Effectiveness of non-pharmaceutical intervention on sperm quality: a systematic review and network meta-analysis
Abstract
Infertility affects about 10% of the world’s population and has been recognized by the WHO as a global public health problem. The aim of this network meta-analysis was to investigate the efficacy of non-pharmaceutical interventions on sperm quality. All randomized clinical trials (RCTs) from the PubMed, MEDLINE, Embase, China national knowledge infrastructure (CNKI), Wanfang database, and Cochrane Library databases evaluating the effectiveness of non-pharmaceutical interventions on semen parameters using network meta-analyses. Results of the ω-3 fatty acid, lycopene, acupuncture, and vitamin suggested evident advantages in improving sperm concentration (MD, 9.93 (95% CI, 7.21 to 12.65)), (MD, 8.79 (95% CI, 2.67 to 14.91)), (MD, 5.40 (95% CI, 2.32 to 8.49)) and (MD, 3.82 (95% CI, 0.70 to 6.94) respectively). Acupuncture has a significant advantage over placebo in improving sperm total motility (MD, 17.81 (95% CI, 10.32 to 25.29)), and the effect of lycopene was obviously greater than that of placebo (MD, 19.91 (95% CI, 2.99 to 36.83)). Lycopene, Coenzyme Q10 (CoQ10), acupuncture, ω-3 fatty acid, and vitamin suggested significant advantages in improving sperm forward motility (MD, 8.64 (95% CI, 1.15 to 16.13), MD, 5.28 (95% CI, 2.70 to 7.86), MD, 3.95 (95% CI, 3.23 to 4.67), MD, 3.50 (95% CI, 2.21 to 4.79)) and (MD, 2.38 (95% CI, 0.96 to 3.80) respectively). This review establishes that non-pharmaceutical interventions, particularly acupuncture, exercise, lycopene, ω-3 fatty acids, CoQ10, zinc, vitamins, selenium, carnitine, or foods rich in these supplements, profitably improve sperm quality that may be used to treat male infertility.
Introduction
The World Health Organization (WHO) has declared infertility to be the major global public health problem for the past few decades [1]. Infertility is the failure to become pregnant after twelve months or more of proper and timed unprotected intercourse [2, 3]. It is estimated that approximately 10 to 15 percent of people worldwide are affected by infertility, thus making it a global concern [4]. There are approximately 186 million infertility cases worldwide, and more than half of them are male infertility [5]. Recently, increasing studies have highlighted the influences of inflammation of the reproductive tract, irregular lifestyles, and nutrition deficiency [6, 7] during the pathogenesis of male infertility. In this regard, being overweight and other conditions, such as alcohol abuse, metabolic syndrome, smoking, and environment are strongly associated with reduced sperm quality and fertility. Therefore, the sperm quality can affect ejaculate competitiveness and influence siring success [8, 9]. Additionally, it has been reported that about 30–80% of male infertility is deemed to be due in part to the negative impacts of oxidative stress on the sperm [10, 11]. Oxidative stress takes place when reactive oxygen species (ROS) overpower semen’s anti-oxidation defenses, which destroy proteins, DNA, and lipids [10, 12]. Sperm DNA damage caused by oxidative stress can lead to decreased sperm motility, acrosomal membrane damage, sperm fertilization ability, and ultimately led to fertility decline [13–15]. One randomized clinical trial (RCT) study suggested that resistance exercise might modulate male infertility through anti-inflammatory and anti-oxidation mechanisms [16].
Treatment and management of male infertility include pharmaceutical, non-pharmaceutical, and surgical interventions. Currently, the primary clinical treatment of male infertility drugs includes antioxidants, hormones, hexanone theobromine, L-carnitine (LC), and other drugs [17], which have several disadvantages, such as uncontrollable side effects, expensiveness, case dependence, and poor outcomes. In the meantime, non-pharmaceutical interventions such as acupuncture, massage, moxibustion, and scraping have been developed and applied in the management and treatment of male infertility [18]. Non-pharmaceutical therapies, especially lifestyle, can improve sperm quality, motility, and morphology through treatments such as lifestyle changes, and can improve the overall physical fitness of men [19, 20]. Although the aforementioned systematic reviews with or without network meta-analysis analyzed the efficacy and safety of different treatments for male infertility, comparisons between the effects of different non-pharmaceutical treatments have not been performed. Therefore, our study aimed to verify the comparative effectiveness of non-pharmaceutical treatments for male infertility by performing the network meta-analysis.
Methods
Data sources and searches
For the network meta-analysis, RCTs were searched in PubMed, Embase, MEDLINE, China national knowledge infrastructure (CNKI), Wanfang database, and Cochrane Library databases from initiation to 5 April 2023, using relevant free-text terms. Relevant medical subject terms (MeSH) and text terms were contained in the search terms in the network meta-analysis. This strategic search terms were as follows: “infertility, male” (MeSH Terms) OR (“infertility” (All Fields) AND “male” (All Fields)) OR “male infertility” (All Fields) OR (“male” (All Fields) AND “infertility” (All Fields)) AND (“diet” (MeSH Terms) OR “diet” (All Fields) OR (“exercise” (MeSH Terms) OR “exercise” (All Fields) OR “exercises” (All Fields) OR “exercise therapy” (MeSH Terms) OR (“exercise” (All Fields) AND “therapy” (All Fields)) OR “exercise therapy” (All Fields) OR “exercises” (All Fields) OR “exercised” (All Fields) OR “exerciser” (All Fields) OR “exercisers” (All Fields) OR “exercising” (All Fields)) OR (“acupunctural” (All Fields) OR “acupuncture” (MeSH Terms) OR “acupuncture” (All Fields) OR “acupuncture therapy” (MeSH Terms) OR (“acupuncture” (All Fields) AND “therapy” (All Fields)) OR “acupuncture therapy” (All Fields) OR “acupuncture s” (All Fields) OR “acupunctured” (All Fields) OR “acupunctures” (All Fields) OR “acupuncturing” (All Fields)) OR (“psychotherapies” (All Fields) OR “psychotherapy” (MeSH Terms) OR “psychotherapy” (All Fields) OR “psychotherapies” (All Fields) OR “psychotherapy s” (All Fields)). We then screened a list of references to all the acquired articles including related reviews.
Study selection
After deduplication, each study is assessed for primary qualifications in the title and abstract, and these articles were screened by two independent researchers (ZMH and SJW). Studies published only as summaries without extra data were excluded. In the case of file screening and data extraction as described above, any discrepancies will be solved by submitting them to the third researcher (ZHC). In accordance with the PRISMA guidelines, the screening process for included studies will be shown in a flow chart [21].
Data extraction and quality assessment
All data from the included literature were extracted by two independent researchers using Excel software in the same predetermined table. Any differences will be solved by the third reviewer. The extracted data items are as follows: the first author and published year, age, sample size, intervention, daily dosage, treatment time, and some other outcomes of interest. The primary outcome was the pregnancy rate. Secondary outcomes included sperm parameters, e.g., sperm concentration, sperm total motility, sperm forward motility, sperm quality, and sperm count. For the results of sperm parameters, our study extracted the relevant metrics applied to evaluate the inclusion study. These studies included both fresh and cryopreserved semen. For these fresh and cryopreserved samples, the control groups for each study had the same sample collection conditions as the intervention groups. There are, therefore, minor effects on the conclusions.
Risk-of-bias assessment
The risk of bias (ROB) for the inclusion study was confirmed for each result by two independent reviewers. The ROB for included studies was evaluated by the Cochrane ROB tool [22]. Differences will be solved by the third reviewer. For RCTs, each ROB project was classified as “low risk” if bias was suspected to significantly alter these results; if the expectation bias caused some uncertainty in the outcomes, it would be “unclear”; alternatively, it would be “high risk” if the expected bias would completely change the outcome. A funnel plot adjusted for comparison was drawn to test any main publication bias in this study [23].
Data synthesis and analysis
This study performed a network meta-analysis (NMA) using STATA 14. The comparative effect of all interventions was assessed by computing the mean difference by applying the random effects model. The estimated significance of the resulting p-value was set at < 0.05 with a 95% confidence interval (CI). The subgroup analyses of clinical results for diverse interventions were performed to elucidate heterogeneity between studies. Moreover, our study conducted a network diagram of the result; the node size represented the number of patients randomly assigned to each intervention; the nodal line thickness corresponded to the number of researchers evaluating each comparison [24]. The ranking probability of each intervening measure in each possible ranking was assessed. Our study concluded the intervention hierarchy and reported that it is the surface under the cumulative ranking curve (SUCRA); the intervention with a SUCRA value of 100 is definitely the best, and the intervention with a value of 0 is definitely the worst [23].
Results
Study characteristics
The screening process for the included studies is indicated in Figure 1. A total of 2508 records were screened, and 27 RCTs (n = 4008 patients) were included in this analysis [25–47]. The network plots of pregnancy rate, sperm concentration, sperm total motility, sperm forward motility, sperm quality, and sperm count analysis are indicated in Figure 2. In the analysis of primary outcomes disaggregated by intervention, there were 11 interventions: CoQ10, zinc, ω-3 fatty acid, carnitine, selenium, lycopene, vitamin, zinc + vitamin, exercise, acupuncture, and placebo. Exercise, acupuncture, and vitamins were the most commonly investigated interventions in all comparisons (4 trials, respectively). With regard to acupuncture, the included studies were divided into two categories: acupuncture (2 trials) and electroacupuncture (2 trials). In addition, the most frequent interventions were CoQ10 (3 trials), selenium (3 trials), and zinc + vitamin (3 trials).
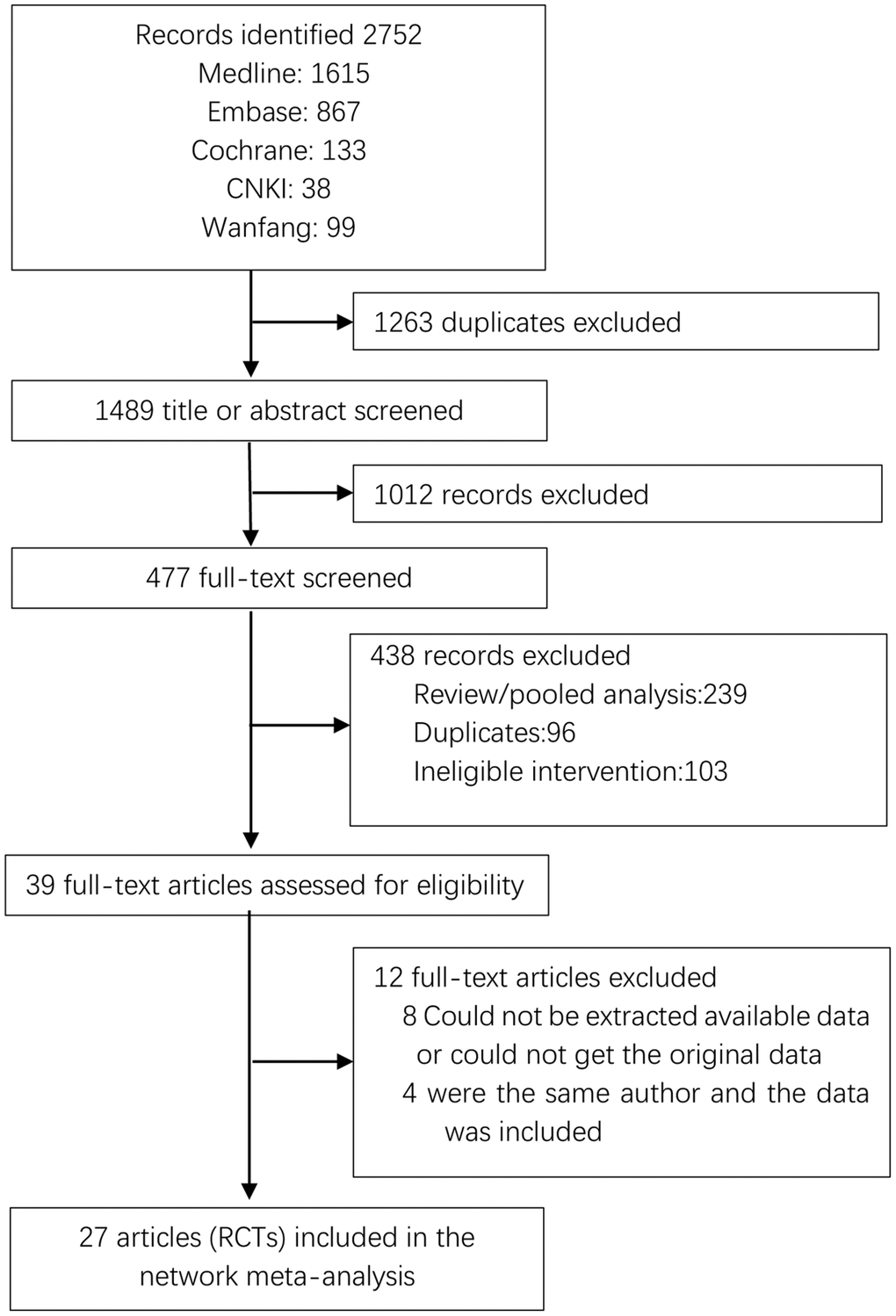
Figure 1. PRISMA flow diagram showing study search and selection. Abbreviation: RCTs: randomized controlled trials.
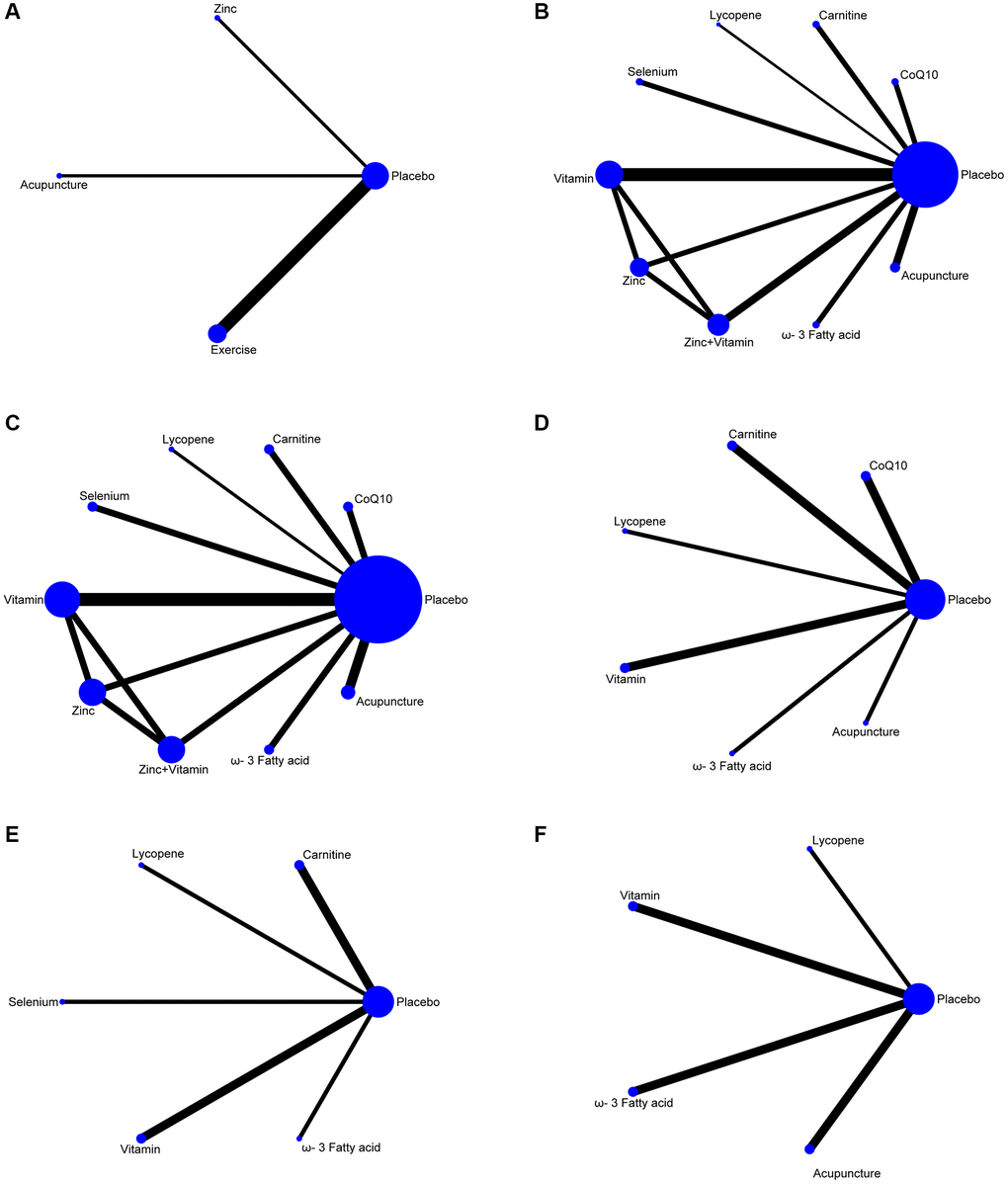
Figure 2. Network plots of non-pharmaceutical interventions on sperm parameters. (A) Pregnancy rate. (B) Sperm concentration. (C) Sperm total motility. (D) Sperm forward motility. (E) Sperm quality. (F) Sperm count.
The baseline and critical features of the included studies are indicated in Table 1. Twenty-two of the included studies lasted longer than three months. The average number of people included in the study was 148 individuals, ranging from 8 to 521 patients. In all trials, patients were aged 18–45 years. Eleven (40.7%) trials recruited patients from Europe, three (11.1%) from Asia, one (3.7%) from North America, one (3.7%) from Australia, one (3.7%) from South America, and one (3.7%) from South Africa. In the rest of the trials, nine (33.3%) proceeded in the Middle East. Figure 3 SUCRA ranking of the interventions based on their treatment effect and cumulative probability plot indicates that all interventions can treat male infertility compared with placebo. The funnel plots of these comparisons show evidence of fundamental symmetry.
Table 1. Summary of the RCT studies investigating the effect of non-pharmaceutical interventions on sperm parameters.
| Study | Country | Age | Sample size | Intervention | Daily dosage | Treatment time | Outcome | References |
| Intervention | Intervention |
| Ketabchi et al. 2018 | Iran | 33.4 (7.6)/32 (6.5) | 51 | Acupuncture | Mock acupuncture | NA | 180d | Improved sperm quality | [27] |
| Sun et al. 2016 | China | 32 (3)/31 (3) | 100 | Acupuncture | Mock acupuncture | 40 min daily | 70d | Improved sperm density, motility and viability | [42] |
| Jin et al. 2017 | China | 32.68 (0.95)/30.33 (0.91) | 72 | Acupuncture | Mock acupuncture | 30 min daily | 60d | Increasing sperm motility and vitality | [34] |
| Yu et al. 2019 | China | 31.45 (0.84)/29.52 (0.81) | 121 | Acupuncture | Mock acupuncture | 30 min daily | 60d | Increasing sperm motility and count | [45] |
| Nadjarzadeh et al. 2012 | Iran | 34.17 (4.52)/34.67 (6.69) | 47 | CoQ10 | Placebo | 200 mg/d | 90d | Improved sperm functions | [25] |
| Balercia et al. 2009 | Italy | 27–39 | 57 | CoQ10 | Placebo | 200 mg/d | 180d | Improved sperm kinetic features | [32] |
| Safarinejad et al. 2012 | Iran | 31/32 | 228 | CoQ10 | Placebo | 200 mg/d | 182d | Improved sperm density, motility and morphology | [38] |
| Lenzi et al. 2004 | Italy | 20–40 | 56 | Carnitine | Placebo | 3 g/d | 180d | Increasing sperm motility | [28] |
| Balercia et al. 2005 | Italy | 20–40 | 59 | Carnitine | Placebo | 3 g/d | 180d | Improved sperm kinetic features | [47] |
| Rolf et al. 1999 | Germany | NA | 31 | Vitamin | Placebo | 1000 mg/800 mg/d | 56d | Improved sperm concentration, motility and morphology | [29] |
| Kessopoulou et al. 1995 | UK | 26–49/25–37 | 30 | Vitamin | Placebo | 600 mg/d | 712d | Increasing sperm motility | [30] |
| Greco et al. 2005 | France | NA | 64 | Vitamin | Placebo | 1000 mg/d | 60d | Improved sperm concentration and motility | [31] |
| Haghighian et al. 2015 | Iran | 32.98 (5.35)/34.12 (4.79) | 54 | Vitamin | Placebo | 600 mg/d | 84d | Improved sperm motility | [33] |
| Omu et al.1997 | Kuwait | 37.8 (7.9)/38.12 (8.2) | 97 | Zinc | Placebo | 500 mg/d | 90d | Improved sperm quality and count | [26] |
| Ebisch et al. 2005 | Netherlands | 32.3–37.0/31.0–38.0 | 40 | Zinc + Vitamin | Placebo | 71 mg/d | 182d | Improved sperm concentration | [46] |
| Wong et al. 2002 | South Africa | 34.4 (4.7)32.9 (4.6) | 211 | Zinc + Vitamin | Placebo | 71 mg/d | 182d | Increasing sperm variables | [43] |
| Raigani et al. 2013 | Iran | NA | 83 | Zinc + Vitamin | Placebo | 270 mg/d | 112d | No differences | [39] |
| Martínez-Soto et al. 2016 | Spain | 35 (0.8)/35.6 (1.0) | 74 | ω-3 Fatty acid | Placebo | 1500 mg/d | 70d | No differences | [35] |
| Safarinejad et al. 2009 | Iran | 32 (9)/32 (10) | 211 | ω-3 Fatty acid | Placebo | 1.84 g/d | 224d | Improved sperm count and motility | [37] |
| Safarinejad et al. 2008 | Iran | 31 (9)/31 (9) | 468 | Selenium | Placebo | 200 ug/d | 182d | Improved sperm quality | [36] |
| Scott et al. 1998 | UK | 32.6 (1.1)/32.9 (1.5) | 34 | Selenium | Placebo | 100 ug/d | 90d | Improved sperm motility | [41] |
| Hawkes et al. 2009 | California | 18–45 | 42 | Selenium | Placebo | 300 mg/d | 336d | No differences | [44] |
| Nouri et al. 2019 | Iran | 32.89 (2.33)/32.15 (2.16) | 44 | Lycopene | Placebo | 25 mg/d | 84d | Improved sperm count and concentration | [40] |
| Behzad et al. 2017 | Germany | 25–40 | 386 | Exercise | No-exercise | / | 168d | Increasing pregnancy rate | [68] |
| Behzad et al. 2017 | Germany | 25–40 | 521 | Exercise | No-exercise | / | 168d | Increasing pregnancy rate | [69] |
| Behzad et al. 2018 | Germany | 25–40 | 407 | Exercise | No-exercise | / | 168d | Increasing pregnancy rate | [16] |
| Behzad et al. 2020 | Germany | 25–40 | 420 | Exercise | No-exercise | / | 252d | Increasing pregnancy rate | [70] |
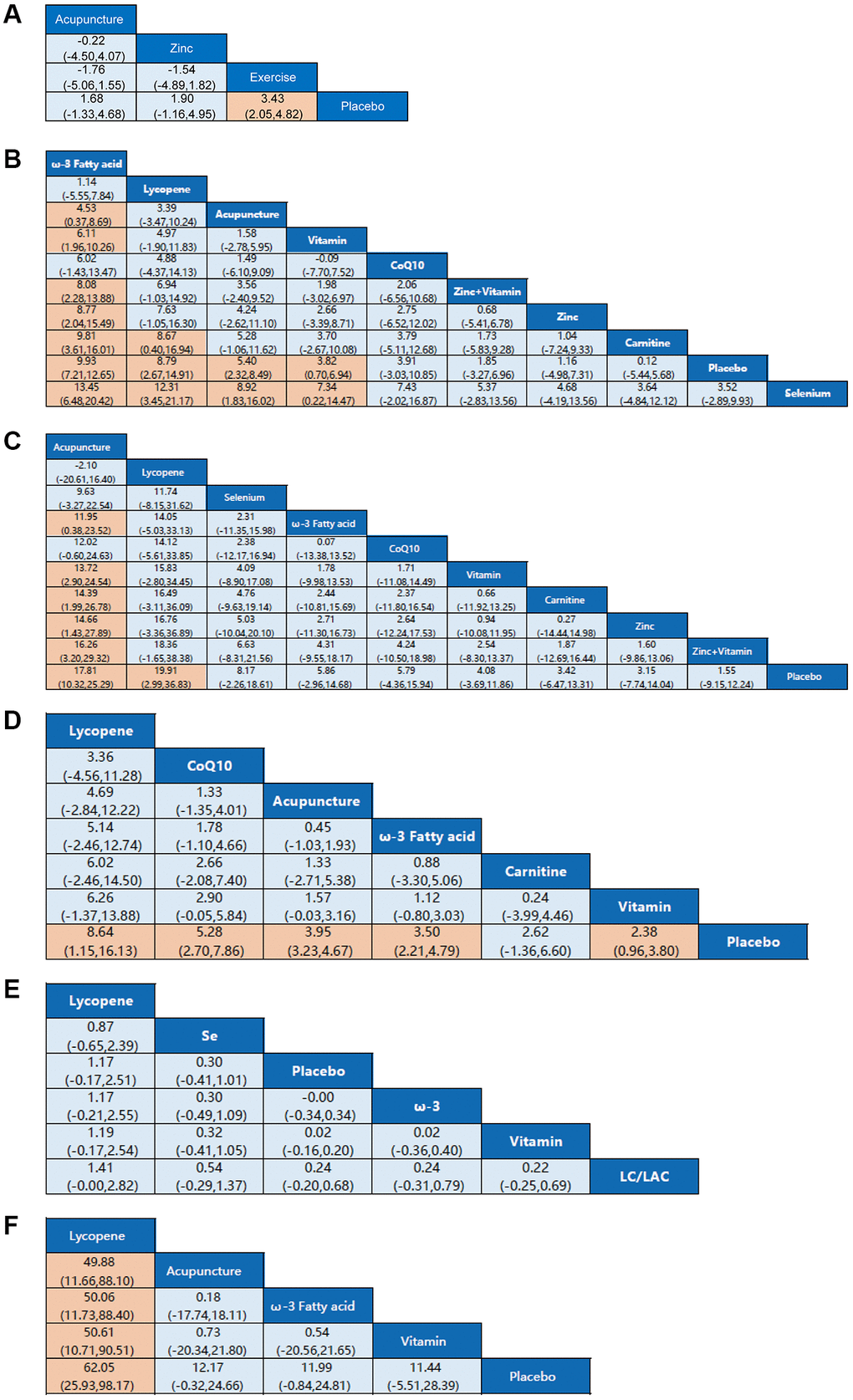
Figure 3. Network meta-analysis estimates for efficacy of non-pharmaceutical interventions on sperm parameters. (A) Pregnancy rate. (B) Sperm concentration. (C) Sperm total motility. (D) Sperm forward motility. (E) Sperm quality. (F) Sperm count. Non-pharmaceutical interventions are listed in order of efficacy ranking according to SUCRAs. Comparisons should be read from left to right. Statistically significant differences are shown in orange.
Network meta-analysis
To assess the influence of non-pharmaceutical treatments on sperm quality, twenty-six studies were included. Usage of CoQ10, zinc, omega-3 fatty acids, carnitine, selenium, lycopene, vitamin, zinc + vitamin, exercise, and acupuncture are relatively frequent in these RCTs. The number of RCTs and the homogeneity between them make our network meta-analysis suitable to test the effectiveness of the various non-pharmaceutical interventions on semen parameters, such as pregnancy rate, sperm concentration, sperm total motility, sperm forward motility, sperm quality, and sperm count. The network diagrams demonstrate the correlation of all available evidence. The line thickness represents the number of included studies, and the node size indicates the sample size (Figure 2).
Pregnancy rate
Four studies resulted in the network meta-analysis for the non-pharmaceutical interventions on pregnancy rate (Figure 2A). The SUCRA rankings suggest that the top three interventions are exercise, zinc, and acupuncture (Table 2). The network meta-analyses outcomes are indicated in Figure 3A. Compared with placebo, acupuncture indicated significant advantages in treating pregnancy rate (MD, 1.68 (95% CI, −1.33 to 4.68)). Zinc was obviously more effective than the placebo (MD, 1.90 (95% CI, −1.16 to 4.99)). Exercise had better efficacy than the placebo (MD, 3.43 (95% CI, 2.05 to 4.82)). We found no detectable differences in any of the other comparisons (Figure 3A).
Table 2. SUCRA ranking probabilities of different treatments.
| Treatment | SUCRA | Rank | | Treatment | SUCRA | Rank |
| Pregnancy rate | Exercise | 88.7 | 1 | Sperm total motility | Acupuncture | 91.6 | 1 |
| Zinc | 53.3 | 2 | | Lycopene | 89.8 | 2 |
| Acupuncture | 49.9 | 3 | | Selenium | 60.8 | 3 |
| Placebo | 8.2 | 4 | | ω-3 Fatty acid | 50 | 4 |
| Sperm concentration | ω-3 Fatty acid | 95 | 1 | | CoQ10 | 48.8 | 5 |
| Lycopene | 87.2 | 2 | | Vitamin | 41.4 | 6 |
| Acupuncture | 70.1 | 3 | | Carnitine | 37.4 | 7 |
| Vitamin | 57.9 | 4 | | Zinc | 36.8 | 8 |
| CoQ10 | 56.5 | 5 | | Zinc + Vitamin | 27.8 | 9 |
| Zinc + Vitamin | 40.7 | 6 | | Placebo | 15.5 | 10 |
| Zinc | 34.8 | 7 | Sperm quality | Lycopene | 94.3 | 1 |
| Carnitine | 27.2 | 8 | | Selenium | 68.3 | 2 |
| Placebo | 23.1 | 9 | | Placebo | 43.6 | 3 |
| Selenium | 7.5 | 10 | | ω-3 Fatty acid | 42.1 | 4 |
| Sperm forward motility | Lycopene | 91.1 | 1 | | Vitamin | 38.9 | 5 |
| CoQ10 | 79.5 | 2 | | Carnitine | 12.7 | 6 |
| Acupuncture | 62.1 | 3 | Sperm count | Lycopene | 99.6 | 1 |
| ω-3 Fatty acid | 50.2 | 4 | | Acupuncture | 50.2 | 2 |
| Carnitine | 37.1 | 5 | | ω-3 Fatty acid | 49.5 | 3 |
| Vitamin | 28.2 | 6 | | Vitamin | 47 | 4 |
| Placebo | 1.9 | 7 | | Placebo | 3.7 | 5 |
| Abbreviation: SUCRA: surface under the cumulative ranking curve. |
Sperm concentration
Twenty studies resulted in the network meta-analysis for the non-pharmaceutical interventions on sperm concentration (Figure 2B). The top four ranked interventions were ω-3 fatty acid, lycopene, acupuncture, and vitamin, as the SUCRA rank indicated (Table 2). The outcomes of the network meta-analyses are shown in Figure 3B. The ω-3 fatty acid, lycopene, acupuncture, and vitamin suggested evident advantages in treating the sperm concentration compared with placebo (MD, 9.93 (95% CI, 7.21 to 12.65)), (MD, 8.79 (95% CI, 2.67 to 14.91)), (MD, 5.40 (95% CI, 2.32 to 8.49)) and (MD, 3.82 (95% CI, 0.70 to 6.94)). ω-3 fatty acid, lycopene, acupuncture, and vitamin were statistically significantly better than the selenium (MD, 13.45 (95% CI, 6.48 to 20.42)), (MD, 12.31 (95% CI, 3.45 to 21.17)), (MD, 8.92 (95% CI, 1.83 to 16.02)) and (MD, 7.34 (95% CI, 0.22 to 14.47)). The ω-3 fatty acid and lycopene had significantly greater effects than carnitine (MD, 9.81 (95% CI, 3.61 to 16.01)) and (MD, 8.67 (95% CI, 0.40 to 16.94)). The ω-3 fatty acid was also statistically significantly better than acupuncture, vitamin, zinc + vitamin, and zinc. In addition, we found no detectable differences in any of the other comparisons (Figure 3B).
Sperm total motility
Sixteen studies resulted in the network meta-analysis for the non-pharmaceutical interventions on sperm total motility (Figure 2C). As the SUCRA rankings show, the top two interventions are acupuncture and lycopene (Table 2). The results are indicated in Figure 3C. Acupuncture has a significant advantage over placebo in treating sperm total motility (MD, 17.81 (95% CI, 10.32 to 25.29)). The effect of lycopene was obviously greater than that of placebo (MD, 19.91 (95% CI, 2.99 to 36.83)). The acupuncture was also statistically significantly better than ω-3 fatty acid (MD, 11.95 (95% CI, 0.38 to 23.52)), vitamin (MD, 13.72 (95% CI, 2.90 to 24.54)), carnitine (MD, 14.39 (95% CI, 1.99 to 26.78)), zinc (MD, 14.66 (95% CI, 1.43 to 27.89)) and zinc+vitamin (MD, 16.26 (95% CI, 3.20 to 29.32)). We found no detectable differences in any of the other comparisons (Figure 3C).
Sperm forward motility
Nine studies resulted in the network meta-analysis for the non-pharmaceutical interventions on sperm forward motility (Figure 2D). The SUCRA rankings suggest that the top three interventions are lycopene, CoQ10 and acupuncture (Table 2). The network meta-analyses outcomes are suggested in Figure 3D. Compared with placebo, lycopene suggested significant advantages in treating sperm forward motility (MD, 8.64 (95% CI, 1.15 to 16.13)). CoQ10 was obviously more effective than the placebo (MD, 5.28 (95% CI, 2.70 to 7.86)). Acupuncture had a better efficacy than the placebo (MD, 3.95 (95% CI, 3.23 to 4.67)). ω-3 fatty acid and vitamin were also statistically obviously better than placebo (MD, 3.50 (95% CI, 2.21 to 4.79)) and (MD, 2.38 (95% CI, 0.96 to 3.80)). We found no detectable differences in any of the other comparisons (Figure 3D).
Sperm quality
Seven resulted in the network meta-analysis for the non-pharmaceutical interventions on sperm quality (Figure 2E). The top two ranked interventions were lycopene and selenium, as the SUCRA rank indicated (Table 2). The main outcomes are indicated in Figure 3E. Lycopene, selenium, ω-3 fatty acid, vitamin, and carnitine were not also statistically obviously better than the placebo. We found no detectable differences in any of the other comparisons (Figure 3E).
Sperm count
Seven resulted in the network meta-analysis for the non-pharmaceutical interventions on sperm count (Figure 2F). The number one intervention is lycopene, as indicated in the SUCRA rankings (Table 2). The results are indicated in Figure 3F. Lycopene had a better efficacy than acupuncture (MD, 49.88 (95% CI, 11.66 to 88.10)), ω-3 fatty acid (MD, 50.06 (95% CI, 11.73 to 88.40)), vitamin (MD, 50.61 (95% CI, 10.71 to 90.51)) or placebo (MD, 62.05 (95% CI, 25.93 to 98.17)). Besides, we found no detectable differences in any of the other comparisons (Figure 3F).
Quality assessment and publication bias
Twenty-seven articles were included in the network meta-analysis based on inclusion and exclusion criteria. Funnel plots, Begg’s test and Egger’s test were used to assess the quality and publication bias of the included studies. The funnel plot of logarithmic WMD in the included studies was symmetric, indicating no significant publication bias (Figure 4).
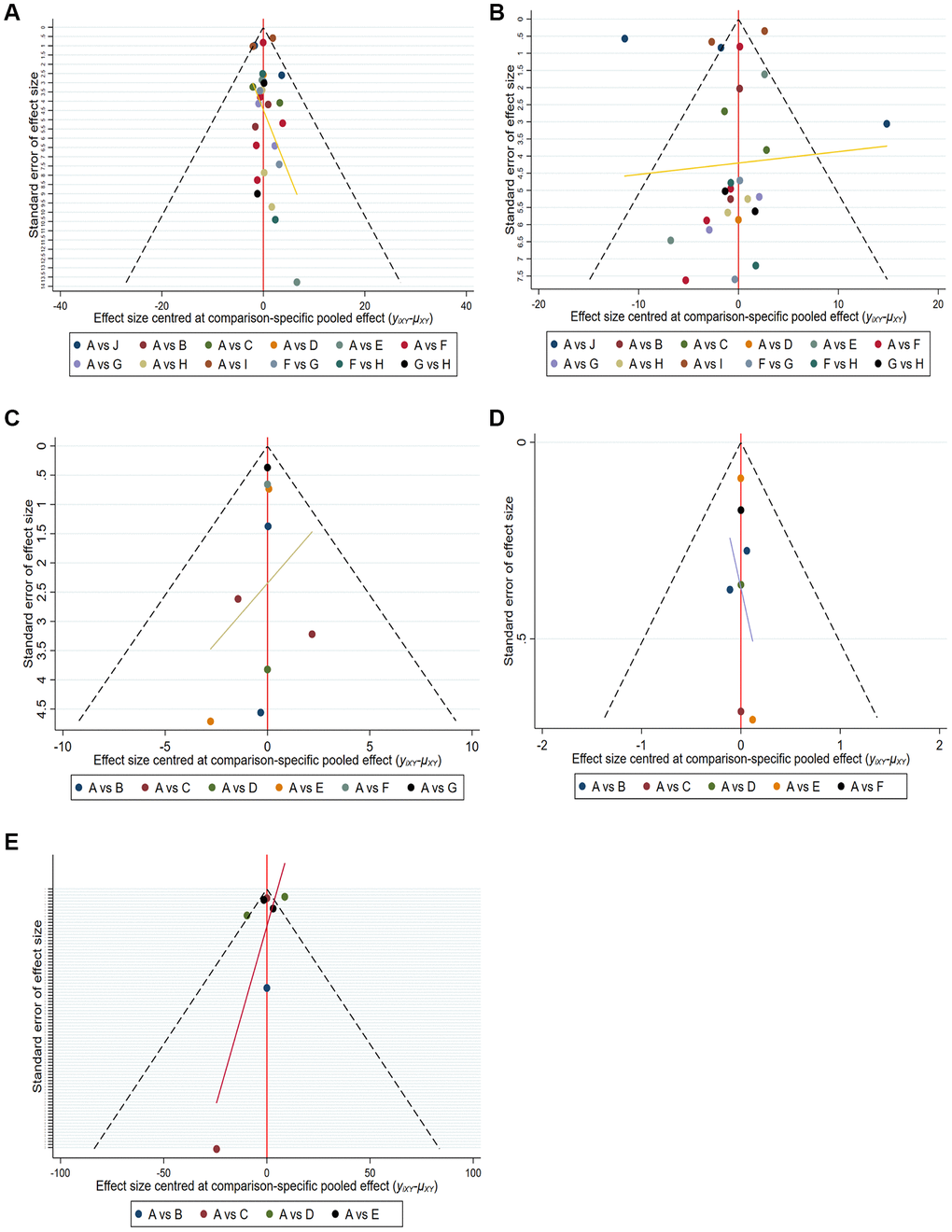
Figure 4. Comparison-adjusted funnel plot for mean overall change in sperm parameters in all comparisons. (A) Pregnancy rate. (B) Sperm concentration. (C) Sperm total motility. (D) Sperm forward motility. (E) Sperm quality.
Discussion
This RCTs network meta-analysis provides the most extensive analysis to date of the influences of non-pharmaceutical interventions on sperm parameters, conducted worldwide, in different healthcare settings, and under different experimental practices (Figure 5). The network meta-analysis indicates the beneficial effects of exercise, zinc, and acupuncture on pregnancy rates. Acupuncture and supplementations with omega-3 fatty acids, lycopene, and vitamins had significant beneficial effects on sperm concentration. Acupuncture and lycopene supplementation also had significant beneficial effects on total sperm motility. Acupuncture and supplementation with lycopene or CoQ10 had significant beneficial effects on sperm forward motility. Lycopene supplementation significantly improved sperm count. The network meta-analysis also indicated that some other dietary supplements might help improve male fertility.
Figure 5. All interventions were able to improve male infertility.
The ultimate goal of male infertility treatment is to improve semen quality and eventually increase pregnancy rates. These RCTs concluded that these non-pharmaceutical interventions were effective in improving several measures of male fertility, including semen quality parameters as well as pregnancy and live birth rates [48, 49]. However, all of these showed positive results for reproductive function and were significant from a fertility perspective. Diet, exercise, and acupuncture have been associated with increased fertility in pregnant women [48, 50]. It has also been associated with increased endometrial thickness, embryo implantation, and live birth in women undergoing in vitro fertilization [49, 51]. The results of these studies are consistent with our results, which further confirmed that exercise, zinc, and acupuncture are positively associated with increased clinical pregnancy rates.
Assessing the fertilizing potential of an ejaculate generally includes tests of sperm function, as well as evaluation of sperm morphology, motility profiles, concentration, viability ability to acrosome-react and to penetrate oocytes [52]. Thus, assessment of sperm quality in a semen sample is the only and standard method to disclose its potential fertility [53]. Omega-3 fatty acids have antioxidant and anti-inflammatory properties that may alter the function of cell membranes through the integration into cell membrane [54], which may explain this mechanism by which Omega-3 fatty acids affect spermatogenesis. Meanwhile, successful sperm fertilization has been shown to depend on the lipid composition of the sperm membrane [55]. Consistent with these findings, this current RCTs meta-analysis indicates a positive impact of Omega-3 fatty acid supplementation on sperm concentration. Nevertheless, other RCTs with large participant samples are needed to confirm the beneficial influences of omega-3 supplementation on sperm parameters. Lycopene, one of the most powerful antioxidants, is an aliphatic hydrocarbon with antioxidant defense against lipid peroxidation [56], which is reported to be present in high concentrations in male testes. It may play an important role in spermatogenesis as an important substance [57]. It was reported to affect several biological processes in prostate disease. In addition, it is also shown to prevent structural and functional injury to the testes and changes in sperm quality caused by oxidative stress [58].
As a traditional treatment, acupuncture may be an underlying treatment option for asthenospermia. Recent studies have shown that acupuncture is effective in treating most kinds of male infertility [59, 60]. The main acupoints selected include Guanyuan, Qihai, Zusanli, Sanyinjiao and Baihui. Guanyuan is where the vitality is, filling the strong true Qi and strong congenital. Guanyuan and Qihai are closely related to reproduction. Taking Sanyinjiao to regulate and replenish the three Yin meridians is a common acupoint for invigorating the spleen and removing dampness. It has the function of nourishing Yin and blood, activating blood circulation, and unblocking collaterals. Zusanli and Yinlingquan have the effect of tonifying the spleen and stomach, regulating Qi and blood, cultivating the acquired essence, and nourishing the congenital kidney Qi. The Bai hui belongs to the Dumai, which is the meeting of all Yang. It can replenish Qi and promote Yang. It is compatible with other acupoints to harmonize Yin and Yang. When all acupoints are used together, the kidney Yang can be strengthened, the kidney Yin can be cultivated, the kidney essence can be enriched, and the innate and acquired complement can promote the growth of sperm and enhance the vitality of sperm. At the same time, the essence can be vigorous and strong, and sometimes, the two essences can fight together, which guarantees pregnancy. A Norwegian study showed that about 20 percent of infertile males prefer alternative treatments such as acupuncture [61]. Summary data on sperm concentration and motility indicated that acupuncture could be used in patients with oligozoospermia and asthenospermia. A variety of mechanisms could elucidate the effectiveness of acupuncture. Siterman et al. [62] showed that acupuncture may decrease lipid peroxidation in sperm or genital inflammation by increasing immune responses. Therefore, our results are consistent with the above previous studies.
CoQ10 is an antioxidant that plays a key effect on the electron transport systems. As Balercia and Safarinejad et al. pointed out, CoQ10 suppresses the formation of organic peroxides in the semen and thus may decrease the oxidative stress of sperm cells [32, 63]. This is consistent with our research results, CoQ10 may improve sperm quality and sperm motility through antioxidants. Over the past few years, interest in the molecule has grown as a supplement to treat fertility. In this study, sperm parameters showed an overall improvement. All content types of carnitines, e.g., LC, L-acetylcarnitine (LAC), or a combination of both carnitines (LAC and LC) had all been demonstrated to augment sperm motility. This study indicated that carnitine supplementation also had certain profitable functions on sperm concentration and sperm total motility. Furthermore, carnitine is participated in the transport of long-chain fatty acids to the mitochondrial matrix for the β-oxidation and exerts antioxidative activity by enhancing the antioxidant enzyme expression [47]. Research data suggest that carnitine therapy is effective in ameliorating fertilization ability and sperm function. The primary antioxidants measured as supplements with beneficial effects on sperm quality parameters were zinc, selenium, and vitamins. On one side, selenium is crucial for spermatogenesis and plays a key function in augmenting the expression and activity of glutathione peroxidase-1 [64]. On the other side, zinc is also an antioxidant that has membrane stabilizing activity through inhibition of membrane-bound oxidases [65]. In the end, in a cross-sectional study, higher vitamin C and E intake were associated with higher sperm count and motility [66]. Our network meta-analysis also indicated that zinc, selenium, and vitamin supplementation may observably add to the sperm quality of infertile males.
According to the fifth edition of the World Health Organization's semen quality analysis standards, the normal reference value of sperm survival rate is above 58%, and the probability of pregnancy for women will be relatively high [67]. Whether or not to conceive normally is also related to various factors such as sperm count, concentration, motility, morphology, and quality of seminal plasma [8]. Of course, the higher the amount of semen, the better the quality of the sperm, and the higher the success rate of pregnancy.
Limitations
However, there are some limitations to the network meta-analysis conducted. Firstly, some non-English works of literature were not reviewed because they could not be translated into common languages. Still, combined data from non-English studies could change current analyses of male infertility. Secondly, a few studies involve different subgroups, making it difficult to conduct an overall comparison. Moreover, the elimination of low-quality studies and subgroup analyses from the network meta-analysis addressed this problem to some extent, but this remained a limitation of this study. Third, the results of this study show that non-pharmaceutical interventions can improve sperm quality, and further research is needed on the direct effect of non-pharmaceutical interventions on fertility. Fourth, although sperm quality is an important indicator of the pregnancy rate, there are many factors that affect the pregnancy rate. Therefore, the pregnancy rate cannot be used as the most important index and gold standard to judge the clinical effect of male infertility. Fifth, the reference standard of semen detection varies from research unit to research unit, so further standardized research is needed. Finally, due to the variety of male infertility types and interventions, non-pharmaceutical interventions were selected for all interventions to be further classified. In addition to all the limitations, our network meta-analysis evaluated and established studies comparing all non-pharmaceutical interventions in male infertility, which is an important aspect of this work.
Conclusion
The study conducts the most extensive analysis to date of the influences of non-pharmaceutical interventions, such as acupuncture, exercise, food, supplements, and nutrients on sperm quality parameters. This study summarizes that the dietary supplementation with some antioxidants, notably selenium, zinc, omega-3 fatty acids, CoQ10, carnitine, acupuncture, exercise, and some foods rich in these supplements can profitably regulate pregnancy rate and sperm quality parameters and impact male fertility. The small number of included studies on supplements and acupuncture resulted in a small sample size for inclusion, and the high heterogeneity of the findings, meaning that further research may lead to changes in the effect estimates outlined in the meta-analysis. Therefore, more RCTs with larger samples size are needed to verify how these supplements influence sperm parameters and fertility.
Author Contributions
ZLC and ZMH designed the manuscript. ZLC wrote the manuscript. SJW searched databases performed the selection of studies. JFQ, QW and YLZ revised the manuscript. HWW and YLZ critically evaluated the review and commented on it. All authors approved the manuscript for publication.
Conflicts of Interest
The authors declare no conflicts of interest related to this study.
Funding
The work was supported by the National Natural Science Foundation of China (Grant No. 82174392), the TCM Clinical Research Project of Shenzhen Traditional Chinese Medicine Hospital (No. G3030202112), and Key Specialty construction project of Traditional Chinese Medicine in Shenzhen, Guangdong Province (2019-2023).
References
-
1.
Cairo Consensus Workshop Group. The current status and future of andrology: A consensus report from the Cairo workshop group. Andrology. 2020; 8:27–52. https://doi.org/10.1111/andr.12720 [PubMed]
-
2.
Lin S, Ke M, Zhang Y, Yan Z, Wu J. Structure of a mammalian sperm cation channel complex. Nature. 2021; 595:746–50. https://doi.org/10.1038/s41586-021-03742-6 [PubMed]
-
3.
Yan X, Dong L, Liu Y, Yang F, Tan K, Li J, Chang D, Yu X. Effects of physical exercises on semen quality and reproductive outcomes in male infertility: A protocol for systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2019; 98:e17494. https://doi.org/10.1097/MD.0000000000017494 [PubMed]
-
4.
Winters BR, Walsh TJ. The epidemiology of male infertility. Urol Clin North Am. 2014; 41:195–204. https://doi.org/10.1016/j.ucl.2013.08.006 [PubMed]
-
5.
Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update. 2015; 21:411–26. https://doi.org/10.1093/humupd/dmv016 [PubMed]
-
6.
Lotti F, Maggi M. Ultrasound of the male genital tract in relation to male reproductive health. Hum Reprod Update. 2015; 21:56–83. https://doi.org/10.1093/humupd/dmu042 [PubMed]
-
7.
Deshpande SSS, Nemani H, Balasinor NH. Diet-induced- and genetic-obesity differentially alters male germline histones. Reproduction. 2021; 162:411–25. https://doi.org/10.1530/REP-21-0034 [PubMed]
-
8.
Minhas S, Bettocchi C, Boeri L, Capogrosso P, Carvalho J, Cilesiz NC, Cocci A, Corona G, Dimitropoulos K, Gül M, Hatzichristodoulou G, Jones TH, Kadioglu A, et al, and EAU Working Group on Male Sexual and Reproductive Health. European Association of Urology Guidelines on Male Sexual and Reproductive Health: 2021 Update on Male Infertility. Eur Urol. 2021; 80:603–20. https://doi.org/10.1016/j.eururo.2021.08.014 [PubMed]
-
9.
Tourmente M, Archer CR, Hosken DJ. Complex interactions between sperm viability and female fertility. Sci Rep. 2019; 9:15366. https://doi.org/10.1038/s41598-019-51672-1 [PubMed]
-
10.
Tremellen K. Oxidative stress and male infertility--a clinical perspective. Hum Reprod Update. 2008; 14:243–58. https://doi.org/10.1093/humupd/dmn004 [PubMed]
-
11.
Agarwal A, Parekh N, Panner Selvam MK, Henkel R, Shah R, Homa ST, Ramasamy R, Ko E, Tremellen K, Esteves S, Majzoub A, Alvarez JG, Gardner DK, et al. Male Oxidative Stress Infertility (MOSI): Proposed Terminology and Clinical Practice Guidelines for Management of Idiopathic Male Infertility. World J Mens Health. 2019; 37:296–312. https://doi.org/10.5534/wjmh.190055 [PubMed]
-
12.
Ferramosca A, Zara V. Diet and Male Fertility: The Impact of Nutrients and Antioxidants on Sperm Energetic Metabolism. Int J Mol Sci. 2022; 23:2542. https://doi.org/10.3390/ijms23052542 [PubMed]
-
13.
Seshadri S, Bates M, Vince G, Jones DI. The role of cytokine expression in different subgroups of subfertile men. Am J Reprod Immunol. 2009; 62:275–82. https://doi.org/10.1111/j.1600-0897.2009.00736.x [PubMed]
-
14.
Fraczek M, Szumala-Kakol A, Dworacki G, Sanocka D, Kurpisz M. In vitro reconstruction of inflammatory reaction in human semen: effect on sperm DNA fragmentation. J Reprod Immunol. 2013; 100:76–85. https://doi.org/10.1016/j.jri.2013.09.005 [PubMed]
-
15.
Barati E, Nikzad H, Karimian M. Oxidative stress and male infertility: current knowledge of pathophysiology and role of antioxidant therapy in disease management. Cell Mol Life Sci. 2020; 77:93–113. https://doi.org/10.1007/s00018-019-03253-8 [PubMed]
-
16.
Hajizadeh Maleki B, Tartibian B. Resistance exercise modulates male factor infertility through anti-inflammatory and antioxidative mechanisms in infertile men: A RCT. Life Sci. 2018; 203:150–60. https://doi.org/10.1016/j.lfs.2018.04.039 [PubMed]
-
17.
Wang M, Wang Q, Du Y, Jiang H, Zhang X. Vitamins combined with traditional Chinese medicine for male infertility: A systematic review and meta-analysis. Andrology. 2020; 8:1038–50. https://doi.org/10.1111/andr.12787 [PubMed]
-
18.
Jia W, Wang C, Yin Y. Acupuncture for oligospermia and asthenozoospermia: A systematic review and meta-analysis. Medicine (Baltimore). 2021; 100:e27816. https://doi.org/10.1097/MD.0000000000027816 [PubMed]
-
19.
Balawender K, Orkisz S. The impact of selected modifiable lifestyle factors on male fertility in the modern world. Cent European J Urol. 2020; 73:563–8. https://doi.org/10.5173/ceju.2020.1975 [PubMed]
-
20.
Hunter E, Avenell A, Maheshwari A, Stadler G, Best D. The effectiveness of weight-loss lifestyle interventions for improving fertility in women and men with overweight or obesity and infertility: A systematic review update of evidence from randomized controlled trials. Obes Rev. 2021; 22:e13325. https://doi.org/10.1111/obr.13325 [PubMed]
-
21.
Moher D, Liberati A, Tetzlaff J, Altman DG, and PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010; 8:336–41. https://doi.org/10.1016/j.ijsu.2010.02.007 [PubMed]
-
22.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019; 366:l4898. https://doi.org/10.1136/bmj.l4898 [PubMed]
-
23.
Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011; 64:163–71. https://doi.org/10.1016/j.jclinepi.2010.03.016 [PubMed]
-
24.
Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One. 2013; 8:e76654. https://doi.org/10.1371/journal.pone.0076654 [PubMed]
-
25.
Nadjarzadeh A, Shidfar F, Amirjannati N, Vafa MR, Motevalian SA, Gohari MR, Nazeri Kakhki SA, Akhondi MM, Sadeghi MR. Effect of Coenzyme Q10 supplementation on antioxidant enzymes activity and oxidative stress of seminal plasma: a double-blind randomised clinical trial. Andrologia. 2014; 46:177–83. https://doi.org/10.1111/and.12062 [PubMed]
-
26.
Omu AE, Dashti H, Al-Othman S. Treatment of asthenozoospermia with zinc sulphate: andrological, immunological and obstetric outcome. Eur J Obstet Gynecol Reprod Biol. 1998; 79:179–84. https://doi.org/10.1016/s0301-2115(97)00262-5 [PubMed]
-
27.
Ketabchi AA, Salajegheh S. The Effects of Acupuncture Treatment in Infertile Patients with Clinical Varicocele. Nephro-Urol Mon. 2018; 10:e65451. https://doi.org/10.5812/numonthly.65451
-
28.
Lenzi A, Sgrò P, Salacone P, Paoli D, Gilio B, Lombardo F, Santulli M, Agarwal A, Gandini L. A placebo-controlled double-blind randomized trial of the use of combined l-carnitine and l-acetyl-carnitine treatment in men with asthenozoospermia. Fertil Steril. 2004; 81:1578–84. https://doi.org/10.1016/j.fertnstert.2003.10.034 [PubMed]
-
29.
Rolf C, Cooper TG, Yeung CH, Nieschlag E. Antioxidant treatment of patients with asthenozoospermia or moderate oligoasthenozoospermia with high-dose vitamin C and vitamin E: a randomized, placebo-controlled, double-blind study. Hum Reprod. 1999; 14:1028–33. https://doi.org/10.1093/humrep/14.4.1028 [PubMed]
-
30.
Kessopoulou E, Powers HJ, Sharma KK, Pearson MJ, Russell JM, Cooke ID, Barratt CL. A double-blind randomized placebo cross-over controlled trial using the antioxidant vitamin E to treat reactive oxygen species associated male infertility. Fertil Steril. 1995; 64:825–31. https://doi.org/10.1016/s0015-0282(16)57861-3 [PubMed]
-
31.
Greco E, Iacobelli M, Rienzi L, Ubaldi F, Ferrero S, Tesarik J. Reduction of the incidence of sperm DNA fragmentation by oral antioxidant treatment. J Androl. 2005; 26:349–53. https://doi.org/10.2164/jandrol.04146 [PubMed]
-
32.
Balercia G, Buldreghini E, Vignini A, Tiano L, Paggi F, Amoroso S, Ricciardo-Lamonica G, Boscaro M, Lenzi A, Littarru G. Coenzyme Q10 treatment in infertile men with idiopathic asthenozoospermia: a placebo-controlled, double-blind randomized trial. Fertil Steril. 2009; 91:1785–92. https://doi.org/10.1016/j.fertnstert.2008.02.119 [PubMed]
-
33.
Haghighian HK, Haidari F, Mohammadi-Asl J, Dadfar M. Randomized, triple-blind, placebo-controlled clinical trial examining the effects of alpha-lipoic acid supplement on the spermatogram and seminal oxidative stress in infertile men. Fertil Steril. 2015; 104:318–24. https://doi.org/10.1016/j.fertnstert.2015.05.014 [PubMed]
-
34.
Jin ZR, Liu BH, Tang WH, Jiang H, Zhang R, Han JS, Xing GG. [Transcutaneous electrical acupoint stimulation for asthenozoospermia]. Zhonghua Nan Ke Xue. 2017; 23:73–7. [PubMed]
-
35.
Martínez-Soto JC, Domingo JC, Cordobilla B, Nicolás M, Fernández L, Albero P, Gadea J, Landeras J. Dietary supplementation with docosahexaenoic acid (DHA) improves seminal antioxidant status and decreases sperm DNA fragmentation. Syst Biol Reprod Med. 2016; 62:387–95. https://doi.org/10.1080/19396368.2016.1246623 [PubMed]
-
36.
Safarinejad MR, Safarinejad S. Expression of Concern: Efficacy of Selenium and/or N-Acetyl-Cysteine for Improving Semen Parameters in Infertile Men: A Double-Blind, Placebo Controlled, Randomized Study. J Urol. 2023. [Epub ahead of print]. https://doi.org/10.1097/JU.0000000000003113 [PubMed]
-
37.
Safarinejad MR. Effect of omega-3 polyunsaturated fatty acid supplementation on semen profile and enzymatic anti-oxidant capacity of seminal plasma in infertile men with idiopathic oligoasthenoteratospermia: a double-blind, placebo-controlled, randomised study. Andrologia. 2011; 43:38–47. https://doi.org/10.1111/j.1439-0272.2009.01013.x [PubMed]
-
38.
Safarinejad MR, Safarinejad S, Shafiei N, Safarinejad S. Effects of the reduced form of coenzyme Q10 (ubiquinol) on semen parameters in men with idiopathic infertility: a double-blind, placebo controlled, randomized study. J Urol. 2012; 188:526–31. https://doi.org/10.1016/j.juro.2012.03.131 [PubMed]
-
39.
Raigani M, Yaghmaei B, Amirjannti N, Lakpour N, Akhondi MM, Zeraati H, Hajihosseinal M, Sadeghi MR. The micronutrient supplements, zinc sulphate and folic acid, did not ameliorate sperm functional parameters in oligoasthenoteratozoospermic men. Andrologia. 2014; 46:956–62. https://doi.org/10.1111/and.12180 [PubMed]
-
40.
Nouri M, Amani R, Nasr-Esfahani M, Tarrahi MJ. The effects of lycopene supplement on the spermatogram and seminal oxidative stress in infertile men: A randomized, double-blind, placebo-controlled clinical trial. Phytother Res. 2019; 33:3203–11. https://doi.org/10.1002/ptr.6493 [PubMed]
-
41.
Scott R, MacPherson A, Yates RW, Hussain B, Dixon J. The effect of oral selenium supplementation on human sperm motility. Br J Urol. 1998; 82:76–80. https://doi.org/10.1046/j.1464-410x.1998.00683.x [PubMed]
-
42.
Sun YM, Li SD, Li Y. Clinical observation of acupuncture for idiopathic oligoasthenozoospermia. Shanghai J Acu-mox. 2016; 35:691–3.
-
43.
Wong WY, Merkus HM, Thomas CM, Menkveld R, Zielhuis GA, Steegers-Theunissen RP. Effects of folic acid and zinc sulfate on male factor subfertility: a double-blind, randomized, placebo-controlled trial. Fertil Steril. 2002; 77:491–8. https://doi.org/10.1016/s0015-0282(01)03229-0 [PubMed]
-
44.
Hawkes WC, Alkan Z, Wong K. Selenium supplementation does not affect testicular selenium status or semen quality in North American men. J Androl. 2009; 30:525–33. https://doi.org/10.2164/jandrol.108.006940 [PubMed]
-
45.
Yu Y, Sha SB, Zhang B, Guan Q, Liang M, Zhao LG, Zhang QY, Wen J, Sun W. Effects and mechanism of action of transcutaneous electrical acupuncture point stimulation in patients with abnormal semen parameters. Acupunct Med. 2019; 37:25–32. https://doi.org/10.1136/acupmed-2017-011365 [PubMed]
-
46.
Ebisch IM, Pierik FH, DE Jong FH, Thomas CM, Steegers-Theunissen RP. Does folic acid and zinc sulphate intervention affect endocrine parameters and sperm characteristics in men? Int J Androl. 2006; 29:339–45. https://doi.org/10.1111/j.1365-2605.2005.00598.x [PubMed]
-
47.
Balercia G, Regoli F, Armeni T, Koverech A, Mantero F, Boscaro M. Placebo-controlled double-blind randomized trial on the use of L-carnitine, L-acetylcarnitine, or combined L-carnitine and L-acetylcarnitine in men with idiopathic asthenozoospermia. Fertil Steril. 2005; 84:662–71. https://doi.org/10.1016/j.fertnstert.2005.03.064 [PubMed]
-
48.
Kellow NJ, Le Cerf J, Horta F, Dordevic AL, Bennett CJ. The Effect of Dietary Patterns on Clinical Pregnancy and Live Birth Outcomes in Men and Women Receiving Assisted Reproductive Technologies: A Systematic Review and Meta-Analysis. Adv Nutr. 2022; 13:857–74. https://doi.org/10.1093/advances/nmac023 [PubMed]
-
49.
Hajizadeh Maleki B, Tartibian B, Chehrazi M. Effectiveness of Exercise Training on Male Factor Infertility: A Systematic Review and Network Meta-analysis. Sports Health. 2022; 14:508–17. https://doi.org/10.1177/19417381211055399 [PubMed]
-
50.
Willis SK, Wise LA, Wesselink AK, Rothman KJ, Mikkelsen EM, Tucker KL, Trolle E, Hatch EE. Glycemic load, dietary fiber, and added sugar and fecundability in 2 preconception cohorts. Am J Clin Nutr. 2020; 112:27–38. https://doi.org/10.1093/ajcn/nqz312 [PubMed]
-
51.
Gaskins AJ, Chiu YH, Williams PL, Keller MG, Toth TL, Hauser R, Chavarro JE, and EARTH Study Team. Maternal whole grain intake and outcomes of in vitro fertilization. Fertil Steril. 2016; 105:1503–10.e4. https://doi.org/10.1016/j.fertnstert.2016.02.015 [PubMed]
-
52.
Rodríguez-Martínez H. Can we increase the estimated value of semen assessment? Reprod Domest Anim. 2006 (Suppl 2); 41:2–10. https://doi.org/10.1111/j.1439-0531.2006.00764.x [PubMed]
-
53.
Sutkeviciene N, Riskeviciene V, Januskauskas A, Zilinskas H, Andersson M. Assessment of sperm quality traits in relation to fertility in boar semen. Acta Vet Scand. 2009; 51:53. https://doi.org/10.1186/1751-0147-51-53 [PubMed]
-
54.
Salas-Huetos A, Rosique-Esteban N, Becerra-Tomás N, Vizmanos B, Bulló M, Salas-Salvadó J. The Effect of Nutrients and Dietary Supplements on Sperm Quality Parameters: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Adv Nutr. 2018; 9:833–48. https://doi.org/10.1093/advances/nmy057 [PubMed]
-
55.
Safarinejad MR, Safarinejad S. The roles of omega-3 and omega-6 fatty acids in idiopathic male infertility. Asian J Androl. 2012; 14:514–5. https://doi.org/10.1038/aja.2012.46 [PubMed]
-
56.
Bhuvaneswari V, Nagini S. Lycopene: a review of its potential as an anticancer agent. Curr Med Chem Anticancer Agents. 2005; 5:627–35. https://doi.org/10.2174/156801105774574667 [PubMed]
-
57.
Durairajanayagam D, Agarwal A, Ong C, Prashast P. Lycopene and male infertility. Asian J Androl. 2014; 16:420–5. https://doi.org/10.4103/1008-682X.126384 [PubMed]
-
58.
Gupta NP, Kumar R. Lycopene therapy in idiopathic male infertility--a preliminary report. Int Urol Nephrol. 2002; 34:369–72. https://doi.org/10.1023/a:1024483520560 [PubMed]
-
59.
Wu X, Chen D, Zhou Y, Xia T. Efficacy of electroacupuncture for the treatment of asthenozoospermia: A protocol for systematic review and meta-analysis. Medicine (Baltimore). 2021; 100:e23350. https://doi.org/10.1097/MD.0000000000023350 [PubMed]
-
60.
Jin ZR, Fang D, Liu BH, Cai J, Tang WH, Jiang H, Xing GG. Roles of CatSper channels in the pathogenesis of asthenozoospermia and the therapeutic effects of acupuncture-like treatment on asthenozoospermia. Theranostics. 2021; 11:2822–44. https://doi.org/10.7150/thno.51869 [PubMed]
-
61.
Oldereid NB, Rui H, Purvis K. Male partners in infertile couples. Personal attitudes and contact with the Norwegian health service. Scand J Soc Med. 1990; 18:207–11. https://doi.org/10.1177/140349489001800309 [PubMed]
-
62.
Siterman S, Eltes F, Wolfson V, Lederman H, Bartoov B. Does acupuncture treatment affect sperm density in males with very low sperm count? A pilot study. Andrologia. 2000; 32:31–9. [PubMed]
-
63.
Safarinejad MR. Efficacy of coenzyme Q10 on semen parameters, sperm function and reproductive hormones in infertile men. J Urol. 2009; 182:237–48. https://doi.org/10.1016/j.juro.2009.02.121 [PubMed]
-
64.
Schnabel R, Lubos E, Messow CM, Sinning CR, Zeller T, Wild PS, Peetz D, Handy DE, Munzel T, Loscalzo J, Lackner KJ, Blankenberg S. Selenium supplementation improves antioxidant capacity in vitro and in vivo in patients with coronary artery disease The SElenium Therapy in Coronary Artery disease Patients (SETCAP) Study. Am Heart J. 2008; 156:1201.e1–11. https://doi.org/10.1016/j.ahj.2008.09.004 [PubMed]
-
65.
Prasad AS. Clinical, immunological, anti-inflammatory and antioxidant roles of zinc. Exp Gerontol. 2008; 43:370–7. https://doi.org/10.1016/j.exger.2007.10.013 [PubMed]
-
66.
Gaskins AJ, Colaci DS, Mendiola J, Swan SH, Chavarro JE. Dietary patterns and semen quality in young men. Hum Reprod. 2012; 27:2899–907. https://doi.org/10.1093/humrep/des298 [PubMed]
-
67.
Baker KC, Steiner AZ, Hansen KR, Barnhart KT, Cedars MI, Legro RS, Diamond MP, Krawetz SA, Usadi R, Baker VL, Coward RM, Sun F, Wild R, et al. Poor reproducibility of percentage of normally shaped sperm using the World Health Organization Fifth Edition strict grading criteria. F S Rep. 2022; 3:110–5. https://doi.org/10.1016/j.xfre.2022.03.003 [PubMed]
-
68.
Hajizadeh Maleki B, Tartibian B. Moderate aerobic exercise training for improving reproductive function in infertile patients: A randomized controlled trial. Cytokine. 2017; 92:55–67. https://doi.org/10.1016/j.cyto.2017.01.007 [PubMed]
-
69.
Hajizadeh Maleki B, Tartibian B. Combined aerobic and resistance exercise training for improving reproductive function in infertile men: a randomized controlled trial. Appl Physiol Nutr Metab. 2017; 42:1293–306. https://doi.org/10.1139/apnm-2017-0249 [PubMed]
-
70.
Hajizadeh Maleki B, Tartibian B. High-intensity interval training modulates male factor infertility through anti-inflammatory and antioxidative mechanisms in infertile men: A randomized controlled trial. Cytokine. 2020; 125:154861. https://doi.org/10.1016/j.cyto.2019.154861 [PubMed]