Alternation of brain intrinsic activity in patients with hypertensive retinopathy: a resting-state fMRI study
Abstract
Objective: To investigate the changes of amplitude of low-frequency fluctuation (ALFF) in brain regions of patients with hypertensive retinopathy by using resting-state functional magnetic resonance imaging (rs-fMRI) and change in the relationship of ALFF value with potential emotional and psychological changes.
Methods: Thirty-one patients with hypertensive retinopathy (HR) (16 men and 15 women) and 31 healthy controls (HCs; 16 men and 15 women) matched for age, sex, and weight were enrolled in the research. The changes in mean ALFF values could reflect brain activity between HR patients and HCs. We used the independent samples t-test to evaluate different demographic and general information between the two groups. Two-sample t-test was used to detect differences of mean ALFF values in the brain region between the two groups using the same software.
Results: The ALFF values in the brain areas of HR and HCs were different. HR patients had lower ALFF value in the left medial superior frontal gyrus and left middle frontal gyrus than the HCs. The higher ALFF values were found in the cerebellum (left inferior and right superior lobes, vermis) and left inferior temporal gyrus of the HR patients than the controls.
Conclusion: Our findings showed fluctuations in ALFF values in the HR patients’ brain regions. ALFF values reflect over or reduced activity in brain regions. Abnormal ALFF values in these brain areas can predict early HR development, preventing the malignant transformation of hypertensive microangiopathy.
Introduction
Hypertension is the most common cardiovascular disease and can cause atherosclerosis, resulting in narrowing of the arterial lumen, thereby hindering blood circulation [1]. In the early stages of hypertension, the eye’s fundus is often normal. When the blood pressure continues to rise, it can cause systemic arteriosclerosis, which occurs in patients with retinopathy, known as hypertensive retinopathy [2]. The degree of hypertensive retinopathy is positively correlated with the degree of systemic major organ disease and is a clinical manifestation that precedes all other complications. More than 70 percent of patients with primary hypertension have different degrees of retinopathy, [3] depending on age, degree of blood pressure elevation, and disease duration. Studies have shown that patients with hypertension of <5 years have a significantly increased risk of retinopathy. The fundus changes in hypertension are mainly related to the degree of increased blood pressure [4]. The shift in fundus is aggravated with the increase of systolic and diastolic blood pressure, which is more closely related to the rise in diastolic blood pressure [4]. Fundus photography and fluorescence imaging may show typical signs of hemorrhage, lipid exudation, and fluorescein leakage, and in severe cases, may show optic papilledema [5] (Figure 1). Hypertensive retinopathy (HR) is the ocular manifestation of systemic vascular disease caused by hypertension. Fundus retinal blood vessels belong to the category of microvessels, which is the only blood vessel that can be directly visualized [6]. It is clinically essential for estimating the course of the disease, its severity, and prognosis.
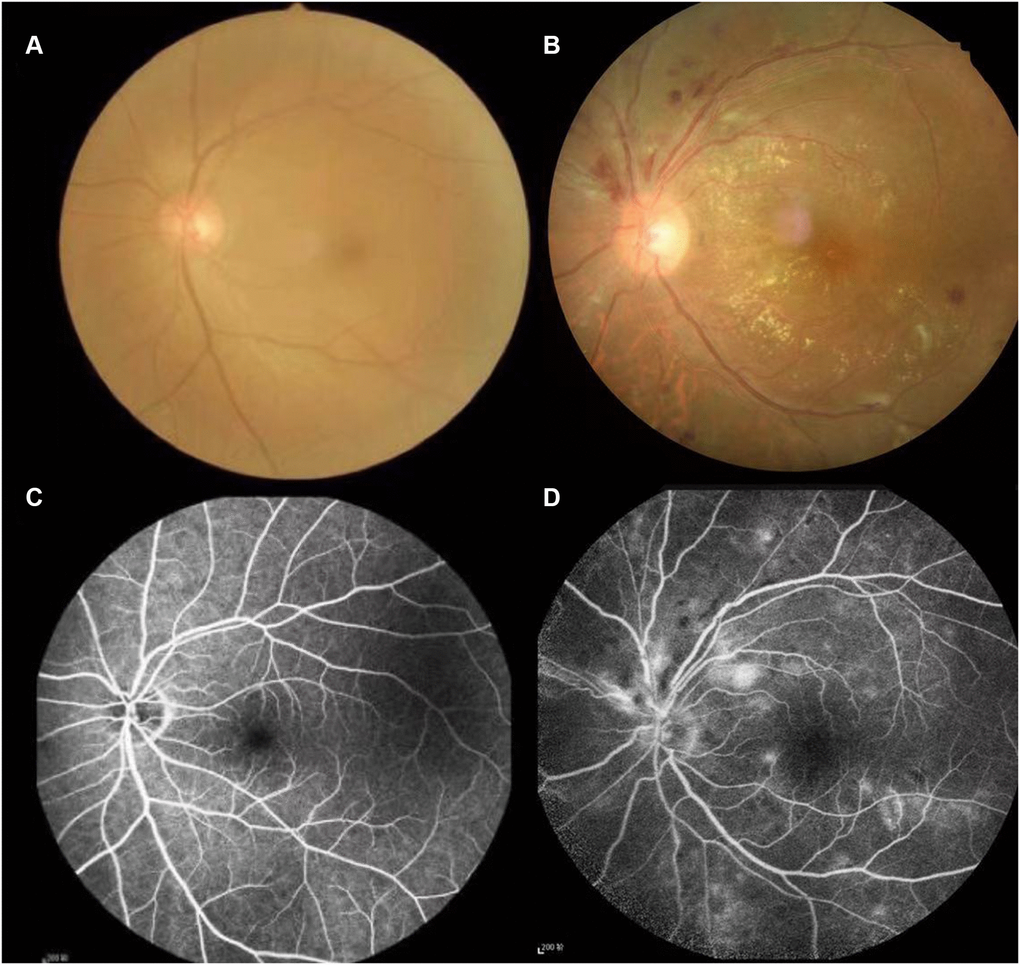
Figure 1. Typical examples of fundus camera and fluorescence fundus angiography in healthy people and patients with Hypertensive retinopathy. (A and C) of Figure 1 shows the left retinal fundus photos and corresponding fluorescence fundus angiography of healthy people. (B and D) of Figure 1 shows the left retinal fundus photos and fluorescence fundus angiography of patients with hypertensive retinopathy. Retinal arteriosclerosis, stenosis, wall light reflection enhancement, silver filiform, artery and vein cross compression phenomenon, the distal vein and capillary dilatation, retinal edema, bleeding and exudation.
Functional magnetic resonance imaging (fMRI) for localization of brain function is a very effective and non-invasive technique for studying fluctuations in functionally related brain areas and has become widely used to study brain function [7]. Although it is a non-invasive technique, it can accurately and reliably locate specific cortical areas of cerebral activity with a spatial resolution of 2 mm. FMRI can track signal changes in real time with a temporal resolution of 1 second, during which the participant's unique brain activation areas can be quickly assessed before identifying functional brain connections. It can be repeated in a variety of ways to scan the subject. FMRI data can be used as a preliminary reflection of mental representations and information processing [8]. Briefly, fMRI technology can display slight changes in MR signals caused by blood oxygenation in venous capillaries in various brain regions. The functional localization of brain activity can be realized on a standard living body without being damaged. As a new non-invasive method, rs-fMRI can be more comfortably implemented clinically. The continuous development of rs-fMRI technology and analytical methods have provided a way for in-depth exploration of pathophysiological mechanisms of nervous system diseases. Clinicians can detect brain abnormalities as early as possible by assessing the function of regional and neural circuits in HR patients’ brains in the resting state.
Amplitude of low-frequency fluctuation (ALFF) reflects the average intensity of the signal in the low-frequency part of each voxel in the blood-oxygen-level-dependent (BOLD) signal. Generally, low frequency refers to 0.01–0.08 Hz. The amplitude can represent the activity intensity of the brain. The BOLD signal intensity can reflect the activation and deactivation of the brain. Each voxel in the brain can calculate an ALFF value, and each ALFF value represents the activity intensity of the voxel in the brain. The ALFF is an effective fMRI analytic technique to evaluate the brain regions’ resting-state activity [9]. Existing research shows that the excellent performance of ALFF is mainly dependent on its test-retest reliability [10–11]. The ALFF value is used to apply rs-fMRI data for disease characterization, depending on its general computability and reliability [12]. In recent years, the application of ALFF technology has revealed spontaneous abnormal activity of local cerebral regions in ophthalmic diseases. The similarity between the functional connection mode in the resting state and the task state’s activation mode is nearly 80%. The resting state’s functional connection is used to explore individual differences in cognitive functions and attention and explore individual differences in complex social decisions [13]. Thus, using this technology, we can easily explain the pathophysiological mechanism of these diseases from different dimensions, which can provide brand-new ideas for guiding the diagnosis, treatment, and evaluation of disease prognosis.
Most ophthalmic studies of diseases use ALFF to analyze the correlation between intrinsic brain activities and mental diseases, such as primary angle-closure glaucoma (PACG), late monocular blindness (MB), adult comitant exotropia strabismus (CES), acute unilateral open globe injury (OGI), and high myopia [14–18] (Table 1). Because of the scarcity of HR patients’ clinical data, previous studies have not extended to HR. To address this issue, we investigated functional changes in the brain network and their relationship with HR patients’ behavioral performance by using rs-fMRI and ALFF technology.
Table 1. ALFF method applied in ophthalmology-related diseases.
| Authors | Year | Diseases |
| Huang et al. [14] | 2015 | Primary angle-closure glaucoma |
| Li et al. [15] | 2016 | Late monocular blindness |
| Tan et al. [16] | 2016 | Adult comitant exotropia strabismus |
| Tan et al. [17] | 2016 | Acute unilateral open globe injury |
| Yu et al. [18] | 2020 | Myopia before and after Lasik surgery |
| Abbreviation: ALFF: the amplitude of low-frequency fluctuation. |
Methods
Subjects
From August 2018 to October 2019, we recruited 31 patients with HR (16 men and 15 women) from the Ophthalmology Department of the First Affiliated Hospital of Nanchang University. The criteria for diagnosing HR were as follows: 1) fundus examination performed based on a systematic diagnosis of stage 2 or above hypertension (systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg); and 2) a record of microaneurysms, retinal hemorrhage, cotton thread spots, exudate, arteriovenous crossing, arteriolar stenosis, and papilledema, which were used to classify retinopathy. Retinopathy was graded according to the Keith–Wagener classification [19] as follows: Grade I, mild retinal artery stenosis or sclerosis; Grade II, moderate arterial stenosis with arteriovenous crossing; Grade III, arterial stenosis and large arteriovenous crossover changes accompanied by bleeding, exudates, and cotton spots; and Grade IV, severe grade III with papilledema. The exclusion criteria for HR patients were as follows: 1) history of eye surgery, diabetes, and nervous system disease; 2) unable to undergo MRI scanning for subjective and objective reasons; 3) alcohol intake >30 g/day; and 4) old or multiple cerebral infarctions in the MRI scan. We recruited 31 healthy age- and sex-matched volunteers (16 males and 15 females) from various communities in Nanchang City, Jiangxi Province, China, as the healthy control (HC) group. All HCs fulfilled the following conditions: 1) no neurologic- or eye-related illness; 2) head MRI scan showing normal brain parenchyma, and 3) no contraindications for MRI. The Medical Ethics Committee of the First Affiliated Hospital of Nanchang University approved this study. The research method conforms to the tenets of the Declaration of Helsinki. All participants were aware of the research purposes, the procedure, and provided voluntary signed informed consent.
Parameters
All MRI examinations were generated by a 3T MR scanner (Trio; Siemens, Munich, Germany). During the scanning, all participants were required to be in the supine position, keep awake, quiet, close their eyes, and not indulge in active thinking. Using a three-dimensional spoiled gradient recalled echo sequence to obtain T1-weighted images, the following specific scan parameters were used: For 176 structural image scans, the field of view was 250 × 250 mm; thickness, 1.0 mm; gap, 0.5 mm; repetition time, 1900 ms; echo time, 2.26 ms; flip angle, 9°; and acquisition matrix, 256 × 256. For the 240 functional image scans, the field of view was 220 × 220 mm; gap, 1.2 mm; thickness, 4.0 mm; echo time, 30 ms; repetition time, 2000 ms; flip angle, 90°; and acquisition matrix, 64 × 64. The scanning duration from start of completion was about 15 min.
Data analysis of fMRI
fMRI data processing mainly includes two steps of raw data preprocessing and statistical analysis of image data. MRIcro software (http://www.mricro.com) was used to classify the available data to eliminate defective data. Owing to the magnetization equilibration, the first 10 volumes were abandoned to eliminate potential artifacts from scanner instability or the surrounding environment. The remaining data were all preprocessed using the DPARSFA (http://rfmri.org/DPARSF). Interlayer scanning is generally adopted for brain scanning. Given the large difference in scanning time of the same brain region, slice timing is required to correct these differences for scanning at the same time point. Rigid body transformation is used to fix the brain in all the images at the same Target. Because the subject’s head could move in the MRI machine, Realign was used to adjust the orientation for head movement correction. Exclude subjects with maximum displacement in the X, Y or Z plane exceeding 1.5-mm and angular motion exceeding 1.5 degrees. To reduce the error, the effects of head motion were cancelled out by Friston six head-motion parameters; the latest research shows that the high-order models have advantages in offsetting head motion [20–21].
Upon completing head motion correction, the next step was to co-register the structure image and function image. The functional and structural images were manually adjusted, including head rotation, translation, and origin positioning, to increase the accuracy of co-registration, segmentation, and normalization. Each person’s brain has individual differences, and hence it needs unified standardization to the same space before they can be compared with each other (voxel size default: 3 mm × 3 mm × 3 mm). The T1-weighted image was registered into the fMRI space and the Montreal Neurological Institute (MNI) standard template was used to convert the image into the MNI space.
Before statistical analysis, spatial smoothing was performed on the acquired data to eliminate artifacts, making the data more consistent with distributional assumptions. In accordance with the analysis requirements of gaussian kernel. There is a linear drift due to increased temperature or subject acclimation, the time sequence of each voxel must eliminate the linear drift [22]. Operate the machine to improve the accuracy of the functional diagram and reduce the influence of other factors. Resting fMRI signals filtered at low frequencies (0.01 ~ 0.08Hz) show significant physiological significance, reflecting spontaneous neural activity Previous studies have described in detail the process of calculating the value of ALFF [23]. Normalized ALFF was obtained by dividing ALFF by the average ALFF of all voxels in the whole brain.
Mass control
Researchers who have received consistency training perform clinical scale scoring, and neither MRI nor scale evaluators know the grouping of patients. To ensure that the fMRI images conformed to the standards, the physician asks the subject to remain as calm as possible before the scan and to keep the body still during the scan. During scanning, the doctor observed the original image and removed or rescued any image with noticeable artefacts or deformation.
Data analysis
Demographic and clinical variables of HR and HC groups were analyzed by SPSS v20.0 software (SPSS Inc, Chicago, IL, USA). The relevant data of the subjects were tested for normality. Differences in the HR and HC groups were evaluated with the independent samples t-test (statistical threshold with P < 0.05). The differences of average ALFF values in the brain region between the HR and HCs using the same software with the two-sample t-test; after Gaussian correction, the statistical significance threshold was set to P < 0.05. We analyzed different ALFF values in the two groups’ brain regions and speculated that differences in ALFF values could distinguish HR patients from HCs.
Ethical statement
All research methods were approved by the committee of the medical ethics of the First Affiliated Hospital of Nanchang University and were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All subjects were explained the purpose, method, potential risks and signed an informed consent form.
Results
Characteristics of the study population
The clinical course of HR in patients was 1.66 ± 12.65 years. The HR and HC groups showed statistically significant differences in Best Corrected Visual Acuity (BCVA), diastolic blood pressure, and systolic blood pressure (P > 0.05). There was no noticeable difference in the following aspects: sex (P > 0.99), age (P = 0.756), weight (P = 0.804), dominant hand (P > 0.99), or heart rate (P = 0.067) (Table 2).
Table 2. Conditions of participants included in the study.
| Condition | HR | HCs | t | P-value* |
| Male/female | 16/15 | 16/15 | N/A | >0.99 |
| Age (years) | 54.35 ± 6.87 | 51.36 ± 6.86 | 0.178 | 0.756 |
| Weight (kg) | 69.64 ± 4.42 | 65.57 ± 5.75 | 0.202 | 0.804 |
| Handedness | 31R | 31R | N/A | >0.99 |
| Duration of HR (years) | 31.66 ± 12.65 | N/A | N/A | N/A |
| Best-corrected VA-left eye | 0.66 ± 0.17 | 1.05 ± 0.25 | –3.764 | 0.007 |
| Best-corrected VA-right eye | 0.57 ± 0.21 | 1.10 ± 0.20 | –3.835 | 0.003 |
| Confrontation VF | Full | Full | –N/A | N/A |
| SBP (mmHg) | 166 ± 25 | 129 ± 18 | 2.864 | 0.023 |
| DBP (mmHg) | 101 ± 15 | 76 ± 11 | 2.142 | 0.016 |
| HR1 (beats per minute) | 69 ± 12 | 62 ± 14 | 0.825 | 0.067 |
| IOP (mmHg) | 16.23 ± 3.12 | 15.69 ± 4.19 | 0.725 | 0.21 |
| *p < 0.05 Independent t-tests comparing two groups, Data shown as mean ± standard deviation. Abbreviations: DBP: diastolic blood pressure; HR: Hypertensive retinopathy; HR1: heart rate; HCs: normal controls; VA: visual acuity; N/A: not applicable; SBP: systolic blood pressure; VF: Visual field; IOP: intra ocular pressure. |
ALFF Differences
Upon comparing the HR and HC groups, HR patients’ ALFF was lower in the left medial superior frontal gyrus(LMSFG) and left middle frontal gyrus (LMFG) than that of the HCs (Figure 2A and 2B [blue]; Table 3). Higher ALFF values in the brain areas were the left inferior cerebellar lobe, cerebellar vermis, left inferior temporal gyrus (LITG), and right superior cerebellar lobe (Figure 2A and 2B [red and yellow]; Table 3). The ROI signal value of brain areas between the HR group and HCs are presented in Figure 3.
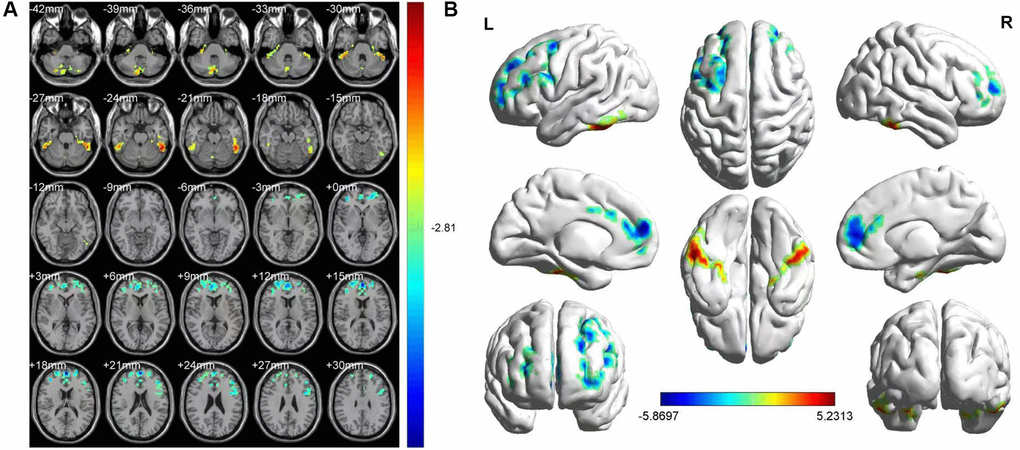
Figure 2. FMRI showed brain regions where the ALFF values' difference was statistically significant between the HR and HC groups. The difference of ALFF value is shown in (A, B) shows the ALFF changes in the cerebral cortex. Different is shown in the left medial SFG, left MFG, left ICL, vermis, left ITG and right SCL. The yellow and red areas represent an increase in ALFF values; the blue regions reduce ALFF values.
Table 3. Brain regions with significant difference in ALFF between HR patients and HCs.
| Brain areas | MNI coordinates | BA (aal) | Number of voxels | T value |
| X | Y | Z |
| HC > patient | | | | | | |
| Frontal_Sup_Medial_L | 0 | 57 | 18 | 23 | 595 | −5.8697 |
| Frontal_Mid_L | −39 | 6 | 57 | 7 | 325 | −5.0923 |
| HC < patient | | | | | | |
| Cerebelum_8_L | −15 | −60 | −48 | 103 | 162 | 4.2122 |
| Vermis_8 | 3 | −72 | −36 | 114 | 200 | 4.1573 |
| Temporal_Inf_L | −54 | −45 | −24 | 89 | 222 | 5.2313 |
| Cerebelum_6_R | 42 | −33 | −30 | 100 | 153 | 4.3988 |
| Notes: The statistical threshold was set at voxel with P < 0.01 for multiple comparisons using false discovery rate. |
| Abbreviations: ALFF: amplitude of low-frequency fluctuation; BA: Brodmann area; HR: hypertensive retinopathy; HC: healthy control; MNI: Montreal Neurological Institute. |
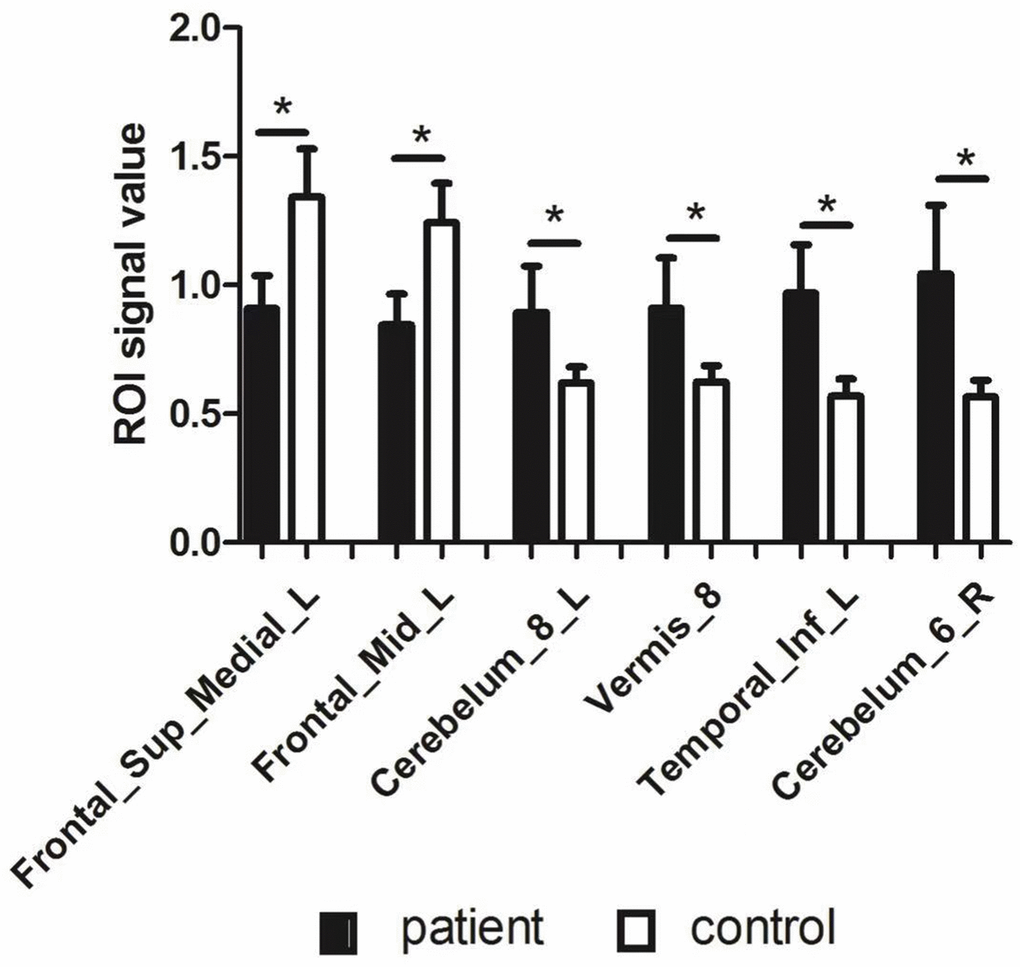
Figure 3. The ROI signal value of brain areas between patients with HR and healthy control. Abbreviations: ALFF: amplitude of low-frequency fluctuation; L: left; R: right; Frontal_Sup_Medial_L: left medial superior frontal gyrus; Frontal_Mid_L: left middle frontal gyrus; Temporal_Inf_L: left inferior temporal gyrus.
Discussion
Rs-fMRI uses BOLD signal changes in local brain tissue to explore the overall or local brain activity and metabolism in a task-free state. ALFF reflects the correlation of neural activity between brain regions and information transmission characteristics, and communication under pathological or physiological conditions. Our research aimed to determine whether there were changes in ALFF values between HR and HCs patients’ brain regions. The rs-fMRI results showed that compared with HCs, HR patients in the LMSFG and LMFG had a decreased ALFF value. The left inferior cerebellar lobe, vermis, LITG, and right superior cerebellar lobe showed increased ALFF value in the HR group.
The middle frontal gyrus (MFG) occupies most of the frontal lobe between the superior frontal sulcus and participates in visual-spatial working memory [24] and attention control [25]. The posterior part of the MFG is the cortical side-viewing center, which controls the bilateral eyeball’s simultaneous contralateral gaze and head and neck rotation. When it is stimulated, both eyes look to the opposite side, with dilated eyelids and pupils, and the head rotates to the opposite side. When it is injured, both eyes are temporarily deflected to the affected side, and there is contralateral gaze paralysis. The early stages of blindness reduce functional connectivity between the temporal and frontal regions during motion processing [26]. In other words, blindness makes it difficult for the frontal lobe of the brain to be activated. It’s difficult for the patients to concentrate because of the impaired eyesight. Early studies showed that the middle frontal gyrus was damaged in patients with clinical depression [27–28]. Recent studies by Quinn et al. have certified that the MFG is indeed related to depression [29]. In our study, the ALFF value in the LMSFG of the HR group was lower than that of the HCs group, indicating frontal lobe injury in HR patients, which may be related to depression and reduced vision.
The medial section of the superior frontal gyrus (SFG), located in front of the paravertebral central lobule, passes forward through the cingulate gyrus [30]. It is mainly responsible for executive process and functions and is the basis of decision-making [31]. The medial frontal cortex is a portion of the default mode network (DMN). In the resting state, DMN is a network composed of brain regions that are still continuously performing certain functional activities in the non-task resting state of the brain, closely related to cognitive function and conscious state [32–33]. The DMN activity is almost unaffected when performing tasks that do not require cognitive involvement. However, when performing difficult cognitive tasks, related brain activities would affect the network’s activities. The more complex the cognition, the more pronounced the effect, and the two showed negative correlation. DMN was negatively activated when performing external tasks but positively activated when performing internal psychology tasks. The activation state is correlated to the task load [34]. In coping with the external environment’s dynamic changes, DMN integrates internal resources and monitors the external environment, which is more conducive to making decisions and plans and can better adapt to various environmental changes. Existing research shows that the DMN brain area is closely related to multiple psychiatric diseases such as depression and anxiety [35–36]. In the present study, the left SFG values were significantly lower in HR patients than in HCs, suggesting that HR may result in DMN damage. Therefore, it can be speculated that patients with HR may suffer from depressive symptoms due to damaged brain nodes of DMN.
The cerebellum is situated in the posterior fossa behind the medulla oblongata. It is an essential center of motor regulation, and it participates in coordinating autonomous movement and controlling balance [37]. For a long time, clinicians’ and researchers’ understanding of the cerebellum was often related to body balance, muscle stretching, and motor regulation. Nevertheless, with the rapid development of imaging technology in recent years, progress in cerebellar functional research has been extended to cognitive ability and emotional and visual processing [38]. More recent research indicates that the cerebellum may also be related to more advanced brain functions. A previously positron emission tomography (PET) imaging study showed that people with social anxiety disorder have abnormal signals in their cerebellum which increased blood flow to the brain, thus indicating that the cerebellum plays a vital role in the occurrence and development of anxiety and depression [39]. Our research show that the ALFF value increased significantly in the HR group’s cerebella, which means that cerebellar activity changes are the basis for depression in HR patients.
The cerebellum has a longitudinally narrow structure that curls up like a worm and is referred to as the “vermis” [18], which is the center of balance and motor control [40]. The vermis is closely related to the spinal cord and the vestibular nuclei, and if lesions occur, a balance disorder may result. Existing research shows that the cerebellum and posterior hemispheres’ vermis specifically activate eye and hand movement [41]. The lobular region of the cerebellar vermis is proprioception, which can recognize the relative position of body parts used for movement. This part of the cerebellum accomplishes proprioception by comparing movement commands from the brain with the spine’s sensations. Moreover, in patients with vision-guided saccade and convergence defects (a kind of systematic binocular vision disorder), the activity of vermis changes [42–43]. The increase of ALFF value in the HR group indicated more significant vermiform activity that may lead to the damage of HR patients’ motor control function.
The inferior temporal gyrus (ITG) is located below the middle temporal gyrus. The ITG is part of the ventral visual pathway that controls natural scene coding in the visual cortex and efficient neural coding for information processing [44]. In addition to this role, the ITG participates in advanced cognitive functions such as visual perception, cognitive language, and decision-making [45]. Furthermore, the ITG is a crucial area to promote cognitive processing and emotional regulation [12]. Early studies have found that ophthalmic diseases such as PAOG and blindness show dysfunction in this area [46, 47]. According to our research, the ALFF value of the left ITG was significantly higher in the HR group than in the HCs, manifesting the enhancement of ITG activity. Thus, we consider that HR patients might be associated with dorsal visual pathway dysfunction.
Based on our above findings, we constructed a table and picture of the functions corresponding to various brain regions and the effects of alterations associated with HR on functional activities (Table 4 and Figure 4).
Table 4. Changes in specific brain regions and their potential functional effects.
| Brain regions | Experimental result | Brain function | Anticipated results |
| Cerebellum posterior lobe | HRs > HCs | Motor control, cognitive, emotional and visual processing | Social and emotional problems, depression and anxiety |
| Vermis | HRs > HCs | Balance and motor control, oculomotor processing | Binocular visual impairment |
| Left inferior temporal gyrus | HRs > HCs | visual perception, cognitive language and memory | dysfunction of the dorsal visual pathway. |
| Left superior frontal gyrus, medial | HRs < HCs | Controls spontaneous eye movements, part of the default model network | Mental disorders, including depression and anxiety |
| Left middle frontal gyrus | HRs < HCs | working memory and attention control | Inattention, Depression and anxiety |
| Abbreviations: HR: hypertensive retinopathy; HC: healthy control. |
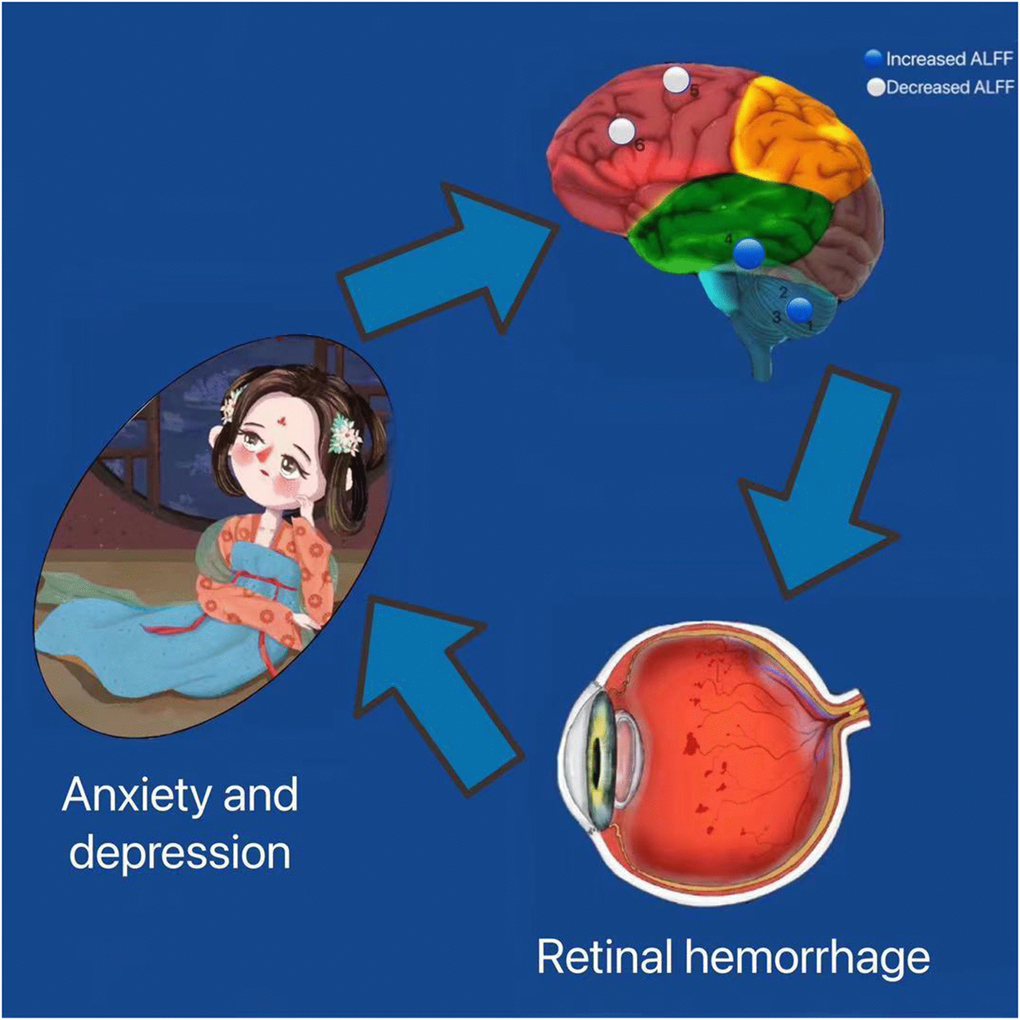
Figure 4. Correlation between functional MRI and clinical manifestation and psychological emotion of HR patients. The ALFF values of the medial LSFG and the LMFG in patients with HR were decreased. HR patients performed more anxiety and depression. Abbreviations: HR: hypertensive retinopathy.
Summary and prospect
This study shows that the ALFF value of specific brain areas in HR patients fluctuated significantly. Changes in ALFF values reflect over- or reduced activity in brain regions. In recent years, research on the pathogenesis of metabolic encephalopathy by rs-fMRI has gradually increased. The research on hypertension-related cognitive dysfunction has become a hot spot. Abnormal ALFF values in these brain regions can predict early HR development and help prevent hypertensive microangiopathy malignant transformation. Significant progress has been made in the etiology, pathology, diagnosis, and treatment of hypertensive retinopathy, which has important guiding significance for clinical practice. However, further experimental and treatment-related investigations are needed to validate these results.
Author Contributions
Jin-Yu Hu, Hui-Ye Shu and Qiu-Yu Li analyzed the data and draft the manuscript; Shi-Nan Wu, Rong-Bin Liang and Qian-Min Ge assisted with data interpretation and figure composing; Li-Juan Zhang and Yi-Cong Pan collected the data; Yi Shao conceived, designed and directed the study, and final revised and approved the manuscript.
Conflicts of Interest
This was not an industry supported study. The authors report no conflicts of interest in this work.
Funding
The Central Government Guides Local Science and Technology Development Foundation (No: 20211ZDG02003); Key Research Foundation of Jiangxi Province (No: 20181BBG70004, 20203BBG73059); Excellent Talents Development Project of Jiangxi Province (No: 20192BCBL23020); Natural Science Foundation of Jiangxi Province (No: 20181BAB205034); Grassroots Health Appropriate Technology “Spark Promotion Plan” Project of Jiangxi Province (No:20188003); Health Development Planning Commission Science Foundation of Jiangxi Province (No: 20201032,202130210); Health Development Planning Commission Science TCM Foundation of Jiangxi Province (No: 2018A060,2020A0087); Education Department Foundation of Jiangxi Province (No: GJJ200157, GJJ200159, GJJ200169).
References
-
1.
Kinoshita N, Kakehashi A, Yasu T, Katayama T, Kuroki M, Tsurimaki Y, Ono R, Yamagami H, Saito M, Kawakami M. A new form of retinopathy associated with myocardial infarction treated with percutaneous coronary intervention. Br J Ophthalmol. 2004; 88:494–96. https://doi.org/10.1136/bjo.2003.027136 [PubMed]
-
2.
Wong TY, Mitchell P. The eye in hypertension. Lancet. 2007; 369:425–35. https://doi.org/10.1016/S0140-6736(07)60198-6 [PubMed]
-
3.
Wang JG, Li Y. Characteristics of hypertension in the Chinese population. Curr Hypertens Rep. 2012; 14:410–15. https://doi.org/10.1007/s11906-012-0288-1 [PubMed]
-
4.
Wong TY, Klein R, Klein BE, Meuer SM, Hubbard LD. Retinal vessel diameters and their associations with age and blood pressure. Invest Ophthalmol Vis Sci. 2003; 44:4644–50. https://doi.org/10.1167/iovs.03-0079 [PubMed]
-
5.
Modi P, Arsiwalla T. Cranial Nerve III Palsy. In: StatPearls. Treasure Island (FL): StatPearls Publishing. 2021. [PubMed]
-
6.
Fraser-Bell S, Symes R, Vaze A. Hypertensive eye disease: a review. Clin Exp Ophthalmol. 2017; 45:45–53. https://doi.org/10.1111/ceo.12905 [PubMed]
-
7.
Brown HD, Woodall RL, Kitching RE, Baseler HA, Morland AB. Using magnetic resonance imaging to assess visual deficits: a review. Ophthalmic Physiol Opt. 2016; 36:240–65. https://doi.org/10.1111/opo.12293 [PubMed]
-
8.
Liu WF, Shu YQ, Zhu PW, Li B, Shi WQ, Lin Q, Liu YX, Zhang MY, Min YL, Yuan Q, Shao Y. The Cerebellum Posterior Lobe Associates with the Exophthalmos of Primary Hyperthyroidism: A Resting-State fMRI Study. Int J Endocrinol. 2019; 2019:8135671. https://doi.org/10.1155/2019/8135671 [PubMed]
-
9.
Zhang Y, Zhu C, Chen H, Duan X, Lu F, Li M, Liu F, Ma X, Wang Y, Zeng L, Zhang W, Chen H. Frequency-dependent alterations in the amplitude of low-frequency fluctuations in social anxiety disorder. J Affect Disord. 2015; 174:329–35. https://doi.org/10.1016/j.jad.2014.12.001 [PubMed]
-
10.
Dai XJ, Liu CL, Zhou RL, Gong HH, Wu B, Gao L, Wang YX. Long-term total sleep deprivation decreases the default spontaneous activity and connectivity pattern in healthy male subjects: a resting-state fMRI study. Neuropsychiatr Dis Treat. 2015; 11:761–72. https://doi.org/10.2147/NDT.S78335 [PubMed]
-
11.
Zuo XN, Di Martino A, Kelly C, Shehzad ZE, Gee DG, Klein DF, Castellanos FX, Biswal BB, Milham MP. The oscillating brain: complex and reliable. Neuroimage. 2010; 49:1432–45. https://doi.org/10.1016/j.neuroimage.2009.09.037 [PubMed]
-
12.
Shi WQ, Wu W, Ye L, Jiang N, Liu WF, Shu YQ, Su T, Lin Q, Min YL, Li B, Zhu PW, Shao Y. Altered spontaneous brain activity patterns in patients with corneal ulcer using amplitude of low-frequency fluctuation: An fMRI study. Exp Ther Med. 2019; 18:125–32. https://doi.org/10.3892/etm.2019.7550 [PubMed]
-
13.
Kaiser RH, Whitfield-Gabrieli S, Dillon DG, Goer F, Beltzer M, Minkel J, Smoski M, Dichter G, Pizzagalli DA. Dynamic Resting-State Functional Connectivity in Major Depression. Neuropsychopharmacology. 2016; 41:1822–30. https://doi.org/10.1038/npp.2015.352 [PubMed]
-
14.
Huang X, Zhong YL, Zeng XJ, Zhou F, Liu XH, Hu PH, Pei CG, Shao Y, Dai XJ. Disturbed spontaneous brain activity pattern in patients with primary angle-closure glaucoma using amplitude of low-frequency fluctuation: a fMRI study. Neuropsychiatr Dis Treat. 2015; 11:1877–83. https://doi.org/10.2147/NDT.S87596 [PubMed]
-
15.
Li Q, Huang X, Ye L, Wei R, Zhang Y, Zhong YL, Jiang N, Shao Y. Altered spontaneous brain activity pattern in patients with late monocular blindness in middle-age using amplitude of low-frequency fluctuation: a resting-state functional MRI study. Clin Interv Aging. 2016; 11:1773–80. https://doi.org/10.2147/CIA.S117292 [PubMed]
-
16.
Tan G, Huang X, Zhang Y, Wu AH, Zhong YL, Wu K, Zhou FQ, Shao Y. A functional MRI study of altered spontaneous brain activity pattern in patients with congenital comitant strabismus using amplitude of low-frequency fluctuation. Neuropsychiatr Dis Treat. 2016; 12:1243–50. https://doi.org/10.2147/NDT.S104756 [PubMed]
-
17.
Tan G, Huang X, Ye L, Wu AH, He LX, Zhong YL, Jiang N, Zhou FQ, Shao Y. Altered spontaneous brain activity patterns in patients with unilateral acute open globe injury using amplitude of low-frequency fluctuation: a functional magnetic resonance imaging study. Neuropsychiatr Dis Treat. 2016; 12:2015–20. https://doi.org/10.2147/NDT.S110539 [PubMed]
-
18.
Yu YJ, Liang RB, Yang QC, Ge QM, Li QY, Li B, Shi WQ, Shao Y. Altered Spontaneous Brain Activity Patterns in Patients After Lasik Surgery Using Amplitude of Low-Frequency Fluctuation: A Resting-State Functional MRI Study. Neuropsychiatr Dis Treat. 2020; 16:1907–17. https://doi.org/10.2147/NDT.S252850 [PubMed]
-
19.
Keith NM, Wagener HP, Barker NW. Some different types of essential hypertension: their course and prognosis. Am J Med Sci. 1974; 268:336–45. https://doi.org/10.1097/00000441-197412000-00004 [PubMed]
-
20.
Li HJ, Dai XJ, Gong HH, Nie X, Zhang W, Peng DC. Aberrant spontaneous low-frequency brain activity in male patients with severe obstructive sleep apnea revealed by resting-state functional MRI. Neuropsychiatr Dis Treat. 2015; 11:207–14. https://doi.org/10.2147/NDT.S73730 [PubMed]
-
21.
Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, Eickhoff SB, Hakonarson H, Gur RC, Gur RE, Wolf DH. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013; 64:240–56. https://doi.org/10.1016/j.neuroimage.2012.08.052 [PubMed]
-
22.
Yan CG, Cheung B, Kelly C, Colcombe S, Craddock RC, Di Martino A, Li Q, Zuo XN, Castellanos FX, Milham MP. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage. 2013; 76:183–201. https://doi.org/10.1016/j.neuroimage.2013.03.004 [PubMed]
-
23.
Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, Tian LX, Jiang TZ, Wang YF. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007; 29:83–91. https://doi.org/10.1016/j.braindev.2006.07.002 [PubMed]
-
24.
Morgan HM, Jackson MC, van Koningsbruggen MG, Shapiro KL, Linden DE. Frontal and parietal theta burst TMS impairs working memory for visual-spatial conjunctions. Brain Stimul. 2013; 6:122–29. https://doi.org/10.1016/j.brs.2012.03.001 [PubMed]
-
25.
Japee S, Holiday K, Satyshur MD, Mukai I, Ungerleider LG. A role of right middle frontal gyrus in reorienting of attention: a case study. Front Syst Neurosci. 2015; 9:23. https://doi.org/10.3389/fnsys.2015.00023 [PubMed]
-
26.
Dormal G, Rezk M, Yakobov E, Lepore F, Collignon O. Auditory motion in the sighted and blind: Early visual deprivation triggers a large-scale imbalance between auditory and "visual" brain regions. Neuroimage. 2016; 134:630–44. https://doi.org/10.1016/j.neuroimage.2016.04.027 [PubMed]
-
27.
Chang CC, Yu SC, McQuoid DR, Messer DF, Taylor WD, Singh K, Boyd BD, Krishnan KR, MacFall JR, Steffens DC, Payne ME. Reduction of dorsolateral prefrontal cortex gray matter in late-life depression. Psychiatry Res. 2011; 193:1–6. https://doi.org/10.1016/j.pscychresns.2011.01.003 [PubMed]
-
28.
Nelson JD, Craig JP, Akpek EK, Azar DT, Belmonte C, Bron AJ, Clayton JA, Dogru M, Dua HS, Foulks GN, Gomes JAP, Hammitt KM, Holopainen J, et al. TFOS DEWS II Introduction. Ocul Surf. 2017; 15:269–75. https://doi.org/10.1016/j.jtos.2017.05.005 [PubMed]
-
29.
Quinn ME, Stange JP, Jenkins LM, Corwin S, DelDonno SR, Bessette KL, Welsh RC, Langenecker SA. Cognitive control and network disruption in remitted depression: a correlate of childhood adversity. Soc Cogn Affect Neurosci. 2018; 13:1081–90. https://doi.org/10.1093/scan/nsy077 [PubMed]
-
30.
Wang Y, Shao Y, Shi WQ, Jiang L, Wang XY, Zhu PW, Yuan Q, Gao G, Lv JL, Wang GX. The predictive potential of altered spontaneous brain activity patterns in diabetic retinopathy and nephropathy. EPMA J. 2019; 10:249–59. https://doi.org/10.1007/s13167-019-00171-4 [PubMed]
-
31.
Talati A, Hirsch J. Functional specialization within the medial frontal gyrus for perceptual go/no-go decisions based on "what," "when," and "where" related information: an fMRI study. J Cogn Neurosci. 2005; 17:981–93. https://doi.org/10.1162/0898929054475226 [PubMed]
-
32.
Andrews-Hanna JR, Smallwood J, Spreng RN. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci. 2014; 1316:29–52. https://doi.org/10.1111/nyas.12360 [PubMed]
-
33.
Zald DH, McHugo M, Ray KL, Glahn DC, Eickhoff SB, Laird AR. Meta-analytic connectivity modeling reveals differential functional connectivity of the medial and lateral orbitofrontal cortex. Cereb Cortex. 2014; 24:232–48. https://doi.org/10.1093/cercor/bhs308 [PubMed]
-
34.
Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005; 102:9673–78. https://doi.org/10.1073/pnas.0504136102 [PubMed]
-
35.
Lemogne C, Delaveau P, Freton M, Guionnet S, Fossati P. Medial prefrontal cortex and the self in major depression. J Affect Disord. 2012; 136:e1–11. https://doi.org/10.1016/j.jad.2010.11.034 [PubMed]
-
36.
Vicentini JE, Weiler M, Almeida SRM, de Campos BM, Valler L, Li LM. Depression and anxiety symptoms are associated to disruption of default mode network in subacute ischemic stroke. Brain Imaging Behav. 2017; 11:1571–80. https://doi.org/10.1007/s11682-016-9605-7 [PubMed]
-
37.
Ferrari C, Oldrati V, Gallucci M, Vecchi T, Cattaneo Z. The role of the cerebellum in explicit and incidental processing of facial emotional expressions: A study with transcranial magnetic stimulation. Neuroimage. 2018; 169:256–64. https://doi.org/10.1016/j.neuroimage.2017.12.026 [PubMed]
-
38.
Adamaszek M, D'Agata F, Ferrucci R, Habas C, Keulen S, Kirkby KC, Leggio M, Mariën P, Molinari M, Moulton E, Orsi L, Van Overwalle F, Papadelis C, et al. Consensus Paper: Cerebellum and Emotion. Cerebellum. 2017; 16:552–76. https://doi.org/10.1007/s12311-016-0815-8 [PubMed]
-
39.
Kilts CD, Kelsey JE, Knight B, Ely TD, Bowman FD, Gross RE, Selvig A, Gordon A, Newport DJ, Nemeroff CB. The neural correlates of social anxiety disorder and response to pharmacotherapy. Neuropsychopharmacology. 2006; 31:2243–53. https://doi.org/10.1038/sj.npp.1301053 [PubMed]
-
40.
Herzfeld DJ, Kojima Y, Soetedjo R, Shadmehr R. Encoding of action by the Purkinje cells of the cerebellum. Nature. 2015; 526:439–42. https://doi.org/10.1038/nature15693 [PubMed]
-
41.
Nitschke MF, Arp T, Stavrou G, Erdmann C, Heide W. The cerebellum in the cerebro-cerebellar network for the control of eye and hand movements--an fMRI study. Prog Brain Res. 2005; 148:151–64. https://doi.org/10.1016/S0079-6123(04)48013-3 [PubMed]
-
42.
Hayakawa Y, Nakajima T, Takagi M, Fukuhara N, Abe H. Human cerebellar activation in relation to saccadic eye movements: a functional magnetic resonance imaging study. Ophthalmologica. 2002; 216:399–405. https://doi.org/10.1159/000067551 [PubMed]
-
43.
Alvarez TL, Jaswal R, Gohel S, Biswal BB. Functional activity within the frontal eye fields, posterior parietal cortex, and cerebellar vermis significantly correlates to symmetrical vergence peak velocity: an ROI-based, fMRI study of vergence training. Front Integr Neurosci. 2014; 8:50. https://doi.org/10.3389/fnint.2014.00050 [PubMed]
-
44.
Baddeley R, Abbott LF, Booth MC, Sengpiel F, Freeman T, Wakeman EA, Rolls ET. Responses of neurons in primary and inferior temporal visual cortices to natural scenes. Proc Biol Sci. 1997; 264:1775–83. https://doi.org/10.1098/rspb.1997.0246 [PubMed]
-
45.
Dien J, Brian ES, Molfese DL, Gold BT. Combined ERP/fMRI evidence for early word recognition effects in the posterior inferior temporal gyrus. Cortex. 2013; 49:2307–21. https://doi.org/10.1016/j.cortex.2013.03.008 [PubMed]
-
46.
Li C, Cai P, Shi L, Lin Y, Zhang J, Liu S, Xie B, Shi Y, Yang H, Li S, Du H, Wang J. Voxel-based morphometry of the visual-related cortex in primary open angle glaucoma. Curr Eye Res. 2012; 37:794–802. https://doi.org/10.3109/02713683.2012.683506 [PubMed]
-
47.
Ma G, Yang D, Qin W, Liu Y, Jiang T, Yu C. Enhanced Functional Coupling of Hippocampal Sub-regions in Congenitally and Late Blind Subjects. Front Neurosci. 2017; 10:612. https://doi.org/10.3389/fnins.2016.00612 [PubMed]