The choice between p53-induced senescence and quiescence is determined in part by the mTOR pathway
Abstract
Transient induction of p53 can cause reversible quiescence and irreversible senescence. Using nutlin-3a (a small molecule that activates p53 without causing DNA damage), we have previously identified cell lines in which nutlin-3a caused quiescence. Importantly, nutlin-3a caused quiescence by actively suppressing the senescence program (while still causing cell cycle arrest). Noteworthy, in these cells nutlin-3a inhibited the mTOR (mammalian Target of Rapamycin) pathway, which is known to be involved in the senescence program. Here we showed that shRNA-mediated knockdown of TSC2, a negative regulator of mTOR, partially converted quiescence into senescence in these nutlin-arrested cells. In accord, in melanoma cell lines and mouse embryo fibroblasts, which easily undergo senescence in response to p53 activation, nutlin-3a failed to inhibit mTOR. In these senescence-prone cells, the mTOR inhibitor rapamycin converted nutlin-3a-induced senescence into quiescence. We conclude that status of the mTOR pathway can determine, at least in part, the choice between senescence and quiescence in p53-arrested cells.
Introduction
Depending on the cell type and other
factors p53 activation can result in apoptosis, reversible (quiescence) and
irreversible (senescence) cell cycle arrest [1-8]. While the choice between
apoptosis and cell cycle arrest has been intensively scrutinized, the choice
between quiescence and senescence was not systematically addressed and remains
elusive. In order to observe whether p53 activation causes either senescence
or quiescence, others and we employed nutlin-3a. Nutlin-3a, a small molecular
therapeutic, inhibits Mdm2/p53 interaction and induces p53 at physiological
levels without causing DNA damage [9-11]. It was reported that nutlin-3a caused
senescent morphology and permanent loss of proliferative potential [12,13].
However, in other cell lines nutlin-3a caused quiescence so that cells resumed
proliferation, when nutlin-3a was removed [14-16]. Moreover, we
recently reported that in human
fibroblasts (WI-38tert) and fibrosarcoma cells (HT-1080-p21-9), in which
nutlin-3a caused quiescence [16], p53 acted as a suppressor of senescence [17].
Thus, ectopic expression of p21 in these cells caused senescence, while
simultaneous induction of p53 converted senescence into quiescence [17]. In
agreement with previous reports [18-20], we found that p53 inhibited the mTOR
pathway [17]. Importantly, the mTOR pathway is involved in cellular senescence
[21-26]. We suggested that p53-mediated arrest remains reversible as long as
p53 inhibits mTOR. If this model is correct, then senescence would occur in
those cells, in which p53 is incapable of suppressing mTOR. Here we provide
experimental evidence supporting this prediction and demonstrate that
irreversibility of p53-mediated arrest may result from its failure to suppress
the mTOR pathway.
Results
Depletion of TSC2 favors senescence by p53
We have shown that nutlin-3a caused quiescence in
HT-p21-9 cells and WI-38tert cells [16]. In these cells, nutlin-3a actively
suppressed senescence and this suppression was associated with inhibition of
the mTOR pathway by p53 [17]. Next, we investigated whether nutlin-3a can
cause senescence in cells lacking tuberous sclerosis 2 (TSC2) (Figure 1A),
given that regulation of mTOR by p53 requires TSC2 [18]. The transduced cells
were transiently treated with nutlin-3a as shown (Figure 1B). The Tsc2-depleted
cells acquired a large/flat morphology and could not resume proliferation,
whereas cells treated with vector and nutlin-3a did not become senescent and
resumed proliferation, forming colonies after removal of nutlin-3a (Figure 1C-D). The potency of shTSC2 with different sequences varied and two other
shTSC2 were less potent but still depleted TSC2 at some time points
(Supplemental Figure 1) and partially decreased the proliferative potential in nutlin-3a-arrested
cells (Supplemental Figure 1).
We next extended this observation
to WI-38tert cells transduced with shTSC2 (Figure 2A). In control, nutlin- 3a caused a lean morphology, a
characteristic of quiescence [16].
Depletion of TSC2 by shTSC2 converted quiescent morphology to senescent
morphology (Figure 2B). Furthermore, this was associated with permanent loss
of proliferative potential (Figure 2C). In control, cells resumed proliferation
after removal of nutlin-3a, whereas nutlin-3a caused permanent loss of
proliferative potential in shTSC2-treated cells (Figure 2C). In agreement with
our results, it was previously observed that knockout of Tsc2 cooperates with
p53 in induction of cellular senescence in MEFs [27].
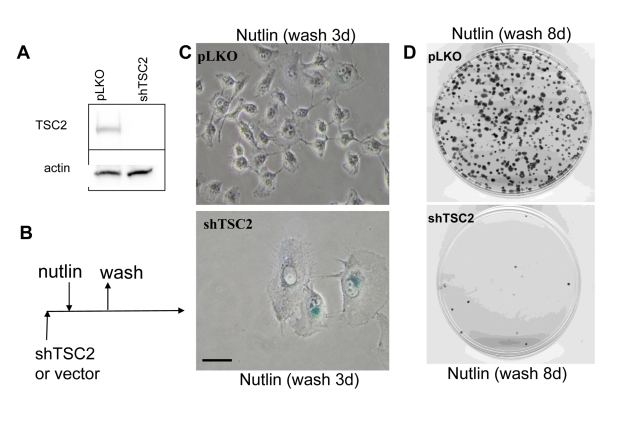
Figure 1. Depletion of TSC2 converts quiescence into senescence in HT-p21-9 cells. (A) HT-p21-9 cells
were transduced with control lentivirus (pLKO) or lentivirus expressing
shTSC2 (sequence # 10) and selected with puromycin for 5 days and then
immunoblot was performed. (B)
Schema: Testing the reversibility of nutlin-3a effects. (C) HT-p21-9 cells were transduced
with control pLKO or shTSC2 and 5000 cells were plated in 24-well plates
and, the next day, were treated with 10 uM nutlin-3a for 3 days. Then
nutlin-3a was washed out and the cells were cultivated in fresh medium for
3 days and then stained for beta-Gal and microphotographed. Bars 50 um. (D) HT-p21-9 cells were transduced
with control pLKO or shTSC2 (and selected for 4 days with puromycin). Then
1000 cells were plated per 60-mm dishes and, the next day, were treated with
nutlin-3a for 3 days. Then nutlin-3a was washed out and cells were
cultivated in fresh medium for 8 days. Colonies were stained with crystal
violet.
Nutlin-3 causes senescence in Mel-10 and -9 cells
We next wished to identify
senescence-prone cells, which undergo senescence in response to nutlin-3a. In
MEL-10 and Mel-9, two melanoma-derived cell lines, nutlin-3a induced p53 and
p21 (Figure 3A) and caused senescent morphology (Figure 3B) and cells did not
resume proliferation, when nutlin-3a was removed (Supplemental Figure 2). In
contrast, rapamycin did not cause senescent morphology and cells resumed
proliferation, when rapamycin was removed (Figure 3B and Supplemental Figure 2). Unlike rapamycin, nutlin-3a did not inhibit S6 phosphorylation (Figure 3A),
a marker of rapamycin-sensitive mTOR activity.
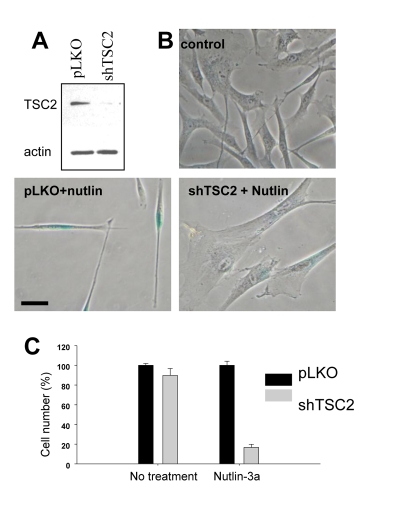
Figure 2. Depletion of TSC2 converts quiescence into senescence in WI-38tert cells. (A)Immunoblot.
WI-38tert cells were transduced with shTSC or control pLKO and cultured for
5 days. (B) WI-38tert
cells were transduced with lentiviruses. Next day, medium was replaced and
Nutlin (10 uM) with our without rapamycin was
added. After 4 days cells were washed and stained for beta-Gal. Bars 50 um.
(C) WI-38tert
cells were transduced with lentiviruses. Next day, medium was replaced and
Nutlin (10 uM) was added. After 4 days cells were washed and counted after
6 days.
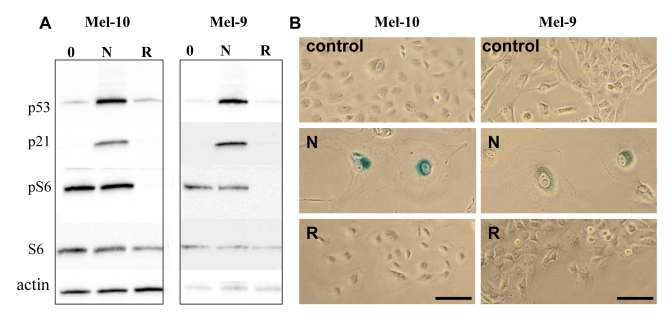
Figure 3. Effects of nutlin-3a and rapamycin on melanoma cells. (A) Mel-10 and Mel-9
cells were incubated with 10 uM nutlin (N) and 500 nM rapamycin (R) for 1
day and immunoblot was performed. (B) Mel-10 and Mel-9 cells were
incubated with 10 uM nutlin and 500 nM rapamycin for 4 days, then drugs
were washed out and cells were incubated for additional 4 days and stained
for beta-Gal. Bars 50 um.
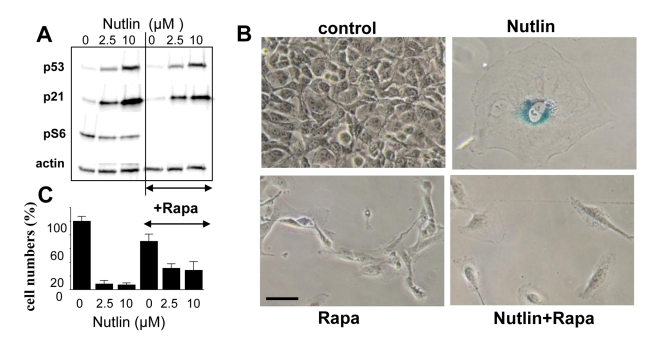
Figure 4. EEffect of rapamycin on nutlin-induced senescence in melanoma cells. (A) Mel-10 cells were incubated with 2.5 and
10 uM nutlin with or without 500 nM rapamycin for 1 day and then immunoblot
was performed. (B) Beta-Gal staining. Mel-10 cells were incubated
with 10 M nutlin alone and 500 nM rapamycin for 4 days, then drugs were
washed out and cells were incubated for additional 3 days and stained for
beta-Gal. Bars 50 um.
Rapamycin suppresses nutlin-3a-induced senescence
To establish a causal link between mTOR and
senescence, we next investigated whether inhibition of the mTOR pathway by
rapamycin could convert nutlin-3a-induced senescence into quiescence. Rapamycin
did not affect p53 and p21 induction caused by nutlin-3a but abrogated S6
phosphorylation (Figure 4A), associated with conversion from senescent
morphology to quiescent morphology (Figure 4B). Importantly, cells were
capable to resume proliferation following removal of nutlin-3a and rapamycin,
indicating that the condition was reversible (Figure 4C). Similar results were
obtained with Mel-9 cells (data not shown).
Next, we extended this observation to cells of
different tissue and species origin. As shown previously, nutlin-3a caused
senescence in mouse embryonic fibroblasts (MEFs) [13]. Here we showed that
nulin-3a failed to inhibit mTOR pathway in MEF (Figure 5A), and caused senescence
(Figure 5B). Rapamycin inhibited the mTOR pathway and converted senescent
morphology to quiescent morphology (Figure 5). This suggests that
failure to suppress a rapamycin-sensitive pathway determines nutlin-3a-induced
senescence instead of quiescence.
Discussion
The role of p53 in organismal aging and
longevity is complex [28-32], indicating that p53 may act as anti-aging factor
in some conditions. We have recently demonstrated that p53 can suppress cellular senescence,
converting it into quiescence [17]. In these quiescence-prone cells, p53
inhibited the mTOR pathway, which is involved in senescence program (Figure 6A).
Still p53 induces senescence in numerous cell types. Here we showed that in
those cell types, in which nutlin-3a caused senescence, it failed to inhibit
the mTOR pathway (Figure 6B). The role of active mTOR as a senescence-inducing
factor in these cells was demonstrated by using rapamycin, which partially
converted nutlin-3a-induced senescence into quiescence (Figure 6B, lower
panel). This indicates that rapamycin-sensitive mTOR activity is necessary for
senescence during nutlin-3a-induced cell cycle arrest. And vice versa, in
quiescence-prone cells, depletion of TSC2 converted quiescence into senescence
(Figure 6A, lower panel). Taken together, data suggest that activation of the mTOR pathway favors senescence (Figure 7). In
agreement, Ras accelerated senescence in nutlin-arrested cells [13]. Similarly,
activation of Ras and MEK in murine fibroblasts converted p53-induced
quiescence into senescence [33]. Interestingly, p53 levels did not correlate
with the senescence phenotype, suggesting that factors other than p53 may
determine senescence [33]. These important observations are in agreement with
our model that senescence requires two factors: cell cycle arrest caused by p53
and simultaneous activation of the growth-promoting mTOR pathway (Note: Ras is
an activator of the mTOR pathway). And vice versa it was observed that
induction of p53 maintains quiescence upon serum starvation, without causing
senescence [34]. In agreement, our model predicts that, by deactivating mTOR,
serum starvation prevents senescence.
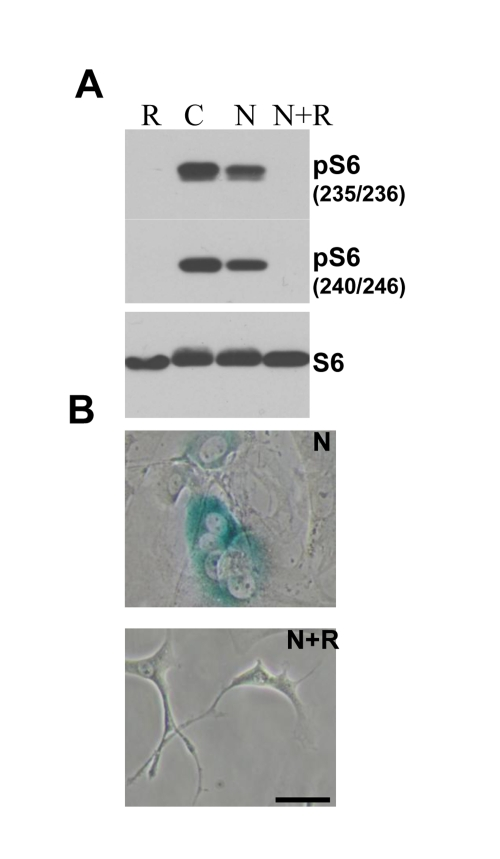
Figure 5. Effect of rapamycin on nutlin-induced senescence in melanoma cells . (A. ) ) Immunoblot.
MEF cells were incubated with 10 nutlin-3a with or without 10 nM rapamycin
for 1 day and immunoblot using rabbit anti-phospho-S6 (Ser240/244) and
(Ser235/236) and mouse anti-S6 was performed. (B) Beta-Gal staining. MEF cells were incubated
with 10 uM nutlin alone or with 500 nM rapamycin for 4 days, then drugs
were washed out and cells were incubated for additional 4 days and stained
for beta-Gal. Bars 50 um.
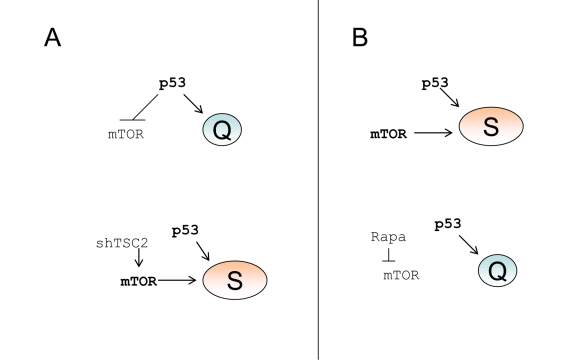
Figure 6. p53 causes senescence by failing to suppress senescence. (A) Quiescence-prone
cells. Upper panel.
P53 causes cell cycle arrest and inhibits the mTOR
pathway, thus ensuring quiescence. Lower panel.
Transduction of cells with
shTSC2 activates mTOR thus converting quiescence into senescence. (B)
Senescence-prone cells. Upper panel.
P53 causes cell cycle arrest without
inhibiting the mTOR pathway, thus ensuring senescence. Lower panel.
Rapamycin inhibits mTOR thus converting senescence into quiescence.
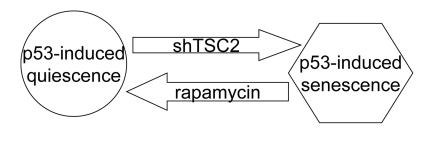
Figure 7. Activation of the mTOR pathway favors senescence in nutlin-3a-arrested cells.
Another factor that favors senescence is the duration
of cell cycle arrest [13,35]. Importantly, the duration of the arrest may
exceed the duration of treatment with nutlin-3a because of persistent induction
of p21 even after removal of nutlin-3a in some cancer cell lines [35].
Additional pathways may be involved in the senescence program. For example,
nutlin-3a induces cytoskeletal rearrangement [36]. We speculate that p53
affects not only rapamycin-sensitive mTORC1 but also the mTORC2 complex, given
that mTORC2 controls the actin cytoskeleton [37]. Also, p53 inhibits downstream
branches of the mTOR pathway [38,39]. P53 stimulates autophagy [18,40], which
in turn is essential for life-extension by pharmacological manipulations (see
[41-44]). Finally, p53 affects cellular metabolism [45-48] and this effect may
contribute to suppression of cellular senescence and synergistically potentate
metabolic changes caused by mTOR inhibition. The relative contribution of all
these mutually dependent factors needs further investigations. The key role of
mTOR in cellular senescence links cellular and organismal aging and age-related
diseases.
Material and methods
Cell lines and reagents.
HT-p21-9 cells are derivatives of HT1080 human
fibrosarcoma cells, where p21 expression can be turned on or off using a
physiologically neutral agent isopropyl--thio-galactosidase (IPTG) [16,49-51].
HT-p21-9 cells express GFP. WI-38-Tert, WI-38 fibroblasts immortalized by
telomerase were described previously [16,17]. Melanoma cell lines, MEL-9
(SK-Mel-103) and MEL-10 (SK-Mel-147), were described previously [52,53]. RPE
cells were described previously [21,22]. MEF, mouse fibroblasts isolated from
13-day embryos, were provided by Marina Antoch (RPCI) and maintained in DMEM
supplemented with 10% FCS. Rapamycin (LC
Laboratories, MA, USA), IPTG (Sigma- Aldrich, St. Louis, MO), nutlin-3a (Sigma-Aldrich)
were used as previously described [17].
Lentiviral shRNA construction
. Bacterial glycerol stocks [clone
NM_000548.2-1437s1c1 (#10), NM_000548.x-4581s1c1 (#7) and NM_000548.2-4551s1c1
(#9)] containing lentivirus plasmid vector pLKO.1-puro with shRNA specific for
TSC2 was purchased from Sigma. The targeting sequences are:
CCGGGCTCATCAACAGGCAGTTCTACTCGAGTAGAACTGCCTGTTGATGAGCTTTTTG (#10), CCGG CAATGAGTCACAGTCCTTTGACTCGAGTCAAAGGACTGTGACTCATTGTTTTTG
(#7) and CCGGCGACGAGTCAAACAAGCCAATCTCGAGATTGGCTTGTTTGACTCGTCGTTTTTG
(#9).
pLKO.1-puro lentiviral vector
without shRNA was used as a control. Lentiviruses were produced in HEK293T
cells after co-transfection of lentivirus plasmid vector with shRNA or control
vector with packaging plasmids using Lipofectamine2000 (Invitrogen). After 48h
and 72h medium containing lentivirus was collected, centrifuged at 2000g and
filtered through 0.22 uM filter. Filtered virus containing medium was used for
cell infection or stored at -80 C. Cells were transduced with lentivirus in the
presence of 8 mg/ml polybrene and selected with puromycin (1-2 mg/ml) for 4-6
days. Cells were treated with drugs either 24h after transduction or after
puromycin selection for infected cells.
Colony formation assay.
Plates were fixed and stained with 1.0 % crystal
violet (Sigma-Aldrich).
Immunoblot analysis.
The following antibodies were used: anti-p53 and anti-p21 antibodies
from Cell signaling and anti-actin antibodies from Santa Cruz Biotechnology,
rabbit anti-phospho-S6 (Ser240/244) and (Ser235/236), mouse anti-S6, mouse
anti-phospho- p70 S6 kinase (Thr389), mouse anti-p21, rabbit
anti-phospho-4E-BP1 (Thr37/46) from Cell Signaling; mouse anti-4E-BP1 from
Invitrogen; mouse anti-p53 (Ab-6) from Calbiochem.
Beta-galactosidase staining.
beta-Gal staining was performed using Senescence
-galactosidase staining kit (Cell Signaling Technology) according to
manufacturer's protocol.
Supplementary Materials
Depletion of TSC2 converts quiescence into senescence in HT-p21-9 cells. (A) HT-p21-9 cells were transduced
with control lentivirus (pLKO) or lentivirus expressing shTSC2
(sequence # 7, 8, 9) and selected with puromycin for 10 days and then
immunoblot was performed. (B) HT-p21-9 cells were transduced with
control pLKO or shTSC2 (and selected for 4 days with puromycin). Then
1000 cells were plated per 60-mm dishes and, the next day, were treated
with nutlin-3a for 3 days. Then nutlin-3a was washed out and cells were
cultivated in fresh medium for 8 days. Colonies were stained with crystal
violet.
Irreversible and reversible effects of nutlin-3a and rapamycin:. Mel-10 and Mel-9 cells were incubated with 10 uM
nutlin (N) and 500 nM rapamycin (R) for 4 day and then nutlin-3a was washed.
After a week, cells were counted.
Conflicts of Interest
The authors of this manuscript have no conflict of
interests to declare.
References
-
1.
Vogelstein
B
, Lane
DP
and Levine
AJ.
Surfing the p53 network.
Nature.
2000;
408:
307
-310.
[PubMed]
.
-
2.
Itahana
K
, Dimri
G
and Campisi
J.
Regulation of cellular senescence by p53.
Eur J Biochem.
2001;
268:
2784
-2791.
[PubMed]
.
-
3.
Vousden
KH
Outcomes of p53 activation--spoilt for choice.
J Cell Sci.
2006;
119:
5015
-5020.
[PubMed]
.
-
4.
Vousden
KH
and Prives
C.
Blinded by the Light: The Growing Complexity of p53.
Cell.
2009;
137:
413
-431.
[PubMed]
.
-
5.
Levine
AJ
and Oren
M.
The first 30 years of p53: growing ever more complex.
Nat Rev Cancer.
2009;
9:
749
-758.
[PubMed]
.
-
6.
Brown
CJ
, Lain
S
, Verma
CS
, Fersht
AR
and Lane
DP.
Awakening guardian angels: drugging the p53 pathway.
Nat Rev Cancer.
2009;
9:
862
-873.
[PubMed]
.
-
7.
Liebermann
DA
, Hoffman
B
and Vesely
D.
p53 induced growth arrest versus apoptosis and its modulation by survival cytokines.
Cell Cycle.
2007;
6:
166
-170.
[PubMed]
.
-
8.
Paris
R
, Henry
RE
, Stephens
SJ
, McBryde
M
and Espinosa
JM.
Multiple p53-independent gene silencing mechanisms define the cellular response to p53 activation.
Cell Cycle.
2008;
7:
2427
-2433.
[PubMed]
.
-
9.
Vassilev
LT
Small-molecule antagonists of p53-MDM2 binding: research tools and potential therapeutics.
Cell Cycle.
2004;
3:
419
-421.
[PubMed]
.
-
10.
Vassilev
LT
, Vu
BT
, Graves
B
, Carvajal
D
, Podlaski
F
, Filipovic
Z
, Kong
N
, Kammlott
U
, Lukacs
C
, Klein
C
, Fotouhi
N
and Liu
EA.
In vivo activation of the p53 pathway by small-molecule antagonists of MDM2.
Science.
2004;
303:
844
-848.
[PubMed]
.
-
11.
Huang
B
and Vassilev
LT.
Reduced transcriptional activity in the p53 pathway of senescent cells revealed by the MDM2 antagonist nutlin-3.
Aging.
2009;
1:
845
-854.
[PubMed]
.
-
12.
Van
Maerken T
, Speleman
F
, Vermeulen
J
, Lambertz
I
, De
Clercq S
, De
Smet E
, Yigit
N
, Coppens
V
, Philippé
J
, De
Paepe A
, Marine
JC
and Vandesompele
J.
Small-molecule MDM2 antagonists as a new therapy concept for neuroblastoma.
Cancer Res.
2006;
66:
9646
-9655.
[PubMed]
.
-
13.
Efeyan
A
, Ortega-Molina
A
, Velasco-Miguel
S
, Herranz
D
, Vassilev
LT
and Serrano
M.
Induction of p53-dependent senescence by the MDM2 antagonist nutlin-3a in mouse cells of fibroblast origin.
Cancer Res.
2007;
67:
7350
-7357.
[PubMed]
.
-
14.
Huang
B
, Deo
D
, Xia
M
and Vassilev
LT.
Pharmacologic p53 Activation Blocks Cell Cycle Progression but Fails to Induce Senescence in Epithelial Cancer Cells.
Mol Cancer Res.
2009;
7:
1497
-1509.
[PubMed]
.
-
15.
Cheok
CF
, Kua
N
, Kaldis
P
and Lane
DP.
Combination of nutlin-3 and VX-680 selectively targets p53 mutant cells with reversible effects on cells expressing wild-type p53.
Cell Death Differ.
2010;
In press
.
-
16.
Korotchkina
LG
, Demidenko
ZN
, Gudkov
AV
and Blagosklonny
MV.
Cellular quiescence caused by the Mdm2 inhibitor nutlin-3a.
Cell Cycle.
2009;
8:
3777
-3781.
[PubMed]
.
-
17.
Demidenko
ZN
, Korotchkina
LG
, Gudkov
AV
and Blagosklonny
MV.
Paradoxical suppression of cellular senescence by p53.
Proc Natl Acad Sci U S A.
2010;
9660-4:
9660
-9664.
[PubMed]
.
-
18.
Feng
Z
, Zhang
H
, Levine
AJ
and Jin
S.
The coordinate regulation of the p53 and mTOR pathways in cells.
Proc Natl Acad Sci U S A.
2005;
102:
8204
-8209.
[PubMed]
.
-
19.
Budanov
AV
and Karin
M.
p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling.
Cell.
2008;
134:
451
-460.
[PubMed]
.
-
20.
Matthew
EM
, Hart
LS
, Astrinidis
A
, Navaraj
A
, Dolloff
NG
, Dicker
DT
, Henske
EP
and El-Deiry
WS.
The p53 target Plk2 interacts with TSC proteins impacting mTOR signaling, tumor growth and chemosensitivity under hypoxic conditions.
Cell Cycle.
2009;
8:
4168
-4175.
[PubMed]
.
-
21.
Demidenko
ZN
and Blagosklonny
MV.
Growth stimulation leads to cellular senescence when the cell cycle is blocked.
Cell Cycle.
2008;
7:
3355
-3361.
[PubMed]
.
-
22.
Demidenko
ZN
, Zubova
SG
, Bukreeva
EI
, Pospelov
VA
, Pospelova
TV
and Blagosklonny
MV.
Rapamycin decelerates cellular senescence.
Cell Cycle.
2009;
8:
1888
-1895.
[PubMed]
.
-
23.
Demidenko
ZN
, Shtutman
M
and Blagosklonny
MV.
Pharmacologic inhibition of MEK and PI-3K converges on the mTOR/S6 pathway to decelerate cellular senescence.
Cell Cycle.
2009;
8:
1896
-1900.
[PubMed]
.
-
24.
Demidenko
ZN
and Blagosklonny
MV.
At concentrations that inhibit mTOR, resveratrol suppresses cellular senescence.
Cell Cycle.
2009;
8:
1901
-1904.
[PubMed]
.
-
25.
Demidenko
ZN
and Blagosklonny
MV.
Quantifying pharma-cologic suppression of cellular senescence: prevention of cellular hypertrophy versus preservation of proliferative potential.
Aging.
2009;
1:
1008
-1016.
[PubMed]
.
-
26.
Pospelova
TV
, Demidenko
ZN
, Bukreeva
EI
, Pospelov
VA
, Gudkov
AV
and Blagosklonny
MV.
Pseudo-DNA damage response in senescent cells.
Cell Cycle.
2009;
8:
4112
-4118.
[PubMed]
.
-
27.
Zhang
H
, Cicchetti
G
, Onda
H
, Koon
HB
, Asrican
K
, Bajraszewski
N
, Vazquez
F
, Carpenter
CL
and Kwiatkowski
DJ.
Loss of Tsc1/Tsc2 activates mTOR and disrupts PI3K-Akt signaling through downregulation of PDGFR.
J Clin Invest.
2003;
112:
1223
-1233.
[PubMed]
.
-
28.
Matheu
A
, Maraver
A
, Klatt
P
, Flores
I
, Garcia-Cao
I
, Borras
C
, Flores
JM
, Vina
J
, Blasco
MA
and Serrano
M.
Delayed ageing through damage protection by the Arf/p53 pathway.
Nature.
2007;
448:
375
-379.
[PubMed]
.
-
29.
Waskar
M
, Landis
GN
, Shen
J
, Curtis
C
, Tozer
K
, Abdueva
D
, Skvortsov
D
, Tavare
S
and Tower
J.
Drosophila melanogaster p53 has developmental stage-specific and sex-specific effects on adult life span indicative of sexual antagonistic pleiotropy.
Aging.
2009;
1:
903
-936.
[PubMed]
.
-
30.
Biteau
B
and Jasper
H.
It's all about balance: p53 and aging.
Aging.
2009;
1:
884
-886.
[PubMed]
.
-
31.
Hur
JH
and Walker
DW.
p53, sex, and aging: lessons from the fruit fly.
Aging.
2009;
1:
881
-883.
[PubMed]
.
-
32.
Donehower
LA
Longevity regulation in flies: a role for p53.
Aging.
2009;
1:
6
-8.
[PubMed]
.
-
33.
Ferbeyre
G
, de Stanchina
E
, Lin
AW
, Querido
E
, McCurrach
ME
, Hannon
GJ
and Lowe
SW.
Oncogenic ras and p53 cooperate to induce cellular senescence.
Mol Cell Biol.
2002;
22:
3497
-3508.
[PubMed]
.
-
34.
Itahana
K
, Dimri
GP
, Hara
E
, Itahana
Y
, Zou
Y
, Desprez
PY
and Campisi
J.
A role for p53 in maintaining and establishing the quiescence growth arrest in human cells.
J Biol Chem.
2002;
277:
18206
-18214.
[PubMed]
.
-
35.
Shen
H
and Maki
CG.
Persistent p21 expression after Nutlin-3a removal is associated with senescence-like arrest in 4N cells.
J Biol Chem.
2010;
285:
23105
-23114.
[PubMed]
.
-
36.
Moran
DM
and Maki
CG.
Nutlin-3a induces cytoskeletal rearrangement and inhibits the migration and invasion capacity of p53 wild-type cancer cells.
Mol Cancer Ther.
2010;
9:
895
-905.
[PubMed]
.
-
37.
Jacinto
E
, Loewith
R
, Schmidt
A
, Lin
S
, Ruegg
MA
, Hall
A
and Hall
MN.
Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive.
Nat Cell Biol.
2004;
6:
1122
-1128.
[PubMed]
.
-
38.
Constantinou
C
and Clemens
MJ.
Regulation of the phosphorylation and integrity of protein synthesis initiation factor eIF4GI and the translational repressor 4E-BP1 by p53.
Oncogene.
2005;
24:
4839
-4850.
[PubMed]
.
-
39.
Constantinou
C
, Elia
A
and Clemens
MJ.
Activation of p53 stimulates proteasome-dependent truncation of eIF4E-binding protein 1 (4E-BP1).
Biol Cell.
2008;
100:
279
-289.
[PubMed]
.
-
40.
Maiuri
MC
, Malik
SA
, Morselli
E
, Kepp
O
, Criollo
A
, Mouchel
PL
, Carnuccio
R
and Kroemer
G.
Stimulation of autophagy by the p53 target gene Sestrin2.
Cell Cycle.
2009;
8:
1571
-1576.
[PubMed]
.
-
41.
Morselli
E
, Galluzzi
L
, Kepp
O
, Criollo
A
, Maiuri
MC
, Tavernarakis
N
, Madeo
F
and Kroemer
G.
Autophagy mediates pharmacological lifespan extension by spermidine and resveratrol.
Aging.
2009;
1:
961
-970.
[PubMed]
.
-
42.
Alvers
AL
, Wood
MS
, Hu
D
, Kaywell
AC
, Dunn
WA Jr
and Aris
JP.
Autophagy is required for extension of yeast chronological life span by rapamycin.
Autophagy.
2009;
5:
847
-849.
[PubMed]
.
-
43.
Bjedov
I
, Toivonen
JM
, Kerr
F
, Slack
C
, Jacobson
J
, Foley
A
and Partridge
L.
Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster.
Cell Metab.
2010;
11:
35
-46.
[PubMed]
.
-
44.
Hands
SL
, Proud
CG
and Wyttenbach
A.
mTOR's role in ageing: protein synthesis or autophagy.
Aging.
2009;
586
-597.
[PubMed]
.
-
45.
Vousden
KH
and Ryan
KM.
p53 and metabolism.
Nat Rev Cancer.
2009;
9:
691
-700.
[PubMed]
.
-
46.
Feng
Z
and Levine
AJ.
The regulation of energy metabolism and the IGF-1/mTOR pathways by the p53 protein.
Trends Cell Biol.
2010;
.
-
47.
Hu
W
, Zhang
C
, Wu
R
, Sun
Y
, Levine
A
and Feng
Z.
Glutaminase 2, a novel p53 target gene regulating energy metabolism and antioxidant function.
Proc Natl Acad Sci U S A.
2010;
107:
7455
-7460.
[PubMed]
.
-
48.
Suzuki
S
, Tanaka
T
, Poyurovsky
MV
, Nagano
H
, Mayama
T
, Ohkubo
S
, Lokshin
M
, Hosokawa
H
, Nakayama
T
, Suzuki
Y
, Sugano
S
, Sato
E
, Nagao
T
, Yokote
K
, Tatsuno
I
and Prives
C.
Phosphate-activated glutaminase (GLS2), a p53-inducible regulator of glutamine metabolism and reactive oxygen species.
Proc Natl Acad Sci U S A.
2010;
107:
7461
-7466.
[PubMed]
.
-
49.
Chang
BD
, Broude
EV
, Dokmanovic
M
, Zhu
H
, Ruth
A
, Xuan
Y
, Kandel
ES
, Lausch
E
, Christov
K
and Roninson
IB.
A senescence-like phenotype distinguishes tumor cells that undergo terminal proliferation arrest after exposure to anticancer agents.
Cancer Res.
1999;
59:
3761
-3767.
[PubMed]
.
-
50.
Chang
BD
, Broude
EV
, Fang
J
, Kalinichenko
TV
, Abdryashitov
R
, Poole
JC
and Roninson
IB.
p21Waf1/Cip1/Sdi1-induced growth arrest is associated with depletion of mitosis-control proteins and leads to abnormal mitosis and endoreduplication in recovering cells.
Oncogene.
2000;
19:
2165
-2170.
[PubMed]
.
-
51.
Broude
EV
, Swift
ME
, Vivo
C
, Chang
BD
, Davis
BM
, Kalurupalle
S
, Blagosklonny
MV
and Roninson
IB.
p21(Waf1/Cip1/Sdi1) mediates retinoblastoma protein degradation.
Oncogene.
2007;
26:
6954
-6958.
[PubMed]
.
-
52.
Mannava
S
, Grachtchouk
V
, Wheeler
LJ
, Im
M
, Zhuang
D
, Slavina
EG
, Mathews
CK
, Shewach
DS
and Nikiforov
MA.
Direct role of nucleotide metabolism in C-MYC-dependent proliferation of melanoma cells.
Cell Cycle.
2008;
7:
2392
-2400.
[PubMed]
.
-
53.
Zhuang
D
, Mannava
S
, Grachtchouk
V
, Tang
WH
, Patil
S
, Wawrzyniak
JA
, Berman
AE
, Giordano
TJ
, Prochownik
EV
, Soengas
MS
and Nikiforov
MA.
C-MYC overexpression is required for continuous suppression of oncogene-induced senescence in melanoma cells.
Oncogene.
2008;
27:
6623
-6634.
[PubMed]
.