Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1α in skeletal muscle
Abstract
SIRT3 is a member of the sirtuin family of NAD+-dependent deacetylases, which is localized to the mitochondria and is enriched in kidney, brown adipose tissue, heart, and other metabolically active tissues. We report here that SIRT3 responds dynamically to both exercise and nutritional signals in skeletal muscle to coordinate downstream molecular responses. We show that exercise training increases SIRT3 expression as well as associated CREB phosphorylation and PGC-1α up-regulation. Furthermore, we show that SIRT3 is more highly expressed in slow oxidative type I soleus muscle compared to fast type II extensor digitorum longus or gastrocnemius muscles. Additionally, we find that SIRT3 protein levels in skeletal muscle are sensitive to diet, for SIRT3 expression increases by fasting and caloric restriction, yet it is decreased by high-fat diet. Interestingly, the caloric restriction regimen also leads to phospho-activation of AMPK in muscle. Conversely in SIRT3 knockout mice, we find that the phosphorylation of both AMPK and CREB and the expression of PGC-1α are down regulated, suggesting that these key cellular factors may be important components of SIRT3-mediated biological signals in vivo.
Introduction
The sirtuin family of
proteins possesses NAD+-dependent deacetylase activity and/or ADP ribosyltransferase activity. The seven mammalian sirtuins
(SIRT1-7) sirtuins (SIRT1-7) are localized
differentially within the cell and have a variety of functions [1,2]. SIRT1 is the most extensively studied member of the family and
regulates diverse biological processes ranging from DNA repair and genome
stability to glucose and lipid homeostasis [3,4]. Although three specific sirtuins, SIRT3-5, are found in the
mitochondria [5,6], not much is known about their function in vivo [7]. SIRT4 has been shown to regulate amino acid-stimulated insulin
secretion by targeting glutamate
dehydrogenase [8], and it was recently demonstrated that SIRT5 participates in the
urea cycle [9]. Among the mitochondrial sirtuins, SIRT3 possesses the most
robust deacetylase activity [10-12]. Indeed, significantly higher levels of mitochondrial
protein acetylation were detected in the livers of SIRT3-null mice, compared to
those of SIRT4 or SIRT5 knockout animals [13]. However,
little is known about the physiological role of SIRT3 despite the fact that a
number of SIRT3 substrates and co-precipitating proteins have been identified:
acetyl-CoA synthetase 2 [14], Ku70 [15], FOXO3a [16], subunit 9
of mitochondrial Complex I(NDUFA9) [17], glutamate
dehydrogenase [13,18] and isocitrate dehydrogenase 2 [18].
SIRT3 has been linked to longevity in men [19,20] and aberrant expression of this sirtuin correlates with
node-positive breast cancer in clinical biopsies from women [21]—suggesting
that SIRT3 serves as an important diagnostic and therapeutic target in human
health/aging and disease, affecting men and women in unique ways. In human
cells, we have shown that SIRT3, along with SIRT4, is required for
Nampt-mediated cell survival after genotoxic stress, wherein maintenance of
mitochondrial NAD+ levels inhibits apoptosis [22]. Previously
we also reported in murine brown adipose tissue that the RNA level of SIRT3
increases by cold exposure and caloric restriction (CR) and that constitutive
expression of SIRT3, in brown pre-adipocytes, stimulates downstream
CREB-mediated expression of PGC-1α and other
mitochondrial-related genes [10]. In this
study, we investigated the physiological conditions that regulate SIRT3 in
skeletal muscle, a metabolically active organ vital for insulin-mediated
glucose disposal and lipid catabolism. Notably, skeletal muscle strongly
influences whole-body lipid metabolism, as lipid catabolism provides up to 70%
of the energy usage for resting muscle [23]. In this
tissue, the balance between fatty acid availability and fatty acid oxidation
rates plays an important role in regulating insulin responses, and
intramuscular fatty acid metabolites like diacylglycerol may cause insulin
resistance [24]. Therefore, studying the role of molecular factors and
pathways acting in muscle under various dietary and environmental conditions
will be critical for better understanding metabolism, health, and disease.
In skeletal muscle, the peroxisome proliferator-activated receptor
gamma coactivator-1α (PGC-1α), a nuclear receptor co-activator, plays
multiple roles in metabolic regulation [25,26]. It stimulates mitochondrial biogenesis [27], induces muscle fiber-type switch, and increases oxidative
capacity in skeletal muscle cells [28]. In addition to transcriptional activation by CREB [29], it has been shown that
AMP-activated protein kinase (AMPK) also increases
PGC-1α expression [30,31] and
activates it by direct phosphorylation [32]. AMPK is also a key molecular sensor and regulator of
muscle metabolism.
AMPK is a ubiquitous heterotrimeric
serine/threonine protein kinase, which functions as a fuel sensor in many
tissues, including skeletal muscle [33]. AMPK is allosterically activated by AMP and by phosphorylation
at Thr172 in the catalytic α-subunit, mainly by
an upstream AMPK kinase, LKB1 [34,35]. Importantly, AMPK is stimulated by cellular stresses that
deplete ATP and elevate AMP, such as diet restriction/hypoglycemia [36], exercise [37], and muscular contraction [38]. Activated AMPK stimulates ATP-generating catabolic pathways,
such as cellular glucose uptake and fatty acid α-oxidation.
AMPK activation also represses ATP-consuming processes, such as lipogenesis, to
restore intracellular energy balance [33,39].
Our work seeks to further
elucidate the role of sirtuins within health and disease, with particular focus
on muscle tissue in this study. We report here that expression of SIRT3 in skeletal
muscle is sensitive to various signals from both diet and exercise, leading to
downstream activation of AMPK and up-regulation of PGC-1α. SIRT3 is therefore a potential
key regulator of skeletal muscle biology, responding to important environmental
cues and activating cellular factors in vivo.
Results
SIRT3 is regulated in
skeletal muscle by exercise
training
We first assayed the SIRT3 expression
profile in vivo to compare the whole-body distribution of SIRT3,
specifically across muscles to tissues like adipose and kidney, where SIRT3 has
been previously described. As predicted,
the SIRT3 tissue distribution pattern mirrors that of SIRT3 mRNA [11]. Indeed,
SIRT3 exhibits high expression in important metabolically active tissues like
kidney, brown fat, liver, and brain (Figure 1). When comparing expression
across muscle samples, we noticed that SIRT3 protein levels were higher in the
slow-twitch soleus muscle compared to the fast-twitch muscles like extensor
digitorum longus and gastrocnemius, in agreement with higher mitochondrial
content and the oxidative feature of the soleus muscle.
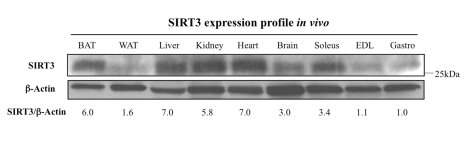
Figure 1. Tissue distribution of SIRT3 protein.
The SIRT3
protein is abundantly expressed in the brown adipose tissue (BAT), liver,
kidney, heart, brain, and soleus muscle, but very low in white adipose
tissue (WAT), the extensor digitorum longus muscle (EDL), or the
gastrocnemius muscle (Gastro). For each sample, 50 μg of protein was
loaded into a 10% acrylamide gel, electrophoresed, and transferred to a
nitrocellulose membrane. The membrane was probed using an anti-SIRT3 serum
or an anti-β-actin antibody. Blots were quantified with ImageQuant and
SIRT3/actin ratios are provided; since gastrocnemius (Gastro) has the
lowest SIRT3 expression in vivo, normalization (l.0) was set with
respect to this tissue.
To study the role of SIRT3 in muscle
within the context of exercise biology, we next tested if SIRT3 protein levels
were sensitive to an established voluntary exercise protocol [40]. Using a
specific anti-mouse SIRT3 polyclonal antibody, we found that SIRT3 protein increased selectively in triceps, the muscle
that undergoes training in the wheel-caged system, but not in cardiac muscle
samples from those same animals (Figure 2A). In contrast to SIRT3, exercise
training failed to alter SIRT1 protein levels in triceps (data not shown). The
specificity of our antibody for detecting the endogenous ~28kDa SIRT3 protein
was confirmed by using SIRT3 knockout tissue lysates (Supplemental Figure 1).
Notably, induction of SIRT3 in skeletal muscle was higher in female mice when
compared to that of male littermates (Figure 2B). In agreement with this
up-regulation, we also observed increased SIRT3 levels in the gastrocnemius
muscle of rats exercised on a treadmill-based exercise paradigm [41]
(Supplemental Figure 2). Even one week of treadmill training was sufficient to
increase SIRT3 protein amount (Supplemental Figure 2B). The up-regulation of
SIRT3 (Figure 2B) correlated with enhanced downstream phosphorylation of CREB
at Ser133 (Figure 2C) and PGC-1α induction (Figure 2D). Lastly,
citrate synthase activity, a mitochondrial marker for exercise training, was
significantly higher in trained muscles than in the respective sedentary
control group (Figure 2E). Collectively, these data suggest that the up-regulation
of SIRT3 by exercise is an important and conserved molecular consequence of
training.
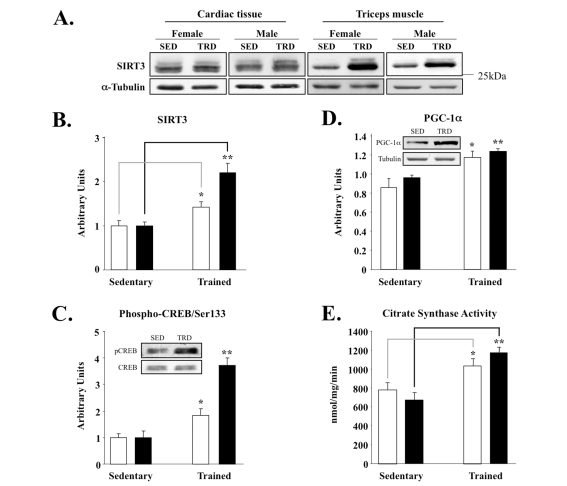
Figure 2. Skeletal muscle-specific induction of SIRT3 and associated factors in exercise-trained mice. (A) Triceps or cardiac
muscle tissue was homogenized and 50 μg of protein was analyzed by
Western blot, using anti-SIRT3 serum (Covance) and α-tubulin control; representative
blots are shown here and throughout. SED = sedentary and TRD = trained. (B)
Quantification of SIRT3 band intensities using ImageQuant from blots with
animals grouped by sex. Males are plotted as clear bars and females as
shaded bars. Total number of animals used per cohort and graphed are as
follows: sedentary males, N = 7; sedentary females, N = 5; exercised males,
N = 8; exercised females, N = 6. (C) Phospho-CREB/Ser133 and total
CREB protein. Band intensities of phospho-CREB and CREB were quantified and
phospho-CREB content was normalized relative to total CREB content; inset
provides sample blots of male triceps tissue. (D) Induction of PGC-1α correlates with enhanced SIRT3
expression in triceps; samples processed and analyzed, as above. Inset
blots are of male triceps tissue. (E) Citrate synthase activity was
measured as a mitochondrial marker from the same triceps samples, as
described previously [40]. N = 2, *P <
0.05, **P < 0.01.
SIRT3 expression in skeletal muscle is sensitive to
dietary intake
Previously
we had demonstrated how CR stimulates the in vivo expression of SIRT3 in
brown fat [10]. Thus we
hypothesized that perhaps SIRT3 expression in muscles is also sensitive to
nutritional signals, especially given how different muscles contain various
levels of SIRT3 (Figure 1) and vary inherently with respect to
energetic/metabolic potential. To test this hypothesis, we measured the SIRT3
levels in leg muscles of mice in either CR or ad libitum (AL) cohorts
after twelve months. CR is an effective
environmental method known to extend lifespan in a number of model organisms,
from yeast and nematodes to rodents, yet the underlying molecular mechanisms by
which this pathway acts in vivo remain largely unknown [42].
Here we
found that the CR diet significantly increased levels of SIRT3 protein in
skeletal muscle, compared to the AL control diet (Figure 3A). In addition,
twenty four hours of fasting was sufficient to induce the muscle expression of
SIRT3 (Figure 3B). Conversely, SIRT3 protein level was significantly decreased
following three months of energy-dense, high-fat feeding (Figure 3C),
indicating that the SIRT3 expression in muscle fluctuates in response to
dietary nutrient uptake. We next measured the effect of CR on AMPK—an enzyme
whose activity is dependent on changes in metabolic/energetic potential [30,31].
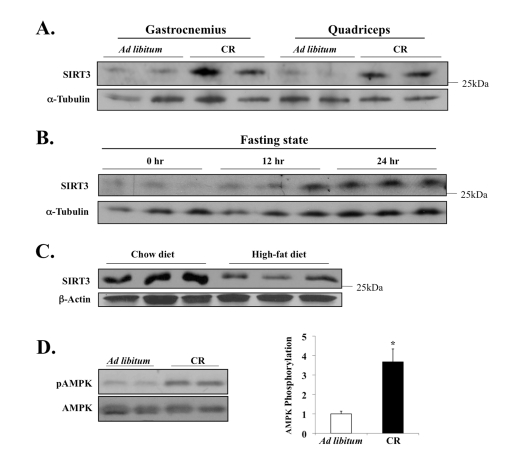
Figure 3. Diet-sensitive expression of SIRT3 and AMPK in muscle tissue. (A) Mice
were fed NIH-31 standard feed ad libitum or NIH-31/NIA-fortified
diet (Harlan Teklad) with a daily food allotment of 60% of the control mice
to establish caloric restriction (CR); twelve months after the onset of CR,
tissues were harvested to examine SIRT3 expression. (B) Mice were
deprived of food for 24 hours, and SIRT3 level in EDL muscle was determined
by Western blot analysis. (C) SIRT3 protein expression is decreased
in murine hind-leg muscle after 3 months of high-fat diet feeding; total
hind-leg tissue protein was isolated and analyzed. (D) AMPK T-172
phosphorylation and AMPK total protein in the quadriceps of the caloric
restricted mice were assayed; AMPK phosphorylation was determined as
phospho-AMPK normalized by total AMPK. N=3, *P < 0.05.
Since AMPK is activated during decreased
energy levels, we hypothesized that AMPK may be activated under CR. In
nematodes, for example, it has recently been shown that AMPK is critical for
mediating key downstream biological effects that enable lifespan extension by
caloric/dietary restriction [43]. Our data here
show that AMPK is hyper-phospho-activated at Thr172
of its catalytic α-subunit, which was quantified and
determined to be three to four times higher than the AL control diet
(Figure 3D). Together these data provided novel connections between caloric
intake, SIRT3 and AMPK that merit more analysis.
Loss of SIRT3 significantly
impacts activation of AMPK, CREB and PGC-1α expression
We next tested if
the lack of SIRT3 would impact AMPK and other
related factors like CREB and/or PGC-1α in skeletal muscle. Consistent
with our previous data, we found that SIRT3-null animals had 50% lower levels
of AMPK phosphorylation compared to the wild-type littermate control group
(Figure 4A). In our exercise model (Figure 2A-D), SIRT3 up-regulation enhanced downstream activation of CREB and
PGC-1α. Accordingly, in
the SIRT3-null mice, activating phosphorylation of CREB at Ser122 was also
reduced (Figure 4B), which correlated with lowered transcriptional activation
of pgc-1α (Figure 4C). This result is consistent with previously published data,
which show that both AMPK and CREB activate pgc-1α
expression in vivo [29].
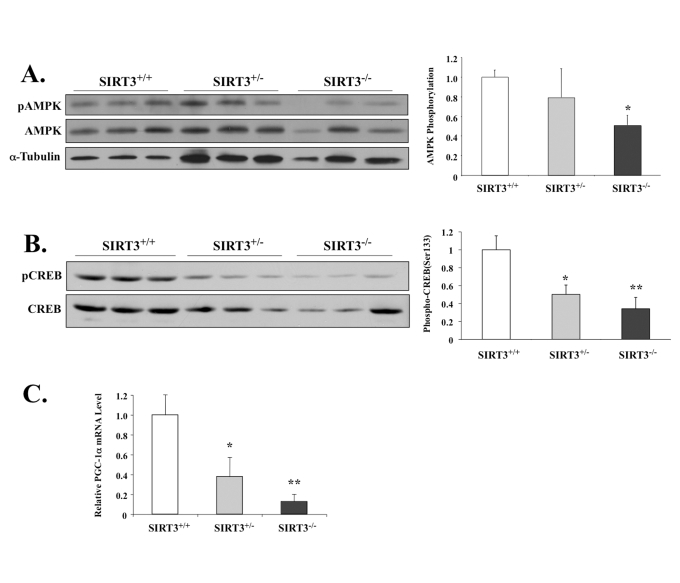
Figure 4. SIRT3-deficient mice have lower phosphorylation levels of AMPK and CREB, as well as decreased PGC-1α mRNA.
(A) AMPK T-172 phosphorylation and AMPK total protein in
the EDL muscles of the male wild-type mice or mice with heterozygous
or homozygous SIRT3 gene deficiency were determined by Western
blot analysis. AMPK phosphorylation was determined as phospho-AMPK
normalized by total AMPK. N=3, *P < 0.05. (B) CREB phosphorylation
and CREB total protein in the EDL muscles of the wild-type mice
or mice with heterozygous or homozygous SIRT3 gene deficiency
were determined by Western blotting analysis. CREB phosphorylation
was determined as phospho-CREB normalized by total CREB. N=3, *P < 0.05, **P<0.01.
(C) Quatitative RT-PCR shows pgc-1α mRNA level reduced
in the gastrocnemius of SIRT3 knockout mice. *P < 0.05, **P<0.01.
Discussion
Here we have found that SIRT3 is
differentially expressed in vivo, with the greatest expression observed
in metabolically active tissues like skeletal muscle, where SIRT3 undergoes
dynamic regulation by different environmental regimens. SIRT3 protein level is
decreased by high-fat feeding, while it is increased by short-term fasting
(24-hour) or long-term nutrient deprivation (12-month CR) and exercise
training. In this study we also show that loss of SIRT3 significantly inhibits
AMPK and CREB phosphorylation, which decreases PGC-1α transcriptional expression in muscle. Consequently, we propose a new
model in which SIRT3 leads to potential downstream changes in response to
important environmental signals (Figure 5). This model suggests that SIRT3 levels may respond to various
nutritional/energetic and physiological challenges by regulating muscle energy
homeostasis via factors like AMPK and PGC-1α.
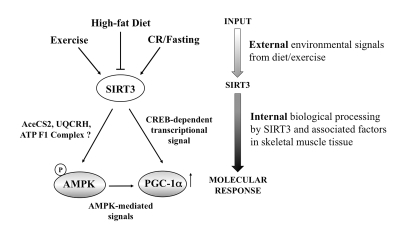
Figure 5. Schematic diagram of potential SIRT3 action in the skeletal myocyte. Collectively,
our data support a working model in which SIRT3 responds dynamically to
various nutritional and physiological signals to potentially impact muscle
energy homeostasis via AMPK and PGC-1α. Since AMPK can also phosphorylate and activate
CREB [52], SIRT3 may activate CREB directly or through AMPK. Given its
dynamic role, SIRT3 action within the skeletal muscle cells may serve as an
important diagnostic and therapeutic target for impacting human health and
disease.
Given our study, it will be interesting to test
whether SIRT3-null animals show any defects under certain environmental
challenges. Despite the hyper-acetylation of mitochondrial proteins in SIRT3
knockout mice, the significance of these biochemical changes is unclear. A
recent study of SIRT3-deficient mice by another group did not find defects in
basal metabolism nor adaptive thermogenesis, while the mice were housed in
standard dietary/sedentary conditions [13]. Similarly,
we found normal treadmill performance in SIRT3 knockout mice while under
standard housing (unpublished observations). Upon challenge with various
environmental signals, however, these animals may respond differently.
Accordingly, we are actively testing how challenges by CR/fasting/high-fat diet
and exercise may affect the SIRT3-null mice and alter key downstream cellular
factors in muscle cells.
The mechanism(s) by which
different environmental variables modulate SIRT3 and activate AMPK in muscle (and other tissues that highly express SIRT3) remains
to be fully elucidated. For example,
activation of acetyl-CoA synthetase 2 (AceCS2) by SIRT3 [44,45] may elevate the AMP/ATP ratio
and consequently activate AMPK. Alternatively, a recent proteomics-based
approach has identified many novel SIRT3-interacting partners in human cells,
including the ATP synthase (mitochondrial F1 complex) alpha/beta subunits and
the ubiquinol-cytochrome
c reductase hinge protein, UQCRH [46]. Since
these proteins (together with NDUFA9 [17]) serve as critical components of
the ATP-generating machinery in cells, SIRT3 may also potentially modulate the
AMP/ATP ratio via these factors to activate AMPK. Moreover, we too have
purified additional putative SIRT3-binding proteins from HeLa cells (using a related
cross-linking/immunoaffinity purification method [47]), which include mitochondrial
acetoacetyl CoA thiolase (also referred to as α-ketothiolase), malate dehydrogenase, thioredoxin
2 (Trx-2), Hsp60, and lactate dehydrogenase (unpublished data). Since some of
these enzymes are important regulators of muscle energy homeostasis, our data
further substantiate that SIRT3 may modulate ATP/energy levels via key
targets to activate AMPK. It is intriguing to note that a study of an
independent line of SIRT3-knockout mice indicated that the ATP level is
significantly reduced in several tissues [17], although the effect of SIRT3
deficiency on muscle ATP level has not been reported.
Interestingly, it has also
been shown that activation of AMPK, upon glucose nutrient restriction of muscle
stem cells, causes an increase in the cellular NAD+/NADH ratio,
consistent with a positive feedback loop needed for prolonged SIRT1 activation [48], as may occur in our SIRT3 model and merits testing. Indeed a
second in vitro study independently validates a similar NAD+/NADH
model via AMPK [49]. Strikingly, AMPK activation (as occurs with CR) may also result in
lifespan extension [50-52], and future study will reveal if SIRT3 is involved in this
process. It is known that activated AMPK
directly phosphorylates PGC-1α[32] and CREB [53]—and that
both AMPK and CREB are involved in the transactivation of PGC-1a [54,55].
Lastly, both SIRT3 and SIRT1 promote mitochondrial
biogenesis and fatty acid oxidation via PGC-1α but in different ways. SIRT3 promotes PGC-1α expression while SIRT1 activates PGC-1α by direct deacetylation [56]. However, we have found that exercise training regulates SIRT3
but not SIRT1 expression in muscle. At present, it remains to be considered how
these two key sirtuin enzymes may work cooperatively within certain tissues in
response to environmental signals.
Furthermore, it is important to consider
that a phenotype may be tissue-specific, especially if SIRT3 has different
biological roles in the body. For example, in the rennin-angiotensin system,
which plays a key role in the pathophysiology of cardiac and renal disease in
humans, targeted disruption of the angiotensin receptor (Agtr1a
gene in mice) yields animals with less cardiac and vascular injury, prolonged
lifespan, increased number of mitochondria, and dramatic up-regulation of SIRT3
in kidney tissue—a possible site of SIRT3 action that may contribute, at least
in part, to the phenotype that is observed [57]. Another
interesting place of molecular action is in brown adipose tissue (BAT), in
which SIRT3 has been previously shown to respond dynamically to CR and regulate
fat cell physiology via PGC-1α [10]. With the
recent discovery of BAT in humans (reviewed in [58]), there are
now new opportunities to explore the role of SIRT3 in diabetes and obesity
research [59]. Moreover,
after intense swimming, it has been reported that the expression of SIRT3 and
PGC-1α increases in white blood cells to activate the
antioxidant response [60]. Lastly, in
human skeletal muscle, it has been reported that SIRT3 and PGC-1α expression decline with age and correlate with a sedentary proteomic
profile found in people with decreased metabolic output [61]. With
exercise, however, these authors observed that the effect is reversed.
Collectively, these data suggest that SIRT3 function is perhaps varied
throughout the body and specialized to meet the unique metabolic/energetic
capacities found within various tissues, particularly in response to
environmental cues.
Thus it will be interesting
to test whether inducible tissue-specific SIRT3-null mice show global metabolic
defects from exercise and/or diet regimens in various parts of the body,
especially with aging. This inducible genetic approach will also allow us to
bypass potential compensatory effects resulting from the lack of SIRT3 during
development. Additionally, a mouse model with increased SIRT3 over-expression
in muscle (and/or other specific tissues) will also be a valuable tool for
further elucidating the biological role(s) of this sirtuin in vivo. All
of this work will be important as we fight against aging and associated
disorders ranging from type 2 diabetes (and other metabolic diseases) to breast
cancer, in which expression of SIRT3 is aberrant. Therefore, small-molecule activators of SIRT3, currently in
development and testing [62], may provide novel and key therapeutic routes for the treatment
of a variety of common diseases, perhaps by mimicking the beneficial molecular
effects of exercise and/or caloric restriction in vivo.
Materials and Methods
Animals,
diet and exercise.
Ethics statement: Protocols
for animal use were in accordance with the guidelines of the Institutional
Animal Care and Use Committees of Baylor College of Medicine and the Joslin
Diabetes Center and the National Institutes of Health. For the caloric
restriction experiment, C57BL/6 male mice were singly caged. At 8 weeks of age,
control mice were fed ad libitum with NIH-31 standard diet (Harlan
Teklad), while food consumption was measured daily. Caloric restricted mice
were fed with NIH-31/NIA-fortified diet (Harlan Teklad) with a daily food
allotment of 90%, 70% and then 60% of the amount consumed by the control
mice—at the first, second, and third week, respectively. From then on, daily
food allotment stabilized at 60% of ad libitum food intake for the
caloric restricted mice. 12 months later, mice were dissected to collect
tissues for analysis. For the fasting experiment, food was removed from 3
months old C57BL/6 male mice at 6pm for 24 hours. For the high-fat diet feeding
experiment, 8-week-old male mice were fed a control diet or a 35% fat-enriched
chow (BioServ) for three months. Various tissueswere also
harvested from mice fed the control diet to examine SIRT3 gene expression by
Western blot analysis at the termination of the study. For the
exercise study [40], 7-week-old
male and female FVB/NJ mice were wheel-cage trained for 6 weeks and fed PicoLab
Mouse Diet 20 (LabDiet/Purina). In brief, mice were housed in individual cages
with or without rodent running wheels (Nalgene,
Rochester, NY) and the animals could exercise voluntarily during a 6-week
training period. At the end of the 6 weeks, mice were euthanized, triceps
muscles were removed and subsequently analyzed for SIRT3, CREB,
phospho-CREB/Ser122, and PGC-1α protein expression [10]. Citrate synthase activity was
measured as a mitochondrial marker post-exercise training from triceps samples,
as described previously [40].
Sirt3
-knockout
mice.
Mice in which the Sirt3 gene
(Accession: NM_022433) was targeted by gene trapping were obtained from the
Texas Institute for Genomic Medicine (Houston, TX, USA). Briefly, these mice
were created by generating embryonic stem (ES) cells (Omnibank No. OST341297) with a retroviral promoter trap that
functionally inactivates one alleleof the Sirt3 gene, as
described previously [63]. Sequenceanalysis indicated that retroviral insertion occurred in the intron
preceding coding exon 2 (Supplemental Figure 1). Targeted 129/SvEvBrd embryonic
stem cells were injected into C57BL/6 albino blastocysts. The chimeras
(129/SvEvBrd) were then crossed with C57BL/6 albinos to produce the
heterozygotes. Heterozygotes were then mated and the offspring were genotyped
using PCR, containing two primers flanking the trapping cassette insertion site
TG0003-5' (ATCTCGCAGATAGGCTATCAGC) and TG0003-3'
(AACCACGTAACCTTACCCAAGG), as well as a third primer LTR
rev, a reverse primer located at the 5' end of the trapping cassette
(ATAAACCCTCTTGCAG TTGCATC). Primer pair TG0003-5' and TG0003-3' amplify
a 336bp fragment from the wild-type allele, while primer pair TG0003-5' and LTR
rev amplify a 160bp fragment from the knockout allele.
Antibodies and Western blots.
The
antibodies used for Western blot analysis included: anti-mouse SIRT3 serum
raised against the C-terminus (DLMQRERGKLD GQDR, Genemed Synthesis, Inc.)
and used for the tissue distribution and high-fat diet analyses; anti-mouse and
anti-rat SIRT3 serum was also developed against the C-terminal regions of each
respective protein (Covance), and the anti-mouse serum was validated for
specificity using brown fat, cardiac tissue, and soleus muscle from SIRT3
knockout mice (Supplemental Fig. 1), then used for analyzing the exercise
samples. Other antibodies used included the following: anti-phospho-CREB/Ser133
(Cell Signaling); anti-CREB (Cell Signaling); anti-phospho-AMPK (Cell
Signaling); AMPK (Cell Signaling); anti-PGC-1α
(Calbiochem); β-actin antibody (Santa Cruz); and α-tubulin (Abcam).
Supplementary Materials
Murine Sirt3 gene structure and inactivation. (A) Annotated
Sirt3 gene structure [62, 52], showing
retroviral insertion site for inactivation of SIRT3 in the null
mice. Lines indicate relative position of known ATG start codons;
the stop codon, TAA, is indicated in exon 7 (E7). Nomenclature
for the exon designations shown here is taken from Cooper et al. [52].
(B) SIRT3 protein levels were assayed from mice tissues
with either homozygous or heterozygous Sirt3 gene deficiency,
using standard Western blot analysis (as before).
SIRT3 up-regulation by exercise is conserved in rodents.
(A) Representative Western blot panels of mice muscle samples
used for quantification in Figure 2, and (B) rat muscle
showing that SIRT3 up-regulation occurs as early as 1-week on
a previously established treadmill-based exercise paradigm [52].
Remarkably, the molecular size of the mouse and rat SIRT3 proteins
is conserved.
Acknowledgments
We thank Dr. E.
O'Brian Smith for assistance with statistical analysis, Dr. Martin Young for valuable
discussions/suggestions, and Margaret Nguyen for technical assistance. O.M.P.
was supported by a National Institutes of Health (NIH) training grant (T32
HD007445) and J.J.C. by a Howard Hughes Medical Institute Predoctoral
Fellowship. L.J.G. received support from a NIH grant (RO1DK068626). This work
was also supported by grants to Q. T. from the U.S. Department of Agriculture
(CRIS 6250-51000-049) and the NIH (RO1DK075978).
Conflicts of Interest
The authors of this article report no conflict of
interest(s).
References
-
1.
Michishita
E
, Park
JY
, Burneskis
JM
, Barrett
JC
and Horikawa
I.
Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins.
Mol Biol Cell.
2005;
16:
4623
-4635.
[PubMed]
.
-
2.
North
BJ
and Verdin
E.
Sirtuins: Sir2-related NAD-dependent protein deacetylases.
Genome Biol.
2004;
5:
224
[PubMed]
.
-
3.
Haigis
MC
and Guarente
LP.
Mammalian sirtuins - emerging roles in physiology, aging, and calorie restriction.
Genes Dev.
2006;
20:
2913
-2921.
[PubMed]
.
-
4.
Michan
S
and Sinclair
D.
Sirtuins in mammals: insights into their biological function.
Biochem J.
2007;
404:
1
-13.
[PubMed]
.
-
5.
Hallows
WC
, Albaugh
BN
and Denu
JM.
Where in the cell is SIRT3? - functional localization of an NAD+-dependent protein deacetylase.
Biochem J.
2008;
411:
e11
-3.
[PubMed]
.
-
6.
Cooper
HM
and Spelbrink
JN.
The human SIRT3 protein deacetylase is exclusively mitochondrial.
Biochem J.
2008;
411:
279
-285.
[PubMed]
.
-
7.
Guarente
L
Mitochondria - a nexus for aging, calorie restriction, and sirtuins.
Cell.
2008;
132:
171
-176.
[PubMed]
.
-
8.
Haigis
MC
, Mostoslavsky
R
, Haigis
KM
, Fahie
K
, Christodoulou
DC
, Murphy
AJ
, Valenzuela
DM
, Yancopoulos
GD
, Karow
M
, Blander
G
, Valenzuela
DM
, Yancopoulos
GD
, Karow
M
, Blander
G
, Wolberger
C
, Prolla
TA
, Weindruch
R
, Alt
FW
and Guarente
L.
SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells.
Cell.
2006;
126:
941
-954.
[PubMed]
.
-
9.
Nakagawa
T
, Lomb
DJ
, Haigis
MC
and Guarente
L.
SIRT5 Deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle.
Cell.
2009;
137:
560
-570.
[PubMed]
.
-
10.
Shi
T
, Wang
F
, Stieren
E
and Tong
Q.
SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes.
J Biol Chem.
2005;
280:
13560
-13567.
[PubMed]
.
-
11.
Onyango
P
, Celic
I
, McCaffery
JM
, Boeke
JD
and Feinberg
AP.
SIRT3, a human SIR2 homologue, is an NAD-dependent deacetylase localized to mitochondria.
Proc Natl Acad Sci U S A.
2002;
99:
13653
-13658.
[PubMed]
.
-
12.
Schwer
B
, North
BJ
, Frye
RA
, Ott
M
and Verdin
E.
The human silent information regulator (Sir)2 homologue hSIRT3 is a mitochondrial nicotinamide adenine dinucleotide-dependent deacetylase.
J Cell Biol.
2002;
158:
647
-657.
[PubMed]
.
-
13.
Lombard
DB
, Alt
FW
, Cheng
HL
, Bunkenborg
J
, Streeper
RS
, Mostoslavsky
R
, Kim
J
, Yancopoulos
G
, Valenzuela
D
, Murphy
A
, Yang
Y
, Chen
Y
, Hirschey
MD
, Bronson
RT
, Haigis
M
, Guarente
LP
, Farese
RV Jr
, Weissman
S
, Verdin
E
and Schwer
B.
Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation.
Mol Cell Biol.
2007;
27:
8807
-8814.
[PubMed]
.
-
14.
Schwer
B
, Bunkenborg
J
, Verdin
RO
, Andersen
JS
and Verdin
E.
Reversible lysine acetylation controls the activity of the mitochondrial enzyme acetyl-CoA synthetase 2.
Proc Natl Acad Sci U S A.
2006;
103:
10224
-10229.
[PubMed]
.
-
15.
Sundaresan
NR
, Samant
SA
, Pillai
VB
, Rajamohan
SB
and Gupta
MP.
SIRT3 is a stress responsive deacetylase in cardiomyocytes that protects cells from stress-mediated cell death by deacetylation of Ku-70.
Mol Cell Biol.
2008;
6384
-6401.
[PubMed]
.
-
16.
Jacobs
KM
, Pennington
JD
, Bisht
KS
, Aykin-Burns
N
, Kim
HS
, Mishra
M
, Sun
L
, Nguyen
P
, Ahn
BH
, Leclerc
J
, Deng
CX
, Spitz
DR
and Gius
D.
SIRT3 interacts with the daf-16 homolog FOXO3a in the mitochondria, as well as increases FOXO3a dependent gene expression.
Int J Biol Sci.
2008;
4:
291
-299.
[PubMed]
.
-
17.
Ahn
BH
, Kim
HS
, Song
S
, Lee
IH
, Liu
J
, Vassilopoulos
A
, Deng
CX
and Finkel
T.
A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis.
Proc Natl Acad Sci U S A.
2008;
105:
14447
-144452.
[PubMed]
.
-
18.
Schlicker
C
, Gertz
M
, Papatheodorou
P
, Kachholz
B
, Becker
CF
and Steegborn
C.
Substrates and Regulation Mechanisms for the Human Mitochondrial Sirtuins Sirt3 and Sirt5.
J Mol Biol.
2008;
790
-801.
[PubMed]
.
-
19.
Bellizzi
D
, Dato
S
, Cavalcante
P
, Covello
G
, Di Cianni
F
, Passarino
G
, Rose
G
and De
Benedictis G.
Characterization of a bidirectional promoter shared between two human genes related to aging: SIRT3 and PSMD13.
Genomics.
2007;
89:
143
-150.
[PubMed]
.
-
20.
Bellizzi
D
, Rose
G
, Cavalcante
P
, Covello
G
, Dato
S
, De
Rango F
, Greco
V
, Maggiolini
M
, Feraco
E
, Mari
V
, Franceschi
C
, Passarino
G
and De
Benedictis G.
A novel VNTR enhancer within the SIRT3 gene, a human homologue of SIR2, is associated with survival at oldest ages.
Genomics.
2005;
85:
258
-263.
[PubMed]
.
-
21.
Ashraf
N
, Zino
S
, Macintyre
A
, Kingsmore
D
, Payne
AP
, George
WD
and Shiels
PG.
Altered sirtuin expression is associated with node-positive breast cancer.
Br J Cancer.
2006;
95:
1056
-1061.
[PubMed]
.
-
22.
Yang
H
, Yang
T
, Baur
JA
, Perez
E
, Matsui
T
, Carmona
JJ
, Lamming
DW
, Souza-Pinto
NC
, Bohr
VA
, Rosenzweig
A
, de Cabo
R
, Sauve
AA
and Sinclair
DA.
Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival.
Cell.
2007;
130:
1095
-1107.
[PubMed]
.
-
23.
Smith
AG
and Muscat
GE.
Skeletal muscle and nuclear hormone receptors: implications for cardiovascular and metabolic disease.
Int J Biochem Cell Biol.
2005;
37:
2047
-2063.
[PubMed]
.
-
24.
Yu
C
, Chen
Y
, Cline
GW
, Zhang
D
, Zong
H
, Wang
Y
, Bergeron
R
, Kim
JK
, Cushman
SW
, Cooney
GJ
, Atcheson
B
, White
MF
, Kraegen
EW
and Shulman
GI.
Mechanism by Which Fatty Acids Inhibit Insulin Activation of Insulin Receptor Substrate-1 (IRS-1)-associated Phosphatidylinositol 3-Kinase Activity in Muscle.
J Biol Chem.
2002;
277:
50230
-50236.
[PubMed]
.
-
25.
Finck
BN
and Kelly
DP.
PGC-1 coactivators: inducible regulators of energy metabolism in health and disease.
J Clin Invest.
2006;
116:
615
-622.
[PubMed]
.
-
26.
Handschin
C
and Spiegelman
BM.
Peroxisome Proliferator-Activated Receptor {gamma} Coactivator 1 Coactivators, Energy Homeostasis, and Metabolism.
Endocr Rev.
2006;
728
-735.
[PubMed]
.
-
27.
Wu
Z
, Puigserver
P
, Andersson
U
, Zhang
C
, Adelmant
G
, Mootha
V
, Troy
A
, Cinti
S
, Lowell
B
, Scarpulla
RC
and Spiegelman
BM.
Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1.
Cell.
1999;
98:
115
-124.
[PubMed]
.
-
28.
Lin
J
, Wu
H
, Tarr
PT
, Zhang
CY
, Wu
Z
, Boss
O
, Michael
LF
, Puigserver
P
, Isotani
E
, Olson
EN
, Lowell
BB
, Bassel-Duby
R
and Spiegelman
BM.
Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres.
Nature.
2002;
418:
797
-801.
[PubMed]
.
-
29.
Lopez-Lluch
G
, Irusta
PM
, Navas
P
and de Cabo
R.
Mitochondrial biogenesis and healthy aging.
Exp Gerontol.
2008;
43:
813
-819.
[PubMed]
.
-
30.
Bergeron
R
, Ren
JM
, Cadman
KS
, Moore
IK
, Perret
P
, Pypaert
M
, Young
LH
, Semenkovich
CF
and Shulman
GI.
Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis.
Am J Physiol Endocrinol Metab.
2001;
281:
1340
-1346.
.
-
31.
Zong
H
, Ren
JM
, Young
LH
, Pypaert
M
, Mu
J
, Birnbaum
MJ
and Shulman
GI.
AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation.
Proc Natl Acad Sci U S A.
2002;
99:
15983
-15987.
[PubMed]
.
-
32.
Jager
S
, Handschin
C
, St-Pierre
J
and Spiegelman
BM.
AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1{alpha}.
Proc Natl Acad Sci U S A.
2007;
104:
12017
-12022.
[PubMed]
.
-
33.
Kahn
BB
, Alquier
T
, Carling
D
and Hardie
DG.
AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism.
Cell Metab.
2005;
1:
15
-25.
[PubMed]
.
-
34.
Hawley
SA
, Boudeau
J
, Reid
JL
, Mustard
KJ
, Udd
L
, Makela
TP
, Alessi
DR
and Hardie
DG.
Complexes between the LKB1 tumor suppressor, STRADalpha/beta and MO25alpha/beta are upstream kinases in the AMP-activated protein kinase cascade.
J Biol.
2003;
2:
28
[PubMed]
.
-
35.
Woods
A
, Johnstone
SR
, Dickerson
K
, Leiper
FC
, Fryer
LG
, Neumann
D
, Schlattner
U
, Wallimann
T
, Carlson
M
and Carling
D.
LKB1 is the upstream kinase in the AMP-activated protein kinase cascade.
Curr Biol.
2003;
13:
2004
-2008.
[PubMed]
.
-
36.
Itani
SI
, Saha
AK
, Kurowski
TG
, Coffin
HR
, Tornheim
K
and Ruderman
NB.
Glucose Autoregulates Its Uptake in Skeletal Muscle: Involvement of AMP-Activated Protein Kinase.
Diabetes.
2003;
52:
1635
-1640.
[PubMed]
.
-
37.
Fujii
N
, Hayashi
T
, Hirshman
MF
, Smith
JT
, Habinowski
SA
, Kaijser
L
, Mu
J
, Ljungqvist
O
, Birnbaum
MJ
, Witters
LA
, Thorell
A
and Goodyear
LJ.
Exercise induces isoform-specific increase in 5'AMP-activated protein kinase activity in human skeletal muscle.
Biochem Biophys Res Commun.
2000;
273:
1150
-1155.
[PubMed]
.
-
38.
Hutber
CA
, Hardie
DG
and Winder
WW.
Electrical stimulation inactivates muscle acetyl-CoA carboxylase and increases AMP-activated protein kinase.
Am J Physiol.
1997;
272:
262
-266.
.
-
39.
Kemp
BE
, Stapleton
D
, Campbell
DJ
, Chen
ZP
, Murthy
S
, Walter
M
, Gupta
A
, Adams
JJ
, Katsis
F
, van
Denderen B
, Jennings
IG
, Iseli
T
, Michell
BJ
and Witters
LA.
AMP-activated protein kinase, super metabolic regulator.
Biochem Soc Trans.
2003;
31:
162
-168.
[PubMed]
.
-
40.
Rockl
KS
, Hirshman
MF
, Brandauer
J
, Fujii
N
, Witters
LA
and Goodyear
LJ.
Skeletal muscle adaptation to exercise training: AMP-activated protein kinase mediates muscle fiber type shift.
Diabetes.
2007;
56(8):
2062
-2069.
[PubMed]
.
-
41.
Koh
HJ
, Hirshman
MF
, He
H
, Li
Y
, Manabe
Y
, Balschi
JA
and Goodyear
LJ.
Adrenaline is a critical mediator of acute exercise-induced AMP-activated protein kinase activation in adipocytes.
Biochem J.
2007;
403:
473
-481.
[PubMed]
.
-
42.
Guarente
L
and Picard
F.
Calorie restriction--the SIR2 connection.
Cell.
2005;
120:
473
-482.
[PubMed]
.
-
43.
Greer
EL
, Dowlatshahi
D
, Banko
MR
, Villen
J
, Hoang
K
, Blanchard
D
, Gygi
SP
and Brunet
A.
An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans.
Curr Biol.
2007;
17:
1646
-1656.
[PubMed]
.
-
44.
North
BJ
and Sinclair
DA.
Sirtuins: a conserved key unlocking AceCS activity.
Trends Biochem Sci.
2007;
32:
1
-4.
[PubMed]
.
-
45.
Hallows
WC
, Lee
S
and Denu
JM.
Sirtuins deacetylate and activate mammalian acetyl-CoA synthetases.
Proc Natl Acad Sci U S A.
2006;
103:
10230
-10235.
[PubMed]
.
-
46.
Law
IK
, Liu
L
, Xu
A
, Lam
KS
, Vanhoutte
PM
, Che
CM
, Leung
PT
and Wang
Y.
Identification and characterization of proteins interacting with SIRT1 and SIRT3: implications in the anti-aging and metabolic effects of sirtuins.
Proteomics.
2009;
9:
2444
-2456.
[PubMed]
.
-
47.
Nakatani
Y
and Ogryzko
V.
Immunoaffinity purification of mammalian protein complexes.
Methods Enzymol.
2003;
370:
430
-444.
[PubMed]
.
-
48.
Fulco
M
, Cen
Y
, Zhao
P
, Hoffman
EP
, McBurney
MW
, Sauve
AA
and Sartorelli
V.
Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt.
Dev Cell.
2008;
14:
661
-673.
[PubMed]
.
-
49.
Canto
C
, Gerhart-Hines
Z
, Feige
JN
, Lagouge
M
, Noriega
L
, Milne
JC
, Elliott
PJ
, Puigserver
P
and Auwerx
J.
AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity.
Nature.
2009;
458:
1056
-1060.
[PubMed]
.
-
50.
McCarty
MF
Chronic activation of AMP-activated kinase as a strategy for slowing aging.
Med Hypotheses.
2004;
63:
334
-339.
[PubMed]
.
-
51.
Apfeld
J
, O'Connor
G
, McDonagh
T
, DiStefano
PS
and Curtis
R.
The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans.
Genes Dev.
2004;
18:
3004
-3009.
[PubMed]
.
-
52.
Dagon
Y
, Avraham
Y
, Magen
I
, Gertler
A
, Ben-Hur
T
and Berry
EM.
Nutritional status, cognition, and survival: a new role for leptin and AMP kinase.
J Biol Chem.
2005;
280:
42142
-42148.
[PubMed]
.
-
53.
Thomson
DM
, Herway
ST
, Fillmore
N
, Kim
H
, Brown
JD
, Barrow
JR
and Winder
WW.
AMP-activated protein kinase phosphorylates transcription factors of the CREB family.
J Appl Physiol.
2008;
104:
429
-438.
[PubMed]
.
-
54.
Handschin
C
, Rhee
J
, Lin
J
, Tarr
PT
and Spiegelman
BM.
An autoregulatory loop controls peroxisome proliferator-activated receptor gamma coactivator 1alpha expression in muscle.
Proc Natl Acad Sci U S A.
2003;
100:
7111
-7116.
[PubMed]
.
-
55.
Herzig
S
, Long
F
, Jhala
US
, Hedrick
S
, Quinn
R
, Bauer
A
, Rudolph
D
, Schutz
G
, Yoon
C
, Puigserver
P
, Spiegelman
B
and Montminy
M.
CREB regulates hepatic gluconeogenesis through the coactivator PGC-1.
Nature.
2001;
413:
179
-183.
[PubMed]
.
-
56.
Gerhart-Hines
Z
, Rodgers
JT
, Bare
O
, Lerin
C
, Kim
SH
, Mostoslavsky
R
, Alt
FW
, Wu
Z
and Puigserver
P.
Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha.
Embo J.
2007;
26:
1913
-1923.
[PubMed]
.
-
57.
Benigni
A
, Corna
D
, Zoja
C
, Sonzogni
A
, Latini
R
, Salio
M
, Conti
S
, Rottoli
D
, Longaretti
L
, Cassis
P
, Morigi
M
, Coffman
TM
and Remuzzi
G.
Disruption of the Ang II type 1 receptor promotes longevity in mice.
J Clin Invest.
2009;
119:
524
-530.
[PubMed]
.
-
58.
Farmer
SR
Obesity: Be cool, lose weight.
Nature.
2009;
458:
839
-840.
[PubMed]
.
-
59.
Capel
F
, Viguerie
N
, Vega
N
, Dejean
S
, Arner
P
, Klimcakova
E
, Martinez
JA
, Saris
WH
, Holst
C
, Taylor
M
, Oppert
JM
, Sorensen
TI
, Clement
K
, Vidal
H
and Langin
D.
Contribution of energy restriction and macronutrient composition to changes in adipose tissue gene expression during dietary weight-loss programs in obese women.
J Clin Endocrinol Metab.
2008;
93:
4315
-4322.
[PubMed]
.
-
60.
Ferrer
MD
, Tauler
P
, Sureda
A
, Tur
JA
and Pons
A.
Antioxidant regulatory mechanisms in neutrophils and lymphocytes after intense exercise.
J Sports Sci.
2009;
27:
49
-58.
[PubMed]
.
-
61.
Lanza
IR
, Short
DK
, Short
KR
, Raghavakaimal
S
, Basu
R
, Joyner
MJ
, McConnell
JP
and Nair
KS.
Endurance exercise as a countermeasure for aging.
Diabetes.
2008;
57:
2933
-2942.
[PubMed]
.
-
62.
Jin
L
, Galonek
H
, Israelian
K
, Choy
W
, Morrison
M
, Xia
Y
, Wang
X
, Xu
Y
, Yang
Y
, Smith
JJ
, Hoffmann
E
, Carney
DP
, Perni
RB
, Jirousek
MR
, Bemis
JE
, Milne
JC
, Sinclair
DA
and Westphal
CH.
Biochemical characterization, localization, and tissue distribution of the longer form of mouse SIRT3.
Protein Sci.
2009;
18(3):
514
-525.
[PubMed]
.
-
63.
Zambrowicz
BP
, Friedrich
GA
, Buxton
EC
, Lilleberg
SL
, Person
C
and Sands
AT.
Disruption and sequence identification of 2,000 genes in mouse embryonic stem cells.
Nature.
1998;
392:
608
-611.
[PubMed]
.
-
64.
Cooper
HM
, Huang
JY
, Verdin
E
and Spelbrink
JN.
A new splice variant of the mouse SIRT3 gene encodes the mitochondrial precursor protein.
PLoS ONE.
2009;
4:
e4986
[PubMed]
.