Dual targeting of the antagonistic pathways mediated by Sirt1 and TXNIP as a putative approach to enhance the efficacy of anti-aging interventions
Abstract
The organism's ability to regulate oxidative stress and metabolism is well recognized as a major determinant of longevity. While much research interest in this area is directed towards the study of genes that inhibit oxidative stress and/or improve metabolism, contribution to the aging process of genes with antagonistic effects on these two pathways is still less understood. The present study investigated the respective roles of the histone deacetylase Sirt1 and the thioredoxin binding protein TXNIP, two genes with opposite effects on oxidative stress and metabolism, in mediating the action of putative anti-aging interventions. Experiments were carried out in vitro and in vivo to determine the effect of proven, limited calorie availability, and unproven, resveratrol and dehydroepiandrosterone (DHEA), on the expression of Sirt1 and TXNIP. The results indicated that limited calorie availability consistently inhibited TXNIP in cancer and in normal cells including stem cells, however, it only slightly induced Sirt1expression in cancer cells. In contrast, resveratrol had a biphasic effect, and DHEA inhibited the expression of these two genes in a tissue specific manner, both in vitro and in vivo. Whereas all the three approaches tested inhibited TXNIP through the glycolytic pathway, DHEA acted by inhibiting G6PD and resveratrol through the activation of AMPK. In light of previous reports that Sirt1 induces AMPK-mediated signaling pathway, our findings point to the possibility of a negative relationship between Sirt1 and TXNIP that, if validated, can be exploited to improve the efficacy of putative anti-aging interventions.
Introduction
Manipulation
of metabolism and resistance to oxidative stress has been shown to promote
longevity of small organisms [1,2], and to
some extent, these same mechanisms appear to act also in mammals despite
considerable divergence during evolution [1]. One of the
most described genes that inhibit oxidative stress and improve glucose
metabolism is the histone deacetylase Sirt1 (for Silent information regulator
T1). Evidence has been provided recently that Sirt1
negatively regulates oxidative stress [3-5] and
protects cells against damage induced
by H2O2, UV radiation, chemicals, and high caloric intake
[6,7].
In addition, this gene was found to regulate cellular metabolism through the
stimulation of glucose uptake [8,9] and
insulin secretion [10-12], making
it a promising target of putative anti-aging interventions [6,10,13].
Little is known however about the contribution to the
aging process of genes that act, in an opposite manner to Sirt1, to induce
oxidative stress and disrupt glucose metabolism. An example of such genes is
the thioredoxin interacting protein (TXNIP) [14-16]. Early
reports have demonstrated that TXNIP enhances cellular susceptibility to
oxidative stress through direct interaction with the anti-stress enzyme,
thioredoxin, and inhibition of its detoxifying functions [17].
Over-expression of this gene was postulated to cause the accumulation of reactive
oxygen species and apoptotic cell death [18,19]. More
recently, evidence was provided that TXNIP may also act as a mediator of
cellular metabolism. For instance, this gene was found to mediate
glucose-induced apoptotic death in pancreatic beta cells, suggesting a
causative relationship between TXNIP and type 2 diabetes [20,21]. In
support of this concept, TXNIP deficiency improved glucose uptake and
attenuated diabetic symptoms in animal models [16,22,23].
Also, and as might be expected from these findings, an inverse correlation
between TXNIP expression and longevity was recently reported [24-26]
supporting the notion that this gene may act to negatively regulate the aging
process.
Taking into account this antagonistic relationship
between Sirt1 and TXNIP regarding their effects on oxidative stress and
metabolism (Figure 1), it is important to determine if, and how putative
anti-aging approaches would affect the function of these two genes, and whether
a regulatory relationship might exist between them. To address these possibilities, the
present study investigated the effects of limited glucose availability (used
here to mimic the effects of calorie restriction), the Sirt1 activator
(resveratrol), and the hormone dehydroepiandrosterone (DHEA), on expression of
Sirt1 and TXNIP in different cellular systems, including cancer, stem cells and
a mice model. A potential link between these two genes and the underlying
mechanisms leading to their regulation were also investigated. Our results
indicated that each of the treatment approaches tested exerted effects on the
expression of Sirt1 and TXNIP, however to various degrees. Interestingly
limited glucose availability was the only approach that consistently reduced
TXNIP expression in all the systems studied. Resveratrol and DHEA exerted
inhibitory effects on TXNIP in a tissue specific manner, in addition, they were
found to act on separate branches of the glycolytic pathway mediated
respectively by AMPK and Glucose 6 phosphate dehydrogenase respectively.
Overall, our findings suggest that dual targeting of antagonistic pathways
implicated in the aging process must be considered in the design of anti-aging
strategies. They also shed light on TXNIP as a downstream target of Sirt1 and
therefore as a potential marker to predict or to evaluate the efficacy of
putative anti-aging therapeutics.
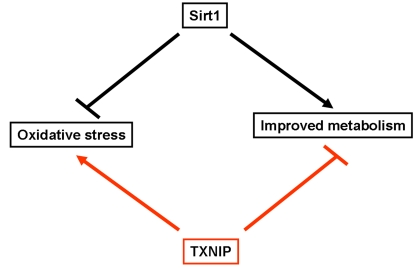
Figure 1. Schematic representation highlighting the opposite effects of Sirt1 and TXNIP on oxidative stress and metabolism. Sirt1 has been shown to inhibit
oxidative stress and improves glucose uptake as well as insulin secretion.
In contrast, TXNIP was found to enhance cellular susceptibility to
oxidative stress and inhibit glucose uptake and insulin secretion.
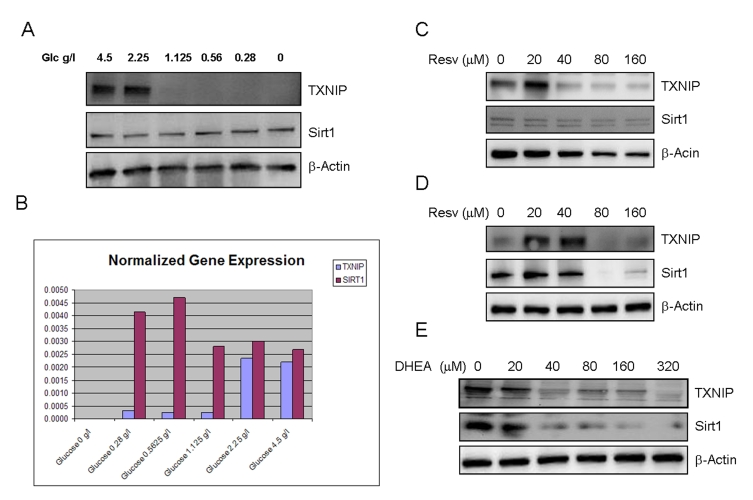
Figure 2. Effects of limited glucose availability, resveratrol and DHEA on expression of TXNIP and Sirt1 in cancer cells. Panel A.
SaOS2 cells were incubated in the presence of reduced glucose levels in the
culture medium for 48 hours. Expression of TXNIP and Sirt1 were determined
by Western blot using specific antibodies. Antibody to β-actin was used
as a loading control. Panel B. Respective expression of TXNIP and
Sirt1 in response to limited glucose availability, measured by quantitative
real time PCR (Q-PCR). SaOS2 cells were incubated in the presence of the
indicated amounts of glucose for 48 hours, QPCR was then performed to
detect expression of TXNIP and Sirt1 using specific primers. Panel C
and D, the RGC (panel C) and SaOS2 (panel D) cells
were subjected to treatment with
increasing concentrations of resveratrol (Resv.) for 48 hours followed by
Western blot as described in Panel A. Panel E, SaOS2 cells
were treated with increasing amounts of DHEA and probed for expression of
Sirt1 and TXNIP, β-actin was used as a loading control.
Results
Respective effects of limited glucose availability,
resveratrol and DHEA on expression of Sirt1 and TXNIP in cancer cells
The effects of limited glucose availability
(used here to mimic the effect of calorie restriction), resveratrol (an
activator of Sirt1) and DHEA (used for hormone replacement therapy), on the
expression of TXNIP and Sirt1 were determined by Western blot using specific
antibodies to the corresponding proteins (Figure 2). The data shows that
cellular incubation with decreasing concentrations of glucose resulted in a
dramatic loss of TXNIP expression (Figure 2A). Similar results were observed by
using other cancer cell lines (Supplemental Data 1). Limited glucose
availability was also found to cause a slight increase in the expression of
Sirt1 (Figure 2A), supporting the notion that this gene acts as a mediator of caloric restriction [27]. PCR
analysis (Figure 2B) confirmed the previous findings and suggests that altered
expression of TXNIP and Sirt1 in response to glucose deprivation occurred at
the transcriptional level.
Curiously, resveratrol had a biphasic effect on the
expression of TXNIP, with a stimulatory action at low concentrations and
inhibition at higher ones (Figure 2C and 2D). Even more intriguing is the
finding that resveratrol suppressed the expression of its own target, Sirt1, in
both RGC cells (Figure 2C) and SaOS2 cells (Figure 2D), at the same
concentrations that inhibit TXNIP. These data suggest that regardless of the
concentration used, resveratrol may exert unwanted effects. At low
concentrations, it induces the expression of TXNIP, a potential antagonist with
regards to Sirt1 effect oxidative stress and metabolism, and at high
concentrations, it inhibits expression of its own target. Based on this, the
dosage at which resveratrol is administered should be carefully determined in
order to achieving beneficial effects of this molecule.
Dehydroepiandrosterone, DHEA is a hormone
thought to play a role in aging based on the observation that its levels
decline dramatically with age to reach values of 30 to 20 % at 70 ~80 years [28]. Although
some studies have suggested that replacement of this hormone at later age may have
beneficial effects on illnesses such as atherosclerosis [29], autoimmune
diseases [30], obesity [31], and
neurodegeneration [32], many
studies failed to demonstrate any beneficial effect of this hormone on aging.
At the molecular level, DHEA was shown to improve glucose uptake [33] and reduce
the formation of reactive oxygen species [34], however,
the putative roles of Sirt1 or TXNIP in mediating these actions are not known.
Here we show that DHEA acts as a dual regulator of these two genes (Figure 2E).
Exposure to increasing concentrations of this hormone resulted in decreased
expression of both TXNIP and Sirt1. While the inhibition of TXNIP by this
hormone is desirable, that of Sirt1 is not, suggesting that the anti-aging
effects of DHEA, if any, would be hindered by its inhibitory effect on Sirt1
and related pathways.
Overall, the findings presented in Figure 2 indicate
that reduced calorie intake would rank the best among three approaches tested in this system. In contrast,
suppression of Sirt1 by both resveratrol and DHEA may represent a major
limitation to their potential use as anti-aging therapeutics. Nevertheless, a
proper understanding of how these two treatments inhibit Sirt1 will help in the
development of approaches to avoid this "side effect" and improve their
efficacy.
Expression of Sirt1 and TXNIP in normal cells
In order to obtain information on the
physiological relevance of targeting Sirt1 and TXNIP in normal tissues, we
investigated the effect of limited glucose availability, resveratrol and DHEA
in differentiated aortic smooth muscle cells, and non-differentiated, human
embryonic stem cells. As shown in Figure 3A, limited glucose availability
inhibited TXNIP expression in smooth muscle cells, without any significant
effect on Sirt1. Similar effects were also observed with resveratrol which
inhibited the expression of TXNIP in a dose dependent manner. DHEA was without
effect on any of the two genes (Figure 3A), raising the possibility that the
glycolytic pathway targeted by this hormone may be altered in normal versus
cancer cells. Of note, no significant changes in Sirt1 expression was
observed in cells treated with any of the approaches tested, further confirming
the difference in response to these treatment between normal and cancer cells.
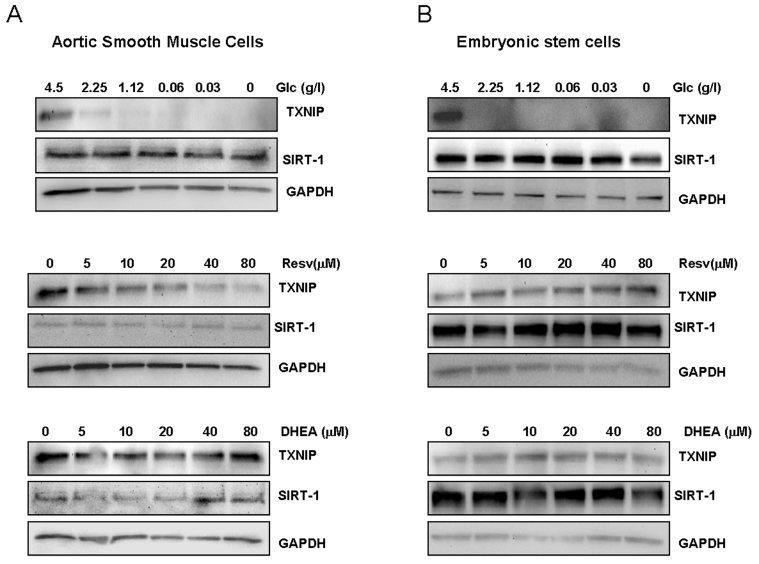
Figure 3. Effects of limited glucose availability, resveratrol and DHEA on expression of TXNIP and Sirt1 in normal cells.
Aortic smooth muscle cells (panel A) and human embryonic stem cells (panel B)
were treated as described in Figure 1 for cancer cells. Expression Sirt1
and TXNIP were analyzed by Western blot using specific antibodies. Antibody
to GAPDH was used as loading control. (Glc: glucose., Resv: Resveratrol).
To test whether cellular
proliferative capacity may account for this difference, we measured expression
of Sirt1 and TXNIP in embryonic stem cells known for rapid self renewal
ability. The human embryonic stem cells,BG01V, were subjected to glucose
restriction or treatment with either resveratrol or DHEA under similar conditions
to those described in Figure 2. As shown in Figure 3B, both Sirt1 and TXNIP
were found to be expressed in human embryonic stem cells; however, none of
these genes was affected by resveratrol or DHEA. In contrast, limited glucose
availability strongly inhibited TXNIP with no significant effect on Sirt1
expression, mirroring its action on cancer (Figure 2) and smooth muscle cells
(Figure 3A). Taken together, these findings suggest that the pathways targeted
by resveratrol and DHEA may be altered in cancer versus normal cells, and that
the effects of these two treatment may be tissue specific. In contrast, the
consistent inhibition of TXNIP by limited glucose availability in cancer cells
as well as in differentiated smooth muscle cells and non-differentia-ted stem
cells, is indicative of a potential role of this gene in mediating the
anti-aging action of calorie restriction.
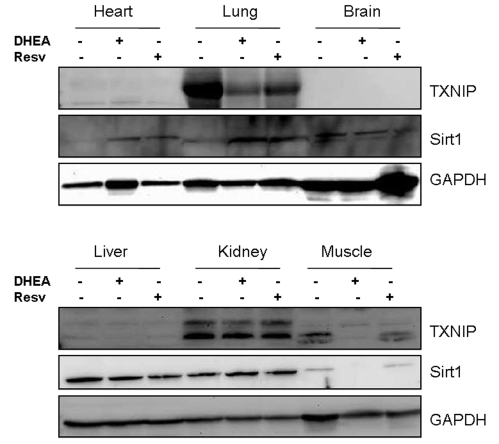
Figure 4. Tissue distribution of Sirt1 and TXNIP in mice and their regulation by resveratrol and DHEA. Mice CD1 strain were injected (i.p.)
with 10 mg/Kg of either resveratrol or DHEA and after 2 days, the
animals were sacrificed and organs harvested and processed by Western blot
for the expression of Sirt1 and TXNIP. Staining with GAPDH was used as a
loading control.
Effect
of resveratrol and DHEA on expression of Sirt1 and TXNIP in vivo
In
order to determine whether the observed effects in vitro could be also
valid in vivo, we have subjected the nude mice (CD1 strain, Charles
River) to treatment with either resveratrol or DHEA (10 mg/Kg each) for 2 days.
The animals were then sacrificed and major organs harvested to analyze
expression of Sirt1 and TXNIP by Western blot. As shown in Figure 4, the
relative expression of these two genes was tissue specific. While Sirt1 is
expressed in most organs tested; TXNIP was found to be expressed mainly in the
lung, kidney and the muscle. Both resveratrol and DHEA inhibited the expression
of TXNIP in the lung, however only DHEA had an inhibitory effect in the muscle.
Sirt1 was not significantly affect in most tissues except in the muscle of
animals treated with DHEA. Taken together, these findings suggest that
resveratrol and DHEA are capable of regulating the expression of TXNIP and
Sirt1, however in a tissue-specific manner.
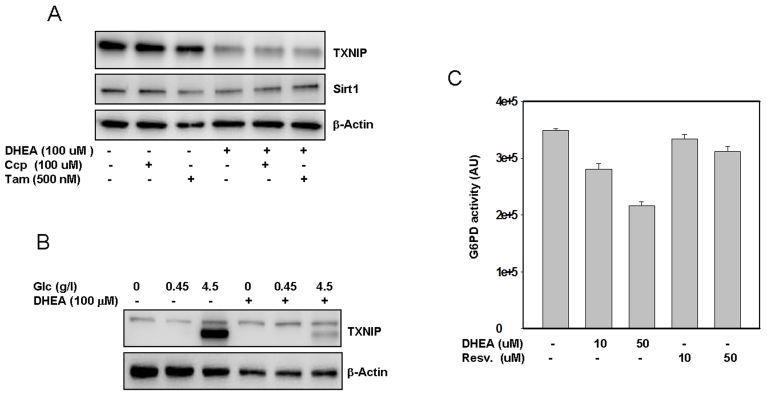
Figure 5. Putative mechanism(s) by which DHEA inhibits TXNIP. SaOS2 (panel A)
were pre-incubated with Tamoxifen or CCP for one hour prior to addition of
DHEA. After an additional incubation for 48 hours, proteins were extracted
and probed by western blot for the expression of TXNIP and Sirt1. Panel B,
The cells were treated with different concentrations of glucose with or
without DHEA. After 48 hours in cultures, proteins were separated by
electrophoresis and probed for TXNIP. Staining with β-actin in
antibody was used as a loading control. Panel C, Effect of DHEA and
resveratrol on G6PD activity. Protein extract (100 μg) was incubated
with DHEA or resveratrol at the indicated concentrations, after 30 min at
37°C, the activity of G6PD was measured as described in the methods
section. Data represent average of four determinations +/- SE.
Putative mechanisms by which DHEA and resveratrol
regulate TXNIP and Sirt1
The observation that TXNIP was consistently inhibited
by all the treatments used in this study suggests that the signaling pathway
leading to its expression may be a common target of these approaches. To define
this pathway, we first investigated the implication of known targets of DHEA in
mediating the effect of this hormone on TXNIP. Previous reports indicated that
at the nuclear level, this hormone acts either through the peroxisome
proliferator activated receptor alpha, pregnane X receptor, or the estrogen
receptor [35]. At the
plasma membrane, NMDA receptors and G-proteins were postulated to mediate the
non-genomic action of DHEA [36,37]. In the
cytoplasm, DHEA was found to act as a non-competitive inhibitor of t he glucose
6 phosphate dehydrogenase [38], a key
glycolytic enzyme with a potential role in regulation of TXNIP.
However, a putative role of these pathways in the regulation of TXNIP and/or of
Sirt1 has not been described.
After subjecting SaOS2 cells to treatment with either
an NMDA antagonist 3-[(+/-)-2-carboxypiperazin-4-yl] propyl-1-phosphonic acid
(CPP), or an anti-estrogen drug (Tamoxifen), followed by cellular exposure to
DHEA, the results (Figure 5A) indicate that neither CCP nor Tamoxifen reversed
the inhibitory action of DHEA on TXNIP. Tamoxifen only partially reversed the
effect of DHEA on Sirt1. Similar results were also obtained by using the RGC
cells (Supplemental data 2), suggesting that these pathways may not play an
important role in mediating the action of DHEA on TXNIP at least.
Comparative effects of DHEA and resveratrol on
activation of the glucose 6 phosphate dehydrogenase
Independently of the putative mechanisms described above,
we have observed that cellular pretreatment with DHEA almost completely
blocked the effects of glucose on TXNIP expression (Figure 5B), suggesting that
this hormone may act, at least in part, by interfering with the glycolityc
pathways that regulate TXNIP expression. Since DHEA has also been shown to
inhibit the glucose 6 phosphate dehydrogenase, G6PD [38], we
investigated whether this possibility may account for the observed effects of
this hormone on TXNIP. The results presented in Figure 5C show that the
activity of G6PD was indeed strongly inhibited and in a dose dependent manner
by DHEA. Of note, since this in vitro assay is based on the production
of NADPH (half of which is produced by G6PD and the rest by other enzymes such
as Malic acid dehydrogenase and isocitrate dehydrogenase [39], the data (Figure 5C) suggest that the inhibitory effect of DHEA on G6PD could be much higher
than the 50% observed. Resveratrol has no effect on G6PD (Figure 5C) suggesting
that it may act through a different pathway to regulate TXNIP.
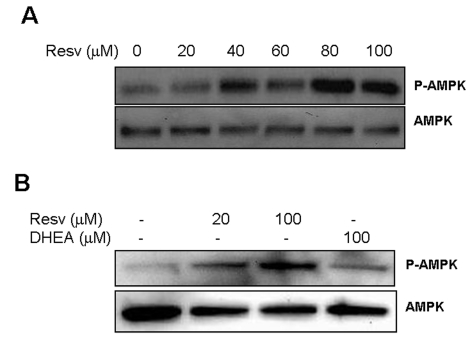
Figure 6. Effect of Resveratrol and DHEA on phosphorylation of AMPK. Panel A,
SaOS2 cells were treated with increasing concentrations of resveratrol for
48 hours, after what, proteins were extracted and probed either with
anti-phospho-AMPK (Thr172), or with antibody to AMPK. Panel B, RGC
cells were incubated with 20 or 100 μM
resveratrol or DHEA 100 μM
for 48 hours, and then probed for phosphor-AMPK and AMPK as described
above.
Comparative effects of DHEA and resveratrol on
AMP-activated protein kinase
Recent investigations of the mechanisms by which
resveratrol improves metabolism revealed that it induces phosphorylation of the
AMP-mediated protein kinase (AMPK) [40], an enzyme
that phosphorylates and inactivates the carbohydrate response element binding
protein ChREB. However, the relevance of this action to the regulation of TXNIP
by resveratrol and the possible implication of DHEA in regulating this pathway
are not yet investigated. The results presented in Figure 6A show that
resveratrol readily activates this enzyme (p-AMPK) in the retinal glial cells
(RGC) without affecting expression of the corresponding gene (AMPK). Resveratrol
also induced AMPK phosphorylation in the osteosarcoma cells SaOS2 (Figure 6B),
however DHEA was without effect. These findings, in addition to those presented
in Figure 5D, suggest that, although both molecules inhibit TXNIP, DHEA and
resveratrol act through separate branches of the glycolytic pathway mediated by
G6PD and AMPK respectively. Also, in light of the recent finding that Sirt1 is
able to activate AMPK [41], our data
suggest that TXNIP may be regulated by Sirt1 through this pathway (Figure 7).
Further confirmation of this link will help establish TXNIP inhibition as an
important determinant for evaluating the efficacy of putative anti-aging
interventions. The finding that limited glucose availability is the only
approach that consistently inhibited expression of TXNIP in the all the systems
used in this study, is in agreement with this concept and suggests that,
regardless of Sirt1 levels, decreased expression of TXNIP may have beneficial
effects for delaying the aging process.
Discussion
The main purpose of this study was to direct attention
to the fact that antagonistic pathways implicated in the aging process must be
simultaneously targeted for optimal anti-aging effects. As a proof of principle
for this concept, we have chosen to analyze the regulation of Sirt1 and TXNIP
in response to proven and unproven approaches thought to affect aging. Due to
the antagonistic roles of these two genes in mediating the pathways that
regulate oxidative stress and metabolism, we hypothesized that approaches that
induce Sirt1 and inhibit TXNIP expression would be the most desirable. By
comparing the effect of limited glucose availability, resveratrol and DHEA in
this system (Figure 2), only limited glucose availability led to the expected
results (induction of Sirt1 and inhibition of TXNIP), providing further support
for the already established notion that calorie restriction is so far the only
proven approach that consistently increase life span. In contrast, we have found
that depending on the dosage and the cell type used, resveratrol produced the
unwanted effects of either inducing TXNIP or inhibiting the expression of its
own target, Sirt1 (Figure 2C and 2D). Based on this, optimal beneficial effects
of resveratrol against aging-associated diseases would necessitate proper understanding
of how this compound induces TXNIP at low doses and inhibits its own target at
higher ones, a knowledge that will then be useful for the development of
strategies to avoid these unwanted effects. In this regards, we have shown that
AMPK is phosphorylated at Thr172 by resveratrol (Figure 6), and in light of
previous findings that AMPK is activated by phosphorylation at Thr172 and
inhibited by phosphorylation at 485/491 [42], one could speculate that resveratrol may induce phosphorylation at
485/491 at low concentrations and at Thr172 at higher ones. This, and the
mechanism(s) by which resveratrol inhibits the expression of its own target
Sirt1, warrants further investigations to determine the conditions under which this compound should be administered without
negatively influencing its own beneficial effects.
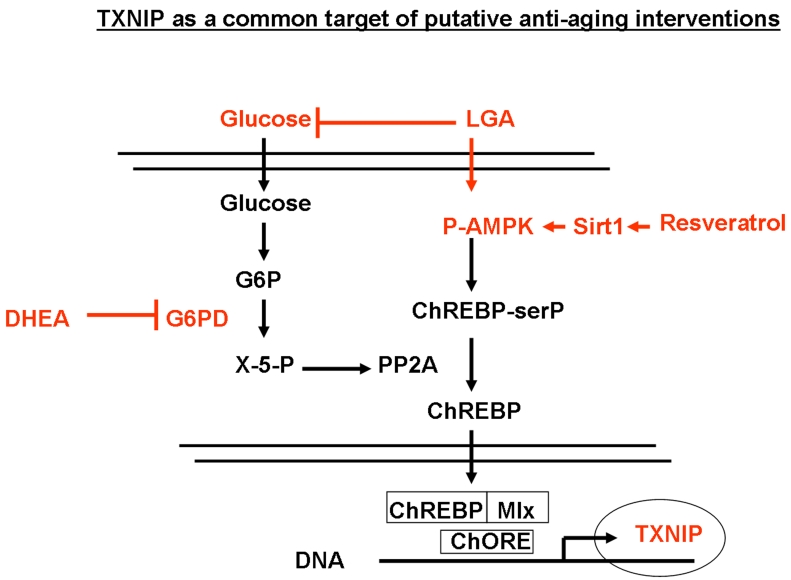
Figure 7. Regulation of TXNIP through the glycolitic pathway and its modulation by limited glucose availability (LGA), resveratrol and DHEA. Down regulation
of glucose levels is known to increase the AMP/ATP ratio which in turn
activates AMPK, leading to increased serine phosphorylation of ChREBP.
Phosphorylated ChREBP is unable to translocate into the nucleus and form a
functional complex with Mlx that is required for TXNIP expression. In
addition, reduced glucose levels would inhibit, the pentose pathway and
lead to the inhibition of the phosphatase PP2A. This will also result in
the accumulation of phosphorylated ChREBP in the cytoplasm and further
inhibition of TXNIP expression. DHEA acts on this glycolytic pathway mainly
through the inhibition of G6PD activity. Resveratrol would act through the
induction of Sirt1-mediated phosphorylation of AMPK, leading to enhanced
phosphorylation ChREBP and inhibition of its nuclear translocation. This
mechanism sheds light on TXNIP as a common downstream target for putative
anti-aging interventions that affect metabolism.
Concerning the action of DHEA,
the finding that this hormone inhibited, in a dose dependent manner, the
expression of both Sirt1 and TXNIP puts it in an unfavorable position regarding
its claimed beneficial effects against aging. Our findings rather provide an
explanation for the controversy surrounding the use of DHEA in hormone
replacement therapy for older population. However, since this hormone has at
least one beneficial effect which is the inhibition of TXNIP, understanding
the mechanism by which it inhibits Sirt1 may be helpful in the design of
strategies to prevent this unwanted effect, and open new avenues for its use
as an anti-aging therapeutic. In this regard, our results point to the
possibility that the estrogen receptor could be a potential mediator of the
undesirable effects of DHEA on Sirt1 (Figure 5A), and suggest that a
combination of DHEA with an estrogen receptor antagonist (i.e. Tamoxifen) may
improve the anti-aging action of this hormone.
While the effects of limited glucose
deprivation were consistently similar in cancer and normal cells, those of
resveratrol and DHEA were surprisingly different. In vitro experiments
indicated that resveratrol but not DHEA affected TXNIP expression in aortic
SMCs (Figure 3A), however, none of these two treatments altered the expression
of this gene in embryonic stem cells (Figure 3B). In vivo the effects of
resveratrol and DHEA were rather tissue specific (Figure 4) as they were
limited mainly to the lung and muscle among all the organs tested. By
comparison with the findings made in Figure 2 using cancer cells, the latter
results suggest that the signaling pathways that regulate expression of Sirt1
and TXNIP may be altered in cancer versus normal tissues, particularly in stem
cells where only limited glucose availability was able to affect the expression
of TXNIP.
The interesting finding that TXNIP was
inhibited by all the treatments approaches tested in this study prompted us to
investigate the underlying mechanism. This led to the observation that both
DHEA and resveratrol utilize the glycolytic pathway to inhibit TXNIP, however,
they acted through two separate pathways mediated respectively by the
inhibition of G6PDH (Figure 5) and the activation of AMPK (Figure 6). As shown
in Figure 7, expression of TXNIP appeared to be regulated, at least in part, by
Sirt1 through activation of the AMPK, which in turn signals for the inhibition
of nuclear translocation of ChREB and its association with Mlx1 [43]. This, in
light of the recent findings that Sirt1 enhances the function of FOXO1α[44] and that
the latter inhibits expression of TXNIP [26], is
indicative of a negative regulatory relationship between Sirt1 and TXNIP
through this pathway. Based on this, and due to its position far downstream of
the glycolytic pathway regulated by Sirt1, TXNIP may represent putative target
to mimic the effects of caloric restriction in a Sirt1-dependend or even
independent manner.
In broader terms, our findings provided
evidence for the concept that the development of strategies to delay the aging
process must take into account the presence of antagonistic pathways such as
those mediated by Sirt1 and TXNIP on oxidative stress and metabolism. The
observation that TXNIP acts as a downstream mediator of Sirt1 and a common
target of various effectors of the glycolytic pathway, suggests that decreased
expression of TXNIP alone may prove to be a reliable marker to evaluate and/or
to predict the efficacy of putative anti-aging therapies.
Materials
and methods
The following drugs and reagents
were obtained from the companies cited: DHEA; (+/-) CPP, Tamoxifen (Sigma, St.
Louis, MO); Antibody to acetylated p53 was obtained from upstate biotechnology
(Lake placide NY); antibody to beta-actin from Sigma (St. Louis, MO);
secondary antibodies conjugated to horseradish peroxidase from BioRad
(Hercules, CA); Enhanced chemiluminescence reagents (ECL) from Amersham
(Arlington Heights, IL); Immobilon-P transfer membrane for western blots from Millipore (Bedford,
MA).
Human monocytic cells (THP-1),
osteosarcoma (SaOS2), breast carcinoma MCF-7 cells, the rat retinal ganglion
cells (RGCs), aortic smooth muscle cells (SMCs) and the human embryonic stem
cells BG01V1 were purchased from ATCC (Rockville MA). Dulbecco's Modified
Eagle's Medium (DMEM), DMEM/F12 (1/1) medium, knockout serum and fetal bovine
serum (FBS) were obtained from Invitrogen (Carlsbad, CA). Cancer cell lines,
HUVEC and SMC cells were maintained in their corresponding culture media and
subjected to treatment with resveratrol or DHEA at concentrations ranging from
0 to 80 μM, or incubated in culture media with decreased glucose
concentrations. After 48 hours of incubation, the cells were washed with PBS
and re-suspended in 100 μl of lysis buffer (50 mM HEPES pH 7.4, 150 mM NaCl,
100 mM NaF, 1 mM MgCl2, 1.5 mM EGTA, 10% glycerol, 1% Triton X100, 1 μg/ml
leupeptin, 1 mM phenyl-methyl-sulfonyl-fluoride). Equal amounts of proteins
were analyzed by Western blot for expression of Sirt1 and TXNIP.
The human embryonic stem cells were
cultivated in DMEM/F12 (1:1) supplemented with 40% knockout serum, 10 mM
L-glutamine, and 1 mM non essential amino acids, and 10 μg/ml bFGF, and grown on mouse embryonic fibroblasts
(MEFs) as feeders. Treatments with resveratrol, DHEA, or reduced glucose
content were carried out as described above. After 48 hours of incubation, the
cells were washed with PBS, treated with collagenase for 5 min at 37°C. The
detached colonies were pelleted by centrifugation at 500xg for 5 min to
separate them from floating MEFS. The pellets were re-suspended in lysis buffer
as described above and proteins processed by Western blot, as described
previously [45], to measure the
expression of Sirt1 and TXNIP. Briefly, equal quantities of protein were
separated by electrophoresis on a 12% SDS‑PAGE gel and transferred to
Immobilon-P membranes. Proteins of interest
were identified by reaction with specific primary and secondary antibodies
linked to horseradish peroxidase and detected by chemiluminescence [45].
PCR
reaction.
TotalRNA was isolated using Qiagen Rneasy
mini kit (QiagenInc., Valencia, CA) as recommended by the supplier.
Total RNAwas quantified by OD at 260 nm. Using equal amount of
total RNA (200ng), stimulated under various conditions, mRNA wasprimed with random hexamers, and complementary DNA (cDNA) wassynthesized
from mRNA by TaqMan reverse transcription withMultiScribe reverse
transcriptase (PE Applied Biosystems, Foster,CT) according to the
manufacturer's description. The finalcDNA product was used for subsequent
cDNA amplification bypolymerase chain reaction.cDNA was
amplified and quantitated by using SYBR Green PCR reagentsfrom PE
Applied Biosystems according to the manufacturer'sinstructions. The
cDNA for GAPDH was amplified by using a specific forwardprimer
(5'-GAA GGT GAA GGT CGG AGT C-3') and a specific reverse primer(5'-GAA
GAT GGT GAT GGG ATT TC-3'). The primers for TXNIP were: ctg gcg taa gct ttt caa gg (forward) agt gca caa agg gga aac ac (reverse). The primers for Sirt1 were: 5'-ATA GCA CAC AAA CAT CAT
GCA-3' (forward) and 5'-TTT ATG CAT AAA ACA CCC AGC-3' (reverse). The PCRreaction
mixture (final volume 25 μl) contained 5 μlof cDNA, 1 μl of 10 μM
forward primer, 1 μlof 10 μM reverse primer, 2.5 μl of PCR 10x SYBRGreen PCR buffer, 3 μl of 25mM MgCl2, 2 μl of dNTPmix
(2.5 mM dATP, 2.5 mM dCTP, 2.5 mM dGTP, and 5 mM dUTP),0.125 μl of
AmpliTag Gold DNA polymerase (5 units/μlAmpliTag Gold DNA
polymerase), and 10.125 μl of H2O. The reaction was
amplified with iCycler iQ MulticolorReal Time PCR Detector
(Bio-Rad) for 37 cycles with meltingat 94 °C for 30 s, an annealing
at 58 °C for 30 s, and extension at 72 °C for 1 min in iCycler iQ PCR 96-wellplates (Bio-Rad).
Glucose 6 phosphate
dehydrogenase activity.
Enzyme activity assays were performed using the Vybrant. Assay Kit
(V-23111, Molecular Probes, Eugene, OR) with minor changes to allow monitoring
of cytosolic enzyme glucose 6-phosphate dehydrogenase in protein extracts.
Briefly cells from a 75 cm2 flask were lysed in (50 mM HEPES pH 7.4,
150 mM NaCl, 100 mM NaF, 1 mM MgCl2, 1.5 mM EGTA, 10% glycerol, 1%
Triton X100, 1 μg/ml
leupeptin, 1 mM phenyl-methyl-sulfonyl-fluoride) and diluted to a final
concentration of 0.5 mg/mg in PBS. Samples containing 50 μl of this solution were added to a
96 well plate and incubated in the absence or the presence of DHEA or
resveratrol for 10 min at room temperature. The 2X reaction mixture containing
the G6PD substrate resazurin was prepared as recommended by the manufacturer.
The reaction was then started by addition of 50 μl of the reaction mixture to the protein samples,
followed by incubation for 10 min at room temperature. The plate is then placed
in a fluorescence microplate reader set up at the excitation 530 and emission
580 nm and fluorescence values recorded.
In vivo experiments
. Mice were
injected with DHEA or resveratrol (10 mg/Kg each) and after 48 hours, the
animals were sacrificed and tissues harvested. To measure the levels of TXNIP
and Sirt1, tissues were homogenized on ice in 300 μl of lysis buffer per gram of tissue. The lysates were then centrifuged
at 1, 000xg for 5 min at 4°C and proteins in the supernatant quantified and
processed for Western blot as described above.
Supplementary Materials
Effect of resveratrol and DHEA on expression of TXNIP and Sirt1 in the breast cancer cells MCF7. Panel A, the retinal ganglion cells RGC.
Panel B, and the monocytic cell line THP-1 Panel C.
Cells were treated with resveratrol or DHEA at the
indicated concentrations for 48 hours, after what,
proteins were extracted and probed by Western blot
using specific antibodies to these two genes. β-actin
was used as a loading control.
Role of NMDA receptor in mediating the action of DHEA in RGC cells. The cells were pre-incubated
with CCP for one hour prior to addition of DHEA. After an
additional incubation for 48 hours, proteins were extracted
and probed by western blot for the expression of TXNIP and
Sirt1. β-actin was used as a loading control.
Acknowledgments
This work was supported in part by funds from the
Children's Memorial Research Center, Chicago, Illinois and the Pharmaceutical
Research Institute at Albany College of Pharmacy and Health Sciences, Albany
NY. We thank Patricia Phillips for help in the preparation of this manuscript.
Conflicts of Interest
The
authors have declared that no competing interests exist.
References
-
1.
Vijg
J
and Suh
Y.
Genetics of longevity and aging.
Annu Rev Med.
2005;
56:
193
-212.
[PubMed]
.
-
2.
Knight
JA
The biochemistry of aging.
Adv Clin Chem.
2000;
35:
1
-62.
[PubMed]
.
-
3.
Smith
J
Human Sir2 and the 'silencing' of p53 activity.
Trends Cell Biol.
2002;
12:
404
-406.
[PubMed]
.
-
4.
Hasegawa
K
, Wakino
S
and Yoshioka
K.
Sirt1 protects against oxidative stress-induced renal tubular cell apoptosis by the bidirectional regulation of catalase expression.
Biochem Biophys Res Commun.
2008;
372:
51
-56.
[PubMed]
.
-
5.
Wang
F
, Nguyen
M
, Qin
FX
and Tong
Q.
SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction.
Aging Cell.
2007;
6:
505
-514.
[PubMed]
.
-
6.
Alcendor
RR
, Gao
S
and Zhai
P.
Sirt1 regulates aging and resistance to oxidative stress in the heart.
Circ Res.
2007;
100:
1512
-1521.
[PubMed]
.
-
7.
Baur
JA
, Pearson
KJ
and Price
NL.
Resveratrol improves health and survival of mice on a high-calorie diet.
Nature.
2006;
444:
337
-342.
[PubMed]
.
-
8.
Trapp
J
and Jung
M.
The role of NAD+ dependent histone deacety-lases (sirtuins) in ageing.
Curr Drug Targets.
2006;
7:
1553
-1560.
[PubMed]
.
-
9.
Milne
JC
, Lambert
PD
and Schenk
S.
Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes.
Nature.
2007;
450:
712
-716.
[PubMed]
.
-
10.
Bordone
L
and Guarente
L.
Calorie restriction, SIRT1 and metabolism: understanding longevity.
Nat Rev Mol Cell Biol.
2005;
6:
298
-305.
[PubMed]
.
-
11.
Bordone
L
, Motta
MC
and Picard
F.
Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells.
PLoS Biol.
2006;
4:
e31
[PubMed]
.
-
12.
Moynihan
KA
, Grimm
AA
and Plueger
MM.
Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice.
Cell Metab.
2005;
2:
105
-117.
[PubMed]
.
-
13.
Guarente
L
Sir2 links chromatin silencing, metabolism, and aging.
Genes Dev.
2000;
14:
1021
-1026.
[PubMed]
.
-
14.
Yamawaki
H
, Haendeler
J
and Berk
BC.
Thioredoxin: a key regulator of cardiovascular homeostasis.
Circ Res.
2003;
93:
1029
-1033.
[PubMed]
.
-
15.
Yamawaki
H
and Berk
BC.
Thioredoxin: a multifunctional antioxidant enzyme in kidney, heart and vessels.
Curr Opin Nephrol Hypertens.
2005;
14:
149
-153.
[PubMed]
.
-
16.
Yoshioka
J
, Imahashi
K
and Gabel
SA.
Targeted deletion of thioredoxin-interacting protein regulates cardiac dysfunction in response to pressure overload.
Circ Res.
2007;
101:
1328
-1338.
[PubMed]
.
-
17.
Patwari
P
, Higgins
LJ
, Chutkow
WA
, Yoshioka
J
and Lee
RT.
The interaction of thioredoxin with Txnip. Evidence for formation of a mixed disulfide by disulfide exchange.
J Biol Chem.
2006;
281:
21884
-21891.
[PubMed]
.
-
18.
Chen
J
, Saxena
G
, Mungrue
IN
, Lusis
AJ
and Shalev
A.
Thioredoxin-interacting protein: a critical link between glucose toxicity and beta-cell apoptosis.
Diabetes.
2008;
57:
938
-944.
[PubMed]
.
-
19.
Chen
J
, Hui
ST
and Couto
FM.
Thioredoxin-interacting protein deficiency induces Akt/Bcl-xL signaling and pancreatic beta-cell mass and protects against diabetes.
Faseb J.
2008;
22:
3581
-3594.
[PubMed]
.
-
20.
Qi
W
, Chen
X
and Gilbert
RE.
High glucose-induced thioredoxin-interacting protein in renal proximal tubule cells is independent of transforming growth factor-beta1.
Am J Pathol.
2007;
171:
744
-754.
[PubMed]
.
-
21.
Stoltzman
CA
, Peterson
CW
, Breen
KT
, Muoio
DM
, Billin
AN
and Ayer
DE.
Glucose sensing by MondoA:Mlx complexes: a role for hexokinases and direct regulation of thioredoxin-interacting protein expression.
Proceedings of the National Academy of Sciences of the United States of America.
2008;
105:
6912
-6917.
[PubMed]
.
-
22.
Parikh
H
, Carlsson
E
and Chutkow
WA.
TXNIP regulates peripheral glucose metabolism in humans.
PLoS Med.
2007;
4:
e158
[PubMed]
.
-
23.
Chutkow
WA
, Patwari
P
, Yoshioka
J
and Lee
RT.
Thioredoxin-interacting protein (Txnip) is a critical regulator of hepatic glucose production.
J Biol Chem.
2008;
283:
2397
-2406.
[PubMed]
.
-
24.
Chondrogianni
N
, de C
M Simoes D
, Franceschi
C
and Gonos
ES.
Cloning of differentially expressed genes in skin fibroblasts from centenarians.
Biogerontology.
2004;
5:
401
-409.
[PubMed]
.
-
25.
Yoshida
T
, Kondo
N
and Oka
S.
Thioredoxin-binding protein-2 (TBP-2): its potential roles in the aging process.
Biofactors.
2006;
27:
47
-51.
[PubMed]
.
-
26.
de Candia
P
, Blekhman
R
, Chabot
AE
, Oshlack
A
and Gilad
Y.
A combination of genomic approaches reveals the role of FOXO1a in regulating an oxidative stress response pathway.
PLoS ONE.
2008;
3:
e1670
[PubMed]
.
-
27.
Guarente
L
and Picard
F.
Calorie restriction - the SIR2 connection.
Cell.
2005;
120:
473
-482.
[PubMed]
.
-
28.
Lamberts
SW
, van den Beld
AW
and van der Lely
AJ.
The endocrinology of aging.
Science.
1997;
278:
419
-424.
[PubMed]
.
-
29.
Fukui
M
, Kitagawa
Y
, Ose
H
, Hasegawa
G
, Yoshikawa
T
and Nakamura
N.
Role of endogenous androgen against insulin resistance and athero- sclerosis in men with type 2 diabetes.
Curr Diabetes Rev.
2007;
3:
25
-31.
[PubMed]
.
-
30.
Gescuk
BD
and Davis
JC Jr.
Novel therapeutic agents for systemic lupus erythematosus.
Curr Opin Rheumatol.
2002;
14:
515
-521.
[PubMed]
.
-
31.
Hernandez-Morante
JJ
, Perez-de-Heredia
F
, Lujan
JA
, Zamora
S
and Garaulet
M.
Role of DHEA-S on body fat distribution: gender- and depot-specific stimulation of adipose tissue lipolysis.
Steroids.
2008;
73:
209
-215.
[PubMed]
.
-
32.
Farr
SA
, Banks
WA
, Uezu
K
, Gaskin
FS
and Morley
JE.
DHEAS improves learning and memory in aged SAMP8 mice but not in diabetic mice.
Life Sci.
2004;
75:
2775
-2785.
[PubMed]
.
-
33.
Perrini
S
, Natalicchio
A
and Laviola
L.
Dehydroepiandrosterone stimulates glucose uptake in human and murine adipocytes by inducing GLUT1 and GLUT4 translocation to the plasma membrane.
Diabetes.
2004;
53:
41
-52.
[PubMed]
.
-
34.
Gutierrez
G
, Mendoza
C
and Zapata
E.
Dehydroepiandrosterone inhibits the TNF-alpha-induced inflammatory response in human umbilical vein endothelial cells.
Atherosclerosis.
2007;
190:
90
-9.
[PubMed]
.
-
35.
Webb
SJ
, Geoghegan
TE
, Prough
RA
and Michael
Miller KK.
The biological actions of dehydroepiandrosterone involves multiple receptors.
Drug Metab Rev.
2006;
38:
89
-116.
[PubMed]
.
-
36.
Debonnel
G
, Bergeron
R
and de Montigny
C.
Potentiation by dehydroepiandrosterone of the neuronal response to N-methyl-D-aspartate in the CA3 region of the rat dorsal hippocampus: an effect mediated via sigma receptors.
J Endocrinol.
1996;
150:
S33
-42.
[PubMed]
.
-
37.
Charalampopoulos
I
, Alexaki
VI
and Lazaridis
I.
G protein-associated, specific membrane binding sites mediate the neuroprotective effect of dehydroepiandrosterone.
Faseb J.
2006;
20:
577
-579.
[PubMed]
.
-
38.
Ziboh
VA
, Dreize
MA
and Hsia
SL.
Inhibition of lipid synthesis and glucose-6-phosphate dehydrogenase in rat skin by dehydroepiandrosterone.
J Lipid Res.
1970;
11:
346
-354.
[PubMed]
.
-
39.
Lawler
JM
and Demaree
SR.
Relationship between NADP-specific isocitrate dehydrogenase and glutathione peroxidase in aging rat skeletal muscle.
Mech Ageing Dev.
2001;
122:
291
-304.
[PubMed]
.
-
40.
Dasgupta
B
and Milbrandt
J.
Resveratrol stimulates AMP kinase activity in neurons.
Proceedings of the National Academy of Sciences of the United States of America.
2007;
104:
7217
-7222.
[PubMed]
.
-
41.
Lan
F
, Cacicedo
JM
, Ruderman
N
and Ido
Y.
SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation.
J Biol Chem.
2008;
283:
27628
-27635.
[PubMed]
.
-
42.
Hurley
RL
, Barre
LK
and Wood
SD.
Regulation of AMP-activated protein kinase by multisite phosphorylation in response to agents that elevate cellular cAMP.
J Biol Chem.
2006;
281:
36662
-36672.
[PubMed]
.
-
43.
Minn
AH
, Hafele
C
and Shalev
A.
Thioredoxin-interacting protein is stimulated by glucose through a carbohydrate response element and induces beta-cell apoptosis.
Endocrinology.
2005;
146:
2397
-2405.
[PubMed]
.
-
44.
Nakae
J
, Cao
Y
and Daitoku
H.
The LXXLL motif of murine forkhead transcription factor FoxO1 mediates Sirt1-dependent transcriptional activity.
J Clin Invest.
2006;
116:
2473
-2483.
[PubMed]
.
-
45.
Rebbaa
A
, Chou
PM
and Mirkin
BL.
Factors secreted by human neuroblastoma mediated doxorubicin resistance by activating STAT3 and inhibiting apoptosis.
Mol Med.
2001;
7:
393
-400.
[PubMed]
.