Abstract
The Mitochondrial Free Radical Theory of Aging (MFRTA) is currently one of the most widely accepted theories used to explain aging. From MFRTA three basic predictions can be made: long-lived individuals or species should produce fewer mitochondrial Reactive Oxygen Species (mtROS) than short-lived individuals or species; a decrease in mtROS production will increase lifespan; and an increase in mtROS production will decrease lifespan. It is possible to add a further fourth prediction: if ROS is controlling longevity separating these parameters through selection would be impossible. These predictions have been tested in Drosophila melanogaster. Firstly, we studied levels of mtROS production and lifespan of three wild-type strains of Drosophila, Oregon R, Canton S and Dahomey. Oregon R flies live the longest and produce significantly fewer mtROS than both Canton S and Dahomey. These results are therefore in accordance with the first prediction. A new transgenic Drosophila model expressing the Ciona intestinalis Alternative Oxidase (AOX) was used to test the second prediction. In fungi and plants, AOX expression regulates both free radical production and lifespan. In Drosophila, AOX expression decreases mtROS production, but does not increase lifespan. This result contradicts the second prediction of MFRTA. The third prediction was tested in flies mutant for the gene dj-1β. These flies are characterized by an age-associated decline in locomotor function and increased levels of mtROS production. Nevertheless, dj-1β mutant flies do not display decreased lifespan, which again is in contradiction with MFRTA. In our final experiment we utilized flies with DAH mitochondrial DNA in an OR nuclear background, and OR mitochondrial DNA in DAH nuclear background. From this, Mitochondrial DNA does not control free radical production, but it does determine longevity of females independently of mtROS production. In summary, these results do not systematically support the predictions of the MFRTA. Accordingly, MFRTA should be revised to accommodate these findings.
Introduction
At present, the
Mitochondrial Free Radical Theory of Aging (MFRTA) is one of the most widely
believed and supported theories of aging. As well as putting forward an
explanation for aging it allows the explanation of inter
and intra species differences. According to this theory, free radicals,
essentially Reactive Oxygen Species (ROS) which are produced as by-products
during normal metabolism inside mitochondria provoke the accumulation of
oxidative damage. The accumulation of this oxidative damage is believed to
disturb cellular homeostasis which, in turn, is responsible for the aging
process. In spite of its attractiveness, MFRTA has received some recent
criticism [1,2,3]. Indeed, some evidence indicates that free radicals are part
of a complex network of cellular signaling, and not just toxic by-products of
metabolism. Their relationship with aging may therefore be far from
straightforward [4].
From MFRTA is it is possible to make three basic
predictions: 1) long-lived individuals or species should produce fewer
mitochondrial ROS (mtROS) than those which are short-lived, 2) a decrease in
mtROS production will increase lifespan and 3) an increase in mtROS production
will decrease lifespan. We can add one more prediction: if mtROS is controlling
aging then both lifespan and ROS production are inherently linked. Most
evidence in support of MFRTA comes from comparative biology and Dietary
restriction (DR) studies, which have attempted to experimentally test the first
prediction. It has been shown in several systems that isolated mitochondria
from long-lived animals produce fewer mtROS than short-lived ones [5]. It is
from these types of studies that a general ‘law' has been proposed, such that
lower mtROS production results in a longer lifespan. However, two important
exceptions to this law have recently beendescribed
[6,7] Ames dwarf mutant mice are the longest-living mouse strain [8], but their
mitochondria produce more free radicals than normal controls. Naked-mole rats
are the longest-living rodents (Maximum Lifespan (MLS) = 28 years), yet they
produce mtROS at the same rate as short-lived mice (MLS= 4 years).
Paradoxically, naked-mole rats also have extraordinarily low levels of glutathione
peroxidase [9], which could be responsible for the accumulation of unusually
elevated levels of oxidative damage in proteins, lipids and nucleic acids
[10].
Dietary
Restriction (DR) is the only non-genetic treatment that has been shown clearly
to increase MLS in most, if not all, species where it has been applied [11].
Since DR decreases mtROS production in isolated mitochondria, a cause and
effect relationship has been proposed (reviewed in [12]). However as several
different physiological parameters are also coordinately altered during DR,
such as insulin signaling [13] and cellular autophagy [14], it is therefore not
possible to attribute exclusively this effect on lifespan to simply the
attenuation of mtROS production. Moreover, moderate exercise or protein
restriction have also been shown to decrease free radical production in a
similar way to DR, but do not increase MLS (reviewed in [2]).
In
summary, MFRTA is currently mainly supported by indirect data which show a
negative correlation between free radical production in isolated mitochondria
and lifespan in several different model organisms. However, correlations can
suggest but not demonstrate causality. In fact, the only definitive way to test
MFRTA is to specifically decrease (or increase) mtROS production and to study
the effect of such a modification on lifespan. In the present study we have
employed a systematic testing of all basic predictions of MFRTA using such a
strategy.
Drosophila melanogaster is an
excellent model organism to study aging due to its short generation time and
lifespan, the availability of the genome sequence and anenormous catalogue of genetic tools. In insects, as in
mammals, there is a negative correlation between free radical production in
isolated mitochondria and lifespan [15]. Thus, the extreme longevity of queen
ants and bees is correlated with a resistance to oxidative stress [16,17].
Evidence from studies in Drosophila melanogasterstrongly supports MFRTA (reviewed in [18]). For example, oxygen tension
modulates Drosophila lifespan and gene expression maps are similar in
old and chronically hyperoxic flies [19]. Moreover, antioxidant therapies
appear to be effective in delaying aging in Drosophila [20,21] ,
although some authors claim that the increase in lifespan is only produced in
short-lived lines [22] or that it is not related to oxidative damage directly
but through the activation of survival-signaling pathways [23]. However, there
is also data from Drosophila studies that appears to contradict MFRTA.
For example, DR has been shown to increase lifespan in Drosophila [24]
without altering free radical production [25]. It is for these reasons that we
have chosen Drosophila as model organism to test MFRTA.
We
first studied free radical production in three independent wild-type strains ofD. melanogaster which show a substantial variation in longevity. The
results of our study show that the longest-lived strain produces the fewest
mtROS which is consistent with MFRTA. However, the longest-lived flies could
have characteristics independent of mtROS that might confer the superior
longevity. A more rigorous way to examine MFRTA is to test its second
prediction by directly manipulating mtROS production. If MFRTA applies,
individuals producing fewer mtROS should be long-lived. Unfortunately, the
exact location and mechanism by which free radicals are produced in the
electron transport chain (ETC) remains unclear. This means that any genetic
modification to the ETC would most likely result in an increase in free radical
production and therefore deleterious effects. However, nature provides some
potential solutions to by-pass this problem.
Fungi and plants modulate mitochondrial free radical levels through the
expression of an enzyme named the Alternative Oxidase (AOX). AOX can by-pass
the mitochondrial ETC at complexes III and IV, concomitantly decreasing mtROS
generation [26]. Its expression has been shown to increase lifespan, at least
in some fungi [27]. Our group has recently introduced a copy of the AOX gene
from the urochordate Ciona intestinalis into human cells [28,29] and
into Drosophila melanogaster [30]. AOX expression confers new
physiological properties to cells and animals, such as resistance to ETC
inhibitors and partial rescue of metabolic alterations caused by genetic
disruption of the ETC complexes or their biosynthesis. We hypothesized that AOX
expression would decrease mitochondrial free radical production in
Drosophila and, if MFRTA is correct, that AOX expression should therefore
also increase the lifespan of individuals expressing it. The third prediction
was tested using a Drosophila mutant, dj-1β, which has
previously been shown to have increased mitochondrial free radical production
in aged flies, manifesting as a severe impairment of locomotive function [30].
If MFRTA is correct, dj-1β mutant flies should also be short-lived.
Finally, we have selected flies with mitochondrial DNA from the OR long-lived
background in DAH short-lived nuclear background (and vice versa), and we have
measured both mtROS and lifespan. If MFRTA is correct both parameters should be
inherently linked and therefore related such that an alteration of one
parameter would translate into a direct effect on the other.
Results
Testing prediction #1:
"Long-lived individuals should produce fewer mtROS"
In order to test the first prediction we investigated the
relationship between levels of mitochondrial ROS production and lifespan in
three different wild-type strains of Drosophila melanogaster (OR, CS and
DAH).
Mitochondrial ROS Production in wild-type strains
mtROS
production was measured in 10 day old flies using two different substrates to
identify which ETC complex or complexes (if any) are implicated in variation of
mtROS production. Using a (pyruvate + proline) substrate cocktail,
significant differences were detected between groups (p < 0.001,
Figure 1A). OR flies (both males and females) produced fewer mtROS than the
other groups. CS males produced fewer mtROS than DAH males, whereas there were
no differences between DAH females and CS flies. OR and DAH females produced significantly fewer mtROS than the
corresponding males. Using SP3G as a substrate significant differences were
also detected (p < 0.001; Figure 1A), but these were essentially a result of
a lower mtROS production of OR males with respect to the other groups. Most of
the mtROS production detected using S3PG as substrate is generated during the
reverse transfer of electrons between the ubiquinone pool and complex I or by
complex III [31]. In relation to aging, complex I seems to be more relevant
than complex III (reviewed in [5]). Therefore, to study in detail the role of
complex III in mtROS production, rotenone was added and experiments using S3PG
were repeated. When rotenone was present, no significant differences were
detected between groups (p = 0.05; Figure 1A). These results indicate that
differences between groups are due to variation in ROS produced by complex I,
but only when electrons flow in the forward direction. This is in accordance
with most published data, supporting an instrumental role of complex I, but not
complex III, in mediating variation of mtROS production related to longevity
(e.g. [6,34]).
Mitochondrial oxygen consumption
Mitochondrial
oxygen consumption was studied in parallel with ROS measurements in order to
investigate whether differences in ROS production are related to overall oxygen
consumption or to coupling. No differences were detected either in state 4 or
state 3 respiration when pyruvate + proline was used as substrate (p >
0.05; Table 1). However, RCI was significantly different between the groups (p< 0.05), being consistently lower in males than females. Using S3PG
(+rotenone) a similar trend was seen (Table 1); no significant differences were
found in state 4 or state 3 respiration (p > 0.05), but OR males have
a lower RCI (p < 0.05) males compared to other groups.
Lifespan studies in wild-type strains
The
mean and MLS of females was found to be extended in comparison with males in
all the strains studied (Figure 1B). OR males lived 51% longer than DAH males
and 40% longer (p < 0.001) than CS males. Strikingly, OR males also
produced only 53% of the mtROS produced by DAH males and 38% of that produced
by CS males when pyruvate + proline was the substrate. OR females lived longer
(p < 0.001) than DAH and CS females, although differences were much
smaller (12% and 8% respectively). Our data are consistent with a direct
(inverse) correlation between ROS production at complex I and lifespan in
wild-type Drosophila strains. In order to confirm such a relationship,
we looked at further possible correlations between
different parameters associated with ROS generation, oxidative metabolism and
MLS. The only significant correlation found was with mtROS production using
pyruvate + proline as substrate (Supplementary Figure 1). Interestingly, antioxidants levels analyzed by qPCR (Supplementary Figure 2)
negatively correlate with lifespan. This is in agreement with the idea that
long-lived strains decrease the generation of damage rather than increase
defense or repair in order to increase longevity.
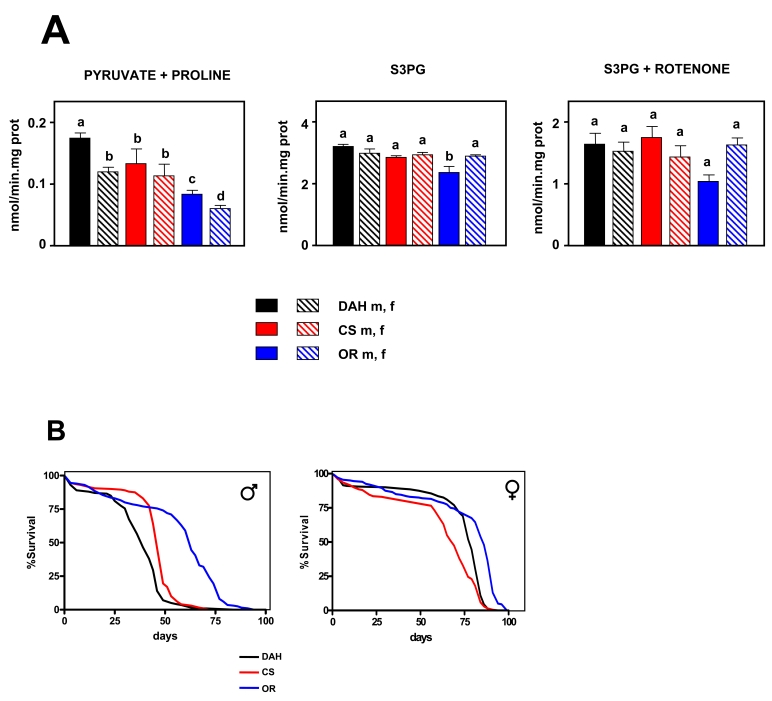
Figure 1. Mitochondrial ROS production versus lifespan in three wild type strains of Drosophila melanogaster.
(A) Rate of mtROS production (assayed as H2O2,
mean +
SEM). a, b, c and d indicate
statistically significant differences between groups (ANOVA, p <
0.05, n = 5-9 samples per group), m: male, f: female. (B) Survival
curves. Combined data from two independent experiments using 100
flies per group per experiment. Mean, maximum lifespans (d) were: DAH males
[39,49]; CS males (46, 53); OR males (63, 74); DAH females (79, 84); CS
females (69, 81); OR females (86, 91).
Table 1. Mitochondrial oxygen consumption (nmol O 2/min.mg
prot) in three wild type strains of Drosophila melanogaster.
Results are presented as mean ±SEM. Number of independent samples in parentheses.
Different letters (a, b) denote statically significant differences between
groups. DAH = Dahomey, CS = Canton S, OR = Oregon R.
| DAH | CS | OR | ANOVA |
| males | females | males | females | males | females | |
|
Pyruvate +
Proline
|
| State 4
|
30 ± 3 (6)
|
29 ± 4 (6)
|
28 ± 4 (6)
|
25 ± 1 (6)
|
36 ± 3 (6)
|
23 ± 4 (6)
|
NS
|
| State 3
|
313 ± 24 (6)
|
390 ± 24 (6)
|
366 ± 16 (6)
|
393 ± 25 (6)
|
316 ± 24 (6)
|
324 ± 24 (6)
|
NS
|
| RCI
|
10.6 ± 1.1 (6)a |
15.2 ± 2.1 (6)b |
13.8 ± 1.1 (6)a |
15.7 ± 1 (6)b |
9 ± 1 (6)a |
14.9 ± 1.7 (6)b | p <
0.01
|
|
sn-glycerol-3-Phosphate
+ rotenone
|
| State 4
|
77 ± 10 (8)
|
73 ± 9 (6)
|
78 ± 10 (8)
|
70 ± 7 (7)
|
74 ± 11 (7)
|
67 ± 6 (8)
|
NS
|
| State 3
|
153 ± 19 (8)
|
187 ± 25 (6)
|
156 ± 32 (8)
|
152 ± 19 (7)
|
116 ± 15 (7)
|
158 ± 18 (8)
|
NS
|
| RCI
|
2.2 ± 0.1 (8)a |
3 ± 0.1 (6)a |
2.3 ± 0.2 (8)a |
2.6 ± 0.2 (7)a |
1.9 ± 0.2(7)b |
2.6 ± 0.1 (8)a | p < 0.001
|
Table 2. Mitochondrial oxygen consumption (nmol O 2/min.mg prot) in in wild type flies (wt)
and flies expressing (AOX/da-GAL4), or not expressing AOX (AOX/–).
AOX flies are from line F6. For equivalent data for line F24 see Supplementary Table 1. Results are presented as
mean ± SEM.
Number of independent samples in parentheses.
| wt | AOX/- | AOX/da-GAL4 | ANOVA |
| males | females | males | females | males | females | |
|
Pyruvate +
Proline
|
| State 4
|
35 ± 7 (9)
|
42 ± 9 (10)
|
32 ± 7 (8)
|
35 ± 6 (9)
|
35 ± 5 (7)
|
46 ± 5 (10)
|
NS
|
| State 3
|
410 ± 45 (9)
|
493 ± 48 (10)
|
419 ± 41 (8)
|
495 ± 40 (9)
|
488 ± 54 (7)
|
491±35 (10)
|
NS
|
| RCI
|
16 ± 5 (9)
|
17 ± 5 (10)
|
15 ± 3 (8)
|
19 ± 4 (9)
|
15 ± 2 (7)
|
15 ± 1 (10)
|
NS
|
|
sn-glycerol-3-Phosphate
+ rotenone
|
| State 4
|
65 ± 16 (6)
|
88 ± 27 (6)
|
132 ± 28
(8)
|
112 ± 12
(7)
|
98± 29 (5)
|
104 ± 14
(8)
|
NS
|
| State 3
|
255 ± 58
(8)
|
273 ± 35
(6)
|
254 ± 27
(8)
|
288 ± 45
(7)
|
241 ± 26
(5)
|
276 ± 44
(8)
|
NS
|
| RCI
|
2.1 ± 0.2 (8)
|
2.2 ± 0.4 (6)
|
2.3 ± 0.7 (8)
|
2.3 ± 0.4 (7)
|
2.4 ±
0.7(5)
|
2.9 ± 0.5
(8)
|
NS
|
Testing prediction #2: "A decrease in
mtROS production should increase MLS"
Expression
of AOX in DAH background
In
order to check the second prediction of MFRTA we expressed the alternative
oxidase (AOX) of Ciona intestinalis in flies, after backcrossing to the
DAH background for 11 generations (the flies expressing the daughterless-GAL4 driver were also backcrossed in the same
conditions). AOX is able to regulate mtROS generation in plants and fungi, and
its expression has been related to an increase in longevity in fungi [26,27].
Firstly, we performed some routine experiments to check the presence and
functionality of AOX in vivo, in the backcrossed flies. We tested
resistance to three different inhibitors of the ETC: 1) rotenone (Complex I),
2) antimycin A (Complex III) and 3) KCN (Complex IV). Flies expressing AOX
showed an increased resistance to antimycin A and KCN compared to
non-expressing flies (Figure 2A and Supplementary Figure 3). Differences in survival were
observed after only 10 min of exposure to drugs inhibiting either complex III
or IV. After 24 h of exposure only flies expressing AOX survived. However, no
difference was observed when a complex I inhibitor (rotenone) was employed. In
order to confirm that these observations were a result of AOX expression we
tested the effect of the inhibitors also on mitochondrial bioenergetics and ROS
production. Using isolated mitochondria we observed that AOX is able to support
state 3 oxygen consumption in the presence of antimycin
or KCN, but not in the presence of rotenone (Figure 2B). Moreover, AOX decreased mtROS production in the presence of complex III or
IV inhibitors, but not in the presence of an inhibitor of complex I (Figure 2C).
These data imply that AOX is expressed and is functional in vivo.
Additionally AOX behaves as theoretically
expected, e.g. AOX-expressing flies are resistant to blocks in complex III or
IV, but not I.
Effects of AOX on mtROS production and oxygen consumption
Having
established that AOX was functional in vivo, we studied mtROS production
in isolated mitochondria in normal conditions (i.e. without inhibitors). The
same experiments carried out in wild-type strains were repeated in AOX
transgene-expressing and non-expres-sing flies from two independent transgenic
lines and in wild type (DAH) controls. AOX was found to decrease mtROS
production in 2-3 day old flies when either pyruvate + proline (by 32-34%) or
S3PG (by 16-20%) was used as a substrate (Figure 3A, S4). When rotenone was
also present in the assay medium AOX flies still produced fewer (27-37%) mtROS
than controls with S3PG as substrate (Figure 3A). However; AOX did not modify
oxygen consumption in state 3 nor state 4 (Tables 2, S1). We also studied the
effects of AOX expression in aged flies. We repeated the same measurements in
30 day old males and 50 day old females, representing equivalent time points in
normal male and female lifespan in the DAH background, but under which
conditions more than 50% of flies are still alive, thus avoiding the selection
of a sub-population.
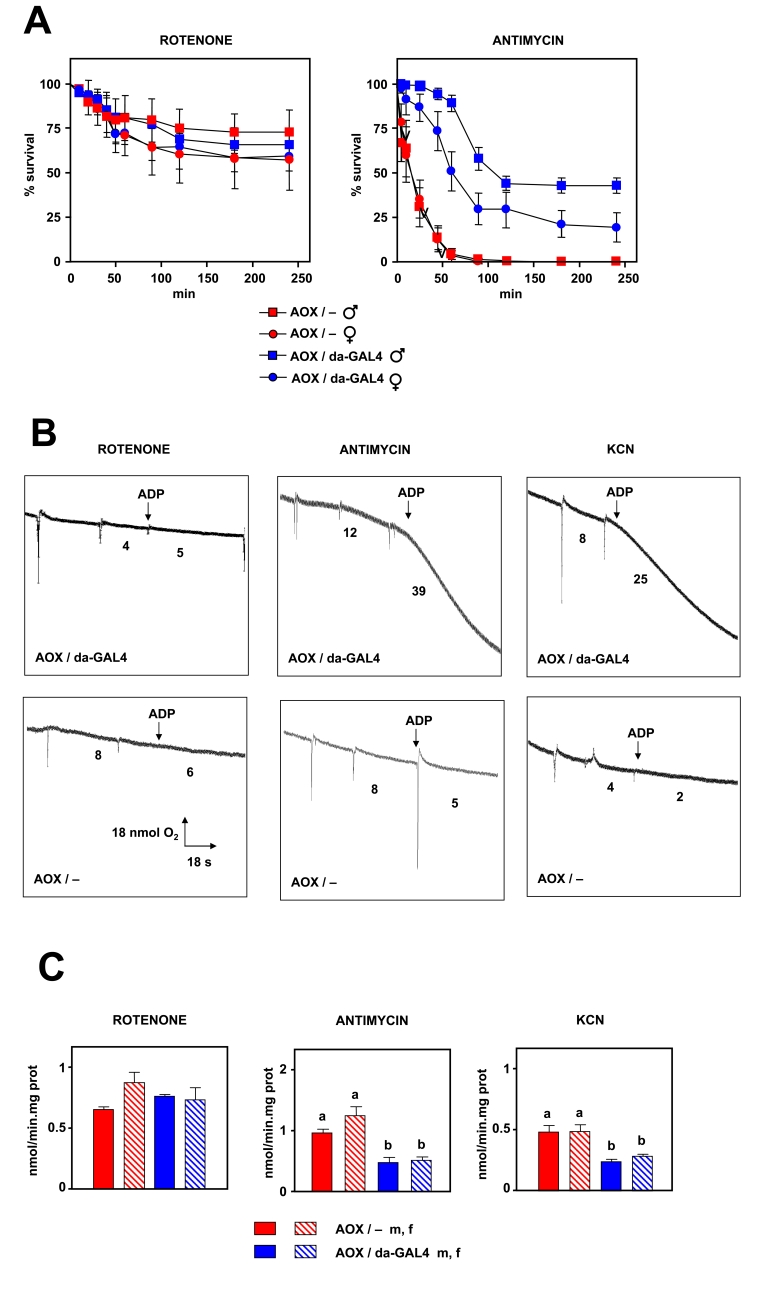
Figure 2. Effects of AOX expression on resistance to respiratory chain inhibitors. (A) Survival
after exposure to 3
mM rotenone or 3 mM antimycin A, of flies of strains and sexes indicated
(AOX / -, flies transgenic for UAS-AOX in absence of GAL4 driver; AOX /
da-GAL4, flies transgenic for AOX in presence of da-GAL4 driver). (B)
Representative oxygraph traces of mitochondrial suspensions (0.5 mg/ml in
state 3) in presence of inhibitors shown. Inferred oxygen consumption rates
(nmol/min) as indicated. Pyruvate+proline was used as substrate in all
experiments. (C) mtROS production (mean +
SEM) in presence of
inhibitors (at least 4 independent samples per experiment, a, b denote
significantly different groups, ANOVA, p < 0.05).
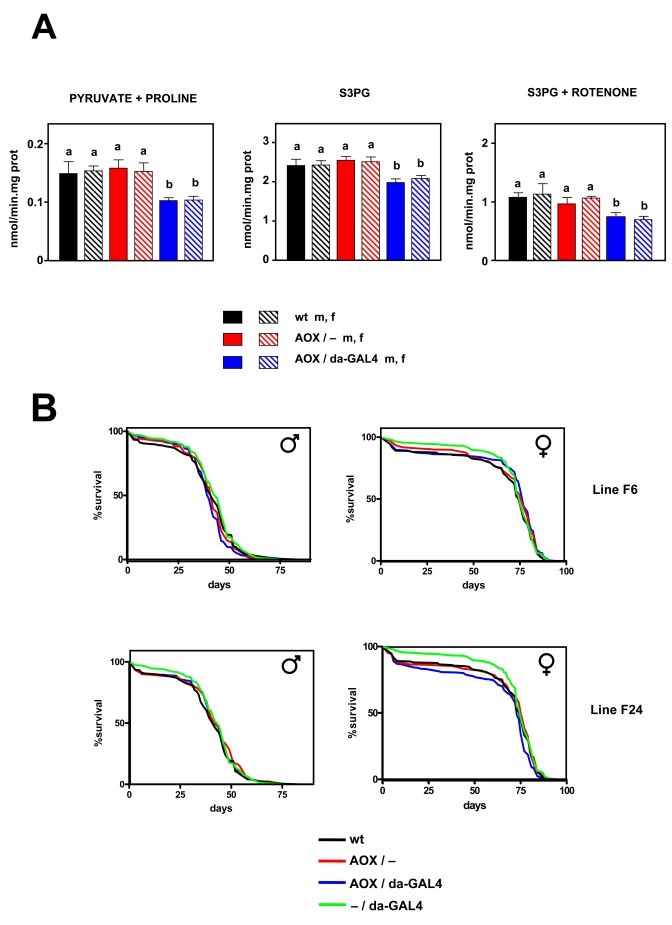
Figure 3. Effect of AOX expression on mtROS production and lifespan. (A) mtROS
production (mean +
SEM). a, b: statistically significant differences
between groups (ANOVA, p < 0.05, n=4-8 samples per group) m:
male, f: female. (B) Survival curves for wild
type (wt), AOX non-expressing (AOX / -), AOX expressing (AOX / da-GAL4 +),
and driver only (- / da-GAL4) flies, all in the DAH (w-)
background. Flies of AOX transgenic lines F6 and F24 as indicated. Combined
data from two independent experiments using 200 flies per group per
experiment. Mean, maximum life spans (d) were: wt males (42,51); wt
females (75, 82); - / daGAL4 males (44, 54); -
/ da-GAL4 females (75, 81), F6 AOX / - females (77, 82); F6 AOX / - males
(42,51); F6 AOX / da-GAL4 males (40,47); F6 AOX / da-GAL4 females (82,
51); F24 AOX / - males (42, 54); F24 AOX / - females (77, 81); F24 F24 AOX /
da-GAL4 males (42, 54); AOX / da-GAL4 females (73, 80).
At the ages studied, AOX also decreased mtROS production,
both in the presence of KCN and absence of ETC inhibitors (Supplementary Figure 5). During
aging mitochondrial oxygen consumption was strongly decreased and AOX was not
able to compensate this decrease (Figure 4A, B). At the same time mtROS
generation was increased, but AOX was able to negate the increase in such a way
that mtROS production in old AOX-expressing flies was similar to that in young
control flies (Figure 4C, D).
Lifespan and AOX
Lifespan was studied in the same two AOX transgenic- lines
(F6 & F24). Both lines have an AOX insertion in an intergenic region, but
on different chromosomes (2 and 3, respectively, [30]). Two independent experi-ments
each with 200 flies per group were carried out. AOX did not significantly
increase lifespan in any of the lines studied (Figure 3B). In males, AOX had a
slightly deleterious effect on longevity in line F6 (MLS decreased by around
9%; p < 0.05), but none at all in line F24 (p > 0.05),
whereas in females the opposite was found: no differences were observed in line
F6 (p > 0.05), but it in line F24 a small decrease (1-3%, p
< 0.05) was seen. In summary, AOX expression did not consistently or
significantly modify lifespan in Drosophila.
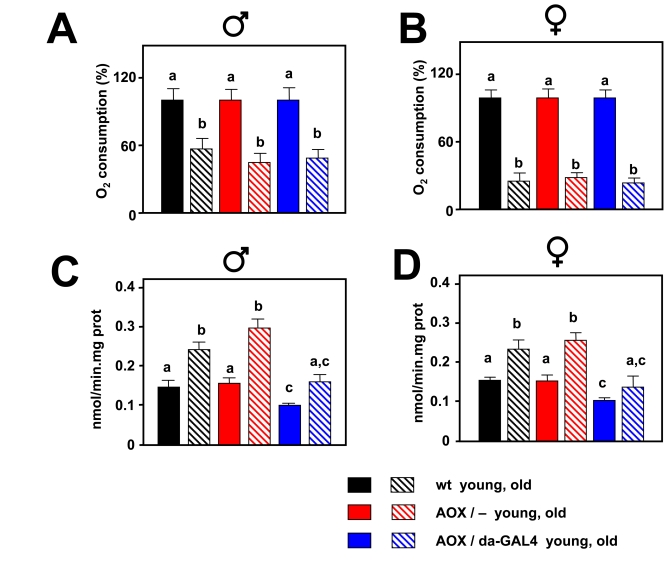
Figure 4. Effect of AOX on mitochondrial bioenergetics and mtROS during aging. Oxygen
consumption in state 3 (% of that in the young group) in 30 d old males (A)
and 50 d old females. (B) AOX expression is not able to compensate
the decrease in oxygen consumption associated with aging. mtROS generation
(nmol H2O2/min.mg.prot) in 30 d old males (C)
and 50 d old females (D). AOX expression diminishes mtROS production
in both young and aged flies, and compensates for the age-associated
increase. a, b and c denote statistically significant differences between
groups (ANOVA, p < 0.05, n = 4-10 samples per group).
Pyruvate+proline was used as substrate in all experiments. Plotted data are
means ± SEM.
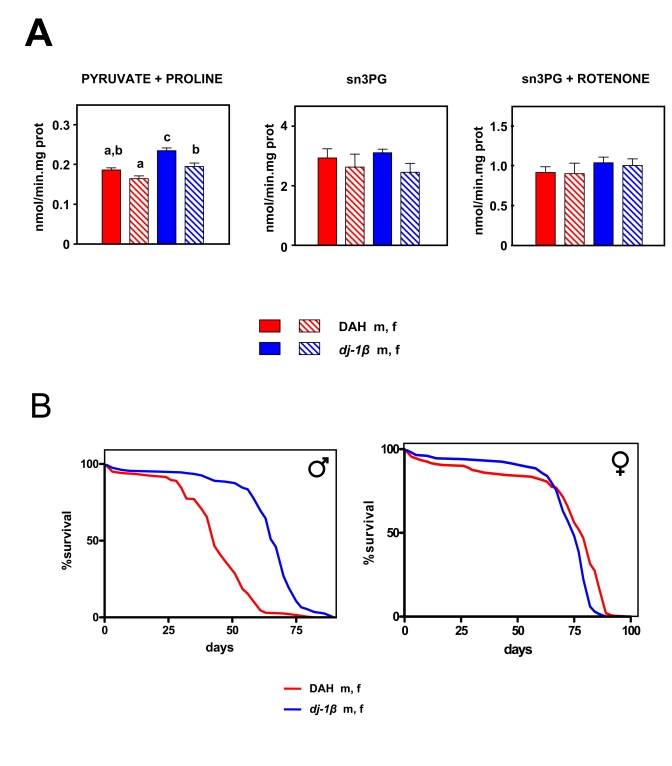
Figure 5. Effects of the dj-1β mutation on mtROS production and lifespan in Drosophila melanogaster. (A) mtROS production, assayed as H2O2,
(mean + SEM). a, b and c denote statistically significant differences between
groups (ANOVA, p<0.05, n = 4-6 samples per group), m: male, f: female. (B)
Survival curves. Combined data from two independent experiments using 100 flies per
group per experiment. Mean, maximum lifespans (d) were: DAH males (43, 58); DAH
females (75, 79); dj-1β mutant males (67, 75); dj-1β mutant females (79, 86).
Testing
prediction #3: "An increase in mtROS production should decrease MLS"
Mitochondrial free radical production and oxygen
consumption in dj-1β mutant flies
We measured mitochondrial free radical
production in 10 day old dj-1β mutant flies using flies from the
DAH background as controls. As expected, dj-1β mutant flies
produced more mtROS than wild-type controls with pyruvate + proline as
substrate (Figure 5A; p <
0.001), although no significant
differences were observed when S3PG was used as a substrate (Figure 5A; p > 0.05). Differences in free radical production were not
reflected in oxygen consumption (Table 3). Recently, we showed that dj-1β
mutant flies produce more mtROS than wild-type flies at 3 weeks of age [30].In our previous report only pyruvate + prolinewas used as a substrate. Our present findings confirm
these results and clarify the mechanism whereby the dj-1β mutation
alters mtROS production. Only when electrons flow in the forward direction
through complex I are differences detected between mutants and controls.
Together, these data support the idea that dj-1β works as a peroxiredoxin [35]. When pyruvate + proline
is used as substrate most of the ROS generated are directed to the
mitochondrial matrix where dj-1β can exert its detoxifying action, whereas when SP3G is
used as the substrate ROS production is split between the matrix and the
inter-membrane space [31], decreasing the potential role of dj-1β in the
detoxification process.
Lifespan
of dj-1β mutant flies
In spite of increased levels of mtROS production dj-1β mutant flies were found to have a longer, not
shorter lifespan than DAH flies of the corresponding sex: by 30%
in males and 9% in females (Figure 5B, p < 0.001). Moreover, even
after seven generations of backcrossing in to the DAH background (reducing
background effects to a minimum)
differences in lifespan between mutants and non-mutants for the dj-1β
were maintained (Supplementary Figure 7A).
Table 3. Mitochondrial oxygen consumption (nmol O 2/min.mg prot) in DAH wild-type and dj-1β mutant.
| DAH | dj-1β |
| males
(6) | females
(7) | males
(7) | females
(10) |
| State 4 |
33 ± 5
|
33 ± 8
|
38 ± 4
|
28 ± 5
|
| State 3 |
322 ± 16
|
396 ± 58
|
279 ± 37
|
281 ± 22
|
| RCI |
13± 2
|
12±1
|
9± 1
|
13 ± 2
|
Lifespan
of dj-1β mutant flies
In spite of increased levels of mtROS production dj-1β
mutant flies were found to have a longer, not shorter lifespan than DAH flies
of the corresponding sex: by 30% in males and 9% in females (Figure 5B, p
< 0.001). Moreover, even after seven generations of backcrossing in to the
DAH background (reducing background effects to a minimum) differences in
lifespan between mutants and non-mutants for the dj-1β were
maintained (Supplementary Figure 7A).
Testing
prediction #4: "An increase in mtROS production should decrease MLS"
Analysis of mitochondrial DNA
The nucleotide sequence of mitochondrial gene coI was
analyzed in three different wild type strains of Drosophila melanogaster
(OR, DAH and CS) as described in material and methods. We found 10 different
polymorphic sites (Supplementary Table 3), although none of the modifications in the gene
analyzed caused changes in the amino acid sequence of the protein (all of them
were synonymous substitutions). The presence of so many polymorphisms indicates
that they could play an important role in Drosophila physiology
including aging and ROS production and so we investigated the question in more
detail.
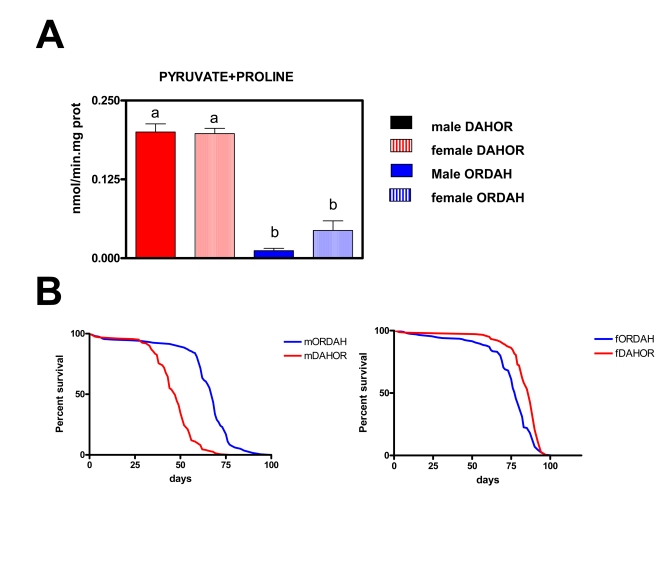
Figure 6. Effects of the changes on mtDNA content on mtROS production and lifespan in new wild type strains of Drosophila melanogaster (DAHOR and ORDAH).
(A) mtROS production, assayed as H2O2,
(mean + SEM). a and b denote statistically significant differences between
groups (ANOVA, p <0.05, n = 4-5 samples per group), m: male, f: female.
(B) Survival curves. Combined data from two independent experiments
using between 80-100 flies per group per experiment. Mean, maximum lifespans
(d) were: ORDAH males (68, 76); ORDAH FEMALES (78, 87); DAHOR males (47,59);
DAHOR females (87,92).
ROS production
Initially we wanted
to know if mitochondrial ROS production was modulated by polymorphisms in mtDNA
so we decided to create new strains of Drosophila melanogaster putting the
mitochondrial genome of OR flies in a DAH nuclear background (and vice versa).
We measured ROS production in 2/3 days old flies (Figure 6A). At this age we
did not find any significant differences between males and females, this
mirrors what is seen in the original DAH background where sex differences are
only detected after 10 days. ROS production was lower (around 86%) in flies
with an OR nuclear background independently of the DAH mitochondrial DNA
background. In fact no differences in ROS were found between OR and ORDAH flies
or between DAH and DAHOR flies (data not shown). This data clearly shows that
mitochondrial DNA does not control free radical production or at least the free radical production
related with longevity in these wild type Drosophila strains. Similar
results were obtained when OR mtDNA was expressed in a CS nuclear background and
vice versa (data not shown), this indicates that the phenomenon is not
restricted to the DAH/OR strains.
Life span of DAHOR and ORDAH strains
Males and females answered differently to changes in the
mitochondrial DNA composition. In the males (Figure 6B) the OR background
resulted in a longer lifespan (45% mean and 29% MLS) independently of the
mitochondrial DNA. This is in agreement with the lower levels of ROS generation
in ORDAH flies. However, in females the results were totally opposite, mtDNA
determines longevity independently of either nuclear DNA or levels of ROS
generation. According to this females with OR mtDNA live longer than females
with DAH mtDNA. Mean and maximum lifespan were 12% and 6% (p<0,001)
respectively longer in DAHOR females than in ORDAH females in spite of this ROS
production was 4.5-fold times higher in the DAHOR females (p<0.001).
Discussion
The MFRTA is one of themost
widely invoked hypotheses accounting for aging, yet the evidence in support of
it is almost entirely indirect. In this study we set out to test its
predictions experimentally. Although we found a negative correlation between
mtROS production and lifespan in 3 wild-type strains of Drosophila
melanogaster (further support for low level of expression of antioxidants
in long-lived individuals) lifespan was not modified as predicted, as a
result of genetic manipulations designed to alter mtROS levels. Moreover, we
were able to dissociate lifespan and ROS production in wild type strains
through changes in the mitochondrial DNA.
A negative correlation between mtROS production in
isolated mitochondria and lifespan in flies, mammals and birds [6,15,36] and
under conditions of DR [12] has been previously reported. We found a similar
relationship, with a long-lived strain (OR) producing fewer mtROS than
short-lived strains (DAH and CS), without a major alteration in oxygen
consumption. This was more pronounced in males, and held up in females only
using a complex I-linked substrate mix. Our findings thus resolves
contradictions of previous studies and emphasizes the importance of studying
both sexes and using both complex I- and III-linked substrates. For example,
Miwa et al. [25] found no correlation between longevity and mtROS in flies
subjected to DR, but their study only looked at females using S3PG as a
substrate. Conversely, Sohal et al. [15] did find a correlation using S3PG in
males. However, all such correlations provide only indirect support for MFRTA.
Interestingly, the expression of 4 antioxidants negatively correlates with
lifespan. This supports the idea that long-lived species produce fewer mtROS
and consequently need lower levels of antioxidants.
AOX
in plants and fungi has been shown to decrease mtROS production when the
cytochrome segment of the respiratory chain, but not complex I, is inhibited.
When C. intestinalis AOX was expressed in Drosophila (this study
and [30]) mtROS production was similarly diminished in the presence of
antimycin or cyanide, but not rotenone. Furthermore, AOX expression sustained
a substantial cyanide- or antimycin-resistant substrate oxidation in
mitochondrial suspensions. AOX expression also decreased mtROS production under
basal conditions, using either pyruvate + proline or S3PG (with or without
rotenone) as a substrate. In plants, the ability of AOX to decrease mtROS
production depends on its ability to keep the ubiquinone pool oxidized [37]. The same could apply in Drosophila, where
semi-ubiquinone at complex I is considered a major site of mtROS generation
[31]. We were unable to detect differences in oxygen consumption under basal
conditions (i.e. without inhibitors) in AOX-expressing flies, suggesting that
AOX does not exert a major effect on respiration in vivo. However, it is
possible that AOX has a subtle effect on normal respiration that cannot be
detected by polarography. The effect on mtROS production of AOX expression in
the DAH background was to diminish it to levels similar to those of wild-type
OR flies. In addition, AOX suppressed the age-associated increase in mtROS
production, but not the age-associated decrease in substrate oxidation by
isolated mitochondria [38,39]. Since AOX expression produced no significant
effect on lifespan, this is consistent with the idea that a mitochondrial
parameter other than ROS production could be a determinant of aging, as
proposed e.g. by Trifunovic and Larsson [40]. Our data showing that mtDNA
composition could regulate lifespan supports such an idea.
Previous
attempts to test the MFRTA by disruption of complex II, both in Drosophila [41]
and Caenorhabditis [42] are limited by the fact that this treatment
clearly produces pleiotropic effects on energy metabolism and development. In
contrast, the dj-1β mutation produces no negative effect on
development or fecundity, its only known phenotypes being age-associated loss
of locomotor function and a hypersensitivity to paraquat. Both of these have
been attributed to the deficit of mitochondrial antioxidant capacity, as
manifested by increased mtROS production compared with that of wild-type
strains, which we confirmed in vitro, using flies of different ages.
Indeed, the locomotor deficiency is corrected by AOX expression [30], which
correlates with decreased mtROS production. Nevertheless, the dj-1β
mutation did not result in shortened lifespan. Surprisingly, the opposite was
observed, with the lifespan dj-1β flies comparable with that of
wild-type OR flies. Moreover, even after backcrossing the dj-1β
mutants for seven generations into a DAH background differences in longevity
were still present. Surprising, the level of expression of antioxidants in dj-1β mutants is reduced when compared
to controls. This indicates that compensation in antioxidant levels can not
account for the long lifespan of dj-1β mutants. Even more
surprising is the fact that correlation between mtROS and lifespan is lost when dj-1β flies are included, but the correlation between antioxidant
levels and lifespan becomes more significant when dj-1β are
included in the correlation (Supplementary Figure 8B,C). DAH background was used as a
control for dj-1β (in experiments using
either isogenic or non-isogenic lines) in order to keep the flies in
experiments increasing (dj-1b mutants) or decreasing (AOX) ROS the same.
For this reason, it may be argued that a decrease in lifespan may be found when dj-1β mutation is expressed in a long-lived background (e.g. OR).
In any case, ROS themselves are bad predictors of lifespan as both OR and dj-1β are long-lived (compared to DAH) in spite of opposite levels of ROS.
One
possible objection to our conclusion that AOX does not increase lifespan
despite diminishing mtROS production would be that the enzyme may not be
functional under normal physiological conditions. However, its ability to
complement several mutations affecting cytochrome oxidase function, as well as
the toxicity of cyanide and antimycin in vivo and the overproduction of
ROSin vitro and in vivo caused by
the dj-1β mutation [30] suggests
otherwise. It can also be argued that an "exogenous" protein cannot increase
the lifespan of the host organism. However, it has been previously shown that
the expression of human UCP-2 increases Drosophila lifespan. And
moreover, we and others have recently demonstrated that it is possible to
increase Drosophila lifespan by expressing: NDI1 that as AOX is not
encoded in the animal host genome [43,44]. Interestingly, the expression of
NDI1 (a protein that can by-pass mitochondrial complex I) significantly
increases lifespan without decreasing the basal rate of ROS production.
We
also cannot exclude that AOX has other, undetected effects influencing
lifespan, which over-ride those mediated through decreased mtROS production.
Note, however, that AOX expression has only a minimal effect on the development
or physiology of wild-type flies [30] and it does not alter the expression of
major antioxidants (Supplementary Figure 6). Certainly, we cannot discard either that the
expression of AOX in certain tissues (e.g. the nervous system) or during
different life stages (e.g. in the last part of life) may have a different
effect on longevity. A similar point could be
made with regard to dj-1β mutant flies, i.e. that increased mtROS
production in vitro and paraquat sensitivity in vivo do not
reflect a systematic effect on mtROS levels in vivo under normal
physiological conditions. Thus, the over-production of ROS in dj-1βmutant flies
could be compensated by the alteration of another function of the protein. DJ-1
participates in RNA metabolism and transcription [45] so its effects on gene
expression could compensate for the over-generation of ROS. However, all these
caveats do not apply to our fourth experimental approach. Where mitochondrial
DNA of OR is expressed in a DAH nuclear background (and vice versa) ROS
production is not altered (it is totally determined by the nuclear background),
but lifespan of females is significantly changed depending on the mitochondrial
background. The fact that DAHOR females are a long-lived strain in spite of
high levels of mtROS production is a strong argument against MFRTA. Moreover,
this demonstrates that it is possible to separate lifespan and ROS production
in wild type strains of Drosophila.
Drosophila melanogaster strains selected for long or
short lifespan [46] exhibit differences in several physiological parameters (including mtROS
production and the levels of antioxidant proteins). It has been suggested that
longevity evolves through coordinated changes in multiple genes and biochemical
pathways [47], which could accommodate our results by postulating that altered
mtROS production or detoxification cannot have a material effect on lifespan
without concomitant changes in other pathways, such as protein acetylation,
insulin signaling or alterations in the degree of un-saturation of lipids in
biological membranes. Moreover, a lifespan-increasing effect in one parameter,
such as a decrease in mtROS production, could result in a compensatory change
in another, such as the repair proteins of DNA, resulting in no net alteration
in lifespan. Once time more the results of DAHOR and ORDAH females support such
hypothesis.
Regardless of the molecular reasons, our findings
indicate that mtROS production is not and cannot be the sole determinant of
lifespan in Drosophila, strengthening similar conclusions arrived at
recently in studies of naked mole rats [9,10], long-lived Ames dwarf mice [7] and C. elegans [48]. However, our findings are subject to two important caveats. First,
the use of in vitro assays to measure mtROS production, which may not reflect
the situation in vivo. And second, the assumption that AOX expression or the
mutational downregulation of dj-1β does not produce pleiotropic or
off-target effects that negate or over-ride effects on mtROS (see above).
The first caveat is one shared
with the great majority (if not all) of studies supporting MFRTA. In fact, it
is still not known if long-lived animals produce fewer free radicals than
short-lived ones in vivo. Further, it has only been demonstrated that isolated
mitochondria from long-lived animals produce fewer molecules of H2O2(data about superoxide are contradictory). Additionally, differences in
ROS generation in isolated mitochondria are only observable under certain
experimental conditions. For example, it is currently assumed that dietary
restricted animals produce fewer mtROS than ad libitum-fed animals. However,
differences are only observed when pyruvate (+malate) (or glutamate (+malate))
are used as a substrate [12]. On the other hand, the use of isolated
mitochondria is required due to the lack of a sensitive method that would allow
quantification of specific free radical species in cells (reviewed in [49]). As
an example of this type of situation we could mention the work carried out in
Brian Merry's laboratory. His laboratory had previously reported differences in
ROS production between ad libitum and caloric restricted rats using isolated
liver mitochondria [50]. However, no differences were observed in the same
experimental model when intact hepatocytes were used for these measurements
[51].
In summary, in order to test
MFRTA we chose an original strategy of trying to modulate the generation of
damage and not just increase antioxidant defense or repair mechanisms. With
this we avoid the drawbacks and caveats of the latter approach [5]. In fact,
even if our results do not reflect the situation in vivo, they are enormously
relevant since they clearly dissociate -for the first time- levels of mtROS
production in isolated mitochondria and longevity in Drosophila melanogaster,
indicating that other mitochondrial factors such as the presence of
polymorphisms in mitochondrial DNA may act as longevity regulators.
Materials
and methods
Flies.
Drosophila wild-type strains Dahomey (DAH),
Canton S (CS) and Oregon R (OR) were obtained from stock-centers or
collaborators. The dj-1βGE23381 mutant [31]: and
AOX-transgenic lines F6 and F24 [30] were as described previously. Flies were
maintained in a standard medium [30], collected using CO2 anesthesia
within 24 h of eclosion, and then kept at a density of 20 flies per vial at 25
ºC in a controlled 12 h light:-dark cycle. Vials were changed every 2-3 days.
We have created two new wild type strains of Drosophilamelanogaster
backcrossing for eleven generations DAH virgin females with OR males and OR
virgin females with DAH males. The new strains of Drosophila melanogaster are
called DAHOR (flies with nuclear DAH DNA and mitochondrial OR DNA) and ORDAH
(flies with nuclear OR DNA and mitochondrial DAH DNA).
Lifespan studies.
Between
180 and 400 flies were used for each study. Each independent study was repeated
twice: data were pooled and analysed together. Flies were collected within 24 h
after eclosion using CO2 anaesthesia and kept at a density of 20
flies per vial at 25 ºC in a controlled 12 h light:-dark cycle.
Every 2-3 days vials were changed and the number of dead flies was counted,
from which mean and maximum lifespan (MLS, the last 10% of surviving flies)
were calculated. Prism GraphPad software was utilized to build survival curves
that were further analysed using the Kaplan Meier Log-Rank Test.
Mitochondrial
biochemistry.
Mitochondria were isolated according to Miwa et al.[31] with some minor modifications [30]. Mitochondrial respiration rates
were measured by polarography using a Clark-type oxygen electrode as previously
[30], in the absence or presence of KCN (100 μM), antimycin
A (10 μM) or rotenone (5 μM). Mitochondrial
ROS production was assayed according to the method described by [32] adapted to
flies [30].
RNA
quantification.
Total RNA was extracted from 10 days old flies
according to [30]. For cDNA synthesis, 13
μl reaction mixes containing 2 μg RNA, 1 μl DEPC 10 mM dNTP mix (Fermentas),
0.4μl Random Primers (0.5ug/μl Promega) and DEPC-treated water were
incubated at 90°C for 3 min, then transferred to ice, where 4 μl 5x M-MuLV
reaction buffer (Fermentas) and 1μl 40U/μl RNase inhibitor
(Fermentas) were added. The reactions were mixed and incubated at 25°C for 10
min. On ice, 2μl of 20U/μl M-MuLV reverse transcriptase (Fermentas)
was added, and the reaction was incubated for a further 10 min at 25°C, 1 h at
37°C and 70°C for 10 min. mRNA levels were analyzed by Q-RT-PCR. The transcript
levels of RpL32, Catalase, Superoxide dismutase 1 and 2 and Glutathione
Peroxidase were measured using primers pairs shown in supplementary Table 2. All RNA extractions were performed in triplicate, with each used as a
template for three separate cDNA synthesis reactions which were then pooled.
Each cDNA pool was itself analysed in triplicate. Expression of the target
genes was measured relative to that of RpL32 (rp49), in order to
normalize for sample and run to run variations. A series of 10-fold dilutions
of an external standard was used in each run to produce a standard curve.
Analytical reactions were performed using 20-fold diluted cDNA samples, in 25
μl reaction volume consisting of 2 μl of the cDNA template,
0.4μl of 20 μM forward and reverse primers, and 12.5ul of 2x MAXIMA
SYBR GREEN Master Mix (Fermentas). The PCR program consisted of a 10 min
pre-incubation at 95°C, 40 cycles of 35 secs denaturation at 95°C, 30 secs
annealing at 60°C and 30 secs extension at 72°C. Melting curve analysis,
consisting of a 15 secs denaturation step at 95°c followed by a 1 min
annealing step at 60°C and a 0.3°C/s denaturation ramp to 95 °C, was performed
after the amplification step to verify that only a single, specific extension
product had been amplified. Data were extracted and analysed using Applied
Biosystems StepOne software version 2.0.
Resistance to inhibitors of the ETC.
To
check the expression and activity of AOX in vivo experiments with a
variety of ETC inhibitors were performed. 20 flies
were kept (males and females separately) in fresh vials. To measure resistance
to KCN, the drug was dissolved in water at varying concentrations and added
directly to the food vial. Resistance to antimycin and rotenone was assayed
essential as described by Fridell et al. [33]. In brief, 2-3 day old flies were
starved for two hours in empty vials, following this flies were placed in vials
containing Whatman paper (3 mm x 1 mm) impreg-nated with 5% (w/v) sucrose
solution and the appropriate drug (3 mM antimycin or rotenone). Under these
conditions without any drug, flies are able to survive more than 72 h so any
effect before this time should be considered to be provoked by exposure to the
drug. The proportion of flies surviving was recorded over 24 h.
Sequencing
of Mitochondrial gene cytochrome c oxidase subunit I.
Mitochondrial
DNA was extracted using standard procedures from mitochondria isolated from
around 150 flies according to Miwa et al. [31] High fidelity PCR using
specific primers CoIF2 and CoIR5 (Supplementary Table 2) were used to amplify a 2.6 kb
fragment containing the cytochrome c oxidase subunit I (CG34067, CoI). PCR
products were purified using a Machary - Nagel PCR purification kit according
to manufacturer's instructions. Products were sequenced using Big dye
Terminator Chemistry 3.1v (Applied Biosystems) and a 3130 AB genetic analyser.
AB sequencing analysis software was used for analysis of electropherograms.
Statistical analysis.
Data were
analysed using GraphPad Prism 4 and one-way ANOVA was used for statistical
testing. When ANOVA was significant (p < 0.05) Newman-Keuls Multiple
Comparison test was also used. Lifespan data were analysed using the Kaplan
Meier Log-Rank Test. The statistically significant value was established as p
< 0.05.
Acknowledgments
Our
work is supported by funding from the Academy of Finland, Tampere Hospital
Medical Research Fund, Juselius Foundation, the European Union and EMBO
(long-term fellowship to AS). We thank Dr. J Chung for supplying the dj-1beta
mutant stock.
Conflicts of Interest
The authors of this manuscript have no conflict of interest to declare.
References
-
1.
Linnane
AW
, Kios
M
and Vitetta
L.
Coenzyme Q(10)—its role as a prooxidant in the formation of superoxide anion/hydrogen peroxide and the regulation of the metabolome.
Mitochondrion.
2007;
7:
S51
-S61.
[PubMed]
.
-
2.
Sanz
A
and Stefanatos
R.
The Mitochondrial Free Radical Theory of Aging: a critical view.
Current Aging Science.
2008;
1:
10
-21.
[PubMed]
.
-
3.
Perez
VI
, Bokov
A
, Van
Remmen H
, Mele
J
, Ran
Q
, Ikeno
Y
and Richardson
A.
Is the oxidative stress theory of aging dead.
Biochim Biophys Acta.
2009;
1790:
1005
-1014.
[PubMed]
.
-
4.
de Magalhaes
JP
and Church
GM.
Cells discover fire: employing reactive oxygen species in development and consequences for aging.
Exp Gerontol.
2006;
41:
1
-10.
[PubMed]
.
-
5.
Sanz
A
, Pamplona
R
and Barja
G.
Is the mitochondrial free radical theory of aging intact.
Antioxid Redox Signal.
2006;
8:
582
-599.
[PubMed]
.
-
6.
Lambert
AJ
, Boysen
HM
, Buckingham
JA
, Yang
T
, Podlutsky
A
, Austad
SN
, Kunz
TH
, Buffenstein
R
and Brand
MD.
Low rates of hydrogen peroxide production by isolated heart mitochondria associate with long maximum lifespan in vertebrate homeotherms.
Aging Cell.
2007;
6:
607
-618.
[PubMed]
.
-
7.
Csiszar
A
, Labinskyy
N
, Perez
V
, Recchia
FA
, Podlutsky
A
, Mukhopadhyay
P
, Losonczy
G
, Pacher
P
, Austad
SN
, Bartke
A
and Ungvari
ZI.
Endothelial function and vascular oxidative stress in long-lived GH/IGF-deficient Ames dwarf mice.
Am J Physiol Heart Circ Physiol.
2008;
295:
H1882
-1894.
[PubMed]
.
-
8.
Bartke
A
, Wright
JC
, Mattisson
JA
, Ingram
DK
, Miller
RA
and Roth
GS.
Dietary restriction and lifespan.
Science.
2002;
296:
319
[PubMed]
.
-
9.
Andziak
B
, O'Connor
TP
and Buffenstein
R.
Antioxidants do not explain the disparate longevity between mice and the longest-living rodent, the naked mole-rat.
Mech Aging Dev.
2005;
126:
1206
-1212.
[PubMed]
.
-
10.
Andziak B, O'Connor TP, Qi W, DeWaal EM, Pierce A, Chaudhuri AR, Van Remmen H, Buffenstein R. High oxidative damage levels in the longest-living rodent, the naked mole-rat.
Aging Cell.
2006;
5:
463
-471.
[PubMed]
.
-
11.
Weindruch
R
and Sohal
RS.
Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging.
N Engl J Med.
1997;
337:
986
-994.
[PubMed]
.
-
12.
Gredilla
R
and Barja
G.
Minireview: the role of oxidative stress in relation to caloric restriction and longevity.
Endocrinology.
2005;
146:
9:3713
-3719.
.
-
13.
Droge
W
Oxidative aging and insulin receptor signalling.
J Gerontol A Biol Sci Med Sci.
2005;
60:
1378
-85.
[PubMed]
.
-
14.
Wohlgemuth
SE
, Julian
D
, Akin
DE
, Fried
J
, Toscano
K
, Leeuwenburgh
C
and Dunn
WA Jr.
Autophagy in the heart and liver during normal aging and calorie restriction.
Rejuvenation Res.
2007;
10:
281
-292.
[PubMed]
.
-
15.
Sohal
RS
, Sohal
BH
and Orr
WC.
Mitochondrial superoxide and hydrogen peroxide generation, protein oxidative damage, and longevity in different species of flies.
Free Rad Biol Med.
1995;
19:
499
-504.
[PubMed]
.
-
16.
Parker
JD
, Parker
KM
, Sohal
BH
, Sohal
RS
and Keller
L.
Decreased expression of Cu-Zn superoxide dismutase 1 in ants with extreme lifespan.
Proc Natl Acad Sci USA.
2004;
101:
3486
-3489.
[PubMed]
.
-
17.
Haddad LS, Kelbert L, Hulbert AJ. Extended longevity of queen honey bees compared to workers is associated with peroxidation-resistant membranes.
Exp Gerontol.
2007;
42:
601
-609.
[PubMed]
.
-
18.
Muller
FL
, Lustgarten
MS
, Jang
Y
, Richardson
A
and Van
Remmen H.
Trends in oxidative aging theories.
Free Rad Biol Med.
2007;
43:
477
-503.
[PubMed]
.
-
19.
Landis
GN
, Abdueva
D
, Skvortsov
J
, Yang
BE
, Rabin
J
, Carrick
S
, Tavare
S
and Tower
J.
Similar gene expression patterns characterize aging and oxidative stress in Drosophila melanogaster.
Proc Natl Acad Sci USA.
2004;
101:
7663
-7668.
[PubMed]
.
-
20.
Parkes
TL
, Elia
AJ
, Dickinson
D
, Hilliker
AJ
, Phillips
JP
and Boulianne
GL.
Extension of Drosophila lifespan by overexpression of human SOD1 in motorneurons.
Nat Genet.
1998;
19:
171
-174.
[PubMed]
.
-
21.
Orr
WC
, Radyuk
SN
, Prabhudesai
L
, Toroser
D
, Benes
JJ
, Luchak
JM
, Mockett
RJ
, Rebrin
I
, Hubbard
JG
and Sohal
RS.
Overexpression of glutamate-cysteine ligase extends lifespan in Drosophila melanogaster.
J Biol Chem.
2005;
280:
37331
-37338.
[PubMed]
.
-
22.
Orr
WC
, Mockett
RJ
, Benes
JJ
and Sohal
RS.
Effects of overexpression of copper-zinc and manganese superoxide dismutases, catalase, and thioredoxin reductase genes on longevity in Drosophila melanogaster.
J Biol Chem.
2003;
278:
26418
-26422.
[PubMed]
.
-
23.
Curtis
C
, Landis
GN
, Folk
D
, Wehr
NB
, Hoe
N
, Waskar
M
, Abdueva
D
, Skvortsov
D
, Ford
D
, Luu
A
, Badrinath
A
, Levine
RL
, Bradley
TJ
, Tavare
S
and Tower
J.
Transcriptional profiling of MnSOD-mediated lifespan extension in Drosophila reveals a species-general network of aging and metabolic genes.
Genome Biol.
2007;
8:
R262
[PubMed]
.
-
24.
Mair
W
, Goymer
P
, Pletchers
SD
and Partridge
L.
Demography of dietary restriction and death in Drosophila.
Science.
2003;
301:
1731
-1733.
[PubMed]
.
-
25.
Miwa
S
, Riyahi
K
, Partridge
L
and Brand
MD.
Lack of correlation between mitochondrial reactive oxygen species production and lifespan in Drosophila.
Ann N Y Acad Sci.
2004;
1019:
388
-391.
[PubMed]
.
-
26.
Gredilla
R
, Grief
J
and Osiewacz
HD.
. Mitochondrial free radical generation and lifespan control in the fungal aging model Podospora anserina.
Exp Gerontol.
2006;
41:
439
-447.
[PubMed]
.
-
27.
Dufour
E
, Boulay
J
, Rincheval
V
and Sainsard-Chanet
A.
A causal link between respiration and senescence in Podospora anserina.
Proc Natl Acad Sci USA.
2000;
97:
4138
-4143.
[PubMed]
.
-
28.
Hakkaart
FA
, Dassa
EP
, Jacobs
HT
and Rustin
P.
Allotopic expression of a mitochondrial alternative oxidase confers cyanide resistance to human cell respiration.
Embo Rep.
2006;
7:
341
-345.
[PubMed]
.
-
29.
Dassa
EP
, Dufour
E
, Goncalves
S
, Paupe
V
, Hakkaart
GAJ
, Jacobs
HT
and Rustin
P.
Expressing the alternative oxidase complements cytochrome c oxidase deficiency in humans cells.
EMBO Mol Med.
2009;
1:
30
-36.
[PubMed]
.
-
30.
Fernandez-Ayala
DJM
, Sanz
A
, Vartiainen
S
, Kemppainen
KK
, Babusiak
M
, Mustalahti
E
, Costa
R
, Tuomela
T
, Zeviani
M
, Chung
J
, O'Dell
KMC
, Rustin
P
and Jacobs
HT.
Expression of the Ciona intestinalis alternative oxidase (AOX) in Drosophila complements defects in mitochondrial oxidative phosphorylation.
Cell Metab.
2009;
9:
449
-460.
[PubMed]
.
-
31.
Miwa
S
, St-Pierre
J
, Partridge
L
and Brand
MD.
Superoxide and hydrogen peroxide production by Drosophila mitochondria.
Free Radic Biol Med.
2003;
35:
938
-948.
[PubMed]
.
-
32.
Sanz
A
and Barja
G.
Academic Press
New York
Estimation of the rate of production of oxygen radicals at mitochondria. In Handbook of models for the study of human aging (M. Conn ed.).
2006;
.
-
33.
Fridell
YW
, Sanchez-Blanco
A
, Silvia
BA
and Helfand
SL.
Targeted expression of the human uncoupling protein 2 (hUCP2) to adult neurons extends lifespan in the fly.
Cell Metab.
2005;
1:
145
-152.
[PubMed]
.
-
34.
Lambert
AJ
, Buckingham
JA
, Boysen
HM
and Brand
MD.
Low complex I content explains the low hydrogen peroxide production rate of heart mitochondria from the long-lived pigeon, Columba livia.
Aging Cell.
2010;
9:
78
-91.
[PubMed]
.
-
35.
Andres-Mateos
E
, Perier
C
, Zhang
L
, Blanchard-Fillion
B
, Greco
TM
, Thomas
B
, Ko
HS
, Sasaki
M
, Ischiropoulos
H
, Przedborski
S
, Dawson
TM
and Dawson
VL.
DJ-1 gene deletion revels that DJ-1 is an atypical peroxiredoxin-like peroxidase.
PNAS.
2008;
104:
14807
-14812.
[PubMed]
.
-
36.
Ku
HH
and Sohal
RS.
Comparison of mitochondrial pro-oxidant generation and anti-oxidant defenses between rat and pigeon: possible basis of variation in longevity and metabolic potential.
Mech Aging Dev.
1993;
72:
67
-76.
[PubMed]
.
-
37.
Millenaar
FF
, Benschop
JJ
, Wagner
AM
and Lambers
H.
The role of the alternative oxidase in stabilizing the in vivo reduction state of the ubiquinone pool and the activation state of the alternative oxidase.
Plant Physiol.
1998;
118:
599
-607.
[PubMed]
.
-
38.
Ross
RE
Age-specific decrease in aerobic efficiency associated with increase in oxygen free radical production in Drosophila melanogaster.
J Insect Physiol.
2000;
46:
1477
-1480.
[PubMed]
.
-
39.
Melvin
RG
and Ballard
JW.
Intraspecific variation in survival and mitochondrial oxidative phosphorylation in wild-caught Drosophila simulans.
Aging Cell.
2006;
5:
225
-233.
[PubMed]
.
-
40.
Trifunovic
A
and Larsson
NG.
Mitochondrial dysfunction as a cause of aging.
J Inter Med.
2008;
263:
167
-178.
.
-
41.
Walker
DW
, Hajek
P
, Muffat
J
, Knoepfle
D
, Cornelison
S
, Attardi
G
and Benzer
S.
Hypersensitivity to oxygen and shortened lifespan in a Drosophila mitochondrial complex II mutant.
Proc Natl Acad Sci USA.
2006;
103:
16382
-16387.
[PubMed]
.
-
42.
Ishii
N
, Ishii
T
and Hartman
PS.
The role of the electron transport gene SDHC on lifespan and cancer.
Exp Gerontol.
2006;
41:
952
-956.
[PubMed]
.
-
43.
Bahadorani
S
, Cho
J
, Lo
JrT
, Contreras
H
, Lawal
HO
, Krantz
DE
, Bradley
TJ
and Walker
DW.
Neuronal expression of a single-subunit yeast NADH-ubiquinone oxidoreductase (Ndi1) extends Drosophila lifespan.
Aging Cell .
2010;
9:
191
-202.
[PubMed]
.
-
44.
Sanz
A
, Soikkeli
M
, Portero-Otin
M
, Wilson
A
, Kemppainen
E
, McIlroy
G
, Ellilä
S
, Kemppainen
KK
, Tuomela
T
, Lakanmaa
M
, Kiviranta
E
, Stefanatos
R
, Dufour
E
, Hutz
B
, Naudi
A
, Jove
M
, Zeb
A
, Vartiainen
S
, Matsuno-Yagi
A
, Yagi
T
, Rustin
P
, Pamplona
R
and Jacobs
HT.
Expression of the yeast NADH dehydrogenase Ndi1 in Drosophila confers increased lifespan independently of dietary restriction.
PNAS.
2010;
In press
.
-
45.
Abeliovich
A
and Beal
MF.
Parkinsonism genes: culprits and clues.
J Neurochemistry.
2006;
99:
1062
-1072.
.
-
46.
Arking
R
, Burde
V
, Graves
K
, Hari
R
, Feldman
E
, Zeevi
A
, Soliman
S
, Saraiya
A
, Buck
S
, Vettraino
J
, Sathrasala
K
, Wehr
N
and Levine
RL.
Forward and reverse selection for longevity in Drosophila is characterized by alteration of antioxidant gene expression and oxidative damage patterns.
Exp Gerontol.
2000;
35:
167
-185.
[PubMed]
.
-
47.
Barja
G
The gene cluster hypothesis of aging and longevity.
Biogerontology.
2008;
9:
57
-66.
[PubMed]
.
-
48.
Doonan
R
, McElwee
JJ
, Matthijssens
F
, Walker
GA
, Houthoofd
K
, Back
P
, Matscheski
A
, Vanfleteren
JR
and Gems
D.
Against the oxidative damage theory of aging: superoxide dismutase protect against oxidative stress but have little or not effect on lifespan in Caenorhabditis elegans.
Genes Dev.
2008;
22:
3236
-3241.
[PubMed]
.
-
49.
Zielonka
J
and Kalyaraman
B.
"ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis "—a critical commentary.
Free Radic Biol Med.
2008;
45:
1217
-1219.
[PubMed]
.
-
50.
Lambert
AJ
and Merry
BJ.
Effect of caloric restriction on mitochondrial reactive oxygen species production and bioenergetics: reversal by insulin.
Am J Physiol Regul Integr Comp Physiol.
2004;
286:
R71
-R79.
[PubMed]
.
-
51.
Lambert
AJ
and Merry
BJ.
Lack of effect of caloric restriction on bioenergetics and reactive oxygen species production in intact rat hepatocytes.
J Gerontol A Biol Sci Med Sci.
2005;
60:
175
-180.
[PubMed]
.