NSun2 represses p27 translation by methylating the 5′UTR of p27 mRNA
The present study was prompted by our earlier findings that high levels of NSun2 lowered p27 abundance. As shown in Fig. 1A, overexpression of NSun2 in HeLa cells by transfection of plasmid pNSun2 reduced p27 protein levels by ~70% while silencing of NSun2 by transfection of small interfering (si) RNA increased p27 protein levels by ~4.2 fold. However, neither overexpression nor knockdown of NSun2 influenced p27 mRNA levels (Fig. 1B). These results suggested that the regulation of p27 by NSun2 may not involve altered p27 mRNA transcription or turnover. Because NSun2 had been shown to regulate the expression of p53, p16, E2F3, Bak1, ErbB2, and CDK1 by methylating the mRNAs encoding these proteins [26–28], we tested if NSun2 was capable of methylating p27 mRNA. To this end, the p27 mRNA fragments described in Fig. 2A were used for in vitro methylation assays (Materials and Methods). The p27 cDNA and p16-CR (coding region of p16 mRNA) were included as negative controls, while bacterial tRNA served as a positive control. As shown in Fig. 2B (left and right), tRNA, 5′UTR, 5′UTRa, and 5′UTRa1 were methylated, while p27 cDNA, p16-CR, p27-CR, p27-3′UTR, p27-5′UTRa2, and p27-5′UTRb were not methylated. Accordingly, the methylation site was located at the p27 5′UTR (positions 1-140).
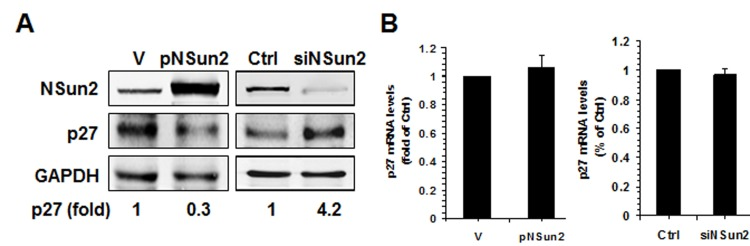
Figure 1. NSun2 regulates p27 expression. (A) HeLa cells were transfected with a vector expressing NSun2 (pNSun2) or with a siRNA targeting NSun2. Forty-eight hours later, cell lysates were prepared and subjected to Western blot analysis to assess the levels of proteins NSun2, p27, and GAPDH. (B) RNA prepared from cells described in Fig. 1A was used for RT-qPCR analysis to assess the levels of p27 mRNA. Data represent the means ± SD from 3 independent experiments.
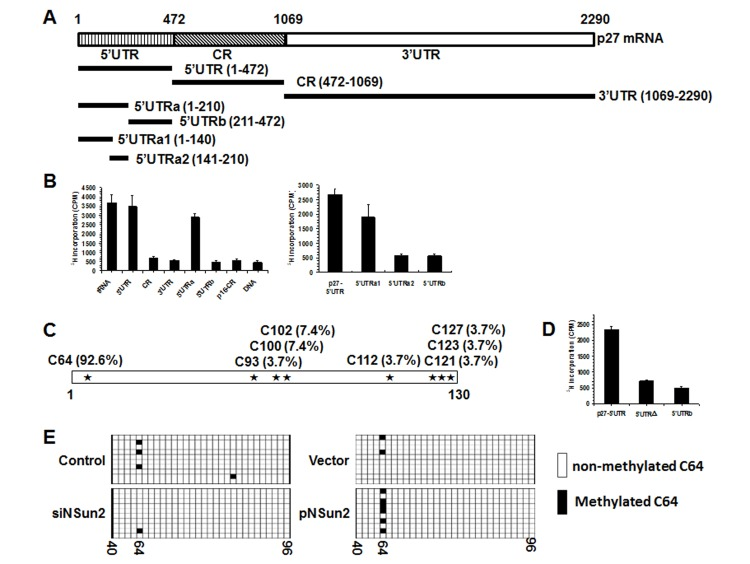
Figure 2. NSun2 methylates p27 5′UTR in vitro and in cells. (A) Schematic representation depicting the p27 mRNA fragments used for in vitro methylation assays. (B) Incorporation of 3H-labeled SAM into p27 5′UTR, CR, 3′UTR, 5′UTRa, and 5′UTRb fragments (left) as well as 5′UTR, 5′UTRa1, 5′UTRa2, and 5′UTRb fragments (right). The incorporation of 3H-labeled SAM into p27 cDNA (DNA) and p16-CR (coding region) served as negative controls. The incorporation of 3H-labeled SAM into bacteria tRNA served as a positive control. (C)In vitro methylated 5′UTRa1 fragment was subjected to bisulfate RNA sequencing analysis to identify the methylation sites, as described in “Materials and Methods”. The percent of methylation at different sites is indicated. (D) Incorporation of 3H-labeled SAM into the 5′UTR variant mutating C64 (5′UTRΔ). 5′UTR and 5′UTRb served as negative and positive controls, respectively. (E) HeLa cells were transfected with a pGL3-derived reporter bearing the p27 5′UTR; 24 h later, cells were further transfected with a vector expressing NSun2 or with NSun2 siRNA and cultured for an additional 48 h. RNA was isolated and subjected to bisulfate sequencing analysis to assess the rate of C64 methylation. Open boxes indicate cytosine-to-uracil conversion, read as thymidine in the cDNA (unmethylated), and filled boxes indicate a retained cytosine (methylated). The numbers below the columns refer to cytosine positions in the p27 5′UTR.
To determine the formation of m5C or m6A in the methylated fragments, p27-5′UTRa1 methylated in vitro by using nonisotopic S-Adenosyl methionine (SAM) and NSun2, and unmethylated (same reaction without adding NSun2) p27-5′UTRa1 were subjected to MS-HPLC analysis. As shown, m5C was detected in the methylated p27-5′UTRa1 fragment (Supplemental Fig. S1). Identification of m6A from the methylated p27-5′UTRa1 did not show any positive results (not shown). To further identify the methylation site by NSun2, in vitro methylated p27-5′UTRa1 was subjected to bisulfate sequencing analysis. As shown in Fig. 2C, among all of the clones sequenced, C64 was detected in ~92.6% of positive clones. Mutation of C64 (p27 5′UTRΔ) nearly abolished the effect of NSun2 on methylating the p27 5′UTR (Fig. 2D), indicating that C64 is a major methylation site.
To test whether NSun2 was capable of methylating p27 5′UTR in cells, HeLa cells were transfected with a pGL3-derived reporter bearing the p27 5′UTR. Twenty-four hours later, cells were further transfected with a vector expressing NSun2 or with NSun2 siRNA and cultured for an additional 48 h. RNA was isolated and subjected to bisulfate sequencing analysis to assess the rate of C64 methylation. As shown, knockdown of NSun2 reduced the methylation of C64, whereas overexpression of NSun2 increased the rate of C64 methylation (Fig. 2E). These results indicate that NSun2 was able to methylate p27 mRNA in cells.
To further test whether methylation of p27 5′UTR by NSun2 influenced p27 expression levels, pGL3-derived reporters bearing fragments of p27 mRNA were constructed (Fig. 3A, schematic). HeLa cells were transfected with each of these reporters and 24 h later, cells were transfected with control or NSun2-directed siRNAs and cultured for an additional 48 h. Cellular lysates then were collected and subjected to Western blot analysis to assess NSun2, Firefly luciferase, Renilla luciferase, and GAPDH protein levels. As shown in Fig. 3B, knockdown of NSun2 elevated the levels of reporter protein expressed from pGL3-5′UTR, but not that expressed from pGL3, pGL3-CR, pGL3-3′UTR, or pGL3-5′UTRΔ, indicating that methylation by NSun2 at C64 selectively repressed the expression of p27. Because NSun2 regulates the expression of p27 without influencing the levels of p27 mRNA (Fig. 1), we further asked if methylation by NSun2 regulated the translation of p27. To this end, in vitro-transcribed reporter transcripts Luciferase (Luc), Luc-5′UTR, and Luc-5′UTRΔ were methylated in vitro by NSun2 or kept unmethylated. These transcripts then were used for in vitro translation assays and reporter activity was used as readout of the efficiency of translation. As shown in Fig. 3C (left), methylation by NSun2 reduced the reporter activity of Luc-5′UTR (by ~59%), but not that of Luc and Luc-5′UTRΔ. Therefore, methylation of p27 5′UTR by NSun2 represses the expression of p27 at the level of translation.
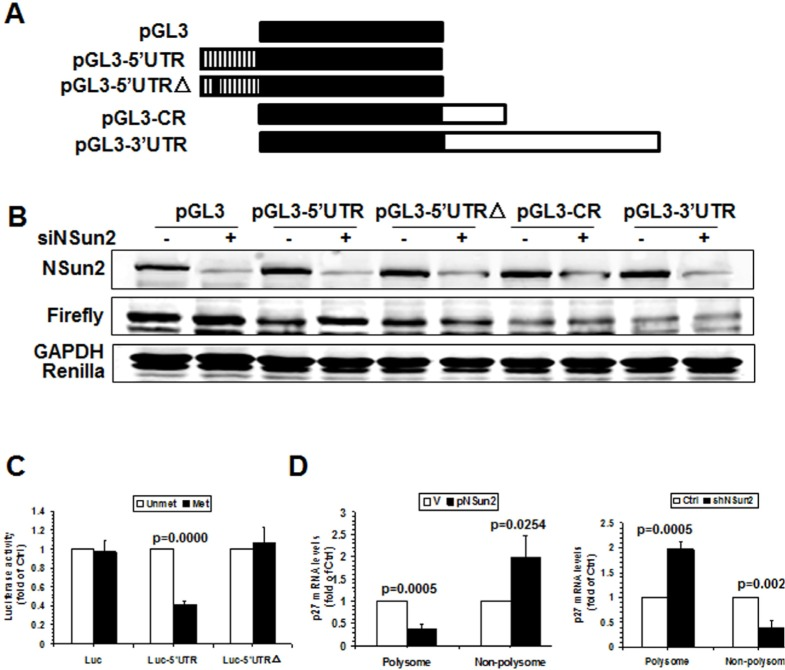
Figure 3. Methylation by NSun2 is functional for regulating the translation of p27. (A) Schematic representation of the pGL3-derived reporter vectors used for reporter gene assays. (B) HeLa cells were transfected with each of the reporter vectors described in Fig. 3A together with a pRL-CMV control reporter. Twenty-four hours later, cells were further transfected with NSun2 siRNA and cultured for an additional 48 h. Firefly luciferase activity against Renilla luciferase activity was analyzed. Data represent the means ± SD from 3 independent experiments; significance was analyzed by Student's t test. (C)In vitro methylated (Met) or unmethylated (Unmet) Luciferase (Luc), luc-5′UTR, and luc-5′UTRΔ reporter transcripts were used for in vitro translation assays. Firefly luciferase activity was measured to determine the translation efficiency. Data represent the means ± SD from 3 independent experiments; significance was analyzed by Student's t test. (D) Cells described in Fig. 1A were used for isolating the polysomal and non-polysomal fractions. RNA prepared from the fractions was subjected to RT-qPCR analysis to assess the presence of p27 mRNA in the polysomal and non-polysomal fractions. Data represent the means ± SD from 3 independent experiments; significance was analyzed by Student's t test.
To further investigate the mechanisms underlying the translational regulation of p27 by NSun2, the polysomal presence of p27 mRNA in cells with overexpressed or silenced NSun2 was examined. As shown in Fig. 3D, overexpression of NSun2 decreased the presence of p27 mRNA in the polysomal fraction (by ~66%, p=0.0005) but increased it in the non-polysomal fraction (by ~2.0 fold, p=0.0254). On the other hand, knockdown of NSun2 increased the presence of p27 mRNA in the polysomal fraction (by ~60%, p=0.0005) but decreased it in the non-polysomal fraction (by ~2.0 fold, p=0.0024). Taken together, these findings indicate that the assembly of p27 mRNA in the polysome was repressed by NSun2-mediated methylation, in turn repressing the translation of p27.
By binding to the p27 5′UTR, RNA-binding proteins HuR and CUGBP1 repress p27 translation [15–16]. These findings raised the question that HuR or CUGBP1 might influence the function of NSun2 in repressing the translation of p27. As shown in Supplemental Fig. S2A by RNA pull-down assays, HuR associated with the p27 5′UTR, 5′UTRb, and 3′UTR, while CUGBP1 only associated with the p27 5′UTR and 5′UTRb, in agreement with previous findings [15, 16]. NSun2 interacted with the p27 5′UTR and 5′UTRa, consistent with the results that NSun2 methylated p27 5′UTR and 5′UTRa (Fig. 2B). Although NSun2 was capable of interacting with the p27 3′UTR, this interaction did not lead to the methylation of p27 3′UTR (Fig. 2B). Furthermore, methylation by NSun2 did not influence the association of HuR or CUGBP1 with the p27 mRNA (Supplemental Fig. S2B), and the association of NSun2 with p27 mRNA did not influence the association of HuR and CUGBP1 with p27 mRNA or vice versa (Supplemental Fig. S2C-E). Moreover, knockdown of NSun2 increased the activities of reporters pGL3-5′UTR and pGL3-5′UTRa, but not those of pGL3-5′UTRb or pGL3-5′UTRΔ (Supplemental Fig. S2F). In contrast, knockdown of HuR or CUGBP1 increased the activities of reporters pGL3-5′UTR, pGL3-5′UTRb, and pGL3-5′UTRΔ, but not that of pGL3-5′UTRa (Supplemental Fig. S2F). These results suggest that the translational repression of p27 by NSun2-mediated mRNA methylation is independent of the effects elicited by HuR or CUGBP1.
NSun2 regulation of p27 and CDK1 impacts upon replicative senescence
Apart from p27, NSun2 was also found to regulate the expression of senescence-associated proteins p53, p16, CDK1, and CDC25C [26–28]. To further investigate whether NSun2 regulated the process of replicative senescence, we assessed the levels of proteins NSun2, p53, p16, CDK1, p27, CDC25C, and GAPDH in early-passage [proliferating, ‘Young’ (Y), ~PDL 28], middle-passage [middle (M), ~PDL 40], and late-passage [senescent (S), ~PDL 55] human diploid fibroblasts (2BS) by Western blot analysis. As shown in Fig. 4A (left), the levels of p16 and p27 increased with replicative replicative senescence, while the levels of p53 remained unchanged. However, the levels of proteins NSun2, CDK1, and CDC25C were reduced with replicative senescence. The inverse correlation between NSun2 and p27 levels was also observed in another model of senescence, proliferating and senescent IDH4 cells (Fig. 4A, right). In addition, the levels of CDK1 mRNA decreased (by ~45% in 2BS cells, p=0.0000; by ~63% in IDH4 cells, p=0.0000) but p27 mRNA levels remained unchanged during senescence in both cell types (Fig. 4B). Both endogenous p53 and p16 are constitutively expressed in IDH4 cells, regardless of whether they are maintained in a proliferative state or they are induced to undergo senescence (Fig. S3). Methylated RNA-specific PCR analysis revealed that the levels of methylated CDK1 and p27 mRNAs decreased in the process of replicative senescence (by ~76% for p27 mRNA; by ~46% for CDK1 mRNA) (Fig. 4C), while the levels of unmethylated CDK1 and p27 mRNAs increased in replicative senescence (by ~2.7 fold for p27 mRNA, p=0.0023; by ~5.9 fold for CDK1 mRNA). These results indicate that NSun2-mediated RNA methylation may be able to regulate the expression levels of CDK1, p27, and CDC25C, but not those of p53 and p16, as cells progress to senescence.
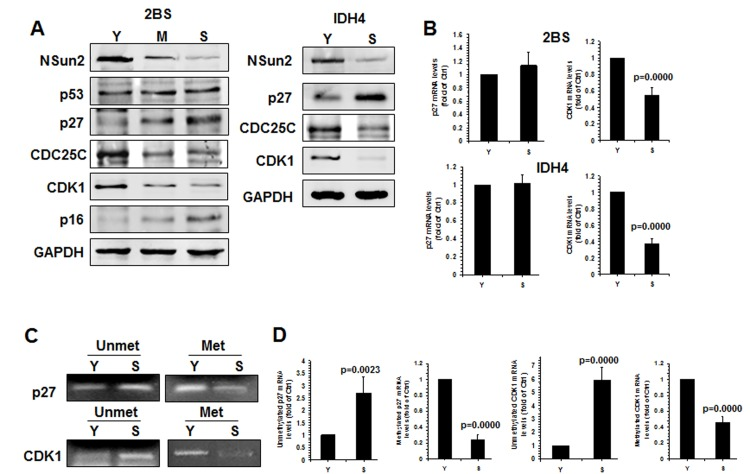
Figure 4. Inverse correlation between p27 levels and NSun2 levels in replicative senescence. (A)Left, the levels of proteins NSun2, p53, p16, p27, CDC25C, CDK1, and GAPDH in early-passage (Proliferating ‘Young’, ‘Y’, ~PDL 28), middle-passage (middle, ~PDL 40), and late-passage (Senescent, ‘S’, ~PDL 55) human diploid fibroblasts (2BS) were assessed by Western blotting. Right, the levels of proteins NSun2, p27, and GAPDH in Proliferating (Y) and Senescent (S) IDH4 cells were analyzed by Western blot analysis. (B) RNA was prepared from young (Y, ~PDL 28) and senescent (S, ~PDL 55) 2BS cells as well as young (Y) and senescent (S) IDH4 cells and RT-qPCR analysis was performed to assess the levels of p27 and CDK1 mRNAs. (C) RNA described in Fig. 4B was subjected to methylation-specific PCR analysis to assess the methylation of p27 and CDK1 mRNA. Data are representative from 3 independent experiments. (D) The density of the methylation-specific PCR (% of Ctrl) in Fig. 4C relative to that of the mRNA levels (% of Ctrl) shown in Fig. 4B is shown. Data represent the means ± SD from 3 independent experiments; significance was analyzed by Student's t test.
To test this idea using a different approach, 2BS cells were stably infected with lentiviruses expressing either NSun2 or NSun2 shRNA. As shown in Fig. 5A, infection with the NSun2-expressing lentiviruses reduced the levels of p27 by ~70%, while infection with NSun2 shRNA-expressing lentiviruses increased the levels of p27 by ~5.1 fold. In contrast, the levels of proteins CDK1 and CDC25C increased in cells with overexpressed NSun2 (by ~2.8 fold for CDK1; by ~1.5 fold for CDC25C) but decreased in cells with silenced NSun2 (by ~70% for both CDK1 and CDC25C). However, the levels of proteins p53 and p16 increased moderately in cells with overexpressed NSun2 and decreased only moderately in cells with silenced NSun2 (Fig. 5A). These modest changes may be an indirect reflection of the fact that 2BS cells with overexpressed NSun2 or silenced NSun2 have a constitutively altered senescence phenotype, perhaps as a result of compensatory changes in response to the permanent alterations in NSun2 levels. In agreement with the findings in Fig. 1B and in previous studies [28], neither overexpression of NSun2 nor knockdown of NSun2 altered the levels of p27 and CDK1 mRNAs in a measurable manner (Fig. 5B). In addition, the levels of cellular methylated p27 mRNA increased in cells with overexpressed NSun2 but decreased in cells with silenced NSun2 (Supplemental Fig. S4).
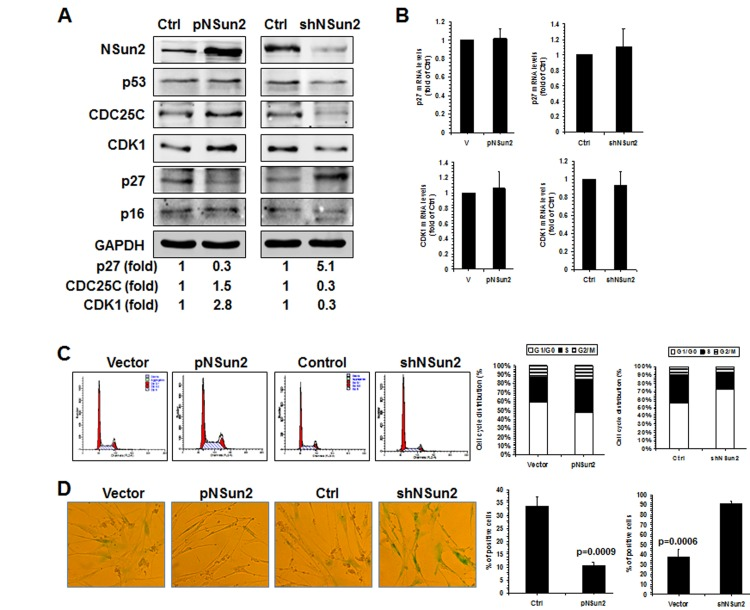
Figure 5. NSun2-p27 regulatory process impacts on the progression of replicative senescence. (A) 2BS cells were stably infected with a lentivirus bearing a pHBLV-NSun2 vector (pNSun2) or a pHBLV-shNSun2 vector (shNSun2). Cell lysates were prepared and subjected to Western blot analysis to assess the levels of proteins NSun2, p53, CDC25C, p27, p16, CDK1, and GAPDH. (B) RNA prepared from cells described in Fig. 5A was subjected to RT-qPCR analysis to assess the levels of p27 and CDK1 mRNAs. Data represent the means ± SD from 3 independent experiments. (C) Cells described in Fig. 5A were analyzed for cell cycle distribution (left). The percentage of cells in each cell cycle compartment is presented (right). (D) Cells described in Fig. 5A were analyzed for the activity of SA-β-gal (left). The means ± SD from 3 independent experiments are presented; significance was analyzed by Student's t test (right).
Finally, knockdown of NSun2 inhibited cell growth and increased the proportions of cells displaying the protein marker senescence-associated (SA)-β-galactosidase (~37.5% vs. ~91.0%), while overexpression of NSun2 promoted cell growth and reduced SA-β-galactosidase activity (~33.5% vs. ~10.6%). We next tested if overexpression of the p27 5′UTR fragment could function as a decoy fragment and rescue the effect of NSun2 overexpression in modulating p27, CDK1, and CDC25C levels, in promoting cell growth, and in delaying replicative senescence. As shown in Figure 6, overexpression of the p27 5′UTR fragment in 2BS cells by infecting 2BS cells with a lentivirus expressing luc-5′UTR (which increased p27 5′UTR levels by ~944 fold) rescued the effect of NSun2 overexpression in influencing the expression of p27, CDK1, and CDC25C (Figure 6A), in promoting DNA replication (progression through the S phase; Figure 6B), and in delaying replicative senescence (Control vs. pNSun2, ~46.4% vs. ~20.0%; control+pHBLV-5′UTR vs. pNSun2+pHBLV-5′UTR, ~51.5% vs. ~46.8%) (Figure 6C). Overexpression of the p27 5′UTR modestly increased the SA-β-galactosidase activity in control vector-transfected cells (~46.4% vs. ~51.5%), although the alteration was not statistically significant. In sum, the influence of NSun2 on the expression of p27, CDK1, and CDC25C has a robust impact upon replicative senescence.
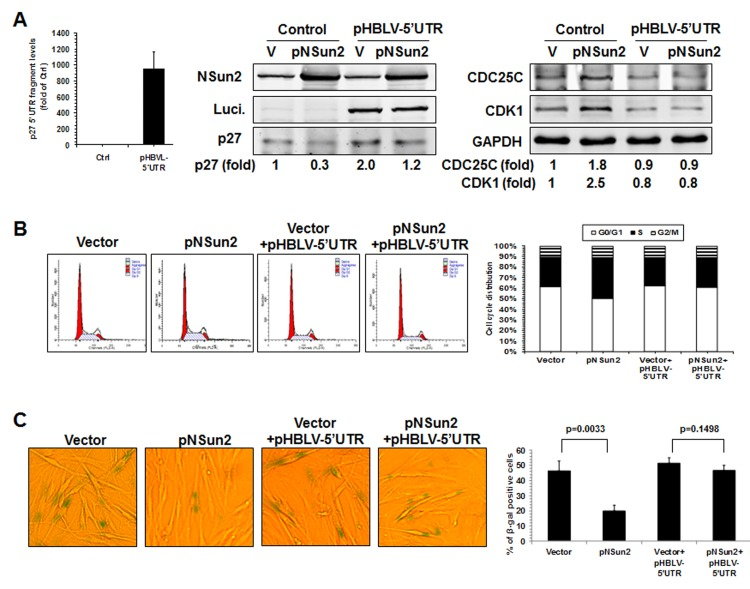
Figure 6. NSun2 delays replicative senescence by regulating p27, CDK1, and CDC25C. (A) 2BS cells were stably infected with a lentivirus bearing a pHBLV-NSun2 vector (pNSun2) or with a control lentivirus (Vector), or stably co-infected with a lentivirus bearing a pHBLV-luc-5′UTR vector (Luc-5′UTR) or kept untreated (Control). Cell lysates were prepared and subjected to Western blot analysis to assess the levels of proteins NSun2, luciferase, p27, CDK1, CDC25C, and GAPDH. (B) The cells described in Fig. 6A were analyzed for cell cycle distribution (left) and the percentage of cells in each cell cycle compartment represented (right). (C) Cells described in Fig. 6A were used for analysis of SA-β-gal activity (left). The means ± SD from 3 independent experiments are presented and the significance was assessed by using Student's t test (right).