Oncogenic viral protein HPV E7 up-regulates the SIRT1 longevity protein in human cervical cancer cells
Abstract
Senescence is blocked in human cervical keratinocytes infected with high risk human papillomavirus (e.g. HPV type16). Viral oncoproteins HPV E6 and HPV E7 access the cell cycle via cellular p53 and retinoblastoma proteins respectively. Previously we have shown that HPV E7, not HPV E6, is also responsible for cervical cancer cell survival (SiHa cells; HPV type16). We now present evidence that SIRT1, an aging-related NAD-dependent deacetylase, mediates HPV E7 survival function in SiHa cervical cancer cells. Moreover, HPV E7 up-regulates SIRT1 protein when expressed in primary human keratinocytes. Conversely, SIRT1 levels decrease following RNAi-mediated silencing of HPV E7 in SiHa cells. Silencing HPV E6 has no effect on SIRT1 but, as expected, causes marked accumulation of p53 protein accompanied by p53-mediated up-regulation of p21. However, p53 acetylation (K382Ac) was barely detectable. Since p53 is a known SIRT1 substrate we propose that elevated SIRT1 levels (induced by HPV E7) attenuate p53 pro-apoptotic capacity via its de-acetylation. Our discovery that HPV E7 up-regulates SIRT1 links a clinically important oncogenic virus with the multi-functional SIRT1 protein. This link may open the way for a more in-depth understanding of the process of HPV-induced malignant transformation and also of the inter-relationships between aging and cancer.
Introduction
The processes of aging and cancer share
many characteristics despite their opposing phenotypes of senescence versus
immortalisation [1]. In both
cases for example there is accumulation of DNA damage and the loss of genomic
integrity [1,2]. Two
major players which impinge upon chromatin structure and the maintenance of
genomic integrity in mammalian systems are p53 [3-6] and SIRT1 [7-9].
Reciprocal regulation occurs between SIRT1 and p53 in which SIRT1 binds and
de-acetylates activated p53 [10-12] whilst
activated p53 down-regulates SIRT1 translation via miR-34a [13]. The p53 protein is best known as a tumour suppressor but is
becoming increasingly recognized as a factor also involved in senescence and
aging [14,15].
Conversely SIRT1, one of seven mammalian sirtuins and an NAD-dependent
de-acetylase [16], first
emerged as a potential anti-aging factor (reviewed in [17,18]) but is
now also implicated in a number of age-related disease processes including
tumour development (which it suppresses) [9,19] and
cancer cell survival (which it supports) [20].
The yeast model provided
the initial evidence linking SIRT1 with aging. Thus Sir2, the ancestral
homologue of SIRT1, is a yeast NAD-dependent deacetylase and longevity factor [16,17]. In
budding yeast Sir2-mediated silencing at rDNA loci is crucial for chromatin
compaction and suppresses homologous recombination [16]. In this
way Sir2 suppresses the formation and accumulation of extra-chromosomal rDNA
circles, one of the primary causes of yeast aging [21]. Sir2 thus
functions as a longevity factor in yeast in which it also silences mating type
loci, is responsive to growth conditions and calorie restriction [16]. Recent evidence
now indicates that, in mammals, its homologue SIRT1 also functions as a
longevity factor [8,17,22-24]
and is responsive to diverse stresses many of which also activate the p53
tumour suppressor [8,17,25,26].
Much of our understanding
of the molecular biology of cancer derives from early studies in which DNA
tumour viruses were employed and the mechanisms by which they induce cell
transformation from normal to cancerous growth were elucidated. Indeed studies
with DNA tumour viruses led to the initial discovery of p53, the major tumour
suppressor in humans [27]. One of
these viruses was the human papillomavirus (HPV) which targets p53 via the HPV
E6 protein. High risk HPV types are now recognised as the cause of human
cervical cancer (see for example [28]) and
responsible for some 500,000 newly diagnosed cases worldwide each year. High
risk HPV types include HPV16 and HPV18 [28]. Although
vaccination strategies are now employed to protect uninfected children, there
remain millions of HPV-infected women who are at risk of developing cervical
cancer. The switch from HPV latency to malignancy is poorly understood. This
transition appears to involve integration of the viral episome into the host
cell genome together with enhanced expression of two viral oncoproteins HPV E6
and HPV E7 [29]. Presumably
some event favours stable integration of viral chromatin into host chromatin.
Understanding this crucial switch may enable the development of novel therapies
designed to protect HPV-positive patients from progression to malignancy.
HPV E6 and E7 are expressed
as bicistronic mRNA. In this current work we have exploited a previous
unexpected observation, namely that in HPV16-positive SiHa cells it is possible
to selectively and individually silence E6 and E7 by RNA interference (RNAi)
despite the contiguous nature of their mRNAs [30]. Here we
have confirmed and extended this important observation, and studied the
individual effects of HPV E6 and E7 expression in human cervical cancer cells
(SiHa) and in primary human epithelial keratinocytes (the cell type infected by
HPV in vivo).
It is already established that HPV16 E6
targets the p53 protein for rapid degradation with consequential loss of p53
tumour suppressor functions, including maintenance of chromosomal integrity. We
have now discovered that the second HPV viral oncogene, HPV16 E7, targets
SIRT1. Specifically we demonstrate (i) that exogenous expression of HPV E7 (but
not HPV E6) in primary human keratinocytes induces abnormally high levels of
the SIRT1 protein, similar to those observed in human cervical cancer cells,
and (ii) that HPV E7 (but not HPV E6) is required to maintain the abnormally
high levels of SIRT1 protein expressed in cervical cancer cells. The ability of
HPV E7 to up-regulate SIRT1 appears to be linked with HPV E7-mediated
suppression of apoptosis in cervical cancer cells [30] since our
current work also demonstrates that SIRT1 suppresses apoptosis in SiHa cells.
In addition to up-regulating SIRT1 we show that HPV E7 induces global
site-specific histone H3 modifications and, in synergy with the kinase aurora
B, up-regulates the anti-apoptotic survivin protein. These various changes are
predicted to affect chromatin structure and to promote cell survival.
Our discovery that HPV E7
influences the expression of SIRT1 provides the first link between an oncogenic
virus and the aging-related SIRT1 protein, and enables access to diverse
SIRT1-dependent cellular regulatory systems. This link may open the way for a
more in-depth understanding of the inter-relationships between aging and
cancer. It may also provide insight into the mechanism of chromosomal
re-distribution during the switch from latent to malignant HPV viral infection,
and also for the maintenance of HPV-induced malignancy.
Results and Discussion
Individual
knock-down of HPV16 E6 and E7 in SiHa cells
For
this study we exploited the ability of RNAi to separately knockdown the viral
oncogenes HPV16 E6 and HPV16 E7 in HPV16-positive SiHa cervical cancer cells.
Individual knockdown of HPV E6 and E7 by their respective siRNAs was verified
by quantitative RT-PCR (Figure 1A, left panel), thus confirming our previous
observations [30]. This
effect appears peculiar to SiHa cells since similar individual knock-down of E6
and E7 was not obtained for HPV16-positive CaSki cervical cancer cells in which
E7 siRNA induced knock-down of both HPV E6 and E7 expression (Figure 1A, right
panel, asterix).
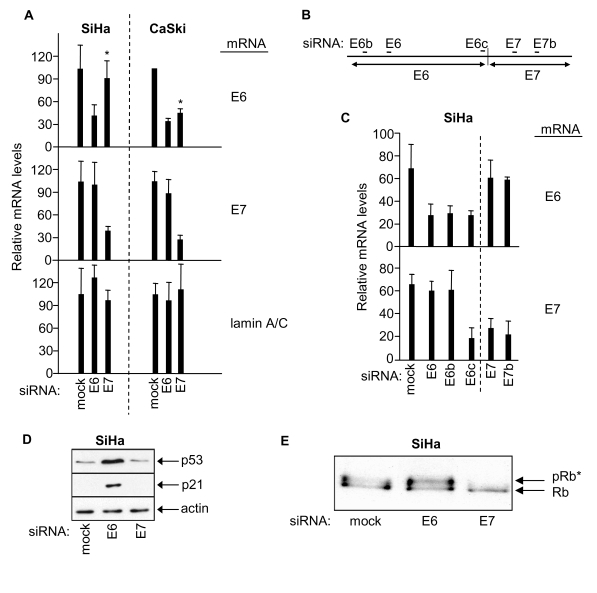
Figure 1. RNAi-mediated knock-down of HPV E6 and HPV E7 in SiHa and CaSki cells, and effects on p53 and retinoblastoma protein. (A) mRNA qRT-PCR determinations 48h
post-transfection as indicated, mean ± s.d. of three determinations.
Asterix indicates differential effect of E7 siRNA on E6 mRNA levels in SiHa
versus CaSki cells. (B) Relative positions of siRNA sequences along
the bicistronic E6/E7 transcript. (C) Relative levels of E6 and E7
mRNAs 48h post-transfection of SiHa cells with the indicated siRNAs. (D,
E) Immunoblots showing effects of E6 or E7 depletion on levels of p53,
p21 and hyperphosphorylated Rb (pRb*) in SiHa cells.
The
mechanistic basis for the differential specificity of E7 siRNA in SiHa versus
CaSki cells is at present unclear. However, it is noteworthy that the
bicistronic E6/E7 transcripts produced in SiHa and CaSki cells differ in their
junctional organisation [29,31] and in
their predicted secondary structures [29]. Secondary
structure could affect accessibility and/or progression of RNAi-associated
machinery along the bicistronic transcript. Thus it may be that an E6/E7 mRNA
boundary effect is imposed by RNA secondary structure which segregates E6 and
E7 RNAi-mediated silencing of the bicistronic transcript in SiHa cells.
RNAi-mediated
knockdown of HPV E6 and E7 in SiHa cells was further investigated using
additional siRNAs (Figure 1B). Selective knockdown of E7 mRNA was obtained
without effect on E6 mRNA levels using two independent E7 siRNAs (E7 and E7b
siRNAs, Figure 1B and 1C). Conversely, two of three E6 siRNAs selectively
targeted E6 mRNA for degradation without effect on E7 mRNA (E6 and E6b siRNAs, Figure 1B and 1C). Interestingly, a third E6 siRNA (E6c), designed to target the
junction region of the E6/E7 bi-cistronic transcript, induced knock down of
both E6 and E7 mRNA sequences (Figure 1B and 1C). Overall these results support
the concept of a boundary effect influencing RNAi-mediated degradation at the
HPV E6/E7 mRNA junction in SiHa cells.
HPV
E6 is known to target p53 for degradation and, as expected, E6 knockdown
resulted in elevated p53 protein levels and up-regulation of p53-dependent p21
expression (Figure 1D; see also [30]) but had no
effect on the phosphorylation status of the retinoblastoma protein (Rb; Figure 1E). HPV E7 on the other hand is known to induce hyperphosphorylation of Rb
and, as expected, depletion of E7 via E7 siRNA resulted in loss of
hyper-phosphorylated Rb (Rb*, Figure 1E; see also [30]) but was
without effect upon p53 protein levels or p21 expression (Figure 1D). These
combined observations are absolutely consistent with selective and individual
RNAi-mediated knock-down of HPV E6 and E7 in human cervical cancer SiHa cells.
E7 silencing has site-specific effects on global histone H3 modifications
We
next examined the epigenetic effects of HPV E6 and E7 upon global modifications
of histone H3. Such modifications represent prime targets for deregulation in
cancer and/or aging [4,5,32,33].
Depleted levels of phosphorylated S10 of histone H3 by ~3 fold were observed
24h following transfection with E7 siRNA and by 48h histone H3 S10P was barely
detectable (Figure 2A, left panel and 2D). Thus HPV16 E7 appears, directly or
indirectly, to increase global histone H3 S10 phosphorylation. In contrast,
levels of S10P H3 were unchanged 24h following transfection with E6 siRNA (Figure 2A, right panel and 2D). However, a decrease was observed by 48h and 72h and
inversely correlated with increasing p53 levels (Figure 2D). Since p53
decreases global histone H3 S10P [4,34] the
observed decrease in histone H3 S10P following HPV E6 RNAi is likely to be
attributable to p53 induction. Levels of acetylated K18 of histone H3 also fell
in response to E7 silencing (Figure 2A, left panel) but did not change in
response to E6 silencing (Figure 2A, right panel). Thus histone H3 K18
acetylation levels are selectively up-regulated by HPV E7. The changes in S10P
and K18Ac of histone H3 precede apoptosis in HPV E7-depleted cells (Figure 2A, Figure 2C for sub-G1 content; see also [30]).
Phosphorylation of histone H2AX at S139 is associated with apoptotic events [35] and levels
peaked transiently 48h post-transfection with E7 siRNA (Figure 2A) co-incident
with the onset of apoptosis ([30]; Figure 2C
and data not shown). There was no change in histone H2AX following
RNAi-mediated silencing of HPV E6 (Figure 2A).
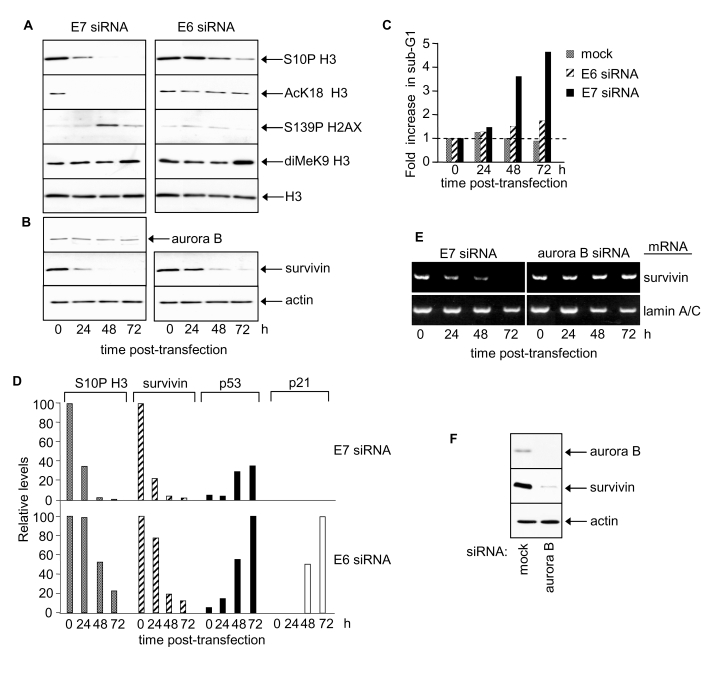
Figure 2. HPV E7 maintains survivin protein and induces site-specific changes in histone H3 modifications.
(A) Histone H3 modifications at 24, 48 and 72 h post-transfection
with E6 or E7 siRNA. Equivalent exposures for each pair of immunoblots
shown. (B) Aurora B and survivin protein levels following E6 or E7
silencing. (C) Cell death after E6 or E7 siRNA treatment. (D)
Relative changes in S10P H3, survivin, p53 and p21 proteins in response to
E7 or E6 siRNA. (E) RT-PCR determinations of survivin and lamin A/C mRNA
levels in SiHa cells as indicated. (F) Immunoblots showing effect of
aurora B silencing on survivin protein levels at 48h post siRNA
transfection.
Acetylated K9 and K14 histone H3 levels
were very low in SiHa cells and no change was detected following transfection
with either E6 or E7 siRNA (data not shown).
Treatment with trichostatin A, a class I and II histone deacetylase inhibitor,
induced a dramatic increase in both K9Ac and K14Ac of histone H3 (data not
shown) indicating high deacetylase activity at these sites in SiHa cells.
Neither E6 nor E7 significantly affected K9 methylation of histone H3 in SiHa
cells (Figure 2A).
HPV
E7 maintains elevated survivin levels in cervical cancer cells
To explore how E7 silencing might decrease S10P we examined levels of aurora B and
survivin. Aurora B is the principal histone H3 S10 kinase in human cells [36] and its
activity is regulated by survivin [37]. Aurora B
protein levels did not change in response to E7 silencing (Figure 2B).
Interestingly, however, E7 silencing depleted survivin protein levels ~5 fold by
24h post-transfection (Figure 2B and 2D), with little, if any, detectable
protein by 48 and 72h. These results identify survivin as a cellular target of
HPV16 E7 and suggest that E7 can, directly or indirectly, upregulate survivin
expression and/or stabilise survivin protein.
In
the mouse model for BRCA1 tumor suppressor function there is evidence that wild
type BRCA1 suppresses survivin expression via SIRT1-dependent epigenetic
modification of histone H3 [38]. Our own
future studies will determine if, in human cervical cancer, HPV16 E7 impacts
upon survivin expression via a SIRT1-dependent mechanism. This will be
particularly interesting given our discovery that HPV E7 up-regulates SIRT1
protein levels in human cervical cancer SiHa cells (see below).
E6
silencing caused a delayed reduction in survivin protein levels (Figure 2B and
2D). The decrease in survivin in response to E6 silencing was inversely
correlated with p53 protein levels, suggesting that this effect is mediated by
p53 (Figure 2D) [39-41]. Lamin
A/C silencing, a negative control, had no effect on survivin protein levels
(data not shown).
HPV E7 up-regulates survivin mRNA whilst aurora B sustains survivin protein levels
HPV E7 silencing also resulted in reduced mRNA levels of survivin (Figure 2E)
indicating that HPV E7 increases host cell survivin transcription and/or mRNA
half-life. Interestingly, although aurora B had no effect on survivin mRNA
levels (Figure 2E) it appeared to sustain survivin protein levels (Figure 2F).
It is possible that phosphorylation by aurora B [37,42]
stabilises survivin protein.
Growth effects of survivin and aurora B in SiHa cells
Survivin knockdown in SiHa cells resulted
in reduced cell growth but failed to induce apoptosis (Figure 3A, 3B; data not
shown). Progressive reduction in G1 cells from ~60% to ~11% was
observed 72h post-transfection (Figure 3C, quantitated by histogram
deconvolution, see Methods)
and cells accumulated in G2/M. There was also a significant increase
in the proportion of cells with >G2/M DNA content (Figure 3C,
dotted circle; and 3D), suggesting that a proportion of the G2/M
cells are still cycling but had failed to undergo cytokinesis. Indeed, two or
more nuclei were visible in many of the enlarged cells resulting from
transfection with survivin siRNA (Figure 3D).
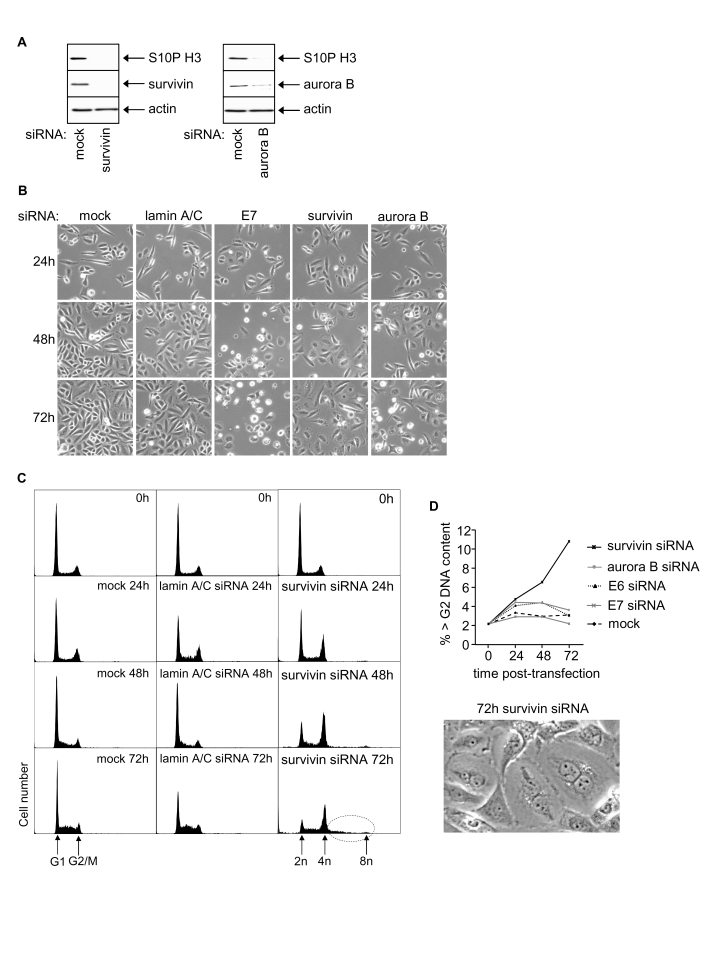
Figure 3. Effects of RNAi-mediated silencing of survivin and aurora B on S10P histone H3 and SiHa cell phenotype. (A) Survivin, aurora B and S10P
histone H3 protein levels 48h post-transfection. (B) Phase contrast
images of SiHa cells as indicated. (C) Cell cycle distribution at 0,
24, 48 and 72h post-transfection with indicated siRNAs. (D)
Induction of polyploidy in SiHa cells following survivin depletion
indicated by percentage of >G2 cells and appearance of
multinuclear cells.
HPV16 E7 up-regulates SIRT1 protein levels in cervical cancer cells
Previously we have shown that SIRT1 acts
as a cancer-specific survival factor in a range of epithelial cancer cell lines
[20]. This
raised the possibility that SIRT1 might mediate the anti-apoptotic effects of
HPV16 E7 [30]. Moreover
SIRT1 protein levels are abnormally elevated in a range of human epithelial
cell lines, including SiHa cervical cancer cells [25]. We now
demonstrate that RNAi-mediated silencing of HPV E7 in SiHa cells down-regulates
SIRT1 protein levels by ~50% 48h post-transfection with E7 siRNA (Figure 4A and
4B). In these same cells the levels of the pro-apoptotic E3 ligase Itch and anti-apoptotic c-FLIP (c-FLIPS
and c-FLIPL) were unaffected by HPV E7 knock-down (data not shown)
demonstrating that the reduction in SIRT1 protein levels reflects a selective effect
of HPV E7 depletion. In contrast to HPV E7 depletion, the depletion of HPV E6
had no effect on levels of SIRT1 protein (Figure 4A and 4B). We conclude that HPV E7, but not HPV E6, is required to maintain the abnormally
high levels of SIRT1 protein characteristically observed in SiHa cervical
cancer cells. Our results thus identify the longevity protein SIRT1 as a novel
cellular target of the viral oncogene HPV16 E7 in human cervical cancer cells.
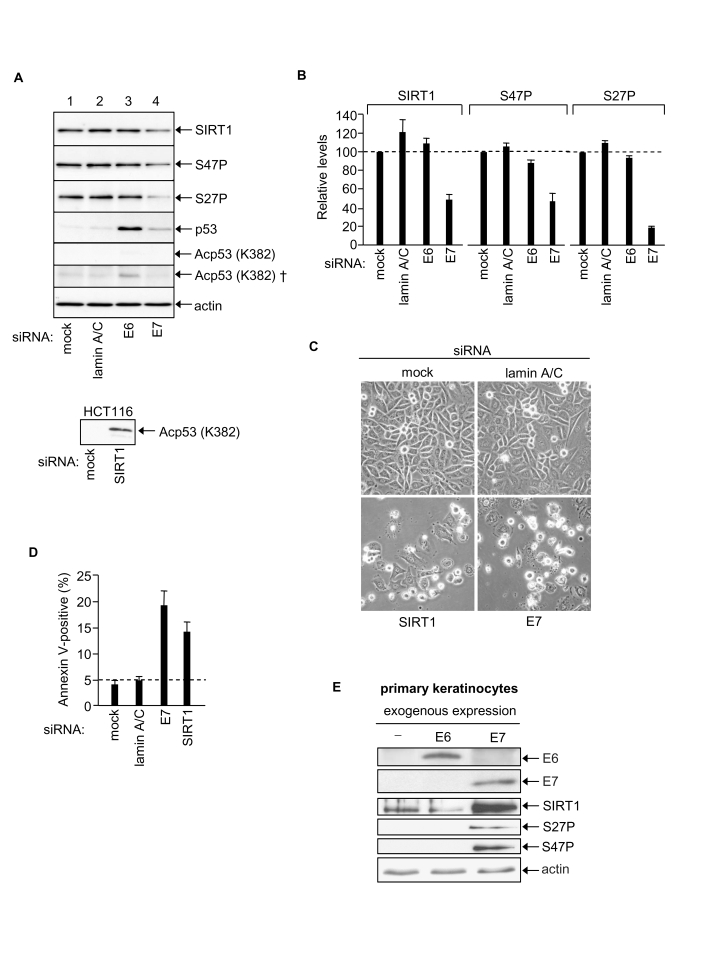
Figure 4. HPV E7 enables SiHa cervical cancer cell survival via up-regulation of SIRT1 protein levels. (A)
Equal amounts of protein analysed by immunoblotting as indicated, upper
panels SiHa cells (50 μg protein). Bottom panel HCT116 cell positive control for p53 K382Ac
detection (40 μg protein, 2 minute exposure). Note that p53 K382Ac is undetectable
in SiHa cells (5 min exposure) and requires 2 h exposure for detection (†).
(B) Relative levels of SIRT1, SIRT1 S47P and SIRT1 S27P 48h
post-transfection with indicated siRNAs, mean of two experiments. (C)
Phase contrast images of SiHa cells post-transfection with the indicated
siRNAs. (D) Apoptotic SiHa cells 48h post-transfection with the
indicated siRNAs. (E) Primary human keratinocytes 48h
post-transfection with expression vectors for HPV E6 and HPV16 E7 and
equivalent samples immunoblotted for HPV E6, HPV E7, SIRT1, SIRT1 S27P and
SIRT1 S47P.
The expression of SIRT1 in mammalian cells is subject to multiple control
mechanisms operating at the levels of transcription, transcript half-life,
translation and protein half-life [13,25,26,43].
In the present study we found that depletion of HPV E7 in SiHa cells had no
significant effect on SIRT1 mRNA levels (data not shown) suggesting that the
effect of HPV E7 on SIRT1 protein expression levels is post-transcriptional.
Recently, we have reported that SIRT1 protein stability in human colorectal
cancer cells (HCT116) is dependent upon JNK2 and is linked with SIRT1
phosphorylation at S27 [25]. A similar
effect may contribute to the enhanced level of SIRT1 in SiHa cervical cancer
cells since HPV E7 silencing caused ~ 80% reduction in SIRT1 S27P, whereas
levels of SIRT1 phosphorylation at a second site, S47, paralleled the decrease
in total SIRT1 (Figure 4A and 4B).
Depletion
of SIRT1 in SiHa cells induces apoptosis
Depletion
of SIRT1 in SiHa cells induced apoptosis (Figure 4C and 4D) demonstrating that
cellular SIRT1 enables SiHa cervical cancer cell survival. This is consistent with
our previous observation that SIRT1 functions as a cancer-specific survival
factor in a range of human epithelial cancer cell lines [20]. Our present
results indicate that HPV E7-dependent elevation of SIRT1 protein levels plays
an essential pro-survival role in human cervical cancer cells. SIRT1-mediated
suppression of apoptosis would thus explain the survival function of HPV E7 in
SiHa cells (this work and Ref. 30). Moreover, since SIRT1 is an NAD-dependent
de-acetylase with multiple cellular targets, the discovery that HPV E7
up-regulates SIRT1 protein levels identifies a new mechanism by which HPV can
access diverse SIRT1-dependent regulatory systems in human cervical
keratinocytes.
Exogenous
expression of HPV E7 increases SIRT1 protein levels in primary human
keratinocytes
The above results strongly indicate that
HPV E7 in some way targets the cellular SIRT1 protein and maintains its
expression at abnormally high levels in cervical cancer cells. The effect is
specific for HPV E7 since no effects on SIRT1 were observed following
RNAi-mediated silencing of HPV E6 under identical conditions (see above). The
HPV E7 effect on SIRT1 could be a late event in the process of malignant cell
transformation by the HPV virus. Alternatively HPV E7-mediated up-regulation of
SIRT1 might play a causative role in malignant cell transformation. In favour
of the latter alternative we show that SIRT1 suppresses apoptosis in SiHa human
cervical cancer cells (SiHa; Figure 4C and 4D).
If
HPV E7-mediated up-regulation of SIRT1 plays a causative role in malignant cell
transformation we reasoned that this effect should be detected as an early
event in the process of keratinocyte transformation. To test this primary human
keratinocytes were exposed to high level HPV E7 expression: i.e. as occurs
following integration of the HPV viral genome into host cell chromatin during
malignant transformation in vivo. For this purpose we constructed individual
expression vectors using HPV E6 and HPV E7 freshly cloned from the SiHa cells employed
throughout this study (see Materials and Methods). Primary human keratinocytes
were transfected with HPV E6 or HPV E7 expression vectors and exogenously
expressed E6 and E7 proteins were detected by immunoblotting (Figure 4E).
Samples were also probed for SIRT1 protein, SIRT1 S27P and SIRT1 S47P (in SiHa
cells SIRT1 protein is phosphorylated at both S27 and S47; [25]).
The
results clearly show that SIRT1 protein levels increase dramatically within 48h
expression of exogenous HPV E7. We have previously noted lack of detectable
SIRT1 S27 and S47 phosphorylation in primary human keratinocytes [25] and this is
confirmed here (Figure 4E). However both S27P and S47P became detectable
following exogenous expression of HPV E7. This may simply reflect a detection
threshold or, alternatively, be mechanistically linked with SIRT1 protein
accumulation (S27P correlates with SIRT1 protein half-life in HCT116 colorectal
cancer cells) [25]. It is also
possible that SIRT1 protein accumulation in response to HPV E7 involves its
relocation within the cell, and/or novel protein-protein interactions, and/or
other post-translational modifications such as sumoylation. Further studies are
required to elucidate the mechanism of SIRT1 protein accumulation in response
to HPV E7.
In
contrast to HPV E7 the exogenous expression of HPV E6 failed to induce a change
in SIRT1 protein level in primary human keratinocytes (Figure 4E; although p53
levels were depleted, data not shown). Thus increased SIRT1 protein expression
levels induced by HPV E7 (i) cannot be attributable to a cellular stress
response induced by expression of a foreign, viral protein, and (ii) cannot be
induced by HPV E6.
We
therefore conclude that HPV E7 selectively induces increased SIRT1 protein
levels and that this occurs within 48h of exogenous E7 expression in primary
human keratinocytes. Conversely, in SiHa cells naturally expressing HPV E7 via
the integrated HPV viral genome, E7 silencing induces a decrease in SIRT1
protein. Thus our overall results indicate that HPV E7 positively regulates
SIRT1 protein levels and that SIRT1 functions as a down-stream mediator of HPV
E7 in sustaining malignant cell survival.
A
new model for cell transformation by HPV E6 and E7
Previous
studies have presumed that HPV E6 is the major survival determinant in human
cervical cancer and this concept has directed anti-cancer pharmaceutical
research towards identification of agents that block the functions of HPV E6.
This has been a reasonable presumption given that HPV E6 targets the p53 tumour
suppressor protein. However, our own previous [30] and present observations
indicate that up-regulation of p53 following HPV E6 silencing is not sufficient
to induce apoptosis in human cervical cancer cells despite induction of cell
growth arrest. HPV E7 silencing, on the other hand, induces apoptosis.
Apoptosis of HPV E7-silenced SiHa cells proceeds despite the continued
suppression of p53 by HPV E6 ([30,44] and Figure 5 schematic). The ability of HPV E7 to up-regulate SIRT1 expression now
provides a mechanistic explanation for this effect since SIRT1 is
anti-apoptotic in epithelial cancer cells (Figure 5).
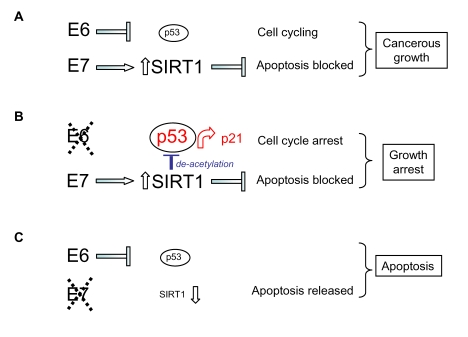
Figure 5.
Model for the
respective effects of HPV E6 and HPV E7 on human cervical cancer cell
survival and proliferation taking into account (A) up-regulation of SIRT1
protein by HPV E7, (B) SIRT1-mediated de-acetylation of p53 and (C)
SIRT1 cervical cancer cell survival functions (see text).
Moreover,
up-regulation of SIRT1 by HPV E7 also explains
the attenuated functioning of p53 following selective HPV E6 depletion since
the acetylation status of p53 under these conditions is low (Figure 4A; p53
K382Ac), consistent with its deacetylation by abnormally high levels of the
SIRT1 de-acetylase (maintained by HPV E7; Figure 5). HPV E6 silencing
nonetheless permitted up-regulation of the p53 target gene p21 ([30]; Figure 1D
and 2D) and this would account for the cell growth arrest induced by selective
silencing of HPV E6 in SiHa cells [30] (see Figure 5).
SIRT1
can function as a cancer-specific survival factor in cell lines derived from
human epithelial and other cancers. Here we report that SIRT1 is targeted for
up-regulation by the viral oncoprotein HPV E7. It is well established that HPV
E6 and E7 function as co-operating onco-proteins and drive malignancy in cervical
epithelial cells. Based on our present observations we suggest a new model for
this co-operation (Figure 5) in which targeting of cellular p53 and SIRT1 by
HPV E6 and E7 respectively enable dual access to the regulatory machinery
normally involved in cellular homeostasis and chromosomal stability. In this
way high risk HPV types subvert the SIRT1/p53 regulatory machinery from the
process of aging to the process of cancerous malignancy.
Materials and Methods
Cell
lines.
SiHa and CaSki cell lines contain the HPV16 viral
episome stably integrated into the host cell genome and were maintained and
subcultured as described [30]. SiHa
contain 1-2 integrated copies of the HPV16 episome per cell; CaSki have ~600
integrated copies per cell. Primary human keratinocytes were cultured in
defined keratinocyte media (Gibco) with appropriate supplements.
siRNA
transfection.
Cells were transfected with HPLC-purified synthetic
siRNAs (Qiagen) formulated into liposomes as described [20,30,45,46].
E6 and E7 siRNA sequences were as follows: E6 siRNA =
5'-GAGGUAUAUGACUUUGCUU(dTdT)-3'; E7 siRNA = 5'-AGGAGGAUGAAAUAGAUGG(dTdT)-3' as pub-lished
[30]. E6b siRNA
= 5'-GUUACCACAGUUAUG CACA(dTdT)-3';
E6c siRNA = 5'-AUCAUCAAGAAC ACGUAGA(dTdT)-3';
E7b siRNA = 5'-CAGAGCCCA UUACAAUAUU(dTdT)-3'.
Other siRNA sequences were: survivin siRNA = 5'-GAGCCAAGAACAAAAU UGC(dTdT)-3';
aurora B siRNA = 5'-GGUGAUGGAG AAUAGCAGU(dTdT)-3',
Itch siRNA = 5'-CCACAAC ACACGAAUUACA(dTdT)-3',
c-FLIP siRNA = 5'-GCAGUCUGUUCAAGGAGCA(dTdT)-3'. Lamin A/C and SIRT1 siRNA
sequences were as published [20].
Selectivity of the siRNAs and efficiency of silencing was confirmed as
described previously [20,25,30,45,46].
E6
and E7 cDNA cloning and exogenous expression.
Mammalian
expression vectors for HPV16 E6 and HPV16 E7 were generated by subcloning HPV16
E6 cDNA or E7 cDNA, freshly cloned from low passage SiHa cells, into the
multiple cloning site of pcDNA3.1. Generated constructs were verified by
sequencing. DNA transfection was performed using lipofectamine (Invitrogen)
according to the manufacturer's instructions as previously described [25].
mRNA
quantification.
Total cellular RNA was isolated using RNeasy kit
(Qiagen) [46]. 100ng RNA
was used for RT-PCR using Qiagen 1-Step RT-PCR kit or 50ng RNA for quantitative
real-time RT-PCR on a DNA Engine Opticon2 system (MJ) using Quantitect SYBR
Green RT-PCR kit (Qiagen). For survivin mRNA amplification primers
5'-GCATGGGTGCCCCGACGT TG-3'
and 5'-TCAATCCATGGCAGCCAGCTG-3' were used in the thermal cycle: 500C
for 30min; 950C for 15 min; 30 cycles of 940C for 30 sec,
580C for 45 sec, 720C for 1 min; followed by 720C
for 5 min. For aurora B, primers 5'-ACAGACGGCTCCATCTGGCCT -3' and 5'-TCAGGCGACAGATTGAAGGGCA-3' were
used in the thermal cycle: 500C for 30min; 950C for 15
min; 50 cycles of 940C for 30 sec, 580C for 45 sec, 720C
for 1 min; and then 720C for 5 min. Cycle parameters and primers for
HPV E6, HPV E7, SIRT1 and lamin A/C mRNAs were as previously described [20,30].
Immunoblotting.
Total cell
extracts were prepared from transfected cells [4] and
equivalent amounts resolved by 15% SDS-PAGE and electroblotted onto
nitrocellulose for immunoblotting. Antibodies used to detect specific
post-translational histone modifications were: anti-phospho S10 H3 (Cell
Signalling), anti-acetyl K18 H3 (Cell Signalling), anti-acetyl K9 H3 (Upstate
Technology), anti-acetyl K14 H3 (Upstate), anti-dimethyl K9 H3 (Upstate) and
anti-phospho S139 H2AX (Upstate). Other antibodies used were: anti-HPV16 E6
(ab70, Abcam), anti-HPV16 E7 (ED17, Santa Cruz), anti-p53 (DO1, Santa Cruz),
anti-acetylated K382 p53 (Cell Signalling), anti-SIRT1 (Abcam), anti-S27P SIRT1
(Cell Signalling), anti-S47P SIRT1 (Cell Signalling), anti-survivin
(NB500-201, Novus Biologicals), anti-aurora B (BD Biosciences), anti-p21 (BD),
anti-RB (G3-245, BD), anti-lamin A/C (636, Santa Cruz), and anti-actin
(MAB1501, Chemicon). Actin was used as a loading reference control in all
experiments. Visualisation of bound antibody was by enhanced chemiluminescence
(Roche). The intensity of bands was quantitated by densitometry using Quantity
One software (Biorad) as previously [4]. Comparative
analyses shown in Figure 2 employed data obtained from equivalent exposures
with the selected antibody (eg. S10P, survivin). We thank Dr Jack Ford for the
K382Ac antibody control (Figure 4A, bottom panel).
Cell
cycle analysis and quantitation of apoptosis.
Cell cycle
analysis, and cell aggregate discrimination and histogram deconvolution using
Cylchred software, were as previously described [4,20,45,46]. Apoptotic cells were identified by
flow cytometry using Annexin V-Fluos (Roche) following the manufacturer's
protocol [30].
Acknowledgments
This
work was funded by a Yorkshire Cancer Research programme grant (to JM).
Conflicts of Interest
The authors have no conflict of interests to declare.
References
-
1.
Finkel
T
, Serrano
M
and Blasco
MA.
The common biology of cancer and ageing.
Nature.
2007;
448:
767
-774.
[PubMed]
.
-
2.
Oberdoerffer
P
and Sinclair
DA.
The role of nuclear architecture in genomic instability and ageing.
Nat Rev Mol Cell Biol.
2007;
8:
692
-702.
[PubMed]
.
-
3.
Rubbi
CP
and Milner
J.
p53 is a chromatin accessibility factor for nucleotide excision repair of DNA damage.
EMBO J.
2003;
22:
975
-986.
[PubMed]
.
-
4.
Allison
SJ
and Milner
J.
Loss of p53 has site-specific effects on histone H3 modification, including serine 10 phosphorylation important for maintenance of ploidy.
Cancer Res.
2003;
63:
6674
-6679.
[PubMed]
.
-
5.
Allison
SJ
and Milner
J.
Remodelling chromatin on a global scale: a novel protective function of p53.
Carcinogenesis.
2004;
25:
1551
-1557.
[PubMed]
.
-
6.
Rubbi
CP
, Milner
J Gatekeeper
and caretaker
or both? In 25 years of p53 Research.
Hainaut P and Wiman KG.
Gatekeeper, caretaker or both?
25 years of p53 Research.
2005;
Springer
233
-255.
.
-
7.
Vaquero
A
, Scher
M
, Lee
D
, Erdjument-Bromage
H
, Tempst
P
and Reinberg
D.
Human SirT1 interacts with histone H1 and promotes the formation of facultative heterochromatin.
Mol Cell.
2004;
16:
93
-105.
[PubMed]
.
-
8.
Oberdoerffer
P
, Michan
S
, McVay
M
, Mostoslavsky
R
, Vann
J
, Park
S-K
, Hartlerode
A
, Stegmuller
J
, Hafner
A
, Loerch
P
, Wright
SM
, Mills
KD
and Bonni
A.
SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging.
Cell.
2008;
135:
907
-918.
[PubMed]
.
-
9.
Wang
R-H
, Sengupta
K
, Li
C
, Kim
H-S
, Cao
L
, Xiao
C
, Kim
S
, Xu
X
, Zheng
Y
, Chilton
B
, Jia
R
, Zheng
Z-M
and Appella
E.
Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice.
Cancer Cell.
2008;
14:
312
-323.
[PubMed]
.
-
10.
Luo
J
, Nikolaev
AY
, Imai
S
, Chen
D
, Su
F
, Shiloh
A
, Guarente
L
and Gu
W.
Negative control of p53 by Sir2a promotes cell survival under stress.
Cell.
2001;
107:
137
-148.
[PubMed]
.
-
11.
Vaziri
H
, Dessain
SK
, Ng
Eaton E
, Imai
SI
, Frye
RA
, Pandita
TK
, Guarente
L
and Weinberg
RA.
hSIR2(SIRT1) functions as a NAD-dependent p53 deacetylase.
Cell.
2001;
107:
149
-159.
[PubMed]
.
-
12.
Langley
E
, Pearson
M
, Faretta
M
, Bauer
UM
, Frye
RA
, Minucci
S
, Pelicci
PG
and Kouzarides
T.
Human SIR2 deacetylates p53 and antagonises PML/p53-induced cellular senescence.
EMBO J.
2002;
21:
2383
-2396.
[PubMed]
.
-
13.
Yamakuchi
M
, Ferlito
M
and Lowenstein
CJ.
miR-34a repression of SIRT1 regulates apoptosis.
Proc Natl Acad Sci USA.
2008;
105:
13421
-13426.
[PubMed]
.
-
14.
Campisi
J
Aging and cancer cell biology, 2008.
Aging Cell.
2008;
7:
281
-284.
[PubMed]
.
-
15.
Rodier
F
, Campisi
J
and Bhaumik
D.
Two faces of p53: aging and tumor suppression.
Nucleic Acids Res.
2007;
35:
7475
-7484.
[PubMed]
.
-
16.
Blander
G
and Guarente
L.
The Sir2 family of protein deacetylases.
Annu Rev Biochem.
2004;
73:
417
-435.
[PubMed]
.
-
17.
Haigis
MC
and Guarente
LP.
Mammalian sirtuins - emerging roles in physiology, aging, and calorie restriction.
Genes & Dev.
2006;
20:
2913
-2921.
[PubMed]
.
-
18.
Brooks
CL
and Gu
W.
How does SIRT1 affect metabolism, senescence and cancer.
Nat Rev Cancer.
2009;
9:
123
-128.
[PubMed]
.
-
19.
Firestein
R
, Blander
G
, Michan
S
, Oberdoerffer
P
, Ogino
S
, Campbell
J
, Bhimavarapu
A
, Luikenhuis
S
, de Cabo
R
, Fuchs
C
, Hahn
WC
, Guarente
LP
and Sinclair
DA.
The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth.
PLoS ONE.
2008;
3:
e2020
[PubMed]
.
-
20.
Ford
J
, Jiang
M
and Milner
J.
Cancer-specific functions of SIRT1 enable human epithelial cancer cell growth and survival.
Cancer Res.
2005;
65:
10457
-10463.
[PubMed]
.
-
21.
Sinclair
DA
and Guarente
L.
Extrachromosomal rDNA circles - a cause of aging in yeast.
Cell.
1997;
91:
1033
-1042.
[PubMed]
.
-
22.
Cohen
HY
, Miller
C
, Bitterman
KJ
, Wall
NR
, Hekking
B
, Kessler
B
, Howitz
KT
, Gorospe
M
, de Cabo
R
and Sinclair
DA.
Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase.
Science.
2004;
305:
390
-392.
[PubMed]
.
-
23.
Bordone
L
, Cohen
D
, Robinson
A
, Motta
MC
, van
Veen E
, Czopik
A
, Steele
AD
, Crowe
H
, Marmor
S
, Luo
J
, Gu
W
and Guarente
L.
SIRT1 transgenic mice show phenotypes resembling calorie restriction.
Aging Cell.
2007;
6:
759
-767.
[PubMed]
.
-
24.
Vijg
J
, Maslov
AY
and Suh
Y.
Aging: a sirtuin shake-up.
Cell.
2008;
135:
797
-798.
[PubMed]
.
-
25.
Ford
J
, Ahmed
S
, Allison
S
, Jiang
M
and Milner
J.
JNK2-dependent regulation of SIRT1 protein stability.
Cell Cycle.
2008;
7:
3091
-3097.
[PubMed]
.
-
26.
Kwon
H-S
and Ott
M.
The ups and downs of SIRT1.
Trends Biochem Sci.
2008;
33:
517
-525.
[PubMed]
.
-
27.
Levine
AJ
The common mechanism of transformation by the small DNA tumor viruses: The inactivation of tumor suppressor gene products: p53.
Virology.
2009;
384:
285
-293.
[PubMed]
.
-
28.
zur Hausen
H
Papillomaviruses causing cancer: evasion from host-cell control in early events in carcinogenesis.
J Natl Cancer Inst.
2000;
92:
690
-698.
[PubMed]
.
-
29.
Jeon
S
and Lambert
PF.
Integration of human papillomavirus type 16 DNA into the human genome leads to increased stability of E6 and E7 mRNAs: implications for cervical carcinogenesis.
Proc Natl Acad Sci USA.
1995;
92:
1654
-1658.
[PubMed]
.
-
30.
Jiang
M
and Milner
J.
Selective silencing of viral gene expression in HPV-positive human cervical carcinoma cells treated with siRNA, a primer of RNA interference.
Oncogene.
2002;
21:
6041
-6048.
[PubMed]
.
-
31.
Baker
CC
, Phelps
WC
, Lindgren
V
, Braun
MJ
, Gonda
MA
and Howley
PM.
Structural and transcriptional analysis of human papillomavirus type 16 sequences in cervical carcinoma cell lines.
J Virol.
1987;
61:
962
-971.
[PubMed]
.
-
32.
Ting
AH
, McGarvey
KM
and Baylin
SB.
The cancer epigenome - components and functional correlates.
Genes & Dev.
2006;
20:
3215
-3231.
[PubMed]
.
-
33.
Fraga
MF
and Esteller
M.
Epigenetics and aging: the targets and the marks.
Trends in Genetics.
2007;
23:
413
-418.
[PubMed]
.
-
34.
Warnock
LJ
, Adamson
R
, Lynch
CJ
and Milner
J.
Crosstalk between site-specific modifications on p53 and histone H3.
Oncogene.
2008;
27:
1639
-1644.
[PubMed]
.
-
35.
Rogakou
EP
, Nieves-Niera
W
, Boon
C
, Pommier
Y
and Bonner
WM.
Initiation of DNA fragmentation during apoptosis induces phosphorylation of H2AX histone at serine 139.
J Biol Chem.
2000;
275:
9390
-9395.
[PubMed]
.
-
36.
Ota
T
, Suto
S
, Katayama
H
, Han
Z-B
, Suzuki
F
, Maeda
M
, Tanino
M
, Terada
Y
and Tatsuka
M.
Increased mitotic phosphorylation of histone H3 attributable to AIM-1/Aurora B overexpression contributes to chromosome number instability.
Cancer Res.
2002;
62:
5168
-5177.
[PubMed]
.
-
37.
Chen
J
, Jin
S
, Tahir
SK
, Zhang
H
, Liu
X
, Sarthy
AV
, McGonigal
TP
, Liu
Z
, Rosenberg
SH
and Ng
S-C.
Survivin enhances aurora-B kinase activity and localizes aurora-B in human cells.
J Biol Chem.
2003;
278:
486
-490.
[PubMed]
.
-
38.
Wang
R-H
, Zheng
Y
, Kim
H-S
, Xu
X
, Cao
L
, Luhusen
T
, Lee
M-H
, Xiao
C
, Vassilopoulos
A
, Chen
W
, Gardner
K
, Man
Y-G
and Hung
M-C.
Interplay among BRCA1, SIRT1, and survivin during BRCA1-associated tumorigenesis.
Mol Cell.
2008;
32:
11
-20.
[PubMed]
.
-
39.
Hoffman
WH
, Biade
S
, Zilfou
JT
, Chen
J
and Murphy
M.
Transcriptional repression of the anti-apoptotic survivin gene by wild-type p53.
J Biol Chem.
2002;
277:
3247
-3257.
[PubMed]
.
-
40.
Mirza
A
, McGuirk
M
, Hockenberry
TN
, Wu
Q
, Ashar
H
, Black
S
, Wen
SF
, Wang
L
, Kirschmeier
P
, Bishop
WR
, Nielsen
LL
, Pickett
CB
and Liu
S.
Human survivin is negatively regulated by wild-type p53 and participates in p53-dependent apoptotic pathway.
Oncogene.
2002;
2002:
2613
-2622.
[PubMed]
.
-
41.
Zhou
M
, Gu
L
, Li
F
, Zhu
Y
, Woods
WG
and Findley
HW.
DNA damage induces a novel p53-survivin signaling pathway regulating cell cycle and apoptosis in acute lymphoblastic leukemia cells.
J Pharmacol Exp Ther.
2002;
303:
124
-131.
[PubMed]
.
-
42.
Wheatley
SP
, Henzing
AJ
, Dodson
H
, Khaled
W
and Earnshaw
WC.
Aurora-B phosphorylation in vitro identifies a residue of survivin that is essential for its localization and binding to inner centromere protein (INCEP) in vivo.
J Biol Chem.
2004;
279:
5655
-5660.
[PubMed]
.
-
43.
Milner
J
Cellular regulation of SIRT1.
Curr Pharm Des.
2009;
15:
39
-44.
[PubMed]
.
-
44.
Nauenburg
S
, Zwerschke
W
and Jansen-Durr
P.
Induction of apoptosis in cervical carcinoma cells by peptide aptamers that bind to the HPV-16 E7 oncoprotein.
FASEB J.
2001;
15:
592
-594.
[PubMed]
.
-
45.
Jiang
M
and Milner
J.
Bcl-2 constitutively suppresses p53-dependent apoptosis in colorectal cancer cells.
Genes & Dev.
2003;
17:
832
-837.
[PubMed]
.
-
46.
Allison
SJ
and Milner
J.
SIRT3 is pro-apoptotic and participates in distinct basal apoptotic pathways.
Cell Cycle.
2007;
6:
2669
-2678.
[PubMed]
.
-
47.
Tang
Y
, Zhao
W
, Chen
Y
, Zhao
Y
and Gu
W.
Acetylation is indispensable for p53 activation.
Cell.
2008;
133:
612
-626.
[PubMed]
.