Abstract
We have recently reported that a novel muscle-specific inositide phosphatase (MIP/MTMR14) plays a critical role in [Ca2+]i homeostasis through dephosphorylation of sn-1-stearoyl-2-arachidonoyl phosphatidylinositol (3,5) bisphosphate (PI(3,5)P2). Loss of function mutations in MIP have been identified in human centronuclear myopathy. We developed a MIP knockout (MIPKO) animal model and found that MIPKO mice were more susceptible to exercise-induced muscle damage, a trademark of muscle functional changes in older subjects. We used wild-type (Wt) mice and MIPKO mice to elucidate the roles of MIP in muscle function during aging. We found MIP mRNA expression, MIP protein levels, and MIP phosphatase activity significantly decreased in old Wt mice. The mature MIPKO mice displayed phenotypes that closely resembled those seen in old Wt mice: i) decreased walking speed, ii) decreased treadmill activity, iii) decreased contractile force, and iv) decreased power generation, classical features of sarcopenia in rodents and humans. Defective Ca2+ homeostasis is also present in mature MIPKO and old Wt mice, suggesting a putative role of MIP in the decline of muscle function during aging. Our studies offer a new avenue for the investigation of MIP roles in skeletal muscle function and as a potential therapeutic target to treat aging sarcopenia.
Introduction
Aging
is a complex biological process marked by the gradual decline of a multitude of
physiological processes/functions that ultimately results in death [1-5]. Normal
aging results in sarcopenia, the decreased muscle mass and function that
develops despite interventions such as increased physical activity and improved
diet [6,7]. While
these interventions have proven to be effective in ameliorating the loss of
muscle function with age, there is no intervention that can completely prevent
or reverse sarcopenia.
The decline in muscle function (force and power) that results from
sarcopenia is a major cause of restricted activity, muscle injuries, and loss
of independence in older individuals. As populations age and live longer, this
problem will continue to grow. The world wide cost of managing the consequences
of sarcopenia is astronomical estimated in the hundreds of billions of dollars.
Research designed to reveal the
cellular mechanisms that contribute to sarcopenia and other age-related muscle
disorders is essential for the development of effective treatments that can
improve health outcomes for older adults.
It
has been shown that the decrease in force and power that functionally
characterize sarcopenia cannot be completely explained by atrophy alone [4,8,9]. Some of
the mechanisms suggested to explain the discrepancy between atrophy-dependent
vs. atrophy-independent loss of muscle function in aging include decreased myosin
force and/or actin-myosin cross-bridge stability [8,9] and
defective excitation-contraction coupling (ECC) [4;10]. Our
research groups have contributed to the field of muscle aging by demonstrating
that specific aspects of the excitation-contraction coupling (ECC) process are
compromised with age [11,12].
While
aging is a multigene phenomenon [13-15], we have
focused our most recent studies on a new protein, muscle-specific inositide
phosphatase (MIP), also known as myotubularin-related protein 14 (MTMR14) [16]. In a
recent report, we characterized its basic functions in skeletal muscle [16]. Our
studies showed that MIP is important in the ECC process of skeletal muscle
(particularly influencing store-operated calcium entry (SOCE), calcium (Ca2+)
storage and Ca2+ release from the sarcoplasmic reticulum (SR).
In the current study, we have used a
combination of approaches to phenotypically compare mature mice lacking MIP
(MIPKO) with old wild type (Wt) mice. We also measured the cellular expression,
concentration, and activity of MIP within muscle fibers with age. These
findings were correlated with functional outcomes and revealed that key
features of sarcopenia manifest in the MIPKO much earlier (12-14 months) than
in wild-type mice (22-24 months). The significant decrease in MIP mRNA
expression, MIP protein content and MIP activity in normal, old Wt mice along
with the striking phenotypic similarities between mature MIPKO and old Wt mice,
suggest a putative role of MIP in the aging decline in muscle function.
Results
In
vivo studies of activity: young and mature MIPKO mice behave like old WT mice
In our recently published study [16], we showed that in a rotarod function test, the
latency of MIPKO mice to fall off the rotating rod was decreased. In the
inclined screen test, the percentage of MIPKO mice that could reach to the top
of the inclined screen was greatly decreased compared to that of Wt
littermates. These findings are very similar to results obtained in old Wt
mice. To broaden the phenotypic comparison between MIPKO and Wt mice, we used
the force-plate actimeter measurements [17]. All mice tested remained in the actimeter for 40
min, and we found that young Wt mice (4-6 month, n=58) walked 280 ± 27 meters,
mature Wt (12-14 month, n=20) walked 283 ± 23 meters, and old Wt (22-24 month,
n=12) walked 175 ± 32 meters. In contrast, young MIPKO mice (4-6 month, n = 12)
walked 240 ± 18 meters, mature MIPKO walked 200 ± 22 (12-14 month, n=12), and
old MIPKO (18-20 month, n=12) walked 155 ± 13 meters. These studies show that
mature MIPKO mice behave like old Wt mice with respect to levels of spontaneous
physical activity.
Treadmill
stress test reveals additional similarities between the mature MIPKO and old Wt
mice
These series of experiments were designed to test the
effects of stress of running until exhaustion in a treadmill. Thus, untrained
Wt and MIPKO mice were ran in a rodent treadmill until exhausted as previously
described by our group [18]. The differences we found were dramatic. Young (n
=12), mature (n = 18), and old Wt (n = 12) respectively ran 33 ± 5, 38 ± 3, and 13 ± 5 min. In contrast, young (n
=12), mature (n = 18), and old MIPKO (n = 12) respectively ran 22 ± 4, 10 ± 3, and 8 ± 5 min. These results are very
intriguing, particularly when we contrasted the average running time of ~10 min
in mature MIPKO mice with the running time of old (22-24 month) Wt mice of 13 ± 5 min. These results strongly
suggest that locomotor dysfunction has an earlier onset in MIPKO mice. Furthermore, it illustrates the dramatic similarities
between mature MIPKO mice and old Wt littermates. We have also consistently
observed that at this age, MIPKO mice are significantly less active in their
cages as further substantiated by our actimeter studies.
Accelerated muscle wasting in MIPKO mice
might explain defective in vivo function and suggest earlier onset of
sarcopenia
We previously demonstrated that an 18-month old MIP KO
mouse has ~40-50% less body mass as compared to the Wt littermates (See Figure 2 of our recent publication [16]). As seen
in Figure 1, we now show that hindlimb muscle mass is also significantly
reduced in the MIPKO mice when compared with Wt littermates, suggesting
premature development of sarcopenia when MIP is ablated. These data might also
help in explaining the reduced physical vigor encountered in MIPKO mice.
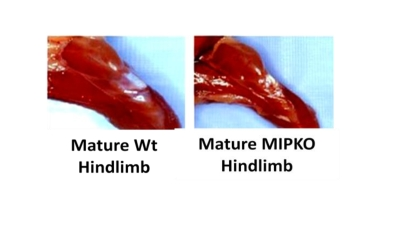
Figure 1. Muscle wasting develops prematurely in the MIPKO mice. Digital
photography demonstrates the typical hindlimb size difference between
18-month old Wt and 18-month old MIPKO mice.
Contractile force and power generation are reduced in
skeletal muscles from mature MIPKO in a fashion similar to that of old Wt mouse
A
functional corollary of sarcopenia in humans is the presence of decreased force
and power. Figure 2A illustrates that the cross sectional area of skeletal
muscle fibers from old Wt and mature MIPKO mice is reduced compared with mature
Wt mice. Figures 2B and 2D illustrate that the maximal contractile force and
power in old Wt, mature MIPKO, and old MIPKO mice are significantly reduced
from values found in mature Wt mice. Atrophy can account for a reduction in
force and power of approximately 25% (the dotted line in Figures 2B and2D) in the old and MIPKO animals. When maximal force and power are
normalized to cross sectional area (Figures 2C and 2E), it is clear that there
is a reduction in force and power that are independent of muscle wasting and
result from changes in the contractile properties of the muscle fibers.
Strikingly, the same functional changes that manifest at 24 months in muscles from
old Wt mice manifest by 12 months of age in MIPKO mice, suggesting that MIP is
an important modulator of contractile function during aging.
Downregulation
of MIP expression and function with normal muscle aging
Figure 3 shows
down-regulation of MIP in Wt mice during aging using 3 different approaches
(MIP gene expression, MIP protein content, and MIP phosphatase activity). These
data show that MIP is tightly regulated at all levels, from transcription to
translation to activity levels, suggesting an important physiological role for
this muscle phosphatase. These data also provide strong evidence for the
involvement of MIP in the development of sarcopenia.
These new data in Figure 3 not only address a specific concern from the review panel,
but are equally exciting for the future of our proposed studies and the field
of sarcopenia.
Dysfunctional Ca2+ homeostasis in old WT and mature MIPKO
Figure 4 shows that Ca2+ homeostasis is disrupted in both old Wt and
mature MIPKO FDB muscle fibers. Three key features of intracellular Ca2+
homeostasis were similarly affected by old age and MIP ablation. First, resting levels
of Ca2+ were higher in old Wt and MIPKO muscle fibers. Second, SR
Ca2+ release triggered by caffeine was reduced in muscle fibers from both
old Wt and mature MIPKO mice. Third, while the Ca2+ transient has a fast
relaxation recovery in young Wt, it is significantly delayed in old Wt and MIPKO. We
previously showed that intracellular elevation of PI(3,5)P2
due to the absence of MIP (see Figure 5) led to chronic activation of the ryanodine
receptor (RyR1) and inhibition of store-operated Ca2+ entry (SOCE) [16],
which supports the present findings of altered Ca2+ homeostasis with age
or in the MIPKO subjects when MIP is downregulated and therefore PI(3,5)P2
is elevated. Undoubtedly, with chronic activation of the RyR1 by PI(3,5)P2,
one predicts that resting levels of Ca2+ will be higher and Ca2+ storage
and Ca2+ transients reduced. This combination of effects is certainly
detrimental to skeletal muscle function and will contribute to the decline in
muscle strength with aging.
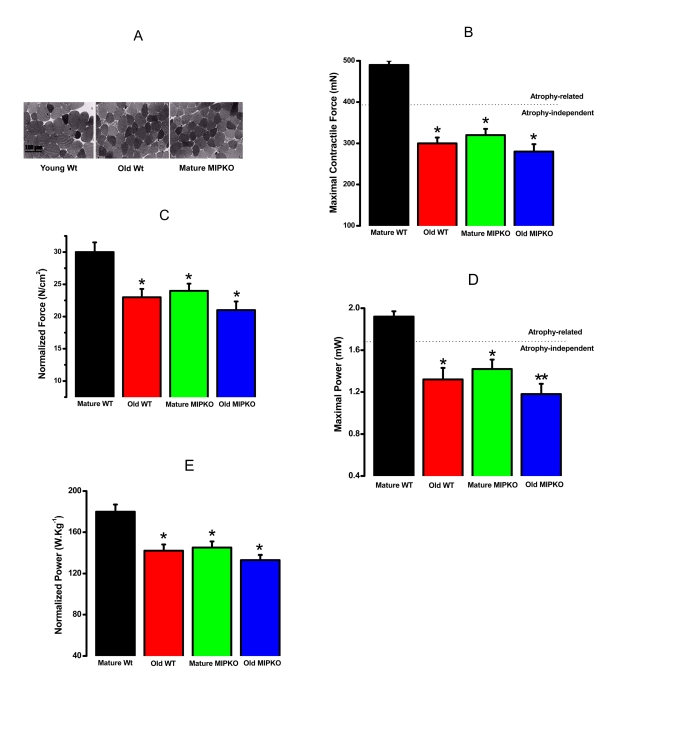
Figure 2. Evidence for muscle atrophy, decreased contractile force, and reduced power in skeletal muscles suggested similarity from old WT and MIPKO mice. In all figures,
the black bars are mature, wild type mice, the red bars are old, wild type
mice, the green bars are mature MIPKO mice, and the blue bars are old,
MIPKO mice. (A) Typical Toluidine blue-stained cross sections of
EDL muscles from young Wt, old Wt, and mature MIPKO mice. The cross-sectional
areas of old Wt and MIPKO cells are significantly reduced compared with those of
the young Wt. (B) Maximal contractile force in EDL muscle for each genotype.
Atrophy (decrease in muscle cross-sectional area) can explain ~ 1/2 of the drop
in total force (note the dotted horizontal line), but does not account for the
complete decrease in contractile force. (C) Data from B, except
that force is normalized per cross-sectional area (N/cm2). This figure illustrates
the atrophy-independent component of contractile dysfunction. (D) Maximal
power in EDL muscle from all four animal models. (E) Data from panel D
was normalized per cross sectional area of muscles. It shows that a significant drop
in power is atrophy-independent. Data is the average ± SE of 24 EDL muscles from 12
mice for each genotype. * indicates a significant difference (p < 0.01) between the
control muscles and a particular genotype. ** indicates a significant difference
(p < 0.01) between the old MIPKO mice and the old Wt and mature MIPKO mice.
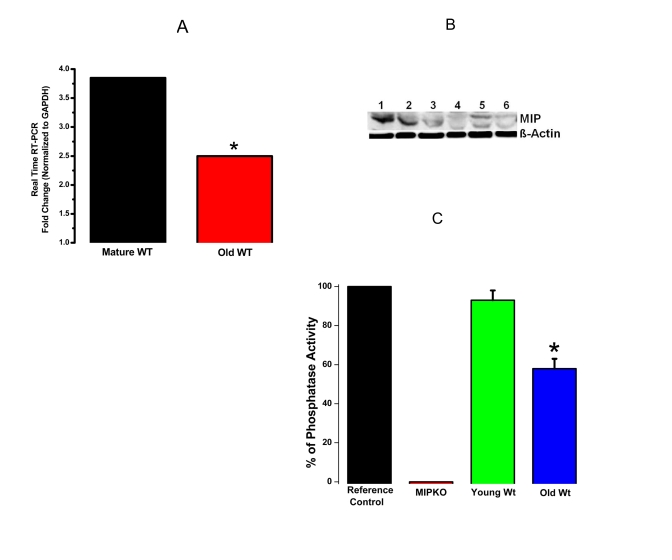
Figure 3. Reduced MIP gene expression, MIP protein levels and MIP phosphatase activity in old skeletal muscles. (A) Significant reduction in MIP
expression in EDL muscles from old Wt mice (red bar) compared with mature Wt mice
(black bar). (B) MIP protein content decreased drastically in old skeletal
muscle. Lanes 1-3, mature Wt EDL; Lanes 4-6, old Wt EDL; β-actin as controls. (C)
MIP enzymatic phosphatase activity reduced by ~ 30% in old Wt EDL muscles as
compared to young Wt EDL muscles. * indicates a significant difference (p < 0.01)
between the control muscles and a particular genotype.
Discussion
This communication and our previous study
[16] elevate the
role of MIP as a potent regulator of skeletal muscle function under normal
conditions. The fact that MIP expression, concentration, and function are
decreased with age also shows that MIP is important for, or at least
contributes to, the development of sarcopenia. Furthermore, we have
demonstrated that PI(3,5)P2 is the major substrate for MIP and that reduction
in MIP levels leads to accumulation of PI(3,5)P2 within the membrane of the muscle SR.
Thus, the significant decrease in MIP phosphatase activity during aging
should induce accumulation of intracellular PI(3,5)P2, leading to Ca2+
homeostasis defects, which is precisely the phenotype identified in both old Wt
and mature MIPKO muscle fibers.
Phospholipids
and phosphoinosites were once thought to play only structural and energetic
functions. They are now recognized as signaling molecules, particularly as second
messengers [19-26].
PIPI(3,5)P2, discovered only 10 years ago, is an isomer of the well-characterized
phosphoinosite, PI(3,5)P2. PI(3,5)P2 has been extensively studied and found to
modulate the properties of many membrane channels. The seven known PIPs (see Figure 5) are thought to form complex signal transduction networks in organisms
spanning yeasts to humans. The broadness of cellular functions controlled and
modulated by lipids seems almost unlimited with defined roles in cell signal
transduction, proliferation, growth, apoptosis, immune response, and adaptation
to stress, modulation of ionic channels and cell transporters [27].
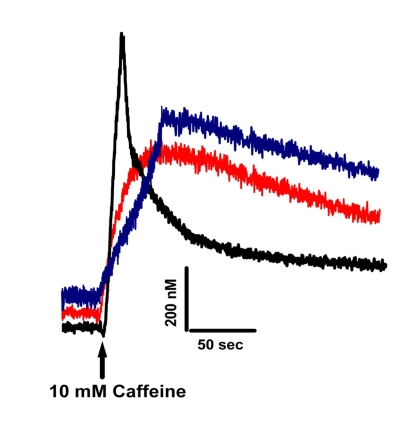
Figure 4. Altered Ca+2
homeostasis is present in muscle fibers from old Wt and MIPKO mice. Original traces
representative of caffeine-induced Fura-2 Ca+2 transients
in mature Wt (black trace), old Wt (red trace), and mature MIPKO FDB muscle
fibers (blue trace). Examples
shown are representative of 6-12 muscle fibers from 3 mice, and data were
normalized to the intracellular Ca+2 concentrations in nM.
Each PIP binds to a distinctive set of
effector proteins and, thereby, regulates a characteristic suite of cellular
processes, including membrane trafficking, cell survival/growth, cell division,
and cellular motility [28] (See also Figure 5). Importantly, aberrant lipid metabolism often leads to the onset of
pathology, and thus the precise balance of signaling lipids and their effectors
can serve as biomarkers for health and disease [19,22,27].
Therefore, the increased intracellular levels of PI(3,5)P2 that result from
either MIP ablation or from the natural decrease in MIP function with aging
might induce significant imbalances in signaling pathways.
The
potent signaling properties of PIPs depend on their localization as well as
abundance, which are determined by the collective actions of PIP kinases, PIP
phosphatases, and phospholipases [29,30]. PIP
phosphatases (such as MIP) are a subfamily of phosphatases that hydrolyze PIPs [31] (Figure 5).
For example, PTEN phosphatase dephosphorylates PI(3,4,5)P3
on the plasma membrane and is critical for a variety of cellular processes [32]. Loss of
PTEN function is associated with the tumorigenesis of many types of cancers [32]. The role
of other PIP phosphatases, such as myotubularin- and myopathy- related phosphatases
(MTMR) have not been well characterized. PIPs are also involved in bipolar
disorder, myopathies, acute myeloid leukemia, and type-2 diabetes. Importantly,
loss-of-function mutations in several MTMR phosphatases (MTM1, MTMR2, and
MTMR13) have been identified in genetic conditions, namely X-linked myotubular
myopathy (a muscle degenerative disease that shares some similarities with sarcopenia)
and Type-4B Charcot-Marie-Tooth disease (a neurodegenerative condition) [33-36].
However, the molecular mechanisms by which the mutations in these phosphatases
induce such diseases remain largely unknown [19,24]. As
shown in Figure 5, the quick interconversion of seven different PIPs creates a
dynamic signaling network that might underlie molecular mechanisms relevant for
a myriad of diseases.
We
believe that aging must be seen as the result of a multitude of long-term,
cumulative adaptations and not only due to changes resulting from the ablation
or the downregulation of a single gene. Nevertheless, changes in MIP function
could cause a cascade of effects with consequences that could be far more
serious and broader than the specific change in MIP itself. The reason for such
assertion is rather simple. MIP controls the intracellular levels of PI[3,5]P2,
which in turn binds to a multitude of proteins [28-30],
therefore working as a principal molecule in the coordination of intracellular
networks [28,31].
Intriguingly, we have recently obtained preliminary evidence that indicates
that mature MIPKO develops cardiovascular diseases and osteoporosis, both in
agreement of a broader and age-related role of MIP (Wacker, Andresen, Bonewald,
Johnson & Brotto; unpublished observations). Furthermore, we previously
showed that intracellular elevation of PI(3,5)P2 is able to activate the
opening of the RyR1 at contracting or resting levels of Ca2+. Such
an effect can lead to a vast amount of secondary changes as Ca2+ itself
is a second messenger that controls cellular life and death. For example,
chronically elevated resting levels of intracellular Ca2+ as
observed in mature MIPKO and old Wt muscle cells, might activate proteolytic
enzymes or enhance the production of reactive oxygen species and free radicals,
which in turn may contribute to muscle wasting.
In summary, our studies suggest new roles of MIP and its
major substrate, PI(3,5)P2, in the decline in muscle function during aging. We
believe that the loss of MIP activity with age could contribute to the loss of
muscle function due to the buildup of PI(3,5)P2 within the muscle SR membrane,
and consequently the increased conductance of the ryanodine receptor channel,
loss of Ca2+ from
the SR, and also inhibition of SOCE into the cell [16]. While additional studies will be required to
better define the cell biological functions of MIP and PI(3,5)P2, we propose
that the MIP-PI(3,5)P2 signaling pathway is an important contributor for aging
sarcopenia, and as such could be explored as a new therapeutic option for the
treatment of not only muscle myopathies, but also for aging sarcopenia. In
addition, the MIPKO model seems suitable for understanding of some aspects of
muscle aging and sarcopenia.
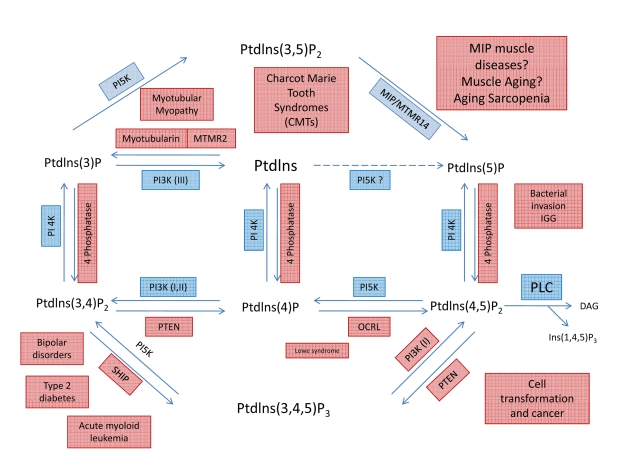
Figure 5. Metabolic pathways showing the interconversion of PIPs and putative roles of MIP and PI(3,5)P2. Through the
specific action of different phosphatases, seven phosphoinosites molecules
are generated. Note that a loss of MIP activity will lead to an increase in
PI(3,5)P2 concentration and activity. Even small changes in content or
activity of phosphatases and/or their substrates can lead to complex
pathologies, including several muscle diseases.
Materials and Methods
Force
Plate Actimeter (FPA).
To complement
our in vivo muscle function approaches we have selected the FPA because
it provides an efficient, accurate, and highly reproducible means of assessing
many aspects of locomotor behavior [17].
Ex-vivo,
isolated muscle contractility protocols.
These studies followed protocols established by Brotto & Nosek [37-39]. Intact
extensor digitorum longus (EDL) muscle of male and female WT and MIPKO mice of
all age groups were removed from tendon to tendon and immediately placed in a
dissecting dish containing a modified bicarbonate Ringer solution with 2.5 mM
extracellular Ca2+ or with 0 mM Ca plus 0.1 mM EGTA to test the
effects of extracellular Ca2+ and SOCE in muscle function. The pH
was adjusted to 7.4 with NaHCO3, followed by the addition of fetal
bovine serum (to 0.2%) to increase viability of the dissected muscle [37,40].The solution was continuously aerated with a
gas-mixture consisting of 95% O2 and5% CO2.
EDL and SOL muscles were mounted vertically between two Radnoti (Monrovea, CA,
USA) stimulating platinum electrodes and immersed in a 20 ml bathing chamber
containing the incubation medium. Via the tendons, the muscles were suspended
from movable isometric force transducers above the chambers and secured to the
base of the tissue support within the chambers. The analog output of the force
transducer were digitized, stored and analyzed with PowerLab Software (Colorado
Springs, CO, USA). For each muscle, the resting tension and the stimulatory
voltage was provided by a Grass S8800 digital stimulator (West Warwick, RI,
USA) and adjusted to produce a maximal isometric tetanic force (Tmax).
EDL muscles were the muscle choice since effects of Sarcopenia are known to be
exacerbated in fast-twitch muscles such as the EDL. Equilibration: The
intact muscles were allowed a 20-minute equilibration period after which time
they were stimulated with pairs of alternating high (that produced Tmax)
and low (that produced 1/2 Tmax) frequency pulse-trains administered
with a periodicity of 1 minute. Utilization of the proposed paradigm of
stimulation helps with the study of the relative contributions of the
contractile proteins (Tmax) and the SR (1/2 Tmax) to
contractile function [37].
Force vs. frequency relationship.
Following equilibration, the muscles are subjected to
stimulation with frequencies ranging from 1-200 Hz to generate the force vs.
frequency (FF) relationship. Force Normalization: All force data are
normalized as either the absolute force (force per cross sectional area) or as
a percentage of the maximum tetanic force (%Tmax) measured before
the beginning of the fatiguing protocol.
Fura-2
monitoring of intracellular Ca2+.
For quantitative measurements of intracellular [Ca2+], flexor
digitorum brevis (FDB) musclefibers were utilized. FDB muscle
fibers were enzymatically isolated in a 0 Ca Tyrode solution containing 2 mg/mL
type I collagenase for 2 hours in a shaking bath at 37°C. FDB muscle fibers
were then transferred to a 0 Ca2+ Tyrode solution without
collagenase and gently triturated with a pipette. The fibers were then be
loaded with 5 μM Fura-2-AM for 40 minutes, after which the Fura-2 AM was washed
off and be allowed to de-esterify. As fiber motion artifacts are associated with
intracellular Ca2+ release, 20 μM N-benzyl-p-toluene
sulfonamide (Sigma), a specific myosin II inhibitor, was then applied for 20
minutes. A dual-wavelength(excitation at 340 nm and 380 nm) PTI
spectrofluorometer (PhotonTechnology International, Birmingham, NJ)
was used to determinethe magnitude and kinetic changes of
caffeine-induced intracellular Ca2+ transients. Ratiometric changes
were converted into relative levels of Ca2+ in nM as previously
detailed by Brotto et al [41].
Biochemical
profiling of skeletal muscles.
Freshly isolated EDL muscles were dissected from all mice and saved for
biochemical analyses. Gene expression, MIP protein content, and MIP activity
assays were performed on these muscles as previously described by Zhao et al.
[11] and recently modified by Shen et al [16].
MIP
gene expression and MIP protein expression.
These procedures were performed as described in Zhao et al, and as
recently modified in Shen et al [16]. Briefly,
the mRNA expression level of MIP gene was determined by qPCR in freshly isolated
EDL muscles after mRNA was extracted using a RNeasy mini kit (Qiagen, Valencia,
CA, USA) and transcribed into cDNA by M-MLV Reverse Transcriptase (Promega,
Madison, WI, USA). qPCR was performed using SYBR Green PCR supermix
(Invitrogen, Carlsbad, CA, USA) on a Bio-Rad MyIQ 96-well PCR detection system.
The glyseraldehyde-3-phosphate dehydrogenase (GAPDH) gene was used as the
reference gene. Quality of the amplicons was confirmed by detection of uniform
melting curve peaks for each gene. One hundred nanograms of cDNA were added per
reaction and the final primer concentration was 200 nM. Experiments were run in
triplicate. Relative Ct values were calculated as 2CtGAPDH-CtTarget.
Protein concentrations were determined by DC protein assay (Bio-Rad) and 10 μg per sample was separated by
SDS-polyacrylamide gel electrophoresis
at room temperature on 4-12% Tris-glycine gradient gels for 2h at 60 mAmps on a
Mini PROTEAN II gel system (Bio-Rad). Gels were loaded in parallel and one set
was stained with Novex Colloidal Blue stain (Invitrogen), per manufacturer's
instructions. Equivalent loading was confirmed using monoclonal β-actin
antibody (Sigma), 0.2 μg mL−1. Results were visualized with an ECL +
kit (GE Healthcare, Piscataway, NJ, USA) following the manufacturer's
directions.
MIP phosphatase activity.
To determine the lipid phosphatase activity, Di-C8
phosphoinositides (Echelon Biosciences Inc., Salt Lake City, UT) and
dioleoyl-phosphatidylserine (Sigma, St. Louis, MO) were resuspended via
sonication in the assay buffer (100 mM sodium acetate, 50 mM bis-Tris, 50 mM
Tris pH 5.5, and 10 mM dithiothreitol) to final concentrations of 100 and 1000 μM, respectively. Equal volumes of di-C8 phosphoinositides
and dioleoyl-phosphatidylserine were added into 1.5 ml microcentrifuge tubes
and the mixtures were prewarmed at 37°C for 5 min.
Reactions were initiated by the addition of 500 ng of GST-MIP fusion protein
diluted in the assay buffer containing 1.0 mg/ml gelatin. This reaction step
provided the control values seen in Figure 4. Next, muscle homogenate reactions
were initiated by the addition of 2000 ng of total muscle protein diluted in
the assay buffer containing 1.0 mg/ml gelatin. Reactions were quenched after 30
min by the addition of 20 μl of 0.1 M N-ethylmaleimide
and spun at 18,000 × g for 10 min to sediment the lipid aggregates. The
supernatant (25 μl) was added to a 96-well plate and 100 μl of Malachite green reagent (Echelon Biosciences Inc., Salt Lake City,
UT) was added to each well. After incubation at room temperature for 15 min,
the color development was measured at 620 nm. Inorganic phosphate release was
quantitated using a standard curve generated with KH2PO4
in distilled H2O.
Statistical analyses.
Values are mean ± SEM. Significance was determined by
ANOVA followed by either Tukey's or Bonferroni's tests. ANOVA on Ranks followed
by Kruskal-Wallis test was used for non-parametric data. A value of P
< 0.05 was used as criterion for statistical significance.
Acknowledgments
This work was supported by
pilot grant from the NIH-National Institutes of Cancer-Center for Trans-disciplinary
Research on Energetics and Cancer to T.M.N., C.K.Q. and M.B.; a Grand
Opportunities (GO) NIH-1RC2AR058962-01
grant to M.B., an American Heart Association grant
0535555N to M.B; NIH grants HL068212 and HL082670 to C.K.Q., NIH grant HL55438
to H.H.V.
Conflicts of Interest
The authors of this manuscript have no conflict of
interests to declare.
References
-
1.
Faulkner
JA
, Brooks
SV
and Zerba
E.
Muscle atrophy and weakness with aging: Contration-induced injury as an underlying mechanism.
J Gerontol.
1995;
50A:
124
-129.
.
-
2.
Gonzalez
E
, Messi
ML
and Delbono
O.
The Specific Force of Single Intact Extensor Digitorum Longus and Soleus Mouse Muscle Fibers Declines with Aging.
J Membr Biol.
2000;
178:
175
-183.
[PubMed]
.
-
3.
Faulkner
JA
, Brooks
SV
and Zerba
E.
Muscle atrophy and weakness with aging: contraction-induced injury as an underlying mechanism.
J Gerontol A Biol Sci Med Sci.
1995;
50 Spec No:
124
-129.
[PubMed]
.
-
4.
Delbono
O
Molecular mechanisms and therapeutics of the deficit in specific force in ageing skeletal muscle.
Biogerontology.
2005;
3:
265
-270.
[PubMed]
.
-
5.
Zahn
JM
, Sonu
R
, Vogel
H
, Crane
E
, Mazan-Mamczarz
K
, Rabkin
R
, Davis
RW
, Becker
KG
, Owen
AB
and Kim
SK.
Transcriptional profiling of aging in human muscle reveals a common aging signature.
PLoS Genet.
2006;
2:
e115
[PubMed]
.
-
6.
Bouchard
C
, Shephard
RJ
, Stephens
T
, Sutton
JR
and McPherson
BD.
Human Kinetics Books
Champaign
3
-28.
Exercise, fitness, and health: A consensus statement. In: Bouchard C, Shephard RJ, Stephens T, Sutton JR, McPherson BD, eds. Exercise, Fitness, and Health: A Consensus of Current Knowledge.
1990;
.
-
7.
Bortz
WM
How fast do we age? Exercise performance over time as a biomarker.
J Gerontol A Biol Sci Med Sci.
1996;
51:
M223
-M225.
[PubMed]
.
-
8.
Lowe
DA
, Husom
AD
, Ferrington
DA
and Thompson
LV.
Myofibrillar myosin ATPase activity in hindlimb muscles from young and aged rats.
Mech Ageing Dev.
2004;
125:
619
-627.
[PubMed]
.
-
9.
Lowe
DA
, Thomas
DD
and Thompson
LV.
Force generation, but not myosin ATPase activity, declines with age in rat muscle fibers.
Am J Physiol Cell Physiol.
2002;
283:
C187
-C192.
[PubMed]
.
-
10.
Brotto
M
, Weisleder
N
and Ma
J.
Store-operated Ca2+ entry in muscle physioloy.
Current Chemical Biology.
2007;
1:
87
-95.
.
-
11.
Zhao
X
, Weisleder
N
, Thornton
A
, Oppong
Y
, Campbell
R
, Ma
J
and Brotto
M.
Compromised store-operated Ca(2+) entry in aged skeletal muscle.
Aging Cell.
2008;
7:
561
-568.
[PubMed]
.
-
12.
Brum
G
, Rios
E
and Stefani
E.
Effects of extracellular calcium on calcium movements of excitation-contraction coupling in frog skeletal muscle fibres.
J Physiol.
1998;
398:
441
-73.
[PubMed]
.
-
13.
Doran
P
, Donoghue
P
, O'Connell
K
, Gannon
J
and Ohlendieck
K.
Proteomics of skeletal muscle aging.
Proteomics.
2009;
9:
989
-1003.
[PubMed]
.
-
14.
Gannon
J
, Staunton
L
, O'Connell
K
, Doran
P
and Ohlendieck
K.
Phosphoproteomic analysis of aged skeletal muscle.
Int J Mol Med.
2008;
22:
33
-42.
[PubMed]
.
-
15.
O'Connell
K
, Gannon
J
, Doran
P
and Ohlendieck
K.
Proteomic profiling reveals a severely perturbed protein expression pattern in aged skeletal muscle.
Int J Mol Med.
2007;
20:
145
-153.
[PubMed]
.
-
16.
Shen
J
, Yu
WM
, Brotto
M
, Scherman
JA
, Guo
C
, Stoddard
CJ
, Yu
WM
, Brotto
M
, Scherman
JA
, Guo
C
, Stoddard
C
, Nosek
TM
, Valdivia
HH
and Qu
CK.
Deficiency of MIP/MTMR14 phosphatase induces a muscle disorder by disrupting Ca(2+) homeostasis.
Nat Cell Biol.
2009;
11:
769
-776.
[PubMed]
.
-
17.
Fowler
SC
, Zarcone
TJ
, Chen
R
, Taylor
MD
and Wright
DE.
Low grip strength, impaired tongue force and hyperactivity induced by overexpression of neurotrophin-3 in mouse skeletal muscle.
Int J Dev Neurosci.
2002;
20:
303
-308.
[PubMed]
.
-
18.
Zhao
X
, Yoshida
M
, Brotto
L
, Takeshima
H
, Weisleder
N
, Hirata
Y
, Nosek
TM
, Ma
J
and Brotto
M.
Enhanced resistance to fatigue and altered calcium handling properties of sarcalumenin knockout mice.
Physiol Genomics.
2005;
23:
72
-78.
[PubMed]
.
-
19.
Sudhahar
CG
, Haney
RM
, Xue
Y
and Stahelin
RV.
Cellular membranes and lipid-binding domains as attractive targets for drug development.
Curr Drug Targets.
2008;
9:
603
-613.
[PubMed]
.
-
20.
Eaton
S
Multiple roles for lipids in the Hedgehog signalling pathway.
Nat Rev Mol Cell Biol.
2008;
9:
437
-445.
[PubMed]
.
-
21.
Rivera
R
and Chun
J.
Biological effects of lysophospholipids.
Rev Physiol Biochem Pharmacol.
2008;
160:
25
-46.
[PubMed]
.
-
22.
Deigner
HP
and Hermetter
A.
Oxidized phospholipids: emerging lipid mediators in pathophysiology.
Curr Opin Lipidol.
2008;
19:
289
-294.
[PubMed]
.
-
23.
Sagin
FG
and Sozmen
EY.
Lipids as key players in Alzheimer disease: alterations in metabolism and genetics.
Curr Alzheimer Res.
2008;
5:
4
-14.
[PubMed]
.
-
24.
Serhan
CN
, Yacoubian
S
and Yang
R.
Anti-inflammatory and proresolving lipid mediators.
Annu Rev Pathol;.
2008;
3:
279
-312.
.
-
25.
Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids.
Nat Rev Mol Cell Biol.
2008;
9:
139
-150.
[PubMed]
.
-
26.
Muoio
DM
and Koves
TR.
Skeletal muscle adaptation to fatty acid depends on coordinated actions of the PPARs and PGC1 alpha: implications for metabolic disease.
Appl Physiol Nutr Metab.
2007;
32:
874
-883.
[PubMed]
.
-
27.
Sang
N
and Chen
C.
Lipid signaling and synaptic plasticity.
Neuroscientist.
2006;
12:
425
-434.
[PubMed]
.
-
28.
Balla
T
Phosphoinositide-derived messengers in endocrine signaling.
J Endocrinol.
2006;
188:
135
-153.
[PubMed]
.
-
29.
Krauss
M
and Haucke
V.
Phosphoinositide-metabolizing enzymes at the interface between membrane traffic and cell signalling.
EMBO Rep.
2007;
8:
241
-246.
[PubMed]
.
-
30.
Krauss
M
and Haucke
V.
Phosphoinositides: regulators of membrane traffic and protein function.
FEBS Lett.
2007;
581:
2105
-2111.
[PubMed]
.
-
31.
Robinson
FL
and Dixon
JE.
Myotubularin phosphatases: policing 3-phosphoinositides.
Trends Cell Biol.
2006;
16:
403
-412.
[PubMed]
.
-
32.
Yin
Y
and Shen
WH.
PTEN: a new guardian of the genome.
Oncogene.
2008;
27:
5443
-5453.
[PubMed]
.
-
33.
Laporte
J
, Hu
LJ
, Kretz
C
, Mandel
JL
, Kioschis
P
, Coy
JF
, Klauck
SM
, Poustka
A
and Dahl
N.
A gene mutated in X-linked myotubular myopathy defines a new putative tyrosine phosphatase family conserved in yeast.
Nat Genet.
1996;
13:
175
-182.
[PubMed]
.
-
34.
Bolino
A
, Muglia
M
, Conforti
FL
, LeGuern
E
, Salih
MA
, Georgiou
DM
, Christodoulou
K
, Hausmanowa-Petrusewicz
I
, Mandich
P
, Schenone
A
, Gambardella
A
, Bono
F
, Quattrone
A
, Devoto
M
and Monaco
AP.
Charcot-Marie-Tooth type 4B is caused by mutations in the gene encoding myotubularin-related protein-2.
Nat Genet.
2000;
25:
17
-19.
[PubMed]
.
-
35.
Azzedine
H
, Bolino
A
, Taieb
T
, Birouk
N
, Di
DM
, Bouhouche
A
, Benamou
S
, Mrabet
A
, Hammadouche
T
, Chkili
T
, Gouider
R
, Ravazzolo
R
, Brice
A
, Laporte
J
and LeGuern
E.
Mutations in MTMR13, a new pseudophosphatase homologue of MTMR2 and Sbf1, in two families with an autosomal recessive demyelinating form of Charcot-Marie-Tooth disease associated with early-onset glaucoma.
Am J Hum Genet.
2003;
72:
1141
-1153.
[PubMed]
.
-
36.
Senderek
J
, Bergmann
C
, Weber
S
, Ketelsen
UP
, Schorle
H
, Rudnik-Schoneborn
S
, Buttner
R
, Buchheim
E
and Zerres
K.
Mutation of the SBF2 gene, encoding a novel member of the myotubularin family, in Charcot-Marie-Tooth neuropathy type 4B2/11p15.
Hum Mol Genet;.
2003;
12:
349
-356.
.
-
37.
Brotto
MA
, Nosek
TM
and Kolbeck
RC.
Influence of ageing on the fatigability of isolated mouse skeletal muscles from mature and aged mice.
Exp Physiol.
2002;
87:
77
-82.
[PubMed]
.
-
38.
Nosek
TM
, Brotto
MA
, Essig
DA
, Mestril
R
, Conover
RC
, Dillmann
WH
and Kolbeck
RC.
Functional properties of skeletal muscle from transgenic animals with upregulated heat shock protein 70.
Physiol Genomics.
2000;
4:
25
-33.
[PubMed]
.
-
39.
Weisleder
N
, Brotto
M
, Komazaki
S
, Pan
Z
, Zhao
X
, Nosek
T
, Parness
J
, Takeshima
H
and Ma
J.
Muscle aging is associated with compromised Ca2+ spark signaling and segregated intracellular Ca2+ release.
J Cell Biol.
2006;
174:
639
-645.
[PubMed]
.
-
40.
Kolbeck
RC
and Spear
WA.
Diaphragm contactility as related to cellular calcium metabolism: Influence of theophylline and fatigue.
1989;
139 ed
.
-
41.
Brotto
MAP
and Creazzo
TL.
Ca2+ transients in embryonic chick heart: Contributions from Ca2+ channels and the sarcoplasmic reticulum.
Am J Physiol Heart Circ Physiol.
1996;
270:
H518
-H525.
.