CYB5R3: a key player in aerobic metabolism and aging?
Abstract
Aging results from a complex and not completely understood chain of processes that are associated with various negative metabolic consequences and ultimately leads to senescence and death. The intracellular ratio of pyridine nucleotides (NAD+/NADH), has been proposed to be at the center stage of age-related biochemical changes in organisms, and may help to explain the observed influence of calorie restriction and energy-sensitive proteins on lifespan in model organisms. Indeed, the NAD+/NADH ratios affect the activity of a number of proteins, including sirtuins, which have gained prominence in the aging field as potential mediators of the beneficial effects of calorie restriction and mediating lifespan. Here we review the activities of a redox enzyme (NQR1 in yeast and CYB5R3 in mammals) that also influences the NAD+/NADH ratio and may play a regulatory role that connects aerobic metabolism with aging.
Aging involves multiple
processes that render cells, tissues and organs vulnerable to stress, damage
and ultimately death. Aging itself is not a disease, but there are a number of
diseases that become exponentially more prevalent with advancing age such as
cancer, cardiovascular disease, metabolic syndrome and neurodegenerative
diseases. Energy sensing, food intake and caloric utilization must be kept in
equilibrium to preserve appropriate fat stores to prevent the deregulation of
glucose homeostasis and other obesity-related disorders.
Calorie restriction (CR) is an
intervention aimed to produce undernutrition without malnutrition. CR increases
healthspan and lifespan in almost all species tested such as yeast, insects,
nematodes and mammals [1], including
nonhuman primates [2]. CR has been studied extensively with consistent
results showing its beneficial effects on longevity, age-associated diseases, attenuation of functional
decline, and carcinogenesis across a variety of species and diet formulations [3]. Among mammals mice have been the most
heavily-researched model with CR eliciting myriad behavioral, physiological,
and metabolic changes that include decreased body temperature, blood glucose,
insulin and fat mass, and increased physical activity, glucose tolerance and
insulin sensitivity [4]. Studies in Saccharomyces cerevisiae and Drosophila
melanogaster have demonstrated that SIR2, which encodes for a NAD+-dependent
histone deacetylase, plays a central role in mediating the increase in
longevity associated with CR in these species [5-7]. The involvement of SIR2 in lifespan extension
by CR may relate to its responsiveness to nicotinamide levels and the NAD+/NADH
ratio, both indicators of cellular energy status [8-10]. A growing body of evidence indicates that the
mammalian homologue of SIR2, SIRT1, also plays a significant role
in responding to CR. For example, CR elevates SIRT1 expression in a
number of tissues [11], and transgenic mice that overexpress SIRT1
exhibit a phenotype mirroring some aspects of CR [12]. SIRT1 has also been shown to improve insulin
sensitivity [13], another consequence of a CR diet [14].
CR promotes a healthy aging
phenotype through a myriad of mechanisms, one of which is thought to be its
ability to increase mitochondrial efficiency and biogenesis. Increases in
mitochondrial biogenesis are driven by eNOS and PGC-1α expression and activation. Furthermore, these changes in mitochondria
following CR are accompanied by a decrease in production of reactive oxygen
species (ROS) without a net reduction of ATP biosynthesis, which indicates a higher
bioenergetics efficiency [15,16]. There are
several reports on how CR induces the deacetylation of PGC-1α by SIRT1[17]. Sirtuins
are NAD+-dependent deacetylases [18], and this
dependence has led researchers to propose that sirtuins are at the center of
the regulatory nexus between energy metabolism and aging because NAD+
is a primary marker for intracellular energy status. It has also been
demonstrated that CR activates sirtuins and thereby increases both the
stability of chromatin [19] and cell
survival [11]. Given the
dependence of sirtuins on NAD+ and the published activities of
sirtuins under CR conditions, it has been hypothesized that NAD+
levels and its metabolism are at the center of the regulatory mechanisms behind
the beneficial effects of CR [17].
Furthermore, the conversion of NADH to its reduced form NAD+ in
mitochondria, a reaction that is supported by coenzyme Q (CoQ), is also thought
to protect mitochondria during aging [20]. We
propose, therefore, mechanisms that affect the NAD+/NADH ratio and
thereby modulate sirtuins and other NAD+-dependent enzymes are key
players in the regulation of the aging process.
We have recently described the role of NQR1,
a gene that encodes cytochrome b5 reductase, a protein that
uses both NADH and CoQ as substrates, in chronological and replicative lifespan
in Saccharomyces cerevisiae [21]. This
enzyme is located at the plasma membrane and is homologous to the mammalian
enzyme encoded by CYB5R3, which can also be found in plasma membranes
and uses exclusively NADH and CoQ as substrates [22]. This
enzyme is a key component of the trans-plasma membrane redox system (PMRS). The
PMRS provides both protection against extracellular oxidants [23] and
prevention of apoptosis initiated by the activation of the neutral sphingo-myelinase
at the plasma membrane [24]. CR induces
the expression of NQR1 in yeast, increasing the cytosolic NADH oxidation
rate [21]. Similarly, CR increases the presence of this enzyme in the plasma
membranes of both the liver and brain of rats, improving the antioxidant
protection of phospholipids in these membranes [25,26]. This
antioxidant system is also activated in mitochondrial DNA-deficient (ρ°) mammalian
cells [27,28], and in vitamin E-deficient rat livers [29]. In the case
of mammalian ρ° cells, cell survival is dependent on the redox homeostasis maintained
by NADH oxidation by the PMRS. As indicated above, the increase of aerobic
metabolism induced by CR also requires the cytosolic cooperation of CYB5R3
to maintain the NAD+/NADH ratio. Thus, any intervention that induces
membrane instability or alters respiratory metabolism will evoke the
transcription of CYB5R3 and activation of its enzymatic product.
Similar to the case in
mammals, yeast NQR1 is upregulated by CR in parallel with an activation
of respiration. Given that the same conditions activate the CoQ biosynthesis
pathway [30], this may
indicate a connection between CoQ biosynthesis and respiration. Interestingly,
over-expression of NQR1 in yeast requires respiration to maintain cell
survival. The mitochondrial mutant strains ΔATP2 and ΔCOR1
cannot grow under anaerobic conditions when NQR1 is overexpressed. The ΔATP2strain has a defective ATP synthase complex and the ΔCOR1 strain
is defective in the bc1 complex. Similar results are obtained when
the ΔCOQ2 strain, in which the CoQ biosynthesis pathway is
inoperable, is used to overexpress NQR1. However, the addition of
external CoQ6 restored both respiration and growth in the latest
strain. These results indicate that NQR1 effect acts through
the respiratory metabolism in yeast [21].
Over-expression of NQR1
extends chronological lifespan in the
absence of SIR2, perhaps acting through a pathway dependent on NAD+/NADH
balance that requires respiration [31], but not SIR2
[32]. NQR1
over-expression also extends replicative lifespan in a SIR2-dependent
manner that mimics CR[8]. NQR1
promotes oxygen consumption while inhibiting ethanol production and this shift
occurs alongside an increase in respiratory chain enzyme activities. NQR1
thus causes a shift from fermentative to respiratory metabolism that may help
explain its role in longevity. Yeast
growing in low glucose (CR) media also shows the increase of both chronological
and replicative lifespan through the activation of respiration [8,31].
We can hypothesize then
that NQR1 in yeast and CYB5R3 in mammals play a regulatory role
connecting aerobic metabolism and aging processes through their ability to
alter the NAD+/NADH ratio. Cytosolic NAD+/NADH must be balanced with
that of mitochondria. We expect that NQR1 would partially prevent the de
novo biosynthesis of NAD+ most likely by increasing the
recycling of the redox state of nucleotides and maintaining the availability
of NAD+ to consumer enzymes.
It is assumed that sirtuins
connect metabolism to aging because they use NAD+ as substrate [18]. This
rationale can also be applied to CYB5R3 because the enzyme consumes NADH
as an obligatory substrate. This enzyme would then be an essential component of
the NAD+/NADH-dependent metabolic pathways in cooperation with the
mitochondrial respiratory chain (Figure 1), which both contribute to the
maintenance of the NAD+/NADH ratio and, as a consequence, regulate
the function of sirtuins and other downstream NAD+ consumers. The
NADH consumers and NAD+ consumers may participate in a regulatory
loop, as a decrease of NAD+ availability will activate NAD+
biosynthesis as has been shown to occur under stress such as in
nutrient-dependent survival mechanisms [33].
Mammalian CYB5R3 may
also connect aerobic metabolism and aging. CYB5R3 encodes for a
membrane-bound form of cytochrome b5reductase in
somatic cells that is N-myristoylated and thereby anchored to the plasma
membrane, mitochondrial outer membrane and endoplasmic reticulum. This isoform
participates in cholesterol biosynthesis [34], fatty acid
elongation and desaturation [35], P-450 mediated hydroxylation of drugs and
steroid hormones [36] and the
PMRS [22]. There is
also a soluble isoform, which lacks the N-terminal binding domain and exists in
the cytoplasm of erythrocytes where its main function is to reduce
methaemoglobin [37]. Both
isoforms come from alternative splicing of the same CYB5R3 gene.Deficiencies of
cytochrome b5reductase cause recessive congenital
methaemoglobulinemia (RCM), which presents with two distinct clinical forms.
RCM type I is benign and limited to red blood cells. RCM type II is severe,
affects all cells in the organism, and can lead to neurological dysfunction
(for review see [38]).
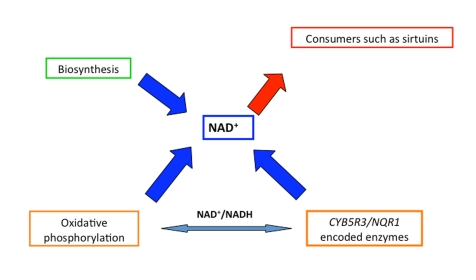
Figure 1. Role of the different characters to guarantee the availability of NAD+ to consumers maintaining at the same time the cellular redox homeostasis through a balanced NAD +/NADH ratio.
Recently, the proteomic
profile of metabolic proteins in the invasive glioblastoma phenotype has been
studied by applying a functional analysis using the Ingenuity Pathway Knowledge
Base (Ingenuity Systems, Redwood City, CA) [39]. The
results identified oxidative phosphorylation, mitochondrial dysfunction and
ubiquinone biosynthesis as canonical pathways of the cancerous phenotype and CYB5R3
is identified as a protein associated with the mitochondrial dysfunction
pathway. Furthermore, the relationship between mitochondrial dysfunction and CYB5R3
has also reported in a study carried out to analyze gene expression induced by
bromide exposure using the Ingenuity Pathway Analysis[40].
Data
from our laboratory seem to indicate a positive role for mammalian CYB5R3
in mitochondrial respiration. We have used siRNA technology to silence CYB5R3
in cultured human cells (Figure 2). Preliminary results indicate that CYB5R3
KO cells exhibit an apparent senescent phenotype based on the accumulation of β-galactosidase. These cells also
show a reduction in the mitochondrial respiration rate based on analysis of
oxygen consumption. Biochemical analysis of these cells also revealed an
increase in the expression of PGC-1α that indicates increased
recycling or de novo biogenesis of mitochondria. In a recently-reported
global analysis of lysine-acetylated proteins, a posttranslational modifica-tion
of CYB5R3-encoded protein by lysine acetylation in its FAD-binding
domain has been identified [41]. Lysine acetylation is necessary
for the interaction between SIRT1 and other sirtuins their targets
before deacetylation can
occur. Though conclusive experimental data still need to be shown, we
hypothesize that SIRT1 regulates the cytosolic NAD+/NADH
ratio by influencing CYB5R3 activity (Figure 3). Conditions of high NADH
would lead to partial inactivation of SIRT1, leading to an accumulation
of the acetylated form of CYB5R3. This active form of CYB5R3
would increase NADH oxidation and release NAD+ that, in turn, would
activate SIRT1. CYB5R3 would be then deacetylated, causing a
decrease in its activity and thereby maintaining the NAD+/NADH ratio
in proper balance. PGC-1α activity will
be also affected by this cycle through its interaction with SIRT1. Taken
together, our preliminary data indicate CYB5R3 could play an essential
role in the mitochondrial metabolism by its contribution to cellular redox
homeostasis. A coordination of the redox balance in both the cytosol and
mitochondria appears to be necessary for optimum cellular health, and may be of
consequence to healthy aging as well.
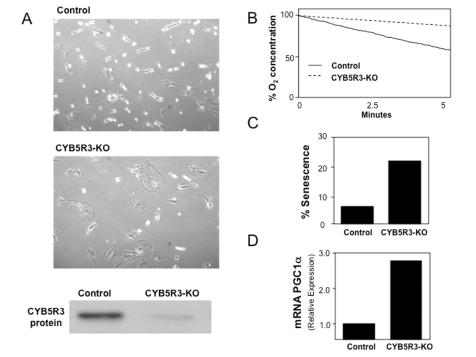
Figure 2. MRC-5 normal human diploid fibroblasts were CYB5R3-silenced (KO cells) and cultured in DMEM medium supplemented with FBS 10%. (A) Cell growth and CYB5R3 protein
levels after five days of CYB5R3-silencing are shown. (B)
Oxygen consumption was measured in parallel in both control and CYB5R3-KO
cells. (C) Percentage of senescence was determined by
senescence-associated-β-galactosidase activity. (D)
Total RNA was extracted in both control and CYB5R3-KO cells and PGC1α mRNA
levels were obtained by real time PCR.
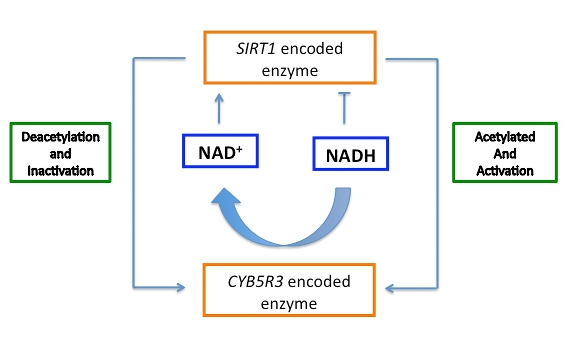
Figure 3. Hypothesis of the regulatory connection between cytochrome b5 reductase and sirtuin to maintain SIRT1 dependent respiration and cytosolic NAD+/NADH ratio.
Acknowledgments
This paper has been partially supported by Spanish
FIS Grant PI080500, NIH Grant 1R01AG028125-01A1, and the Intramural Research
Program of the NIH, National Institute on Aging.
Conflicts of Interest
The authors of this
manuscript have no conflict of interest to declare.
References
-
1.
Bartke
A
, Wright
JC
, Mattison
JA
, Ingram
DK
, Miller
RA
and Roth
GS.
Dietary restriction and life-span.
Science.
2002;
296:
2141
-2142.
[PubMed]
.
-
2.
Mattison
JA
, Roth
GS
, Lane
MA
and Ingram
DK.
Dietary restriction in aging nonhuman primates.
Interdiscip Top Gerontol.
2007;
35:
137
-158.
[PubMed]
.
-
3.
Weindruch
R
and Sohal
RS.
Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging.
N Engl J Med.
1997;
337:
986
-994.
[PubMed]
.
-
4.
Canto
C
and Auwerx
J.
Caloric restriction, SIRT1 and longevity.
Trends Endocrinol Metab.
2009;
20:
325
-331.
[PubMed]
.
-
5.
Lin
SJ
, Defossez
PA
and Guarente
L.
Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae.
Science.
2000;
289:
2126
-2128.
[PubMed]
.
-
6.
Rogina
B
and Helfand
SL.
Sir2 mediates longevity in the fly through a pathway related to calorie restriction.
Proc Natl Acad Sci U S A.
2004;
101:
15998
-16003.
[PubMed]
.
-
7.
Chen
J
, Zhou
Y
, Mueller-Steiner
S
, Chen
LF
, Kwon
H
, Yi
S
, Mucke
L
and Gan
L.
SIRT1 protects against microglia-dependent beta amyloid toxicity through inhibiting NF-kappa B signaling.
J Biol Chem.
2005;
280:
40364
-40374.
[PubMed]
.
-
8.
Lin
SJ
, Kaeberlein
M
, Andalis
AA
, Sturtz
LA
, Defossez
PA
, Culotta
VC
, Fink
GR
and Guarente
L.
Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration.
Nature.
2002;
418:
344
-348.
[PubMed]
.
-
9.
Anderson
RM
, Bitterman
KJ
, Wood
JG
, Medvedik
O
and Sinclair
DA.
Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae.
Nature.
2003;
423:
181
-185.
[PubMed]
.
-
10.
Anderson
RM
, Latorre-Esteves
M
, Neves
AR
, Lavu
S
, Medvedik
O
, Taylor
C
, Howitz
KT
, Santos
H
and Sinclair
DA.
Yeast life-span extension by calorie restriction is independent of NAD fluctuation 1.
Science.
2003;
302:
2124
-2126.
[PubMed]
.
-
11.
Cohen
HY
, Miller
C
, Bitterman
KJ
, Wall
NR
, Hekking
B
, Kessler
B
, Howitz
KT
, Gorospe
M
, de Cabo
R
and Sinclair
DA.
Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase.
Science.
2004;
305:
390
-392.
[PubMed]
.
-
12.
Bordone
L
and Guarente
L.
Calorie restriction, SIRT1 and metabolism: understanding longevity.
Nat Rev Mol Cell Biol.
2005;
6:
298
-305.
[PubMed]
.
-
13.
Sung
B
, Park
S
, Yu
BP
and Chung
HY.
Modulation of PPAR in aging, inflammation, and calorie restriction.
J Gerontol A Biol Sci.
2004;
59A:
997
-1006.
.
-
14.
Lane
MA
, Ingram
DK
and Roth
GS.
Calorie restriction in nonhuman primates: effects on diabetes and cardiovascular disease risk.
Toxicol Sci.
1999;
52:
41
-48.
[PubMed]
.
-
15.
Nisoli
E
, Tonello
C
, Cardile
A
, Cozzi
V
, Bracale
R
, Tedesco
L
, Falcone
S
, Valerio
A
, Cantoni
O
, Clementi
E
, Moncada
S
and Carruba
MO.
Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS.
Science.
2005;
310:
314
-317.
[PubMed]
.
-
16.
Lopez-Lluch
G
, Hunt
N
, Jones
B
, Zhu
M
, Jamieson
H
, Hilmer
S
, Cascajo
MV
, Allard
J
, Ingram
DK
, Navas
P
and de Cabo
R.
Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency.
Proc Natl Acad Sci U S A.
2006;
103:
1768
-1773.
[PubMed]
.
-
17.
Canto
C
and Auwerx
J.
PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure.
Curr Opin Lipidol.
2009;
20:
98
-105.
[PubMed]
.
-
18.
Finkel
T
, Deng
CX
and Mostoslavsky
R.
Recent progress in the biology and physiology of sirtuins.
Nature.
2009;
460:
587
-591.
[PubMed]
.
-
19.
Vaquero
A
and Reinberg
D.
Calorie restriction and the exercise of chromatin.
Genes Dev.
2009;
23:
1849
-1869.
[PubMed]
.
-
20.
Olgun
A
Converting NADH to NAD+ by nicotinamide nucleotide transhydrogenase as a novel strategy against mitochondrial pathologies during aging.
Biogerontology.
2009;
10:
531
-534.
[PubMed]
.
-
21.
Jimenez-Hidalgo
M
, Santos-Ocana
C
, Padilla
S
, Villalba
JM
, Lopez-Lluch
G
, Martin-Montalvo
A
, Minor
RK
, Sinclair
DA
, de Cabo
R
and Navas
P.
NQR1 controls lifespan by regulating the promotion of respiratory metabolism in yeast.
Aging Cell.
2009;
8:
140
-151.
[PubMed]
.
-
22.
Villalba
JM
, Navarro
F
, Cordoba
F
, Serrano
A
, Arroyo
A
, Crane
FL
and Navas
P.
Coenzyme Q reductase from liver plasma membrane: purification and role in trans-plasma-membrane electron transport.
Proc Natl Acad Sci U S A.
1995;
92:
4887
-4891.
[PubMed]
.
-
23.
Navas
P
, Villalba
JM
and de Cabo
R.
The importance of plasma membrane coenzyme Q in aging and stress responses.
Mitochondrion 7 Suppl.
2007;
7:
S34
-40.
.
-
24.
Villalba
JM
and Navas
P.
Plasma membrane redox system in the control of stress-induced apoptosis.
Antioxid Redox Signal.
2000;
2:
213
-230.
[PubMed]
.
-
25.
De
Cabo R
, Cabello
R
, Rios
M
, Lopez-Lluch
G
, Ingram
DK
, Lane
MA
and Navas
P.
Calorie restriction attenuates age-related alterations in the plasma membrane antioxidant system in rat liver.
Exp Gerontol.
2004;
139:
297
-304.
.
-
26.
Hyun
DH
, Emerson
SS
, Jo
DG
, Mattson
MP
and de Cabo
R.
Calorie restriction up-regulates the plasma membrane redox system in brain cells and suppresses oxidative stress during aging.
Proc Natl Acad Sci U S A.
2006;
103:
19908
-19912.
[PubMed]
.
-
27.
Gomez-Diaz
C
, Villalba
JM
, Perez-Vicente
R
, Crane
FL
and Navas
P.
Ascorbate stabilization is stimulated in rho(0)HL-60 cells by CoQ10 increase at the plasma membrane.
Biochem Biophys Res Commun.
1997;
234:
79
-81.
[PubMed]
.
-
28.
Hyun
DH
, Hunt
ND
, Emerson
SS
, Hernandez
JO
, Mattson
MP
and de Cabo
R.
Up-regulation of plasma membrane-associated redox activities in neuronal cells lacking functional mitochondria.
J Neurochem.
2007;
100:
1364
-1374.
[PubMed]
.
-
29.
Navarro
F
, Navas
P
, Burgess
JR
, Bello
RI
, De
Cabo R
, Arroyo
A
and Villalba
JM.
Vitamin E and selenium deficiency induces expression of the ubiquinone-dependent antioxidant system at the plasma membrane.
FASEB J.
1998;
12:
1665
-1673.
[PubMed]
.
-
30.
Padilla
S
, Tran
UC
, Jimenez-Hidalgo
M
, Lopez-Martin
JM
, Martin-Montalvo
A
, Clarke
CF
, Navas
P
and Santos-Ocana
C.
Hydroxylation of demethoxy-Q6 constitutes a control point in yeast coenzyme Q6 biosynthesis.
Cell Mol Life Sci.
2009;
66:
173
-186.
[PubMed]
.
-
31.
Bonawitz
ND
, Chatenay-Lapointe
M
, Pan
Y
and Shadel
GS.
Reduced TOR signaling extends chronological life span via increased respiration and upregulation of mitochondrial gene expression.
Cell Metab.
2007;
5:
265
-277.
[PubMed]
.
-
32.
Smith
DL Jr
, McClure
JM
, Matecic
M
and Smith
JS.
Calorie restriction extends the chronological lifespan of Saccharomyces cerevisiae independently of the Sirtuins.
Aging Cell.
2007;
6:
649
-662.
[PubMed]
.
-
33.
Yang
H
, Yang
T
, Baur
JA
, Perez
E
, Matsui
T
, Carmona
JJ
, Lamming
DW
, Souza-Pinto
NC
, Bohr
NA
, Rosenzweig
A
, de Cabo
R
, Sauve
AA
and Sinclair
DA.
Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival.
Cell.
2007;
130:
1095
-1107.
[PubMed]
.
-
34.
Reddy
VV
, Kupfer
D
and Caspi
E.
Mechanism of C-5 double bond introduction in the biosynthesis of cholesterol by rat liver microsomes.
J Biol Chem.
1977;
252:
2797
-2801.
[PubMed]
.
-
35.
Keyes
SR
and Cinti
DL.
Biochemical properties of cytochrome b5-dependent microsomal fatty acid elongation and identification of products.
J Biol Chem.
1980;
255:
11357
-11364.
[PubMed]
.
-
36.
Passon
PG
and Hultquist
DE.
Soluble cytochrome b5 reductase from human erythrocytes.
Biochim Biophys Acta.
1972;
275:
62
-73.
[PubMed]
.
-
37.
Jaffé
ER
Methemoglobin pathophysiology.
Progress Clin Biol Res.
1981;
51:
133
-151.
.
-
38.
Percy
MJ
and Lappin
TR.
Recessive congenital methaemoglobinemia: cytochrome b5 reductase deficiency.
Br J Haematol.
2008;
141:
298
-308.
[PubMed]
.
-
39.
Rajcevic
U
, Petersen
K
, Knol
JC
, Loos
M
, Bougnaud
S
, Klychnikov
O
, Li
KW
, Pham
TV
, Wang
J
, Miletic
H
, Peng
Z
, Bjerkvig
R
, Jimenez
CR
and Niclou
SP.
iTRAQ-based proteomics profiling reveals increased metabolic activity and cellular cross-talk in angiogenic compared with invasive glioblastoma phenotype.
Mol Cell Proteomics.
2009;
8:
2595
-2612.
[PubMed]
.
-
40.
Price
JA
, Rogers
JV
, McDougal
JN
, Shaw
MQ
, Reid
FM
, Kiser
RC
and Graham
JS.
Gene expression analysis of bromine-induced burns in porcine skin.
Toxicol Lett.
2008;
182:
69
-78.
[PubMed]
.
-
41.
Choudhary
C
, Kumar
C
, Gnad
F
, Nielsen
ML
, Rehman
M
, Walther
TC
, Olsen
JV
and Mann
M.
Lysine acetylation targets protein complexes and co-regulates major cellular functions.
Science.
2009;
325:
834
-840.
[PubMed]
.