Abstract
Dyslipidemia is characterized by increased triglyceride and low-density lipoprotein (LDL) levels, and decreased high-density lipoprotein (HDL) levels. Such an atherogenic lipid profile often predisposes an at risk individual to coronary artery disease with incompletely understood mechanisms. Apolipoprotein D (apoD) is an atypical apolipoprotein. Unlike canonical apolipoproteins that are produced mainly in liver and intestine, apoD is expressed widely in mammalian tissues. ApoD does not share significant degrees of homology in amino acid sequence with other apolipoproteins. Instead, apoD is structurally similar to lipocalins, a diverse family of lipid-binding proteins that are responsible for transporting lipids and other small hydrophobic molecules for metabolism. Plasma ApoD is present mainly in HDL and to a lesser extent in low density lipoproteins (LDL) and very low-density lipoproteins (VLDL). Genetic variants of apoD are associated with abnormal lipid metabolism and increased risk of developing metabolic syndrome. Increased apoD deposition is detectable in atherosclerotic lesions of humans with established cardiovascular disease as well as mice with premature atherosclerosis. Moreover, apoD is associated with anti-oxidation and anti-stress activities, contributing to lifespan expansion in fruit flies. Elderly subjects and patients with Alzheimer exhibit markedly elevated apoD production in the brain. Thus, apoD is emerged as a significant player in lipid metabolism and aging. Here we focus our review on recent advances toward our understanding of apoD in lipid metabolism and address whether apoD dysregulation contributes to the pathogenesis of dyslipidemia and atherosclerosis. We will also discuss the functional implication of apoD in aging.
Atherosclerosis
Coronary
artery disease (CAD) is the leading cause of death in America. It happens when
the arteries that supply blood to cardiac muscle become hardened and narrowed.
This is due to excessive deposition of cholesterol, fatty substances and
cellular waste products in the inner lining of coronary artery. Such a
pathological condition, termed atherosclerosis, can happen in men and women,
particularly at a later age. Aside from genetic predisposition, factors that
account for the risk of
atherosclerosis include dyslipidemia, hypertension, obesity, diabetes and
smoking. These factors alone or in combination can hasten the progression of
atherosclerosis and development of CAD. Although progress has been made in
elucidating the pathophysiology of atherosclerosis, the exact cause and
mechanism underlying the development of atherosclerosis still remain obscure [1-3].
Cholesterol homeostasis
plays an important role in atherosclerosis. Cholesterol is an essential
component of cellular membrane and also a precursor for the synthesis of
steroid hormones and bile acids. Cholesterol in the body derives from two
different sources, dietary intake and de novo synthesis in tissue such
as liver - the major organ for endogenous cholesterol supply. Unlike fatty
acids and triglyceride, cholesterol cannot be catabolized. Excessive
cholesterol must be rid itself of the body via its transportation to liver for
biliary excretion. This pathway, termed "reverse cholesterol transport", is
facilitated by high-density lipoprotein (HDL) and is viewed as the primary
mechanism by which HDL protects against the development of atherosclerosis [4-7]. Clinical data
and preclinical studies have conclusively demonstrated that lower HDL levels
constitute an independent risk factor for coronary artery disease. However, the
molecular basis underlying the cardioprotective action of HDL remains
incompletely understood. For better clinical management of CAD, further studies
are warranted to better understand cholesterol metabolism and pathogenesis of
atherosclerosis.
Apolipoprotein D (apoD)
is a component of HDL. Due to its relative low abundance in HDL particles, apoD
has received considerably less attention in the research area of
HDL-cholesterol metabolism and atherosclerosis. Recent data indicate that
aberrant apoD expression is associated with altered lipid metabolism and risk
of coronary artery disease. This has spurred us to conduct a comprehensive
review of apoD function in triglyceride and cholesterol metabolism to address
the question of whether apoD is another significant player in the pathogenesis
of atherosclerosis.
ApoD production in health
and disease
In humans, plasma apoD levels
range from 3 to 11 μmol/L. This level is equivalent to plasma levels (4.9±0.5
μmol/L) of apolipoprotein C-III (apoC-III), an important player in plasma
triglyceride metabolism [8-11].
However, plasma apoD levels are upregulated under certain pathophysiological
conditions, such as in women with gross cystic disease [12]. ApoD levels are also elevated in the brain of subjects with chronic
schizophrenia and in the prefrontal cortex of patients with Alzheimer disease [13-15]. Furthermore, treatment with antipsychotic drugs, expecially
clozapine, also results in elevated apoD expression in rodent brains as well as
in human plasma [13,16,17]. Increased apoD production is seen in the rat brain
following traumatic brain injury [18].
In addition, elevated apoD
production is detected in liver tumors resected from hepatocellular carcinoma [19], as
well as in invasive carcinoma of the breast [20,21].
Elevated apoD levels are present in cyst
fluids of women with gross cystic disease of the breast [12].
Furthermore, increased apoD levels are also detected in the breast nipple
aspirate fluid in women with breast cancer, but nipple fluid apoD levels do not
seem to correlate with the stage of the breast cancer disease [22].
Recently, Rickhag et al. [23] demonstrate in a rat model of stroke that apoD is significantly
elevated in the peri-infarct area during the recovery period. It is suggested
that upregulated apoD production serves the function of transporting
cholesterol and phospholipids, a remodeling process that is required for the
recovery of brain injury. Likewise, high apoD protein levels are found in
patients with failing hearts, compared with non-failing control subjects,
raising the possibility that apoD is potential biomarker in human end-stage
heart failure [24].
Niemann-Pick Type C (NPC)
disease is a human neurodegenerative disorder characterized by impaired
intracellular cholesterol transport [25]. Interestingly, in rodent models of the human NPC disease, apoD
protein levels are markedly elevated by 30-fold in the brain and 6-fold in
plasma, correlating with increased intracellular cholesterol storage [26,27]. These findings implicate apoD in the pathogenesis of NPC disease.
In addition to its altered
expression in the brain, apoD is upregulated in cultured myotubes from patients
with type 2 diabetes [28]. Likewise, enhanced apoD expression is detected in muscle biopsies
from patients with disuse atrophy, a pathological condition that impacts muscle
function and activity of daily living [29]. Interestingly, the induction of apoD mRNA expression is accompanied
by a corresponding increase in leptin receptor mRNA in the immobilized muscle
of patients with disuse atrophy. Immunohistochemistry colocalizes apoD and
leptin receptor in the perinuclear area in the immobilized muscle fibers [29]. These data are consistent with the observation of Liu et al. [30], who show that apoD and leptin receptor physically interact with each
other in the brain. ApoD and leptin receptor expression are coordinately
regulated in the hypothalamus in modulating food intake and energy homeostasis
in mice. Dissociation of apoD with the leptin receptor is linked to the
development of obesity in leptin receptor-deficient db/db mice [30].
Posttranslational modification of apoD
ApoD possesses two N-glycosylation sites (Asn45 and Asn78) [31],
both of which are evolutionally conserved among species (Figure 1A). This raises the hypothesis that apoD is
regulated at the post-translational level. In support of this hypothesis, we
incubated aliquots of sera from normal C57BL/6J mice in the absence and
presence of peptide:N-glycosidase F (PNGase F), an amidase that catalyzes the
removal of carbohydrate moieties from N-linked glycoproteins. As shown in Figure 1B, pre-incubation of plasma apoD with PNGase F resulted in apoD
de-glycosylation, as evidenced by the production of de-glycosylated apoD with
reduced molecular masses. Likewise, serum apoD as well as apoD secreted from
axillary glands in humans are also glycosylated [32,33].
While the physiological significance of this post-translational modification remained
incompletely understood, it is suggested that N-glycosylation modulates apoD
protein folding, resulting in conformational changes favorable for binding to
its physiological ligands or association with HDL. In this context, it would be
of significance to convert the two N-glycosylation sites Asn45 and Asn78 to
alanine residues in apoD by site-directed mutagenesis. The resulting apoD
mutants will be ideal molecules for determining the physiological impact of
N-glycosylation on the ability of apoD to associate with ligands and/or with HDL
in metabolism.
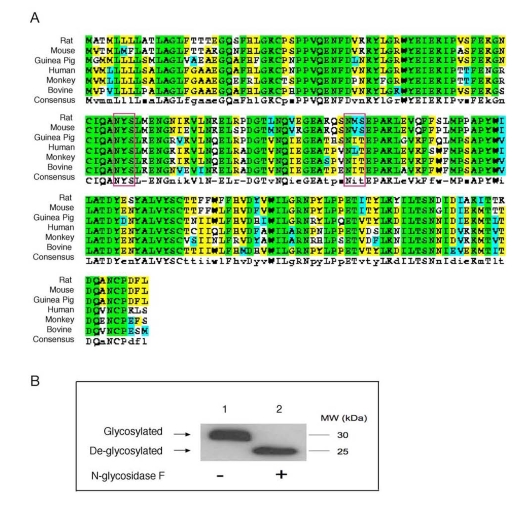
Figure 1. Conservation of N-glycosylation sites in apoD among species. (A). ApoD protein sequences of
different species were aligned using the ClustalW program. Amino acid
residues in box denote two highly conserved N-glycosylation sites in apoD.
The consensus N-glycosylation site is Asn-X-Ser/Thr. (B) Plasma apoD is
N-glycosylated. Aliquots of plasma (20 μg protein) from C57BL/6J mice were
incubated without (-) and with (+) 1,000 U of N-glycosidase F (New England
Biolabs) in a total volume of 30 μl at 37°C for 1 hour to remove N-glycan chains from glycopeptides. The
reaction mixture was resolved on 4-20% SDS-polyacrylamide gels, followed by
immunoblot analysis using anti-apoD. Glycosylated and de-glycosylated forms
of apoD are indicated.
Effect of apoD on HDL-cholesterol metabolism
ApoD is an atypical apolipoprotein of 169 amino acids. Unlike canonical apolipoproteins that are produced mainly in liver and
intestine, apoD is expressed widely in mammalian tissues including brain,
liver, intestine, cardiac and skeletal muscle, adipose tissue, and pancreas [34-36] (Figure 2). ApoD does not share significant degrees of homology in the
amino acid sequence with other apolipoproteins. Instead, apoD is structurally
similar to the lipocalin family of proteins. This superfamily comprises a
diverse class of lipid-binding proteins including fatty acid binding proteins
(FABPs), plasma retinol-binding proteins (RBP) and apolipoprotein M (apoM) [34,37-39]. Despite their dissimilarities in amino acid
sequences, the lipocalin superfamily of proteins share a highly conserved β-barrel structure that is comprised of an
eight-stranded anti-parallel β-sheet [40].
Such a tertiary architecture is predicted to form a ligand-binding pocket that
is thought for binding and transporting lipids and other small hydrophobic
molecules [34,37].
This characteristic lipocalin fold is validated for apoD by
Eichinger et al. [41],
who recently crystalized the human apoD protein in its free form and in complex
with progesterone. Cystallographic studies reveal that the eight-stranded
anti-parallel β-sheets of apoD are connected by four loops in
a pair-wise manner, forming a conically shaped cavity that is capable of
binding hydrophobic ligands [40,41].
Consistent with its structural organization, apoD is shown to associate with a
number of ligands including cholesterol, progesterone, pregnenolone, bilirubin
and arachidonic acid [13,34].
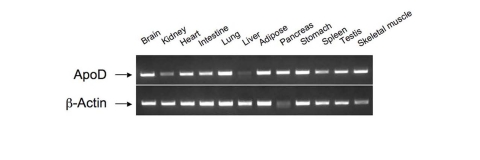
Figure 2. Tissue distribution of apoD mRNA expression.
Total RNA was prepared from different tissues of C57BL/6J mice. Aliquots of purified RNA (100 ng)
were subjected to RT-PCR analysis using apoD sequence-specific primers.
The resulting PCR products were resolved on 1% agarose gel containing ethidiumbromide
and visualized by UV light.
Circulating ApoD is present
mainly in HDL and to a lesser extent in LDL and VLDL [42,43](Figure 3). Nonetheless,
little is known about the role of apoD in lipoprotein metabolism and its impact
on atherosclerosis. Plasma apoD levels are significantly reduced in patients
with Tangier disease, a rare autosomal recessive disorder that is caused by
mutations in the ATP-binding cassette A1 (ABCA1) gene [34,44]. As ABCA1 plays a key role in effluxing cholesterol from cells, ABCA1
loss-of-function results in diminished cholesterol removal from peripheral
tissues, contributing to excessive accumulation of cholesterol in the body and
increased risk of developing atherosclerosis in patients with Tangier disease [45-50].
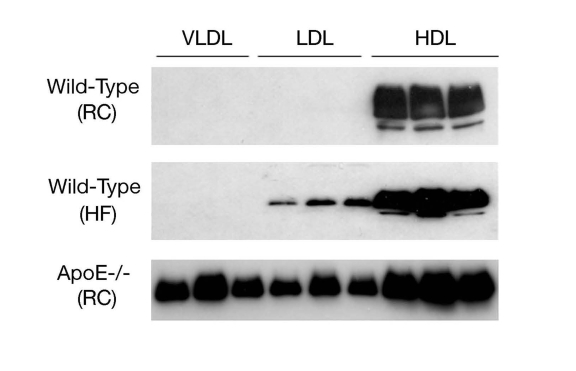
Figure 3. ApoD distribution in lipoproteins. Male
C57BL/6J mice (3-5 weeks old) were fed regular chow (RC) or high fat diet
(HF) for 8 weeks. Aliquots of 250-μl sera of mice (n=5) were fractionated
by gel filtration column chromatography. Fractions (500 μl) were collected
for the determination of cholesterol concentrations. Likewise, aliquots of
sera (250 μl) of male apoE knockout mice (ApoE-/-, 12 weeks old on regular
chow) were fractionated to VLDL, LDL and HDL fractions. Peak fractions of
VLDL, LDL and HDL were subjected to immunoblot assay using anti-ApoD
antibody.
Recently, Vaisar et al. [51]
took a proteomics approach to profile protein composition of HDL particles
isolated from human subjects. Their studies reveal that HDL isolated from
healthy individuals versus subjects with established CAD carry different
protein cargos. Interestingly, apoD is highly enriched in HDL isolated from
seven subjects with CAD, in comparison to six healthy controls. This
observation seems paradoxical, as CAD patients are associated with lower HDL
levels and apoD is mainly bound to HDL in the circulation. An increased apoD
content in HDL may present a pathological marker or constitute a compensatory
response to impaired cholesterol metabolism in subjects with established CAD.
To recapitulate this clinical observation, we determined plasma apoD
expression in normal and atherogenic mice with genetic depletion of apolipoprotein
E (apoE), a commonly used rodent model of atherosclerosis. ApoE knockout mice display a marked increase in total plasma cholesterol
levels and develop atherosclerosis with the deposition of fatty streaks in the
proximal aorta at 3 months of age. We show that plasma apoD levels are markedly
increased in apoE knockout mice (Figure 4). These results together with clinical data presage a potential role of
apoD in the pathogenesis of atherosclerosis.
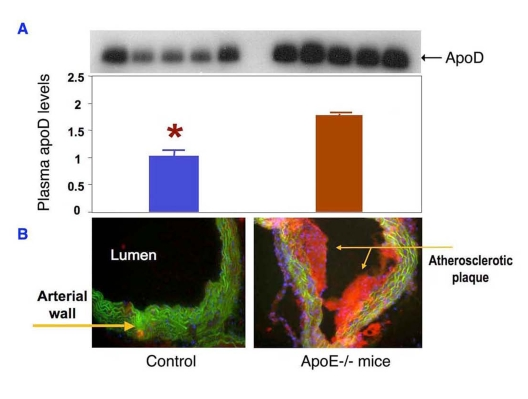
Figure 4. ApoD production is upregulated in atherogenic mice. (A) Sera of ApoE knockout (n=5)
and control mice (n=5) at 12 weeks of age were subjected to immunoblot
analysis using anti-apoD antibody.
(B) Sections of aorta were stained
by oil red O to visualize the atherosclerotic lesions in the aorta of apoE
knockout mice. Data were obtained from 16-wk old mice. *P <0.05
vs. ApoE-/- mice by ANOVA.
Abnormal apoD production in metabolic syndrome
In addition to its role in cholesterol homeostasis, apoD is involved in
triglyceride metabolism. Epidemiological studies identified three distinct
missense mutations, namely Phe36Val, Tyr108Cys and Thr158Lys in the apoD gene
in African populations. Each of these three mutations is associated with
significantly elevated plasma triglyceride levels and reduced HDL-cholesterol
levels, a plasma lipid profile that is characteristic of metabolic syndrome [52,53]. Although the underlying molecular basis remains to be defined, these
clinical data implicate abnormal apoD function in the pathogenesis of metabolic
syndrome.
Consistent with this idea, two
studies demonstrate a linkage between the TaqI polymorphism of the apoD
gene and type 2 diabetes in South Indians and Nauruans [54,55]. Subsequently, Vijayaraghavan et al. [56] report that the TaqI polymorphism of apoD is associated with
the development of obesity, insulin resistance and hyperinsulinemia in the
British Caucasoid population. This effect seems to be independent of body
weight, as no significant association is detected between the apoD polymorphism
and body mass index (BMI) or waist to hip ratio in the same cohort of subjects [56].
Curry et al. [57]
report that plasma apoD levels are significantly lower in patients with hyper-chylomicronemia.
In a separate study to identify the factors that affect lipids and
apolipoproteins at birth, Lane et al. [58] show that significant
reductions in triglyceride and ApoD levels are detected in infants who
subsequently became ill in the postnatal period with problems relating to
carbohydrate metabolism (e.g., infants of diabetic mothers). Together these
clinical data suggest that apoD is another significant player in lipid
metabolism. ApoD dysregulation may contribute to metabolic abnormalities in
insulin resistant subjects with obesity and/or type 2 diabetes.
Further evidence of apoD as a significant player in lipid metabolism
derives from the studies in obese db/db mice. Due to leptin receptor
deficiency, db/db mice are hyperphagic, developing morbid obesity and
type 2 diabetes at about 12 weeks of age. Interestingly, Liu et al. [30] show that apoD and leptin receptor, which are co-expressed in the
hypothalamus, interact with each other in regulating food uptake and body
weight gain. Hypothalamic apoD mRNA is markedly induced in response to high fat
feeding. However, this effect is abolished with a concomitant reduction of apoD
mRNA levels in the hypothalamus of obese db/db mice. These data suggest
that apoD may participate in the regulation of food intake and body fat
accumulation via crosstalking with the leptin receptor.
ApoD in HDL remodeling
There are two lines of evidences suggesting that apoD contributes to
HDL remodeling. First, apoD is
shown to modulate the activity of lecithin:cholesterol
acyltransferase (LCAT), an HDL-bound enzyme that catalyzes the conversion of
free cholesterol to cholesterol ester that is sequently recruited into the core
of HDL. This effect along with apolipoprotein E (apoE) contributes to HDL core
expansion and promotes HDL maturation [59].
Albers et al. report that apoD is a carrier of lysolecithin, a product of the
LCAT reaction [60]. This finding is accordance with the observation that apoD interacts
with LCAT [61]. However, whether apoD acts as an activator or
inhibitor of LCAT activity still remains controversial. Studies by Kostner et
al.[62]
suggest that apoD is an activator of LCAT, which is at variance with the data
of Albers et al. [63], who show that apoD is an inhibitor of LCAT.
Steyrer et al. [64]
studied the activation of LCAT activity by apoD in comparison to apoA-I and
apoC-I in reconstituted proteoliposomes. ApoA-I is the most potent activator of
LCAT, followed by apoC-I and apoD. Their studies suggest that apoD modulates
LCAT activity presumbly by stabilizing the enzyme on HDL [64].
Second, apoD contributes to HDL remodeling via its covalent cross-link
with apolipoprotein A-II (apoA-II), a structural component of HDL. Blanco-Vaca
et al. [65] detect the presence of disulfide-linked heterodimers of apoD and
apoA-II in human plasma. Non-reducing polyacrylamide gel electrophoresis
demonstrates that the apoD-apoA-II heterodimer has an apparent molecular mass
of 38 kDa, which is significantly larger than monomeric apoD (MW, 29 kDa).
Sequence analysis reveals the presence of five cysteine residues in the human
apoD protein. Mass Spectrometric
analysis in combination with crystallographic studies of human apoD protein
illustrates that four cysteines (Cys16-Cys41 and Cys8-Cys114) are primed for
forming two intra-molecular disulfide bonds and the remaining unpaired cysteine
(Cys-116) is responsible for inter-molecular covalent cross-link with Cys-6 of apoA-II within HDL [31,41].
Interestingly, the rodent apoD lacks the unpaired Cys-116, as it is replaced by
threonine at the corresponding amino acid residue. Thus, the physiological
significance of this covalent cross-link between apoD and apoA-II in HDL
remodeling and cholesterol metabolism remains elusive [41].
ApoD in oxidative stress and aging
Increased oxidative stress is
closely associated with inflammation, insulin resistance, diabetes and
atherosclerosis. There is accumulating evidence that apoD plays an important role
in oxidative stress. Do Carmo et al. [66]
show in cultured NIH/3T3 fibroblasts that
apoD expression is significantly induced in response to cellular stress, regardless of whether the stress
condition is caused by lipopolysaccharide
(LPS) stimulation, H2O2 treatment or UV-light irradiation. This effect seems to be mediated by the NF-kB pathway, as there are several conserved NF-kB
binding sites in the apoD promoter [66]. Furthermore,
Do Carmo et al. [67]show that apoD confers a neuroprotective effect in the
brain of mice. Their studies demonstrate that mice over-expressing human apoD
inneurons, as opposed to normal controls, are more
resistant with a 3-fold higher survival rate in response to human coronavirus-induced acute encephalitis. Likewise, Ganfornina et al. [68] show that
apoD overexpression in the brain
protects mice from oxidative stress. This effect correlates with the ability of
apoD to prevent lipid peroxidation in cells [68].
Additional
evidence of apoD function against oxidative stress stems from studies in fruit
flies. Sanchez et al. [69] show that
genetically modified Drosophila mutants with loss-of-function of the
human apoD homolog gene (GLaz) exhibit high sensitivity to oxidative
stress and nutrient deprivation. The GLaz mutant flies also have an
increased accumulation of lipid peroxidation products in the body, accompanied
by 10-15% reduction in lifespan. Conversely, Walker et al. [70]show that Drosophila with overexpression of the apoD
homolog GLaz displays enhanced resistance to starvation and oxidative
stress. ApoD overexpression also ameliorates lipid peroxidation with a 30%
extension of lifespan in flies [70]. Similar
observations are made by Muffat et al. [71], who
demonstrate that overproduction of the human apoD are also associated with
significantly reduced lipid peroxidation products, protecting against oxidative
stress and extending lifespan by about 40% in fruit flies. Together these data
demonstrate an evolutionally conserved safeguarding mechanism by which apoD
acts to protect against lipid peroxidation and oxidative stress.
Although
apoD is shown to confer a significant beneficial effect on aging in fruit
flies, there is a lack of evidence that apoD contributes to lifespan expansion
in mammals. It is noteworthy that apoD is
abundantly expressed in the brain. Elderly subjects and patients with Alzheimer
are associated with markedly elevated apoD production in the brain [72-74], but the underlying pathophysiology is unknown. It is
important to understand whether and how apoD affects aging and contributes to
lifespan expansion in mammals.
Impact of apoD on atherosclerosis
Does apoD contribute to atherosclerosis? To address this issue, Sarjeant et al [75] subject thin-sections of
coronary arteries of archived human specimens to anti-apoD
immunohistochemistry. Their studies visualize an increased apoD deposition in
atheromatous plaques. Consistent with this finding, we show that apoD is
localized in atherosclerotic lesions of apoE knockout mice (Figure 5). Thus,
elevated apoD deposition along with excessive cholesterol accumulation is
detectable in atherosclerotic lesions of both human and rodent origins. This is
correlated with the ability of apoD to bind and transport cholesterol, raising
the possibility that apoD may play a significant role in the pathogenesis of
atherosclerosis. It follows that an increased deposition of apoD in
atherosclerotic lesions can derive from a compensatory response of apoD to
facilitate cholesterol removal from peripheral cells or result from the
consequence of defects in apoD-mediated cholesterol trafficking. Further studies
are warranted to distinguish whether apoD contributes to or protects against
the development of atherosclerosis.
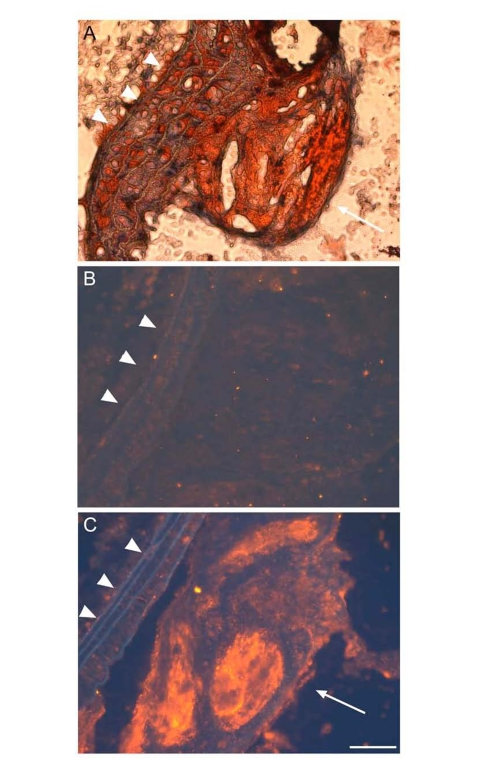
Figure 5. ApoD is localized to atherosclerotic plaques ofapoE?deficient mice.
Proximal aorta sections of male apoE knockout mice were subjected to oil red O staining (A),
and to immunohistochemistry using control rabbit IgG against bacterial β-galactosidase (B)
and rabbit anti-apoD antibody (C). The secondary antibody used in immunostaining is the donkey
anti-rabbit IgG conjugated with Cy3. ApoD was stained red in theatherosclerotic plaque (C),
as indicated by arrow. Elastic fibers ofvessels were auto-fluorescent blue,
as indicated by arrowhead. Bar = 50 μm.
Conclusions and perspectives
ApoD is a 29-kDa glycoprotein of 169 amino acids. ApoD is evolutionally
conserved among species and is expressed in a variety of mammalian tissues.
Although classifed as apolipoprotein, apoD belongs to the lipocalin family due
to its structural adoptation of a β-barrel
structure that is characteristic of lipocalins [40,41].
ApoD is shown to be a multi-ligand binding protein that is capable of
transporting small hydrophobic molecules such as arachidonic acid, steroid
hormones, and cholesterol for metabolism or signaling [34].
Altered apoD expression has been associated with a number of pathological
conditions, including breast carcinoma, prostate cancer, Parkinson's disease,
Alzheimer, schizophrenia, bipolar disorder, etc [13-17,34,72,74,76-80]. Elevated apoD deposition is also detected in amyloid
plaques in the brains of patients with Alzheimer with undefined pathophysiology
[15,79]. These data underscore the importance of apoD in the
pathophysiology of cancer and neurological disorders. However, a comprehensive
survey of apoD function is beyond the scope of this article. Instead, we center
our review on apoD in lipid metabolism in relation to the pathogenesis of
dyslipidemia and atherosclerosis, the two intertwined pathological traits that
consequently predispose an at-risk individual to CAD.
ApoD is categorized as apolipoprotein due to its initial isolation from
human HDL. Indeed, circulating apoD is bound mainly to HDL, correlating with
the ability of apoD to associate via covalent cross-link with apoA-II. Plasma
apoD is also present at a relatively low content in VLDL and LDL, suggesting
that apoD plays significant roles in both triglyceride and cholesterol
metabolism. Consistent with this notion, apoD polymorphism is associated with
lipid disorders, as characterized by elevated plasma triglyceride levels and/or
reduced HDL levels. ApoD is enriched in HDL isolated from patients with
established CAD. Likewise, increased apoD deposition is detected in the
atherosclerotic plaques of both human and rodent origins. However, a cause and
effect relationship between aberrant apoD production and abnormal lipoprotein
metabolism remains unknown. For example, how do apoD mutations result in
elevated plasma triglyceride levels? Does apoD affect hepatic VLDL production
and plasma VLDL clearance? Does apoD protect against or contribute to the pathogenesis
of atherosclerosis? While apoD is present in HDL in dimerization with apoA-II,
it is not clear how this inter-molecular cross-link affects HDL remodeling and
impacts cholesterol metabolism. Obviously, further studies are needed to
characterize the role of apoD in triglyceride and cholesterol metabolism and
decipher the underlying mechanism that links apoD dysregulation to
abnormalities in lipoprotein metabolism, accounting for heightened risk of
developing CAD in subjects with obesity and/or diabetes.
Equally important, apoD is implicated to play a significant role in
aging, as elevated apoD production results in lifespan extension in Drosophila.
Elevated apoD production is seen in aging brains and altered brain apoD
expression is associated with neurological disorders. It is of paramount
importance to define apoD function in the brain and understand the molecular
basis by which apoD affects aging and contributes neurological diseases.
Materials and Methods
Analysis of apoD N-glycosylation.
Aliquots
of plasma (20 μg protein) from C57BL/6J mice (male, 10 weeks old) were
incubated without (-) and with (+) 1,000 U of N-glycosidase F (New England
Biolabs) in a total volume of 30 μl at 37°C for 1 hour. The
reaction mixture was resolved on 4-20% SDS-polyacrylamide gels, followed by
immunoblot analysis using polyclonal rabbit anti-apoD (developed in our own
laboratory).
RNA isolation and RT-PCR
assay.
Total RNA isolation from tissue (20 mg) was performed using the RNeasy
Mini Kit (QIAGEN, Valencia, CA). Aliquots
of purified RNA (100 ng) from were subjected to RT-PCR analysis using apoD
sequence-specific primersflanking the apoD mRNA for forward reaction
(5'-TAAGGCCTCTCCTGCAGCCA-3') and reverse reaction (5'-CTTTACAGGAAGTCCGGGCAG-3'). The resulting PCR products were resolved on 1%
agarose gel containing ethidium bromide and visualized by UV light.
Immunohistochemistry.
Mice
were sacrificed and the proximal aorta of individual mice was dissected free of
adipose and connective tissue, and immediately fixed in 4% paraformaldehyde.
The aorta was mounted in Cryomatrix (Shandon, Pittsburgh, PA) and frozen in
isopentane that has been pre-cooled in liquid nitrogen. Transverse
cryo-sections (10 μm) were cut and stained by oil red O to visualize the
atherosclerotic lesions. Consecutive sections were immunostained using either
rabbit control IgG derived against bacterial β-galactosidase
or polyclonal rabbit anti-apoD, followed by incubation
with the donkey anti-rabbit IgG conjugated with Cy3. All animal studies were approved by the IACUC of
Children's Hospital of Pittsburgh (protocol #30-07).
Acknowledgement
This study was supported in part by
American Diabetes Association and National Health Institute grant DK066301. We
thank Dr. Steve Ringquist and members of the Dong Lab for critical proofreading
of this manuscript.
Conflicts of Interest
The authors in this manuscript have no conflict of interest
to declare.
References
-
1.
Bamba
V
and Rader
DJ.
Obesity and atherogenic dyslipidemia.
Gastroenterology.
2007;
132:
2181
-2190.
[PubMed]
.
-
2.
Kanter
JE
, Johansson
F
, LeBoeuf
RC
and Bornfeldt
KE.
Do glucose and lipids exert independent effects on atherosclerotic lesion initiation or progression to advanced plaques.
Circ Res.
2007;
100:
769
-781.
[PubMed]
.
-
3.
Liang
CP
, Han
S
, Senokuchi
T
and Tall
AR.
The macrophage at the crossroads of insulin resistance and atherosclerosis.
Circ Res.
2007;
100:
1546
-1555.
[PubMed]
.
-
4.
Tall
AR
Cholesterol efflux pathways and other potential mechanisms involved in the athero-protective effect of high density lipoproteins.
J Intern Med.
2008;
263:
256
-273.
[PubMed]
.
-
5.
Rader
DJ
Mechanisms of disease: HDL metabolism as a target for novel therapies.
Nature clinical practice.
2007;
4:
102
-109.
.
-
6.
Joy
T
and Hegele
RA.
Is raising HDL a futile strategy for atheroprotection.
Nature reviews.
2008;
7:
143
-155.
.
-
7.
Valenta
DT
, Bulgrien
JJ
, Banka
CL
and Curtiss
LK.
Overexpression of human ApoAI transgene provides long-term atheroprotection in LDL receptor-deficient mice.
Atherosclerosis.
2006;
189:
255
-263.
[PubMed]
.
-
8.
Shachter
NS
Apolipoproteins C-1 and C-III as important modulators of lipoprotein metabolism.
Curr Opin Lipidol.
2001;
12:
297
-304.
[PubMed]
.
-
9.
Altomonte
J
, Cong
L
, Harbaran
S
, Richter
A
, Xu
J
, Meseck
M
and Dong
HH.
Foxo1 Mediates Insulin Action on ApoC-III and Triglyceride Metabolism.
J Clin Invest.
2004;
114:
1493
-1503.
[PubMed]
.
-
10.
Olivieri
O
, Stranieri
C
, Bassi
A
, Zaia
B
, Girelli
D
, Pizzolo
F
, Trabetti
E
, Cheng
S
, Grow
MA
, Pignatti
PF
and Corrocher
R.
ApoC-III gene polymorphisms and risk of coronary artery disease.
J Lipid Res.
2002;
43:
1450
-1457.
[PubMed]
.
-
11.
Kamagate
A
, Qu
S
, Perdomo
G
, Su
D
, Kim
DH
, Slusher
S
, Meseck
M
and Dong
HH.
FoxO1 mediates insulin-dependent regulation of hepatic VLDL production in mice.
J Clin Invest.
2008;
118:
2347
-2364.
[PubMed]
.
-
12.
Sanchez
LM
, Diez-Itza
I
, Vizoso
F
and Lopez-Otin
C.
Cholesterol and apolipoprotein D in gross cystic disease of the breast.
Clin Chem.
1992;
38:
695
-698.
[PubMed]
.
-
13.
Thomas
EA
and Yao
JK.
Clozapine specifically alters the arachidonic acid pathway in mice lacking apolipoprotein D.
Schizophrenia research.
2007;
89:
147
-153.
[PubMed]
.
-
14.
Hansen
T
, Hemmingsen
RP
, Wang
AG
, Olsen
L
, Timm
S
, Soeby
K
, Jakobsen
KD
, Fenger
M
, Parnas
J
, Rasmussen
HB
and Werge
T.
Apolipoprotein D is associated with long-term outcome in patients with schizophrenia.
Pharmacogenomics J.
2006;
6:
120
-125.
[PubMed]
.
-
15.
Helisalmi
S
, Hiltunen
M
, Vepsalainen
S
, Iivonen
S
, Corder
EH
, Lehtovirta
M
, Mannermaa
A
, Koivisto
AM
and Soininen
H.
Genetic variation in apolipoprotein D and Alzheimer's disease.
J Neurol.
2004;
251:
951
-957.
[PubMed]
.
-
16.
Thomas
EA
, Laws
SM
, Sutcliffe
JG
, Harper
C
, Dean
B
, McClean
C
, Masters
C
, Lautenschlager
N
, Gandy
SE
and Martins
RN.
Apolipoprotein D levels are elevated in prefrontal cortex of subjects with Alzheimer's disease: no relation to apolipoprotein E expression or genotype.
Biol Psychiatry.
2003;
54:
136
-141.
[PubMed]
.
-
17.
Thomas
EA
, George
RC
, Danielson
PE
, Nelson
PA
, Warren
AJ
, Lo
D
and Sutcliffe
JG.
Antipsychotic drug treatment alters expression of mRNAs encoding lipid metabolism-related proteins.
Mol Psychiatry.
2003;
8:
983
-93, 50.
[PubMed]
.
-
18.
Franz
G
, Reindl
M
, Patel
SC
, Beer
R
, Unterrichter
I
, Berger
T
, Schmutzhard
E
, Poewe
W
and Kampfl
A.
Increased expression of apolipoprotein D following experimental traumatic brain injury.
J Neurochem.
1999;
73:
1615
-1625.
[PubMed]
.
-
19.
Vizoso
FJ
, Rodriguez
M
, Altadill
A
, Gonzalez-Dieguez
ML
, Linares
A
, Gonzalez
LO
, Junquera
S
, Fresno-Forcelledo
F
, Corte
MD
and Rodrigo
L.
Liver expression of steroid hormones and Apolipoprotein D receptors in hepatocellular carcinoma.
World J Gastroenterol.
2007;
13:
3221
-3227.
[PubMed]
.
-
20.
Soiland
H
, Janssen
EA
, Korner
H
, Varhaug
JE
, Skaland
I
, Gudlaugsson
E
, Baak
JP
and Soreide
JA.
Apolipoprotein D predicts adverse outcome in women >/=70 years with operable breast cancer.
Breast cancer research and treatment.
2009;
3:
519
-528.
[PubMed]
.
-
21.
Gonzalez
LO
, Corte
MD
, Junquera
S
, Bongera
M
, Rodriguez
JC
and Vizoso
FJ.
Expression of androgen receptor and two androgen-induced proteins (apolipoprotein D and pepsinogen C) in ductal carcinoma in situ of the breast.
Histopathology.
2007;
50:
866
-874.
[PubMed]
.
-
22.
Alexander
H
, Stegner
AL
, Wagner-Mann
C
, Du
Bois GC
, Alexander
S
and Sauter
ER.
Proteomic analysis to identify breast cancer biomarkers in nipple aspirate fluid.
Clin Cancer Res.
2004;
10:
7500
-7510.
[PubMed]
.
-
23.
Rickhag
M
, Deierborg
T
, Patel
S
, Ruscher
K
and Wieloch
T.
Apolipoprotein D is elevated in oligodendrocytes in the peri-infarct region after experimental stroke: influence of enriched environment.
J Cereb Blood Flow Metab.
2008;
28:
551
-562.
[PubMed]
.
-
24.
Wei
YJ
, Huang
YX
, Zhang
XL
, Li
J
, Huang
J
, Zhang
H
and Hu
SS.
Apolipoprotein D as a novel marker in human end-stage heart failure: a preliminary study.
Biomarkers.
2008;
13:
535
-548.
[PubMed]
.
-
25.
Kolodny
EH
Niemann-Pick disease.
Curr Opin Hematol.
2000;
7:
48
-52.
[PubMed]
.
-
26.
Suresh
S
, Yan
Z
, Patel
RC
, Patel
YC
and Patel
SC.
Cellular cholesterol storage in the Niemann-Pick disease type C mouse is associated with increased expression and defective processing of apolipoprotein D.
J Neurochem.
1998;
70:
242
-251.
[PubMed]
.
-
27.
Ong
WY
, Hu
CY
and Patel
SC.
Apolipoprotein D in the Niemann-Pick type C disease mouse brain: an ultrastructural immunocytochemical analysis.
J Neurocytol.
2002;
31:
121
-129.
[PubMed]
.
-
28.
Hansen
L
, Gaster
M
, Oakeley
EJ
, Brusgaard
K
, Damsgaard
Nielsen EM
, Beck-Nielsen
H
, Pedersen
O
and Hemmings
BA.
Expression profiling of insulin action in human myotubes: induction of inflammatory and pro-angiogenic pathways in relationship with glycogen synthesis and type 2 diabetes.
Biochem Biophys Res Commun.
2004;
323:
685
-695.
[PubMed]
.
-
29.
Chen
YW
, Gregory
CM
, Scarborough
MT
, Shi
R
, Walter
GA
and Vandenborne
K.
Transcriptional pathways associated with skeletal muscle disuse atrophy in humans.
Physiol Genomics.
2007;
31:
510
-520.
[PubMed]
.
-
30.
Liu
Z
, Chang
GQ
and Leibowitz
SF.
Apolipoprotein D interacts with the long-form leptin receptor: a hypothalamic function in the control of energy homeostasis.
Faseb J.
2001;
15:
1329
-1331.
[PubMed]
.
-
31.
Yang
CY
, Gu
ZW
, Blanco-Vaca
F
, Gaskell
SJ
, Yang
M
, Massey
JB
, Gotto
AM Jr
and Pownall
HJ.
Structure of human apolipoprotein D: locations of the intermolecular and intramolecular disulfide links.
Biochemistry.
1994;
33:
12451
-12455.
[PubMed]
.
-
32.
Schindler
PA
, Settineri
CA
, Collet
X
, Fielding
CJ
and Burlingame
AL.
Site-specific detection and structural characterization of the glycosylation of human plasma proteins lecithin:cholesterol acyltransferase and apolipoprotein D using HPLC/electrospray mass spectrometry and sequential glycosidase digestion.
Protein Sci.
1995;
4:
791
-803.
[PubMed]
.
-
33.
Zeng
C
, Spielman
AI
, Vowels
BR
, Leyden
JJ
, Biemann
K
and Preti
G.
A human axillary odorant is carried by apolipoprotein D.
Proc Natl Acad Sci U S A.
1996;
93:
6626
-6630.
[PubMed]
.
-
34.
Rassart
E
, Bedirian
A
, Do
Carmo S
, Guinard
O
, Sirois
J
, Terrisse
L
and Milne
R.
Apolipoprotein D.
Biochim Biophys Acta.
2000;
1482:
185
-198.
[PubMed]
.
-
35.
Drayna
D
, Fielding
C
, McLean
J
, Baer
B
, Castro
G
, Chen
E
, Comstock
L
, Henzel
W
, Kohr
W
and Rhee
L.
Cloning and expression of human apolipoprotein D cDNA.
J Biol Chem.
1986;
261:
16535
-16539.
[PubMed]
.
-
36.
Seguin
D
, Desforges
M
and Rassart
E.
Molecular characterization and differential mRNA tissue distribution of mouse apolipoprotein D.
Brain research.
1995;
30:
242
-250.
[PubMed]
.
-
37.
Skerra
A
Lipocalins as a scaffold.
Biochim Biophys Acta.
2000;
1482:
337
-350.
[PubMed]
.
-
38.
Yang
Q
, Graham
TE
, Mody
N
, Preitner
F
, Peroni
OD
, Zabolotny
JM
, Kotani
K
, Quadro
L
and Kahn
BB.
Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes.
Nature.
2005;
436:
356
-362.
[PubMed]
.
-
39.
Wolfrum
C
, Poy
MN
and Stoffel
M.
Apolipoprotein M is required for prebeta-HDL formation and cholesterol efflux to HDL and protects against atherosclerosis.
Nat Med.
2005;
11:
418
-422.
[PubMed]
.
-
40.
Flower
DR
, North
AC
and Sansom
CE.
The lipocalin protein family: structural and sequence overview.
Biochim Biophys Acta.
2000;
1482:
9
-24.
[PubMed]
.
-
41.
Eichinger
A
, Nasreen
A
, Kim
HJ
and Skerra
A.
Structural insight into the dual ligand specificity and mode of high density lipoprotein association of apolipoprotein D.
J Biol Chem.
2007;
282:
31068
-31075.
[PubMed]
.
-
42.
McConathy
WJ
and Alaupovic
P.
Studies on the isolation and partial characterization of apolipoprotein D and lipoprotein D of human plasma.
Biochemistry.
1976;
15:
515
-520.
[PubMed]
.
-
43.
McConathy
WJ
and Alaupovic
P.
Isolation and partial cha-racterization of apolipoprotein D: a new protein moiety of the human plasma lipoprotein system.
FEBS Lett.
1973;
37:
178
-182.
[PubMed]
.
-
44.
Alaupovic
P
, Schaefer
EJ
, McConathy
WJ
, Fesmire
JD
and Brewer
HB Jr.
Plasma apolipoprotein concentrations in familial apolipoprotein A-I and A-II deficiency (Tangier disease).
Metabolism: clinical and experimental.
1981;
30:
805
-809.
[PubMed]
.
-
45.
Bodzioch
M
, Orso
E
, Klucken
J
, Langmann
T
, Bottcher
A
, Diederich
W
, Drobnik
W
, Barlage
S
, Buchler
C
, Porsch-Ozcurumez
M
, Kaminski
WE
, Hahmann
HW
, Oette
K
, Rothe
G
, Aslanidis
C
, Lackner
KJ
and Schmitz
G.
The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease.
Nature genetics.
1999;
22:
347
-351.
[PubMed]
.
-
46.
Brooks-Wilson
A
, Marcil
M
, Clee
SM
, Zhang
LH
, Roomp
K
, van
Dam M
, Yu
L
, Brewer
C
, Collins
JA
, Molhuizen
HO
, Loubser
O
, Ouelette
BF
, Fichter
K
, Ashbourne-Excoffon
KJ
, Sensen
CW
, Scherer
S
, Mott
S
, Denis
M
, Martindale
D
, Frohlich
J
, Morgan
K
, Koop
B
, Pimstone
S
, Kastelein
JJ
, Genest
J Jr
and Hayden
MR.
Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency.
Nature genetics.
1999;
22:
336
-345.
[PubMed]
.
-
47.
Frikke-Schmidt
R
, Nordestgaard
BG
, Jensen
GB
and Tybjaerg-Hansen
A.
Genetic variation in ABC transporter A1 contributes to HDL cholesterol in the general population.
J Clin Invest.
2004;
114:
1343
-1353.
[PubMed]
.
-
48.
Lawn
RM
, Wade
DP
, Garvin
MR
, Wang
X
, Schwartz
K
, Porter
JG
, Seilhamer
JJ
, Vaughan
AM
and Oram
JF.
The Tangier disease gene product ABC1 controls the cellular apolipoprotein-mediated lipid removal pathway.
J Clin Invest.
1999;
104:
R25
-31.
[PubMed]
.
-
49.
Rust
S
, Rosier
M
, Funke
H
, Real
J
, Amoura
Z
, Piette
JC
, Deleuze
JF
, Brewer
HB
, Duverger
N
, Denefle
P
and Assmann
G.
Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1.
Nature genetics.
1999;
22:
352
-355.
[PubMed]
.
-
50.
Zannis
VI
, Chroni
A
, Kypreos
KE
, Kan
HY
, Cesar
TB
, Zanni
EE
and Kardassis
D.
Probing the pathways of chylomicron and HDL metabolism using adenovirus-mediated gene transfer.
Curr Opin Lipidol.
2004;
15:
151
-166.
[PubMed]
.
-
51.
Vaisar
T
, Pennathur
S
, Green
PS
, Gharib
SA
, Hoofnagle
AN
, Cheung
MC
, Byun
J
, Vuletic
S
, Kassim
S
, Singh
P
, Chea
H
, Knopp
RH
, Brunzell
J
, Geary
R
, Chait
A
, Zhao
XQ
, Elkon
K
, Marcovina
S
, Ridker
P
, Oram
JF
and Heinecke
JW.
Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL.
J Clin Invest.
2007;
117:
746
-756.
[PubMed]
.
-
52.
Desai
PP
, Bunker
CH
, Ukoli
FA
and Kamboh
MI.
Genetic variation in the apolipoprotein D gene among African blacks and its significance in lipid metabolism.
Atherosclerosis.
2002;
163:
329
-338.
[PubMed]
.
-
53.
Kamboh
MI
, Albers
JJ
, Majumder
PP
and Ferrell
RE.
Genetic studies of human apolipoproteins. IX. Apolipoprotein D polymorphism and its relation to serum lipoprotein lipid levels.
American journal of human genetics.
1989;
45:
147
-154.
[PubMed]
.
-
54.
Baker
WA
, Hitman
GA
, Hawrami
K
, McCarthy
MI
, Riikonen
A
, Tuomilehto-Wolf
E
, Nissinen
A
, Tuomilehto
J
, Mohan
V
and Viswanathan
M.
Apolipoprotein D gene polymorphism: a new genetic marker for type 2 diabetic subjects in Nauru and south India.
Diabet Med.
1994;
11:
947
-952.
[PubMed]
.
-
55.
Hitman
GA
, McCarthy
MI
, Mohan
V
and Viswanathan
M.
The genetics of non-insulin-dependent diabetes mellitus in south India: an overview.
Ann Med.
1992;
24:
491
-497.
[PubMed]
.
-
56.
Vijayaraghavan
S
, Hitman
GA
and Kopelman
PG.
Apolipoprotein-D polymorphism: a genetic marker for obesity and hyperinsulinemia.
J Clin Endocrinol Metab.
1994;
79:
568
-570.
[PubMed]
.
-
57.
Curry
MD
, McConathy
WJ
and Alaupovic
P.
Quantitative determination of human apolipoprotein D by electro-immunoassay and radial immunodiffusion.
Biochim Biophys Acta.
1977;
491:
232
-241.
[PubMed]
.
-
58.
Lane
DM
and McConathy
WJ.
Factors affecting the lipid and apolipoprotein levels of cord sera.
Pediatr Res.
1983;
17:
83
-91.
[PubMed]
.
-
59.
Zannis
VI
, Chroni
A
and Krieger
M.
Role of apoA-I, ABCA1, LCAT, and SR-BI in the biogenesis of HDL.
J Mol Med.
2006;
84:
276
-294.
[PubMed]
.
-
60.
Albers
JJ
, Cabana
VG
and Dee
Barden Stahl Y.
Purification and characterization of human plasma lecithin:cholesterol acyltransferase.
Biochemistry.
1976;
15:
1084
-1087.
[PubMed]
.
-
61.
Holmquist
L
Separation of free and apolipoprotein D-associated human plasma lecithin: cholesterol acyltransferase.
J Biochem Biophys Methods.
1989;
19:
93
-103.
[PubMed]
.
-
62.
Kostner
G
Studies on the cofactor requirements for lecithin:cholesterol acyltransferase.
Scand J Clin Lab Invest Suppl.
1974;
137:
19
-21.
[PubMed]
.
-
63.
Albers
JJ
, Lin
J
and Roberts
GP.
Effect of human plasma apolipoproteins on the activity of purified lecithin: cholesterol acyltransferase.
Artery.
1979;
5:
61
-75.
[PubMed]
.
-
64.
Steyrer
E
and Kostner
GM.
Activation of lecithin-cholesterol acyltransferase by apolipoprotein D: comparison of proteoliposomes containing apolipoprotein D, A-I or C-I.
Biochim Biophys Acta.
1988;
958:
484
-491.
[PubMed]
.
-
65.
Blanco-Vaca
F
, Via
DP
, Yang
CY
, Massey
JB
and Pownall
HJ.
Characterization of disulfide-linked heterodimers containing apolipoprotein D in human plasma lipoproteins.
J Lipid Res.
1992;
33:
1785
-1796.
[PubMed]
.
-
66.
Do
Carmo S
, Levros
LC Jr
and Rassart
E.
Modulation of apolipoprotein D expression and translocation under specific stress conditions.
Biochim Biophys Acta.
2007;
1773:
954
-969.
[PubMed]
.
-
67.
Do
Carmo S
, Jacomy
H
, Talbot
PJ
and Rassart
E.
Neuroprotective effect of apolipoprotein D against human coronavirus OC43-induced encephalitis in mice.
J Neurosci.
2008;
28:
10330
-10338.
[PubMed]
.
-
68.
Ganfornina
MD
, Do
Carmo S
, Lora
JM
, Torres-Schumann
S
, Vogel
M
, Allhorn
M
, Gonzalez
C
, Bastiani
MJ
, Rassart
E
and Sanchez
D.
Apolipoprotein D is involved in the mechanisms regulating protection from oxidative stress.
Aging Cell.
2008;
7:
506
-515.
[PubMed]
.
-
69.
Sanchez
D
, Lopez-Arias
B
, Torroja
L
, Canal
I
, Wang
X
, Bastiani
MJ
and Ganfornina
MD.
Loss of glial lazarillo, a homolog of apolipoprotein D, reduces lifespan and stress resistance in Drosophila.
Curr Biol.
2006;
16:
680
-686.
[PubMed]
.
-
70.
Walker
DW
, Muffat
J
, Rundel
C
and Benzer
S.
Overexpression of a Drosophila homolog of apolipoprotein D leads to increased stress resistance and extended lifespan.
Curr Biol.
2006;
16:
674
-679.
[PubMed]
.
-
71.
Muffat
J
, Walker
DW
and Benzer
S.
Human ApoD, an apolipoprotein up-regulated in neurodegenerative diseases, extends lifespan and increases stress resistance in Drosophila.
Proc Natl Acad Sci U S A.
2008;
105:
7088
-7093.
[PubMed]
.
-
72.
Kalman
J
, McConathy
W
, Araoz
C
, Kasa
P
and Lacko
AG.
Apolipoprotein D in the aging brain and in Alzheimer's dementia.
Neurological research.
2000;
22:
330
-336.
[PubMed]
.
-
73.
Desai
NM
, Goss
JA
, Deng
S
, Wolf
BA
, Markmann
E
, Palanjian
M
, Shock
AP
, Feliciano
S
, Brunicardi
FC
, Barker
CF
, Naji
A
and Markmann
JF.
Elevated portal vein drug levels of sirolimus and tacrolimus in islet transplant recipients: local immunosuppression or islet toxicity.
Transplantation.
2003;
76:
1623
-1625.
[PubMed]
.
-
74.
Terrisse
L
, Poirier
J
, Bertrand
P
, Merched
A
, Visvikis
S
, Siest
G
, Milne
R
and Rassart
E.
Increased levels of apolipoprotein D in cerebrospinal fluid and hippocampus of Alzheimer's patients.
J Neurochem.
1998;
71:
1643
-1650.
[PubMed]
.
-
75.
Sarjeant
JM
, Lawrie
A
, Kinnear
C
, Yablonsky
S
, Leung
W
, Massaeli
H
, Prichett
W
, Veinot
JP
, Rassart
E
and Rabinovitch
M.
Apolipoprotein D inhibits platelet-derived growth factor-BB-induced vascular smooth muscle cell proliferated by preventing translocation of phosphorylated extracellular signal regulated kinase 1/2 to the nucleus.
Arterioscler Thromb Vasc Biol.
2003;
23:
2172
-2177.
[PubMed]
.
-
76.
Hall
RE
, Horsfall
DJ
, Stahl
J
, Vivekanandan
S
, Ricciardelli
C
, Stapleton
AM
, Scardino
PT
, Neufing
P
and Tilley
WD.
Apolipoprotein-D: a novel cellular marker for HGPIN and prostate cancer.
The Prostate.
2004;
58:
103
-108.
[PubMed]
.
-
77.
Miranda
E
, Vizoso
F
, Martin
A
, Quintela
I
, Corte
MD
, Segui
ME
, Ordiz
I
and Merino
AM.
Apolipoprotein D expression in cutaneous malignant melanoma.
Journal of surgical oncology.
2003;
83:
99
-105.
[PubMed]
.
-
78.
Desai
PP
, Hendrie
HC
, Evans
RM
, Murrell
JR
, DeKosky
ST
and Kamboh
MI.
Genetic variation in apolipoprotein D affects the risk of Alzheimer disease in African-Americans.
Am J Med Genet B Neuropsychiatr Genet.
2003;
116B:
98
-101.
[PubMed]
.
-
79.
Desai
PP
, Ikonomovic
MD
, Abrahamson
EE
, Hamilton
RL
, Isanski
BA
, Hope
CE
, Klunk
WE
, DeKosky
ST
and Kamboh
MI.
Apolipoprotein D is a component of compact but not diffuse amyloid-beta plaques in Alzheimer's disease temporal cortex.
Neurobiology of disease.
2005;
20:
574
-582.
[PubMed]
.
-
80.
Navarro
A
, Del Valle
E
, Astudillo
A
, Gonzalez
del Rey C
and Tolivia
J.
Immunohistochemical study of distribution of apolipoproteins E and D in human cerebral beta amyloid deposits.
Experimental neurology.
2003;
184:
697
-704.
[PubMed]
.