Greater skewness and kurtosis in females
A very obvious new fact emerged when we
plotted all our data classified by sex.
The skewness of the female distribution curve (1.322)
resulting from 115,754 values was greater in extent than the male equivalent
(1.099) resulting from 119,472 values (Figure 2a). The difference is
significant at a level of a = 0.0098. This is all the more
astonishing since the left sides of the curves are almost perfectly
superimposed, meaning that the above sex-specific differences in telomere size
are not due to differences in frequency of short
telomeres but to higher frequencies of long ones in females. Figure 2c
shows skewness and kurtosis classified by sex with skewness equations of y=0.013x+0.98 (r=0.78) for females and
y=0.012x+0.85 (r=0.86) for males and the kurtosis equations of y=0.07x+1.25
(r=0.67) for females and y=0.06x+0.79 (r=0.82) for males. In both criteria, the
female line runs above the male line, corroborating the histograms in
Figure 2a and both lines run nearly parallel, meaning
that skewness and kurtosis do not essentially differ between genders during a
lifetime.
What
was the morphologic correlate of the abnormally high T/C FISH medians?
Re-examination of our basic data, i.e. the hybridized metaphases, revealed an
overall infrequent occurrence of extremely long telomeres of single chromosome
arms. This was most evident in the elderly with pangenomically shorter
telomeres (Figure 3c). These extremely long, single telomeres appeared to be
randomly distributed all over the genome and were singularities, since we could
not relocate an identical event in other metaphases of the same individual.
These elongations, however, often occurred in doublets, meaning that both
chromatids of the same p or q arm were elongated. This strongly suggests that,
once the elongation has been achieved in a single chromatid, this newly
acquired long telomere is the template for the next generation of descendants
of this single individual cell. We coined the term "erratic extensive
elongation" (EEE) of single telomeres to define this phenomenon. Once
identified, we also found EEE of telomeres in younger people (Figure 3b) and
even in newborns, although it was hardly visible and only detectable by means
of the software, (Figure 3a). It is conceivable that
minor differences in telomere lengths of individual chromosome arms might be the
result of EEE that occurred years earlier and was subsequently exposed to
general pangenomic erosion. We think it highly likely that EEE is responsible
for the skewness of the distribution of telomere values (Figure 2a).
Evidence that EEE is propagated to the cells' progeny came from an anecdotal
observation in a middle-aged woman in whose PBL we encountered a considerable
number of endoreduplications (ER), together with an increased frequency of EEE.
ER is the result of a dysregulation in cell-cycle progression that ends not in
normal mitosis but in re-entering of the next cycle,
leading to tetraploidy with descendant chromosomes closely attached [12]
(Figure 3d). Every "tetrad" of chromatids represents 3 generations. If EEE is
detected on two of four telomeres of comparable
length, it should be derived
from the EEE of an ancestor chromatid. In Figure 3e, this is visible at the
telomeres of chromosome 2p, 5p, 6q, and 10p.
A
broadly accepted theory explains accelerated telomere shortening by oxidative
stress (ROS), with men having the greater load [23-28]. Assuming that ROS
causes DNA damage directly, forcing the cell either to repair or to die, the
result should be telomere length distributions in two isomorphic curves with a
parallel shift of the female curve to the right. As we show, this is not the
case. Therefore, the curves we obtained are most likely the result of a
mechanism counteracting telomeric loss which is more active/effective in
females.
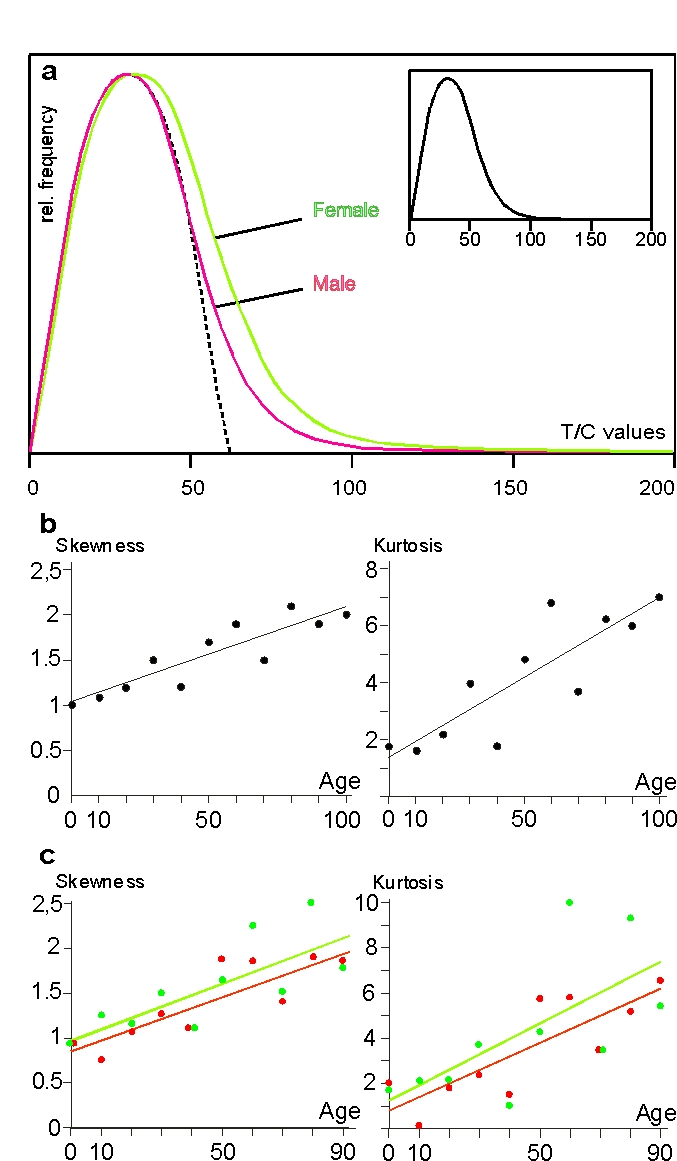
Figure 2. Erratic extensive elongation (EEE) of single telomeres in peripheral lymphocytes. (a) Histogram of telomere
lengths of all p and q arms of chromosomes of females (green)
and males (red). The dotted line represents the theoretical
Gaussian distribution, the histogram in the smaller insert
represents the curve of female and male values together. The
actual curves are skewed to the right. (b) Skewness and kurtosis
of telomere lengths of p and q arms of chromosomes of lymphocytes
in all age groups. Values are limited to 150 T/C values. (c)
Skewness and kurtosis of telomere lengths of p and q arms of
chromosomes of female (filled circles and green line) and
male probands (open circles and red line) ranging from newborns
up to 90 years. Centenarians are excluded because the male
group consists of only 2 persons. Values limited to 150 T/C
values as mentioned above. (see “Statistical analysis” for further details).
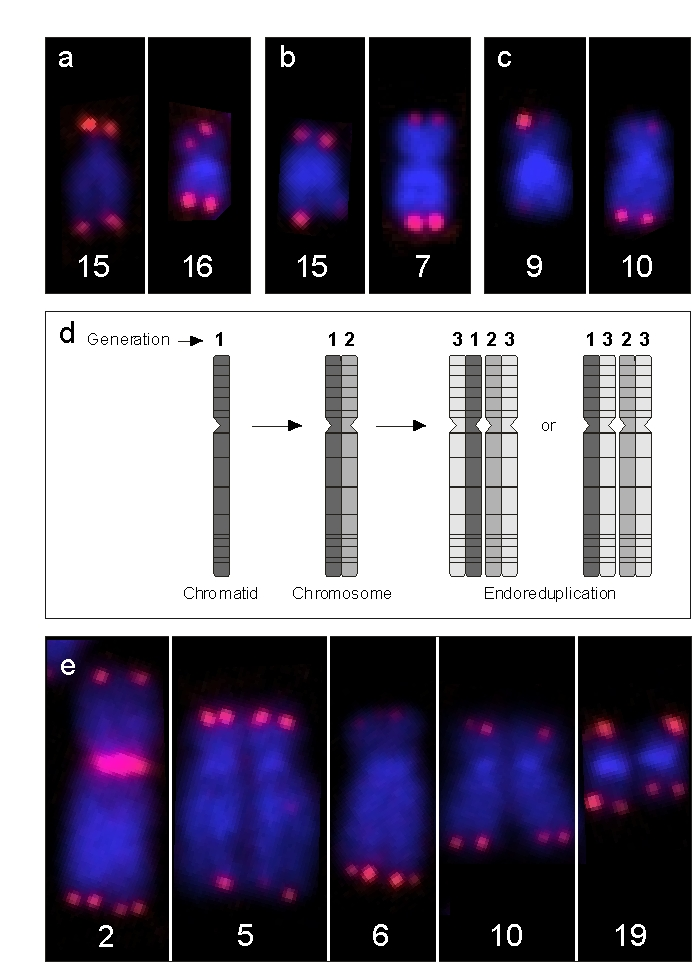
Figure 3. Examples of EEE. (a) Depicts chromosomes of a newborn male
with enhanced signal intensity at one p arm of chromosome 15 and at both
chromatids of chromosome 16, (b). This is also the case at a single
chromatid of 15q and on both chromatids of chromosome 7q in a 50-year-old
male and, (c) at a single chromatid of chromosome 9p and at both
arms of chromosome 10q in a male centenarian. (d) Schematic view of
endoreduplication (ER), the result being a group of four homologous
chromatids which emerged from one single chromatid. Note that the
juxtapositions of the sister and descendant chromatids may be variable,
since their three-dimensional packing is broken up by chromosome spreading.
(e) Single chromosomes of a sporadic ER observed in a 40-year-old
female with features of EEE. Since telomeric EEEs at 2p, 5q, 6q, and 10p
are doublets and not quadruplets, EEE at these positions must have occurred
one cell cycle prior to the ER event. In addition, there is a single
telomeric EEE at 19q, which must have occurred during the S-phase directly
preceding this ER.
Phenomenologically,
EEE is more likely to be a repair of accidental individual telomere damage than
a mechanism operating continuously. Following this line of argument, it is very
unlikely that telomerase is responsible for EEE, since telomerase-stabilized
telomeres show constant lengths of around 4-10 kb in humans (for review,
see [13]). A fitting candidate mechanism is the alternative pathway (ALT). ALT
is involved in maintenance or lengthening of telomeres, uses enzymes operative
in DNA recombination and rep- lication,
and can create extreme heterogeneity in telomere lengths [13] by using
so-called telomeric circles. Theoretically, these circles can arise from
telomeres by means of homologous recombination [14] and might be used by
telomerase as a template to elongate telomeres as has been proposed [15].
ALT
was found to be active in neoplastic cells and immortalized cell lines with or without
telomerase [14]. A physiological role of ALT is suggested by the observation
that telomerase-null mice show some degree of telomere elongation in
B-lymphocytes during germinal center reaction [16]. Furthermore, Rad54-null
mice have significantly shorter telomeres than wild animals, in spite of
unimpaired telomerase activity [17]. Rad54 is involved in DNA recombination and
may be part of ALT. We frequently found telomeric EEE of around 150 T/C values
(corresponding to 33 kb) and, in rare cases, even up to 300 T/C value (63 kb).
Our observations thus match data of mean telomere lengths of over 50 kb, as
measured by terminal restriction fragment analysis in so-called human
ALT-positive cell lines [18]. Finally, if ALT is indeed the key player in EEE, which
still has to be proven, the question remains whyfemales
use ALT more effectively.
As
we and others have clearly demonstrated a linear decline in telomere length [4,6,8-11], it seems at least unlikely that sex hormones play a role. It is,
however, well documented that exposure to reactive oxygen species (ROS) leads
to damage in nuclear DNA (see [19] and reviews [20,21]), and to telomere
shortening [22], and a growing body of evidence indicates that males are
subject to higher levels of cellular stress than females [23-28]. A direct
influence of the extracellular environment, the biosphere, on telomere length
was recently shown in a pair of twins of different gender with blood chimerism
[29]: Compared with their "normal" length (i.e. female cells in the woman and
male cells in the man), the telomeres within the male lymphocytes in the female
twin were 33% longer than those in the male twin. By contrast, comparison of
the telomeres within the female lymphocytes in the female with their
counterparts in the male revealed that the latter were shortened to 87% of
their "normal" length.
Apart
from direct damaging influences, ROS can serve as messengers to control other
physiological processes not directly involved in ROS defense [20,30,31]. For
example, ROS reacts with plasma thiol to form disulfides. This extracellular
thiol/disulfide equilibrium has signaling functions on different cell membrane
receptors, e.g. EGFR, and regulates poly ADP-ribose polymerase (PARP) [32,33].
EGFR is involved in the expression of hTERT, which controls telomerase activity
[34], and PARP is a key DNA repair enzyme and is involved in DNA replication,
recombination [35], telomere maintenance (for review, see [36]), and DNA
histone modifications and double-strand break repair [37].
Against
this background, it is tempting to speculate on an interplay between ROS, the
thiol/disulfide system and the control of telomere length. All the more, since
telomere erosion can be slowed by N-acetylcysteine, which changes the
thiol/disulfide system and inhibits ROS formation [20,38,39]. In conclusion,
this study provides evidence that the gender-specific differences in telomere
attrition depend on factors leading, in the female, to enhanced
repair/recombination processes in the lymphocytes (visible by EEE) and,
finally, to longer telomeres (evident by greater skewness and kurtosis of the
female histogram). Time will show whether it contributes to the gender
differences in life expectancy.