Native intestinal aging
In this section we focus on the gastrointestinal system and review relevant three-dimensional organotypic culture models. The small intestine is the primary organ for nutrient absorption from food, while the colon (or large intestine) is the primary organ for reabsorption of water [154]. Here, we focus on the small intestine, due to the larger number of in vitro three-dimensional models, but large intestine models are briefly discussed as well. The small intestine has a complex tissue structure involving crypts (valley points) and villi (mountain points); with the crypts providing a stem cell niche (Figure 2A). Stem cells located within crypts asymmetrically divide and the resultant epithelial cells migrate up toward villi and eventually slough off into the gut lumen. Multiple distinct epithelial populations arise from these stem cells, including microfold cells, enteroendocrine cells, enterocytes, goblet cells, Paneth cells, and tuft cells; this process of continual epithelial renewal and differentiation is integral to a healthy gut barrier. On the epithelial surface there is a brush boarder and single or bi-layered mucus layer depending on location within the gut [155]. Interacting with this surface is the microbiome which is made up of commensal bacteria and pathobionts (resident microbes with pathogenic potential) that constantly interact with the mucin layer of the gut [155]. Diversity of the gut microbiome has been established as an important factor in gut health and host health [156–165]. The diversity of the microbiota presents in different regions of the gastrointestinal tract depend on many factors including pH, host health, mucin composition, bacterial cooperation, nutrient availability, location within the gut, and age of the host [157]. Further, within the subepithelial and stromal tissue there are additional cells, including fibroblasts, smooth muscle cells, microvascular cells, and both circulating and resident immune cells (e.g., monocyte derived macrophages, neutrophils, dendritic cells, T cells). The immune cells are known to interact with and traverse the epithelial surface [166–168]. Given the complexity of the intestinal tissue and the number of host and bacterial cell types, it is unsurprising that many of the cellular interactions are poorly understood, especially in aging tissue where both the host tissue and microbiome can change [169].
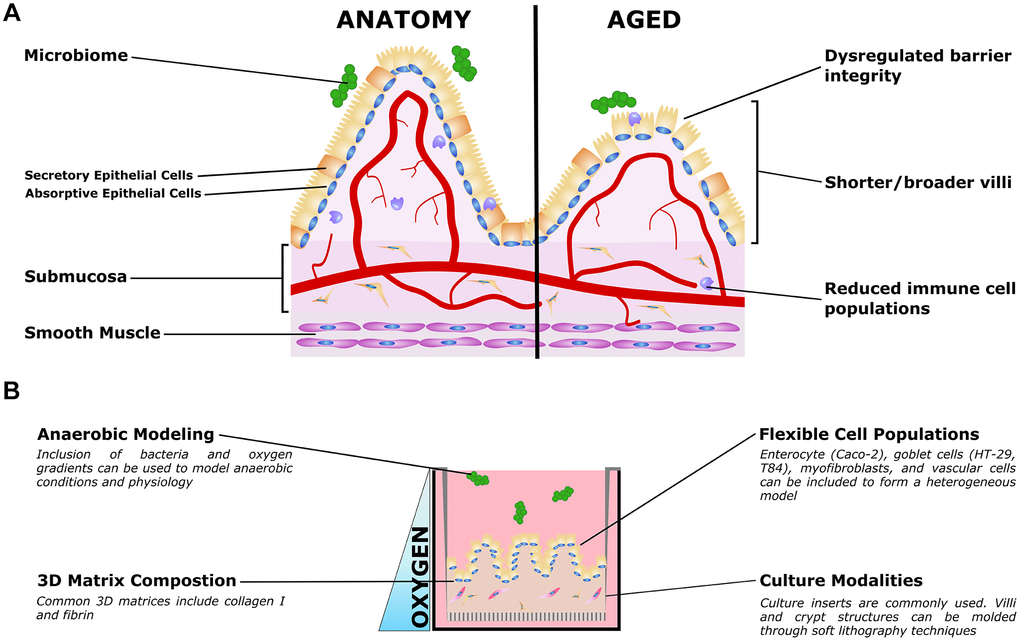
Figure 2. Organotypic models of gut aging. (A) Simplified gut anatomy and aging, focusing on the most commonly modeled components. A mixed epithelial population, described in the text, forms a simple cuboidal epithelial layer with both secretory and absorptive epithelium. A layer of mucus inside the gut lumen supports the host/microbiome interaction. The stroma underneath the epithelium, the submucosa, is host to nerves (not shown) blood vessels, fibroblasts, and immune cells important for gut function. Smooth muscle is required for gut peristalsis. In aging, the macrostructure of villi degrades, with villi becoming shorter and broader. Immune cell populations are disrupted, and reduced epithelial barrier integrity can lead to increased microbial infiltration into the submucosa and vasculature. (B) Organotypic models of the gut typically only model a small subset of these features, and are typically adapted to aspects that are relevant to specific questions. For example, epithelial and immune populations may be co-cultured to study intercellular interactions in a simple format. To study the influence of villous structures, soft lithography can be used to recreate the villi/crypt geometry. Microbiome co-cultures can be included, and microfluidic organ-on-a-chip models have been used to mimic the oxygen gradient from the vascularized submucosa to the anaerobic lumen.
Aging in the gut presents as reductions to nutrient ingestion, the tolerance of resident microbiota, and the response to infection (key aging phenotypes are summarized in Table 2). Often these co-present with dehydration and malnutrition [166]. Generally, there is a lower intake of macronutrients and micronutrients in aged individuals, although this lower intake could be attributed to lower physical activity, problems with teeth, impaired sense of taste and smell, psychological factors, income levels, and drug side effects [170–172]. Together, lessened nutrient intake, dehydration, and malnutrition contribute to overall healthy decline and morbidity in aged individuals [172]. Additionally, there is evidence showing that absorption of glucose and vitamins increases with age while some nutrients such as cholesterol and fatty acid decrease or slow; changes in absorption have been well reviewed in animals [170, 172] but continues to require more investigation in the human gut [172, 173]. It has been suggested that changes in nutrient absorption could also be tied to the changes in morphology found in aged animals and in humans [174].
Table 2. Prominent phenotypes of aging intestine.
| Prominent aging phenotypes | References |
| Increased microbial infiltration into submucosa and vasculature | [180–182] |
| Reductions to nutrient ingestion, tolerance of resident microbiota, and the response to infection. | [166] |
| Villi morphology changes, decreased cells per villus, decreased mucosal surface area, decreased crypt numbers | [174, 177, 178, 183–185] |
| Increased cell apoptosis, reduced cell proliferation and survival, decreased regenerative potential of stem cells | [166, 177, 184–187] |
| Disruption of Wnt Signaling | [177, 188–190] |
Morphologically, as the small intestine ages, numerous structural changes have been observed in several models. These structural changes are coupled to cellular changes, for example, the dynamics of cell life cycle from the crypt to extrusion at the villi [170, 175–177]. In one year old rabbits compared to young rabbits, there are morphological changes in the jejunum and ileum; villi shorten, number of cells/villus drops, and mucosal surface area declines in the jejunum while villus cell size remained constant in both areas [178]. Changes in villous height are associated with mucosal surface area at all ages [178] and these declines in surface area have been related to differences in nutrient absorption of aged individuals [174]. In healthy mice it takes around 4-5 days for a stem cell derived progenitor to move from the crypt, differentiating along the way, to the tip of the villus, where it ultimately undergoes apoptosis and extrusion. Morphological changes such as villi length increase and crypt number decrease lead to larger crypts with more cells and are coupled with less travel of progenitor cells to the tip of the villus as well as increased apoptotic events, decreased cell proliferation, and lower cell survival in aged mice [177]. Aging and how it effects wound healing in the small intestine has also been investigated in mouse models. Martin and colleagues studied the regenerative capacity of small intestinal epithelium after injury in young and old mice using full or partial body irradiation [179]. Authors found that after injury induced by full body irradiation, crypts of old mice were smaller than controls while young mice had larger crypts. After partial body irradiation, the crypts of young animals were found to be smaller, while the number of surviving crypts in old mice was lower than in young mice.
In rats, morphological changes such as increased numbers of crypts and villi are observed with aging, although size and cell production rate changes were not observed [183]. Atrophy of intestinal mucosa also occurs in aged rats and this contributes to decreased number of enterocytes [184, 185]. These changes can be localized to specific tissues; for example, mucosal atrophy in rats has been found in proximal regions of the small intestine, but not in the distal small intestine; similarly, the decline in villi height has been found in the ileum but not the duodenum [184]. Changes in morphology are thought to be closely tied to transport function across the gut barrier and may be tied to malabsorption of nutrients, but more evidence is needed to support this [169, 170, 174, 178]. Further, the association between aging and morphological changes is poorly understood in human intestine. Currently, there are few studies that have examined human intestinal morphology; Webster and colleagues found that elderly people have shorter villi and possibly broader villi when comparing shape and dimensions of proximal jejunal villi in young versus aged humans [174]. The villous changes in humans were not definitively linked to changes in intestinal function, but changes in surface area are thought to contribute to the nutrient absorption decline that aged individuals often experience [174].
Changes in enzyme distribution and brush border membrane makeup have been observed in mice [170], rats [185], and rabbits [178], but the conclusions differ by species and it is unclear whether these changes are associated with aging [170]. Briefly, in adult and aged mice there are similar activities and distribution of enzymes in the brush boarder membrane [170]; while in aged rats lower alkaline phosphatase activities have been found; conversely, higher sucrase/alkaline phosphatase in the brush boarder membrane have been found in adult vs. young rabbits. Differences in mucus structure and chemical composition have been tied to age changes [166, 170, 191]; specifically glycoproteins in the mucus change with age in rats [170, 191]. There is some evidence suggesting that the process of bacterial adhesion to mucus also changes with age, shown with bifidobacterial strains [166, 192–194]. However, gastric and duodenal mucus thickness does not change with age in healthy individuals [166, 195]; mechanical properties of mucus have been found to remain stable as well [166].
On a cellular level, differences have been observed with aging. Most prominently, stem cell changes have been observed in aged animal studies and in organoid cultures [177, 188]. In small intestinal tissue from mice, the intestinal stem cell markers Lgr5 and Olfm4 were examined but found to be similar in young and old samples, while the quiescent intestinal stem cell markers Lrig1 and Tert were reduced [177]. However, when examining numbers of stem cells in young versus old cultures, no difference was found [177]. Wnt signaling, an important aspect of self-renewal and proliferation in intestinal stem cells, is altered in aging gut [188–190]. Elevated Wnt activation can lead to intestinal tumorigenesis [196] and malformed crypts (less lobes and buds per crypt) in small intestine mouse organoid cultures [189]. However, there is conflicting literature on how elevated or lowered Wnt signaling effects stem cells in aged mice. Nalapareddy and colleagues found that during aging, intestinal stem cells, Paneth cells, and mesenchyme secrete less Wnt ligands which leads to overall reduced Wnt signaling and lower regenerative potential of stem cells [177]. Using organoid models derived from duodenal (proximal) crypts in mice, the decreased stem cell function can be rescued by endogenous Wnt in vitro [177]. There is evidence that the stem cells may lose fitness in maintaining differentiated cell populations; specifically Paneth cells, responsible for generating anti-microbial peptides [166]. The amount of Paneth cells and their secretory functions have been found to decline with age [166, 187], and this may be due to the age-related stem cell decline and reduced ability to generate Paneth cells [166, 179, 197].
The mucus is the site of antibody production (specifically, secretory immunoglobulin A; IgA) and is the first defense against harmful microorganisms [166]. Goblet cells, the primary contributor to the mucus layer, have a stable population in aging mice [166, 198]. As previously reviewed, the literature remains unclear on the effect of aging on IgA response, migration, and production [166]. Aging has been found to decrease secretory IgA amounts in animals (mice, rat, non-human primates) when exposed to cholera toxin [166, 199–202] and increase somatic hypermutation in mice [166, 203]. In contrast, other studies have shown no changes in serum or intestinal amounts of IgA in aged rats and mice; some results suggest that the lower levels of IgA are due to an overall homing decline rather than changes in amounts of IgA [166, 201, 204–207]. Dendritic cells present antigens to B and T cells in the intestinal immune system, and evidence points to decreasing cell numbers and function in aged mice [186]. Further, this plays a role in decline of regulatory immune functioning [166, 208, 209] and may play a role in low grade inflammation observed in the aging gut [166, 169, 210, 211].
The microbiome plays an important role in digestion, absorption, and nutrient processing [212], but it remains incompletely understood how the intestinal barrier and immune system interact with microbiota and how this system is affected by aging. In the study of microbiota, it remains unclear how gut diversity affects the aging process and how gut diversity changes with age. There is not enough evidence or investigation on age-related associations and gut health to determine causes/effects of gut on old age [164, 165], although there are many health practices that correlate with perturbations of the gut microbiome including drug/antibiotic usage and diet [164, 213]. There is evidence that the gut microbiome is affected by sex differences [212, 214–217], and this may be implicated in sex differences in aging-associated disease. Sex differences in the microbiome affect gut health but also risk of disease development including atherosclerosis, diabetes, hypertension, dyslipidemia, and obesity [212]. In general, aging and its relation to sex and hormonal differences requires more investigation, but there are indications that changes in the aging gut are sex-linked due to hormonal differences during early life, adulthood, and aging [214, 215]. In aging males, testosterone levels drop slightly from levels during adulthood while in aging females, there is a dramatic drop in estrogen from the oscillation range of adulthood [215]. The general effects of hormonal supply decline to the gut microbiome are unknown, but are likely sex-specific [215] and may be associated with the immune component of the gut [216].
Tissue engineered gut models
There are a few limitations to traditional intestinal models that can be addressed with 3D organotypic gut models (Figure 2B). 2D cultures on culture inserts are often used to model gut, but these cultures are unstable after 4 weeks due to cellular overgrowth and formation of multicellular layers [155]. To study enteric bacterial pathogens, researchers have often used human tissue explants; animal models [218]; and 2D cultures with cell lines such as T84 and HT-29 which mimic goblet cells, and Caco-2 which serve as enterocytes [219]. Although helpful in understanding microbiome-host responses, these models are typically inconsistent with the human anatomy and physiology in the gut [218, 220]. Similarly, mouse transgenic models are often used to study inflammatory gut diseases but mice do not develop some prevalent human diseases, such as ulcerative colitis or Barrett’s esophagus [221]. To address gaps in more traditional models, several 3D models have been established based on organoid, explant cultures, micro-fluidic chips, and organotypic gut models (OGMs) generated through self-assembly and partial villous molding. Intestinal tissue derived organoids are a popular model that has been used to study aging; these are called enteroids for small intestine, or colonoids for large intestine models. Enteroids consist of only epithelial cells and model crypt like populations or are often differentiated to model surface/villous epithelium [218]; these have been studied using monolayers on tissue culture inserts and embedded in extracellular matrix [218, 221]. Human induced pluripotent stem cell (iPSC) derived intestinal organoids, contain both epithelial and mesenchymal lineages and model both crypt and surface villus [218]. Models of differentiated intestinal organoids, although limit appropriate human scale, can include even the rare cells of intestine models including enteroendocrine, tuft, M cells, and Paneth cells [222].
3D cultures have been generated with both primary human cells and commercially available lines. OGMs have been generated with adult human intestinal stem cells [222], iPSC [222], Caco-2 [155, 222, 223], T84 [222], HT-29 [155, 222, 223], and myofibroblasts [155]. OGMs are only recently developed, but they have advantages over 2D models, micro-fluidic chips, explant cultures, and organoid structures because of their ability to mimic appropriate tissue length scales for oxygen diffusion and customizable cell and material properties [218]. Additionally, human based models that include human cells and relevant 3D microenvironments can be used to study diseases such as gastroesophageal reflux disease, Barrett’s esophagus, IBD, and ulcerative colitis; for therapeutic screening; and other aging associated research [221].
Incorporation of 3D villi in OGMs have been demonstrated to model the human system more closely [220] and help to understand the changes in crypt/villi that have been observed in aged animals [177, 178, 183, 184]. Several groups have generated 3D gut models with villous platforms though pre-culture molding of hydrogels and custom plate inserts [219, 220, 224]. These systems have been found to mimic mammalian intestines more closely than 2D cultures facilitating cell differentiation, absorption/metabolism, and have been used to evaluate drug permeability [220]. Yi and colleagues compared absorption and metabolism of enterocyte (Caco-2) 2D monolayer cultures and 3D villous collagen scaffolds covered with enterocytes. They found that in the 3D cultures, cell growth was higher (likely due to more surface area), there were more in vivo phenotypes such as lower expression of P-gp (efflux transporter protein, p-glycoprotein) which is overexpressed in 2D monolayers, and increased alkaline phosphatase expression (a metabolic enzyme and intestinal epithelial differentiation marker) [219]. To generate 3D collagen villi structures, multiple groups have used relatively stiff collagen and an alginate reverse molding method to create villous structures from collagen hydrogel [219, 220]. Yu and colleagues promoted a basement membrane like surface by coating the collagen with laminin. Villous structures were fabricated to match the density and depth of human villi and models were cultured for 14 days; a 21-day duration led to breakdown of villi [220]. Similar pre-culture molding of villous structures has been used in microfluidic-chips [225–227]; and as reviewed by others [225]. These models capture appropriate microanatomy of the intestinal surface and have the potential to elucidate the respective roles of structural and cellular changes in aging.
Organoid models have been used to study several diseases [189, 190, 222, 228, 229]; illustrating how 3D cultures provide a physiologically relevant model without the complexity of fully in vivo studies. Woo and colleagues demonstrate how a 3D model (specifically an intestinal organoid spheroid model) can be used to study the human disease dyskeratosis congenita. Dyskeratosis congenita causes intestinal defects (including stem cell failure) and is characterized by decreases in telomerase, telomere length, telomere capping, and Wnt activity [190]; it is particularly relevant to aging since some of these disease characteristics are similar to what happens in aged intestinal cells [188]. In organoids generated with the dyskeratosis congenita model cell line, there was incomplete and thin epithelia, overgrowth of mesenchymal cells, and inferior E-cadherin and beta-catenin expression; the organoids did not have proper budding crypts or cavitation [190]. Through CRISPR/CAS9-mediated repair and administration of Wnt agonists the authors were able to rescue the disease phenotype and demonstrate normal organoid formation in vitro. In other disease specific models, organoids made with cells derived from inflammatory bowel disease patients maintain characteristics of disease in vitro such as gene expression profiles that regulate absorption and secretion [222, 228]. Disease focused organoid studies [190] and other organoid models generated with aged mice cells [189] demonstrate the potential of more physiologically relevant in vitro models to address aging questions. By building off of these methods and incorporating human cell types, anatomies, and physiology it is possible to develop a human derived organotypic gut model [155] and avoid costly procedures involved in animal colonies [213].
Tissue engineered gut models to study aging
A recent study by Arnold and colleagues demonstrates the physiological relevance of 3D in vitro models for aging [230]. In vivo, older animals have higher ratios of non-saccharolytic v. saccharolytic bacteria and lower amounts of β-galactosidase when compared to younger animals. Pre-biotic galacto-oligosaccharides (GOS) have previously been found to have a positive impact on intestinal health and can be administered through diet. To study the effects of dietary GOS on aging in the gut, using young and old mice models of Clostridiodes difficile were used. In the aged mouse models, dietary GOS promoted changes in microbiome composition and transcriptomic analysis also revealed differences in gene expression. Aged mice that were fed a GOS diet had decreased intestinal permeability and increased mucus abundance and thickness when compared to aged mice not fed the GOS diet. These changes in permeability supported previous findings attributing the leaky gut to increased non-saccharolytic bacteria and lower amounts of key enzymes. Further, these results were additional tested in colonic organoids injected with stool samples from young and old mice. Using the colonic organoids generated from one young mouse and stool sample injection from experimental mouse models, authors showed that they were able to reproduce differences of age, minor differences of the GOS diet, and bifidogenic responses observed in the in vivo mouse models [230]. As the authors already showed a reproduction of aged phenotypes in organoid models, reproducing these characteristics in scalable and humanized organotypic models may be beneficial in research questions of how diet and microbiome affect aged humans.
The ability to culture anaerobic bacteria is an important step in modeling the microbiome of the gut in healthy tissue and to improving the understanding of how aging changes the host-microbiome interaction [158, 163, 164, 231–233]. Most in vitro models, including OGMs, only study a few relevant features of the complex physiology at a time; models that include microbiota are no exception. One study showed their ability to culture 5 different microbe types in vitro on a custom scaffold and evaluated for proliferation and biofilm formation [234]. It is important to recognize, that although this is a human microbiota gut model, it does not incorporate human gut cells or microanatomy. Combining microbiota and human 3D OGMs is an important step in modeling the human gut; some work on the combinations of microbiota and human gut cells has been carried out in microfluidic chips [225], but these tend to lack relevant villous anatomy and appropriate oxygen diffusion scales. These factors have been partially addressed in an innovative upright cylindrical culture system [155]. Authors generated the vertical lumen with an un-patterned surface and a threaded surface to mimic crypt and villi of the intestine. Their model includes epithelial cells (Caco-2 and mucus producing HT-29 cells) and myofibroblasts seeded on and into silk-based scaffolds, respectively. With this design, they achieved proximal-to-distal oxygen gradients and reached anaerobic conditions in patterned lumens. As a proof of concept, they cultured anaerobic bacteria using this model. Importantly, the patterned lumen model was stable for long-term culture (at least 8 weeks); they further showed continuous mucus production and accumulation (~10 μm average thickness of the mucus layer). Although this model does not incorporate aging phenotypes, aged cells, or differences due to aging in the microbiome, it highlights the recent progress in developing organotypic constructs that could be adapted to aging studies.
In vitro organoids are common in the gut/microbiome field of study [188, 218, 222, 235, 236] and have been used to assess intestinal stem cell function during chronological aging [177, 188–190, 237, 238]. Although there is conflicting literature on Wnt signaling in the intestine and how it effects intestinal stem cells, several recent studies have used organoid models to investigate aging and how it changes crypt/villi formation and stem cell function in the gut. Each study also presented a rescue method to restore normal Wnt signaling and gut formations [177, 189]. Cui et al. cultured organoids from aged mice and showed reduced differentiation and increased expression of Wnt target genes (Axin2 and Ascl2). The organoids generated from aged mice presented rounded cysts without typical differentiated cell types, in contrast to organoids generated from young mice, which demonstrated differentiation and formation of villus structures. These phenotypes matched organoid cultures of cells that exhibit overactivation of Wnt signaling (through seeding with adenomatous polyposis coli deficient cells). The decreased differentiation of intestinal stem cells and impaired structure could be rescued by reducing exposure to the Wnt agonist R-spondin-1 and thus reducing Wnt activity. Rescued organoids matched those generated with cells isolated from young mice. Nalpareddy and colleagues generated organoids from duodenal proximal crypts of aged and young mice as well as humans [177]. In humans, organoids were generated from people 12–16 and 62–77 years old. The authors found decreased formation of organoids in the aged group, which was improved by adding Wnt 3a (a Wnt pathway agonist). This data supported their findings in mice organoids where aged mice organoids had lower organoid formation rates after 3 passages and decreased stem cell function (determined by lower lobes and buds per crypt). Adding Wnt 3a increased organoid formation and expression of Wnt target genes (Axin1 and Ascl2) in the aged cultures [177]. While interpreting the apparently contradictory results of these studies is difficult, they do highlight the use of organotypic models in performing detailed signaling studies that would be challenging and expensive in animal models.
In vitro intestinal models have a particularly relevant potential impact on personalized medicine due to the person-to-person variability in gut health. Aside from genetics, variation in local community and world regions as well as day-to-day activities result in microbiome and inflammatory differences that are not yet understood [239]. Personalized medicine and patient derived organotypic models may help to address these parameters. One organotypic microfluidic chip model named iHuMiX has paved the way for personalized gut models [240]. The iHuMiX platform utilizes compartments including microbial, epithelial, and flow chambers and allows for study of specific bacteria on host specific physiology. While microfluidic systems often present technical barriers for non-specialist labs, these results highlight the customizability of organotypic models, including adaption to personalized medicine. As with OSCs described in the prior section, the tradeoff between complexity and capability for organotypic gut models results in several limitations.
Limitations
As with OSCs and other organotypic models, the most prominent limitation is the lack of cell populations and structural features of the in vivo gut. While a great deal of the work described above has extensively modeled epithelial cells and their stem cell niches, the gut is much more complex; immune cells, vasculature, smooth muscle, and neuronal populations all contribute to the gut, and its physiology when aged. Further, the organization of the gut, most notably the crypts and villi, is well understood to influence function and disease; these features are only incompletely reflected in organotypic models [219, 220, 241]. More unique to the gut is the anaerobic microbiome, which is critical to understanding gut and organismal health [158, 163, 164, 231–233]. While there has been demonstrated inclusion of anaerobic microbiome in a gut model [225–227], the complexity of the system makes it challenging to broadly replicate in other labs. Indeed, the general challenges of creating and maintaining hypoxic and anoxic cultures significantly limits the ability of organotypic models to correctly match the lumen environment. Further, there is significant evidence that the microbiome is not restricted to the gut lumen, and translocation of commensal bacteria to surrounding tissues, including lymph nodes, is a driver of disease [242, 243]. While organotypic gut models may be suited to address some questions of bacterial translocation, none have reached the scale or complexity required to include lymphatics. While this is a single example, it does highlight the more general limitations on most organotypic models.
As with other organotypic models, sex differences are understudied. This is despite clear sex differences in aging associated gastrointestinal diseases [244, 245] and cancers [246, 247]. While sex differences local to the cell populations used could, and should, be studied using organotypic models, systemic factors including hormones remain a challenge. As a pertinent example in the gut, sex hormone levels are known to regulate the mucosal surface and barrier integrity [248]. While organotypic models to lend themselves to studying the impact of specific hormone levels, they clearly lack the complexity of overall systemic changes that come with aging and sex differences.