HSCs transdifferentiate primarily to pneumonocytes in radiation-induced lung damage repair
Abstract
Accumulative radiation exposure leads to hematopoietic or tissue aging. Whether hematopoietic stem cells (HSCs) are involved in lung damage repair in response to radiation remains controversial. The aim of this study is to identify if HSC can transdifferentiate to pneumonocytes for radiation-induced damage repair. To this end, HSCs from male RosamT/mG mice were isolated by fluorescence-activated cell sorting (FACS) and transplanted into lethally irradiated female CD45.1 mice. 4 months after transplantation, transplanted HSC was shown to repair the radiation-induced tissue damage, and donor-derived tdTomato (phycoerythrin, PE) red fluorescence cells and Ddx3y representing Y chromosome were detected exclusively in female recipient lung epithelial and endothelial cells. Co-localization of donor-derived cells and recipient lung tissue cells were observed by laser confocal microscopy and image flow cytometry. Furthermore, the results showed HSC transplantation replenished radiation-induced lung HSC depletion and the PE positive repaired lung epithelial cells were identified as donor HSC origin. The above data suggest that donor HSC may migrate to the injured lung of the recipient and some of them can be transdifferentiated to pneumonocytes to repair the injury caused by radiation.
Introduction
Exposure to radiation is an inevitable event in human growth, development, and physiological aging. In addition to nuclear accidents, nuclear leakage and nuclear war, the space radiation following development of aerospace projects, medical radiation or tumor radiotherapy in daily life and radiation sources applied in consumer goods, all have different degrees of radioactivity [1–3]. Irradiation is extremely harmful or lethal to human health, and it also showed that accumulative radiation exposure leads to accelerated hematopoietic aging [4–5] or tissue aging [6–8]. Therefore, enhancement of the body resistance to radiation and improvement of treatment strategies post-radiation have been got great attention. Bone marrow transplantation (BMT) is one of the major choices in saving life and repairing the damaged organs or tissues particularly in high-dose radiation, and there is a tremendous amount of effort in BMT studies in human or animal system [3, 9–11]. Nevertheless, the specific type of contributing cells and their underlying mechanism in tissue repair in response to radiation is not fully understood. Bone marrow (BM) harbors hematopoietic stem cells (HSCs), which differentiate into every type of mature blood cells [12]; endothelial cell progenitors; and marrow stromal cells, also called mesenchymal stem cells (MSCs), which can differentiate into mature cells of multiple mesenchymal tissues including fat, bone, and cartilage [13].
Bone marrow-derived cells were widely studied in their repair abilities responding to different stimuli or stress, in which the plasticity or transdifferentiation was proposed as the mechanism for damage repair, but controversy on the role of HSCs remains. The pioneering transdifferentiation study of HSC from Dr. Krause’s group [14], showed that HSC (stained with PKH26+ Fr25Lin-) from BM was capable of giving rise to multi-organ and multi-lineage engraftment in lethally irradiated mice, including lung, intestine, stomach, skin, bile, et al, especially to lung tissue cells [15–17]. In later studies, a series of similar conclusions were drawn that bone marrow stem cells possess the ability to transdifferentiate to non-hematopoietic tissue cells, such as endothelial precursors [18], hepatic cells [19], intestine cells [20], brain microglia and macroglia [21], skeletal muscle cells [22] and cardiac muscle cells [23, 24]. Notably, most of these studies used BM-derived cells as donor cells, not HSCs alone. On the other hand, opposing opinions were also supported by evidence suggesting that HSCs are incapable of transdifferentiating to non-hematopoietic cells, especially cardiac muscle cells, represented by groups from Dr. Weissman and Dr. Murry [25–27]. There are several aspects that might cause the controversy: purity in donor cell population, reliability in organ damage models, and detection methods. Therefore, further studies are warranted to determine the importance and efficiency in transdifferentiation of HSCs in response to radiation insult. In particular, it is essential to address the dispute by optimizing detection techniques, appropriate donor mouse model and specific HSC purification, suitable recipient mice, and valid controls. The genetically modified RosamT/mG mice [28] offer a new opportunity to track the fate of HSCs. Almost all the HSCs from the mice express high-intensity red fluorescence of tdTotamo. So, in this study, we used RosamT/mG mice for HSC donors to explore HSC transdifferentiation ability in radiation-induced tissue damage.
Results
HSC transplantation repaired the radiation induced lung damage
To examine if HSC transplantation (HSCT) could repair the radiation-induced tissue damage, HSCs (CD48-CD150+Lin-Sca-1+c-Kit+) from normal C57 BL/6 mice and Atg7-/- mice (Atg7f/f;Vav-iCre mice, in which the HSC function was severely diminished) [29, 30] were transplanted into 60Co γ ray 9 Gy lethally irradiated CD45.1 mice (Figure 1A). The survival time of mice irradiated with lethal dose of 9 Gy was 8-11 days. Transplantation of HSCs from normal mice (HSCT) could save the lives of mice irradiated with lethal dose. But HSCs from Atg7f/f;Vav-Cre mice (A7-VAV-T) couldn’t save the lives of irradiated mice, most of which died at about 2 months post transplantation (Figure 1B), indicating that normal HSCs could save the lives of lethally irradiated mice. The lung coefficient in irradiation (IR) group increased as compared to the control group; HSCT returned to normal, but A7-Vav-T group was still increased, like the IR group (Figure 1C). 9 Gy IR induced lung pathological alteration in mice, including smaller alveolar cavity, thickening alveolar septal, inflammatory cell infiltration, bronchial epithelial cell structure destruction and hemorrhage observed in the HE pathological section, while HSCT could alleviate radiation-induced pulmonary inflammatory response, most of which returned to normal (Figure 1D). The inflammatory factors including TNF-α, IL-6, IL-1β, and IL-10 increased significantly in IR group, HSCT reduced this damage; In contrast, these factors remained high in A7-VAV-T group (Figure 1E). ALP (Alkaline phosphatase, assessing lung vascular permeability after radiation [31]) was increased in IR group and returned to normal in HSCT group, but it was getting worse in A7-Vav-T group (Figure 1F). The lung epithelial cells (represented by staining of SP-C and T1-α) and endothelial cells (represented by staining of CD31) decreased significantly in A7-VAV-T group as compared to the control, but returned to normal in HSCT group (Figure 1G). HSCT also repaired other tissue damage induced by radiation (Supplementary Figure 2A–2H). Together, these data suggested that normal HSC could repair the radiation- induced tissue damage.
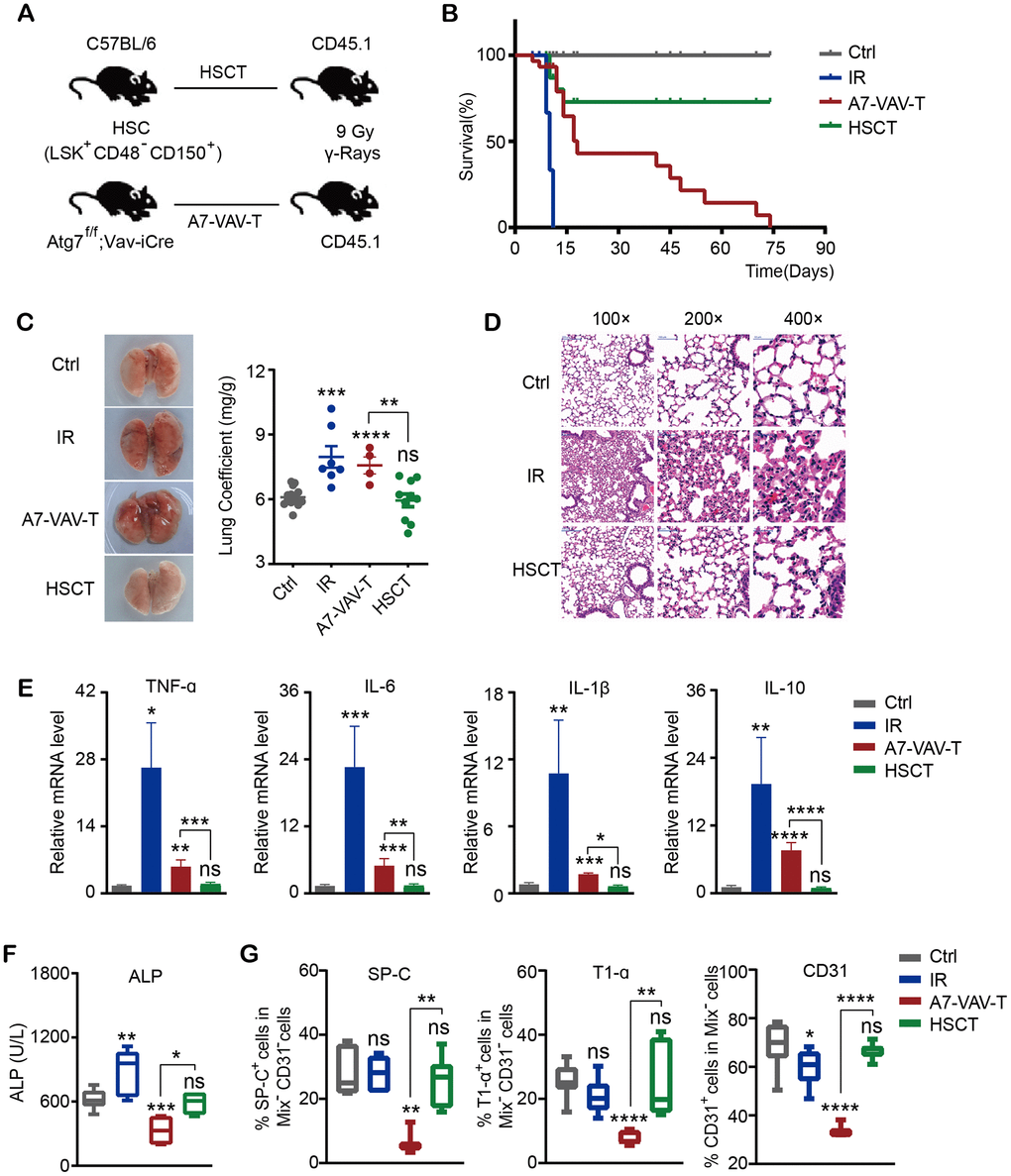
Figure 1. HSC transplantation repaired the radiation-induced lung damage. (A) Transplantation scheme of HSC (CD48-CD150+Lin-Sca-1+c-Kit+) from normal C57BL/6 mice and Atg7-/- mice (Atg7f/f;Vav-iCre mice) into 60Co γ ray 9 Gy lethally irradiated CD45.1 mice. (B) The survival time of mice in each group, including Ctrl (Control non-irradiated mice), IR (Mice irradiated with lethal dose of 9 Gy), HSCT (Transplantation of HSC from normal mice), A7-VAV-T (Transplantation of HSC from Atg7f/f;Vav-iCre mice). N≥10. (C) The lung appearance and coefficient in each group. (D) Lung HE pathological alteration in mice, HSCT alleviated radiation-induced pulmonary inflammatory response. (E) The inflammatory factors including TNF-α, IL-6, IL-1β, IL-10 expression in each group. (F) ALP indicating lung vascular permeability activity in each group. (G) Lung epithelial cells (represented by staining of SP-C and T1-α) and endothelial cells (represented by staining of CD31) percentage in each group. N≥4 in C, D, E, F, G. *: p<0.05; **:p<0.01; ***: p<0.001; ****: p<0.0001.
Male donor-derived red fluorescence cells and Y chromosome were detected in female recipient lung tissue cells
To test whether donor derived cells are present in recipient tissue cells, HSCs from male RosamT/mG mice were sorted and transplanted into lethally irradiated female CD45.1 mice (Figure 2A). 4 months after HSCT, the recipient mice were sacrificed, tdTomato/PE red fluorescence of donor mice and Ddx3y representing Y chromosome were detected in recipient mice. No-irradiated CD45.1 mice were used as negative control (marked as Ctrl); RosamT/mG mice were used as positive control (MTG); transplanted mice were marked as MTG-T.
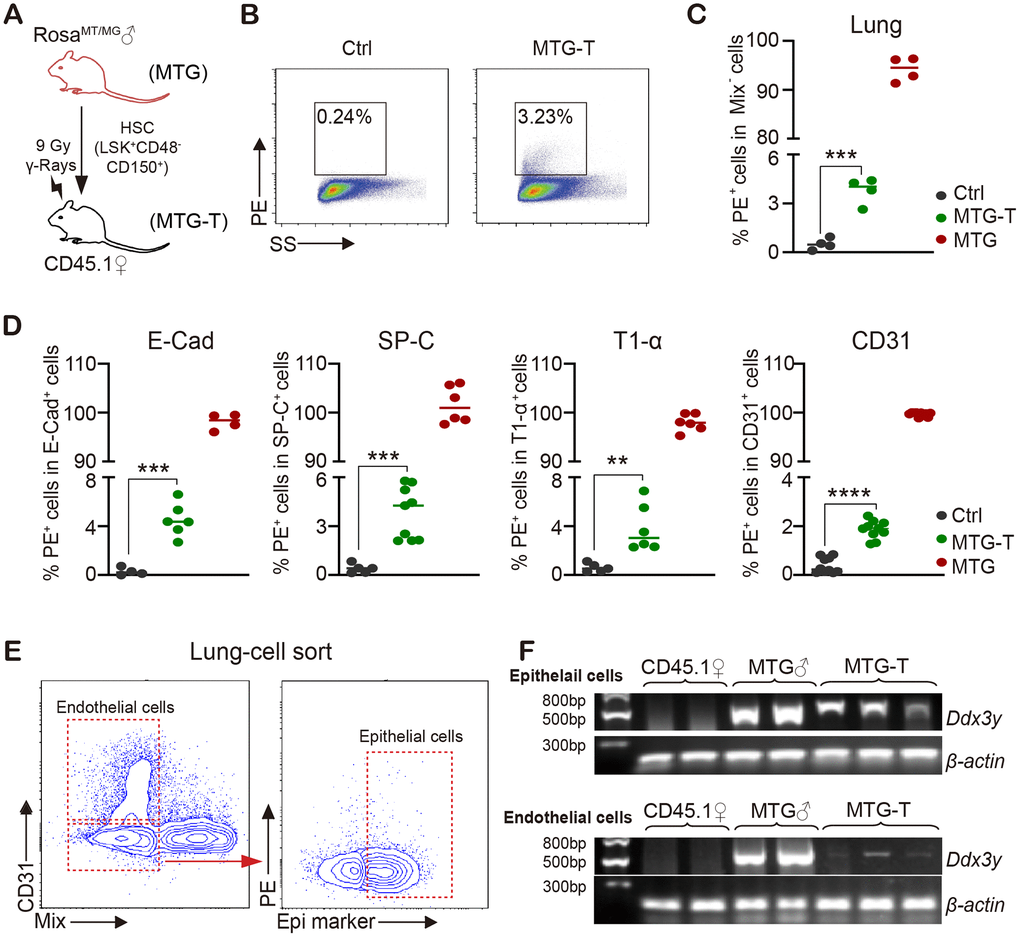
Figure 2. Male donor-derived PE red fluorescence cells and Y chromosome were detected in female recipient lung tissue cells. (A) HSC from male RosamT/mG mice were sorted and transplanted into lethally irradiated female CD45.1 mice. (B) Flow cytometry chart of PE (tdTomato) fluorescence in lung tissue cells with exclusion of blood cells including lineage (CD3, CD8, B220, Gr-1, TER119), macrophage (CD11b, F4/80), megakaryocyte (CD41/CD61) and CD45 (marked as Mix). (C) Statistical analysis of PE positive percentage in lung tissue cells. (D) PE percentage in lung epithelial cells (E-Cadherin, SP-C and T1-α) and endothelial cells (CD31) (after exclusion of blood cells). (E) For Ddx3y detection, lung epithelial cells (Epithelial marker+/blood Mix marker-/CD31-) and endothelial cells (blood Mix marker-/CD31+) were sorted. (F) Representative images of Ddx3y expression by amplification with PCR and detection by nucleic acid electrophoresis. N≥4 in (C, D, E, F). **:p<0.01; ***: p<0.001; ****: p<0.0001.
Exclusion of blood cells was performed by cardiac perfusion and blood cell marker staining. Among the non-hematological tissues, lung tissue cells possessed the highest PE positive percentage of 3.23% (Figure 2B, 2C). With subsequent PE test in lung epithelial cells (E-Cadherin, SP-C and T1-α) and endothelial cells (CD31), it also had around 2%-5% positive PE percentage in different cells (after exclusion of contaminated blood cells) (Figure 2D). For Ddx3y detection, lung epithelial cells (Epithelial marker+/blood Mix FITC-/CD31-) and endothelial cells (blood Mix FITC-/CD31+) were sorted (Figure 2E), DNA of these cells was extracted and then Ddx3y was amplified with quantitative PCR and detected by nucleic acid electrophoresis. The results showed that Ddx3y was expressed in the female recipient lung epithelial cells and it could also be detected in some endothelial cells of the female recipient mice (Figure 2F). PE red fluorescence could also be detected in the liver and intestine tissue cells to some extent, however, not in heart and kidney cells (Supplementary Figure 2I); but Ddx3y could not be tested in any kinds of these tissues (Supplementary Figure 2J).
In order to examine if HSC transdifferentiation occurred in non-radiation setting, we transplanted HSC of RosamT/mG mice into non-irradiated CD45.1 mice, and no PE red fluorescence was detectable in any kind of tissues of the recipient mice (Data not shown). These data together suggested that under radiation injury, transplanted HSCs repaired the lung injury at least in part through transdifferentiation.
Co-localization of donor-derived cells and recipient lung tissue cells were observed
To verify HSC transdifferentiation, confocal immuno-fluorescence and imaging flow cytometry were carried out to observe the morphology of the donor derived cells and recipient lung tissue cells.
The image flow cytometry results showed that in MTG-T group, after exclusion of the blood cells, the PE red fluorescence from donor and the recipient lung epithelial cell marker SP-C or endothelial cell marker CD31 were co-localized, with quantitative analysis showing 10% co-localization of SP-C with PE (Figure 3A), and 2% co-localization of CD31 with PE (Figure 3B). The PE positive cells in the lung were sorted, fixed on the slides and stained with lung specific marker. This single cell confocal microscope result also showed co-localization of PE red fluorescence with lung epithelial and endothelial cells (Figure 3C, 3D). For the in situ lung section, lung epithelial cell marker SP-C, E-cadherin, pan-Keratin, and endothelial cell marker CD31 were stained, and co-localization of PE red fluorescence with these markers were observed by confocal microscopy, with DAPI staining to characterize the nucleus. It showed co-localization or co-expression of PE and SP-C (Figure 3E), PE and CD31 (Figure 3F), PE and T1-α (Supplementary Figure 3A), PE and P-keratin (Supplementary Figure 3B), PE and E-Cadherin (Supplementary Figure 3C). These data suggested that in morphology, the donor-derived HSC cells could be transformed to recipient lung tissue cells. Moreover, the image flow cytometry and single-cell confocal microscope were both at a single cell level, the images showed that the transformed PE positive cells were all one nucleus, no division or fusion shape, which in somewhat excluded cell fusion.
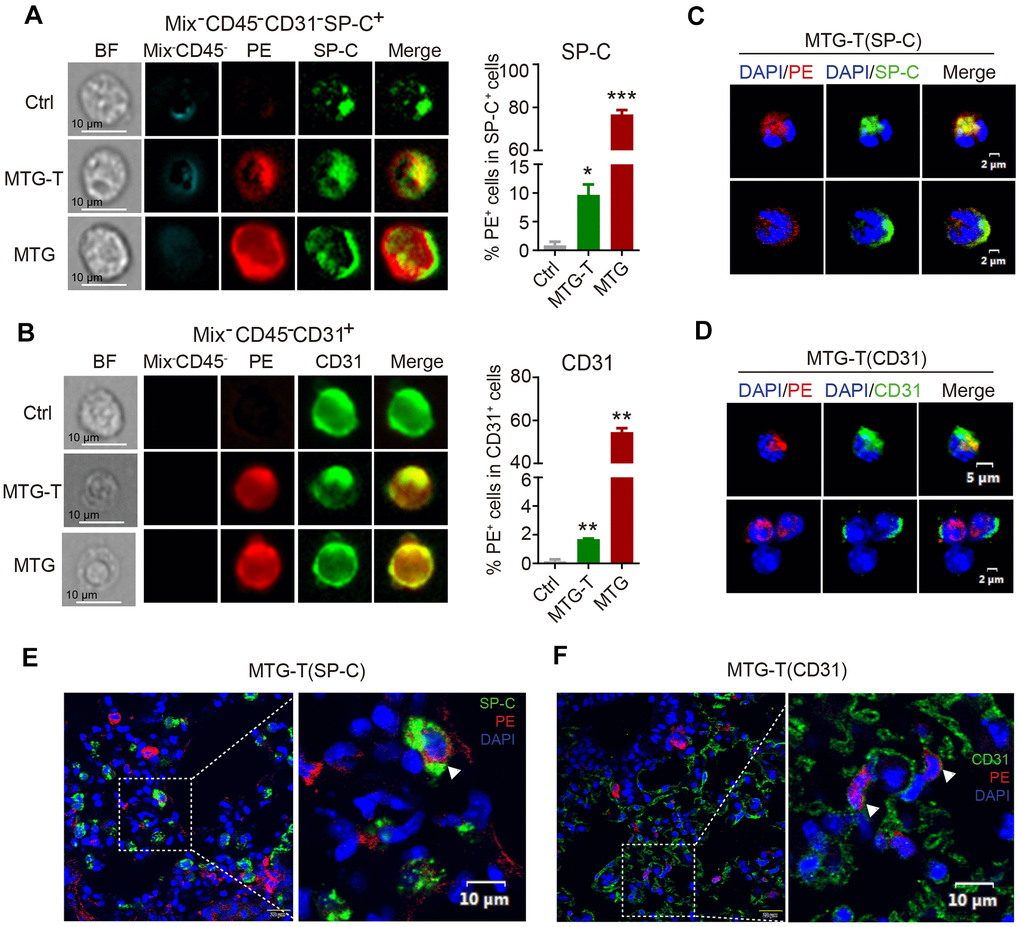
Figure 3. Co-localization of donor-derived cells and recipient lung tissue cells were observed. (A) The representative images by image flow cytometry results of donor derived PE red fluorescence and the recipient lung epithelial cell marker SP-C and (B) endothelial cell marker CD31. The right side showed the quantitative analysis of SP-C with PE, CD31 with PE with exclusion of the blood cells (Blood Mix antibodies and CD45). (C, D) Representative images of single-cell confocal of PE red fluorescence with lung epithelial cell SP-C and endothelial cell CD31 in sorted single PE positive cells of MTG-T group. (E, F) Representative images of confocal co-localizaiton of PE red fluorescence with lung epithelial cell marker SP-C, endothelial cell marker CD31 in situ lung section of MTG-T group, DAPI was stained to characterize the nucleus.
HSC transplantation replenished radiation-induced lung HSC depletion and the repaired epithelial cells were of HSC donor origin
To explore how the BM HSCs repair the injured lung, HSPC (stained against LSK) and HSC residency in the lung were measured by flow cytometry. HSPC and HSC percentage and cell number in the lung all decreased significantly in irradiated group (IR). HSCT restored the HSPCs and HSCs, including LT-HSCs and ST-HSCs, but A7-VAV-T could not restore the injured HSCs in the irradiated lung (Figure 4A, 4B). To determine the source of these restored HSCs, the PE red fluorescence of the HSPCs and HSCs in the lung of MTG-T mice was measured, and it showed that most of the restored HSCs were donor-origin (Figure 4C). To investigate the function or gene expression in these donor-derived lung cells, the Epi+PE+ and Epi+PE- cells in the MTG-T lung were sorted (Figure 4D), and HSPCs from donor were used as control. The Epi+PE+ and Epi+PE- cells both expressed SP-C (alveolar type II epithelial cell marker) and AQP-5 (alveolar type I epithelial cell marker), which confirmed the epithelial characteristics of the PE+ cells in the lung (Figure 4E). Fgf3 and FLT3 (HSC specific genes, [32, 33]) were expressed in the Epi+PE+ cells, suggesting that the cells were derived from donor HSCs. Fgf10 (Fibroblast growth factor 10) and SOX6 (SRY-Box Transcription Factor 6), which are involved in damage repair [34, 35], were only expressed in the Epi+PE+ cells, not in Epi+PE- cells, indicating that these PE+ epithelial cells were repaired cells (Figure 4E). These data further suggested that upon irradiation, HSCs from donor BM may migrate to the lungs of the recipients and some of them can be transdifferentiated into lung epithelial cells to repair the lung injury.
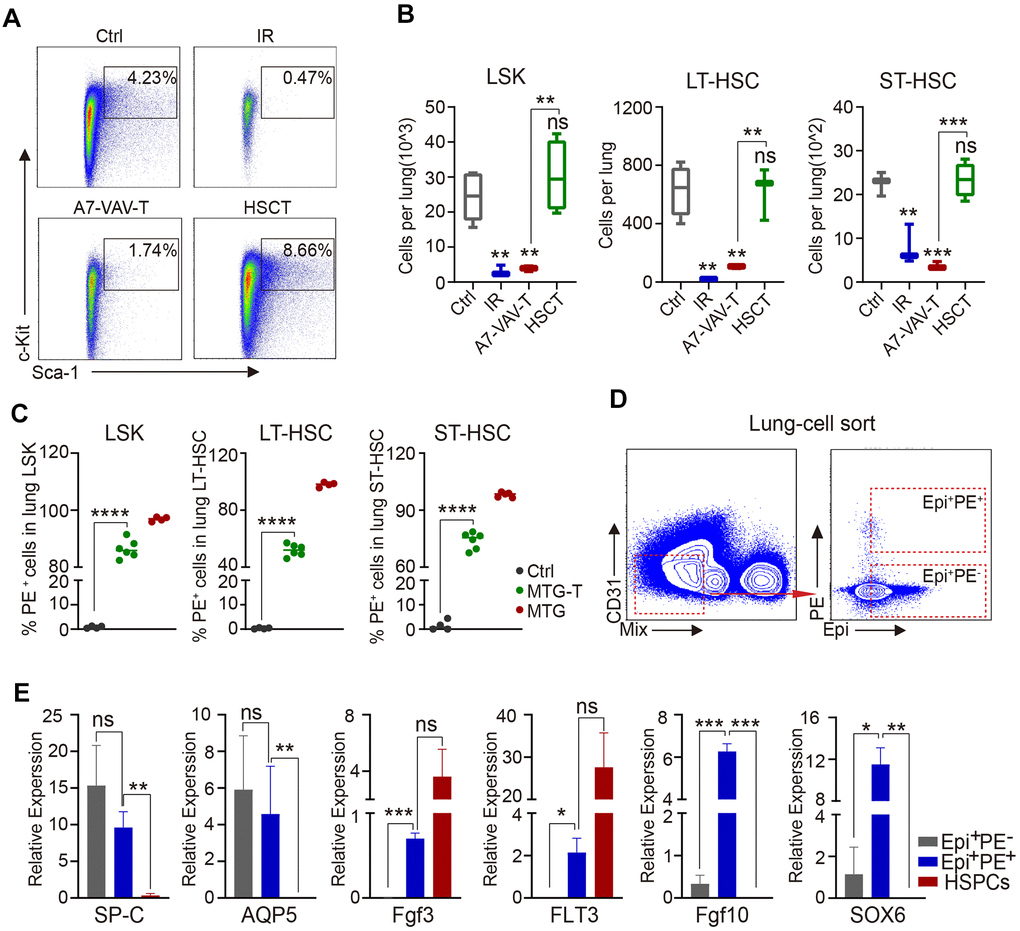
Figure 4. HSC transplantation replenished radiation-induced lung HSC depletion and the repaired epithelial cells were of donor origin. (A) Flow cytometry chart of HSPC residency in the lung cells of each group, including Ctrl, IR, A7-VAV-T, HSCT. (B) HSPC and HSC cell number in the lung in each group, HSCT restored the HSPC and HSC (LT-HSC and ST-HSC), but A7-VAV-T can’t restore the injured HSCs in irradiated lung. (C) PE red fluorescence of the HSPCs and HSCs in the lung of Ctrl, MTG-T and MTG group. (D) To investigate the function or gene expression in the donor-derived lung cells, the Epi+PE+, Epi+PE- cells in the MTG-T lung were sorted after exclusion of blood Mix cells, Epi markers include E-Cadherin, SP-C and T1-α, and HSPCs from donors were used as control for the experiment. (E) Different gene expression including SP-C (alveolar type II epithelial cell marker) and AQP-5 (alveolar type I epithelial cell marker), Fgf3 and FLT3 (hematopoietic stem cell specific genes), Fgf10 and SOX6 (repair genes related) in Epi+PE+, Epi+PE- cells from recipients and HSPC cells from the donors. N≥4. *: p<0.05; **:p<0.01; ***: p<0.001; ****: p<0.0001.
Discussion
Lung is one of the several moderately radio-sensitive organs, and lung epithelial cells are fairly sensitive to radiation rays [36, 37]. Radiation-induced lung injury is a common complication of acute radiation syndrome and chest tumor radiotherapy. Its occurrence is often related to the production of reactive oxygen species (ROS), enhancement of signal transduction such as tumor necrosis factor α, transforming growth factor β and other cytokines. These radiation-triggered factors can result in early radiation pneumonitis [38] and late radiation-induced pulmonary fibrosis [39], in which pulmonary fibrosis is often associated with lung aging [40]. In radiation- induced aging, some small molecules such as ABT263 and piperlongumine have been explored for novel senolytic agents in aged HSCs of mice or in vitro lung fibroblast cells [41–43]. However, for high dose radiation diseases, reduction of the acute radiation pneumonitis and late pulmonary fibrosis is of great importance, especially in the current world facing potential nuclear risk. Bone marrow transplantation is one of the most important methods for the therapy, which could restore hematopoiesis and enhance the repair of damaged tissues and organs [44]. But which group of cells in bone marrow contribute to the repair and how the repair is achieved are still unclear. Many studies showed bone marrow MSCs retain their multi-lineage differentiation capacity; however, studies have also shown that MSCs actually enhance the progression of lung injuries, suggest that MSCs may have dual effects and that may limit the application of MSCs [45].
Plasticity of HSCs is the ability of HSCs to differentiate into a variety of non-hematopoietic tissues. Transdifferentiation represents the irreversible conversion of cells from one differentiated cell type to another. Since 1998, there have been many exciting discoveries indicating that stem cells derived from the BM can differentiate into mature, non-hematopoietic cells of multiple tissues, including epithelial cells of the lung [46, 47, 14–24], although opposing results were reported by several groups [25–27]. Despite the controversy, these positive results suggest that stem cells derived from the bone marrow may become valuable tools for cell replacement strategies and regenerative medicine in the future. But it needs more accurate methods and models to verify the existence of transdifferentiation.
To track the fate of bone marrow stem cells (BMSCs), researchers usually transplant BMSCs from a donor to a recipient that differs genotypically or phenotypically. To date, the most commonly used donor specific markers are the Y chromosome (in sex-mismatched transplantations) and transgenes [48]. Another approach to identify donor-derived cells is to use normal mice with genetic polymorphisms that can be detected in all daughter cells. The transgenic mice with GFP, EGFP and RosamT/mG are mice with genetic fluorescent markers [28, 49, 50]. But the genetic stability and fluorescence strength in the mice might be different, and there might be gene silence occurred. GFP transgenic mice are derived from the CMV enhancer of chicken β-actin promoter, no convincing evidence has shown that all transplanted BM cells or HSCs express high intensity of GFP fluorescence [51]. Likewise, whether the background auto-fluorescence is expressed in the newly differentiated cells is also uncertain, so it is difficult to detect the fluorescence signal in the recipient. However, the high intensity and stable expression of red fluorescence from RosamT/mG mice used in our study provides a reliable system. The genetically modified mice RosamT/mG with a dual fluorescents brought new opportunity to track the fate of BM stem cells. All the cells from the mice express high intensity red fluorescence of tdTotamo (Supplementary Figure 1). Therefore, in this study RosamT/mG mice were used as HSC donor to explore the transdifferentiation repair potential in radiation-induced tissue damage.
As the major and most important stem cells in BM, HSCs are one of the widely studied stem cells. The labeling and purification of HSCs also developed over time. In the 1990s, Weissman et al performed LSK markers to label HSPCs, and later, CD34 and Flk2 were introduced to distinguish long term and short term HSCs [52, 53]. Around 2000s, SLAM family (CD41, CD48 and CD150) was used to enrich the HSCs since SLAM is stable and widely expressed in different mice [54, 55]. Thus, in our study, CD48-CD150+ LSK were used for purification and sorting of LT-HSCs.
To confirm HSC transdifferentiation, it needs to satisfy one or more of the following criteria: (i) the donor derived cell stains with tissue-specific markers; (ii) the donor derived cell does not stain with a monoclonal antibody to hematopoietic CD45; and (iii) the cell exhibits distinctive morphology, indicative of a differentiated, nonhematopoietic cell fate [25, 56].
With these guidelines, we validated the HSC transdifferentiation from several aspects, including donor derived transgenic tdTomato/PE red fluorescence and genetic marker of Y chromosome in the recipient mice, cell morphology of confocal colocalization between donor cells and recipient cells, with exclusion of the interference of blood cells and cell fusion. First, we validated that HSCs from normal mice repaired the tissue injury induced by irradiation, including saving life of lethally irradiated mice, alleviating radiation-induced inflammatory response and fibrosis, recovering of vascular permeability. With HSC transplantation from male RosamT/mG mice to female mice, PE red fluorescence and Y chromosome from donor mice were detected in recipient lung epithelial cells and endothelial cells. The lung epithelial cell marker including E-cadherin, SP-C of alveolar type II cells, T1-α of alveolar type I cells were stained by exclusion of hematopoietic cells (lineage cells, CD45 blood cells, macrophage and megakaryocytes) and endothelial cells (CD31). CD31 of endothelial cells were stained by exclusion of hematopoietic cells and lung epithelial cells [57]. Ddx3y representing Y chromosome was detected in the female recipient lung epithelial cells and endothelial cells. The co-localizaiton of donor derived PE fluorescence and recipient lung tissue cells were also observed by confocal microscopy. These data suggested that donor derived HSCs might differentiate to lung epithelial or endothelial cells. To exclude the co-localization by cell fusion, single cell confocal and image flow cytometry were performed. The transdifferentiated donor derived lung epithelial cells were proved to be single cells and single nucleus from the results. Last, the donor-derived epithelial cells were verified from HSC origin and characterized of epithelial cells and repaired cells. These data suggested that HSC transdifferentiated to lung pneumonocytes, which contributes to the repair of the lung injury imposed by radiation.
Controversies about the transdifferentiation of hematopoietic stem cells are mainly resulted from different animal systems and methodologies that researchers applied, such as the type and age of donor mice, the limitations of the analysis methods, and the status of the recipients (the source and degree of injury). The use of older mice may limit the ability of HSC transdifferentiation. Studies have shown that younger mice have stronger differentiation ability than old mice [58]. The purification of HSCs is another critical point. The marker used by Krause's team (which supported transdifferentiation) at that time might not be very accurate (PKH26+Fr25Lin- marker) for HSCs [14], so the extensive transdifferentiation phenomenon detected might be false positive. Kotton DN et al used SP cells to mark HSCs, they didn’t observe the reconstitution [59]. Third, the injury of recipients may be an essential point for triggering transdifferentiation. The type and degree of injury may affect the transdifferentiation ability of HSCs, and there might be a threshold injury required for triggering transdifferentiation [60–62]. So donor- derived cells were undetectable in recipient mice without irradiation.
Up to all, we speculate that the transdifferentiation of hematopoietic stem cells may not be a normal physiological phenomenon, but more likely to be one of the repair mechanisms of tissues and organs in the non-physiological injury of the body, and the transdifferentiation may be limited to certain tissues such as the lung tissue that possesses special structure. Lung tissue acts as the barrier of blood gas exchange and provides oxygen and fresh arterial blood for the body. There are abundant capillaries in lung tissue. Recently, the lung has been identified as one of the organs for platelet production and as an organ for storing hematopoietic stem and progenitor cells [63, 64]. So we conjecture that in the case of radiation damage (including hematopoietic and non-hematopoietic damage), after HSC transplantation, the donor HSCs reconstitute the hematopoietic system first, and subsequently, the repaired HSC might enter the injured lung, thereby initiating lung repair cascade. In addition, the increase of lung growth factors (such as Fgf10) and transcription factors (such as SOX6) might promote the transdifferentiation of HSC to lung epithelial or endothelial cells through paracrine or nuclear transcription (Figure 5 illustrates the cartoon of HSCs transdifferentiation to pneumonocytes for irradiated lung repair). Nevertheless, the mechanism responsible for HSC transdifferentiation remains to be resolved in our future study.
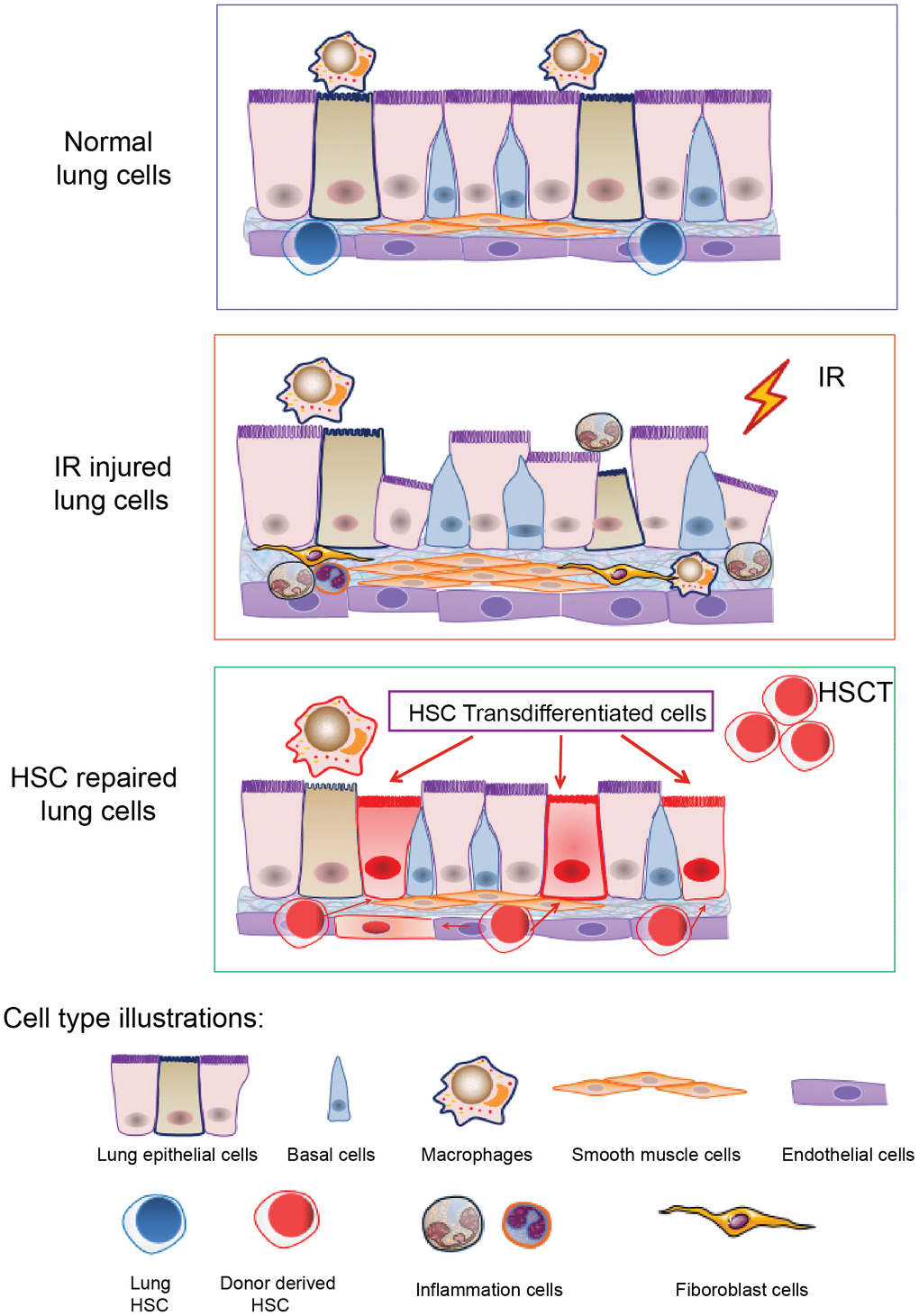
Figure 5. A cartoon illustrating HSCs transdifferentiation to pneumonocytes for irradiated lung repair. The cartoon illustrates HSCs transdifferentiate into the lung cells under certain injury such as irradiation. Irradiation induced lung bronchial epithelial cell structure destruction, inflammatory cell infiltration and fibrous hyperplasia and impaired HSC residency in the lung. After HSCT, donor HSCs migrate into the injured lung, and some of them can be transdifferentiated to lung epithelial and endothelial cells to repair the injury imposed by irradiation.
In summary, we conclude that donor HSCs from bone marrow may migrate to the injured lung of the recipient, and some of them can be transdifferentiated to lung epithelial and endothelial cells to repair the injury caused by radiation. Future study is still warranted to illustrate the underlying mechanism for HSC transdifferentiation.
Materials and Methods
Mice and HSC transplantation
C57BL/6, Atg7f/f;Vav-iCre, RosamT/mG mice were used as donors, CD45.1 mice were used as recipients in this study. Atg7f/f;Vav-iCre mice were generated by crossing Atg7f/f mice (kindly from Dr. Komatsu, Japan and breded in our lab) [65, 66] and Vav-iCre mice (purchased from the Jackson Laboratory). RosamT/mG mice were kindly from Dr. Yulong He lab in Hematology Center of Cyrus Tang Medical Institue, Soochow University of China. The recipients and donor mice were all 6-8 weeks while experiments. Before HSC transplantation, the recipient CD45.1 mice were irradiated with 60Co γ-ray lethal dose of 9 Gy. HSC (CD48-CD150+LSK) from the donor mice were sorted by FACS (BD FACS Aria III, USA) and a total of 2000 HSC were transplanted into one recipient mouse within 4 hours after irradiation. For lung damage repair detection, C57BL/6 and Atg7f/f;Vav-iCre mice were as HSC donors (HSCT and A7-VAV-T), non-irradiated CD45.1 were used as negative control (Ctrl), irradiated CD45.1 mice positive control (IR). For trans-differentiation study, male RosamT/mG mice were used as HSC donors (MTG-T), non-irradiated CD45.1 were used as negative control(Ctrl), RosamT/mG mice were used as positive control (MTG). All experimental procedures with animals were approved by Soochow University Institutional Animal Care and Use Committee. Reagents used in this study were listed in Supplementary Table 1.
Lung damage repair detection
For lung damage repair detection, C57BL/6 and Atg7f/f; iVav-Cre mice were as HSC donors. Survival of each group (Ctrl, IR, HSCT and A7-VAV-T) was recorded. For other experiments, eight weeks after transplantation (the IR group was 7 days after irradiation), the mice were sacrificed. Lung coefficient was calculated by lung weight to mice weight. The pathological changes of lung in each group were observed by HE staining. RNA of lung tissue was extracted and the relative expression levels of inflammatory factors IL-1β, IL-6, TNF-α, IL-10 in lung tissues of each group were quantified using Q-PCR (LightCycler 480 II, Roche, Switzerland) to measure radiation-induced inflammatory response. Primer sequence were seen in Supplementary Table 2. ALP was measured to assess lung vascular permeability after radiation by biochemical test. Lung tissue cell (SP-C of alveolar type II cells, T1-α of alveolar type I cells, CD31 of endothelial cells) percentage alteration was examined by flow cytometry (Beckman Coulter, USA).
PE fluorescence detection by flow cytometry
To test whether donor derived cells are present in recipient tissue, flow cytometry was performed to detect the red tdTomato or PE fluorescence in recipient mice. 4 months after HSCT, the recipient mice were sacrificed, cardiac perfusion were performed in mice in order to remove the influence of blood cells in circulation. Bone marrow, heart, liver, lung, kidney, small intestine of mice in each group were taken and digested into single cells by collagenase, dispase II and DNase I incubation in 37° C for 1 h. Antibodies of blood cells (marked as Mix) including lineage (CD3, CD8, B220, Gr-1, TER119), macrophage (CD11b, F4/80), megakaryocyte (CD41/CD61) and CD45 were stained to subsequently exclude the influence of blood cells. After exclusion of these cells, PE (tdTomato) fluorescence in tissue single cells were detected by flow cytometry (Beckman Coulter). PE fluorescence in alveolar type II cells (SP-C), alveolar type I cells (T1-α), other epithelial cells (E-Cadherin), endothelial cells (CD31) was also measured.
Y chromosome detection
To test whether donor-derived Y chromosome are present in recipient tissue, Ddx3y represented Y chromosome was examined by PCR in recipients lung epithelial cells and endothelial cells. Lung epithelial cells (Epithelial marker+/blood Mix marker-/CD31-) and endothelial cells (blood Mix marker-/CD31+) were sorted by FACS, DNA of these cells were extracted and then Ddx3y was amplified with PCR and detected by nucleic acid electrophoresis. Epithelial markers for sorting include SP-C, T1-α and E-Cadherin. Ddx3y primer sequence were seen in Supplementary Table 2.
Confocal microscope and image flow cytometry
Confocal microscope (Olympus FV1000MPE, Japan) and imaging flow cytometry (Amnis ImageStream MarkII, Merck Millipore, USA) were utilized to analyze the co-expression between donor marker (tdTomato-red fluorescence) and lung epithelial cell markers. For confocal microscope, frozen section of lung tissue was made after the cardiac perfusion of mice. Lung epithelial cell and endothelial cell markers (including Pan-keratin, T1-α, SP-C, E-Cadherin and CD31) conjugated with FITC and DAPI were stained respectively and observed in laser confocal microscope. The co-localization of PE and recipient lung cells were inspected. For image flow cytometry, lung tissue was digested into single cells and stained with lung epithelial and endothelial cell marker (including T1-α, SP-C and CD31) conjugated with APC, DAPI, and exclusion with blood cells (described as above Mix and CD45). The co-localization of PE fluorescence and lung epithelial/endothelial cells were quantified by the image flow wizard software.
Statistical analysis
Statistical analyses were performed using SPSS version 22.0. The statistical significance of the observed differences was determined by unpaired t tests. Data were expressed as mean ± standard error of the mean (SEM). P<0.05 was considered to indicate a statistically significant difference.
Author Contributions
LL, CG, SZ and JW designed the study. LL and CG performed the majority of the experiments. LJ, YL, CZ, JZ, CS, JC performed the animal breeding and genotyping. WW, LX, YF, NY, SZ, JW analyzed the data. SZ and JW wrote the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This work was supported by grants from National Natural Science Foundation of China (No. 81673093, No. 31201073, No. 82000117, No. 91649113, No. 81800152, No. 31771640, No. 81570126,), Jiangsu Science and Technology Department (No. BK20130333 and BK20160330, BK20200191), by The Postdoctor Science Foundation of Jiangsu Province (No. 2020Z064), and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
References
-
1.
Ohnishi K, Ohnishi T. The biological effects of space radiation during long stays in space. Biol Sci Space. 2004; 18:201–05. https://doi.org/10.2187/bss.18.201 [PubMed]
-
2.
Valentin J, and International Commission on Radiological Protection. Protecting people against radiation exposure in the event of a radiological attack. A report of The International Commission on Radiological Protection. Ann ICRP. 2005; 35:1–110, iii-iv. https://doi.org/10.1016/j.icrp.2005.01.001 [PubMed]
-
3.
Battiwalla M, Fakhrejahani F, Jain NA, Klotz JK, Pophali PA, Draper D, Haggerty J, McIver Z, Jelinek J, Chawla K, Ito S, Barrett J. Radiation exposure from diagnostic procedures following allogeneic stem cell transplantation—how much is acceptable? Hematology. 2014; 19:275–79. https://doi.org/10.1179/1607845413Y.0000000131 [PubMed]
-
4.
Chang J, Feng W, Wang Y, Luo Y, Allen AR, Koturbash I, Turner J, Stewart B, Raber J, Hauer-Jensen M, Zhou D, Shao L. Whole-body proton irradiation causes long-term damage to hematopoietic stem cells in mice. Radiat Res. 2015; 183:240–48. https://doi.org/10.1667/RR13887.1 [PubMed]
-
5.
Shao L, Feng W, Li H, Gardner D, Luo Y, Wang Y, Liu L, Meng A, Sharpless NE, Zhou D. Total body irradiation causes long-term mouse BM injury via induction of HSC premature senescence in an Ink4a- and Arf-independent manner. Blood. 2014; 123:3105–15. https://doi.org/10.1182/blood-2013-07-515619 [PubMed]
-
6.
Qin W, Liu B, Yi M, Li L, Tang Y, Wu B, Yuan X. Antifibrotic agent pirfenidone protects against development of radiation-induced pulmonary fibrosis in a murine model. Radiat Res. 2018; 190:396–403. https://doi.org/10.1667/RR15017.1 [PubMed]
-
7.
Golla S, Golla JP, Krausz KW, Manna SK, Simillion C, Beyoğlu D, Idle JR, Gonzalez FJ. Metabolomic analysis of mice exposed to gamma radiation reveals a systemic understanding of total-body exposure. Radiat Res. 2017; 187:612–29. https://doi.org/10.1667/RR14592.1 [PubMed]
-
8.
Richardson RB. Ionizing radiation and aging: rejuvenating an old idea. Aging (Albany NY). 2009; 1:887–902. https://doi.org/10.18632/aging.100081 [PubMed]
-
9.
Mortazavi SM, Shekoohi-Shooli F, Aghamir SM, Mehrabani D, Dehghanian A, Zare S, Mosleh-Shirazi MA. The healing effect of bone marrow-derived stem cells in acute radiation syndrome. Pak J Med Sci. 2016; 32:646–51. https://doi.org/10.12669/pjms.323.9895 [PubMed]
-
10.
Asano S. Current status of hematopoietic stem cell transplantation for acute radiation syndromes. Int J Hematol. 2012; 95:227–31. https://doi.org/10.1007/s12185-012-1027-8 [PubMed]
-
11.
Saha S, Bhanja P, Kabarriti R, Liu L, Alfieri AA, Guha C. Bone marrow stromal cell transplantation mitigates radiation-induced gastrointestinal syndrome in mice. PLoS One. 2011; 6:e24072. https://doi.org/10.1371/journal.pone.0024072 [PubMed]
-
12.
Mosaad YM. Hematopoietic stem cells: an overview. Transfus Apher Sci. 2014; 51:68–82. https://doi.org/10.1016/j.transci.2014.10.016 [PubMed]
-
13.
Nicolay NH, Lopez Perez R, Debus J, Huber PE. Mesenchymal stem cells – a new hope for radiotherapy-induced tissue damage? Cancer Lett. 2015; 366:133–40. https://doi.org/10.1016/j.canlet.2015.06.012 [PubMed]
-
14.
Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001; 105:369–77. https://doi.org/10.1016/s0092-8674(01)00328-2 [PubMed]
-
15.
Kotton DN, Ma BY, Cardoso WV, Sanderson EA, Summer RS, Williams MC, Fine A. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development. 2001; 128:5181–88. [PubMed]
-
16.
Wong AP, Keating A, Lu WY, Duchesneau P, Wang X, Sacher A, Hu J, Waddell TK. Identification of a bone marrow-derived epithelial-like population capable of repopulating injured mouse airway epithelium. J Clin Invest. 2009; 119:336–48. https://doi.org/10.1172/JCI36882 [PubMed]
-
17.
Dooner MS, Aliotta JM, Pimentel J, Dooner GJ, Abedi M, Colvin G, Liu Q, Weier HU, Johnson KW, Quesenberry PJ. Conversion potential of marrow cells into lung cells fluctuates with cytokine-induced cell cycle. Stem Cells Dev. 2008; 17:207–19. https://doi.org/10.1089/scd.2007.0195 [PubMed]
-
18.
Hess DC, Hill WD, Martin-Studdard A, Carroll J, Brailer J, Carothers J. Bone marrow as a source of endothelial cells and NeuN-expressing cells after stroke. Stroke. 2002; 33:1362–68. https://doi.org/10.1161/01.str.0000014925.09415.c3 [PubMed]
-
19.
Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000; 6:1229–34. https://doi.org/10.1038/81326 [PubMed]
-
20.
Chang YH, Lin LM, Lou CW, Chou CK, Ch’ang HJ. Bone marrow transplantation rescues intestinal mucosa after whole body radiation via paracrine mechanisms. Radiother Oncol. 2012; 105:371–77. https://doi.org/10.1016/j.radonc.2012.10.005 [PubMed]
-
21.
Recio JS, Álvarez-Dolado M, Díaz D, Baltanás FC, Piquer-Gil M, Alonso JR, Weruaga E. Bone marrow contributes simultaneously to different neural types in the central nervous system through different mechanisms of plasticity. Cell Transplant. 2011; 20:1179–92. https://doi.org/10.3727/096368910X552826 [PubMed]
-
22.
Haond C, Drouet M, Derdouch S, Bonnet ML, Norol F, Mayol JF, Vainchenker W, LeGrand R, Turhan AG, Herodin F. In vitro and in vivo evaluation of the haematopoietic potential of skeletal muscle in a non-human primate model. Bone Marrow Transplant. 2008; 41:579–84. https://doi.org/10.1038/sj.bmt.1705941 [PubMed]
-
23.
Stocum DL. Stem cells in CNS and cardiac regeneration. Adv Biochem Eng Biotechnol. 2005; 93:135–59. https://doi.org/10.1007/b99969 [PubMed]
-
24.
Sahara M, Sata M, Matsuzaki Y, Tanaka K, Morita T, Hirata Y, Okano H, Nagai R. Comparison of various bone marrow fractions in the ability to participate in vascular remodeling after mechanical injury. Stem Cells. 2005; 23:874–78. https://doi.org/10.1634/stemcells.2005-0012 [PubMed]
-
25.
Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002; 297:2256–59. https://doi.org/10.1126/science.1074807 [PubMed]
-
26.
Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004; 428:664–8. https://doi.org/10.1038/nature02446 [PubMed]
-
27.
Chien KR, Frisén J, Fritsche-Danielson R, Melton DA, Murry CE, Weissman IL. Regenerating the field of cardiovascular cell therapy. Nat Biotechnol. 2019; 37:232–37. https://doi.org/10.1038/s41587-019-0042-1 [PubMed]
-
28.
Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007; 45:593–605. https://doi.org/10.1002/dvg.20335 [PubMed]
-
29.
Fang Y, Zhu L, An N, Jiang G, Qian J, Zhao R, Yuan N, Zhang S, Wang J. Blood autophagy defect causes accelerated non-hematopoietic organ aging. Aging (Albany NY). 2019; 11:4910–22. https://doi.org/10.18632/aging.102086 [PubMed]
-
30.
Fang Y, An N, Zhu L, Gu Y, Qian J, Jiang G, Zhao R, Wei W, Xu L, Zhang G, Yao X, Yuan N, Zhang S, et al. autophagy-Sirt3 axis decelerates hematopoietic aging. Aging Cell. 2020; 19:e13232. https://doi.org/10.1111/acel.13232 [PubMed]
-
31.
Verma S, Kalita B, Bajaj S, Prakash H, Singh AK, Gupta ML. A combination of podophyllotoxin and rutin alleviates radiation-induced pneumonitis and fibrosis through modulation of lung inflammation in mice. Front Immunol. 2017; 8:658. https://doi.org/10.3389/fimmu.2017.00658 [PubMed]
-
32.
Anderson MJ, Southon E, Tessarollo L, Lewandoski M. Fgf3-Fgf4-cis: a new mouse line for studying Fgf functions during mouse development. Genesis. 2016; 54:91–98. https://doi.org/10.1002/dvg.22913 [PubMed]
-
33.
Buza-Vidas N, Cheng M, Duarte S, Charoudeh HN, Jacobsen SE, Sitnicka E. FLT3 receptor and ligand are dispensable for maintenance and posttransplantation expansion of mouse hematopoietic stem cells. Blood. 2009; 113:3453–60. https://doi.org/10.1182/blood-2008-08-174060 [PubMed]
-
34.
Yuan T, Volckaert T, Chanda D, Thannickal VJ, De Langhe SP. Fgf10 signaling in lung development, homeostasis, disease, and repair after injury. Front Genet. 2018; 9:418. https://doi.org/10.3389/fgene.2018.00418 [PubMed]
-
35.
Llontop P, Santana-Rodríguez N, Clavo B, Quintana A, Fiuza MD, Camacho R, Santana-Rodríguez A, Santana C, Ruíz-Caballero JA. Stem cells protect the bronchial stump in rat, increasing Sox6, Col2a1, and Agc1 expression. Lung. 2014; 192:441–48. https://doi.org/10.1007/s00408-014-9569-6 [PubMed]
-
36.
Almeida C, Nagarajan D, Tian J, Leal SW, Wheeler K, Munley M, Blackstock W, Zhao W. The role of alveolar epithelium in radiation-induced lung injury. PLoS One. 2013; 8:e53628. https://doi.org/10.1371/journal.pone.0053628 [PubMed]
-
37.
Down JD, Yanch JC. Identifying the high radiosensitivity of the lungs of C57L mice in a model of total-body irradiation and bone marrow transplantation. Radiat Res. 2010; 174:258–63. https://doi.org/10.1667/RR2149.1 [PubMed]
-
38.
Theise ND, Henegariu O, Grove J, Jagirdar J, Kao PN, Crawford JM, Badve S, Saxena R, Krause DS. Radiation pneumonitis in mice: a severe injury model for pneumocyte engraftment from bone marrow. Exp Hematol. 2002; 30:1333–38. https://doi.org/10.1016/s0301-472x(02)00931-1 [PubMed]
-
39.
Giridhar P, Mallick S, Rath GK, Julka PK. Radiation induced lung injury: prediction, assessment and management. Asian Pac J Cancer Prev. 2015; 16:2613–17. https://doi.org/10.7314/apjcp.2015.16.7.2613 [PubMed]
-
40.
Gulati S, Thannickal VJ. The aging lung and idiopathic pulmonary fibrosis. Am J Med Sci. 2019; 357:384–89. https://doi.org/10.1016/j.amjms.2019.02.008 [PubMed]
-
41.
Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J, Janakiraman K, Sharpless NE, Ding S, Feng W, Luo Y, Wang X, Aykin-Burns N, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. 2016; 22:78–83. https://doi.org/10.1038/nm.4010 [PubMed]
-
42.
Wang Y, Chang J, Liu X, Zhang X, Zhang S, Zhang X, Zhou D, Zheng G. Discovery of piperlongumine as a potential novel lead for the development of senolytic agents. Aging (Albany NY). 2016; 8:2915–26. https://doi.org/10.18632/aging.101100 [PubMed]
-
43.
Zhang X, Zhang S, Liu X, Wang Y, Chang J, Zhang X, Mackintosh SG, Tackett AJ, He Y, Lv D, Laberge RM, Campisi J, Wang J, et al. Oxidation resistance 1 is a novel senolytic target. Aging Cell. 2018; 17:e12780. https://doi.org/10.1111/acel.12780 [PubMed]
-
44.
Porada CD, Atala AJ, Almeida-Porada G. The hematopoietic system in the context of regenerative medicine. Methods. 2016; 99:44–61. https://doi.org/10.1016/j.ymeth.2015.08.015 [PubMed]
-
45.
Yao Y, Zheng Z, Song Q. Mesenchymal stem cells: a double-edged sword in radiation-induced lung injury. Thorac Cancer. 2018; 9:208–17. https://doi.org/10.1111/1759-7714.12573 [PubMed]
-
46.
Geiger H, Sick S, Bonifer C, Müller AM. Globin gene expression is reprogrammed in chimeras generated by injecting adult hematopoietic stem cells into mouse blastocysts. Cell. 1998; 93:1055–65. https://doi.org/10.1016/s0092-8674(00)81210-6 [PubMed]
-
47.
Van Arnam JS, Herzog E, Grove J, Bruscia E, Ziegler E, Swenson S, Krause DS. Engraftment of bone marrow-derived epithelial cells. Stem Cell Rev. 2005; 1:21–27. https://doi.org/10.1385/SCR:1:1:021 [PubMed]
-
48.
Trotman W, Beckett T, Goncz KK, Beatty BG, Weiss DJ. Dual Y chromosome painting and in situ cell-specific immunofluorescence staining in lung tissue: an improved method of identifying donor marrow cells in lung following bone marrow transplantation. Histochem Cell Biol. 2004; 121:73–79. https://doi.org/10.1007/s00418-003-0598-0 [PubMed]
-
49.
Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. ’Green mice’ as a source of ubiquitous green cells. FEBS Lett. 1997; 407:313–19. https://doi.org/10.1016/s0014-5793(97)00313-x [PubMed]
-
50.
Fujiki Y, Tao K, Bianchi DW, Giel-Moloney M, Leiter AB, Johnson KL. Quantification of green fluorescent protein by in vivo imaging, PCR, and flow cytometry: comparison of transgenic strains and relevance for fetal cell microchimerism. Cytometry A. 2008; 73:11–118. https://doi.org/10.1002/cyto.a.20533 [PubMed]
-
51.
Yokota T, Kawakami Y, Nagai Y, Ma JX, Tsai JY, Kincade PW, Sato S. Bone marrow lacks a transplantable progenitor for smooth muscle type alpha-actin-expressing cells. Stem Cells. 2006; 24:13–22. https://doi.org/10.1634/stemcells.2004-0346 [PubMed]
-
52.
Randall TD, Weissman IL. Characterization of a population of cells in the bone marrow that phenotypically mimics hematopoietic stem cells: resting stem cells or mystery population? Stem Cells. 1998; 16:38–48. https://doi.org/10.1002/stem.160038 [PubMed]
-
53.
Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc Natl Acad Sci USA. 2001; 98:14541–46. https://doi.org/10.1073/pnas.261562798 [PubMed]
-
54.
Weksberg DC, Chambers SM, Boles NC, Goodell MA. CD150- side population cells represent a functionally distinct population of long-term hematopoietic stem cells. Blood. 2008; 111:2444–51. https://doi.org/10.1182/blood-2007-09-115006 [PubMed]
-
55.
Chen J, Ellison FM, Keyvanfar K, Omokaro SO, Desierto MJ, Eckhaus MA, Young NS. Enrichment of hematopoietic stem cells with SLAM and LSK markers for the detection of hematopoietic stem cell function in normal and Trp53 null mice. Exp Hematol. 2008; 36:1236–43. https://doi.org/10.1016/j.exphem.2008.04.012 [PubMed]
-
56.
Herzog EL, Chai L, Krause DS. Plasticity of marrow-derived stem cells. Blood. 2003; 102:3483–93. https://doi.org/10.1182/blood-2003-05-1664 [PubMed]
-
57.
Singer BD, Mock JR, D’Alessio FR, Aggarwal NR, Mandke P, Johnston L, Damarla M. Flow-cytometric method for simultaneous analysis of mouse lung epithelial, endothelial, and hematopoietic lineage cells. Am J Physiol Lung Cell Mol Physiol. 2016; 310:L796–801. https://doi.org/10.1152/ajplung.00334.2015 [PubMed]
-
58.
Lynch K, Pei M. Age associated communication between cells and matrix: a potential impact on stem cell-based tissue regeneration strategies. Organogenesis. 2014; 10:289–98. https://doi.org/10.4161/15476278.2014.970089 [PubMed]
-
59.
Kotton DN, Fabian AJ, Mulligan RC. Failure of bone marrow to reconstitute lung epithelium. Am J Respir Cell Mol Biol. 2005; 33:328–34. https://doi.org/10.1165/rcmb.2005-0175RC [PubMed]
-
60.
Hudson D, Kovalchuk I, Koturbash I, Kolb B, Martin OA, Kovalchuk O. Induction and persistence of radiation-induced DNA damage is more pronounced in young animals than in old animals. Aging (Albany NY). 2011; 3:609–20. https://doi.org/10.18632/aging.100340 [PubMed]
-
61.
Herzog EL, Van Arnam J, Hu B, Krause DS. Threshold of lung injury required for the appearance of marrow-derived lung epithelia. Stem Cells. 2006; 24:1986–92. https://doi.org/10.1634/stemcells.2005-0579 [PubMed]
-
62.
Pesaresi M, Sebastian-Perez R, Cosma MP. Dedifferentiation, transdifferentiation and cell fusion: in vivo reprogramming strategies for regenerative medicine. FEBS J. 2019; 286:1074–93. https://doi.org/10.1111/febs.14633 [PubMed]
-
63.
Lefrançais E, Ortiz-Muñoz G, Caudrillier A, Mallavia B, Liu F, Sayah DM, Thornton EE, Headley MB, David T, Coughlin SR, Krummel MF, Leavitt AD, Passegué E, Looney MR. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature. 2017; 544:105–09. https://doi.org/10.1038/nature21706 [PubMed]
-
64.
Borges I, Sena I, Azevedo P, Andreotti J, Almeida V, Paiva A, Santos G, Guerra D, Prazeres P, Mesquita LL, Silva LS, Leonel C, Mintz A, Birbrair A. Lung as a niche for hematopoietic progenitors. Stem Cell Rev Rep. 2017; 13:567–74. https://doi.org/10.1007/s12015-017-9747-z [PubMed]
-
65.
Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y, Kominami E, Tanaka K, Chiba T. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol. 2005; 169:425–34. https://doi.org/10.1083/jcb.200412022 [PubMed]
-
66.
Cao Y, Zhang S, Yuan N, Wang J, Li X, Xu F, Lin W, Song L, Fang Y, Wang Z, Wang Z, Zhang H, Zhang Y, et al. Hierarchal Autophagic Divergence of Hematopoietic System. J Biol Chem. 2015; 290:23050–63. https://doi.org/10.1074/jbc.M115.650028 [PubMed]