Introduction
The aging process represents progressive
changes in a cell or an organism which culminate in death due to accumulated
defects in function leading to system failure [1]. These
defects result in part from accumulated damage to DNA. Such damage may result from
environmental insults such as ultraviolet (UV) and ionizing radiation,
exogenous chemical and biological genotoxins, as well as endogenous mutagens (e.g.,
reactive oxygen intermediates). The accumulated changes lead to deficiencies in
enzymes involved in necessary metabolic and maintenance processes, over time
causing an escalating loss of function with an inability to maintain
replicative fidelity of the genome [2-4]. Thus,
organisms with mutations to genes directly involved in basic genome structure,
maintenance and replicative fidelity would understandably have an accelerated
aging phenotype and/or shortened life spans.
Individuals
with a progeroid syndrome have a premature aging phenotype and, depending on
the specific mutations involved, the effects on lifespan may range from
moderate to severe. Examples include Werner syndrome (WS), Bloom syndrome
(BLM), Cockayne syndrome (CS), ataxia-telangiectasia (AT), Hutchinson-Gilford
progeria syndrome (HGPS), and restrictive dermopathy (RD). They arise from
mutations in one or several genes involved in DNA metabolism or in its
regulation. Accelerated aging also may result from partial genome imbalances
as seen in the chromosomal disorders of Down, Klinefelter and Turner syndromes.
WS or BLM arise from mutations in the WRN or BLM genes which encode RecQ DNA helicase proteins [5-7] while CS
stems from mutations to the E
xcision R
epair C
ross-C
omplementing
group 6
or 8
proteins (ERCC-6 or -8, also called CSB or CSA,
respectively) [8]. Mutations
to the ATM (a
taxia-t
elangiectasia m
utated) gene
cause AT; ATM encodes a phosphatidylinositol-3-kinase involved in the
cell cycle checkpoint signaling pathway for detection of DNA damage and its
subsequent repair [9,10]. Thus,
the WRN, BLM, ERCC6/8 and ATM proteins are involved directly in DNA repair
processes and their mutations cause elevated levels of genome instability,
premature aging phenotypes and for ERCC8 and ATM cancer susceptibilities.
Interestingly, HGPS and RD are laminopathy-based diseases; they arise not from
mutated DNA metabolism genes but from mutations causing altered
processing/maturation of lamin A, an intermediate-filament protein component of
the nuclear lamina [6,11-16].
Nevertheless, HGPS and RD are the most severe forms of progeria; HGPS
individuals have an average life span of 13.5 years while RD individuals suffer
perinatal death [13,15,17].
While lamin A is not involved directly in DNA metabolism, particularly DNA
repair and damage responses, DNA double-strand breaks (DSBs) are found to
accumulate in HGPS and RD cells [18-20]. Similar
DSB accumulation also appears to happen in physiological aging for healthy
individuals who have intact DNA metabolism genes [21]. Thus, an
interesting question concerns how altered lamin A proteins cause disruption of
the normal organization of the nuclear genome and how such spatial disruptions
cause deficiencies in DNA repair processes even though DNA repair or metabolism
genes are not defective. This review will consider the epigenetic effects of
lamin A abnormalities and their perturbation of DNA damage recognition and its
repair, leading to genome instability in HGPS and RD patients.
Laminopathies
in Hutchinson-Gilford progeria syndrome & restrictive dermopathy
The
lamins are filamentous protein components of the nuclear lamina and, to a
lesser extent, they form foci within the nucleoplasm in performing dynamic
structural roles in the nucleus [22-24]. Lamin
proteins also interact directly with histone H2A [25]. There are four major lamin proteins (A-type and B-type) in humans. Lamins A and C (A-type) derive from alternative mRNA
splicing products of the LMNA gene; exons 1-10 encode the N-terminal 566
amino acids of lamins A and C; however, exons 11 and 12 are unique to lamin A
mRNA and code for an additional 98-amino acid C-terminal region which contains
functionally important post-translational modification sites. Lamin B1 and B2
(B-type) are encoded by LMNB1 and LMNB2 genes and are expressed
throughout development and in adult cells. In contrast, LMNA expression
occurs in differentiated cell types. Lamins A and B differ from lamin C in that
they are post-translationally modified in their C-terminal regions (Figure 1).
The lamin B proteins retained the added farnesyl and carboxy methyl groups
which are critical for their nuclear function [26]. In
contrast, these prosthetic groups are removed by proteolytic cleavage in the
final step of lamin A maturation processing (Figure 1). Genetic disruptions of
this final proteolytic step form the basis for HGPS and RD [15,23,27].
Prelamin
A is the translation product of the mature LMNA mRNA in normal
individuals. This 664-amino-acid protein is post-translationally processed
into lamin A by two transfer reactions and two proteolytic cleavages (Figure 1). A farnesyl transferase specifically directs the transfer of the
hydrophobic 15-carbon chain from farnesyl pyrophosphate to the cysteine at the
C-terminal CAAX motif of prelamin A. The terminal tripeptide is then
proteolytically removed by either Rce-1 (Ras converting enzyme-1) or the zinc
metallo-proteinase Zmpste24 (also known as FACE-1). The terminal cysteine then
is carboxy-methylated. Prelamin B is similarly post-translationally modified
to this stage. For prelamin A the 15-amino acid C-terminal peptide containing
the two modifications then is removed by a 2nd Zmpste24 cleavage to
generate mature lamin A [28].
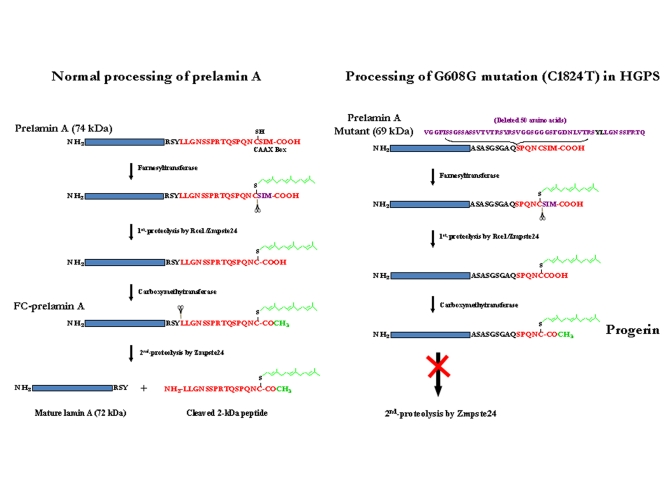
Figure 1. Maturation of lamin A and formation of progerin or LA∆50. (A) Normal processing of prelamin A. (B)
Processing of G608G mutation (C1824T) in HGPS cells. Underline LY
(in black) in the deleted 50 AAs: Zmpste24 cleavage site
The
HGPS and RD laminopathies arise from deficiencies in these post-translational
modifications of prelamin A. All Zmpste24 enzymatic activity is lost in individuals with RD
(Zmpste24-/-);
the farnesylated and carboxy-methylated prelamin A (FC-prelamin A) is
toxic, especially with the absence of normal lamin A, causing perinatal death [29,30]. HGPS
individuals are heterozygous for a mutation within the LMNA gene
itself. The dominant mutation is a CàT base
substitution at position 1824 within exon 11. Although there is no amino acid
change (G608G) a cryptic splice donor site is activated within exon 11.
Sporadic use of this cryptic site in splicing of LMNA pre-mRNA removes
an additional 150 base-pair sequence, causing a 50-amino acid deletion (Figure 1) within the prelamin A protein though mature lamin A is still largely
produced. The missing region includes the second Zmpste24 cleavage site
(Figure 1). Thus, a slightly smaller farnesylated and carboxy-methylated
mutant prelamin A protein (termed progerin or LAΔ50) forms and
accumulates though at a much slower rate than for FC-prelamin A formed with the
homozygous Zmpste24 mutation in RD. While the farnesyl and carboxy-methyl
moieties are necessary for lamin B functions their persistence in progerin and
FC-lamin A causes multiple abnormalities in nuclear structure and function [11,16,20,23,27,31,32]. The hydrophobic farnesyl chain
gives progerin a greater affinity for the inner nuclear membrane (INM),
redistributing progerin away from nucleoplasmic foci. This association with
the INM also deforms the membrane. During interphase, the dysmorphic nuclei
are lobulated, the nuclear lamina thickens, and there is a loss of heterochromatin
and nucleoplasmic lamin A foci. The nucleoplasmic foci normally contain the
replicative proteins PCNA and polymerase δ and appear to be critical for
ordered initiation of genome replication in early S-phase [32,33].
Functionally, histone modification and gene expression patterns change [8,34], and DNA
damage increases with a loss of DNA repair efficiency [12,18]. Cell
division also is modified during nuclear envelope dissolution and reassembly.
During mitosis progerin plus normal lamin A mis-localize into insoluble
cytoplasmic aggregates and membranes, delaying their return to the INM and
lamina of the reformed nucleus. This causes spatial and functional disruption
of interphase G1 chromatin and may lead to formation of bi-nucleate
cells [35,36]. These structural, spatial and DNA damage/repair
changes lead to increased genome instability and cytotoxicity as progerin
protein accumulates in aging HGPS cells [11,23].
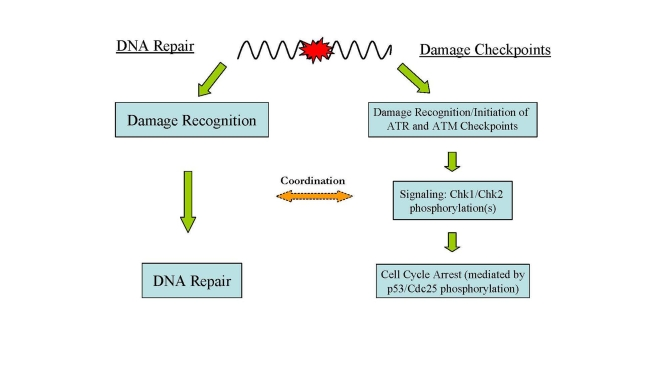
Figure 2. Major DNA damage responses in human cells. In
response to DNA damage, two major cellular pathways, DNA damage checkpoints
and DNA repair, are activated for maintaining genome integrity and
stability.
DNA
damage and accumulation in HGPS and RD cells
It
is generally believed that cellular DNA damage accumulation is a hallmark step leading to premature aging and the aging phenotypes
featured with genome instability. Indeed,
like other types of progeroid cells, HGPS and RD cells accumulate DNA damage,
in particular DSBs, with continued passage in culture [12,18,19],
indicating that DNA repair activity is impaired in these cells. The DSB
accumulation causes genome instability, eventually leading to cellular
senescence. However, unlike most types of progeria, the DNA damage accumulation
in HGPS and RD is not caused by genetic deficiency in DNA repair pathways,
making the laminopathy-based diseases a unique type of progeria in terms of the
cause of genome instability and DNA repair dysfunction. Some insights into the
molecular mechanisms responsible for DSB accumulation in HGPS and RD cells
recently have been revealed and are discussed in following sections.
The laminopathy-based progeroid cells
also were found to be sensitive to various DNA damaging agents. In particular,
Zmpste24-/- mouse embryonic fibroblasts (MEFs) are extremely
sensitive to DSB inducers such as camptothecin (CPT) and etoposide [12], which is
consistent with the observation of DSB accumulation in aging HGPS and RD
patient cells. Interestingly, however,
MEFs are also hypersensitive to UV irradiation which typically induces bulky
DNA adducts exclusively removed by the nucleotide excision repair pathway (NER)
[12].
In addition, MEFs are sensitive to mitomycin C, a carcinogen inducing
interstrand crosslinks in DNA. However, MEFs show very limited sensitivity to
the alkylating agent methyl methane-sulfonate (MMS) [12]. These
cytotoxicity phenotypes reflect the deficiency in maintaining genome stability
in the Zmpste-24 deficient mouse cells.
DNA
damage response signaling in HGPS and RD
HGPS
and RD cells in culture exhibit limited growth potential relative to BJ normal
human primary fibroblast cells. Young HGPS and RD cells grow quite well but
the cells senesce quickly relative to normal fibroblasts and growth stops, much
sooner for RD than HGPS [18]. As the
growth rate slows the frequency of dysmorphic nuclei increases as does the
number with γ-H2AX (a marker of DNA DSBs) foci detected by
immunofluorescence microscopy [11,19,37].
H2AX is a variant of histone H2A and represents a minor component of that
histone in cell nuclei [38]. Histone
H2AX is phosphorylated to γ-H2AX in response to DSBs in interphase cells via ATM signaling [39,40]. Thus,
γ-H2AX has been used in immunomicroscopy to cytologically mark nuclear
sites of DNA DSBs and is employed biochemically to isolate chromatin fragments containing
DSBs using the Ch
romatin I
mmuno-P
recipitation (ChIP)
procedure [19]. A
combination of culture ‘aging' and the specific tracking approaches of immunofluorescence
microscopy, the ChIP assay and Western blotting now allow mechanistic questions
to be asked concerning the deficiencies in DNA damage recognition and repair in
aging progeroid cells.
DNA damage in cells evokes a checkpoint response which
moderates cell cycle progression for repair of the damage [41] (Figure 2). The first part of this process is recognition of the DNA damage and
initiation of the damage response which includes activation of cell cycle
checkpoints and the phosphorylation of H2AX. The response begins with the
activation of ATM and ATR (ATM- and
Rad3-related) which play central roles in DNA damage checkpoints. ATR is
activated by a wide spectrum of DNA damages inducing replication stress while
ATM is activated primarily by DNA DSBs [9,42,43].
Signal-transducing kinases Chk1 and Chk2 are then phosphorylated by activated
ATM and ATR leading to a cascade of further down-stream activating signals (i.e., phosphorylation of p53) via the kinase activities of Chk1 and Chk2 [41,43].
Culture-aged
HGPS and RD cells contain accumulated DNA damage and compromised genome
integrity. Liu et al. examined these cells to determine if the damage
checkpoint pathways were persistently activated [18]. They
found that aged HGPS and RD cells contained higher levels of γ-H2AX than
did normal BJ fibroblasts indicating more frequent DNA DSBs. The progeroid
cells also exhibited high levels of phosphorylated Chk1 and Chk2 due to ATM and
ATR activation. Phospho-rylated p53 is a downstream product of Chk1 and Chk2
activation and it also was increased significantly in the HGPS and RD cells.
These findings demonstrate that ATR and ATM checkpoint pathways were
persistently activated by the damaged DNA in the progeroid cells. While ATM and
ATR were diffusely distributed in the nuclei of BJ cells, they clustered into
distinct foci in nuclei of the HGPS and RD cells [18]. These
foci were identical to those observed in BJ cells treated with UV irradiation
(for ATR) or CPT (for ATM) [12].
Liu
et al. also determined biochemically whether ATM and ATR activities were
responsible for the reduced replicative capacity of HGPS cells. Caffeine
inhibits both ATM and ATR, and caffeine-treated HGPS cells demonstrated a
significant restoration of replicative activity. Knockdown of ATM and ATR
protein levels by siRNA silencing also restored significant replicative
activity [18]. Thus, the
decreased cell cycling observed in aged progeroid cells is one response to the
accumulated DNA damage which is mediated by ATM and ATR checkpoint pathways.
Are the activation and sub-nuclear clustering of ATM
and ATR in progeroid cells directly related to the accumulated progerin
protein? This question was addressed by investigating the effects of progerin
expression in normal cells and, alternatively, the inhibition of the prelamin A
processing in progeroid cells [18]. It was
observed that HeLa cells transfected with a progerin-expressing plasmid
exhibited ATR nuclear foci formation, demonstrating that foci formation is
progerin-dependent. Inhibition of the prenylation of G608G mutant prelamin A
with the farnesyl transferase inhibitor
L-744832 restored normal nuclear shape. Interestingly, however, the levels of γ-H2AX
and phosphorylated Chk1 and Chk2 in HGPS cells were not reduced. Thus,
reversal of dysmorphic nuclei formation has no effect on cell cycle checkpoint
activation from existing DNA DSBs.
Deficiencies in DNA damage recognition and repair in
HGPS and RD
Genome
instability can arise from multiple causes; one of the most obvious being an
increased sensitivity to DNA damage due to genetic or epigenetic deficiencies
in DNA repair. The persistent activation of ATM/ATR checkpoint pathways in
HGPS and RD reflects a delay in DNA repair efficiency in these cells [18]. The DSB
accumulation in these cells is particularly puzzling since HGPS and RD cells
are genetically defective in prelamin A and related processing pathways rather
than in DNA repair proteins.
It is expected that multiple DSB repair
proteins would be recruited to the DNA damage sites for repair as part of the
damage response. Surprisingly, such was not the case. Employing
immunofluorescence tracking of γ-H2AX foci and neutral single-cell
electrophoretic (comet) assays to measure DNA DSBs Zou's group observed a
significant parallel increase in nuclear γ-H2AX foci and DSB frequency in
HGPS cells relative to BJ fibroblasts. Cellular progerin levels exhibited
similar increases in the aged progeroid cells [19]. Although
elements of the damage response system (i.e., ATR, ATM, Chk1, Chk2 and
p53) were activated [18],
immunofluorescence studies indicated that nuclear foci of Rad50 or Rad51 did
not colocalize with the γ-H2AX foci in HGPS and RD cells [19]. This was
unexpected since Rad50 (part of the MRN complex of Mre11/Rad50/Nbs1) and Rad51
are components critical for repair of DNA DSBs [41,44-46] and
for the restart of stalled replication forks [47]. In
contrast, DSBs induced in normal BJ cells by CPT showed colocalization of
γ-H2AX with Rad50 or Rad51 foci. The failed recruitment of repair factors
to the laminopathy-induced DSBs made the DNA damage unrepairable in HGPS and RD
cells [19]. Impaired recruitment to DSB foci of Rad51 and 53BP1 (p53-binding
protein 1) also was observed in bone marrow cells of Zmpste24-/-mice and in HGPS cells treated with γ-irradiation [12]. These data
raise the question of why these repair proteins were not recruited to the DSB
sites.
Xeroderma
pigmentosum group A (XPA) protein is
a specific and essential factor for NER but is not involved in the repair of
DSBs [41]. The role
of XPA in NER is believed to include DNA damage recogni-tion/verification, NER
nuclease recruiting, and stabilization of repair intermediates [41,48-51]. NER
does not process DSBs nor does it introduced DSB intermediates during the
repair process. Surprisingly, XPA colocalized with the γ-H2AX sites of
DSBs in HGPS and RD cells [19]. XPC is the
major DNA damage recognition protein in NER [41] but did not
exhibit nuclear foci in HGPS and RD cells
indicating that the colocalization of XPA and γ-H2AX was specific and not
related to NER [19].
Furthermore, in HGPS and RD cells treated with CPT (a DSB-inducer) XPA did not
colocalize to these CPT-induced DSBs though it still colocalized to the
endogenous laminopathy-induced DSB foci. Also, the CPT-induced foci were
repaired in HGPS and RD cells, though at a slower rate than in the BJ cells.
The latter result demonstrates that the DSB repair system per se in HGPS
and RD cells is functional, and, also that the XPA behaves normally in not
binding to genotoxin-induced DSBs.
How
does the binding of XPA to laminopathy-generated DSBs relate to the lack of
Rad50 and Rad51 binding? Is the XPA association with the DSBs sufficient to
exclude these proteins? Zou's group employed the ChIP assay and siRNA
knockdown of XPA to resolve these questions. XPA was found in the
γ-H2AX-associated chromatin fragments from HGPS cells but not from normal
BJ cells, even when DSBs were induced in the latter by CPT [19]. Nuclease
treatment of the chromatin before immunoprecipitation released the XPA from the
γ-H2AX chromatin complex. Thus, DNA mediates the association of XPA and
γ-H2AX-marked chromatin containing DNA DSBs.
If
this XPA association with DSBs in progeroid chromatin is sufficient to exclude
Rad50 and Rad51, this exclusion should be reversible with XPA depletion by
knockdown with RNAi. Lui et al. observed that XPA depletion partially restored the
recruitment of Rad50, Rad51 and Ku70 to γ-H2AX chromatin containing DNA
DSBs [19,52]. This confirms that the binding of XPA to
laminopathy-induced DSBs in HGPS and RD cells disrupted recruitment of factors
normally involved in their repair. This is further supported by their finding
that XPA depletion significantly reduced the level of DSBs in HGPS cells but
had no effect on CPT-induced DSB level in BJ cells. Thus, XPA binding to DNA
DSBs in progeroid cells may explain the absence of appropriate repair proteins
at these sites and the genome instability observed in these cells due to
failure to execute DNA repair.
Bomgarden et al. found that of the multiple NER
factors XPA specifically was needed for ATR signaling of DNA damage during
S-phase and that XPA knockdown compromised the normal response to UV damage [53]. This is
consistent with the role of XPA in verifying the presence of bulky lesions in
NER [54-56]. The proportion of HGPS cells
in S-phase increases with cell age as does the level of accumulated DSBs.
Thus, it would be interesting to see if the localization of XPA to these damage
sites is required for activation of ATM and ATR checkpoint pathways in HGPS and
RD cells [18].
Lamin A and C proteins form nucleoplasmic
foci which organize proteins for initiation of replication in early S-phase,
including the colocalization of PCNA [32].
Microinjection of an N-terminal mutant lamin A protein (ΔNLA) disrupts the
nuclear lamina organization in mammalian cells and causes a redistribution of
the replication elongation proteins PCNA and RFC [57,58]. The
absence of PCNA at replication centers due to its sequestration in
ΔNLA-lamins aggregates in a dominant-negative manner may lead to stalled
replication forks; collapse of the replication forks may result in DSBs [59]. Shumaker et al. also
observed that the Ig-fold domain of all lamin proteins bound directly to PCNA
and that excess amounts of the Ig-fold domain sequestered the PCNA and
inhibited DNA replication [60]. The
Ig-fold domain occurs just before the CAAX-box which is modified in the
laminopathies (see Figure 1). Progerin and FC-prelamin A, the mutant forms of
lamin A in HGPS and RD cells, respectively (Figure 1; [6,11-16]), are
known to disrupt normal nuclear structure including the perinucleolar lamin A/C
granules containing the replicative proteins PCNA and polymerase δ [33]. If these
progeroid proteins generate a redistribution of PCNA and/or RFC, they also
would cause replication fork stalling followed by DNA DSB formation. During
this process, the replication fork and its damage intermediates, now PCNA- and
RFC-deficient, may become accessible for XPA binding. The bound XPA then
blocks association of DSBs with the repair proteins Rad50, Rad51 and 53BP1 [12,19] (Figure 3). PCNA forms discrete nuclear foci in early-passage HGPS cells [61] when no XPA
foci were seen. However, PCNA foci were not seen in late-passage cells
(unpublished data) when there is an increase in XPA foci colocalizing with γ-H2AX
and in DNA DSBs [19].
Why
does XPA colocalize with the laminopathy-induced DSBs marked by γ-H2AX in
aging progeroid cells? Stalled replication forks may result in S-phase arrest
via persistent ATM/ATR activation [18,53]. DSBs
can be generated at stalled forks [59,62-64] that
contain strand termini of double-stranded/single-stranded DNA (ds-ssDNA)
junctions, mostly from Okazaki fragments. A recent study indicated that XPA
exhibits an affinity for these ds-ssDNA junctions even higher than its affinity
for the DNA damage processed by NER [51]. In HGPS
cells, the possible sequestration of PCNA at functioning replication forks and
in progerin aggregates may leave the strand termini of ds-ssDNA junctions
unprotected, allowing access to XPA for binding (Figure 3). Thus, the amount
of progerin increases with age in progeroid cells, as does the number of
nuclear γ-H2AX foci and measurable DSBs as well as XPA foci [19]. In
addition, the unexpected translocation of XPA to the DSB sites in progeroid
cells may trap this NER protein at the collapsed replication forks, which
subsequently may silence NER activity for repair of bulky DNA adducts such as
the photoproducts induced by UV irradiation. This may explain the observed
hypersensitivity of progeroid cells to UV damage in addition to DSB damage [12].
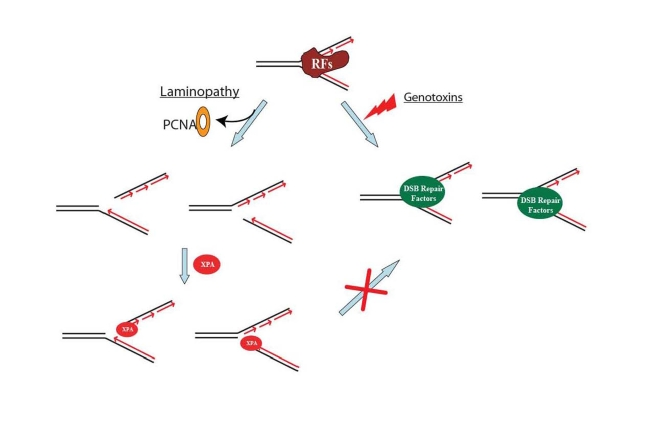
Figure 3. A proposed model showing that DNA double-strand break repair activity is impaired in HGPS and RD cells. Unlike the replication fork
collapse induced by genotoxins, laminopathy-induced replication fork
collapse may be characterized with a possible loss of PCNA at replication
forks. The subsequent possible binding of XPA to the "naked" replication
forks with DNA double-strand breaks (DSBs) blocks the access of DSB repair
proteins to the damage sites. RFs stands for replication factors.
Therapeutic
strategies for treatment of HPGS
Farnesyl transferase inhibitors (FTIs) have been
applied to progeroid cells and to Zmpste24-/- mice to block the
prenylation reaction since it is believed that a major phenotype-inducing
element of progerin and FC-prelamin A is the farnesyl moiety [14,29]. FTI
treatment did reduce farnesylated forms of progerin and FC-prelamin A and
correct the nuclear dysmorphology [65,66].
However, FTI treatment of progeroid cells did not reduce the frequency of DNA
DSBs nor the levels γ-H2AX protein and its nuclei foci [12,19,52].
Consistently these proteins were prenylated instead by geranylgeranyl addition
and some of the laminopathy conditions persisted [67,68]. The
prenyl groups are derived from the cholesterol biosynthetic pathway; statins
and amino-bisphosphonates are common drugs for treatment of
hypercholesterolemia [29]. These
drugs also appear more effective than FTIs in reducing phenotypic markers of
laminopathy in model mice and cellular (HGPS, RD) assays [29,67,68].
It will be of interest to determine whether the statin/amino-bisphosphonate
drug combination will be more effective in reducing aberrant nuclear morphology
and genome instability phenotypes.
HGPS
and normal aging
Great
interest in understanding HGPS has been promoted by recent findings that linked
normal aging to the laminopathy disease. The connection is supported by several
lines of evidence and observation. First, the same mechanism responsible for
HGPS is also active in normal aging cells [21]. Cells from healthy individuals also express low levels
of progerin from sporadic use of the cryptic splice site [21], resulting
in similar phenotypes. For instance, the level of γ-H2AX increases with an
individual's age in tissue samples and with time in culture for primary cell
explants [21,37,39],
which is concomitant with a parallel increase in laminopathy-induced DNA damage
and the pathological changes in nuclear morphology and chromatin structures.
Secondly, like in HGPS, DNA damage accumulation in healthy aging cells is not
caused by a genetic deficiency in DNA repair. It is quite likely that the same
sporadic abnormal splicing of prelamin A mRNA is responsible for the genome
instability in both HGPS and normal aging.
Finally, like in HPGS, DSBs formed in normal human aging also are unrepairable
although genotoxin-induced DSBs in the same cells can be efficiently repaired [2]. All these
mechanistic similarities strongly support the use of HGPS or related
laminopathies as an excellent model for the study of normal human aging.
Grant
sponsors: National Cancer Institute (NCI) of National Institutes of Health
(NIH) (to Y.Z.); grant number: CA86927; and National Institute on Aging (NIA)
of NIH (to Y.Z.); grant number: AG031503
The authors of this manuscript have no conflict of interests to declare.