p65 null MEFs are resistant to
genotoxin-induced death To address how NF-κB
deficiency alters p53-dependent apoptosis in transformed cells we examined the
ability of p65 null MEFs transformed with the adenoviral oncogene E1A to
undergo apoptosis induced by genotoxic agents. As a control for these studies
NF-κB function was reconstituted by retroviral
gene transfer of p65 (Figure 1A). To control for this manipulation p65 null
MEFs were mock infected with an empty retroviral vector. Thus these two cell
types differ only in p65 expression and are a closer genetic match than
available wild type MEFs. When the sensitivity to apoptosis induced by
genotoxic agents of the p65 null and reconstituted MEFs was compared cells
lacking p65 were resistant to both a topoisomerase inhibitor (etoposide) and
UV-irradiation, two DNA-damaging agents that activate cell death. However, reconstitution
of p65 greatly increased sensitivity to induction of apoptosis by these
agents. Apoptosis was assessed by the appearance of cells with hypodiploid DNA
content (Figure 1B) and also by activation of caspases (Figure 1C), therefore
two criteria confirmed that loss of p65 confers resistance to genotoxin-induced
apoptosis. In contrast, p65 null cells are extremely sensitive to TNFα induced apoptosis, while p65 reconstitution confers
resistance, consistent with the p65-dependent activation of anti-apoptotic
genes by TNFa [13,41-47].
Gene profiling of p65 null mefs reveals lack of noxa expression
Since p65 is a transcription factor, we reasoned that there may be
one or more genes whose expression is compromised in p65 null MEFs and whose
function is necessary for p53-dependent death, most likely controlling
cytochrome c release from mitochondria.
Therefore, we compared the expression profile of p65 null versus
p65 reconstituted cells using gene microarray analyses. Strikingly, expression
profiling revealed that one of the genes upregulated by p65 expression was
Noxa, a pro-apoptotic BH3-only protein of the Bcl-2 family [50] (Table 1). Previously, this protein was reported to be induced by
p53 and to be required for p53-induced death by controlling cytochrome c
release [38,51], making it a candidate for
immediate study. Of the other Bcl-2 family members screened none
belonged to the top most differentially regulated genes (ratio p65
reconstituted/null >1.7; Table 1). To validate the microarray data RT-PCR
assays were performed to compare the expression level of several other Bcl-2
family members and the ones not represented in the array. As shown in Figure 4A, Noxa expression was absent in p65 null cells but expression was restored by
re-introducing p65 (Figure 4A). In contrast, expression of other Bcl-2 family
member was not impaired in p65 null cells. Likewise, immunoblot analysis showed
Bax expression was not altered by the p65 status of the cells (Figure 4A). To
assess whether Noxa expression could be induced by genotoxins, levels of Noxa
mRNA from etoposide or UV-irradiation treated p65 null and reconstituted cells
were evaluated by Northern blot. Noxa mRNA could not be detected in untreated
p65 null cells but was present in the p65 reconstituted cells, consistent with
the microarray analysis (Figure 4B). Moreover, Noxa mRNA expression could be
induced by etoposide or UV-treatment in the reconstituted cells but not in the
p65 null cells (Figure 4B). Thus, p65 is necessary for Noxa expression and for
genotoxin-dependent induction of Noxa.
Table 1. Comparison of expression of several genes present in the array.
Expression of caspases is shown as constitutive genes. Average Ratio (n=5)
| P65/vector
| stdev
| Gene
| |
| 0.7
| 0.2
| Bad
| Bcl-2 family
|
| 0.6
| 0.1
| Bag1
| |
| 1.2
| 0.3
| Bag3
| |
| 0.7
| 0.1
| Bak1
| |
| 0.9
| 0.2
| Bax
| |
| 0.6
| 0.1
| Bcl2l
| |
| 1.4
| 0.5
| Bcl2l10
| |
| 1.4
| 0.5
| Bcl2l2
| |
| 1.0
| 0.1
| Biklk
| |
| 1.0
| 0.1
| Bnip2
| |
| 0.6
| 0.2
| Bnip3l
| |
| 0.8
| 0.3
| Bok
| |
| 1.9
| 0.1
| Noxa
| |
| 0.8
| 0.3
| Casp1
| Caspases
|
| 1.3
| 0.2
| Casp2
| |
| 0.8
| 0.3
| Casp3
| |
| 1.2
| 0.2
| Casp6
| |
| 1.1
| 0.2
| Casp7
| |
| 1.4
| 0.4
| Casp8
| |
| 0.7
| 0.1
| Casp9
| |
| 1.2
| 0.5
| Casp11
| |
| 0.9
| 0.4
| Casp12
| |
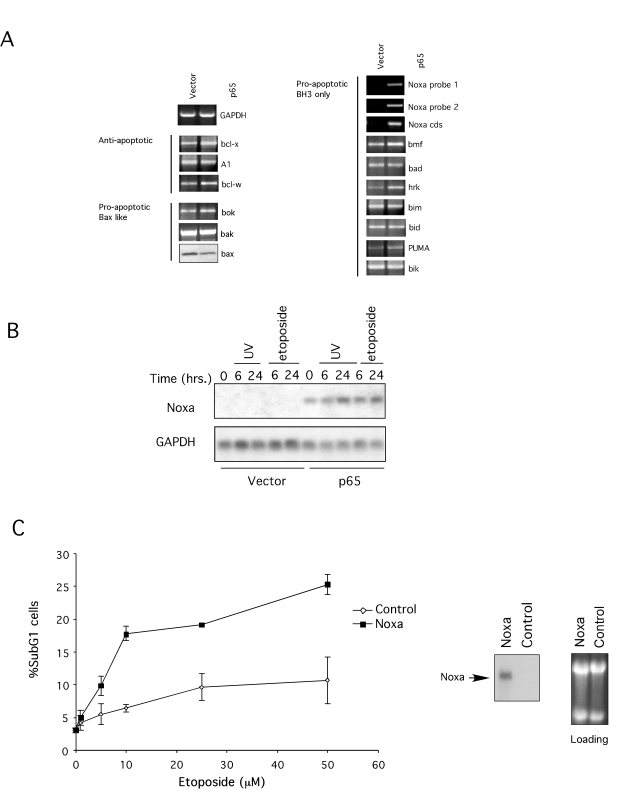
Figure 4. Expression of Bcl2 family members.
(A) RT-PCR of bcl2 family members. cDNA was prepared from total RNA
from p65 null (vector) and reconstituted cells (p65). Specific
oligonucleotides for each gene (and three pairs for Noxa) were used to determine
expression. GAPDH expression was used as a control. Bax expression was
detected by immunoblot. (B) Northern Blot for Noxa after genotoxic
treatments. Total RNA was extracted from p65 null and reconstituted cells
after treatment with 10 μM
etoposide or 5 mJ UV-irradiation for the times indicated. Expression of
Noxa, and GAPDH as control, was revealed by blotting with specific
radio-labeled probes. (C) Expression of Noxa sensitizes p65
null MEFs to genotoxic agents. Cloned murine Noxa was introduced into p65
null cells by retroviral transfer and sensitivity to etoposide and
UV-irradiation compared. Noxa cloned in the anti-sense orientation was used
as a control. After selection cells were treated with 10 μM etoposide or 5 mJ UV-irradiation for 24 hr and
apoptosis assessed by flow cytometry as described in Figure 1. Results are
representative of three different viral clones for both control and Noxa.
Northern blotting confirmed Noxa expression.
Noxa
has been shown to be necessary for p53-dependent apoptosis [38,51], however, it is not clear whether expression of Noxa
is sufficient to explain sensitivity to genotoxic agents. To address this issue
Noxa was re-introduced in p65 null cells by retroviral transfer. Expression of
the exogenous Noxa was confirmed by Northern blot analysis (Figure 4C). As
depicted in Figure 4C, reconstitution of Noxa expression was not sufficient to
promote death; however, Noxa expressing cells were more sensitive to genotoxic
treatment than control cells. Thus, expression of Noxa in p65 null cells
restored sensitivity to genotoxic agents.
p53 is mutant in p65 null and reconstituted cells
Noxa
has been shown to be necessary for p53-dependent death and its expression is
indeed induced by p53 [38,51]. MEFs
and, particularly, transformed cell lines derived from MEFs, very frequently
acquire mutations in the p53 tumour suppressor. A trivial explanation of our
data is that the p65 null cells but not the p65 reconstituted cells had
acquired a p53 mutation during serial passage and immortalization, thus
explaining the lower apoptotic sensitivity of p65 null cells to DNA damaging
agents. To test this possibility, the DNA sequence of the p53 gene from both
p65 null and reconstituted cells was compared. Both cell lines showed
identical sequence for the p53 gene, excluding the possibility that a p53
mutation would explain the observed difference in sensitivity to genotoxins.
However, these data revealed that p53 was mutant: there was a silent mutation
at codon 82 (c to t at base 246) and a missense mutation at codon 275 (c to g
at base 824) that results in a Proline to Arginine substitution. This position
corresponds to codon 278 in human p53 within the DNA binding domain, an
extremely well conserved region. This particular Pro278Arg mutation has been
found in human tumours although the functionality of this mutant had not been
previously tested. Moreover, no wild type allele was detected in our sequencing
and Southern Blotting revealed that both p65 null and reconstituted cells had
only one copy of p53 (data not shown).
To
test the function of this mutant p53 its ability to activate a reporter gene
was tested. Mutant p53 was first cloned from p65 null cells by RT-PCR. To
control for the activity of this mutant codon 275 was reverted to wild type by
site directed mutagenesis. Mutant or wild type p53 was then expressed in the
p53 null cell line SaOS-2 along with a PG13 p53-responsive reporter construct
(Figure 5A) or the Noxa promoter -183 to +146 in front of the luciferase
reporter gene construct (Figure 5B). The data clearly showed that wild type
p53 activated both the p53 reporter and the Noxa promoter reporter constructs
while P275R mutant failed to do so. Immunoblotting showed that this lack of
activity could not be explained by differences in p53 expression (Figure 5C).
Expression of a well known p53 target, p21, was also assessed by immunoblotting
and again, while wild type p53 induced expression of p21, mutant p275R failed
to do so (Figure 5C). In further experiments the ability of the P275R mutation
to interfere with wild type p53 was tested. However, no interference was
observed (data not shown) indicating that the P275R mutation, unlike some other
p53 mutations, did not generate a dominant negative p53.
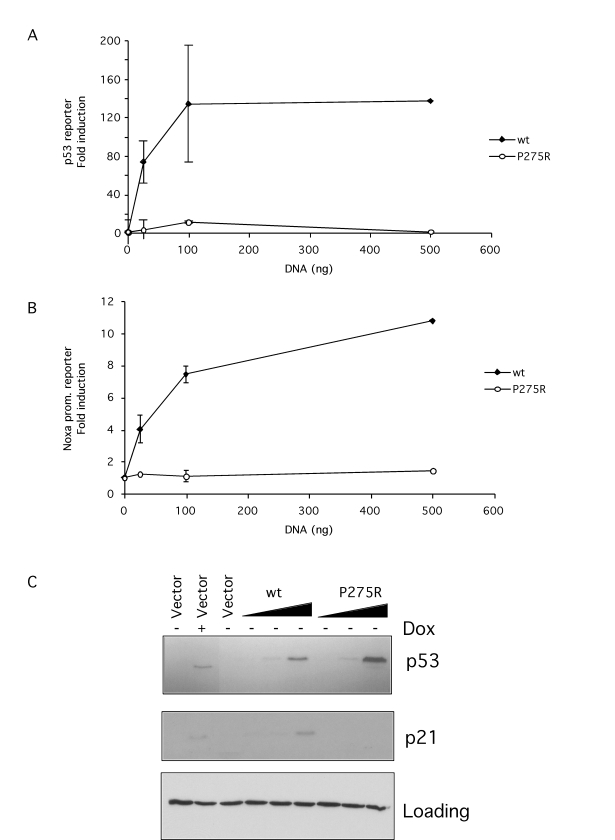
Figure 5. p53 in p65 null and reconstituted cells is a non-functional mutant. (A-B) 0.5
μg of PG13-Luc
p53 luciferase reporter (A) or Noxa promoter luciferase reporter (B) were
co-transfected into SaOS-2 cells along with increasing amounts of the wild
type or P275R mutant p53 vectors. 48 hr after transfection luciferase
activity was compared. Results are expressed as fold induction above mock
(empty pcDNA3 vector) control. (C) p53 P275R or wild type expression
was demonstrated by immunoblotting in extracts derived from SaOS-2 p53
tet-on cells transfected as described for A-B. As a control, p53 was
induced by doxocycline treatment. Endogenous p21 induction was assessed by
immunoblotting from the same extracts. A non-specific band detected with the p21 antibody was used as loading control.
Thus p53 status cannot explain the difference in sensitivity of the p65 null cells
and reconstituted. Moreover, genotoxin induced death and induction of Noxa
expression in these cells is p53 independent.
Control of Noxa expression and apoptosis induction by p73
p73 is a member of the p53 family that
has been shown to promote apoptosis and to activate p53 target genes through
the p53 elements in their promoters [52-56]. Recently, E1A
activation of p73 and induction of Noxa expression in the absence of p53 in an
osteosarcome cell line has been shown [57]. To assess whether Noxa can be induced by p73, a
reporter approach was used.
p73
expression vectors were transiently transfected into SaOS-2 cell line along
with the Noxa promoter reporter. Both p73α and p73β activated the expression of the reporter and they also activated
expression of the PG13 p53 reporter (Figure 6A). To test if p73 was activating
the Noxa promoter through the p53 element, a mutant promoter was used were the
p53 element had been eliminated. Neither p73α nor p73β activated the expression of this reporter indicating that Noxa
expression by p73 uses the p53 element in its promoter, consistent with
previous reports. As a control, p73 did not activate transcription of a NF-κB dependent reporter (Figure 6A). A p73 inducible
SaOS-2 cell line was also used to demonstrate Noxa reporter induction by p73.
Induction of p73 expression by Doxicycline indeed activated Noxa reporter but
failed to activate the mutant promoter for the p53 element (data not shown),
thus corroborating that p73 controls Noxa promoter.
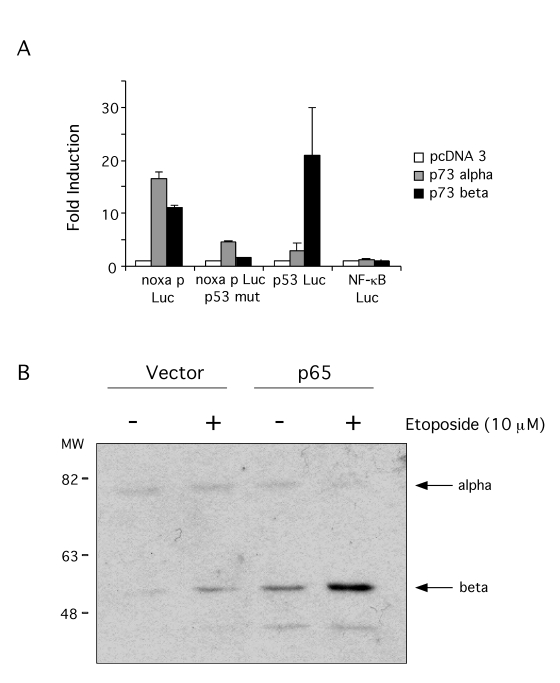
Figure 6. p73 induces Noxa promoter.
(A) SaOS-2 cells were transfected with 1 μg of pcDNA3 control vector,
p73α or p73β expression vectors
along with the following luciferase reporter plasmids: Noxa promoter
reporter, Noxa promoter p53 mutant reporter, PG13-Luc p53 reporter or
NF3TK-Luc NF-κB reporter (as control). 48 hr after transfection luciferase
activity was compared. Results are expressed as fold induction above mock
(empty pcDNA3 vector) control. (B) p73 activation in p65 null and
reconstituted cells. Cells were treated with 10μM etoposide for 24 hr
and p73 levels determined by immunoblotting with a pan-p73 antibody.
Having confirmed that p73 can control Noxa promoter, we tested next if DNA damage can
induce p73 in p65 null and reconstituted cells. Cells were treated with etoposide for 24 hr and p73 levels assessed by
immuno-blotting. This revealed the induction of a 52 kDa protein, consistent
with the p73β isoform (Figure 6B). While induction was seen in both cell types, the
levels of p73 in the p65 reconstituted cells were markedly higher than in the
p65 null cells. p73 levels in etoposide treated p65 null cells were comparable
to basal levels in the reconstituted cells, which were significantly induced
by etoposide treatment.
Dominant negative p73β blocks genotoxin-induced apoptosis and Noxa
expression
The
previous data suggest that the reason p65 null cells are less sensitive to DNA
damage is the failure to induce sufficiently high levels of p73. To test this
possibility, a dominant negative form of p73β (ΔN-p73β) was expressed in the p65 reconstituted cells by
retroviral transfer and the level of apoptosis following etoposide treatment
determined. Apoptosis induction, assessed both by hypodiploid DNA content
(Figure 7A) and caspase activation (Figure 7B), showed that dominant negative
p73β effectively blocked apoptosis. This result indicates
that p73 activation is necessary for DNA damage-induced apoptosis in this
context. Over-expression of wild type p73β in the p65 null
cells, however, did not induce apoptosis. Moreover, etoposide treatment of p65
null cells expressing wild type p73β did not cause
apoptosis (Figure 7A and B), suggesting that although necessary, p73 expression
was not sufficient for etoposide-induced apoptosis in the absence of p65.
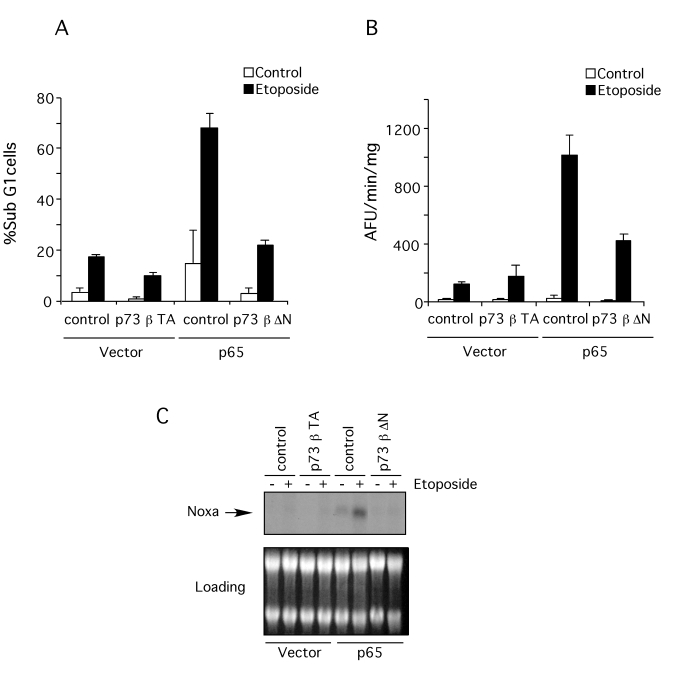
Figure 7. Dominant negative p73β blocks apoptosis induction and Noxa expression.
p73β wild type was introduced into p65 null cells and p73β dominant negative
was introduced into p65 reconstituted cells by retroviral transfer. Then
apoptosis induction and Noxa expression was assessed after treatment with
10 μM etoposide for 18
hr. (A)
Floating and attached cells were then collected and stained with propidium
iodide (PI). DNA content was analyzed by flow cytometry. Results are
presented as percentage of cells with sub-G1 DNA content. The data shown represent the mean and SEM of three
independent experiments. (B) S-100 extracts from p65 null
(vector) and reconstituted cells (p65) were used to assess caspase activity
by cleavage (arbitrary fluorescence units per minute [AFU/min]) of the
fluorogenic substrate, Ac-DEVD-afc. The data
shown represent the mean and SEM of three independent experiments. (C)
Northern Blot for Noxa expression. Total RNA was extracted from p65 null
(vector) and reconstituted (p65) cells after treatment with etoposide.
Expression of Noxa was revealed by blotting with a specific radio-labeled
probe. As loading control the ethidium bromide stained gel previous to
transfer onto membrane is shown. This blot is representative of three
independent experiments.
We used Northern blotting in order to test whether p73β controls Noxa expression in p65 null cells. As shown in Figure 7C,
over-expression of wild type p73β did not restore
Noxa expression in p65 null cells, even after etoposide treatment. However,
expression of a dominant negative form of p73β in p65
reconstituted cells successfully
blocked expression of Noxa in these cells and prevented Noxa induction by
etoposide treatment. These results are in accordance with the effect of dominant
negative p73β expression in apoptosis induction indicating that
Noxa expression is the key regulator of apoptosis induced by genotoxins in the
absence of p65.
Discussion
DNA damage-induced apoptosis has been reported to require NF-κB [15], although
which step of the apoptotic process is NF-κB
dependent and which gene(s) are involved was not known. To investigate this
issue, we characterized p65-dependent apoptosis using p65 null MEFs, which are
resistant to genotoxin-induced apoptosis. These experiments uncovered a defect
in the release of cytochrome c from mitochondria. We then performed
expression profiling to identify candidate mediators of this effect. Our
microarray analysis showed that Noxa -which acts to trigger cytochrome c
release and is a known DNA damage-induced gene- was missing in the absence of
p65. Noxa is a pro-apoptotic BH3-only member of the Bcl2 family that binds and
blocks the anti-apoptotic function of Bcl-2 protein, thus promoting cytochrome c
release from the mitochondria and death [38].
Therefore, lack of Noxa expression was likely to explain the apoptotic defect
in p65 null cells. Subsequent experiments confirmed that p65 was indeed
required for genotoxin-dependent induction of Noxa mRNA. Importantly,
re-introducing Noxa into p65 null cells sensitized them to apoptosis induced by
genotoxic agents. Interestingly, despite Noxa being
necessary for genotoxin-induced apoptosis, ectopic expression of Noxa alone did
not induce apoptosis. These findings suggest that Noxa expression alone is not
sufficient for cell death and other factors may be required.
Noxa is known to be a transcriptional target of p53, a tumour suppressor that is
activated by both oncogenes and genotoxic chemotherapeutic drugs. The experi-ments
described in this work were performed using transformed and immortalized MEFs,
which frequently acquire p53 mutations during cell culture. Clearly, our
interpretation would be invalidated if the p65 reconstituted cells acquired a
mutation that com-promised p53 but the p65 null cells did not. When we
sequenced p53 we found that both cell types had identical p53 sequence,
excluding the possibility that a differential
p53 function explained the p65 resistant phenotype. However, p53 had acquired
two mutations, a silent mutation in codon 82 and a substitution in codon 275,
from Proline to Arginine. This position is within the DNA binding domain of
p53 and corresponds to P278R in human p53, a mutation that is found in a subset
of human tumours. The effect on p53 function of this mutation was unknown. Our
analysis showed that this mutation compromised p53 ability to transactivate
target genes, including Noxa. Therefore p53 is not responsible for Noxa
induction in our cells.
p63
and p73 are proteins functionally and structurally related to p53, constituting
a family of related transcription factors. The overall structure of the three
proteins is quite similar, producing remarkably similar effects when
over-expressed in cells [58,59]. E1A
transformed MEFs deficient in both p63 and p73 are resistant to
genotoxin-induced apoptosis, even in the presence of p53, therefore suggesting
that these three genes might act together or p63/p73 act in an independent
pathway to activate DNA damage-induced apoptosis [60]. Moreover,
p73 activation is induced by a subset of DNA damaging drugs and blocking its
function with a dominant negative mutant or siRNA led to
apoptosis resistance of transformed human cell lines, irrespective
of p53 status [61].
Activation of p73 alone can induce apoptosis suggesting a pro-apoptotic role on
its own. Several of the p53 dependent genes involved in apoptosis have been
demonstrated to be significantly regulated by p73, such as Bax, DR5 and PUMA [52], and p53
binding to the promoters of its pro-apoptotic targets PERP, Noxa and Bax
required the presence of p73 and/or p63 [60]. Moreover,
Noxa was recently shown to be a p73 target to trigger E1A-induced apoptosis in
p53 deficient cells [57].
Consequently, we investigated
the role of p73 in DNA damage-induced apoptosis in the p65 null cells. Our
data showed that activation of p73 by genotoxins was compromised in the absence
of p65. By using a dominant negative p73 mutant we demonstrated that
genotoxin-induced apoptosis relies on p73 activation and importantly, that p73
activation is required for Noxa expression in our cells. We also provide, for
the first time, formal proof that Noxa is regulated by p73 at the promoter
level through the p53 element.
How
NF-κB participates in this process is unclear. The NF-κB transcription factor is widely accepted as an
anti-apoptotic factor [62] and several
anti-apoptotic genes (for review see [22]) are known to be activated by NF-κB following treatment with TNFα. Moreover, the embryonic lethality in p65 knock-out mice is caused by
extensive TNFα induced apoptosis in the liver [63]. NF-κB activation in
tumor cell lines by chemotherapy has been reported and inhibition of NF-κB activation can enhance apoptosis induced by
chemotherapy in a xenograft model of tumorigenesis [64,65]. These
are strong data consistent with an anti-apoptotic role for NF-κB. However, in other situations NF-κB appears to be pro-apoptotic [15-17,31-34,66].
As suggested by Blagosklonny [67], cellular
responses should be defined in molecular terms where the same signalling
pathways may participate in different, and often contradictory, end-points (in
our case, induction of apoptosis vs. survival). Upstream signaling is initiated
simultaneously and
the cell translates it according to cellular context. Therefore NF-κB may act as a stress response transcription factor
whose effect on a cell is context-dependent. There may also be mechanistic
differences between the pro-apoptotic activity and anti-apoptotic activity of
NF-κB; suppression of steady state but not stimulus-induced
NF-κB activity inhibits Alphavirus-induced apoptosis [68]. This is
consistent with our observations that TNFα, a well-known
activator of NF-κB through the canonical
pathway, had no effect on Noxa expression, even though a NF-κB control reporter was activated (data not shown).
In our system, the pro-apoptotic effect
of NF-κB depends on the activation of p73. How this is
accomplished is not clear. p73 activation is mediated in part by protein
stabilization, as it is for p53, since proteasome inhibitors stabilize the
protein [69]. In
contrast to p53, however, p73 degradation is not mediated by MDM2, although p73
binds to MDM2 and blocks its transcription promoting activity. p73
stabilization and activation by genotoxic stress is also associated with p73
phosphorylation. Several kinases have been implicated in this step. Thus
following γ-irradiation c-Abl phosphorylates p73 at Tyr99
activating p73 and inducing apoptosis [70,71].
Phosphorylation at Tyr120 and Tyr240 were also shown [72]. The
checkpoint kinases, CHK1 and CHK2, which are activated following DNA damage may
also play a role, controlling p73 mRNA induction [73]. Aurora Kinase A regulates p73 dependent apoptosis
in p53 deficient cell lines [74]. It is
possible that absence of p65 DNA damage fails to activate p73 because the
activity of one or all of these kinases is compromised in the absence of p65.
We showed that ectopic expression of p73β alone in the
absence of p65 was not sufficient to reinstate expression of Noxa or restore
apoptosis sensitivity. These data indicate
that the absence of p65 compromises other steps in genotoxin-induced apoptosis
in addition to p73. The simplest model is that p65 is required for the
DNA-damage induced signalling pathways upstream of p73.
Since
inhibition of NF-κB is currently being
explored as a way of potentiating anti-cancer therapy [21,75] it is
essential to define specifically where and when NF-κB shows a preferentially pro- or anti-apoptotic face.
The observation that NF-κB controls expression of
Noxa in the absence of functional p53, and loss of p53 function occurs in
>50% of human tumours, suggests that in some contexts inhibition of NF-κB may compromise, rather than enhance, the efficacy of
conventional anti-cancer therapy.
Materials and methods
Plasmids,
reagents and antibodies.
Murine p65
was cloned into the EcoRI site in the retroviral vector pWZL-Hygro. Murine
Noxa was cloned from cDNA made from p65 reconstituted MEFs as a HA tagged
fusion gene into the BamHI/EcoRI site of pcDNA3.1+ and the retroviral vectors
pWZL-Blast and pBabe-Puro. Mutant P275R p53 was obtained by RT-PCR from
immortalized p65 null cells and cloned directly into pcDNA3-TOPO (Invitrogen).
Wild type p53 was generated by reverting the P275R mutation using the
Quick-change site directed mutagenesis kit (Stratagene) following
manufacturer's instructions. Expression vectors for p73α, DN-p73α, p73β and DN-p73β were kindly provided by Prof. G. Melino (University of Leicester, UK) and were subcloned into pWZLBlast retroviral vector. Recombinant mouse tumor necrosis factor α (TNFα) and etoposide were
purchased from Sigma. For immunoblotting we used antibodies against human Noxa
(Imgenex), Bax, caspase 2, p21 (Santa Cruz Biotechnology), anti-HA (kindly
provided by Dr P. Kaldis, NCI-Frederick, Frederick, MD), Apaf-1 (Alexis),
cytochrome c (BD-Pharmingen), β-actin (Abcam), XIAP (BD-Transduction
Laboratories), p53 (supernatant from culture of the DO-1 hybridoma), p73
(provided by Prof. G. Melino, University of Leicester, UK) and p65 (kindly
provided by Dr N. Rice, NCI-Frederick, Frederick, MD).
Cells,
transfection and retroviral gene transfer.
Mouse fibroblasts and human cancer cells were grown in Dulbecco's
modified minimal essential medium (DMEM, Gibco) supplemented with 10% fetal
bovine serum (FBS, Gibco) at 37 °C in 10% CO2. Cells were transfected with the Lipofectamine Plus reagent
(Gibco) in accordance with the manufacturer's instructions and washed after 3
hr incubation before adding fresh DMEM+10% FBS and incubating for a further 18
hr. For retoviral transfer, viral vectors were transfected into the Phi-NX
ecotropic packaging cell line and after 24 hr the transfection culture medium
was filtered and added to p65 null MEFs. Infected cells were selected using
the appropriate antibiotic.
Electro-Mobility
Shift Assay (EMSA).
Preparation of
nuclear extracts was previously described [35]. The
binding reaction consisted of 10 μg of extracted nuclear protein and 5 μg of
poly dI-dC (Roche) in a total reaction volume of 10 μl containing 6 mM MgCl2.
This mixture was then incubated at room temperature for 10 minutes, after which
2 μl (50,000 cpm) of the NF-κB consensus oligonucleotide (Promega), end-labeled with [32P]-γ-ATP (specific activity = 3,000 Ci/mmol; Amersham), was added. A
control reaction mixture containing a 100-fold molar excess of non-radioactive
NF-κB oligonucleotide was used to verify the specificity
of the binding reaction. After incubation at 4˚C for 15 minutes the
reaction mixtures were run on a 5% PAGE. After drying, the gels were subjected
to autoradiography.
Extract
preparation and caspase activity assay.
2 x 108 p65 and reconstituted MEFs were used to prepare S-100
extracts as described [36]. Briefly,
cells were harvested by trypsinization and washed in PBS. Cells were
resuspended in 10 ml of extract buffer (50 mM PIPES, pH 7.0, 50 mM KCl, 5 mM
EGTA, 2 mM MgCl2, 1 mM DTT, 2 μg/ml each of Leupeptin, Chymostatin,
Antipain and Pepstatin A, 10 μg/ml Cytochalasin B and 100 μM PMSF), centrifuged
and excess buffer immediately removed. Cells were then lysed by three
freeze-thaw cycles in liquid nitrogen and centrifuged at 100,000 x g for
60 minutes to obtain an S-100 extract (≈30 mg/ml protein by Bradford assay). For caspase activity assessment, 30 μg of cell extract
were used to determine conversion of the fluorogenic
caspase substrate Ac-DEVD-afc (Biomol). For caspase activation, equine
cytochrome c (1 μM;
Sigma) was added and extracts incubated at 37 ˚C for 60 minutes with 1 mM
ATP as indicated. After this time caspase activity was determined using a Cytoflour 2000 flourimeter.
Cytochrome c
immuno-localization.
p65 null and
reconstituted MEFs were grown on glass coverslips prior to treatment with
etoposide (10 μM) or UV-irradiation (5
mJ). 18 hr later cells were fixed in 2% formaldehyde and permeabilized with
0.2% Triton. Fixed cells were incubated with an anti-native cytochrome c antibody.
A secondary antibody coupled to Alexa Green (Molecular Probes) was used to
detect cytochrome c.
Flow cytometry.
Floating cells were recovered and pooled
with adherent cells harvested by trypsinization. Cells were resuspended in PBS
containing 1% Triton, 50 μg/ml propidium iodide, and 100 μg/ml RNase A and
stained for 30 minutes. After this time the percentage of cells with sub-G1
DNA content was determined by flow cytometry.
Microarray
analysis.
Total mRNA from p65 null
and reconstituted MEFs was amplified and labeled with Cyanine 3 (Cy3) or
Cyanine 5 (Cy5) dUTP, essentially as described [37] and used
for microarray hybridization onto cDNA microarrays. These arrays were
manufactured at the NCI Microarray Facility (Frederick, MD) by spotting UniGene
mouse cDNA clones (Incyte Genomics) onto glass slides. Data was collected on an
Axon scanner where Cy3 and Cy5 fluorescence was measured and compared. Results
were expressed as ratio of Cy3 to Cy5 for each experiment. The data shown are
averages from 5 independent arrays.
RT-PCR and Northern Blot.
Total RNA from p65 null and reconstituted
MEFs was obtained using Trizol (Gibco BRL) following the manufacturer's
instructions. For RT-PCR 1 μg of RNA
was used to generate cDNA using the GeneAmp RNA PCR kit (Perkin Elmer) which
was then used to amplify the corresponding genes with specific
oligonucleotides. The coding sequence amplified for each gene were; caspase-3,
62-772; caspase-9, 205-1264; GAPDH, 339-865; Bcl-X, 122-487; A1, 133-387,
Bcl-w, 65-503, Bok, 50-482; Bak, 31-585, Noxa(CDS), 1-312; Noxa (probe 1),
1-1040; Noxa (probe 2) 1230-1832; Bmf, 81-434; Bad, 109-366; Hrk, 1-242; Bim,
33-306; Bid, 32-292; PUMA, 85-485; and Bik, 120-399.
For Northern blot 10 μg of RNA
was loaded per lane onto a 1% agarose-formaldehyde gel. The RNA was transferred
to Hybond-N+ membranes (Amersham Pharmacia) and hybridized with [32P]-labeled
cDNA probes using ExpressHyb Hybridization solution (Clontech) following the
manufacturer's instructions. The Noxa probe was generated as a PCR fragment
from the mRNA extending from 1 to 1040. PUMA probe was generated by PCR
amplification of the 85-485 fragment of mouse mRNA. GAPDH probe was purchased
from SeeGene.
Luciferase
assays.
For reporter
assays, Saos-2 p53 Tet-on cells were transfected with 0.5 μg of luciferase reporter and varying amounts
of the appropriate expression vector using Lipofectamine Plus (Invitrogen)
according to manufacturer's instructions. The Noxa reporter was made placing
the -183 to +149 (SacII/SacII) fragment of the murine Noxa promoter [38] in the SmaI site in pGL3-Basic
(Promega). PG13-Luc, containing a generic p53 response element [39] and NF3TK-Luc, containing a trimer of the
NF-kB site in the H2-k promoter [40], were also used. Cells were harvested 48
hr after transfection and luciferase activity was measured in duplicate with
the Optocomp II luminometer (MGM Instruments) using 20 μl cell lysate, 100 μl substrate injection and 10
second count time. Results are expressed as fold induction above control.