Table 1); the variations in the levels of CRP, PCT, IL-6, and IL-10 among these three groups are shown in Supplementary Table 2.
Figure 1. Study flowchart.
Table 1. The baseline characteristics of esophageal cancer patients.
| Variables | Esophageal cancer (n = 438) |
| Age (years) | 57.82 ± 9.88 |
| Sex | |
| Male | 288 |
| Female | 150 |
| Smoking | |
| Yes | 285 |
| No | 153 |
| Drinking | |
| Yes | 215 |
| No | 223 |
| BMI (kg/m2) | 22.49 ± 3.10 |
| Hypertension | |
| Yes | 100 |
| No | 338 |
| Fasting blood glucose | |
| ≥7.0 mmol/L | 67 |
| <7.0 mmol/L | 371 |
| Family history | |
| Yes | 123 |
| No | 315 |
| Pathological grading | |
| Well differentiation | 97 |
| Moderate differentiation | 180 |
| Poorly differentiation | 161 |
| Histological type | |
| Squamous cell carcinoma | 419 |
| Undifferentiated carcinoma | 9 |
| Others | 10 |
| TNM stage | |
| I | 100 |
| II | 257 |
| III | 81 |
| Tumor location | |
| Upper | 53 |
| Middle | 240 |
| Lower | 96 |
| Gastroesophageal junction | 49 |
| Level of anastomosis | |
| Intra thoracic | 69 |
| Neck | 369 |
| Surgical type | |
| Open surgery | 17 |
| Thoracoscopy | 421 |
| Hospital stay time (days) | 13.73 ± 4.34 |
| Abbreviations: BMI: body mass index; TNM: tumor node metastasis. |
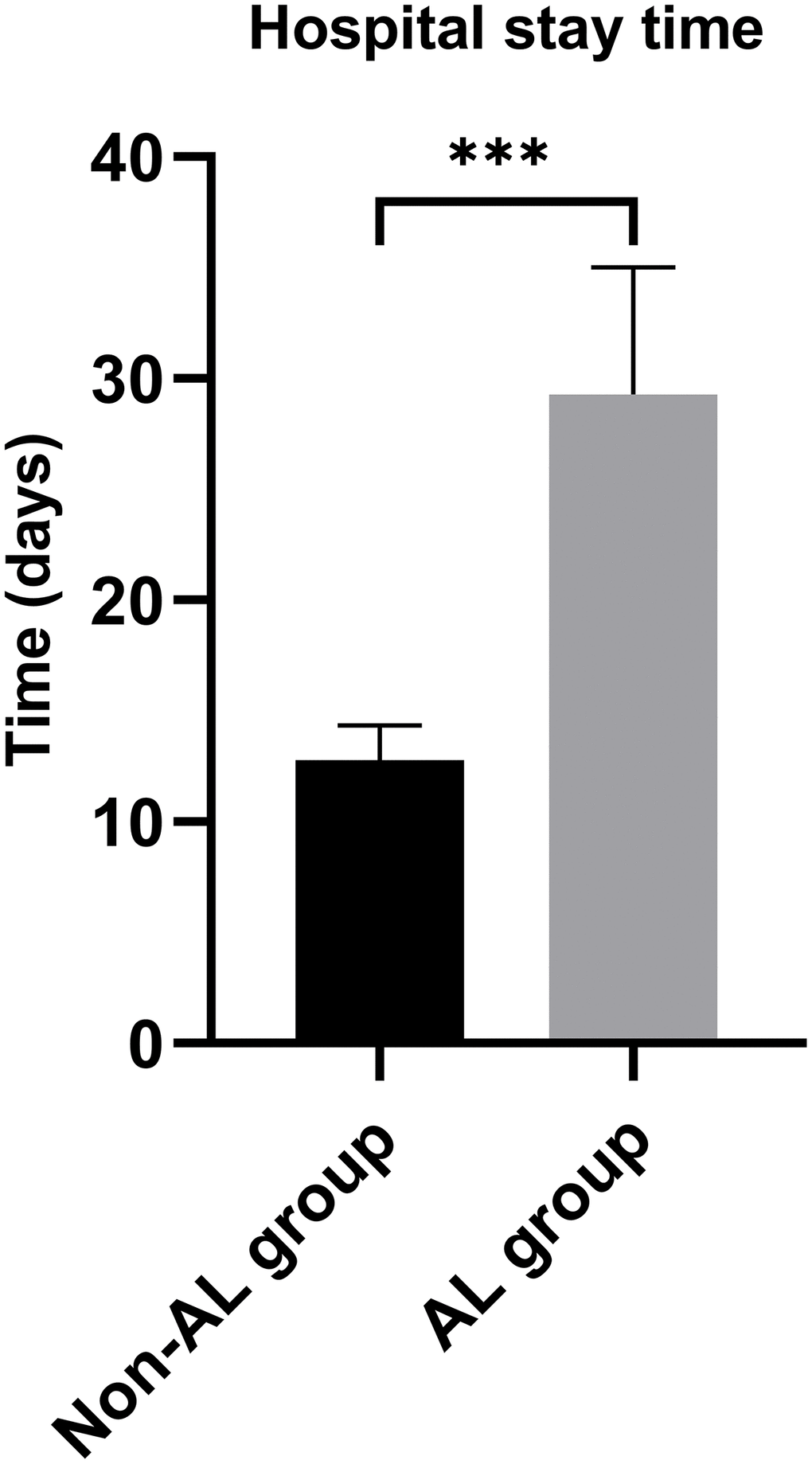
Figure 2. Hospital stay time between anastomotic leakage group and non-anastomotic leakage group.***P < 0.001.
Table 2. The baseline characteristics of esophageal cancer patients between AL and non-AL groups.
| Variables | AL group (n = 25) | Non-AL group (n = 413) | P |
| Age (years) | 62.00 ± 9.77 | 57.56 ± 9.84 | 0.041 |
| Sex | | | 0.532 |
| Male | 15 | 273 | |
| Female | 10 | 140 | |
| Smoking | | | 0.013 |
| Yes | 22 | 263 | |
| No | 3 | 150 | |
| Drinking | | | 0.911 |
| Yes | 12 | 203 | |
| No | 13 | 210 | |
| BMI (kg/m2) | 21.80 ± 2.72 | 22.53 ± 3.12 | 0.253 |
| Hypertension | | | 0.261 |
| Yes | 8 | 92 | |
| No | 17 | 321 | |
| Fasting blood glucose | | | 0.001 |
| ≥7.0 mmol/L | 10 | 57 | |
| <7.0 mmol/L | 15 | 356 | |
| Family history | | | 0.992 |
| Yes | 7 | 116 | |
| No | 18 | 297 | |
| Pathological grading | | | 0.465 |
| Well differentiation | 4 | 93 | |
| Moderate differentiation | 9 | 171 | |
| Poorly differentiation | 12 | 149 | |
| Histological type | | | 0.108 |
| Squamous cell carcinoma | 22 | 397 | |
| Undifferentiated carcinoma | 1 | 8 | |
| Others | 2 | 8 | |
| TNM stage | | | 0.599 |
| I | 4 | 96 | |
| II | 17 | 240 | |
| III | 4 | 77 | |
| Tumor location | | | 0.889 |
| Upper | 3 | 50 | |
| Middle | 13 | 227 | |
| Lower | 5 | 91 | |
| Gastroesophageal junction | 4 | 45 | |
| Level of anastomosis | | | 0.044 |
| Intra thoracic | 8 | 61 | |
| Neck | 7 | 352 | |
| Surgical type | | | 0.012 |
| Thoracotomy | 4 | 13 | |
| Thoracoscopy | 21 | 400 | |
| Hospital stay time (days) | 29.28 ± 5.73 | 12.78 ± 1.58 | <0.001 |
| Abbreviations: BMI: body mass index; TNM: tumor node metastasis; AL: anastomotic leakage. Bold values are statistically significant (P < 0.05). |
Predictive powers of inflammatory biomarkers for AL
On the first day postoperatively, the levels of interleukin-6 (IL-6), IL-10, C-reactive protein (CRP), and procalcitonin (PCT) were assessed in both the AL and non-AL groups, as outlined in Table 3. Remarkably elevated levels of IL-6 (203.01 ± 61.20 vs. 129.20 ± 54.66 pg/ml), IL-10 (15.08 ± 7.77 vs. 8.76 ± 5.02 pg/ml), CRP (186.11 ± 60.60 vs. 144.23 ± 45.27 mg/l), and PCT (4.84 ± 2.98 vs. 2.50 ± 1.03 ng/ml) were observed in the AL group compared to the non-AL group (Table 3). The ROC curve analysis presented in Table 4 indicated that IL-6 exhibited the highest predictive value as an early postoperative indicator for AL, demonstrating a sensitivity of 92.00% and specificity of 61.02% at a cutoff value of 132.13 pg/ml. These findings suggest that IL-6 possesses favorable diagnostic ability for AL. Furthermore, the combination of IL-6 and IL-10 exhibited superior diagnostic capability for AL compared to either cytokine alone or other combinations of inflammatory biomarkers (Figure 3). The area under the curve (AUC) for the combination of IL-6 and IL-10 was 0.899 (95% confidence interval = 0.87–0.93, p < 0.001).
Table 3. Cytokine levels in the AL and non-AL groups on postoperative day 1.
| Variables | AL (n = 25) | Non-AL (n = 413) | P |
| IL-6 (pg/ml) | 203.01 ± 61.20 | 129.20 ± 54.66 | <0.001 |
| IL-10 (pg/ml) | 15.08 ± 7.77 | 8.76 ± 5.02 | <0.001 |
| CRP (mg/l) | 186.11 ± 60.60 | 144.23 ± 45.27 | <0.001 |
| PCT (ng/ml) | 4.84 ± 2.98 | 2.50 ± 1.03 | 0.001 |
| Abbreviations: IL-6: interleukin-6; IL-10: interleukin-10; CRP: C-reactive protein, PCT: Procalcitonin; AL: anastomotic leakage. Bold values are statistically significant (P < 0.05). |
Table 4. ROC curves for predictive values of serum inflammatory cytokines in detecting anastomotic leakage in patients with esophageal cancer.
| Variables | Youden index J | SE | Associated criterion | Sensitivity % | Specificity % | AUC (95%) | 95% CI | Significance level P (Area = 0.5) |
| IL-6 (pg/ml) | 0.530 | 0.040 | >132.13 | 92.00 | 61.02 | 0.818 | 0.78–0.85 | <0.001 |
| IL-10 (pg/ml) | 0.444 | 0.053 | >9.97 | 80.00 | 64.41 | 0.767 | 0.73–0.81 | <0.001 |
| CRP (mg/l) | 0.428 | 0.056 | >162.97 | 76.00 | 66.83 | 0.712 | 0.67–0.75 | <0.001 |
| PCT (ng/ml) | 0.473 | 0.056 | >3.12 | 72.00 | 75.30 | 0.786 | 0.74–0.82 | <0.001 |
| CRP and PCT | 0.564 | 0.049 | >0.056 | 76.00 | 80.39 | 0.840 | 0.80–0.87 | <0.001 |
| IL-10 and CRP | 0.549 | 0.042 | >0.060 | 76.00 | 78.93 | 0.832 | 0.79–0.87 | <0.001 |
| IL-10 and PCT | 0.608 | 0.043 | >0.068 | 76.00 | 84.75 | 0.861 | 0.83–0.89 | <0.001 |
| IL-6 and CRP | 0.615 | 0.043 | >0.057 | 84.00 | 77.48 | 0.844 | 0.81–0.88 | <0.001 |
| IL-6 and IL-10 | 0.652 | 0.025 | >0.043 | 88.00 | 77.24 | 0.899 | 0.87–0.93 | <0.001 |
| IL-6 and PCT | 0.708 | 0.048 | >0.088 | 80.00 | 90.80 | 0.872 | 0.84–0.90 | <0.001 |
| Abbreviations: ROC: Receiver Operating Characteristic; SE: stand error; AUC: Area Under the ROC Curve; 95% CI: Confidence interval; IL-6: interleukin-6; IL-10: interleukin-10; CRP: C-reactive protein, PCT: Procalcitonin. |
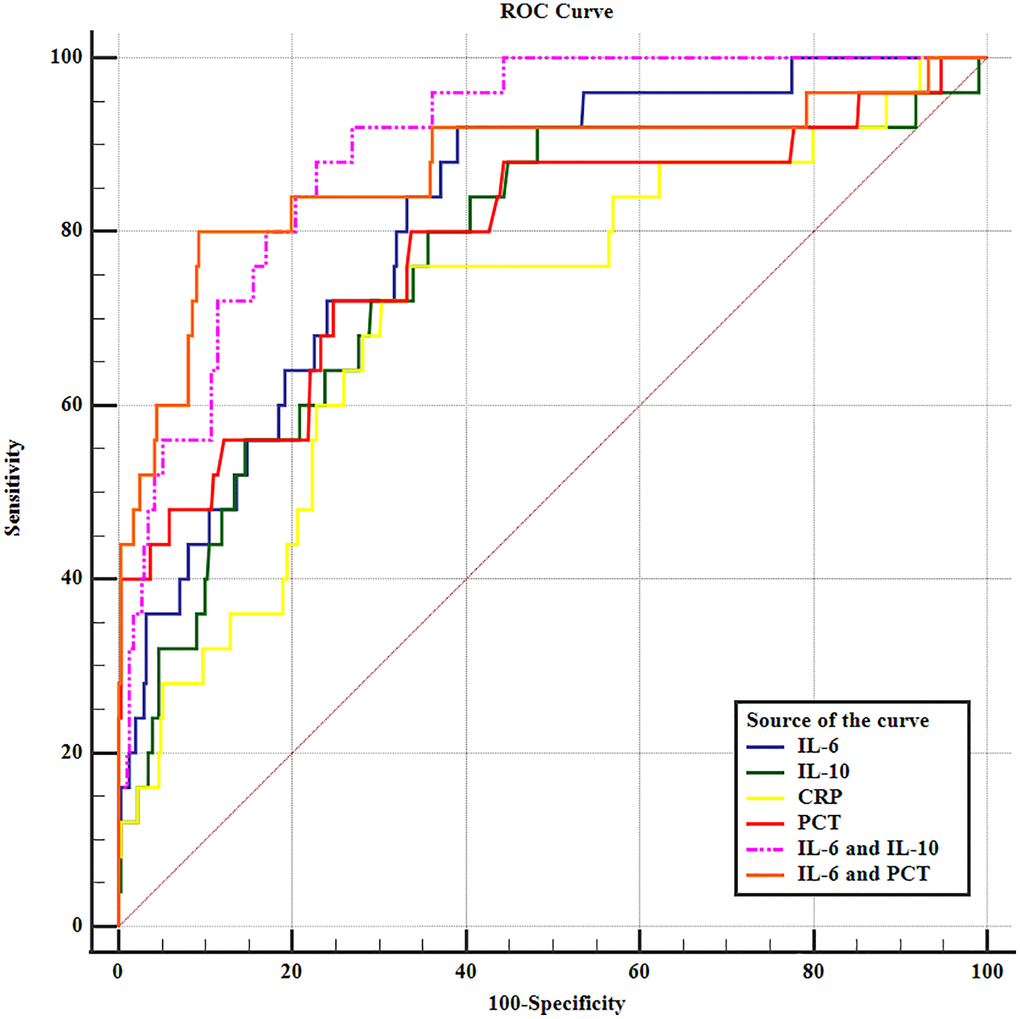
Figure 3. The receiver operating characteristic curve of inflammatory biomarkers in predicting anastomotic leakage in EC patients.
Risk factors for AL
The univariate logistic analysis revealed several factors that were associated with the risk of AL. These factors included older age (>60 vs. ≤60 years), smoking (yes vs. no), FBG (≥7.0 mmol/L vs. <7.0 mmol/L), intrathoracic location (intrathoracic vs. neck), open surgery (open surgery vs. thoracoscopy), and higher levels of IL-10, IL-6, CRP, and PCT (Table 5). Next, we identified five candidate predictors by LASSO regression analysis: FBG, IL-6, IL-10, CRP, and PCT (Supplementary Figure 1). In the multivariate analysis, after adjusting for other variables, it was found that FBG ≥7.0 mmol/L, IL-6 >132.13 pg/ml, IL-10 >9.97 pg/ml, CRP >162.97 mg/l, and PCT >3.12 ng/ml were independent risk factors for AL (Table 6 and Figure 4).
Table 5. Risk factors for anastomotic leakage in patients with esophageal cancer by univariate logistic regression analyses.
| Variables | B | S.E. | Wald | OR (95% CI) | P |
| Age (years) | | | | | |
| >60 vs. ≤60 | 0.884 | 0.421 | 4.418 | 2.42 (1.06–5.52) | 0.036 |
| Sex | | | | | |
| Male vs. Female | −0.262 | 0.421 | 0.388 | 0.77 (0.34–1.76) | 0.533 |
| Smoking | | | | | |
| Yes vs. No | 1.431 | 0.624 | 5.260 | 4.18 (1.23–14.21) | 0.022 |
| Drinking | | | | | |
| Yes vs. No | −0.046 | 0.412 | 0.013 | 0.96 (0.43–2.14) | 0.911 |
| BMI | | | | | |
| >24 vs. ≤24 | −0.824 | 0.556 | 2.194 | 0.44 (0.15–1.31) | 0.139 |
| Hypertension | | | | | |
| Yes vs. No | 0.496 | 0.445 | 1.243 | 1.64 (0.69–3.93) | 0.265 |
| Fasting blood glucose | | | | | |
| ≥7.0 mmol/L vs. <7.0 mmol/L | 1.426 | 0.432 | 10.879 | 4.16 (1.78–9.72) | 0.001 |
| Family history | | | | | |
| Yes vs. No | −0.004 | 0.459 | <0.001 | 0.99 (0.41–2.45) | 0.992 |
| Pathological grading | | | | | |
| Moderate vs. Well | 0.202 | 0.625 | 0.108 | 1.22 (0.37–4.08) | 0.743 |
| Poorly vs. Well | 0.627 | 0.592 | 0.122 | 1.87 (0.59–5.98) | 0.290 |
| Histological type | | | | | |
| Undifferentiated carcinoma vs. Squamous cell carcinoma | 0.813 | 1.083 | 0.564 | 2.26 (0.27–18.84) | 0.453 |
| Others vs. Squamous cell carcinoma | 1.507 | 0.820 | 3.373 | 4.51 (0.90–22.52) | 0.066 |
| TNM stage | | | | | |
| II vs. I | 0.531 | 0.569 | 0.871 | 1.70 (0.56–5.18) | 0.351 |
| III vs. I | 0.221 | 0.723 | 0.093 | 1.25 (0.30–5.15) | 0.760 |
| Tumor location | | | | | |
| Lower vs. Upper | −0.047 | 0.659 | 0.005 | 0.95 (0.26–3.48) | 0.944 |
| Middle vs. Upper | −0.088 | 0.751 | 0.014 | 0.92 (0.21–3.99) | 0.907 |
| Gastroesophageal junction vs. Upper | 0.393 | 0.791 | 0.247 | 1.48 (0.31–6.98) | 0.619 |
| Location of anastomotic leakage | | | | | |
| Intra thoracic vs. Neck | 0.999 | 0.451 | 4.915 | 2.72 (1.12–6.57) | 0.027 |
| Surgical type | | | | | |
| Open surgery vs. Thoracoscopy | 1.768 | 0.614 | 8.293 | 5.86 (1.76–19.53) | 0.004 |
| IL-6 (pg/ml) | | | | | |
| >132.13 vs. ≤132.13 | 2.890 | 0.744 | 15.089 | 18.00 (4.19–77.38) | <0.001 |
| IL-10 (pg/ml) | | | | | |
| >9.97 vs. ≤9.97 | 1.979 | 0.510 | 15.036 | 7.24 (2.66–19.68) | <0.001 |
| CRP (mg/l) | | | | | |
| >162.97 vs. ≤162.97 | 1.853 | 0.480 | 14.916 | 6.38 (2.49–16.34) | <0.001 |
| PCT (ng/ml) | | | | | |
| >3.12 vs. ≤3.12 | 2.059 | 0.460 | 20.057 | 7.84 (3.18–19.31) | <0.001 |
| Abbreviations: IL-6: interleukin-6; IL-10: interleukin-10; CRP: C-reactive protein, PCT: Procalcitonin; BMI: body mass index; TNM: tumor node metastasis. Bold values are statistically significant (P < 0.05). |
Table 6. Risk factors for anastomotic leakage in patients with esophageal cancer by multivariate logistic regression analyses.
| Variables | B | S.E. | Wald | OR (95% CI) | P |
| Fasting blood glucose | | | | | |
| ≥7.0 mmol/L vs. <7.0 mmol/L | 1.237 | 0.567 | 4.760 | 3.45 (1.13-10.48) | 0.029 |
| IL-6 (pg/ml) | | | | | |
| >132.13 vs. ≤132.13 | 2.698 | 0.799 | 11.398 | 14.84 (3.10-71.06) | 0.001 |
| IL-10 (pg/ml) | | | | | |
| >9.97 vs. ≤9.97 | 1.966 | 0.585 | 11.276 | 7.14 (2.27-22.50) | 0.001 |
| CRP (mg/l) | | | | | |
| >162.97 vs. ≤162.97 | 1.809 | 0.560 | 10.438 | 6.11 (2.04-18.30) | 0.001 |
| PCT (ng/ml) | | | | | |
| >3.12 vs. ≤3.12 | 2.182 | 0.548 | 15.839 | 8.87 (3.03-25.97) | 0.000 |
| Abbreviations: IL-6: interleukin-6; IL-10: interleukin-10; CRP: C-reactive protein, PCT: Procalcitonin. Bold values are statistically significant (P < 0.05). |
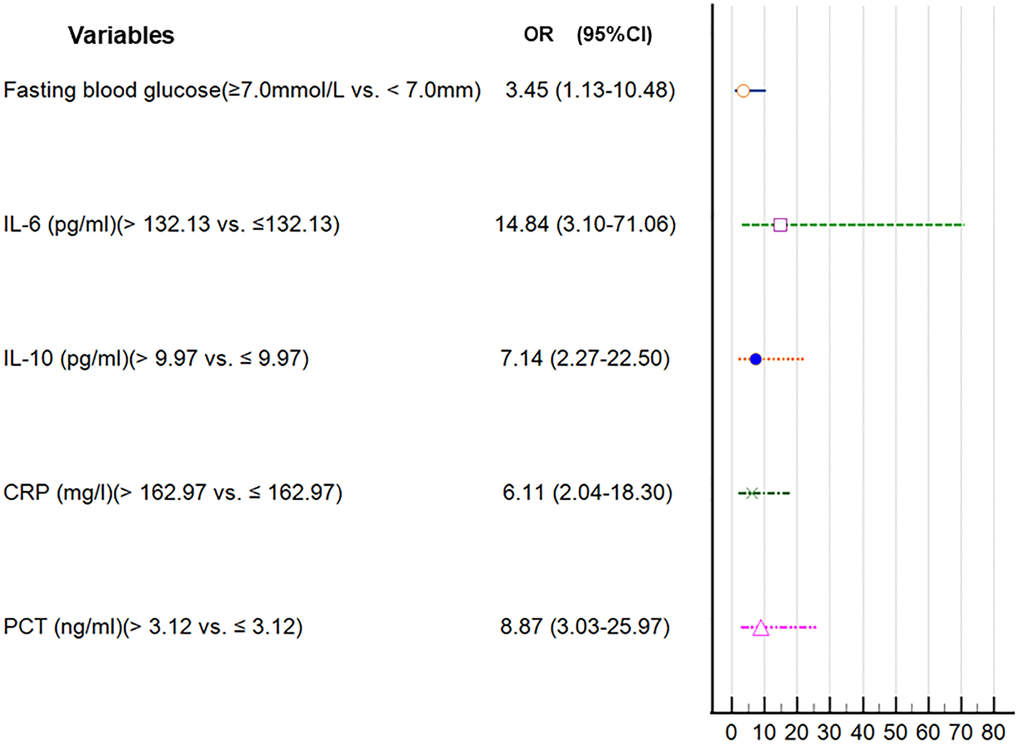
Figure 4. Forest plot of the parameters in the multivariate regression analysis.
Nomogram model of tredicting the AL
A nomogram model was developed based on the results of the multivariate logistic regression analysis to predict the occurrence of AL in patients with EC, as depicted in Figure 5. The C-Index of this predicting nomogram was calculated to be 0.940 (95% CI = 0.901–0.978), indicating a good discriminative ability of the model (Figure 6). Additionally, the decision curve analysis (DCA) demonstrated the clinical utility of this diagnostic nomogram model for making informed clinical decisions (Figure 7).
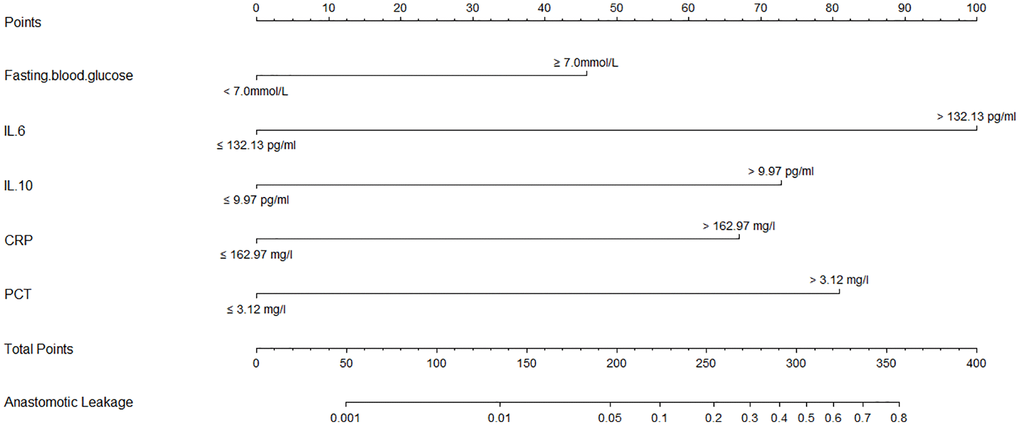
Figure 5. Calibration and clinical use of a diagnostic nomogram for predicting anastomotic leakage in patients with EC.
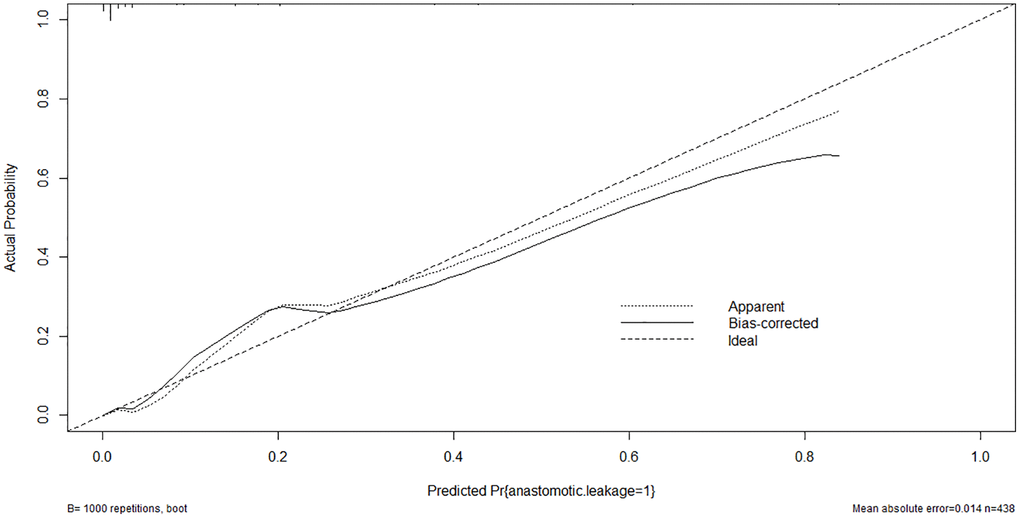
Figure 6. Calibration curve of the nomogram model of anastomotic leakage in patients with EC.
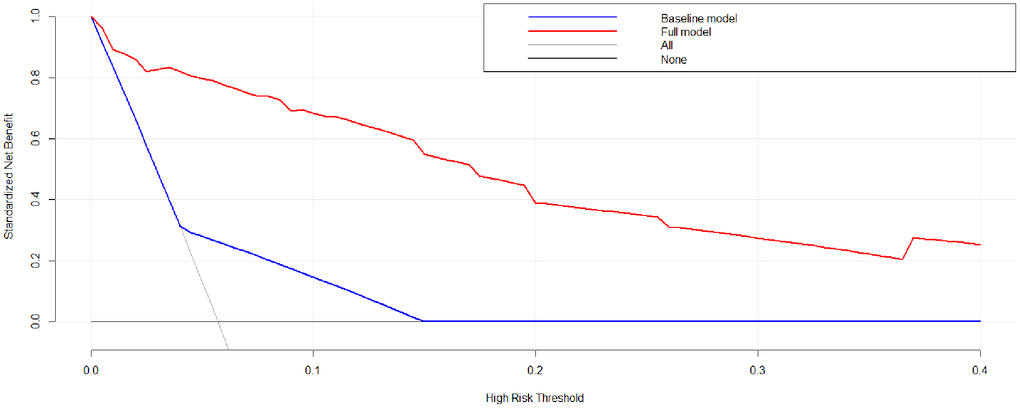
Figure 7. Decision curve analysis of the nomogram model of anastomotic leakage in patients with EC.
Discussion
This study revealed that the levels of IL-10, IL-6, CRP, and PCT in the peripheral blood were significantly elevated in the group of patients who experienced AL following esophagectomy, compared to the group without AL. Among these biomarkers, IL-6 exhibited the highest predictive value for AL, as indicated by the area under the receiver operating characteristic (ROC) curve. Moreover, combining IL-10 and IL-6 resulted in improved predictive power for AL, surpassing the individual cytokines or other combinations of inflammatory biomarkers. Logistic regression analyses demonstrated that FBG ≥7.0 mmol/L and higher levels of IL-10, IL-6, CRP, and PCT were associated with an increased risk of AL. Furthermore, the developed diagnostic nomogram model, based on these identified risk factors, proved to be effective in predicting the occurrence of AL in patients undergoing esophagectomy.
The diagnosis of AL is primarily based on the observation of clinical symptoms and the interpretation of imaging studies. Clinical symptoms may include fever, increased heart rate, chest or abdominal pain, difficulty swallowing, and signs of infection such as wound redness or drainage. Imaging studies, such as computed tomography (CT) scans or contrast studies, are commonly used to visualize the anastomotic site and detect any signs of leakage, such as contrast extravasation or the presence of fluid in areas outside the surgical site. These diagnostic modalities play a crucial role in confirming the presence of AL and guiding subsequent management decisions [11]. The pathophysiology of the disease remains unclear, with the development of AL after esophagectomy being associated with factors such as adequate perioperative preparation, as well as technical and anesthesiological considerations [40, 41]. The diagnosis of AL is often delayed due to the nonspecific and heterogeneous nature of its clinical manifestations [41]. A technical defect may be responsible for the occurrence of early AL, while late AL could be attributed to occult clinical symptoms in the early stages or an increase in oral intake after discharge [26, 42]. The timely diagnosis of AL is crucial, emphasizing the need for new biomarkers to aid in its detection.
CRP and PCT are biomarkers of AL [38, 43–45]. Elevated levels of C-reactive protein (CRP), an acute-phase protein, can be observed in both infectious and non-infectious conditions. However, due to its elevation in both situations, the CRP level lacks effectiveness in effectively distinguishing between infection and surgical complications [46, 47]. PCT, when compared to CRP, demonstrates greater specificity as a marker for severe infections and complications [48–50]. The ability of PCT to differentiate among postoperative complications is currently unknown and requires further investigation [49, 51]. According to the study by Lagoutte et al., the accuracy of PCT in predicting AL was found to be lower compared to CRP [49].
According to the findings of Ellebaek et al., postoperative cytokine levels, specifically IL-10 and IL-6, were observed to increase in patients who developed AL within 5 days after surgery. These elevated levels were found to be predictive of AL [52]. In the study conducted by Zawadzki et al., it was suggested that IL-6 served as a superior predictor of AL compared to CRP, particularly after low anterior resection for rectal cancer [37]. Certain studies have proposed that an increased peritoneal level of IL-6 exhibits stronger predictive capabilities for the occurrence of AL following colorectal surgery [36, 39, 53–56]; however, it is important to note that these studies did not measure the level of IL-6 in peripheral blood. In a study by Song et al., it was reported that plasma levels of IL-10, IL-6, and IL-8 on postoperative day 1 can serve as predictive markers for the development of AL in patients with EC undergoing esophagectomy [24]. Furthermore, IL-10 demonstrated higher predictive accuracy for AL compared to IL-6 or IL-8, as reported by Song et al. [24]. In the present study, peripheral levels of IL-10, IL-6, CRP, and PCT were significantly higher in the AL group than in the non-AL group after esophagectomy. Based on the area under the ROC curve, IL-6 was the best predictor of AL, which was inconsistent with a previous report [24]. This discrepancy may be related to the use of different definitions of AL and/or different cytokine assay methods; it may also be related to heterogeneity in the study populations.
In addition, the combination of IL-10 and IL-6 showed greater predictive power for AL, compared with either cytokine alone or other combinations of cytokines (Table 3). The mechanism underlying the association among IL-6, IL-10, and AL is unclear. The levels of IL-10 and IL-6 are elevated in sepsis [57–59], indicating a mixed hyperinflammatory and immunosuppressed status. AL after esophagectomy is associated with severe infection; thus, it is similar to sepsis. IL-10 promotes survival in mice with septic peritonitis [60], suggesting an anti-inflammatory effect. Peritoneal levels of IL-10 and IL-6 are reportedly predictive of AL after colorectal surgery [55], which was consistent with our findings. Cytokines are primarily divided into anti-inflammatory and proinflammatory cytokines. IL-6 and IL-10 were proinflammatory and anti-inflammatory cytokines, respectively. Greca et al. suggested that IL-6 showed a detrimental influence on the healing of colonic anastomoses [61] and may trigger AL. The serum level of CRP is a good indicator of symptomatic AL [62]; it is elevated in response to an increased level of IL-6. In addition, an elevated peritoneal level of IL-6 is associated with an increased risk of AL [36, 55, 56]. IL-10 induces an immunosuppressive or anti-inflammatory response and maintains inflammatory homeostasis in AL [31, 56]. IL-10 could facilitate innate immune responses to reduce the damage caused by bacterial and viral infections [63]. It also promotes tissue healing after inflammation or infection [64, 65]. IL-10 thus reflects the anti-inflammatory response to postoperative infection and surgical injury, which contributes to postoperative complications such as AL [66].
In addition, patient-related factors including diabetes mellitus, old age, alcohol, obesity, male sex, steroid use, smoking, malnutrition, and radiation therapy are reportedly associated with an elevated risk of AL [67–71]. In this study, univariate, LASSO, and multivariate logistic analyses indicated that FBG ≥7.0 mmol/L, and higher levels of IL-10, IL-6, CRP, and PCT were risk factors for AL. According to the results of logistic regression analysis, a diagnostic nomogram model was developed. This diagnostic nomogram model had a good discriminative ability for AL with a C-index value of 0.940. In addition, DCA showed that this diagnostic nomogram model was useful for making clinical decisions.
This study had several limitations. First, we evaluated the predictive powers of IL-6 and IL-10 for AL in EC patients; other cytokines should be investigated, especially for IL-8. Second, this study had a retrospective design. Third, subclinical AL was not routinely evaluated. Fourth, we did not investigate whether AL was associated with survival in EC patients. Last, we did not divide the EC patients into two separate sets (one for training set and one for validation set) due to limited sample size.
Conclusions
In conclusion, this study addresses a critical gap in the preoperative management of patients with esophageal cancer by developing a novel nomogram model to assess the risk of anastomotic leakage (AL) following esophagectomy. Our findings demonstrate the effectiveness of this diagnostic nomogram, which incorporates key risk factors, in accurately predicting the occurrence of AL in this patient population. By leveraging clinical data and patient characteristics, our nomogram offers clinicians a valuable tool for risk stratification and early intervention, ultimately improving patient outcomes and optimizing postoperative care. Moving forward, further validation and implementation of this nomogram in clinical practice hold the potential to enhance surgical decision-making, personalize treatment strategies, and reduce the burden of AL-associated complications in patients with esophageal cancer. However, due to the limited sample size, patients were not divided into training sets and validation sets, which we will improve in the future.
Author Contributions
Chenglin Li, Wei Song and Jialing Zhang designed the study. Chenglin Li, Yonggang Luo and Jialing Zhang were involved in database search and statistical analyses. Chenglin Li and Jialing Zhang were involved in the writing of manuscript and its critical revision. All authors were responsible for the submission of the final version of the paper. All authors approved the final version. All authors agree to be accountable for all aspects of the work.
Conflicts of Interest
The authors declare no conflicts of interest related to this study.
Ethical Statement and Consent
Informed consent was obtained from all participants, and the study protocol obtained approval from the Ethics Committee of Huaian No. 1 People’s Hospital, adhering to the principles outlined in the Helsinki Declaration (No. KY-2024-051-01).
Funding
No funding was used for this paper.
References
-
1.
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016; 66:115–32. https://doi.org/10.3322/caac.21338 [PubMed]
-
2.
Yang YM, Hong P, Xu WW, He QY, Li B. Advances in targeted therapy for esophageal cancer. Signal Transduct Target Ther. 2020; 5:229. https://doi.org/10.1038/s41392-020-00323-3 [PubMed]
-
3.
van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, Cuesta MA, Blaisse RJ, Busch OR, et al, and CROSS Group. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012; 366:2074–84. https://doi.org/10.1056/nejmoa1112088 [PubMed]
-
4.
Ruol A, Castoro C, Portale G, Cavallin F, Sileni VC, Cagol M, Alfieri R, Corti L, Boso C, Zaninotto G, Peracchia A, Ancona E. Trends in management and prognosis for esophageal cancer surgery: twenty-five years of experience at a single institution. Arch Surg. 2009; 144:247–54. https://doi.org/10.1001/archsurg.2008.574 [PubMed]
-
5.
He S, Xu J, Liu X, Zhen Y. Advances and challenges in the treatment of esophageal cancer. Acta Pharm Sin B. 2021; 11:3379–92. https://doi.org/10.1016/j.apsb.2021.03.008 [PubMed]
-
6.
Vellayappan BA, Soon YY, Ku GY, Leong CN, Lu JJ, Tey JC. Chemoradiotherapy versus chemoradiotherapy plus surgery for esophageal cancer. Cochrane Database Syst Rev. 2017; 8:CD010511. https://doi.org/10.1002/14651858.CD010511.pub2 [PubMed]
-
7.
Teixeira Farinha H, Digklia A, Schizas D, Demartines N, Schäfer M, Mantziari S. Immunotherapy for Esophageal Cancer: State-of-the Art in 2021. Cancers (Basel). 2022; 14:554. https://doi.org/10.3390/cancers14030554 [PubMed]
-
8.
Abdelsattar ZM, Habermann E, Borah BJ, Moriarty JP, Rojas RL, Blackmon SH. Understanding Failure to Rescue After Esophagectomy in the United States. Ann Thorac Surg. 2020; 109:865–71. https://doi.org/10.1016/j.athoracsur.2019.09.044 [PubMed]
-
9.
Kitadani J, Ojima T, Nakamura M, Hayata K, Katsuda M, Takeuchi A, Yamaue H. Impact of Anastomotic Leakage on Survival for Patients with Thoracic Esophageal Cancer Performed with Esophagectomy Followed by Right Colon Interposition. J Gastrointest Surg. 2022; 26:1090–2. https://doi.org/10.1007/s11605-021-05196-7 [PubMed]
-
10.
Eichelmann AK, Ismail S, Merten J, Slepecka P, Palmes D, Laukötter MG, Pascher A, Mardin WA. Economic Burden of Endoscopic Vacuum Therapy Compared to Alternative Therapy Methods in Patients with Anastomotic Leakage After Esophagectomy. J Gastrointest Surg. 2021; 25:2447–54. https://doi.org/10.1007/s11605-021-04955-w [PubMed]
-
11.
Fabbi M, Hagens ERC, van Berge Henegouwen MI, Gisbertz SS. Anastomotic leakage after esophagectomy for esophageal cancer: definitions, diagnostics, and treatment. Dis Esophagus. 2021; 34:doaa039. https://doi.org/10.1093/dote/doaa039 [PubMed]
-
12.
Zhong L, Zhong J, Tan Z, Wei Y, Su X, Wen Z, Rong T, Hu Y, Luo K. An Approach to Accelerate Healing and Shorten the Hospital Stay of Patients With Anastomotic Leakage After Esophagectomy: An Explorative Study of Systematic Endoscopic Intervention. Front Oncol. 2021; 11:657955. https://doi.org/10.3389/fonc.2021.657955 [PubMed]
-
13.
Aurello P, Berardi G, Moschetta G, Cinquepalmi M, Antolino L, Nigri G, D'Angelo F, Valabrega S, Ramacciato G. Recurrence Following Anastomotic Leakage After Surgery for Carcinoma of the Distal Esophagus and Gastroesophageal Junction: A Systematic Review. Anticancer Res. 2019; 39:1651–60. https://doi.org/10.21873/anticanres.13270 [PubMed]
-
14.
Low DE, Kuppusamy MK, Alderson D, Cecconello I, Chang AC, Darling G, Davies A, D'Journo XB, Gisbertz SS, Griffin SM, Hardwick R, Hoelscher A, Hofstetter W, et al. Benchmarking Complications Associated with Esophagectomy. Ann Surg. 2019; 269:291–8. https://doi.org/10.1097/SLA.0000000000002611 [PubMed]
-
15.
Schmidt HM, Gisbertz SS, Moons J, Rouvelas I, Kauppi J, Brown A, Asti E, Luyer M, Lagarde SM, Berlth F, Philippron A, Bruns C, Hölscher A, et al. Defining Benchmarks for Transthoracic Esophagectomy: A Multicenter Analysis of Total Minimally Invasive Esophagectomy in Low Risk Patients. Ann Surg. 2017; 266:814–21. https://doi.org/10.1097/SLA.0000000000002445 [PubMed]
-
16.
Seesing MFJ, Gisbertz SS, Goense L, van Hillegersberg R, Kroon HM, Lagarde SM, Ruurda JP, Slaman AE, van Berge Henegouwen MI, Wijnhoven BPL. A Propensity Score Matched Analysis of Open Versus Minimally Invasive Transthoracic Esophagectomy in the Netherlands. Ann Surg. 2017; 266:839–46. https://doi.org/10.1097/SLA.0000000000002393 [PubMed]
-
17.
van Workum F, van der Maas J, van den Wildenberg FJ, Polat F, Kouwenhoven EA, van Det MJ, Nieuwenhuijzen GA, Luyer MD, Rosman C. Improved Functional Results After Minimally Invasive Esophagectomy: Intrathoracic Versus Cervical Anastomosis. Ann Thorac Surg. 2017; 103:267–73. https://doi.org/10.1016/j.athoracsur.2016.07.010 [PubMed]
-
18.
Li H, Zhuang S, Yan H, Wei W, Su Q. Risk Factors of Anastomotic Leakage After Esophagectomy With Intrathoracic Anastomosis. Front Surg. 2021; 8:743266. https://doi.org/10.3389/fsurg.2021.743266 [PubMed]
-
19.
Chen C, Jiang H. The assessment of intraoperative technique-related risk factors and the treatment of anastomotic leakage after esophagectomy: a narrative review. J Gastrointest Oncol. 2021; 12:207–15. https://doi.org/10.21037/jgo-21-45 [PubMed]
-
20.
van Kooten RT, Voeten DM, Steyerberg EW, Hartgrink HH, van Berge Henegouwen MI, van Hillegersberg R, Tollenaar RAEM, Wouters MWJM. Patient-Related Prognostic Factors for Anastomotic Leakage, Major Complications, and Short-Term Mortality Following Esophagectomy for Cancer: A Systematic Review and Meta-Analyses. Ann Surg Oncol. 2022; 29:1358–73. https://doi.org/10.1245/s10434-021-10734-3 [PubMed]
-
21.
Hagens ERC, Reijntjes MA, Anderegg MCJ, Eshuis WJ, van Berge Henegouwen MI, Gisbertz SS. Risk Factors and Consequences of Anastomotic Leakage After Esophagectomy for Cancer. Ann Thorac Surg. 2021; 112:255–63. https://doi.org/10.1016/j.athoracsur.2020.08.022 [PubMed]
-
22.
Moon SW, Kim JJ, Cho DG, Park JK. Early detection of complications: anastomotic leakage. J Thorac Dis. 2019; 11:S805–11. https://doi.org/10.21037/jtd.2018.11.55 [PubMed]
-
23.
Markar S, Gronnier C, Duhamel A, Mabrut JY, Bail JP, Carrere N, Lefevre JH, Brigand C, Vaillant JC, Adham M, Msika S, Demartines N, Nakadi IE, et al, and FREGAT (French Eso-Gastric Tumors) working group, FRENCH (Fédération de Recherche EN CHirurgie), and AFC (Association Française de Chirurgie). The Impact of Severe Anastomotic Leak on Long-term Survival and Cancer Recurrence After Surgical Resection for Esophageal Malignancy. Ann Surg. 2015; 262:972–80. https://doi.org/10.1097/SLA.0000000000001011 [PubMed]
-
24.
Song JQ, He YZ, Fang Y, Wu W, Zhong M. The predictive value of plasma cytokines on gastroesophageal anastomotic leakage at an early stage in patients undergoing esophagectomy. J Thorac Dis. 2017; 9:2544–50. https://doi.org/10.21037/jtd.2017.07.32 [PubMed]
-
25.
Asti E, Bonitta G, Melloni M, Tornese S, Milito P, Sironi A, Costa E, Bonavina L. Utility of C-reactive protein as predictive biomarker of anastomotic leak after minimally invasive esophagectomy. Langenbecks Arch Surg. 2018; 403:235–44. https://doi.org/10.1007/s00423-018-1663-4 [PubMed]
-
26.
Baker EH, Hill JS, Reames MK, Symanowski J, Hurley SC, Salo JC. Drain amylase aids detection of anastomotic leak after esophagectomy. J Gastrointest Oncol. 2016; 7:181–8. https://doi.org/10.3978/j.issn.2078-6891.2015.074 [PubMed]
-
27.
Dutta S, Fullarton GM, Forshaw MJ, Horgan PG, McMillan DC. Persistent elevation of C-reactive protein following esophagogastric cancer resection as a predictor of postoperative surgical site infectious complications. World J Surg. 2011; 35:1017–25. https://doi.org/10.1007/s00268-011-1002-1 [PubMed]
-
28.
Hoeboer SH, Groeneveld AB, Engels N, van Genderen M, Wijnhoven BP, van Bommel J. Rising C-reactive protein and procalcitonin levels precede early complications after esophagectomy. J Gastrointest Surg. 2015; 19:613–24. https://doi.org/10.1007/s11605-015-2745-z [PubMed]
-
29.
Ip B, Ng KT, Packer S, Paterson-Brown S, Couper GW. High serum lactate as an adjunct in the early prediction of anastomotic leak following oesophagectomy. Int J Surg. 2017; 46:7–10. https://doi.org/10.1016/j.ijsu.2017.08.027 [PubMed]
-
30.
Berkelmans GH, Kouwenhoven EA, Smeets BJ, Weijs TJ, Silva Corten LC, van Det MJ, Nieuwenhuijzen GA, Luyer MD. Diagnostic value of drain amylase for detecting intrathoracic leakage after esophagectomy. World J Gastroenterol. 2015; 21:9118–25. https://doi.org/10.3748/wjg.v21.i30.9118 [PubMed]
-
31.
Su'a B, Milne T, Jaung R, Jin JZ, Svirskis D, Bissett IP, Eglinton T, Hill AG. Detection of Anastomotic Leakage Following Elective Colonic Surgery: Results of the Prospective Biomarkers and Anastomotic Leakage (BALL) Study. J Surg Res. 2022; 273:85–92. https://doi.org/10.1016/j.jss.2021.12.019 [PubMed]
-
32.
Hoek VT, Sparreboom CL, Wolthuis AM, Menon AG, Kleinrensink GJ, D'Hoore A, Komen N, Lange JF, and APPEAL II collaborators. C-reactive protein (CRP) trajectory as a predictor of anastomotic leakage after rectal cancer resection: A multicentre cohort study. Colorectal Dis. 2022; 24:220–7. https://doi.org/10.1111/codi.15963 [PubMed]
-
33.
Liesenfeld LF, Sauer P, Diener MK, Hinz U, Schmidt T, Müller-Stich BP, Hackert T, Büchler MW, Schaible A. Prognostic value of inflammatory markers for detecting anastomotic leakage after esophageal resection. BMC Surg. 2020; 20:324. https://doi.org/10.1186/s12893-020-00995-2 [PubMed]
-
34.
Baeza-Murcia M, Valero-Navarro G, Pellicer-Franco E, Soria-Aledo V, Mengual-Ballester M, Garcia-Marin JA, Betoret-Benavente L, Aguayo-Albasini JL. Early diagnosis of anastomotic leakage in colorectal surgery: prospective observational study of the utility of inflammatory markers and determination of pathological levels. Updates Surg. 2021; 73:2103–11. https://doi.org/10.1007/s13304-021-01082-8 [PubMed]
-
35.
Bertocchi E, Barugola G, Ceccaroni M, Guerriero M, Rossini R, Gentile I, Ruffo G. Laparoscopic colorectal resection for deep infiltrating endometriosis: can we reliably predict anastomotic leakage and major postoperative complications in the early postoperative period? Surg Endosc. 2022; 36:422–9. https://doi.org/10.1007/s00464-021-08301-8 [PubMed]
-
36.
Sparreboom CL, Wu Z, Dereci A, Boersema GS, Menon AG, Ji J, Kleinrensink GJ, Lange JF. Cytokines as Early Markers of Colorectal Anastomotic Leakage: A Systematic Review and Meta-Analysis. Gastroenterol Res Pract. 2016; 2016:3786418. https://doi.org/10.1155/2016/3786418 [PubMed]
-
37.
Zawadzki M, Krzystek-Korpacka M, Gamian A, Witkiewicz W. Serum cytokines in early prediction of anastomotic leakage following low anterior resection. Wideochir Inne Tech Maloinwazyjne. 2018; 13:33–43. https://doi.org/10.5114/wiitm.2018.72785 [PubMed]
-
38.
Daams F, Wu Z, Lahaye MJ, Jeekel J, Lange JF. Prediction and diagnosis of colorectal anastomotic leakage: A systematic review of literature. World J Gastrointest Surg. 2014; 6:14–26. https://doi.org/10.4240/wjgs.v6.i2.14 [PubMed]
-
39.
Cini C, Wolthuis A, D'Hoore A. Peritoneal fluid cytokines and matrix metalloproteinases as early markers of anastomotic leakage in colorectal anastomosis: a literature review and meta-analysis. Colorectal Dis. 2013; 15:1070–7. https://doi.org/10.1111/codi.12192 [PubMed]
-
40.
Vetter D, Gutschow CA. Strategies to prevent anastomotic leakage after esophagectomy and gastric conduit reconstruction. Langenbecks Arch Surg. 2020; 405:1069–77. https://doi.org/10.1007/s00423-020-01926-8 [PubMed]
-
41.
Jones CE, Watson TJ. Anastomotic Leakage Following Esophagectomy. Thorac Surg Clin. 2015; 25:449–59. https://doi.org/10.1016/j.thorsurg.2015.07.004 [PubMed]
-
42.
Bolton JS, Conway WC, Abbas AE. Planned delay of oral intake after esophagectomy reduces the cervical anastomotic leak rate and hospital length of stay. J Gastrointest Surg. 2014; 18:304–9. https://doi.org/10.1007/s11605-013-2322-2 [PubMed]
-
43.
de Mooij CM, Maassen van den Brink M, Merry A, Tweed T, Stoot J. Systematic Review of the Role of Biomarkers in Predicting Anastomotic Leakage Following Gastroesophageal Cancer Surgery. J Clin Med. 2019; 8:2005. https://doi.org/10.3390/jcm8112005 [PubMed]
-
44.
Yeung DE, Peterknecht E, Hajibandeh S, Hajibandeh S, Torrance AW. C-reactive protein can predict anastomotic leak in colorectal surgery: a systematic review and meta-analysis. Int J Colorectal Dis. 2021; 36:1147–62. https://doi.org/10.1007/s00384-021-03854-5 [PubMed]
-
45.
Singh PP, Zeng IS, Srinivasa S, Lemanu DP, Connolly AB, Hill AG. Systematic review and meta-analysis of use of serum C-reactive protein levels to predict anastomotic leak after colorectal surgery. Br J Surg. 2014; 101:339–46. https://doi.org/10.1002/bjs.9354 [PubMed]
-
46.
van Genderen ME, Lima A, de Geus H, Klijn E, Wijnhoven B, Gommers D, van Bommel J. Serum C-reactive protein as a predictor of morbidity and mortality in intensive care unit patients after esophagectomy. Ann Thorac Surg. 2011; 91:1775–9. https://doi.org/10.1016/j.athoracsur.2011.02.042 [PubMed]
-
47.
Warschkow R, Tarantino I, Ukegjini K, Beutner U, Müller SA, Schmied BM, Steffen T. Diagnostic study and meta-analysis of C-reactive protein as a predictor of postoperative inflammatory complications after gastroesophageal cancer surgery. Langenbecks Arch Surg. 2012; 397:727–36. https://doi.org/10.1007/s00423-012-0944-6 [PubMed]
-
48.
Garcia-Granero A, Frasson M, Flor-Lorente B, Blanco F, Puga R, Carratalá A, Garcia-Granero E. Procalcitonin and C-reactive protein as early predictors of anastomotic leak in colorectal surgery: a prospective observational study. Dis Colon Rectum. 2013; 56:475–83. https://doi.org/10.1097/DCR.0b013e31826ce825 [PubMed]
-
49.
Lagoutte N, Facy O, Ravoire A, Chalumeau C, Jonval L, Rat P, Ortega-Deballon P. C-reactive protein and procalcitonin for the early detection of anastomotic leakage after elective colorectal surgery: pilot study in 100 patients. J Visc Surg. 2012; 149:e345–9. https://doi.org/10.1016/j.jviscsurg.2012.09.003 [PubMed]
-
50.
Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis. 2004; 39:206–17. https://doi.org/10.1086/421997 [PubMed]
-
51.
Ito S, Sato N, Kojika M, Yaegashi Y, Suzuki Y, Suzuki K, Endo S. Serum procalcitonin levels are elevated in esophageal cancer patients with postoperative infectious complications. Eur Surg Res. 2005; 37:22–8. https://doi.org/10.1159/000083144 [PubMed]
-
52.
Ellebæk MB, Baatrup G, Gjedsted J, Fristrup C, Qvist N. Cytokine response in peripheral blood indicates different pathophysiological mechanisms behind anastomotic leakage after low anterior resection: a pilot study. Tech Coloproctol. 2014; 18:1067–74. https://doi.org/10.1007/s10151-014-1204-2 [PubMed]
-
53.
Fouda E, El Nakeeb A, Magdy A, Hammad EA, Othman G, Farid M. Early detection of anastomotic leakage after elective low anterior resection. J Gastrointest Surg. 2011; 15:137–44. https://doi.org/10.1007/s11605-010-1364-y [PubMed]
-
54.
Uğraş B, Giriş M, Erbil Y, Gökpinar M, Citlak G, Işsever H, Bozbora A, Oztezcan S. Early prediction of anastomotic leakage after colorectal surgery by measuring peritoneal cytokines: prospective study. Int J Surg. 2008; 6:28–35. https://doi.org/10.1016/j.ijsu.2007.10.001 [PubMed]
-
55.
Sammour T, Singh PP, Zargar-Shoshtari K, Su'a B, Hill AG. Peritoneal Cytokine Levels Can Predict Anastomotic Leak on the First Postoperative Day. Dis Colon Rectum. 2016; 59:551–6. https://doi.org/10.1097/DCR.0000000000000598 [PubMed]
-
56.
Qi XY, Liu MX, Xu K, Gao P, Tan F, Yao ZD, Zhang N, Yang H, Zhang CH, Xing JD, Cui M, Su XQ. Peritoneal Cytokines as Early Biomarkers of Colorectal Anastomotic Leakage Following Surgery for Colorectal Cancer: A Meta-Analysis. Front Oncol. 2022; 11:791462. https://doi.org/10.3389/fonc.2021.791462 [PubMed]
-
57.
Gogos CA, Drosou E, Bassaris HP, Skoutelis A. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J Infect Dis. 2000; 181:176–80. https://doi.org/10.1086/315214 [PubMed]
-
58.
Strunk T, Hibbert J, Doherty D, Nathan E, Simmer K, Richmond P, Currie A, Burgner D. Impaired Cytokine Responses to Live Staphylococcus epidermidis in Preterm Infants Precede Gram-positive, Late-onset Sepsis. Clin Infect Dis. 2021; 72:271–8. https://doi.org/10.1093/cid/ciaa063 [PubMed]
-
59.
Zaghloul N, Addorisio ME, Silverman HA, Patel HL, Valdés-Ferrer SI, Ayasolla KR, Lehner KR, Olofsson PS, Nasim M, Metz CN, Wang P, Ahmed M, Chavan SS, et al. Forebrain Cholinergic Dysfunction and Systemic and Brain Inflammation in Murine Sepsis Survivors. Front Immunol. 2017; 8:1673. https://doi.org/10.3389/fimmu.2017.01673 [PubMed]
-
60.
van der Poll T, Marchant A, Buurman WA, Berman L, Keogh CV, Lazarus DD, Nguyen L, Goldman M, Moldawer LL, Lowry SF. Endogenous IL-10 protects mice from death during septic peritonitis. J Immunol. 1995; 155:5397–401. [PubMed]
-
61.
Greca FH, Souza Filho ZA, Giovanini A, Camargo Junior CA, Rubin MR, Silva RF. Interleukin-6 (IL-6) influence on colonic anastomosis healing in rats. Acta Cir Bras. 2007; 22:110–4. https://doi.org/10.1590/s0102-86502007000200006 [PubMed]
-
62.
Platt JJ, Ramanathan ML, Crosbie RA, Anderson JH, McKee RF, Horgan PG, McMillan DC. C-reactive protein as a predictor of postoperative infective complications after curative resection in patients with colorectal cancer. Ann Surg Oncol. 2012; 19:4168–77. https://doi.org/10.1245/s10434-012-2498-9 [PubMed]
-
63.
Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011; 29:71–109. https://doi.org/10.1146/annurev-immunol-031210-101312 [PubMed]
-
64.
Kole A, Maloy KJ. Control of intestinal inflammation by interleukin-10. Curr Top Microbiol Immunol. 2014; 380:19–38. https://doi.org/10.1007/978-3-662-43492-5_2 [PubMed]
-
65.
Li MO, Flavell RA. Contextual regulation of inflammation: a duet by transforming growth factor-beta and interleukin-10. Immunity. 2008; 28:468–76. https://doi.org/10.1016/j.immuni.2008.03.003 [PubMed]
-
66.
Klava A, Windsor AC, Farmery SM, Woodhouse LF, Reynolds JV, Ramsden CW, Boylston AW, Guillou PJ. Interleukin-10. A role in the development of postoperative immunosuppression. Arch Surg. 1997; 132:425–9. https://doi.org/10.1001/archsurg.1997.01430280099016 [PubMed]
-
67.
Kryzauskas M, Bausys A, Degutyte AE, Abeciunas V, Poskus E, Bausys R, Dulskas A, Strupas K, Poskus T. Risk factors for anastomotic leakage and its impact on long-term survival in left-sided colorectal cancer surgery. World J Surg Oncol. 2020; 18:205. https://doi.org/10.1186/s12957-020-01968-8 [PubMed]
-
68.
Cong ZJ, Fu CG, Wang HT, Liu LJ, Zhang W, Wang H. Influencing factors of symptomatic anastomotic leakage after anterior resection of the rectum for cancer. World J Surg. 2009; 33:1292–7. https://doi.org/10.1007/s00268-009-0008-4 [PubMed]
-
69.
Zarnescu EC, Zarnescu NO, Costea R. Updates of Risk Factors for Anastomotic Leakage after Colorectal Surgery. Diagnostics (Basel). 2021; 11:2382. https://doi.org/10.3390/diagnostics11122382 [PubMed]
-
70.
Sciuto A, Merola G, De Palma GD, Sodo M, Pirozzi F, Bracale UM, Bracale U. Predictive factors for anastomotic leakage after laparoscopic colorectal surgery. World J Gastroenterol. 2018; 24:2247–60. https://doi.org/10.3748/wjg.v24.i21.2247 [PubMed]
-
71.
van Rooijen SJ, Huisman D, Stuijvenberg M, Stens J, Roumen RMH, Daams F, Slooter GD. Intraoperative modifiable risk factors of colorectal anastomotic leakage: Why surgeons and anesthesiologists should act together. Int J Surg. 2016; 36:183–200. https://doi.org/10.1016/j.ijsu.2016.09.098 [PubMed]