SIRT1 performs a balancing act on the tight-rope toward longevity
Abstract
Our recent study defined a new role for SIRT1 as a regulator of hepatic lipid metabolism. In the liver a major target of this sirtuin is the PPARα/PGC-1α signaling axis. Ablation of SIRT1 in the liver results in disrupted fatty acid oxidation, increased cellular stress, and elevations in proinflammatory cytokines. However, contrary to previous studies, we observed no changes in glucose production in the absence of SIRT1, despite impaired PGC-1α signaling. These findings point toward the involvement of other players in SIRT1-regulated hepatic metabolism. Here we discuss our findings, and comment on some of the controversy surrounding this protein in the current literature.
The food we eat has long been linked to the rate we
age. Selective pressures in times of food abundance and scarcity have
influenced our very genetic makeup, instilling in our genome genes believed to
control the delicate balance between metabolism and aging. However, this
balance has been disrupted in western societies with developments in
agriculture and technologies that have promoted the intake of high-calorie
diets and sedentary lifestyles. We are witnessing an alarming increase in the
rate of metabolic syndrome, which consists of a collection of abnormalities
including obesity, type 2 diabetes, dyslipidemia, fatty liver, and a
pro-inflammatory and prothrombotic state [1,2] Currently, one in four adults
in the United States suffers from metabolic syndrome and worldwide estimates
are over 2.1 billion [3,4]. Ultimately, this epidemic threatens human
life-span projections and puts great pressure on our already overburdened
health care system.
The sirtuin family of proteins appears to
be at the crossroads between nutritional status and longevity. Sirtuins are highly conserved NAD+-dependent
protein deacetylases and/or ADP ribosyltransferases that
target histones, transcription factors, and co-regulators to adapt gene
expression in response to the cellular energy state [5]. Many members of this
family, including the founder Sir2, have been shown to impact aging in species
ranging from yeast to fly and it is believed these protective actions result
from the beneficial regulation of stress management, and energy homeostasis.
SIRT1, the mammalian ortholog of Sir2, plays a role in numerous physiological
processes including fat metabolism, glucose homeostasis and immune response.
Because SIRT1 activity is dependent on the energy status of the cell, it
provides a direct link between metabolism, chromosome structure, and metabolic
gene regulation [6].
The liver is a central metabolic organ in charge of
regulating nutrient homeostasis in fed and fasting conditions. It controls key
aspects of lipid and glucose metabolism in response to nutritional and hormonal
signals [7]. Tight regulation of glucose by the liver is essential to ensuring
that glucose-dependent tissues such as brain and red blood cells have ample
energy supply during periods of nutrient deprivation. Recent reports have shown
that SIRT1 protein levels and enzymatic activity are induced in the fasted
liver [8,9]. SIRT1 regulates genes involved in gluconeogenesis through
deacetylation of several key transcription factors and coactivators [8,9,10].
The liver also plays an important role in maintaining lipid homeostasis. In
line with its role as a metabolic mediator, SIRT1 is known to regulate genes
involved in fatty acid oxidation and lipolysis [11]. Interestingly, the SIRT1
activator resveratrol has shown promise as a therapeutic agent for the
treatment of metabolic diseases [12,13]. Mice fed a high-fat diet along with
resveratrol remained lean and healthy compared to over-weight control animals [13].
Additionally, resveratrol significantly increased aerobic capacity, as
evidenced by increased running time and elevated oxygen consumption in muscle
fibers. Resveratrol treatment also protected mice against diet-induced-obesity
and insulin resistance [12]. Groups are now focusing on the development of high
affinity small molecule activators of SIRT1 as a therapeutic approach for
treating diseases of aging such as type-2 diabetes [14].
Although SIRT1 is an important regulator
of metabolism, the tissue-specific and systemic roles of SIRT1 are difficult to
dissect in vivo, primarily due to the complicated developmental defects
in the SIRT1 whole-body knockout mouse [15,16]. In search of further evidence
to identify a tissue-specific role of SIRT1 in the regulation of energy
homeostasis, we developed a knockout mouse model containing hepatic deletion of
SIRT1 (LKO) [17]. Microarray analysis of liver from LKO mice revealed a
striking reduction in expression of genes regulated by the peroxisome proliferators-activated
receptors α (PPARα). This lipid sensing nuclear
receptor is an important mediator of the adaptive response to fasting and
starvation. Deletion of SIRT1 in the liver impairs PPARα signaling and decreases fatty acid β-oxidation, whereas
over-expression of SIRT1 induces expression of PPARα target genes. Furthermore, we found that SIRT1 regulates PPARα signaling by directly interacting with the PPARα nuclear receptor. This interaction appears to be ligand dependent, as SIRT1
is recruited to response elements on promoters of PPARα target genes by agonists as well as by changes of nutritional status.
One mechanism by which SIRT1 regulates PPARα signaling in the
liver appears to be through the hands of PGC-1α, a key
coactivator for PPARα signaling and a direct target of SIRT1 [9,18]. It
has been shown that SIRT1 activates PGC-1α primarily by its
deacetylation [9] (Figure 2).
In keeping with these findings, we observed that
although PGC-1α message levels are lower in SIRT1 LKO livers, PGC-1α protein accumulates
on promoter regions of PPARα target genes but in a less
active hyperacetylated form. These findings suggest that activated PGC-1α is required for promoting transcription of PPARα targets and that SIRT1 may be involved in monitoring the
recruitment/dissociation cycle of PGC-1α. Additionally,
GST-pull down mapping data showed that the
core domain of SIRT1 directly interacts with PPARα.
Therefore, another plausible mechanism underlying our observations is that PPARα may be a bona fide SIRT1 substrate. Further studies are necessary to elucidate weather
SIRT1 indeed deacetylates PPARα, thereby affecting its
activity.
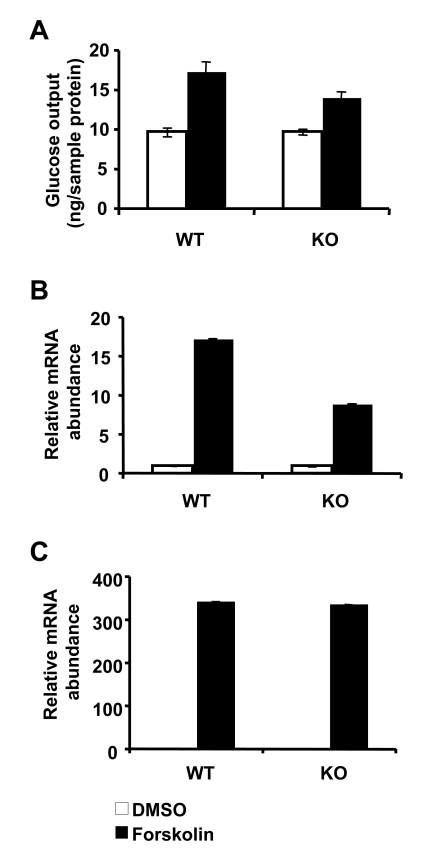
Figure 1. Loss of SIRT1 has minimal impact on gluconeogenesis in primary hepatocytes. (A) Glucose output
from primary hepatocytes isolated from control and SIRT1 LKO mice. Cells
were treated with DMSO (white bars) or 10 μM forskolin (black bars) and
incubated for 6 h in glucose free DMEM supplemented with 20 mM sodium
lactate and 2 mM sodium pyruvate. Glucose output was measured in culture
medium using a glucose oxidase kit (Sigma). Data represent mean +
SD. (B-C) SIRT1 deficiency in primary hepatocytes reduces the
induction of PGC-1α (B) but not
PEPCK (C) message in response to 10μM forskolin treatment. mRNA from
primary hepatocytes treated with DMSO (white bars) or forskolin (black
bars) were analyzed using qPCR. Data represent mean +
SD.
A major focus of our study was to characterize how
disruptions in PPARα signaling affect the physiology of SIRT1 LKO mice [17].
When challenged with a high-fat diet, LKO mice displayed increased hepatic
steatosis and hallmarks of endoplasmic reticulum stress and inflammatory
responses. Interestingly, in a trend very similar to those reported in the PPARα knockout mouse, LKO mice displayed elevated levels of proinflammatory
cytokines. These observations indicate that SIRT1 LKO mice are prone to
development of hepatic inflammation, which has been implicated in the progression
of insulin resistance [9,20]. These
findings provide evidence that solidify SIRT1's role as a key regulator of
metabolic homeostasis and complement previous animal studies using pharmacological
tools [14] or modest SIRT1 overexpression mouse models [21,22].
Several of the metabolic abnormalities we observed in
the SIRT1 LKO mice [17], however, are in direct contrast to those recently
reported by Chen et al. [23].
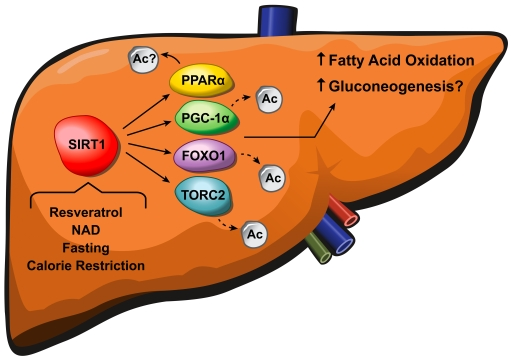
Figure 2. SIRT1 regulates fatty acid oxidation and gluconeogenesis in the liver. Resveratrol, NAD+,
fasting and calorie restriction activate SIRT1, causing deacetylation of
PGC-1α, FOXO1, and
TORC2 which in turn leads to increased fatty acid oxidation and
gluconeogenesis. The exact mechanism underlying how SIRT1 activates PPARα and the precise role of PGC-1α in the SIRT1-mediated glucose
homeostasis remain to be clarified.
Using a similar
hepatic-specific knockout mouse model, Chen et al. observed a reduction in
weight gain and liver fat accumulation in LKO mice when fed a western-style
diet. Additionally, their mice were protected from the physiological impacts of
a western diet with lower blood glucose and insulin levels. Similar to our
study, their group observed minor physiological differences in LKO mice fed a
chow diet. In wake of these findings, Chen et al. proposed that SIRT1 activity
in the liver is directly proportional to calorie intake, and that excess
calories and/or SIRT1 activators may result in elevated synthesis of fat and
cholesterol. One possible factor contributing to the discrepancy between our
observations and those of Chen et al. may be the difference in age of animals
at which the feeding was initiated and data were collected. In our study, mice
were six-week old when high-fat diet feeding was initiated, whereas four-month
old mice were utilized in the study carried
out by Chen et al. The varied responses of SIRT1 LKO mice to a western-style
diet at different ages raises the possibility that hepatic SIRT1 may
selectively regulate alternative metabolic pathways at multiple stages of
development. An inducible SIRT1 knockout model will be helpful to dissect
age-dependent effects of SIRT1. Moreover, since the liver is such a dynamic
metabolic organ, small variations in dietetic components and genetic
backgrounds may also contribute to the inconsistency between these two
studies.
Another surprising phenotype observed in the SIRT1 LKO
mice is their normal gluconeogenesis in response to a 16-h fasting [17]. The
inducible coactivator PGC-1α is an important component of a
number of transcriptional complexes that regulate glucose and lipid metabolism.
Hepatic knockdown of SIRT1 significantly
abrogates the fasting induction of gluconeogenic genes by regulating the
acetylation status of PGC1α [11]. However, we observed no
changes in fasting glucose levels in the absence of hepatic SIRT1 despite
impaired PGC-1α signaling. Liver specific SIRT1 knockout mice had
slightly higher, although not statistically significant, fasting glucose levels
compared to littermate controls upon high-fat feeding. Expression levels of the
two rate-limiting enzymes in the gluconeogenic pathway, PEPCK and G-6Pase, were
also unchanged in the absence of hepatic SIRT1. Consistent with these
observations, forskolin, an intracellular cAMP stimulator, promoted gluconeogenesis
independently of SIRT1 levels in primary hepatocytes (Figure 1A). Additionally,
although the forskolin-mediated induction of PGC1α expression
was decreased in these cells (Figure 1B), the overall message levels of PEPCK
remained similar between control and LKO hepatocytes (Figure 1C).
Gluconeogenesis is regulated by a complex interplay between transcription
factor and hormonal and coregulator signaling. While PGC-1α is known to control hepatic glucose production, other factors such as
FOXO1 and TORC2 are reported to promote gluconeo-genesis [24]. Interestingly,
SIRT1 has been shown to deacetylate and repress both FOXO1 [25] and TORC2 [24].
Therefore, a likely explanation for our findings is that while PGC-1α activity is lower in SIRT1 KO livers, compensatory effects of FOXO1
and TORC2 balance the reduction in PGC-1α signaling (Figure 2). Another possible explanation for the contradiction in these studies may lie
in differences in cell types and method of SIRT1 deletion/knockdown used in the
animal studies. It is important to note that the hepatic-specific albumin-Cre
driven SIRT1 knockout mouse utilized in our study is a permanent knockout
model. Phenotypes observed in these mice may reflect systemic and local
compensatory effects in wake of hepatic deletion of SIRT1. Studies done by Rodger
et al. [11] employed transient knockdown methods using adenovirus-mediated
shRNA which seem to provoke more acute responses to loss of hepatic SIRT1.
In conclusion, while our study defines a
new role for SIRT1 as a key regulator of hepatic lipid metabolism, it also adds
fuel to the fire of controversy surrounding this protein as a central player in
mammalian energy homeostasis. It appears that in the liver, a major target of
this sirtuin is the PPARα/PGC-1α signaling axis. Ablation of
SIRT1 in the liver creates disruptions in fatty acid oxidation, increased
cellular stress, and elevations in proinflammatory cytokines. What remains to
be determined is the precise role SIRT1 plays in regulating gluconeogenesis and
cholesterol metabolism in the liver and how this, in turn, affects systemic
metabolism. Our findings and others suggest that activation of SIRT1 may
provide a therapeutic strategy for treatment of metabolic syndrome.
Acknowledgments
We thank Drs. Sailesh Surapureddi and Anton Jetten for
criticalreading of the manuscript; Dr. Frederic Alt at Harvard
Medical School for providing the SIRT1 exon 4 floxed allele; and NIEHS
Multimedia Services Department for the cartoon graph of Figure 2. This work was
supported by the IntramuralResearch Program of the NIH, National Institute of
EnvironmentalHealth Sciences to X.L. (Z01 ES102205).
Conflicts of Interest
The authors in this
manuscript have no conflict of interests to declare.
References
-
1.
Eckel
RH
, Grundy
SM
and Zimmet
PZ.
The metabolic syndrome.
Lancet.
2005;
65:
1415
-28.
[PubMed]
.
-
2.
Grundy
SM
, HB
Brewer Jr
, Cleeman
JI
, Smith
SC Jr
and Lenfant
C.
Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition.
Arterioscler Thromb Vasc Biol.
2004;
24:
e13
-e18.
[PubMed]
.
-
3.
Flegal
KM
, Carroll
MD
, Ogden
CL
and Johnson
CL.
Prevalence and trends in obesity among US adults, 1999-2000.
Jama.
2002;
288:
1723
-1727.
[PubMed]
.
-
4.
Li
Z
, Bowerman
S
and Heber
D.
Health ramifications of the obesity epidemic.
Surg Clin North Am.
2005;
85:
681
-701.
[PubMed]
.
-
5.
Blander
G
and Guarente
L.
The Sir2 family of protein deacetylases.
Annu Rev Biochem.
2004;
73:
417
-435.
[PubMed]
.
-
6.
Bishop
NA
and Guarente
L.
Genetic links between diet and lifespan: shared mechanisms from yeast to humans.
Nat Rev Genet.
2007;
8:
835
-844.
[PubMed]
.
-
7.
van den Berghe
G
The role of the liver in metabolic homeostasis: implications for inborn errors of metabolism.
J Inherit Metab Dis.
1991;
14:
407
-420.
[PubMed]
.
-
8.
Liu
Y
, Dentin
R
, Chen
D
, Hedrick
S
, Ravnskjaer
K
, Schenk
S
, Milne
J
, Meyers
JD
, Cole
P
, Iii
JY
, Olefsky
J
, Guarente
L
and Montminy
M.
A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange.
Nature.
2008;
456:
269
-273.
[PubMed]
.
-
9.
Rodgers
JT
, Lerin
C
, Haas
W
, Gygi
SP
, Spiegelman
BM
and Puigserver
P.
Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1.
Nature.
2005;
434:
113
-118.
[PubMed]
.
-
10.
Motta
MC
, Divecha
N
, Lemieux
M
, Kamel
C
, Chen
D
, Gu
W
, Bultsma
Y
, McBurney
M
and Guarente
L.
Mammalian SIRT1 represses forkhead transcription factors.
Cell.
2004;
116:
551
-563.
[PubMed]
.
-
11.
Rodgers
JT
and Puigserver
P.
Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1.
Proc Natl Acad Sci U S A.
2007;
104:
12861
-12866.
[PubMed]
.
-
12.
Baur
JA
, Pearson
KJ
, Price
NL
, Jamieson
HA
, Lerin
C
, Kalra
A
, Prabhu
VV
, Allard
JS
, Lopez-Lluch
G
, Lewis
K
, Pistell
PJ
and Poosala
S.
Resveratrol improves health and survival of mice on a high-calorie diet.
Nature.
2006;
444:
337
-342.
[PubMed]
.
-
13.
Lagouge
M
, Argmann
C
, Gerhart-Hines
Z
, Meziane
H
, Lerin
C
, Daussin
F
, Messadeq
N
, Milne
J
, Lambert
P
, Elliott
P
, Geny
B
, Laakso
M
, Puigserver
P
and Auwerx
J.
Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha.
Cell.
2006;
127:
1109
-1122.
[PubMed]
.
-
14.
Milne
JC
, Lambert
PD
, Schenk
S
, Carney
DP
, Smith
JJ
, Gagne
DJ
, Jin
L
, Boss
O
, Perni
RB
, Vu
CB
, Bemis
JE
and Xie
R.
et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes.
Nature.
2007;
450:
712
-716.
[PubMed]
.
-
15.
Cheng
HL
, Mostoslavsky
R
, Saito
S
, Manis
JP
, Gu
Y
, Patel
P
, Bronson
R
, Appella
E
, Alt
FW
and Chua
KF.
Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice.
Proc Natl Acad Sci U S A.
2003;
100:
10794
-10799.
[PubMed]
.
-
16.
McBurney
MW
, Yang
X
, Jardine
K
, Hixon
M
, Boekelheide
K
, Webb
JR
, Lansdorp
PM
and Lemieux
M.
The mammalian SIR2alpha protein has a role in embryogenesis and gametogenesis.
Mol Cell Biol.
2003;
23:
38
-54.
[PubMed]
.
-
17.
Purushotham
A
, Schug
TT
, Xu
Q
, Surapureddi
S
, Guo
X
and Li
X.
Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation.
Cell Metab.
2009;
9:
327
-338.
[PubMed]
.
-
18.
Li
S
, Liu
C
, Li
N
, Hao
T
, Han
T
, Hill
DE
, Vidal
M
and Lin
JD.
Genome-wide coactivation analysis of PGC-1alpha identifies BAF60a as a regulator of hepatic lipid metabolism.
Cell Metab.
2008;
8:
105
-117.
[PubMed]
.
-
19.
Kanda
H
, Tateya
S
, Tamori
Y
, Kotani
K
, Hiasa
K
, Kitazawa
R
, Kitazawa
S
, Miyachi
H
, Maeda
S
, Egashira
K
and Kasuga
M.
MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity.
J Clin Invest.
2006;
116:
1494
-1505.
[PubMed]
.
-
20.
Weisberg
SP
, McCann
D
, Desai
M
, Rosenbaum
M
, Leibel
RL
and Ferrante
Jr.
Obesity is associated with macrophage accumulation in adipose tissue.
J Clin Invest.
2003;
112:
1796
-1808.
[PubMed]
.
-
21.
Banks
AS
, Kon
N
, Knight
C
, Matsumoto
M
, Gutierrez-Juarez
R
, Rossetti
L
, Gu
W
and Accili
D.
SirT1 Gain of Function Increases Energy Efficiency and Prevents Diabetes in Mice.
Cell metabolism.
2008;
8:
333
-341.
[PubMed]
.
-
22.
Pfluger
PT
, Herranz
D
, Velasco-Miguel
S
, Serrano
M
and Tschop
MH.
Sirt1 protects against high-fat diet-induced metabolic damage.
Proc Natl Acad Sci U S A.
2008;
105:
9793
-9798.
[PubMed]
.
-
23.
Chen
D
, Bruno
J
, Easlon
E
, Lin
SJ
, Cheng
HL
, Alt
FW
and Guarente
L.
Tissue-specific regulation of SIRT1 by calorie restriction.
Genes Dev.
2008;
22:
1753
-1757.
[PubMed]
.
-
24.
Liu
Y
, Dentin
R
, Chen
D
, Hedrick
S
, Ravnskjaer
K
, Schenk
S
, Milne
J
, Meyers
DJ
, Cole
P
, Iii
JW
, Olefsky
J
, Guarente
L
and Montminy
M.
A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange.
Nature.
2008;
456:
269
-273.
[PubMed]
.
-
25.
Motta
MC
, Divecha
N
, Lemieux
M
, Kamel
C
, Chen
D
, Gu
W
, Bultsma
Y
, McBurney
M
and Guarente
L.
Mammalian SIRT1 represses forkhead transcription factors.
Cell.
2004;
116:
551
-563.
[PubMed]
.